Conformational Dynamics of the Active Site Loop in Dihydroorotase Highlighting the Limitations of Loop-In Structures for Inhibitor Docking
Abstract
1. Introduction
2. Results
2.1. Identification of 5-FOA and Myricetin as Inhibitiors of Yeast DHOase
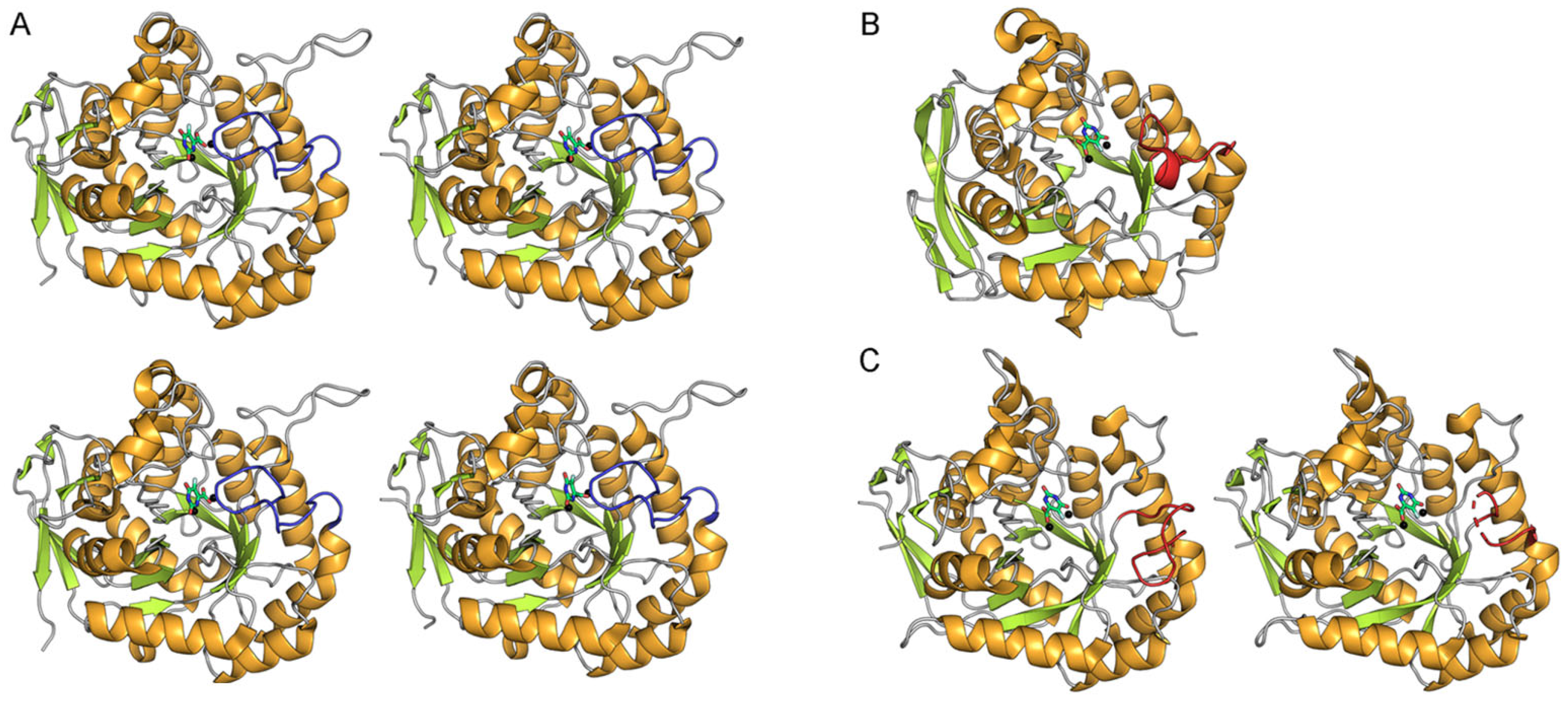
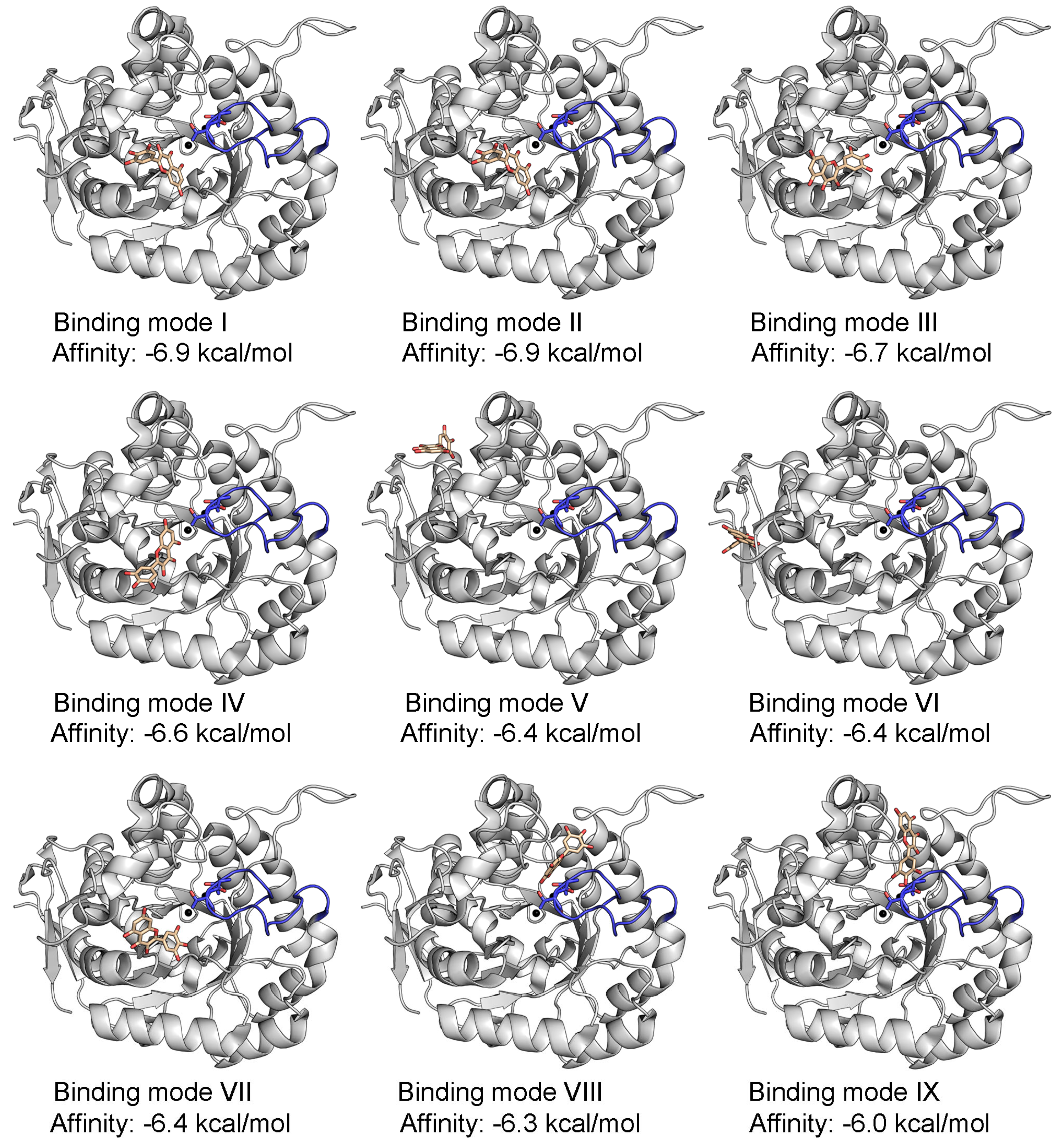
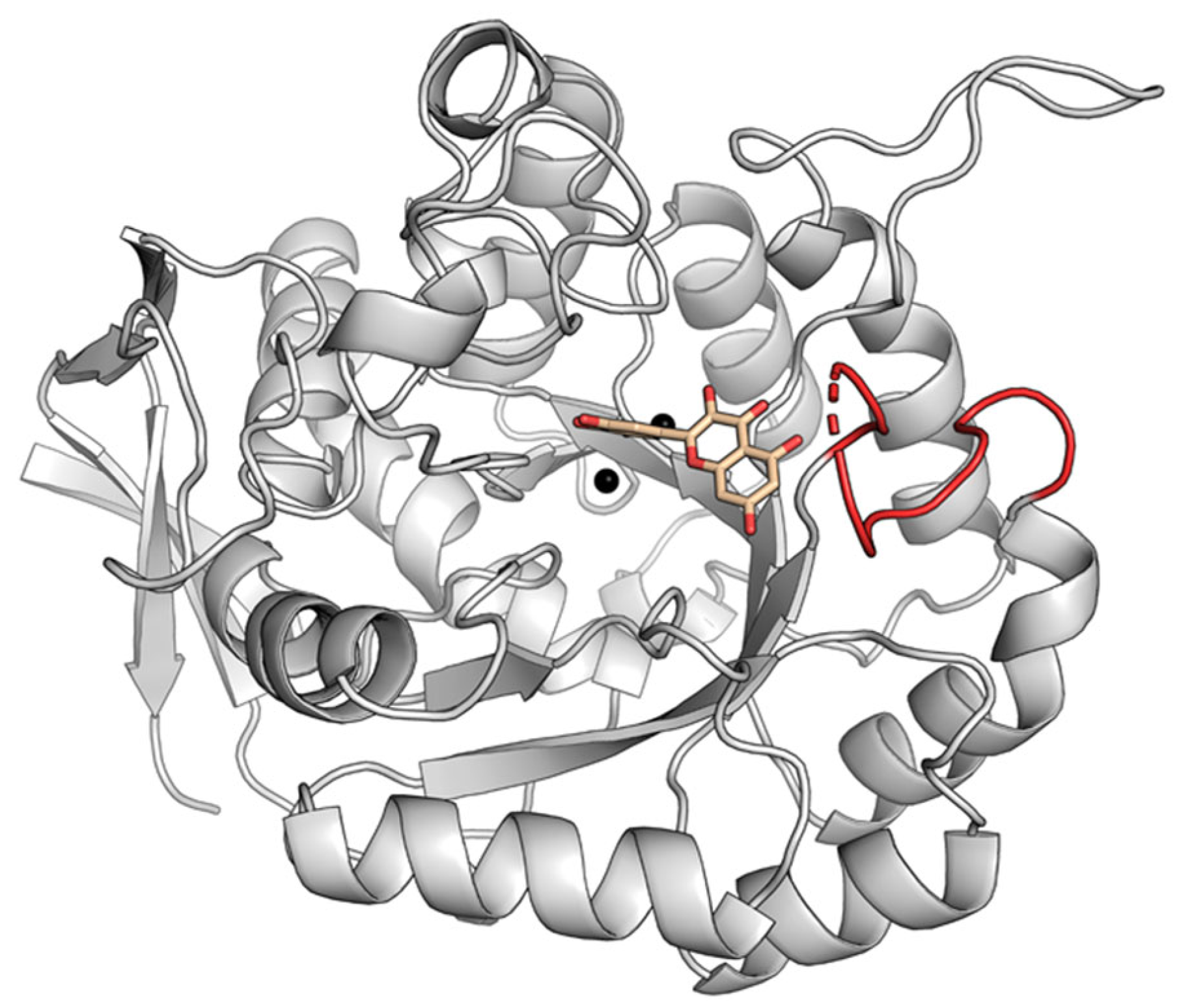
2.2. Dynamic Binding Modes of 5-FOA to DHOase
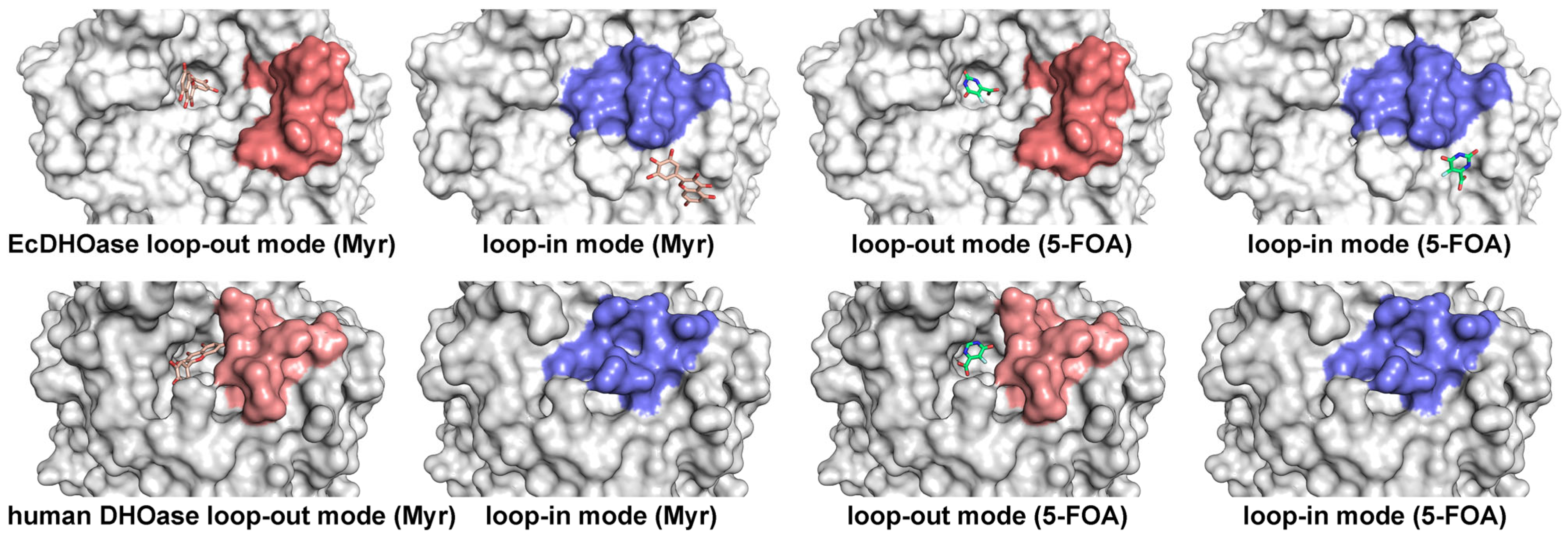
2.3. Docking Analysis of Myricetin and 5-FOA to S. cerevisiae DHOase
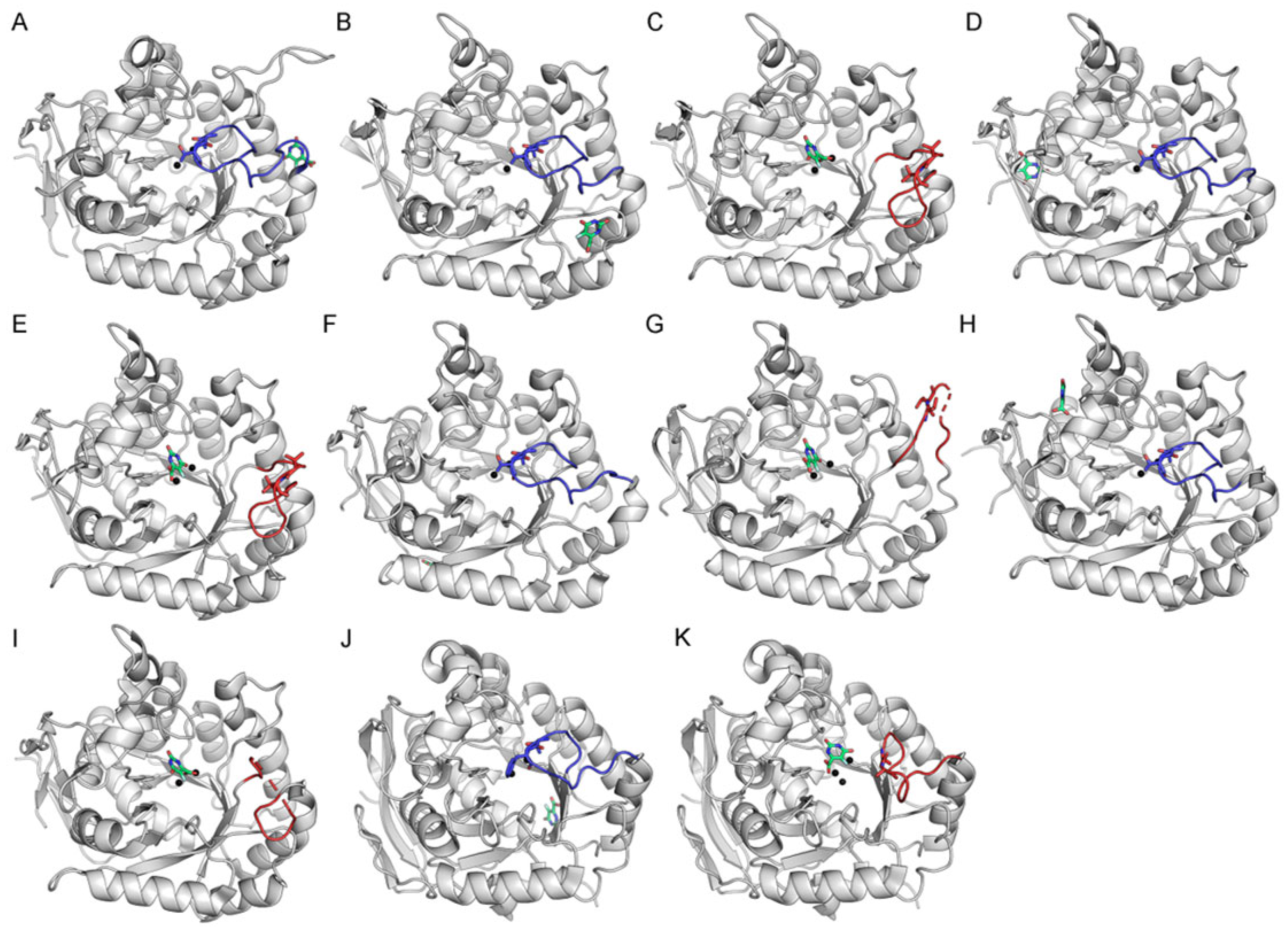
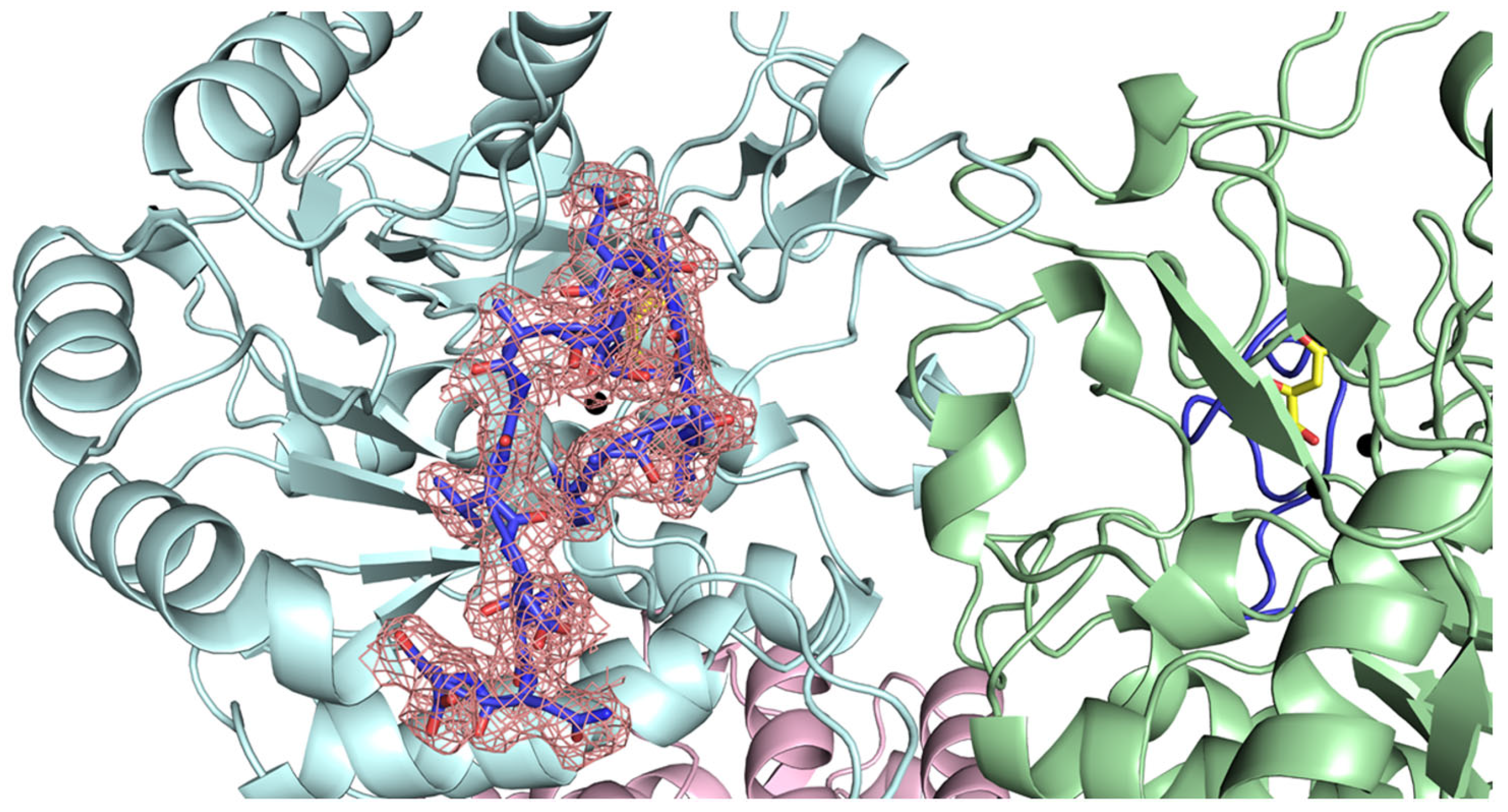
2.4. Docking Analysis of Myricetin and 5-FOA to DHOases Exhibiting Both Loop-In and Loop-Out Conformations
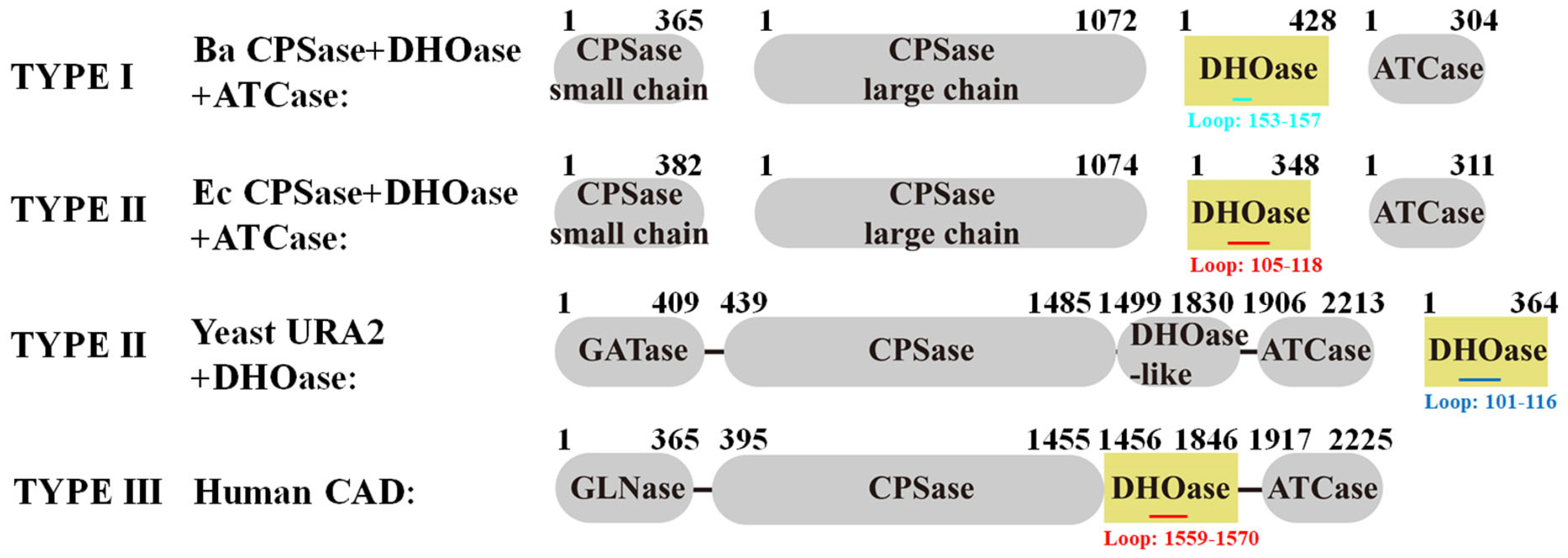
2.5. Comparative Analysis of Active Site Loop Conformations Across DHOase Types Using Crystal Structures from the Protein Data Bank
2.6. Classification of the Loop Conformation States in Type I DHOases
2.7. Species-Dependent Loop Conformation Preferences in DHOase
2.8. Ligand-Bound State Analysis and Its Association with Loop Conformations Across DHOase Types
2.9. pH Conditions Do Not Alter the Active Site Loop Conformation of DHOase
2.10. AlphaFold 3.0 Predictions Reveal Discrepancies Between Predicted and Experimental Loop Conformations in DHOases
3. Discussion
4. Materials and Methods
4.1. Chemicals and Bacterial Strain
4.2. Expression and Purification of the Recombinant Protein
4.3. Enzyme Assay
4.4. Binding Analysis Using AutoDock Vina
4.5. Collection and Analysis of DHOase Structures from PDB for Active Site Loop Conformation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, C.; Zhao, Y.; Wang, L.; Guo, Z.; Ma, L.; Yang, R.; Wu, Y.; Li, X.; Niu, J.; Chu, Q.; et al. De novo pyrimidine biosynthetic complexes support cancer cell proliferation and ferroptosis defence. Nat. Cell Biol. 2023, 25, 836–847. [Google Scholar] [CrossRef]
- del Caño-Ochoa, F.; Ramón-Maiques, S. Deciphering CAD: Structure and function of a mega-enzymatic pyrimidine factory in health and disease. Protein Sci. 2021, 30, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Del Cano-Ochoa, F.; Moreno-Morcillo, M.; Ramon-Maiques, S. CAD, A multienzymatic protein at the head of de novo py-rimidine biosynthesis. Subcell. Biochem. 2019, 93, 505–538. [Google Scholar] [PubMed]
- EJones, M. Pyrimidine Nucleotide Biosynthesis in Animals: Genes, Enzymes, and Regulation of UMP Biosynthesis. Annu. Rev. Biochem. 1980, 49, 253–279. [Google Scholar] [CrossRef]
- AO’DOnovan, G.; Neuhard, J. Pyrimidine metabolism in microorganisms. Microbiol. Mol. Biol. Rev. 1970, 34, 278–343. [Google Scholar] [CrossRef]
- Huang, C.-Y. The Loop-In Binding Mode of Dihydroorotase: Implications for Ligand Binding and Therapeutic Targeting. Int. J. Mol. Sci. 2025, 26, 1359. [Google Scholar] [CrossRef]
- Lin, E.-S.; Huang, C.-Y. Binding Pattern and Structural Interactome of the Anticancer Drug 5-Fluorouracil: A Critical Review. Int. J. Mol. Sci. 2024, 25, 3404. [Google Scholar] [CrossRef]
- Shin, J.; Mir, H.; Khurram, M.A.; Fujihara, K.M.; Dynlacht, B.D.; Cardozo, T.J.; Possemato, R. Allosteric regulation of CAD modulates de novo pyrimidine synthesis during the cell cycle. Nat. Metab. 2023, 5, 277–293. [Google Scholar] [CrossRef]
- Lin, E.-S.; Huang, Y.-H.; Yang, P.-C.; Peng, W.-F.; Huang, C.-Y. Complexed Crystal Structure of the Dihydroorotase Domain of Human CAD Protein with the Anticancer Drug 5-Fluorouracil. Biomolecules 2023, 13, 149. [Google Scholar] [CrossRef]
- del Caño-Ochoa, F.; Ng, B.G.; Rubio-Del-Campo, A.; Mahajan, S.; Wilson, M.P.; Vilar, M.; Rymen, D.; Sánchez-Pintos, P.; Kenny, J.; Martos, M.L.; et al. Beyond genetics: Deciphering the impact of missense variants in CAD deficiency. J. Inherit. Metab. Dis. 2023, 46, 1170–1185. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Huang, Y.-H.; Lin, J.-J.; Huang, C.-Y. Crystal structures of monometallic dihydropyrimidinase and the human dihydroorotase domain K1556A mutant reveal no lysine carbamylation within the active site. Biochem. Biophys. Res. Commun. 2018, 505, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.F.; Huang, C.Y. Allantoinase and dihydroorotase binding and inhibition by flavonols and the substrates of cyclic ami-dohydrolases. Biochimie 2014, 101, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, J.A. Getting CAD in Shape: The Atomic Structure of Human Dihydroorotase Domain. Structure 2014, 22, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Grande-García, A.; Lallous, N.; Díaz-Tejada, C.; Ramón-Maiques, S. Structure, Functional Characterization, and Evolution of the Dihydroorotase Domain of Human CAD. Structure 2014, 22, 185–198. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Rislund, L.; Andersen, B.; Sandrini, M.P.; Cook, P.F.; Schnackerz, K.D.; Piskur, J. Dihydropyrimidine amidohydrolases and dihydroorotases share the same origin and several enzymatic properties. Nucleic Acids Res. 2003, 31, 1683–1692. [Google Scholar] [CrossRef]
- Thoden, J.B.; Phillips, G.N.; Neal, T.M.; Raushel, F.M.; Holden, H.M. Molecular Structure of Dihydroorotase: A Paradigm for Catalysis through the Use of a Binuclear Metal Center. Biochemistry 2001, 40, 6989–6997. [Google Scholar] [CrossRef]
- Simmer, J.P.; EKelly, R.; Rinker, A.G.; Zimmermann, B.H.; Scully, J.L.; Kim, H.; Evans, D.R. Mammalian dihydroorotase: Nucleotide sequence, peptide sequences, and evolution of the dihydroorotase domain of the multifunctional protein CAD. Proc. Natl. Acad. Sci. USA 1990, 87, 174–178. [Google Scholar] [CrossRef]
- Kelly, R.E.; Mally, M.I.; Evans, D.R. The dihydroorotase domain of the multifunctional protein CAD. Subunit structure, zinc content, and kinetics. J. Biol. Chem. 1986, 261, 6073–6083. [Google Scholar] [CrossRef]
- Evans, D.R.; Guy, H.I. Mammalian Pyrimidine Biosynthesis: Fresh Insights into an Ancient Pathway. J. Biol. Chem. 2004, 279, 33035–33038. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.-K.; Mao, F.-F.; Hou, Y.-C.; Zhang, Y.-Q.; Liu, L.-H.; Wei, Q.; Sun, J.-X.; Liu, C.; Shi, J.; et al. Prognostic and Immunotherapeutic Predictive Value of CAD Gene in Hepatocellular Carcinoma: Integrated Bioinformatics and Experimental Analysis. Mol. Biotechnol. 2024, 67, 1240–1255. [Google Scholar] [CrossRef]
- Li, G.; Xiao, K.; Li, Y.; Gao, J.; He, S.; Li, T. CHIP promotes CAD ubiquitination and degradation to suppress the proliferation and colony formation of glioblastoma cells. Cell. Oncol. 2023, 47, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, L.; Wang, T.; Hu, J.; Wang, J.; Tu, R.; Su, H.; Jiang, J.; Qing, G.; Liu, H. Synergistic targeting of CHK1 and mTOR in MYC-driven tumors. Carcinogenesis 2020, 42, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Ridder, D.A.; Schindeldecker, M.; Weinmann, A.; Berndt, K.; Urbansky, L.; Witzel, H.R.; Heinrich, S.; Roth, W.; Straub, B.K. Key Enzymes in Pyrimidine Synthesis, CAD and CPS1, Predict Prognosis in Hepatocellular Carcinoma. Cancers 2021, 13, 744. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Ge, C.; Niu, S.; Duan, Y.; Fan, Y.; Jin, Q.; Chen, X.; Tao, J.; Huang, S. Functional analyses of Toxoplasma gondii dihydroorotase reveal a promising anti-parasitic target. FASEB J. 2023, 38, e23397. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Biological Potential of Carnivorous Plants from Nepenthales. Molecules 2023, 28, 3639. [Google Scholar] [CrossRef]
- Krungkrai, S.R.; Wutipraditkul, N.; Krungkrai, J. Dihydroorotase of human malarial parasite Plasmodium falciparum differs from host enzyme. Biochem. Biophys. Res. Commun. 2008, 366, 821–826. [Google Scholar] [CrossRef]
- Seymour, K.K.; Lyons, S.D.; Phillips, L.; Rieckmann, K.H.; Christopherson, R.I. Cytotoxic Effects of Inhibitors of de Novo Pyrimidine Biosynthesis upon Plasmodium falciparum. Biochemistry 1994, 33, 5268–5274. [Google Scholar] [CrossRef]
- Krungkrai, J.; Krungkrai, S.R.; Phakanont, K. Antimalarial activity of orotate analogs that inhibit dihydroorotase and dihy-droorotate dehydrogenase. Biochem. Pharmacol 1992, 43, 1295–1301. [Google Scholar] [CrossRef]
- Rathod, P.K.; Khatri, A.; Hubbert, T.; Milhous, W.K. Selective activity of 5-fluoroorotic acid against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 1989, 33, 1090–1094. [Google Scholar] [CrossRef]
- Wang, C.-C.; Tsau, H.-W.; Chen, W.-T.; Huang, C.-Y. Identification and Characterization of a Putative Dihydroorotase, KPN01074, from Klebsiella pneumoniae. Protein J. 2010, 29, 445–452. [Google Scholar] [CrossRef]
- Porter, T.N.; Li, Y.; Raushel, F.M. Mechanism of the dihydroorotase reaction. Biochemistry 2004, 43, 16285–16292. [Google Scholar] [CrossRef]
- Washabaugh, M.W.; Collins, K.D. Dihydroorotase from Escherichia coli. Purification and characterization. J. Biol. Chem. 1984, 259, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Yates, R.A.; Pardee, A.B. Pyrimidine biosynthesis in Escherichia coli. J. Biol. Chem. 1956, 221, 743–756. [Google Scholar] [CrossRef]
- del Caño-Ochoa, F.; Ramón-Maiques, S. The multienzymatic protein CAD leading the de novo biosynthesis of pyrimidines localizes exclusively in the cytoplasm and does not translocate to the nucleus. Nucleosides Nucleotides Nucleic Acids 2020, 39, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- del Caño-Ochoa, F.; Ng, B.G.; Abedalthagafi, M.; Almannai, M.; Cohn, R.D.; Costain, G.; Elpeleg, O.; Houlden, H.; Karimiani, E.G.; Liu, P.; et al. Cell-based analysis of CAD variants identifies individuals likely to benefit from uridine therapy. Anesthesia Analg. 2020, 22, 1598–1605. [Google Scholar] [CrossRef]
- Hervé, G. Structural Insight into the Core of CAD. Structure 2017, 25, 819–820. [Google Scholar] [CrossRef]
- Carrey, E.A. Phosphorylation, allosteric effectors and inter-domain contacts in CAD; their role in regulation of early steps of pyrimidine biosynthesis. Biochem. Soc. Trans. 1993, 21, 191–195. [Google Scholar] [CrossRef]
- Lee, L.; EKelly, R.; Pastra-Landis, S.C.; Evans, D.R. Oligomeric structure of the multifunctional protein CAD that initiates pyrimidine biosynthesis in mammalian cells. Proc. Natl. Acad. Sci. USA 1985, 82, 6802–6806. [Google Scholar] [CrossRef]
- Guan, H.-H.; Huang, Y.-H.; Lin, E.-S.; Chen, C.-J.; Huang, C.-Y. Structural Analysis of Saccharomyces cerevisiae Dihydroorotase Reveals Molecular Insights into the Tetramerization Mechanism. Molecules 2021, 26, 7249. [Google Scholar] [CrossRef]
- Guan, H.-H.; Huang, Y.-H.; Lin, E.-S.; Chen, C.-J.; Huang, C.-Y. Complexed Crystal Structure of Saccharomyces cerevisiae Dihydroorotase with Inhibitor 5-Fluoroorotate Reveals a New Binding Mode. Bioinorg. Chem. Appl. 2021, 2021, 2572844. [Google Scholar] [CrossRef]
- Guan, H.-H.; Huang, Y.-H.; Lin, E.-S.; Chen, C.-J.; Huang, C.-Y. Plumbagin, a Natural Product with Potent Anticancer Activities, Binds to and Inhibits Dihydroorotase, a Key Enzyme in Pyrimidine Biosynthesis. Int. J. Mol. Sci. 2021, 22, 6861. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.-H.; Huang, Y.-H.; Lin, E.-S.; Chen, C.-J.; Huang, C.-Y. Structural basis for the interaction modes of dihydroorotase with the anticancer drugs 5-fluorouracil and 5-aminouracil. Biochem. Biophys. Res. Commun. 2021, 551, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Serre, V.; Guy, H.; Penverne, B.; Lux, M.; Rotgeri, A.; Evans, D.; Hervé, G. Half of Saccharomyces cerevisiae Carbamoyl Phosphate Synthetase Produces and Channels Carbamoyl Phosphate to the Fused Aspartate Transcarbamoylase Domain. J. Biol. Chem. 1999, 274, 23794–23801. [Google Scholar] [CrossRef] [PubMed]
- Serre, V.; Guy, H.; Liu, X.; Penverne, B.; Hervé, G.; Evans, D. Allosteric regulation and substrate channeling in multifunctional pyrimidine biosynthetic complexes: Analysis of isolated domains and yeast-mammalian chimeric proteins. J. Mol. Biol. 1998, 281, 363–377. [Google Scholar] [CrossRef]
- Souciet, J.; Nagy, M.; Le Gouar, M.; Lacroute, F.; Potier, S. Organization of the yeast URA2 gene: Identification of a defective dihydroorotase-like domain in the multifunctional carbamoylphosphate synthetase-aspartate transcarbamylase complex. Gene 1989, 79, 59–70. [Google Scholar] [CrossRef]
- Guyonvarch, A.; Nguyen-Juilleret, M.; Hubert, J.-C.; Lacroute, F. Structure of the Saccharomyces cerevisiae URA4 gene encoding dihydroorotase. Mol. Genet. Genom. 1988, 212, 134–141. [Google Scholar] [CrossRef]
- Holm, L.; Sander, C. An evolutionary treasure: Unification of a broad set of amidohydrolases related to urease. Proteins 1997, 28, 72–82. [Google Scholar] [CrossRef]
- Lee, M.; Maher, M.J.; Christopherson, R.I.; Guss, J.M. Kinetic and Structural Analysis of Mutant Escherichia coli Dihydroorotases: A Flexible Loop Stabilizes the Transition State. Biochemistry 2007, 46, 10538–10550. [Google Scholar] [CrossRef]
- Lee, M.; Chan, C.W.; Graham, S.C.; Christopherson, R.I.; Guss, J.M.; Maher, M.J. Structures of Ligand-free and Inhibitor Complexes of Dihydroorotase from Escherichia coli: Implications for Loop Movement in Inhibitor Design. J. Mol. Biol. 2007, 370, 812–825. [Google Scholar] [CrossRef]
- Lipowska, J.; Miks, C.D.; Kwon, K.; Shuvalova, L.; Zheng, H.; Lewiński, K.; Cooper, D.R.; Shabalin, I.G.; Minor, W. Pyrimidine biosynthesis in pathogens–Structures and analysis of dihydroorotases from Yersinia pestis and Vibrio cholerae. Int. J. Biol. Macromol. 2019, 136, 1176–1187. [Google Scholar] [CrossRef]
- Nussinov, R. Pioneer in Molecular Biology: Conformational Ensembles in Molecular Recognition, Allostery, and Cell Function. J. Mol. Biol. 2025, 437, 169044. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.; Arakelov, G.; Dokholyan, N.V. The evolving landscape of protein allostery: From computational and experi-mental perspectives. J. Mol. Biol. 2025, 438, 169060. [Google Scholar]
- Fenwick, R.B.; Esteban-Martín, S.; Salvatella, X. Understanding biomolecular motion, recognition, and allostery by use of conformational ensembles. Eur. Biophys. J. 2011, 40, 1339–1355. [Google Scholar] [CrossRef] [PubMed]
- Bohnuud, T.; Kozakov, D.; Vajda, S.; Briggs, J.M. Evidence of Conformational Selection Driving the Formation of Ligand Binding Sites in Protein-Protein Interfaces. PLOS Comput. Biol. 2014, 10, e1003872. [Google Scholar] [CrossRef]
- Hatzakis, N.S. Single molecule insights on conformational selection and induced fit mechanism. Biophys. Chem. 2014, 186, 46–54. [Google Scholar] [CrossRef]
- Vogt, A.D.; Di Cera, E. Conformational selection is a dominant mechanism of ligand binding. Biochemistry 2013, 52, 5723–5729. [Google Scholar] [CrossRef]
- Shukla, D.; Hernández, C.X.; Weber, J.K.; Pande, V.S. Markov State Models Provide Insights into Dynamic Modulation of Protein Function. Acc. Chem. Res. 2015, 48, 414–422. [Google Scholar] [CrossRef]
- Paul, F.; Weikl, T.R.; Dokholyan, N.V. How to Distinguish Conformational Selection and Induced Fit Based on Chemical Relaxation Rates. PLOS Comput. Biol. 2016, 12, e1005067. [Google Scholar] [CrossRef]
- Vogt, A.D.; Pozzi, N.; Chen, Z.; Di Cera, E. Essential role of conformational selection in ligand binding. Biophys. Chem. 2014, 186, 13–21. [Google Scholar] [CrossRef]
- Redhair, M.; Atkins, W.M. Analytical and functional aspects of protein-ligand interactions: Beyond induced fit and conformational selection. Arch. Biochem. Biophys. 2021, 714, 109064. [Google Scholar] [CrossRef]
- Michel, D. Conformational selection or induced fit? New insights from old principles. Biochimie 2016, 128–129, 48–54. [Google Scholar] [CrossRef]
- Liu, X.; Speckhard, D.C.; Shepherd, T.R.; Sun, Y.J.; Hengel, S.R.; Yu, L.; Fowler, C.A.; Gakhar, L.; Fuentes, E.J. Distinct Roles for Conformational Dynamics in Protein-Ligand Interactions. Structure 2016, 24, 2053–2066. [Google Scholar] [CrossRef]
- Wankowicz, S.A.; de Oliveira, S.H.; Hogan, D.W.; Bedem, H.V.D.; Fraser, J.S. Ligand binding remodels protein side-chain conformational heterogeneity. eLife 2022, 11, e74114. [Google Scholar] [CrossRef]
- Rice, A.J.; Lei, H.; Santarsiero, B.D.; Lee, H.; Johnson, M.E. Ca-asp bound X-ray structure and inhibition of Bacillus anthracis dihydroorotase (DHOase). Bioorg. Med. Chem. 2016, 24, 4536–4543. [Google Scholar] [CrossRef]
- Rice, A.J.; Truong, L.; Johnson, M.E.; Lee, H. A colorimetric assay optimization for high-throughput screening of dihydroorotase by detecting ureido groups. Anal. Biochem. 2013, 441, 87–94. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chiang, W.-Y.; Chen, P.-J.; Lin, E.-S.; Huang, C.-Y. Anticancer and Antioxidant Activities of the Root Extract of the Carnivorous Pitcher Plant Sarracenia purpurea. Plants 2022, 11, 1668. [Google Scholar] [CrossRef]
- Tiwari, K.; Kumar, R.; Dubey, V.K. Biochemical characterization of dihydroorotase of Leishmania donovani: Understanding pyrimidine metabolism through its inhibition. Biochimie 2016, 131, 45–53. [Google Scholar] [CrossRef]
- Garavito, M.F.; Narváez-Ortiz, H.Y.; Zimmermann, B.H. Pyrimidine Metabolism: Dynamic and Versatile Pathways in Pathogens and Cellular Development. J. Genet. Genom. 2015, 42, 195–205. [Google Scholar] [CrossRef]
- Vitali, J.; Nix, J.C.; Newman, H.E.; Colaneri, M.J. Crystal structure of Methanococcus jannaschii dihydroorotase. Proteins Struct. Funct. Bioinform. 2022, 91, 91–98. [Google Scholar] [CrossRef]
- Evans, H.G.; Fernando, R.; Vaishnav, A.; Kotichukkala, M.; Heyl, D.; Hachem, F.; Brunzelle, J.S.; Edwards, B.F.; Evans, D.R. Inter-subunit communication in the dihydroorotase-aspartate transcarbamoylase complex of Aquifex aeolicus. Protein Sci. 2014, 23, 100–109. [Google Scholar] [CrossRef]
- Edwards, B.F.; Fernando, R.; Martin, P.D.; Grimley, E.; Cordes, M.; Vaishnav, A.; Brunzelle, J.S.; Evans, H.G.; Evans, D.R. The mono-nuclear metal center of type-I dihydroorotase from Aquifex aeolicus. BMC Biochem. 2013, 14, 36. [Google Scholar] [CrossRef]
- Zhang, P.; Martin, P.D.; Purcarea, C.; Vaishnav, A.; Brunzelle, J.S.; Fernando, R.; Guy-Evans, H.I.; Evans, D.R.; Edwards, B.F. Dihy-droorotase from the hyperthermophile Aquifex aeolicus is activated by stoichiometric association with aspartate transcar-bamoylase and forms a one-pot reactor for pyrimidine biosynthesis. Biochemistry 2009, 48, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.D.; Purcarea, C.; Zhang, P.; Vaishnav, A.; Sadecki, S.; Guy-Evans, H.I.; Evans, D.R.; Edwards, B.F. The Crystal Structure of a Novel, Latent Dihydroorotase from Aquifex aeolicus at 1.7 Å Resolution. J. Mol. Biol. 2005, 348, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Purcarea, C.; Ebert, R.; Sadecki, S.; Guy, H.I.; Evans, D.R. Aquifex aeolicus dihydroorotase: Association with aspartate transcarbamoylase switches on catalytic activity. J. Biol. Chem. 2004, 279, 53136–53144. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Maher, M.J.; Guss, J.M. Structure of the T109S mutant of Escherichia coli dihydroorotase complexed with the inhibitor 5-fluoroorotate: Catalytic activity is reflected by the crystal form. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2007, 63, 154–161. [Google Scholar] [CrossRef]
- Del Caño-Ochoa, F.; Ramadane-Morchadi, L.; Eixerés, L.; Moreno-Morcillo, M.; Fernández-Leiro, R.; Ramón-Maiques, S. Dis-ruption of CAD oligomerization by pathogenic variants. J. Mol. Biol. 2024, 436, 168832. [Google Scholar] [CrossRef]
- del Caño-Ochoa, F.; Grande-García, A.; Reverte-López, M.; D’aBramo, M.; Ramón-Maiques, S. Characterization of the catalytic flexible loop in the dihydroorotase domain of the human multi-enzymatic protein CAD. J. Biol. Chem. 2018, 293, 18903–18913. [Google Scholar] [CrossRef]
- Ornelas, A.; Korczynska, M.; Ragumani, S.; Kumaran, D.; Narindoshvili, T.; Shoichet, B.K.; Swaminathan, S.; Raushel, F.M. Func-tional annotation and three-dimensional structure of an incorrectly annotated dihydroorotase from cog3964 in the amidohydrolase superfamily. Biochemistry 2013, 52, 228–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Skolnick, J. TM-Align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef]
- Steinkellner, G.; Kroutil, W.; Gruber, K.; Gruber, C.C. AlphaFold 3 is great—But it still needs human help to get chemistry right. Nature 2025, 637, 548. [Google Scholar] [CrossRef]
- Brotzakis, Z.F.; Zhang, S.; Murtada, M.H.; Vendruscolo, M. AlphaFold prediction of structural ensembles of disordered proteins. Nat. Commun. 2025, 16, 1632. [Google Scholar] [CrossRef]
- O’lEary, K. AlphaFold gets an upgrade (and a Nobel). Nat. Med. 2024, 30, 3393. [Google Scholar] [CrossRef] [PubMed]
- McMaster, B.; Thorpe, C.; Ogg, G.; Deane, C.M.; Koohy, H. Can AlphaFold’s breakthrough in protein structure help decode the fundamental principles of adaptive cellular immunity? Nat. Methods 2024, 21, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Luganini, A.; Boschi, D.; Lolli, M.L.; Gribaudo, G. DHODH inhibitors: What will it take to get them into the clinic as antivirals? Antivir. Res. 2025, 236, 106099. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, J.; Zhou, L.; Yi, R.; Chen, Y.; He, S. Targeting vulnerability in tumor therapy: Dihydroorotate dehydrogenase. Life Sci. 2025, 371, 123612. [Google Scholar] [CrossRef]
- Sharma, M.; Pandey, V.; Poli, G.; Tuccinardi, T.; Lolli, M.L.; Vyas, V.K. A comprehensive review of synthetic strategies and SAR studies for the discovery of PfDHODH inhibitors as antimalarial agents. Part 1: Triazolopyrimidine, isoxazolopyrimidine and pyrrole-based (DSM) compounds. Bioorg. Chem. 2024, 146, 107249. [Google Scholar] [CrossRef]
- Gehlot, P.; Vyas, V.K. A Patent Review of Human Dihydroorotate Dehydrogenase (hDHODH) Inhibitors as Anticancer Agents and their Other Therapeutic Applications (1999–2022). Recent Patents Anti-Cancer Drug Discov. 2024, 19, 280–297. [Google Scholar] [CrossRef]
- Backer, N.; Kumar, A.; Singh, A.K.; Singh, H.; Narasimhan, B.; Kumar, P. Medicinal chemistry aspects of uracil containing dUTPase inhibitors targeting colorectal cancer. Drug Discov. Today 2023, 29, 103853. [Google Scholar] [CrossRef]
- Gehlot, P.; Vyas, V.K. Recent advances on patents of Plasmodium falciparum dihydroorotate dehydrogenase (Pf DHODH) inhibitors as antimalarial agents. Expert Opin. Ther. Patents 2023, 33, 579–596. [Google Scholar] [CrossRef]
- Verrier, E.R.; Weiss, A.; Bach, C.; Heydmann, L.; Turon-Lagot, V.; Kopp, A.; El Saghire, H.; Crouchet, E.; Pessaux, P.; Garcia, T.; et al. Combined small molecule and loss-of-function screen uncovers estrogen receptor alpha and CAD as host factors for HDV infection and antiviral targets. Gut 2019, 69, 158–167. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Wu, Q.; Kim, L.J.Y.; Morton, A.R.; Gimple, R.C.; Prager, B.C.; Shi, Y.; Zhou, W.; Bhargava, S.; et al. Targeting py-rimidine synthesis accentuates molecular therapy response in glioblastoma stem cells. Sci. Transl. Med. 2019, 11, eaau4972. [Google Scholar] [CrossRef] [PubMed]
- Madak, J.T.; Bankhead, A., 3rd; Cuthbertson, C.R.; Showalter, H.D.; Neamati, N. Revisiting the role of dihydroorotate dehydro-genase as a therapeutic target for cancer. Pharmacol. Ther. 2019, 195, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Boschi, D.; Pippione, A.C.; Sainas, S.; Lolli, M.L. Dihydroorotate dehydrogenase inhibitors in anti-infective drug research. Eur. J. Med. Chem. 2019, 183, 111681. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.; Lee, H.; Ghassemi, M.; Chen, J.; Cook, J.L.; Mankin, A.S.; Neyfakh, A.A.; Galán, J.E. Nucleotide Biosynthesis Is Critical for Growth of Bacteria in Human Blood. PLOS Pathog. 2008, 4, e37. [Google Scholar] [CrossRef]
- Shang, W.; Li, X.-H.; Zeng, L.-H.; Li, Z.; Hu, Y.; Wen, H.-M.; Cao, F.-J.; Wan, G.-X. Mechanistic Insights into Flavonoid Subclasses as Cardioprotective Agents Against Doxorubicin-Induced Cardiotoxicity: A Comprehensive Review. Drug Des. Dev. Ther. 2025, 19, 5553–5596. [Google Scholar] [CrossRef]
- Scarlata, G.G.M.; Lopez, I.; Gambardella, M.L.; Milanović, M.; Milić, N.; Abenavoli, L. Preventive and Therapeutic Effects of Baicalein, Galangin, and Isorhamnetin in Chronic Liver Diseases: A Narrative Review. Molecules 2025, 30, 1253. [Google Scholar] [CrossRef]
- Pan, C.; Yang, Y.; Zhao, Z.; Hu, J. Combined effects of natural products and exercise on apoptosis pathways in obesity-related skeletal muscle dysfunction. Apoptosis 2025, 30, 537–552. [Google Scholar] [CrossRef]
- Warias, P.; Plewa, P.; Poniewierska-Baran, A. Resveratrol, Piceatannol, Curcumin, and Quercetin as Therapeutic Targets in Gastric Cancer—Mechanisms and Clinical Implications for Natural Products. Molecules 2024, 30, 3. [Google Scholar] [CrossRef]
- Zou, B.; Long, Y.; Gao, R.; Liu, Q.; Tian, X.; Liu, B.; Zhou, Q. Nanodelivery system of traditional Chinese medicine bioactive compounds: Application in the treatment of prostate cancer. Phytomedicine 2024, 135, 155554. [Google Scholar] [CrossRef]
- Pathak, R.; Chandra, P.; Sachan, N. Unveiling the Health Potential of Myricetin: Bio-accessibility, Safety Considerations, and Therapeutic Mechanisms. Curr. Pharm. Des. 2025. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Rahmani, A.H. Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis. Int. J. Mol. Sci. 2025, 26, 4188. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sikarwar, O.; Verma, A.; Solanki, K.; Agrawal, N.; Dubey, N.; Yadav, H.N. Unveiling myricetin’s pharmaco-logical potency: A comprehensive exploration of the molecular pathways with special focus on PI3K/AKT and Nrf2 signaling. J. Biochem. Mol. Toxicol. 2024, 38, e23739. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A. Myricetin: A Significant Emphasis on Its Anticancer Potential via the Modulation of Inflammation and Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 9665. [Google Scholar] [CrossRef]
- Sharma, P.; Khan, M.A.; Najmi, A.K.; Chaturvedi, S.; Akhtar, M. Myricetin-induced apoptosis in triple-negative breast cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Med. Oncol. 2022, 39, 248. [Google Scholar] [CrossRef]
- Kloskowski, P.; Neumann, P.; Kumar, P.; Berndt, A.; Dobbelstein, M.; Ficner, R. Myricetin-bound crystal structure of the SARS-CoV-2 helicase NSP13 facilitates the discovery of novel natural inhibitors. Acta Crystallogr. Sect. D Struct. Biol. 2025, 81, 310–326. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, Y.; Ding, Y.; Cui, Y.; Li, W.; Liu, T. Structure and Inhibition of Insect UDP-N-acetylglucosamine Pyrophosphorylase: A Key Enzyme in the Hexosamine Biosynthesis Pathway. J. Agric. Food Chem. 2024, 72, 19286–19294. [Google Scholar] [CrossRef]
- Griep, M.A.; Blood, S.; Larson, M.A.; Koepsell, S.A.; Hinrichs, S.H. Myricetin inhibits Escherichia coli DnaB helicase but not primase. Bioorg. Med. Chem. 2007, 15, 7203–7208. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Huang, C.-C.; Chen, C.-C.; Yang, K.-J.; Huang, C.-Y. Inhibition of Staphylococcus aureus PriA Helicase by Flavonol Kaempferol. Protein J. 2015, 34, 169–172. [Google Scholar] [CrossRef]
- Huang, C.-Y. Inhibition of a Putative Dihydropyrimidinase from Pseudomonas aeruginosa PAO1 by Flavonoids and Substrates of Cyclic Amidohydrolases. PLoS ONE 2015, 10, e0127634. [Google Scholar] [CrossRef]
- Lin, E.-S.; Luo, R.-H.; Huang, C.-Y. A Complexed Crystal Structure of a Single-Stranded DNA-Binding Protein with Quercetin and the Structural Basis of Flavonol Inhibition Specificity. Int. J. Mol. Sci. 2022, 23, 588. [Google Scholar] [CrossRef]
- Lin, E.-S.; Huang, Y.-H.; Luo, R.-H.; Basharat, Z.; Huang, C.-Y. Crystal Structure of an SSB Protein from Salmonella enterica and Its Inhibition by Flavanonol Taxifolin. Int. J. Mol. Sci. 2022, 23, 4399. [Google Scholar] [CrossRef]
- Huang, C.-Y. Crystal structure of SSB complexed with inhibitor myricetin. Biochem. Biophys. Res. Commun. 2018, 504, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Kuzikov, M.; Costanzi, E.; Reinshagen, J.; Esposito, F.; Vangeel, L.; Wolf, M.; Ellinger, B.; Claussen, C.; Geisslinger, G.; Corona, A.; et al. Identification of Inhibitors of SARS-CoV-2 3CL-Pro Enzymatic Activity Using a Small Molecule in Vitro Repurposing Screen. ACS Pharmacol. Transl. Sci. 2021, 4, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Huang, C.-Y. The complexed crystal structure of dihydropyrimidinase reveals a potential interactive link with the neurotransmitter γ-aminobutyric acid (GABA). Biochem. Biophys. Res. Commun. 2023, 692, 149351. [Google Scholar] [CrossRef]
- Lin, E.-S.; Luo, R.-H.; Yang, Y.-C.; Huang, C.-Y. Molecular Insights into How the Dimetal Center in Dihydropyrimidinase Can Bind the Thymine Antagonist 5-Aminouracil: A Different Binding Mode from the Anticancer Drug 5-Fluorouracil. Bioinorg. Chem. Appl. 2022, 2022, 1817745. [Google Scholar] [CrossRef]
- Huang, C.Y. Structure, catalytic mechanism, posttranslational lysine carbamylation, and inhibition of dihydropyrimidi-nases. Adv. Protein Chem. Struct. Biol. 2020, 122, 63–96. [Google Scholar]
- Basbous, J.; Aze, A.; Chaloin, L.; Lebdy, R.; Hodroj, D.; Ribeyre, C.; Larroque, M.; Shepard, C.; Kim, B.; Pruvost, A.; et al. Dihydro-pyrimidinase protects from DNA replication stress caused by cytotoxic metabolites. Nucleic Acids Res. 2020, 48, 1886–1904. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Ning, Z.-J.; Huang, C.-Y. Crystal structure of dihydropyrimidinase in complex with anticancer drug 5-fluorouracil. Biochem. Biophys. Res. Commun. 2019, 519, 160–165. [Google Scholar] [CrossRef]
- Tzeng, C.-T.; Huang, Y.-H.; Huang, C.-Y. Crystal structure of dihydropyrimidinase from Pseudomonas aeruginosa PAO1: Insights into the molecular basis of formation of a dimer. Biochem. Biophys. Res. Commun. 2016, 478, 1449–1455. [Google Scholar] [CrossRef]
- Barba, M.; Glansdorff, N.; Labedan, B. Evolution of Cyclic Amidohydrolases: A Highly Diversified Superfamily. J. Mol. Evol. 2013, 77, 70–80. [Google Scholar] [CrossRef]
- Kim, G.-J.; Kim, H.-S. Identification of the structural similarity in the functionally related amidohydrolases acting on the cyclic amide ring. Biochem. J. 1998, 330, 295–302. [Google Scholar] [CrossRef]
- Aganyants, H.; Weigel, P.; Hovhannisyan, Y.; Lecocq, M.; Koloyan, H.; Hambardzumyan, A.; Hovsepyan, A.; Hallet, J.-N.; Sakanyan, V. Rational Engineering of the Substrate Specificity of a Thermostable D-Hydantoinase (Dihydropyrimidinase). BioTech 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Hsu, C.-C.; Chen, M.-C.; Yang, Y.-S. Effect of metal binding and posttranslational lysine carboxylation on the activity of recombinant hydantoinase. JBIC J. Biol. Inorg. Chem. 2008, 14, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Yang, P.-C.; Lin, E.-S.; Ho, Y.-Y.; Peng, W.-F.; Lu, H.-P.; Huang, C.-C.; Huang, C.-Y. Crystal Structure of Allantoinase from Escherichia coli BL21: A Molecular Insight into a Role of the Active Site Loops in Catalysis. Molecules 2023, 28, 827. [Google Scholar] [CrossRef]
- Ho, Y.-Y.; Hsieh, H.-C.; Huang, C.-Y. Biochemical Characterization of Allantoinase from Escherichia coli BL21. Protein J. 2011, 30, 384–394. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Huang, C.-Y. Creation of a putative third metal binding site in type II dihydroorotases significantly enhances enzyme activity. Protein Pept. Lett. 2015, 22, 1117–1122. [Google Scholar] [CrossRef]
- Ho, Y.-Y.; Huang, Y.-H.; Huang, C.-Y. Chemical rescue of the post-translationally carboxylated lysine mutant of allantoinase and dihydroorotase by metal ions and short-chain carboxylic acids. Amino Acids 2013, 44, 1181–1191. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Lien, Y.; Chen, J.-H.; Lin, E.-S.; Huang, C.-Y. Identification and characterization of dihydropyrimidinase inhibited by plumbagin isolated from Nepenthes miranda extract. Biochimie 2020, 171–172, 124–135. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with pyrx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]





| No. | PDB ID | Structure | Unique Ligands | Amino Acid Residues | Seq. Id. | TM-Score | RMSD | Type | Loop State in the Monomer |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1J79 | Escherichia coli dihydroorotase | Zn(αβ), NCA orotic acid | 1–348 | 100 | 1 | 0 | II | A: out B: out a |
| 2 | 1XGE | Escherichia coli dihydroorotase | Zn(αβ), DHO, NCA | 1–348 | 98.2 | 0.999 | 0.18 | II | A: out B: in |
| 3 | 1XRF | Aquifex aeolicus dihydroorotase | Zn(α) | 1–422 | 16.1 | 0.722 | 2.79 | I | A: in b |
| 4 | 1XRT | Aquifex aeolicus dihydroorotase | Zn(α) | 1–422 | 15.9 | 0.723 | 2.91 | I | A: in b B: in b |
| 5 | 2E25 | The T109S mutant of Escherichia coli dihydroorotase in complex with FOA | Zn(αβ), FOA | 1–348 | 99.1 | 0.998 | 0.28 | II | A: out |
| 6 | 2EG6 | Escherichia coli dihydroorotase | Zn(αβ) | 1–348 | 99.4 | 0.999 | 0.2 | II | A: out B: out a |
| 7 | 2EG7 | Escherichia coli dihydroorotase in complex with HDDP | Zn(αβ), HDDP | 1–348 | 99.4 | 0.999 | 0.21 | II | A: out B: in |
| 8 | 2EG8 | Escherichia coli dihydroorotase in complex with FOA | Zn(αβ), FOA | 1–348 | 99.4 | 0.999 | 0.22 | II | A: out B: out a |
| 9 | 2GWN | Porphyromonas gingivalis dihydroorotase | Zn(αβ), cacodylate ion | 1–449 | 14.1 | 0.846 | 2.49 | I | A: in |
| 10 * | 2OGJ | Agrobacterium fabrum dihydroorotase | Zn(αβ) imidazole | 1–407 | ND | 0.609 | 3.93 | ND | ND |
| 11 | 2Z00 | Thermus thermophilus dihydroorotase | Zn(αβ) | 1–426 | 16.9 | 0.851 | 2.59 | I | A: in b |
| 12 | 2Z24 | Thr110Ser dihydroorotase from Escherichia coli | Zn(αβ), DHO, NCA | 1–348 | 99.1 | 0.999 | 0.21 | II | A: out B: out a |
| 13 | 2Z25 | Thr110Val dihydroorotase from Escherichia coli | Zn(αβ), DHO, NCA | 1–348 | 99.1 | 0.999 | 0.21 | II | A: out B: in |
| 14 | 2Z26 | Thr110Ala dihydroorotase from Escherichia coli | Zn(αβ), mixed NCA/DHO, NCA | 1–348 | 99.1 | 0.999 | 0.23 | II | A: out B: out a |
| 15 | 2Z27 | Thr109Ser dihydroorotase from Escherichia coli | Zn(αβ), DHO, NCA | 1–348 | 99.1 | 0.999 | 0.22 | II | A: out B: out a |
| 16 | 2Z28 | Thr109Val dihydroorotase from Escherichia coli | Zn(αβ), DHO, NCA | 1–348 | 99.1 | 0.999 | 0.21 | II | A: out B: out a |
| 17 | 2Z29 | Thr109Ala dihydroorotase from Escherichia coli | Zn(αβ), DHO, NCA | 1–348 | 99.1 | 0.999 | 0.22 | II | A: out B: out a |
| 18 | 2Z2A | Thr109Gly dihydroorotase from Escherichia coli | Zn(αβ), NCA, mixed NCA/DHO | 1–348 | 99.1 | 0.999 | 0.22 | II | A: out B: out a |
| 19 | 2Z2B | Deletion 107–116 mutant of dihydroorotase from E. coli | Zn(αβ) | 1–338 | 96.1 | 0.960 | 0.51 | II | A: out |
| 20 | 3D6N | The Aquifex aeolicus dihydroorotase complex | Zn(α), citrate anion | 1–422 | 16.7 | 0.840 | 2.75 | I | A: in |
| 21 | 3GRI | Staphylococcus aureus dihydroorotase | Zn(α) | 1–424 | 15.1 | 0.829 | 2.69 | I | A: out B: out |
| 22 | 3JZE | Dihydroorotase from Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 | Zn(αβ), ACY | 1–348 | 87.5 | 0.980 | 1.3 | II | A: in B: out C: in D: out |
| 23 | 3MJM | His257Ala dihydroorotase from Escherichia coli | Zn(αβ), DHO, NCA | 1–348 | 97.9 | 0.999 | 0.2 | II | A: out B: in |
| 24 | 3MPG | Bacillus anthracis dihydroorotase | Zn(αβ) | 1–428 | 16.6 | 0.840 | 2.80 | I | A: in b B: in b |
| 25 | 3PNU | Campylobacter jejuni dihydroorotase | Zn(αβ) | 1–335 | 36.2 | 0.903 | 1.90 | II | A: out B: out |
| 26 | 4BJH | The Aquifex aeolicus dihydroorotase (H180A, H232A) complex | Zn(α), DHO | 1–422 | 16.1 | 0.841 | 2.75 | I | A: in |
| 27 | 4BY3 | Human dihydroorotase in apo-form obtained recombinantly from E. coli. | Zn(αβγ) | 1456–1822 | 15.7 | 0.818 | 2.94 | III | A: out |
| 28 | 4C6B | Human dihydroorotase with incomplete active site, obtained recombinantly from E. coli | Without metal | 1456–1822 | 15.4 | 0.785 | 2.70 | III | A: out |
| 29 | 4C6C | Human dihydroorotase obtained recombinantly from HEK293 cells | Zn(αβγ) | 1456–1822 | 15.8 | 0.818 | 2.64 | III | A: out |
| 30 | 4C6D | Human dihydroorotase bound to substrate at pH 6.0 | Zn(αβγ), mixed DHO/NCA | 1456–1822 | 15.7 | 0.814 | 2.64 | III | A: in/out |
| 31 | 4C6E | Human dihydroorotase bound to substrate at pH 5.5 | Zn(αβ), mixed DHO/NCA | 1456–1822 | 15.5 | 0.814 | 2.64 | III | A: in/out |
| 32 | 4C6F | Human dihydroorotase bound to substrate at pH 6.5 | Zn(αβγ), mixed DHO/NCA | 1456–1822 | 15.7 | 0.814 | 2.64 | III | A: in/out |
| 33 | 4C6I | Human dihydroorotase bound to substrate at pH 7.0 | Zn(αβγ), mixed DHO/NCA | 1456–1822 | 15.7 | 0.813 | 2.65 | III | A: in/out |
| 34 | 4C6J | Human dihydroorotase bound to substrate at pH 7.5 | Zn(αβγ), mixed DHO/NCA | 1456–1822 | 15.7 | 0.813 | 2.65 | III | A: in/out |
| 35 | 4C6K | Human dihydroorotase bound to substrate at pH 8.0 | Zn(αβγ), mixed DHO/NCA | 1456–1822 | 15.5 | 0.814 | 2.64 | III | A: in/out |
| 36 | 4C6L | Human dihydroorotase bound to the inhibitor fluoroorotate at pH 6.0 | Zn(αβ), FOA | 1456–1822 | 15.5 | 0.819 | 2.64 | III | A: out |
| 37 | 4C6M | Human dihydroorotase bound to the inhibitor fluoroorotate at pH 7.0 | Zn(αβγ), FOA | 1456–1822 | 15.5 | 0.819 | 2.65 | III | A: out |
| 38 | 4C6N | Human dihydroorotase E1637T mutant bound to substrate at pH 6.0 | Zn(αβ), NCA | 1456–1822 | 15.4 | 0.812 | 2.63 | III | A: in |
| 39 | 4C6O | Human dihydroorotase C1613S mutant in apo-form at pH 6.0 | Zn(αβ) | 1456–1822 | 15.7 | 0.818 | 2.64 | III | A: out |
| 40 | 4C6P | Human dihydroorotase C1613S mutant in apo-form at pH 7.0 | Zn(αβγ) | 1456–1822 | 15.5 | 0.819 | 2.64 | III | A: out |
| 41 | 4C6Q | Human dihydroorotase C1613S mutant bound to substrate at pH 7.0 | Zn(αβ), mixed orotic acid/ NCA | 1456–1822 | 15.5 | 0.814 | 2.64 | III | A: in/out |
| 42 | 4LFY | Burkholderia cenocepacia dihydroorotase | Zn(αβ) | 1–364 | 53.9 | 0.969 | 0.94 | II | A: out a B: out a |
| 43 | 4YIW | Bacillus anthracis dihydroorotase | Zn(αβ), NCA | 1–428 | 16.6 | 0.842 | 2.78 | I | A: in B: in |
| 44 # | 5NNL | Inactive dihydroorotase-like domain of Chaetomium thermophilum CAD | Without metal | 1519–1855 | 10 | 0.747 | 3.26 | ND | ND |
| 45 | 5VGM | Vibrio cholerae dihydroorotase | Zn(αβ) | 1–342 | 53 | 0.939 | 0.94 | II | A: out a B: out a |
| 46 | 5YNZ | Human dihydroorotase K1556A mutant | Zn(α) | 1456–1822 | 15.2 | 0.819 | 2.65 | III | A: out |
| 47 | 6CTY | Yersinia pestis dihydroorotase | Zn(αβ), malic acid | 1–348 | 71.1 | 0.971 | 1.32 | II | A: in B: in C: in D: in E: out a F: in |
| 48 | 6GDD | Dihydroorotase from Aquifex aeolicus under 1200 bar of hydrostatic pressure | Zn(α) | 1–422 | 16.0 | 0.744 | 2.91 | I | A: in b |
| 49 | 6GDE | Dihydroorotase from Aquifex aeolicus under 600 bar of hydrostatic pressure | Zn(α) | 422 | 16.1 | 0.727 | 2.63 | I | A: in b |
| 50 | 6GDF | Dihydroorotase from Aquifex aeolicus standard (P,T) | Zn(α) | 1–422 | 16.1 | 0.734 | 2.79 | I | A: in b |
| 51 | 6HFD | Human dihydroorotase mutant F1563L apo structure | Zn(αβγ) | 1456–1822 | 15.7 | 0.818 | 2.65 | III | A: out |
| 52 | 6HFE | Human dihydroorotase mutant F1563T apo structure | Zn(αβγ) | 1456–1822 | 15.7 | 0.819 | 2.63 | III | A: out |
| 53 | 6HFF | Human dihydroorotase mutant F1563Y apo structure | Zn(αβγ) | 1456–1822 | 15.7 | 0.818 | 2.64 | III | A: out |
| 54 | 6HFH | Human dihydroorotase mutant F1563A co-crystallized with carbamoyl aspartate at pH 7.0 | Zn(αβγ), DHO c | 1456–1822 | 15.7 | 0.818 | 2.65 | III | A: out |
| 55 | 6HFI | Human dihydroorotase mutant F1563A apo structure | Zn(αβγ) | 1456–1822 | 15.7 | 0.818 | 2.65 | III | A: out |
| 56 | 6HFJ | Human dihydroorotase mutant F1563A co-crystallized with carbamoyl aspartate at pH 7.5 | Zn(αβγ), DHO c | 1456–1822 | 16.1 | 0.818 | 2.66 | III | A: out |
| 57 | 6HFK | Human dihydroorotase mutant F1563L co-crystallized with carbamoyl aspartate at pH 6.5 | Zn(αβγ), DHO c | 1456–1822 | 15.5 | 0.818 | 2.66 | III | A: out |
| 58 | 6HFL | Human dihydroorotase mutant F1563L co-crystallized with carbamoyl aspartate at pH 7.0 | Zn(αβγ), DHO c | 1456–1822 | 15.7 | 0.818 | 2.65 | III | A: out |
| 59 | 6HFN | Human dihydroorotase mutant F1563L co-crystallized with carbamoyl aspartate at pH 7.5 | Zn(αβγ), DHO c | 1456–1822 | 16.1 | 0.818 | 2.66 | III | A: out |
| 60 | 6HFP | Human dihydroorotase mutant F1563T co-crystallized with carbamoyl aspartate at pH 7.0 | Zn(αβγ), DHO c | 1456–1822 | 15.7 | 0.819 | 2.63 | III | A: out |
| 61 | 6HFQ | Human dihydroorotase mutant F1563T co-crystallized with carbamoyl aspartate at pH 7.5 | Zn(αβγ), DHO c | 1456–1822 | 16.1 | 0.819 | 2.63 | III | A: out |
| 62 | 6HFR | Human dihydroorotase mutant F1563Y co-crystallized with carbamoyl aspartate at pH 7.0 | Zn(αβγ), NCA | 1456–1822 | 15.7 | 0.813 | 2.65 | III | A: in |
| 63 | 6HFU | Human dihydroorotase mutant F1563Y co-crystallized with carbamoyl aspartate at pH 7.5 | Zn(αβγ), NCA | 1456–1822 | 16.1 | 0.813 | 2.65 | III | A: in |
| 64 | 6HFS | Human dihydroorotase mutant F1563Y co-crystallized with carbamoyl aspartate at pH 6.5 | Zn(αβγ), NCA | 1456–1822 | 15.5 | 0.813 | 2.65 | III | A: in |
| 65 | 6HG1 | Hybrid dihydroorotase domain of human CAD with E. coli flexible loop in apo state | Zn(αβ) | 1456–1822 | 19.1 | 0.821 | 2.78 | III | A: out a |
| 66 | 6HG2 | Hybrid dihydroorotase domain of human CAD with E. coli flexible loop, bound to FOA | Zn(αβ), FOA | 1456–1822 | 16.3 | 0.808 | 2.52 | III | A: out a |
| 67 | 6HG3 | Hybrid dihydroorotase domain of human CAD with E. coli flexible loop, bound to dihydroorotate | Zn(αβ), DHO | 1456–1822 | 16.9 | 0.815 | 2.62 | III | A: out a |
| 68 | 6L0A | Saccharomyces cerevisiae dihydroorotase complexed with malate at pH 7 | Zn(αβ), malic acid | 1–364 | 28.2 | 0.926 | 1.92 | II | A: in B: in C: in D: in |
| 69 | 6L0B | Saccharomyces cerevisiae dihydroorotase complexed with 5-fluorouracil | Zn(αβ), 5-fluorouracil | 1–364 | 28.2 | 0.925 | 1.97 | II | A: in B: in C: in D: in |
| 70 | 6L0F | Saccharomyces cerevisiae dihydroorotase complexed with 5-aminouracil | Zn(αβ), 5-aminouracil | 1–364 | 28.2 | 0.926 | 1.96 | II | A: in B: in C: in D: in |
| 71 | 6L0G | Saccharomyces cerevisiae dihydroorotase complexed with malate at pH 6 | Zn(αβ), malic acid | 1–364 | 28.2 | 0.926 | 1.95 | II | A: in B: in C: in D: in |
| 72 | 6L0H | Saccharomyces cerevisiae dihydroorotase complexed with malate at pH 7 | Zn(αβ), malic acid | 1–364 | 28.2 | 0.927 | 1.92 | II | A: in B: in C: in D: in |
| 73 | 6L0I | Saccharomyces cerevisiae dihydroorotase complexed with malate at pH 6.5 | Zn(αβ), malic acid | 1–364 | 28.2 | 0.925 | 1.97 | II | A: in B: in C: in D: in |
| 74 | 6L0J | Saccharomyces cerevisiae dihydroorotase complexed with malate at pH 7.5 | Zn(αβ), malic acid | 1–364 | 28.2 | 0.926 | 1.96 | II | A: in B: in C: in D: in |
| 75 | 6L0K | Saccharomyces cerevisiae dihydroorotase complexed with malate at pH 9 | Zn(αβ), malic acid | 1–364 | 28.2 | 0.924 | 2 | II | A: in B: in C: in D: in |
| 76 | 7CA0 | Saccharomyces cerevisiae dihydroorotase complexed with 5-fluoroorotic acid | Zn(αβ), FOA | 1–364 | 28.2 | 0.926 | 1.95 | II | A: in B: in C: in D: in |
| 77 | 7CA1 | Saccharomyces cerevisiae dihydroorotase complexed with plumbagin | Zn(αβ), plumbagin | 1–364 | 28.2 | 0.925 | 1.98 | II | A: in B: in C: in D: in |
| 78 | 7UOF | Methanococcus jannaschii dihydroorotase | Zn(αβ) | 1–423 | 13.5 | 0.845 | 2.66 | I | A: out |
| 79 | 8GVZ | Human dihydroorotase in complex with the anticancer drug 5-fluorouracil | Zn(αβ), 5-fluorouracil | 1456–1822 | 15.1 | 0.813 | 2.65 | III | A: in |
| 80 | 8GW0 | Human dihydroorotase in complex with malic acid | Zn(αβ), malic acid | 1456–1822 | 15.6 | 0.813 | 2.65 | III | A: in |
| 81 | 8PBE | Human dihydroorotase mutant K1556T bound to the substrate carbamoyl aspartate | Zn(αβγ), NCA | 1456–1822 | 14.8 | 0.813 | 2.67 | III | A: in |
| 82 | 8PBG | Human dihydroorotase mutant K1556T bound to the inhibitor fluoroorotate | Zn(αβγ), FOA | 1456–1822 | 15.2 | 0.814 | 2.64 | III | A: out |
| 83 | 8PBH | Human dihydroorotase mutant R1617Q bound to the substrate carbamoyl aspartate | Zn(αβγ), NCA | 1456–1822 | 15.4 | 0.814 | 2.63 | III | A: in |
| 84 | 8PBI | Human dihydroorotase mutant R1617Q bound to the inhibitor fluoroorotate | Zn(αβγ), FOA | 1456–1822 | 15.1 | 0.818 | 2.64 | III | A: out |
| 85 | 8PBJ | Human dihydroorotase mutant R1722W bound to the substrate carbamoyl aspartate | Zn(αβγ), NCA | 1456–1822 | 15.1 | 0.814 | 2.64 | III | A: in |
| 86 | 8PBK | Human dihydroorotase mutant R1722W bound to the inhibitor fluoroorotate | Zn(αβγ), FOA | 1456–1822 | 15.8 | 0.819 | 2.64 | III | A: out |
| 87 | 8PBM | Human dihydroorotase mutant R1789Q bound to the substrate dihydroorotate | Zn(αβγ), DHO | 1456–1822 | 15.5 | 0.813 | 2.65 | III | A: in/out |
| 88 | 8PBN | Human dihydroorotase mutant R1789Q bound to the inhibitor fluoroorotate | Zn(αβγ), FOA | 1456–1822 | 15.5 | 0.818 | 2.65 | III | A: out |
| 89 | 8PBP | Human dihydroorotase mutant R1785C bound to the substrate carbamoyl aspartate | Zn(αβγ), NCA/DHO | 1456–1822 | 15.5 | 0.814 | 2.64 | III | A: in |
| 90 | 8PBQ | Human dihydroorotase mutant R1810Q bound to the substrate carbamoyl aspartate | Zn(αβγ), NCA | 1456–1822 | 15.8 | 0.814 | 2.64 | III | A: in |
| 91 | 8PBR | Human dihydroorotase mutant R1475Q in apo form | Zn(αβγ) | 1456–1822 | 15.5 | 0.820 | 2.66 | III | A: out |
| 92 | 8PBS | Human dihydroorotase mutant K1482M in apo form | Zn(αβγ) | 1456–1822 | 15.7 | 0.806 | 2.71 | III | A: out |
| 93 | 8PBT | Human dihydroorotase mutant K1482M bound to the substrate dihydroorotate | Zn(αβγ), DHO | 1456–1822 | 16.1 | 0.818 | 2.66 | III | A: out |
| 94 | 8PBU | Human dihydroorotase mutant K1482M bound to the inhibitor fluoroorotate | Zn(αβγ), FOA | 1456–1822 | 16.1 | 0.818 | 2.66 | III | A: out |
| 95 | 9FS1 | Human dihydroorotase mutant S1538L bound to carbamoyl aspartate | Zn(αβγ), NCA | 1460–1821 | 15.1 | 0.813 | 2.65 | III | A: in |
| 96 | 9FS2 | Human dihydroorotase mutant S1538A bound to substrate | Zn(αβγ), DHO/NCA | 1460–1821 | 15.8 | 0.814 | 2.65 | III | A: in/out |
| 97 | 9FS3 | Human dihydroorotase mutant S1538A in apo form | Zn(αβγ) | 1460–1821 | 15.5 | 0.818 | 2.65 | III | A: out |
| Type | DHOase | Loop Length | Loop Composition | In | Out | Note |
|---|---|---|---|---|---|---|
| II | S. cerevisiae DHOase | 16 | PAGVTTNSAAGVDPND | 40 | 0 | 10 structures; 40 monomers (10 tetramers) |
| II | C. jejuni DHOase | 16 | PAGITTNSNGGVSSFD | 0 | 2 | 1 structure; 2 monomers (1 dimer) |
| II | E. coli DHOase | 14 | PANATTNSSHGVTS | 4 | 24 | 15 structures; 28 monomers (2 monomers and 13 dimers) |
| II | S. enterica DHOase | 14 | PANATTNSSHGVTS | 2 | 2 | 1 structure; 4 monomers (1 tetramer) |
| II | Y. pestis DHOase | 14 | PANATTNSTHGVSD | 5 | 1 | 1 structure; 6 monomers (1 hexamer) |
| II | B. cenocepacia DHOase | 14 | PAGATTNSDHGVTD | 0 | 2 | 1 structure; 2 monomers (1 dimer) |
| II | V. cholera DHOase | 14 | PAGATTNSDSGVTS | 0 | 2 | 1 structure; 2 monomers (1 dimer) |
| III | Human DHOase | 12 | LNETFSELRLDS | 21 | 31 | 52 structures; 52 monomers (52 monomers) |
| I | M. jannaschii DHOase | 12 | MVKSVGDLFIED | 0 | 1 | 1 structure; 1 monomer (1 monomer) |
| I | P. gingivalis DHOase | 11 | LGSSTGNMLVD | 1 | 0 | 1 structure; 1 monomer (1 monomer) |
| I | T. thermophilus DHOase | 6 | GRTNED | 1 | 0 | 1 structure; 1 monomer (1 monomer) |
| I | A. aeolicus DHOase | 6 | GSPVMD | 8 | 0 | 7 structures; 8 monomers (6 monomers and 1 dimer) |
| I | S. aureus DHOase | 6 | GVGVQT | 0 | 2 | 1 structure; 2 monomers (1 dimer) |
| I | B. anthracis DHOase | 6 | GVGVQD | 4 | 0 | 2 structures; 4 monomers (2 dimers) |
| Total: 6 type I, 7 type II, and 1 type III DHOases | 6–16; Typically important residues: G for type I; TT for type II; and TF for type III | 86 | 67 | 95 structures; 153 monomers (63 monomers, 20 dimers, 11 tetramers, and 1 hexamer) | ||
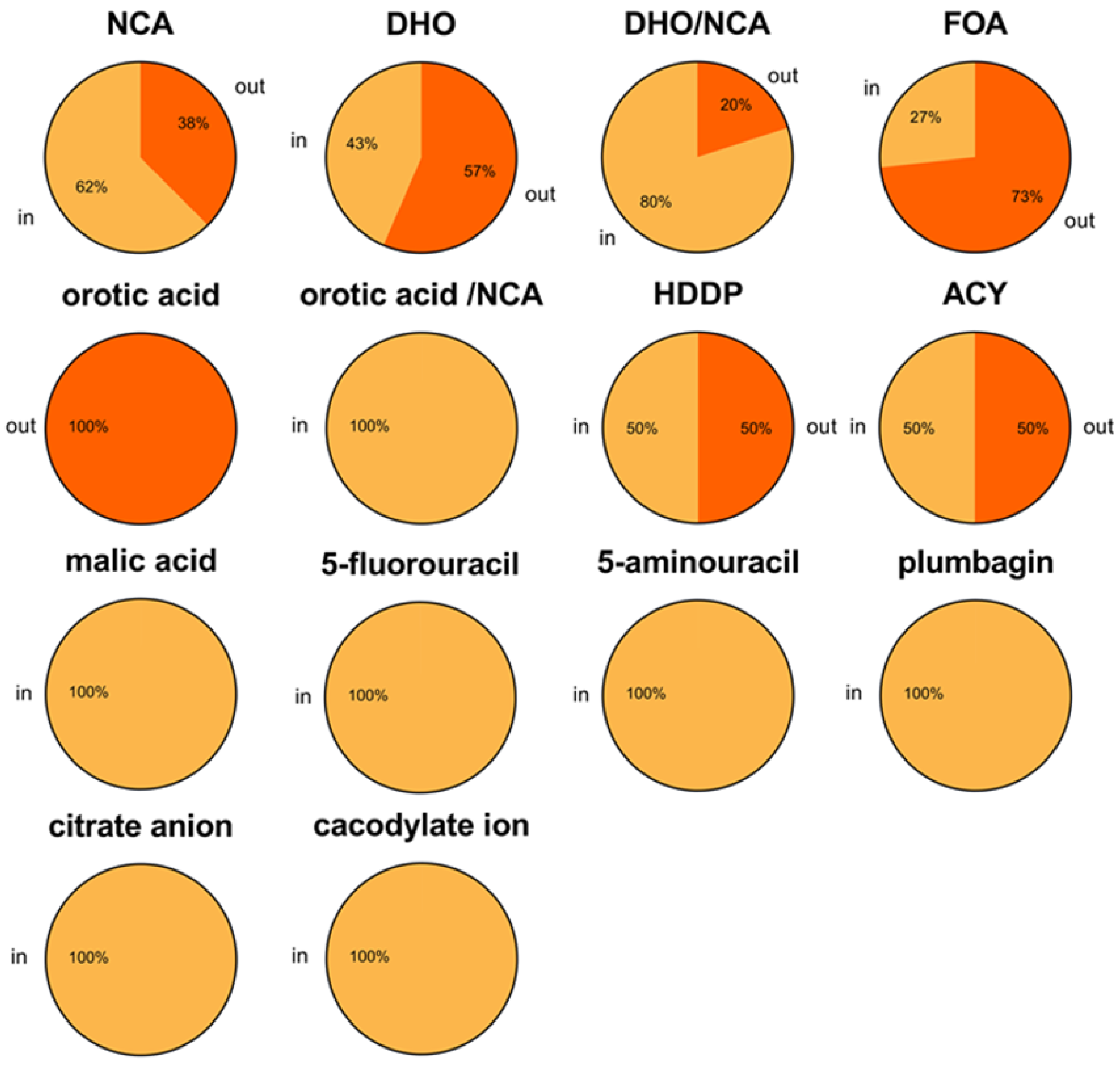
| Type | Loop In (with Ligand) | Loop Out (with Ligand) | Note |
|---|---|---|---|
| I | 14 (5) | 3 (0) | 17 monomers (9 monomers and 4 dimers) |
| II | 51 (51) a | 33 (23) | 84 monomers (2 monomers, 16 dimers, 11 tetramers, 1 hexamer) |
| III | 21 (21) b | 31 (17) | 52 monomers (52 monomers) |
| Total | 86 (77) | 67 (40) | 153 monomers |
| Ligand | Loop In State | Loop Out State | Total |
|---|---|---|---|
| NCA | 10 (Type I: 2, II: 3, III: 5) | 6 (Type II: 6) | 16 |
| DHO | 10 (Type I: 1, III: 9) | 13 (Type II: 9, III: 4) | 23 |
| DHO/NCA a | 8 (Type III: 8) | 2 (Type II: 1, III: 1) | 10 |
| 5-FOA | 4 (Type II: 4) | 11 (Type II: 3, III: 8) | 15 |
| Orotic acid | 0 | 1 (Type II: 1) | 1 |
| Orotic acid/NCA a | 1 (Type III: 1) | 0 | 1 |
| HDDP | 1 (Type II: 1) | 1 (Type II: 1) | 2 |
| Cacodylate ion | 1 (Type I: 1) | 0 | 1 |
| Citrate anion | 1 (Type I: 1) | 0 | 1 |
| Acetic acid | 2 (Type II: 2) | 2 (Type II: 2) | 4 |
| Malic acid | 30 (Type II: 29, III: 1) | 0 | 30 |
| 5-fluorouracil | 5 (Type II: 4, III: 1) | 0 | 5 |
| 5-aminouracil | 4 (Type II: 4) | 0 | 4 |
| Plumbagin | 4 (Type II: 4) | 0 | 4 |
| Total | 81 | 36 | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-H.; Huang, T.-Y.; Wang, M.-C.; Huang, C.-Y. Conformational Dynamics of the Active Site Loop in Dihydroorotase Highlighting the Limitations of Loop-In Structures for Inhibitor Docking. Int. J. Mol. Sci. 2025, 26, 9688. https://doi.org/10.3390/ijms26199688
Huang Y-H, Huang T-Y, Wang M-C, Huang C-Y. Conformational Dynamics of the Active Site Loop in Dihydroorotase Highlighting the Limitations of Loop-In Structures for Inhibitor Docking. International Journal of Molecular Sciences. 2025; 26(19):9688. https://doi.org/10.3390/ijms26199688
Chicago/Turabian StyleHuang, Yen-Hua, Tsai-Ying Huang, Man-Cheng Wang, and Cheng-Yang Huang. 2025. "Conformational Dynamics of the Active Site Loop in Dihydroorotase Highlighting the Limitations of Loop-In Structures for Inhibitor Docking" International Journal of Molecular Sciences 26, no. 19: 9688. https://doi.org/10.3390/ijms26199688
APA StyleHuang, Y.-H., Huang, T.-Y., Wang, M.-C., & Huang, C.-Y. (2025). Conformational Dynamics of the Active Site Loop in Dihydroorotase Highlighting the Limitations of Loop-In Structures for Inhibitor Docking. International Journal of Molecular Sciences, 26(19), 9688. https://doi.org/10.3390/ijms26199688







