Glia Cells Are Selectively Sensitive to Nanosized Titanium Dioxide Mineral Forms
Abstract
1. Introduction
2. Results
2.1. Characterization of Anatase and Rutile Nanoparticles
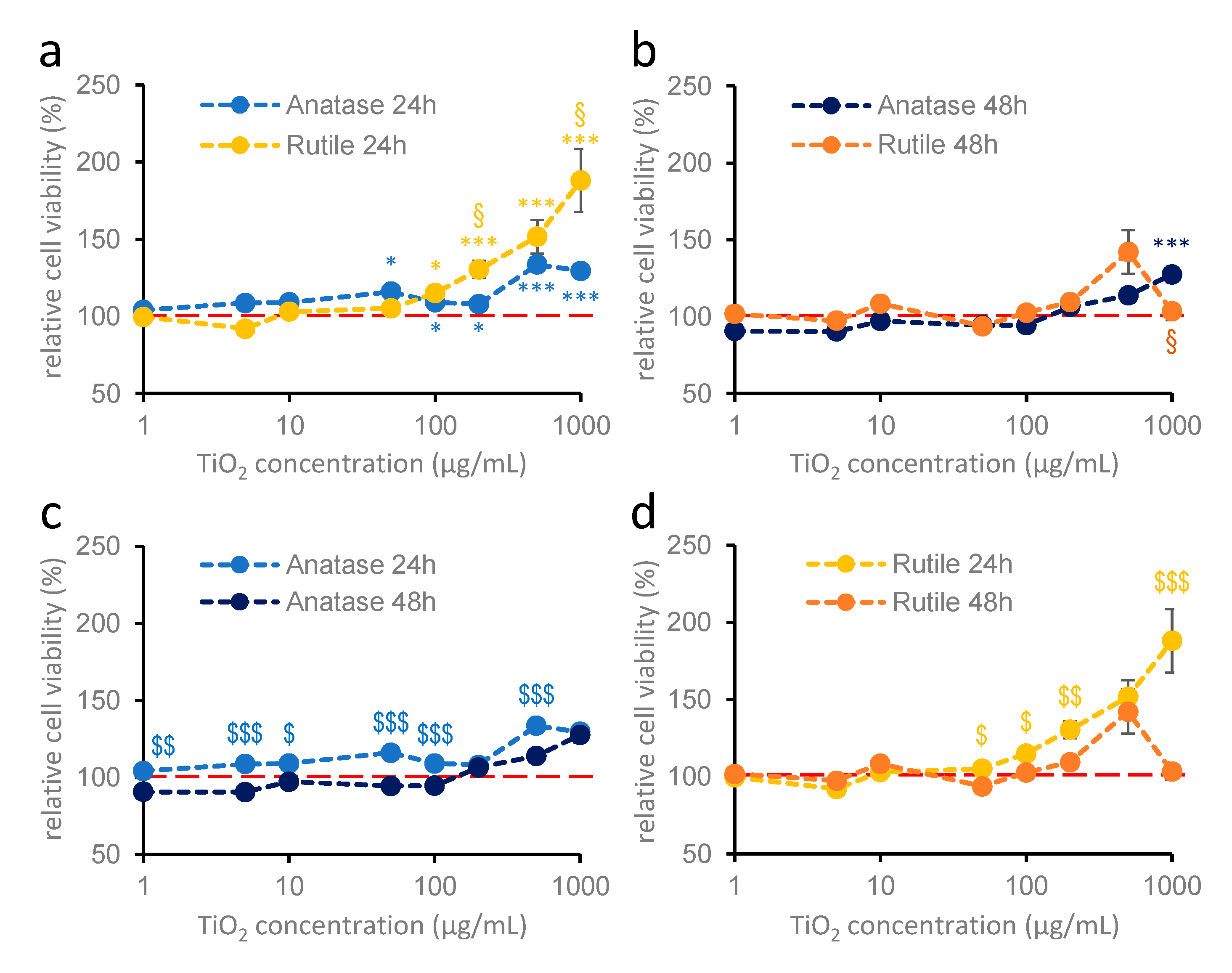
2.2. Effect of TiO2 Nanoparticles on the Viability of Hippocampal Astroglia Cultures
2.3. Effect of TiO2 Nanoparticles on Cortical Astroglia Cultures
2.4. Effects of TiO2 Nanoparticles on Primary Cortical Cultures Containing Both Neurons and Astroglia Cells
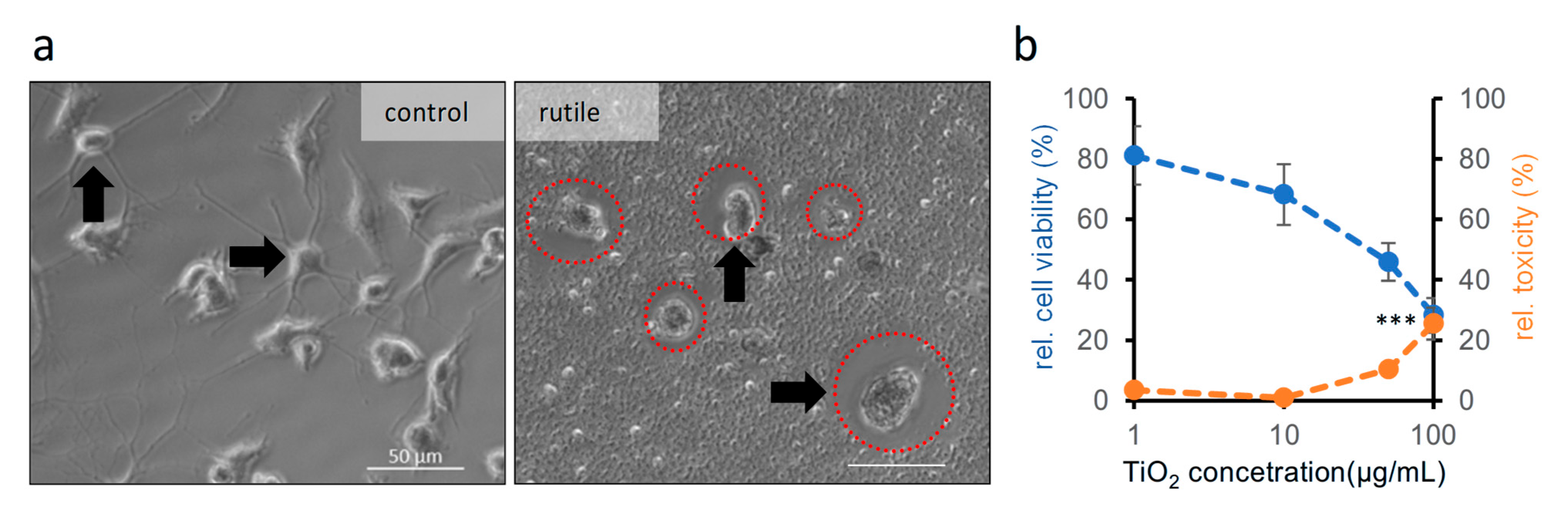
2.5. Effect of TiO2 Nanoparticles on Microglial Cells
3. Discussion
3.1. Effects of Anatase on Different Cell Types
3.2. TiO2 with Rutile Crystal Structure Acts Differently on Different Cell Types
4. Materials and Methods
4.1. Animal Handling
4.2. Gene Expression Analyses Using Public Datasets
4.3. Preparation of Cell Cultures
4.3.1. Preparation of Mixed Neuron and Astroglia and Pure Neuronal Cultures
4.3.2. Preparation of Pure Astroglia Cultures
4.3.3. Preparation of Microglial Cultures
4.4. Preparation, Application, and Verification of Titanium Dioxide Solutions
4.5. Viability Measurement Using MTT Method and Cytotoxicity Measurement Using LDH Assay
4.6. Datasets, Statistics, and Data Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TiO2 | titanium dioxide; |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; |
| LDH | lactate dehydrogenase. |
Appendix A
| Name of Gene Set | Version | ID |
|---|---|---|
| Hallmark Reactive Oxygen Species Pathway | v2025.1.Mm | MM3895 |
| WP Oxidative stress and redox pathway | v2025.1.Mm | MM15823 |
| WP Oxidative stress response | v2025.1.Mm | MM15941 |
| WP Oxidative damage response | v2025.1.Mm | MM15945 |
| GOCC NADPH Oxidase Complex | v2025.1.Mm | MM12311 |
| GOBP Superoxide Metabolic Process | v2025.1.Mm | MM4854 |
| GOBP Regulation of Superoxide Metabolic Process | v2025.1.Mm | MM10142 |
| GOBP NADH Metabolic Process | v2025.1.Mm | MM4836 |
References
- Rahimi, N.; Pax, R.A.; Gray, E. MacA. Review of Functional Titanium Oxides. I: TiO2 and Its Modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Bischoff, N.S.; de Kok, T.M.; Sijm, D.T.H.M.; van Breda, S.G.; Briedé, J.J.; Castenmiller, J.J.M.; Opperhuizen, A.; Chirino, Y.I.; Dirven, H.; Gott, D.; et al. Possible Adverse Effects of Food Additive E171 (Titanium Dioxide) Related to Particle Specific Human Toxicity, Including the Immune System. Int. J. Mol. Sci. 2020, 22, 207. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Y.; Wen, J.; Xu, Y.; Zhu, J.; Bian, Z. Polyaniline-TiO2 Composite Photocatalysts for Light-Driven Hexavalent Chromium Ions Reduction. Sci. Bull. 2020, 65, 105–112. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. [Google Scholar] [CrossRef]
- Tucci, P.; Porta, G.; Agostini, M.; Dinsdale, D.; Iavicoli, I.; Cain, K.; Finazzi-Agró, A.; Melino, G.; Willis, A. Metabolic Effects of TiO2 Nanoparticles, a Common Component of Sunscreens and Cosmetics, on Human Keratinocytes. Cell Death Dis. 2013, 4, e549. [Google Scholar] [CrossRef]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of Titanium Dioxide Nanoparticles in Food Products: Analytical Methods to Define Nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health-a Review. Biol. Trace. Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef]
- Wilson, C.L.; Natarajan, V.; Hayward, S.L.; Khalimonchuk, O.; Kidambi, S. Mitochondrial Dysfunction and Loss of Glutamate Uptake in Primary Astrocytes Exposed to Titanium Dioxide Nanoparticles. Nanoscale 2015, 7, 18477–18488. [Google Scholar] [CrossRef] [PubMed]
- Kischkewitz, J. Pigments, Inorganic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2006; pp. 31–50. ISBN 9783527306732. [Google Scholar]
- Jovanović, B. Critical Review of Public Health Regulations of Titanium Dioxide, a Human Food Additive. Integr. Environ. Assess Manag. 2015, 11, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liu, J.; Feng, X.; Wei, L.; Shao, L. A Review on Potential Neurotoxicity of Titanium Dioxide Nanoparticles. Nanoscale Res. Lett. 2015, 10, 1042. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.; Liu, J.; Feng, X.; Zhou, T.; Shao, L. Unraveling the Neurotoxicity of Titanium Dioxide Nanoparticles: Focusing on Molecular Mechanisms. Beilstein J. Nanotechnol. 2016, 7, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. Identification of the Mechanisms That Drive the Toxicity of TiO2 Particulates: The Contribution of Physicochemical Characteristics. Part. Fibre Toxicol. 2009, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, C.; Chen, A.; Feng, X.; Liang, H.; Yin, S.; Zhang, G.; Shao, L. Neurotoxicity of Nanoparticles Entering the Brain via Sensory Nerve-to-Brain Pathways: Injuries and Mechanisms. Arch. Toxicol. 2020, 94, 1479–1495. [Google Scholar] [CrossRef]
- Yang, J.; Luo, M.; Tan, Z.; Dai, M.; Xie, M.; Lin, J.; Hua, H.; Ma, Q.; Zhao, J.; Liu, A. Oral Administration of Nano-Titanium Dioxide Particle Disrupts Hepatic Metabolic Functions in a Mouse Model. Environ. Toxicol. Pharmacol. 2017, 49, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Onishchenko, G.E.; Erokhina, M.V.; Abramchuk, S.S.; Shaitan, K.V.; Raspopov, R.V.; Smirnova, V.V.; Vasilevskaya, L.S.; Gmoshinski, I.V.; Kirpichnikov, M.P.; Tutelyan, V.A. Effects of Titanium Dioxide Nanoparticles on Small Intestinal Mucosa in Rats. Bull. Exp. Biol. Med. 2012, 154, 265–270. [Google Scholar] [CrossRef]
- Hu, H.; Li, L.; Guo, Q.; Jin, S.; Zhou, Y.; Oh, Y.; Feng, Y.; Wu, Q.; Gu, N. A Mechanistic Study to Increase Understanding of Titanium Dioxide Nanoparticles-Increased Plasma Glucose in Mice. Food Chem. Toxicol. 2016, 95, 175–187. [Google Scholar] [CrossRef]
- Heidari, Z.; Mohammadipour, A.; Haeri, P.; Ebrahimzadeh-Bideskan, A. The Effect of Titanium Dioxide Nanoparticles on Mice Midbrain Substantia Nigra. Iran. J. Basic Med. Sci. 2019, 22, 745–751. [Google Scholar] [CrossRef]
- Rashid, M.M.; Tavčer, P.F.; Tomšič, B. Influence of Titanium Dioxide Nanoparticles on Human Health and the Environment. Nanomaterials 2021, 11, 2354. [Google Scholar] [CrossRef]
- Czajka, M.; Sawicki, K.; Sikorska, K.; Popek, S.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of Titanium Dioxide Nanoparticles in Central Nervous System. Toxicol. Vitr. 2015, 29, 1042–1052. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2022/63 of 14 January 2022 Amending Annexes II and III to Regulation (EC) No 1333/2008 as Regards the Food Additive Titanium Dioxide (E 171); European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Li, J.; Zhang, D.; Hou, C. Application of Nano-Titanium Dioxide in Food Antibacterial Packaging Materials. Bioengineering 2024, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Valentini, X.; Deneufbourg, P.; Paci, P.; Rugira, P.; Laurent, S.; Frau, A.; Stanicki, D.; Ris, L.; Nonclercq, D. Morphological Alterations Induced by the Exposure to TiO2 Nanoparticles in Primary Cortical Neuron Cultures and in the Brain of Rats. Toxicol. Rep. 2018, 5, 878–889. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H. Effects of Titanium Dioxide Nanoparticles on α-Synuclein Aggregation and the Ubiquitin-Proteasome System in Dopaminergic Neurons. Artif. Cells Nanomed. Biotechnol. 2016, 44, 690–694. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and Mechanisms of Action of Titanium Dioxide Nanoparticles in Living Organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Huerta-García, E.; Pérez-Arizti, J.A.; Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; López-Marure, R. Titanium Dioxide Nanoparticles Induce Strong Oxidative Stress and Mitochondrial Damage in Glial Cells. Free Radic. Biol. Med. 2014, 73, 84–94. [Google Scholar] [CrossRef]
- Heneka, M.T.; Rodríguez, J.J.; Verkhratsky, A. Neuroglia in Neurodegeneration. Brain Res. Rev. 2010, 63, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The Role of Astrocytes in Oxidative Stress of Central Nervous System: A Mixed Blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Morel, L.; Chiang, M.S.R.; Higashimori, H.; Shoneye, T.; Iyer, L.K.; Yelick, J.; Tai, A.; Yang, Y. Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J. Neurosci. 2017, 37, 8706–8717. [Google Scholar] [CrossRef]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular Basis of Astrocyte Diversity and Morphology across the CNS in Health and Disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef]
- Batiuk, M.Y.; Martirosyan, A.; Wahis, J.; de Vin, F.; Marneffe, C.; Kusserow, C.; Koeppen, J.; Viana, J.F.; Oliveira, J.F.; Voet, T.; et al. Identification of Region-Specific Astrocyte Subtypes at Single Cell Resolution. Nat. Commun. 2020, 11, 1220. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Xin, W.; Bonci, A. Functional Astrocyte Heterogeneity and Implications for Their Role in Shaping Neurotransmission. Front. Cell Neurosci. 2018, 12, 141. [Google Scholar] [CrossRef]
- Dallérac, G.; Zapata, J.; Rouach, N. Versatile Control of Synaptic Circuits by Astrocytes: Where, When and How? Nat. Rev. Neurosci. 2018, 19, 729–743. [Google Scholar] [CrossRef]
- Kipp, M.; Norkute, A.; Johann, S.; Lorenz, L.; Braun, A.; Hieble, A.; Gingele, S.; Pott, F.; Richter, J.; Beyer, C. Brain-Region-Specific Astroglial Responses in Vitro after LPS Exposure. J. Mol. Neurosci. 2008, 35, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Cragnolini, A.B.; Montenegro, G.; Friedman, W.J.; Mascó, D.H. Brain-Region Specific Responses of Astrocytes to an in Vitro Injury and Neurotrophins. Mol. Cell Neurosci. 2018, 88, 240–248. [Google Scholar] [CrossRef]
- Ernsberger, P.; Iacovitti, L.; Reis, D.J. Astrocytes Cultured from Specific Brain Regions Differ in Their Expression of Adrenergic Binding Sites. Brain Res. 1990, 517, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox Control of Microglial Function: Molecular Mechanisms and Functional Significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Ishihara, Y.; Itoh, K. Microglial Inflammatory Reactions Regulated by Oxidative Stress. J. Clin. Biochem. Nutr. 2023, 72, 23–27. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives; Nutrition and Food Safety; Standards & Scientific Advice on Food Nutrition. Thirteenth of the Joint FAO/WHO Expert Committee on Food Additives. In Thirteenth Report of the Joint FAO/WHO Expert Committee on Food Additives; FAO nutrition meetings report series. WHO technical report series; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 1970; pp. 1–57. ISBN 92-4-120445-1. [Google Scholar]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of Health Safety Aspects of Titanium Dioxide Nanoparticles in Food Application. NanoImpact 2020, 18, 100224. [Google Scholar] [CrossRef]
- Gong, J.-Y.; Holt, M.G.; Hoet, P.H.M.; Ghosh, M. Neurotoxicity of Four Frequently Used Nanoparticles: A Systematic Review to Reveal the Missing Data. Arch. Toxicol. 2022, 96, 1141–1212. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Du, Q.; Ran, B.; Liu, X.; Wang, X.; Ma, X.; Cheng, F.; Sun, B. The Neurotoxicity Induced by Engineered Nanomaterials. Int. J. Nanomed. 2019, 14, 4167–4186. [Google Scholar] [CrossRef] [PubMed]
- Madorran, E.; Stožer, A.; Bevc, S.; Maver, U. In Vitro Toxicity Model: Upgrades to Bridge the Gap between Preclinical and Clinical Research. Bosn. J. Basic Med. Sci. 2020, 20, 157–168. [Google Scholar] [CrossRef]
- Wang, S.; Alenius, H.; El-Nezami, H.; Karisola, P. A New Look at the Effects of Engineered ZnO and TiO2 Nanoparticles: Evidence from Transcriptomics Studies. Nanomaterials 2022, 12, 1247. [Google Scholar] [CrossRef]
- Papp, A.; Horváth, T.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M.; Berkesi, D.S.; Kozma, G.; Kónya, Z.; Wilhelm, I.; Patai, R.; et al. Presence of Titanium and Toxic Effects Observed in Rat Lungs, Kidneys, and Central Nervous System in Vivo and in Cultured Astrocytes in Vitro on Exposure by Titanium Dioxide Nanorods. Int. J. Nanomed. 2020, 15, 9939–9960. [Google Scholar] [CrossRef]
- Hong, F.; Sheng, L.; Ze, Y.; Hong, J.; Zhou, Y.; Wang, L.; Liu, D.; Yu, X.; Xu, B.; Zhao, X.; et al. Suppression of Neurite Outgrowth of Primary Cultured Hippocampal Neurons Is Involved in Impairment of Glutamate Metabolism and NMDA Receptor Function Caused by Nanoparticulate TiO2. Biomaterials 2015, 53, 76–85. [Google Scholar] [CrossRef]
- Ze, Y.; Zheng, L.; Zhao, X.; Gui, S.; Sang, X.; Su, J.; Guan, N.; Zhu, L.; Sheng, L.; Hu, R.; et al. Molecular Mechanism of Titanium Dioxide Nanoparticles-Induced Oxidative Injury in the Brain of Mice. Chemosphere 2013, 92, 1183–1189. [Google Scholar] [CrossRef]
- Ferraro, S.A.; Domingo, M.G.; Etcheverrito, A.; Olmedo, D.G.; Tasat, D.R. Neurotoxicity Mediated by Oxidative Stress Caused by Titanium Dioxide Nanoparticles in Human Neuroblastoma (SH-SY5Y) Cells. J. Trace. Elem. Med. Biol. 2020, 57, 126413. [Google Scholar] [CrossRef] [PubMed]
- Han, S.G.; Newsome, B.; Hennig, B. Titanium Dioxide Nanoparticles Increase Inflammatory Responses in Vascular Endothelial Cells. Toxicology 2013, 306, 1–8. [Google Scholar] [CrossRef]
- Long, T.C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G.V.; Veronesi, B. Nanosize Titanium Dioxide Stimulates Reactive Oxygen Species in Brain Microglia and Damages Neurons in Vitro. Environ. Health Perspect 2007, 115, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Ze, Y.; Wang, L.; Yu, X.; Hong, J.; Zhao, X.; Ze, X.; Liu, D.; Xu, B.; Zhu, Y.; et al. Mechanisms of TiO2 Nanoparticle-Induced Neuronal Apoptosis in Rat Primary Cultured Hippocampal Neurons. J. Biomed. Mater. Res. A 2015, 103, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, I.-L.; Chang, C.-C.; Wu, C.-Y.; Hsieh, Y.-K.; Chuang, C.-Y.; Wang, C.-F.; Huang, Y.-J. Indirect Effects of TiO2 Nanoparticle on Neuron-Glial Cell Interactions. Chem. Biol. Interact 2016, 254, 34–44. [Google Scholar] [CrossRef]
- Chang, X.; Li, J.; Niu, S.; Xue, Y.; Tang, M. Neurotoxicity of Metal-Containing Nanoparticles and Implications in Glial Cells. J. Appl. Toxicol. 2021, 41, 65–81. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.H.; Jiménez-Barrios, N.; Déciga-Alcaraz, A.; Martínez-Cuazitl, A.; Mata-Miranda, M.M.; Vázquez-Zapién, G.J.; Pedraza-Chaverri, J.; Chirino, Y.I.; Orozco-Ibarra, M. Astrocytes Are More Vulnerable than Neurons to Silicon Dioxide Nanoparticle Toxicity in Vitro. Toxics 2020, 8, 51. [Google Scholar] [CrossRef]
- Feng, X.; Chen, A.; Zhang, Y.; Wang, J.; Shao, L.; Wei, L. Central Nervous System Toxicity of Metallic Nanoparticles. Int. J. Nanomed. 2015, 10, 4321–4340. [Google Scholar] [CrossRef]
- Mu, X.; Li, W.; Ze, X.; Li, L.; Wang, G.; Hong, F.; Ze, Y. Molecular Mechanism of Nanoparticulate TiO2 Induction of Axonal Development Inhibition in Rat Primary Cultured Hippocampal Neurons. Environ. Toxicol. 2020, 35, 895–905. [Google Scholar] [CrossRef]
- Gerber, L.-S.; Heusinkveld, H.J.; Langendoen, C.; Stahlmecke, B.; Schins, R.P.; Westerink, R.H. Acute, Sub-Chronic and Chronic Exposures to TiO2 and Ag Nanoparticles Differentially Affects Neuronal Function in Vitro. Neurotoxicology 2022, 93, 311–323. [Google Scholar] [CrossRef]
- He, Q.; Zhou, X.; Liu, Y.; Gou, W.; Cui, J.; Li, Z.; Wu, Y.; Zuo, D. Titanium Dioxide Nanoparticles Induce Mouse Hippocampal Neuron Apoptosis via Oxidative Stress- and Calcium Imbalance-Mediated Endoplasmic Reticulum Stress. Environ. Toxicol. Pharmacol. 2018, 63, 6–15. [Google Scholar] [CrossRef]
- Wu, J.; Sun, J.; Xue, Y. Involvement of JNK and P53 Activation in G2/M Cell Cycle Arrest and Apoptosis Induced by Titanium Dioxide Nanoparticles in Neuron Cells. Toxicol. Lett. 2010, 199, 269–276. [Google Scholar] [CrossRef] [PubMed]
- De Simone, U.; Lonati, D.; Ronchi, A.; Coccini, T. Brief Exposure to Nanosized and Bulk Titanium Dioxide Forms Induces Subtle Changes in Human D384 Astrocytes. Toxicol. Lett. 2016, 254, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arizti, J.A.; Ventura-Gallegos, J.L.; Galván Juárez, R.E.; Ramos-Godinez, M. del P.; Colín-Val, Z.; López-Marure, R. Titanium Dioxide Nanoparticles Promote Oxidative Stress, Autophagy and Reduce NLRP3 in Primary Rat Astrocytes. Chem. Biol. Interact. 2020, 317. [Google Scholar] [CrossRef] [PubMed]
- Coccini, T.; Grandi, S.; Lonati, D.; Locatelli, C.; De Simone, U. Comparative Cellular Toxicity of Titanium Dioxide Nanoparticles on Human Astrocyte and Neuronal Cells after Acute and Prolonged Exposure. Neurotoxicology 2015, 48, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sapolsky, R.M.; Giffard, R.G. Differential Sensitivity of Murine Astrocytes and Neurons from Different Brain Regions to Injury. Exp. Neurol. 2001, 169, 416–424. [Google Scholar] [CrossRef]
- Zhao, G.; Flavin, M.P. Differential Sensitivity of Rat Hippocampal and Cortical Astrocytes to Oxygen-Glucose Deprivation Injury. Neurosci. Lett. 2000, 285, 177–180. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, J.; Sun, J. Four Types of Inorganic Nanoparticles Stimulate the Inflammatory Reaction in Brain Microglia and Damage Neurons in Vitro. Toxicol. Lett. 2012, 214, 91–98. [Google Scholar] [CrossRef]
- Huang, W.; Tao, Y.; Zhang, X.; Zhang, X. TGF-Β1/SMADs Signaling Involved in Alleviating Inflammation Induced by Nanoparticulate Titanium Dioxide in BV2 Cells. Toxicol. Vitr. 2022, 80, 105303. [Google Scholar] [CrossRef]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The Effect of Crystalline Phase (Anatase, Brookite and Rutile) and Size on the Photocatalytic Activity of Calcined Polymorphic Titanium Dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Rouse, I.; Power, D.; Brandt, E.G.; Schneemilch, M.; Kotsis, K.; Quirke, N.; Lyubartsev, A.P.; Lobaskin, V. First Principles Characterisation of Bio-Nano Interface. Phys. Chem. Chem. Phys. 2021, 23, 13473–13482. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Costa, C.; Sharma, V.; Kiliç, G.; Pásaro, E.; Teixeira, J.P.; Dhawan, A.; Laffon, B. Comparative Study on Effects of Two Different Types of Titanium Dioxide Nanoparticles on Human Neuronal Cells. Food Chem. Toxicol. 2013, 57, 352–361. [Google Scholar] [CrossRef]
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Cellular Toxicity of TiO2 Nanoparticles in Anatase and Rutile Crystal Phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of The European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 53, 33–79.
- Szentgyörgyi, V.; Tagscherer-Micska, B.; Rátkai, A.; Schlett, K.; Bencsik, N.; Tárnok, K. Cell Type-Specific Effects of Fusarium Mycotoxins on Primary Neurons and Astroglial Cells. Toxins 2025, 17, 368. [Google Scholar] [CrossRef]
- Tárnok, K.; Kiss, E.; Luiten, P.G.M.; Nyakas, C.; Tihanyi, K.; Schlett, K.; Eisel, U.L.M. Effects of Vinpocetine on Mitochondrial Function and Neuroprotection in Primary Cortical Neurons. Neurochem. Int. 2008, 53, 289–295. [Google Scholar] [CrossRef]
- Tárnok, K.; Szilágyi, L.; Berki, T.; Németh, P.; Gráf, L.; Schlett, K. Anoxia Leads to a Rapid Translocation of Human Trypsinogen 4 to the Plasma Membrane of Cultured Astrocytes. J. Neurochem. 2010, 115, 314–324. [Google Scholar] [CrossRef]
- Tóth, E.; Szuróczki, S.; Kéki, Z.; Kosztik, J.; Makk, J.; Bóka, K.; Spröer, C.; Márialigeti, K.; Schumann, P. Brevundimonas balnearis sp. nov., Isolated from the Well Water of a Thermal Bath. Int. J. Syst. Evol. Microbiol. 2017, 67, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiszelhardt, E.; Tóth, E.; Bóka, K.; Bencsik, N.; Schlett, K.; Tárnok, K. Glia Cells Are Selectively Sensitive to Nanosized Titanium Dioxide Mineral Forms. Int. J. Mol. Sci. 2025, 26, 9684. https://doi.org/10.3390/ijms26199684
Geiszelhardt E, Tóth E, Bóka K, Bencsik N, Schlett K, Tárnok K. Glia Cells Are Selectively Sensitive to Nanosized Titanium Dioxide Mineral Forms. International Journal of Molecular Sciences. 2025; 26(19):9684. https://doi.org/10.3390/ijms26199684
Chicago/Turabian StyleGeiszelhardt, Eszter, Erika Tóth, Károly Bóka, Norbert Bencsik, Katalin Schlett, and Krisztián Tárnok. 2025. "Glia Cells Are Selectively Sensitive to Nanosized Titanium Dioxide Mineral Forms" International Journal of Molecular Sciences 26, no. 19: 9684. https://doi.org/10.3390/ijms26199684
APA StyleGeiszelhardt, E., Tóth, E., Bóka, K., Bencsik, N., Schlett, K., & Tárnok, K. (2025). Glia Cells Are Selectively Sensitive to Nanosized Titanium Dioxide Mineral Forms. International Journal of Molecular Sciences, 26(19), 9684. https://doi.org/10.3390/ijms26199684







