Homologous Recombination in Thyroid Tumor Samples
Abstract
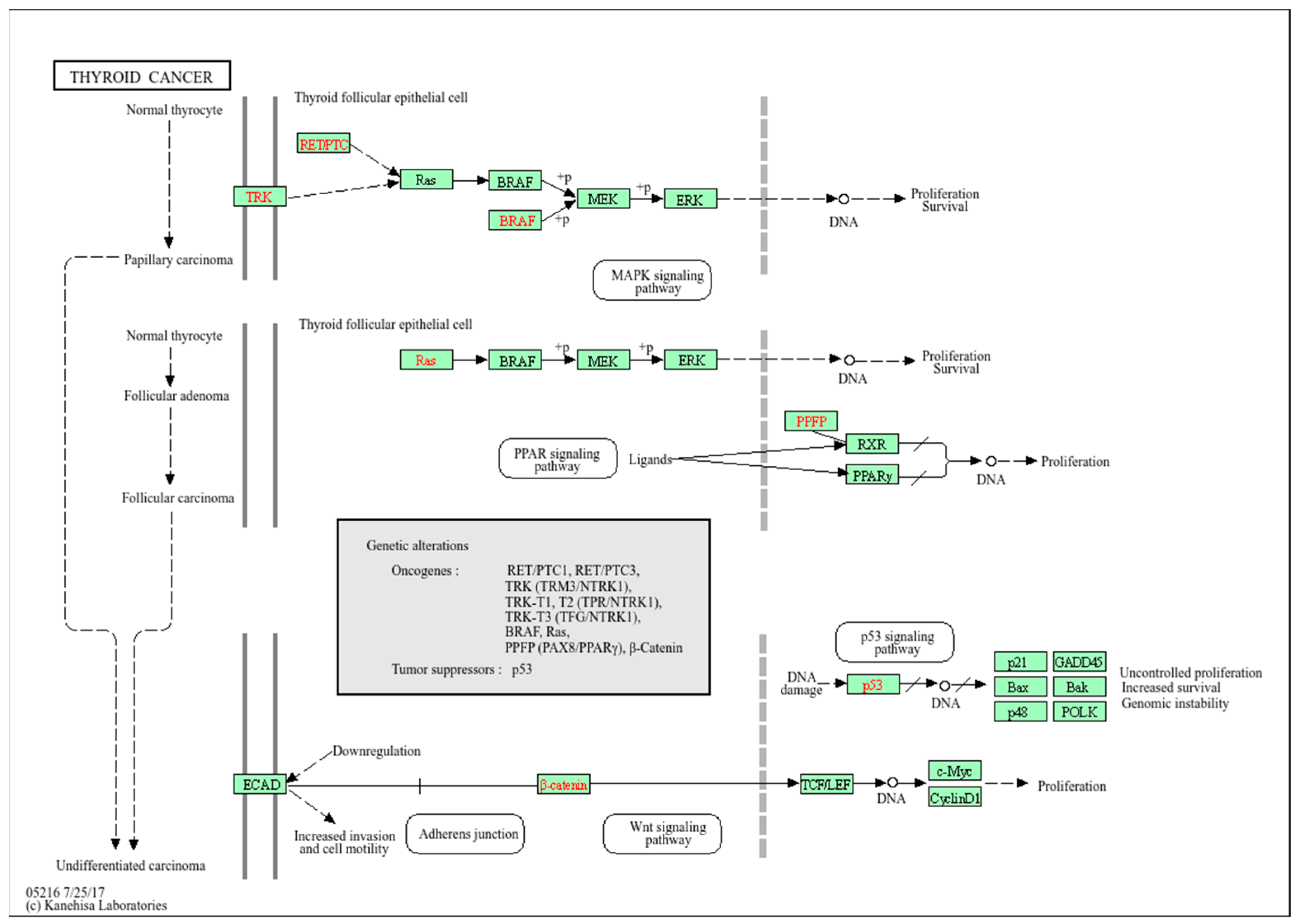
1. Introduction
2. Results
2.1. Bioinformatics Analysis of Genes Associated with PTC
2.2. Protein–Protein Interactions in PTC
2.3. Sequencing of HR Genes
2.4. Limitations of the Study
2.4.1. Small Sample Size
2.4.2. Limited Genetic Analysis
2.4.3. Single-Center Study
2.4.4. Lack of Long-Term Follow-Up
2.4.5. Technical Limitations
2.4.6. Inability to Establish Causality
3. Discussion
4. Materials and Methods
4.1. Bioinformatic Analysis of Proteins
4.2. Experimental Analysis
4.2.1. Material
4.2.2. DNA Extraction
4.2.3. Sequencing of Homologous Recombination Genes
4.2.4. Bioinformatics Analysis in Sequencing
- Reading the alignment to the reference genome was performed using BWA-MEM (v0.7.17) with the following parameters: -t 8-M -R ‘@RG\tID:sample1\tSM:sample1\tPL:ILLUMINA’.
- Filtering and sorting of BAM files were performed using samtools (v1.9) with the following parameters: view -b -q 20 -F 4, sort, index.
- Trimming and filtering of reads were performed by fastp (v0.23.2) with the parameters: -q 20 -u 30—length_required 30—detect_adapter_for_pe -w 8.
- Calling of variants was performed using GATK HaplotypeCaller (v3.3) with the parameters:—min-base-quality-score 20—standard-min-confidence-threshold-for-calling 30—native-pair-hmm-threads 8.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATC | Anaplastic thyroid carcinoma |
| DTC | Differentiated thyroid carcinoma |
| FNMTC | Familial Non Medullary Thyroid Carcinoma |
| HR | Follicular thyroid carcinoma |
| MPS | Massively parallel sequencing |
| PTC | Papillary thyroid cancer |
References
- Song, Y.S.; Park, Y.J. Genomic Characterization of Differentiated Thyroid Carcinoma. Endocrinol. Metab. 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Paz-Ibarra, J.; Concepción-Zavaleta, M.J.; Quiroz-Aldave, J.E. Environmental factors related to the origin and evolution of differentiated thyroid cancer: A narrative review. Expert Rev. Endocrinol. Metab. 2024, 19, 469–477. [Google Scholar] [CrossRef]
- Wójcicka, A.; Czetwertyńska, M.; Świerniak, M.; Długosińska, J.; Maciąg, M.; Czajka, A.; Dymecka, K.; Kubiak, A.; Kot, A.; Płoski, R.; et al. Variants in the ATM-CHEK2-BRCA1 axis determine genetic predisposition and clinical presentation of papillary thyroid carcinoma. Genes Chromosomes Cancer 2014, 53, 516–523. [Google Scholar] [CrossRef]
- Xu, L.; Doan, P.C.; Wei, Q.; Liu, Y.; Li, G.; Sturgis, E.M. Association of BRCA1 functional single nucleotide polymorphisms with risk of differentiated thyroid carcinoma. Thyroid 2012, 22, 35–43. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, L.Y.; Yan, Y.D.; Liao, Y.; Di, Y.S.; Xiao, X.Y. A Multiple Primary Malignancy Patient With FANCA Gene Mutation: A Case Report and Literature Review. Front. Oncol. 2020, 10, 1199. [Google Scholar] [CrossRef]
- Nagano, H.; Matsumoto, H.; Ando, Y.; Nakajo, M.; Yamashita, M. Mutations in Anaplastic Thyroid Carcinoma: An Analysis of the Japanese National Genomic Database. Laryngoscope Investig. Otolaryngol. 2025, 10, e70110. [Google Scholar] [CrossRef] [PubMed]
- Collot, T.; Niogret, J.; Carnet, M.; Chevrier, S.; Humblin, E.; Favier, L.; Bengrine-Lefevre, L.; Desmoulins, I.; Arnould, L.; Boidot, R. PARP inhibitor resistance and TP53 mutations in patients treated with olaparib for BRCA-mutated cancer: Four case reports. Mol. Med. Rep. 2021, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.; Marques, I.J.; Saramago, A.; Moura, M.M.; Pojo, M.; Cabrera, R.; Santos, C.; Rosário, F.; Lousa, D.; Vicente, J.B.; et al. Identification of novel candidate predisposing genes in familial nonmedullary thyroid carcinoma implicating DNA damage repair pathways. Int. J. Cancer 2025, 156, 130–144. [Google Scholar] [CrossRef]
- Borowczyk, M.; Sypniewski, M.; Szyda, J.; Braszka, M.; Ziemnicka, K.; Ruchała, M.; Oszywa, M.; Król, Z.J.; Dobosz, P. Genetic predisposition to differentiated thyroid cancer in the Polish population. Pol. Arch. Intern. Med. 2024, 134, 16654. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Q.; Ni, K.; Zhang, P.; Liu, Y.; Xie, J.; Ji, W.; Cheng, C.; Zhou, Q. Combining single-cell sequencing and spatial transcriptome sequencing to identify exosome-related features of glioblastoma and constructing a prognostic model to identify BARD1 as a potential therapeutic target for GBM patients. Front. Immunol. 2023, 14, 1263329. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Xing, M. BRAF mutation in papillary thyroid. J. Natl. Cancer Inst. 2003, 95, 625–627. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, L.; Wu, J.; Li, H.; Wang, Y.; Liu, B. Prognostic Value of Tumor Multifocality in Pediatric Papillary Thyroid Carcinoma: A Real-Life Multicentric Study. Otolaryngol. Head. Neck Surg. 2023, 169, 1606–1614. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Nikiforov, Y.E. Molecular genetics of thyroid cancer: Implications for diagnosis, treatment and prognosis. Expert Rev. Mol. Diagn. 2008, 8, 83–95. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.Z.; Zhang, Q.; Guan, Y.X.; Chen, Q.J.; Zhu, Q.Y. Meta-Analyses of Association Between BRAF(V600E) Mutation and Clinicopathological Features of Papillary Thyroid Carcinoma. Cell Physiol. Biochem. 2016, 38, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 2007, 14, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Huo, M.; Sun, Y.; Wu, H.; Chen, H.; Wang, Y.; Fu, R. Association between x-ray repair cross-complementing group 3 (XRCC3) genetic polymorphisms and papillary thyroid cancer susceptibility in a Chinese Han population. Tumour Biol. 2016, 37, 979–987. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Junit, S.M.; Ng, K.L.; Jayapalan, J.J.; Karikalan, B.; Hashim, O.H. Papillary Thyroid Cancer: Genetic Alterations and Molecular Biomarker Investigations. Int. J. Med. Sci. 2019, 16, 450–460. [Google Scholar] [CrossRef]
- Spirina, L.V.; Chizhevskaya, S.Y.; Kondakova, I.V. Molecular Profiling of Follicular Variant of Papillary Thyroid Cancer. Bull. Exp. Biol. Med. 2020, 169, 85–88. [Google Scholar] [CrossRef]
- Koen, K.; Robin, P.; Eline, N. CHEK2 mutations and papillary thyroid cancer: Correlation or coincidence? Hered Cancer Clin. Pract. 2022, 20, 5. [Google Scholar] [CrossRef]
- Hawsawi, Y.M.; Shams, A.; Theyab, A.; Abdali, W.A.; Hussien, N.A.; Alatwi, H.E.; Alzahrani, O.R.; Oyouni, A.A.A.; Babalghith, A.O.; Alreshidi, M. BARD1 mystery: Tumor suppressors are cancer susceptibility genes. BMC Cancer 2022, 22, 599. [Google Scholar] [CrossRef] [PubMed]
| Name | Source | Evidence | Confidence |
|---|---|---|---|
| CCDC6 | UniProtKB-RC | CURATED | ★★★★☆ |
| ASCL1 | UniProtKB-RC | CURATED | ★★★★☆ |
| NAA15 | UniProtKB-RC | CURATED | ★★★★☆ |
| ABCA3 | UniProtKB-RC | CURATED | ★★★★☆ |
| PAM | UniProtKB-RC | CURATED | ★★★★☆ |
| MBOAT4 | UniProtKB-RC | CURATED | ★★★★☆ |
| TCIM | UniProtKB-RC | CURATED | ★★★★☆ |
| CALCA | UniProtKB-RC | CURATED | ★★★★☆ |
| CACNA1H | UniProtKB-RC | CURATED | ★★★★☆ |
| GHRL | UniProtKB-RC | CURATED | ★★★★☆ |
| GFRA1 | UniProtKB-RC | CURATED | ★★★★☆ |
| RREB1 | UniProtKB-RC | CURATED | ★★★★☆ |
| SNRPB | UniProtKB-RC | CURATED | ★★★★☆ |
| NAA10 | UniProtKB-RC | CURATED | ★★★★☆ |
| NCOA4 | UniProtKB-RC | CURATED | ★★★★☆ |
| TCIM | UniProtKB-RC | CURATED | ★★★★☆ |
| RET | UniProtKB-RC | CURATED | ★★★★☆ |
| Gene | Mutation Class | Mutation | avsnp150 | Type |
|---|---|---|---|---|
| BRCA1 | Synonymous SNV | c.4308T>A (p.Ser1436) | rs1060915 | Benign |
| BRCA1 | Nonsynonymous SNV | c.3113A>C (p.Glu1038Ala) | rs16941 | Conflicting classifications of pathogenicity |
| BRCA2 | Synonymous SNV | c.4563A>G (p.Leu1521) | rs206075 | Benign/Little Clinical Significance |
| BRCA2 | Nonsynonymous SNV | c.7397= (p.Val2466) | rs169547 | Benign |
| FANCA | Nonsynonymous SNV | c.2426G>A (p.Gly809Asp) | rs7195066 | Benign |
| Gene | Mutation Class | Mutation | avsnp150 | Type |
|---|---|---|---|---|
| ATR | Synonymous SNV | c.7875G>A (p.Gln2625=) | rs1802904 | Benign/Likely_benign |
| ATR | Synonymous SNV | c.5208T>C (p.Tyr1736) | rs2227931 | Benign/Likely_benign |
| BARD1 | Synonymous SNV | c.1518T>C | rs2070093 | Benign |
| BARD1 | Nonsynonymous SNV | c.1519G>T | rs2070094 | Benign/Likely_benign |
| BLM | Synonymous SNV | c.3102G>A (p.Thr1034) | rs2227933 | Benign |
| BLM | Synonymous SNV | c.3531C>A (p.Ala1177) | rs2227934 | Benign |
| BRCA2 | Synonymous SNV | c.4563A>G (p.Leu1521) | rs206075 | Benign |
| BRIP1 | Synonymous SNV | c.3411T>C (p.Tyr1137) | rs4986763 | Benign |
| CHEK1 | Nonsynonymous SNV | c.1411A>G (p.Ile471Val) | rs506504 | Benign |
| FANCE | Synonymous SNV | c.387A>T (p.Pro129=) | rs4713867 | Uncertain significance |
| NBN | Synonymous SNV | c.2016A>C (p.Pro672=) | rs1061302 | Benign |
| RPA1 | Synonymous SNV | c.12A>G | rs5030749 | Benign |
| Code | Gender | Diagnosis | Age | Recurrence | Prognosis | Patients Observation Time |
|---|---|---|---|---|---|---|
| MTEX71 | Female | Diffuse toxic goiter | 41 | No | Alive | 24 |
| BIOQ72 | Female | Papillary thyroid cancer T1N0M0 | 36 | Yes | Alive | 36 |
| DNAR73 | Female | Papillary thyroid cancer T2N0M0 | 74 | Yes | Alive | 36 |
| CELL74 | Female | Nodular colloidal macro-microfollicular goiter, with patches of fibrosis, hyalinosis | 70 | No | Alive | 18 |
| XRAY75 | Female | Macro-microfollicular colloidal goiter with focal regressive changes and focal hyperplasia of the thyroid epithelium. | 66 | No | Alive | 12 |
| GENF76 | Female | Follicular adenomas of the thyroid gland on the background of nodular colloidal goiter | 31 | No | Alive | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spirina, L.V.; Tsyganov, M.M.; Chizhevskaya, S.Y.; Tarasenko, N.V.; Bogdanova, V.A. Homologous Recombination in Thyroid Tumor Samples. Int. J. Mol. Sci. 2025, 26, 9716. https://doi.org/10.3390/ijms26199716
Spirina LV, Tsyganov MM, Chizhevskaya SY, Tarasenko NV, Bogdanova VA. Homologous Recombination in Thyroid Tumor Samples. International Journal of Molecular Sciences. 2025; 26(19):9716. https://doi.org/10.3390/ijms26199716
Chicago/Turabian StyleSpirina, Liudmila V., Matvey M. Tsyganov, Svetlana Yu. Chizhevskaya, Natalia V. Tarasenko, and Veronika A. Bogdanova. 2025. "Homologous Recombination in Thyroid Tumor Samples" International Journal of Molecular Sciences 26, no. 19: 9716. https://doi.org/10.3390/ijms26199716
APA StyleSpirina, L. V., Tsyganov, M. M., Chizhevskaya, S. Y., Tarasenko, N. V., & Bogdanova, V. A. (2025). Homologous Recombination in Thyroid Tumor Samples. International Journal of Molecular Sciences, 26(19), 9716. https://doi.org/10.3390/ijms26199716






