Gene Expression of Nrf2 and KEAP1 in Monocytes of Patients with Chronic Kidney Disease (CKD)
Abstract
1. Introduction
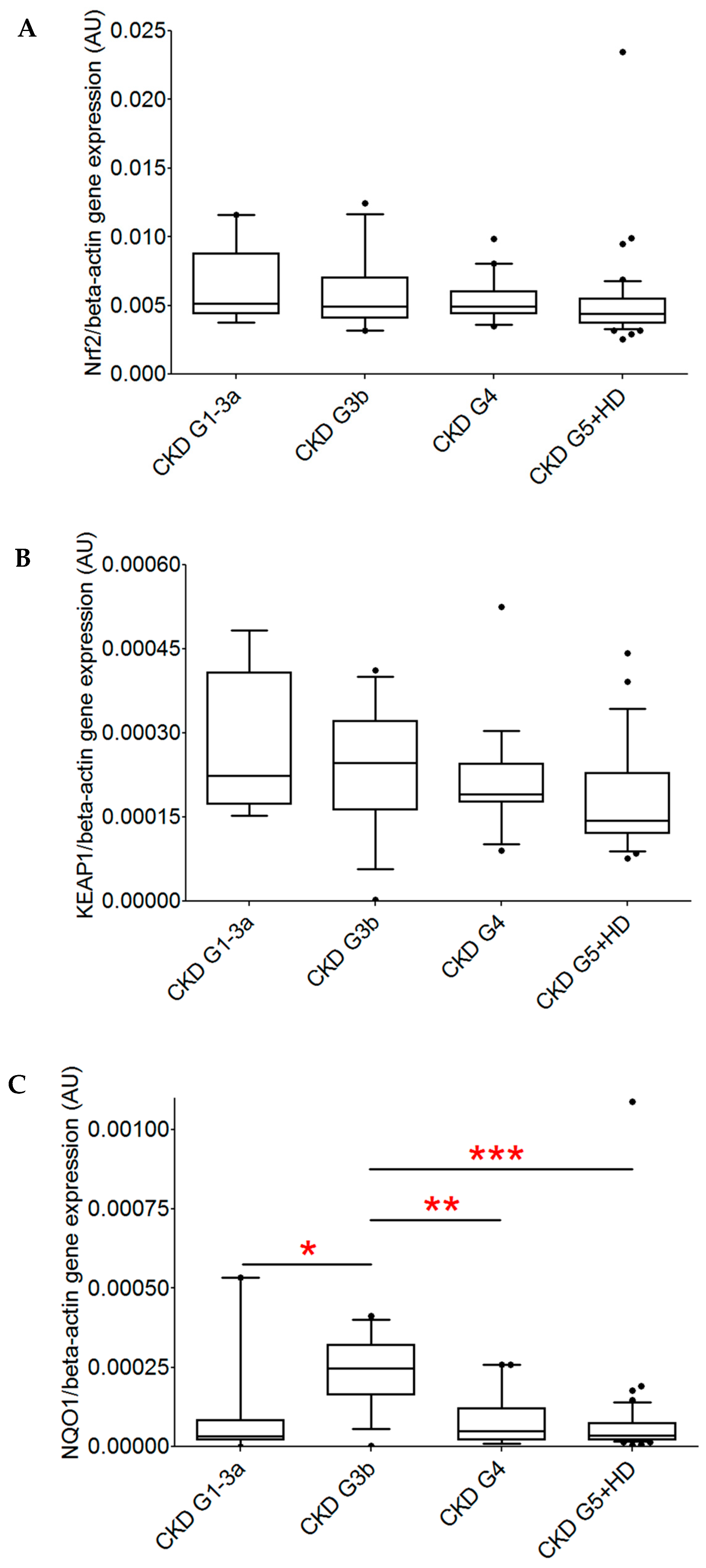
2. Results
3. Discussion
Limitation
4. Materials and Methods
4.1. Study Participants
4.2. Cell Sample Preparation
4.3. RNA Preparation
4.4. cDNA Synthesis and Quantitative Real-Time PCR
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | Beta actin |
| CKD G1–G5 | Chronic kidney disease eGFR stages 1–5 |
| eGFR | Estimated glomerular filtration rate |
| HD | Hemodialysis therapy |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| KRT | Kidney replacement therapy |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| ROS | Reactive oxygen species |
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vallianou, N.G.; Mitesh, S.; Gkogkou, A.; Geladari, E. Chronic Kidney Disease and Cardiovascular Disease: Is there Any Relationship? Curr. Cardiol. Rev. 2019, 15, 55–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tepel, M.; Echelmeyer, M.; Orie, N.N.; Zidek, W. Increased intracellular reactive oxygen species in patients with end-stage renal failure: Effect of hemodialysis. Kidney Int. 2000, 58, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Vondenhoff, S.; Schunk, S.J.; Noels, H. Increased cardiovascular risk in patients with chronic kidney disease. Herz 2024, 49, 95–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ravarotto, V.; Bertoldi, G.; Stefanelli, L.F.; Nalesso, F.; Calò, L.A. Pathomechanism of oxidative stress in cardiovascularrenal remodeling and therapeutic strategies. Kidney Res. Clin. Pr. 2022, 41, 533–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza-Chaverri, J.; Scholze, A. Nrf2 Activation in Chronic Kidney Disease: Promises and Pitfalls. Antioxidants 2022, 11, 1112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Rasmussen, M.; Dong, Q.-R.; Tepel, M.; Scholze, A. Expression of the NRF2 Target Gene NQO1 Is Enhanced in Mononuclear Cells in Human Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2017, 2017, 9091879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juul-Nielsen, C.; Shen, J.; Stenvinkel, P.; Scholze, A. Systematic review of the nuclear factor erythroid 2-related factor 2 (NRF2) system in human chronic kidney disease: Alterations, interventions and relation to morbidity. Nephrol. Dial. Transplant. 2022, 37, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Arefin, S.; Mudrovcic, N.; Hobson, S.; Pietrocola, F.; Ebert, T.; Ward, L.J.; Witasp, A.; Hernandez, L.; Wennberg, L.; Lundgren, T.; et al. Early vascular aging in chronic kidney disease: Focus on microvascular maintenance, senescence signature and potential therapeutics. Transl. Res. 2025, 275, 32–47, Erratum in Transl. Res. 2025, 276, 38. https://doi.org/10.1016/j.trsl.2024.12.003. [Google Scholar] [CrossRef] [PubMed]
- Leal, V.O.; Saldanha, J.F.; Stockler-Pinto, M.B.; Cardozo, L.F.M.F.; Santos, F.R.; Albuquerque, A.S.D.; Jr, M.L.; Mafra, D. NRF2 and NF-κB mRNA expression in chronic kidney disease: A focus on nondialysis patients. Int. Urol. Nephrol. 2015, 47, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gidlund, E.-K.; Witasp, A.; Qureshi, A.R.; Söderberg, M.; Thorell, A.; Nader, G.A.; Barany, P.; Stenvinkel, P.; von Walden, F. Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease. Am. J. Physiol. Physiol. 2019, 317, F1122–F1131. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Hansen, K.H.; Scholze, A. Nrf2 Protein Serum Concentration in Human CKD Shows a Biphasic Behavior. Antioxidants 2023, 12, 932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Y.-L.; Chen, H.; Chen, D.-Q.; Vaziri, N.D.; Su, W.; Ma, S.-X.; Shang, Y.-Q.; Mao, J.-R.; Yu, X.-Y.; Zhang, L.; et al. Activated NF-κB/Nrf2 and Wnt/β-catenin pathways are associated with lipid metabolism in CKD patients with microalbuminuria and macroalbuminuria. Biochim. et Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 2317–2332. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.M. The NRF2 stimulating agent, tin protoporphyrin, activates protective cytokine pathways in healthy human subjects and in patients with chronic kidney disease. Physiol. Rep. 2020, 8, e14566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calabrese, V.; Mancuso, C.; Sapienza, M.; Puleo, E.; Calafato, S.; Cornelius, C.; Finocchiaro, M.; Mangiameli, A.; Di Mauro, M.; Stella, A.M.G.; et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperon- 2007, 12, 299–306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, M.; Mehndiratta, M.; Gupta, S.; Kalra, O.P.; Shukla, R.; Gambhir, J.K. Genetic association of NAD(P)H quinone oxidoreductase (NQO1*2) polymorphism with NQO1 levels and risk of diabetic nephropathy. Biol. Chem. 2016, 397, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Dong, J.; Zhou, Z.; Zhou, H.; Xu, Y.; Zhang, Q.; Chen, C.; Pi, J. The spatiotemporal and paradoxical roles of NRF2 in renal toxicity and kidney diseases. Redox Biol. 2025, 79, 103476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rispoli, R.M.; Popolo, A.; De Fabrizio, V.; Bianca, R.D.d.V.; Autore, G.; Dalli, J.; Marzocco, S. Targeting Inflammatory Imbalance in Chronic Kidney Disease: Focus on Anti-Inflammatory and Resolution Mediators. Int. J. Mol. Sci. 2025, 26, 3072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scutt, G.; Overall, A.; Bakrania, P.; Krasteva, E.; Parekh, N.; Ali, K.; Davies, J.G.; Rajkumar, C. The Association of a Single-Nucleotide Polymorphism in the Nuclear Factor (Erythroid-Derived 2)-Like 2 Gene With Adverse Drug Reactions, Multimorbidity, and Frailty in Older People. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 75, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Atyah, M.; Zhou, C.; Zhou, Q.; Chen, W.; Weng, J.; Wang, P.; Shi, Y.; Dong, Q.; Ren, N. The Age-Specific Features and Clinical Significance of NRF2 and MAPK10 Expression in HCC Patients. Int. J. Gen. Med. 2022, 15, 737–748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro, M.; Alvarenga, L.; Coutinho-Wolino, K.S.; Nakao, L.S.; Cardozo, L.F.; Mafra, D. Sulforaphane upregulates the mRNA expression of NRF2 and NQO1 in non-dialysis patients with chronic kidney disease. Free. Radic. Biol. Med. 2024, 221, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Cardozo, L.F.; Paiva, B.R.; Baptista, B.G.; Fanton, S.; Alvarenga, L.; Lima, L.S.; Britto, I.; Nakao, L.S.; Fouque, D.; et al. Sulforaphane Supplementation Did Not Modulate NRF2 and NF-kB mRNA Expressions in Hemodialysis Patients. J. Ren. Nutr. 2024, 34, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bolati, D.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-κB. BMC Nephrol. 2013, 14, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, P.; Zhong, S.; Wang, G.; Zhang, S.-Y.; Chu, C.; Zeng, S.; Yan, Y.; Cheng, X.; Bao, Y.; Hocher, B.; et al. N-Acetylcysteine Suppresses LPS-Induced Pathological Angiogenesis. Cell. Physiol. Biochem. 2018, 49, 2483–2495. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612, Erratum in Annu. Intern. Med. 2011, 155, 408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| CKD G1–G5 (n = 89) | |
|---|---|
| Age, years | 67 (55–75) |
| Men, n (%) | 53 (60) |
| Kidney disease, underlying cause, n (%) | |
| Glomerulonephritis | 14 (16) |
| Diabetic nephropathy | 6 (7) |
| Hypertensive nephropathy | 6 (7) |
| Other/unknown | 63 (71) |
| eGFR, mL/min/1.73 m2 | 30 (24.3–40.8) * |
| Hemodialysis therapy | 45 (51) |
| Nrf2 Gene Expression | KEAP1 Gene Expression | NQO1 Gene Expression | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age | −0.3 | 0.04 | −0.3 | 0.04 | −0.12 | 0.43 |
| eGFR, mL/min/1.73 m2 | 0.04 | 0.79 | 0.17 | 0.28 | −0.03 | 0.85 |
| Study Target (Gene Name) | Gene Accession Numbers | Forward Primer Reverse Primer |
|---|---|---|
| Nrf2 (NFE2L2) | NM_006164 | 5′-TTCAGCCAGCCCAGCACATC-3′ 5′-CGTAGCCGAAGAAACCTCATTGTC-3′ |
| KEAP1 (Keap1) | NM_203500 | 5′-GGGTCCCCTACAGCCAAG-3′ 5′-TGGGGTTCCAGAAGATAA GC-3′ |
| NQO1 (NQO1) | NM_000903.2 | 5′-CTGCCATCATGCCTGACTAA-3′ 5′-TGCAGATGTACGGTGTGGAT-3′ |
| ACTB (ACTB) | NM_001101.3 | 5′-GGACTTCGAGCAAGAGATGG-3′ 5′-AGCACTGTGTTGGCGTACAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timimi, A.; Nagarajah, S.; Tepel, M.; Scholze, A. Gene Expression of Nrf2 and KEAP1 in Monocytes of Patients with Chronic Kidney Disease (CKD). Int. J. Mol. Sci. 2025, 26, 9693. https://doi.org/10.3390/ijms26199693
Timimi A, Nagarajah S, Tepel M, Scholze A. Gene Expression of Nrf2 and KEAP1 in Monocytes of Patients with Chronic Kidney Disease (CKD). International Journal of Molecular Sciences. 2025; 26(19):9693. https://doi.org/10.3390/ijms26199693
Chicago/Turabian StyleTimimi, Ahmed, Subagini Nagarajah, Martin Tepel, and Alexandra Scholze. 2025. "Gene Expression of Nrf2 and KEAP1 in Monocytes of Patients with Chronic Kidney Disease (CKD)" International Journal of Molecular Sciences 26, no. 19: 9693. https://doi.org/10.3390/ijms26199693
APA StyleTimimi, A., Nagarajah, S., Tepel, M., & Scholze, A. (2025). Gene Expression of Nrf2 and KEAP1 in Monocytes of Patients with Chronic Kidney Disease (CKD). International Journal of Molecular Sciences, 26(19), 9693. https://doi.org/10.3390/ijms26199693






