Staurosporine as an Antifungal Agent
Abstract
1. Staurosporine: An Indolo[2,3-a]carbazole Alkaloid with Anticancer and Antifungal Activity

2. Brief History of Staurosporine: From Discovery to Biological Activity Disclosure
3. Biological Sources and Natural and Synthetic Analogues of Staurosporine
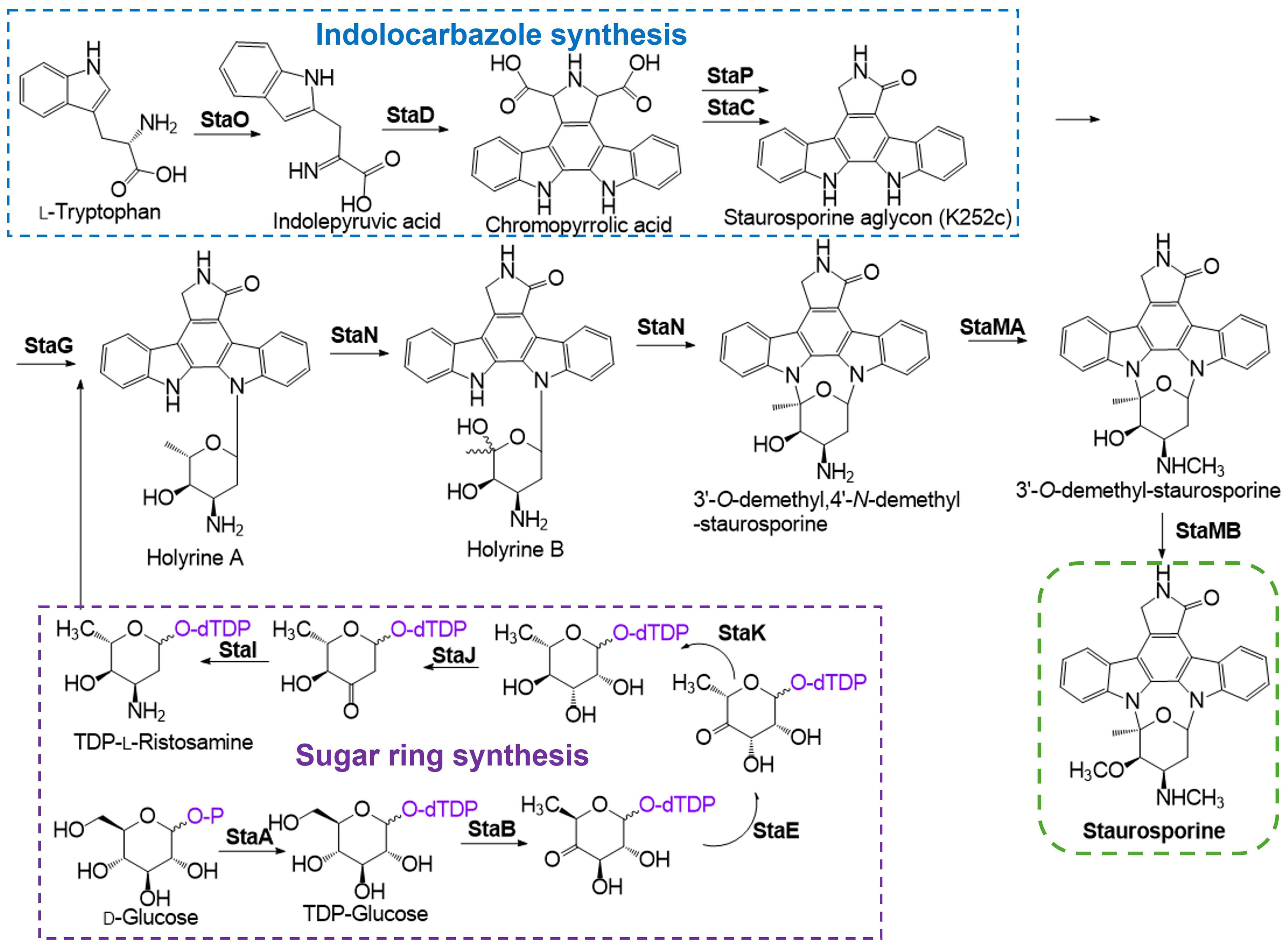
4. STS Biosynthetic Pathway
5. Antifungal Activity of Staurosporine and Related Compounds
5.1. Antifungal Activity of Staurosporine Against Phytopathogens
5.2. Antifungal Activity of Staurosporine Against Human Pathogens
5.3. Strategies to Improve Activity and Overcome Toxicity
6. Antifungal Modes of Action
6.1. Protein Kinase Inhibition and Induction of Apoptosis
6.2. Alterations at the Plasma Membrane Level and in Fungal Developmental Processes
7. Concluding Remarks and Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GSH | Glutathione |
| PCD | Programmed Cell Death |
| PK | Protein Kinase |
| PM | Plasma Membrane |
| PKA | Protein Kinase A (cAMP-dependent protein kinase) |
| PKC | Protein Kinase C (Ca2+/phospholipid-dependent protein kinase) |
| PKG | Protein Kinase G (cGMP-dependent protein kinases) |
| ROS | Reactive Oxygen Species |
| SLEDs | Sphingolipid-Enriched Domains |
| STS | Staurosporine |
| IPA | indole-3-pyruvic acid |
| CPA | Chromopyrrolic acid |
References
- Gulshan, K.; Moye-Rowley, W.S. Multidrug resistance in fungi. Eukaryot. Cell 2007, 6, 1933. [Google Scholar] [CrossRef] [PubMed]
- Shahi, P.; Moye-Rowley, W.S. Coordinate control of lipid composition and drug transport activities is required for normal multidrug resistance in fungi. Biochim. Biophys. Acta 2009, 1794, 852. [Google Scholar] [CrossRef] [PubMed]
- Regional Committee for the Western Pacific. Antimicrobial Resistance; WHO Regional Office for the Western Pacific: Manila, Philippines, 2019.
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; WHO: Geneva, Switzerland, 2022; p. 48. ISBN 978-92-4-006024-1.
- WHO (Ed.) Antifungal Agents in Clinical and Preclinical Development: Overview and Analysis; WHO: Geneva, Switzerland, 2025; p. 88.
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428. [Google Scholar] [CrossRef]
- van Rhijn, N.; Arikan-Akdagli, S.; Beardsley, J.; Bongomin, F.; Chakrabarti, A.; Chen, S.C.; Chiller, T.; Lopes Colombo, A.; Govender, N.P.; Alastruey-Izquierdo, A.; et al. Beyond bacteria: The growing threat of antifungal resistance. Lancet 2024, 404, 1017. [Google Scholar] [CrossRef]
- Kim, H.Y.P.; Nguyen, T.A.M.; Kidd, S.P.; Chambers, J.M.; Alastruey-Izquierdo, A.P.; Shin, J.M.; Dao, A.P.; Forastiero, A.M.; Wahyuningsih, R.M.; Chakrabarti, A.M.; et al. Candida auris-a systematic review to inform the world health organization fungal priority pathogens list. Med. Mycol. 2024, 62, myae042. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Barrero-Garcia, I.; Leon-Moya, C. Fungal infections in immunocompromised critically ill patients. J. Intensive Med. 2024, 4, 299. [Google Scholar] [CrossRef]
- Omura, S. Philosophy of new drug discovery. Microbiol. Rev. 1986, 50, 259. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hwang, B.K. Diversity of antifungal actinomycetes in various vegetative soils of Korea. Can. J. Microbiol. 2002, 48, 407. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clement, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. MMBR 2016, 80, 1–43, https://doi.org/10.1128/MMBR.00019-15; Correction in Microbiol. Mol. Biol. Rev. MMBR 2016, 80, iii. [Google Scholar] [CrossRef]
- Tanaka, Y.; Omura, S. Agroactive compounds of microbial origin. Annu. Rev. Microbiol. 1993, 47, 57. [Google Scholar] [CrossRef]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 OF Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. 1977, 30, 275. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Rabby, S.M.F.; Gupta, D.R.; Rahman, M.; Paul, S.K.; Mahmud, N.U.; Rahat, A.A.M.; Jankuloski, L.; Islam, T. Natural Protein Kinase Inhibitors, Staurosporine, and Chelerythrine Suppress Wheat Blast Disease Caused by Magnaporthe oryzae Triticum. Microorganisms 2022, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, J.Y.; Hwang, I.S.; Yun, B.S.; Kim, B.S.; Hwang, B.K. Isolation and Antifungal and Antioomycete Activities of Staurosporine from Streptomyces roseoflavus Strain LS-A24. J. Agric. Food Chem. 2006, 54, 3041. [Google Scholar] [CrossRef] [PubMed]
- Correa, H.; Aristizabal, F.; Duque, C.; Kerr, R. Cytotoxic and antimicrobial activity of pseudopterosins and seco-pseudopterosins isolated from the octocoral Pseudopterogorgia elisabethae of San Andres and Providencia Islands (Southwest Caribbean Sea). Mar. Drugs 2011, 9, 334. [Google Scholar] [CrossRef]
- Khadayat, K.; Sherpa, D.D.; Malla, K.P.; Shrestha, S.; Rana, N.; Marasini, B.P.; Khanal, S.; Rayamajhee, B.; Bhattarai, B.R.; Parajuli, N. Molecular Identification and Antimicrobial Potential of Streptomyces Species from Nepalese Soil. Int. J. Microbiol. 2020, 2020, 8817467. [Google Scholar] [CrossRef]
- Alam, K.; Mazumder, A.; Sikdar, S.; Zhao, Y.M.; Hao, J.; Song, C.; Wang, Y.; Sarkar, R.; Islam, S.; Zhang, Y.; et al. Streptomyces: The biofactory of secondary metabolites. Front. Microbiol. 2022, 13, 968053. [Google Scholar] [CrossRef]
- Nicault, M.; Zaiter, A.; Dumarcay, S.; Chaimbault, P.; Gelhaye, E.; Leblond, P.; Bontemps, C. Elicitation of Antimicrobial Active Compounds by Streptomyces-Fungus Co-Cultures. Microorganisms 2021, 9, 178. [Google Scholar] [CrossRef]
- Ward, A.C.; Allenby, N.E. Genome mining for the search and discovery of bioactive compounds: The Streptomyces paradigm. FEMS Microbiol. Lett. 2018, 365, fny240. [Google Scholar] [CrossRef]
- Sánchez, C.; Méndez, C.; Salas, J.A. Indolocarbazole natural products: Occurrence, biosynthesis, and biological activity. Nat. Prod. Rep. 2006, 23, 1007. [Google Scholar] [CrossRef]
- Park, B.S.; Abdel-Azeem, A.Z.; Al-Sanea, M.M.; Yoo, K.H.; Tae, J.S.; Lee, S.H. Staurosporine analogues from microbial and synthetic sources and their biological activities. Curr. Med. Chem. 2013, 20, 3872. [Google Scholar] [CrossRef]
- Gani, O.A.; Engh, R.A. Protein kinase inhibition of clinically important staurosporine analogues. Nat. Prod. Rep. 2010, 27, 489. [Google Scholar] [CrossRef]
- Nakatani, S.; Naoe, A.; Yamamoto, Y.; Yamauchi, T.; Yamaguchi, N.; Ishibashi, M. Isolation of bisindole alkaloids that inhibit the cell cycle from Myxomycetes Arcyria ferruginea and Tubifera casparyi. Bioorganic Med. Chem. Lett. 2003, 13, 2879. [Google Scholar] [CrossRef]
- Zhang, L.; Carroll, P.; Meggers, E. Ruthenium complexes as protein kinase inhibitors. Org. Lett. 2004, 6, 521. [Google Scholar] [CrossRef]
- Salas, J.A.; Mendez, C. Indolocarbazole antitumour compounds by combinatorial biosynthesis. Curr. Opin. Chem. Biol. 2009, 13, 152. [Google Scholar] [CrossRef]
- Clark, A.M.; Hufford, C.D. Chapter 2 Antifungal Alkaloids. In The Alkaloids: Chemistry and Pharmacology; Cordell, G.A., Ed.; Academic Press: Cambridge, MA, USA, 1992; Volume 42, p. 117. [Google Scholar]
- Santos, F.C.; Lobo, G.M.; Fernandes, A.S.; Videira, A.; de Almeida, R.F.M. Changes in the Biophysical Properties of the Cell Membrane Are Involved in the Response of Neurospora crassa to Staurosporine. Front. Physiol. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.P.; Monteiro, J.; Lucchi, C.; Kowbel, D.J.; Cordeiro, J.M.; Correia-de-Sa, P.; Rigden, D.J.; Glass, N.L.; Videira, A. Extracellular calcium triggers unique transcriptional programs and modulates staurosporine-induced cell death in Neurospora crassa. Microb. Cell 2014, 1, 289. [Google Scholar] [CrossRef]
- Castro, A.; Lemos, C.; Falcao, A.; Fernandes, A.S.; Glass, N.L.; Videira, A. Rotenone enhances the antifungal properties of staurosporine. Eukaryot. Cell 2010, 9, 906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goncalves, A.P.; Heller, J.; Daskalov, A.; Videira, A.; Glass, N.L. Regulated Forms of Cell Death in Fungi. Front. Microbiol. 2017, 8, 1837. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, T.; Nomoto, H.; Takahashi, I.; Kato, Y.; Morimoto, M.; Tomita, F. Staurosporine, a potent inhibitor of phospholipidCa++dependent protein kinase. Biochem. Biophys. Res. Commun. 1986, 135, 397. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2015, 100, 1–23. [Google Scholar] [CrossRef]
- Xie, J.L.; O’Meara, T.R.; Polvi, E.J.; Robbins, N.; Cowen, L.E. Staurosporine Induces Filamentation in the Human Fungal Pathogen Candida albicans via Signaling through Cyr1 and Protein Kinase A. mSphere 2017, 2, e00056-17. [Google Scholar] [CrossRef] [PubMed]
- LaFayette, S.L.; Collins, C.; Zaas, A.K.; Schell, W.A.; Betancourt-Quiroz, M.; Gunatilaka, A.A.; Perfect, J.R.; Cowen, L.E. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 2010, 6, e1001069. [Google Scholar] [CrossRef]
- Wiesner, D.A.; Dawson, G. Staurosporine induces programmed cell death in embryonic neurons and activation of the ceramide pathway. J. Neurochem. 1996, 66, 1418. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Howe, J.; Tan, K.S.W. Staurosporine-induced programmed cell death in Blastocystis occurs independently of caspases and cathepsins and is augmented by calpain inhibition. Microbiology 2010, 156, 1284. [Google Scholar] [CrossRef]
- Dunai, Z.A.; Imre, G.; Barna, G.; Korcsmaros, T.; Petak, I.; Bauer, P.I.; Mihalik, R. Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells. PLoS ONE 2012, 7, e41945. [Google Scholar] [CrossRef] [PubMed]
- Gescher, A. Staurosporine analogues—Pharmacological toys or useful antitumour agents? Crit. Rev. Oncol./Hematol. 2000, 34, 127. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Goncalves, A.P.; Castro, A.; Lopes, T.A.; Gardner, R.; Glass, N.L.; Videira, A. Modulation of fungal sensitivity to staurosporine by targeting proteins identified by transcriptional profiling. Fungal Genet. Biol. 2011, 48, 1130. [Google Scholar] [CrossRef][Green Version]
- Fernandes, A.S.; Castro, A.; Videira, A. Reduced glutathione export during programmed cell death of Neurospora crassa. Apoptosis 2013, 18, 940. [Google Scholar] [CrossRef]
- Kase, H.; Iwahashi, K.; Matsuda, Y. K-252a, a potent inhibitor of protein kinase C from microbial origin. J. Antibiot. 1986, 39, 1059. [Google Scholar] [CrossRef]
- Wood, J.L.; Stoltz, B.M.; Goodman, S.N. Total Synthesis of (+)-RK-286c, (+)-MLR-52, (+)-Staurosporine, and (+)-K252a. J. Am. Chem. Soc. 1996, 118, 10656. [Google Scholar] [CrossRef]
- Takahashi, I.; Asano, K.; Kawamoto, I.; Tamaoki, T.; Nakano, H. UCN-01 and UCN-02, new selective inhibitors of protein kinase C. J. Antibiot. 1989, 42, 564. [Google Scholar] [CrossRef]
- Takahashi, I.; Saitoh, Y.; Yoshida, M.; Sano, H.; Nakano, H.; Morimoto, M.; Tamaoki, T. UCN-01 and UCN-02, new selective inhibitors of protein kinase C. II. Purification, physico-chemical properties, structural determination and biological activities. J. Antibiot. 1989, 42, 571. [Google Scholar] [CrossRef]
- Han, Z.; Pantazis, P.; Lange, T.S.; Wyche, J.H.; Hendrickson, E.A. The staurosporine analog, Ro-31-8220, induces apoptosis independently of its ability to inhibit protein kinase C. Cell Death Differ. 2000, 7, 521. [Google Scholar] [CrossRef][Green Version]
- Santos, F.C.; Fernandes, A.S.; Antunes, C.A.; Moreira, F.P.; Videira, A.; Marinho, H.S.; de Almeida, R.F. Reorganization of plasma membrane lipid domains during conidial germination. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 156. [Google Scholar] [CrossRef] [PubMed]
- Aresta-Branco, F.; Cordeiro, A.M.; Marinho, H.S.; Cyrne, L.; Antunes, F.; de Almeida, R.F. Gel domains in the plasma membrane of Saccharomyces cerevisiae: Highly ordered, ergosterol-free, and sphingolipid-enriched lipid rafts. J. Biol. Chem. 2011, 286, 5043. [Google Scholar] [CrossRef]
- Santos, F.C.; Marquês, J.T.; Bento-Oliveira, A.; de Almeida, R.F.M. Sphingolipid-enriched domains in fungi. FEBS Lett. 2020, 594, 3698. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Ōmura, S. Chemical biology of natural indolocarbazole products: 30 years since the discovery of staurosporine. J. Antibiot. 2009, 62, 17. [Google Scholar] [CrossRef]
- Furusaki, A.; Hashiba, N.; Matsumoto, T.; Hirano, A.; Iwai, Y.; Omura, S. X-Ray Crystal-Structure of Staurosporine—New Alkaloid from a Streptomyces Strain. J. Chem. Soc. Chem. Comm. 1978. [Google Scholar] [CrossRef]
- Funato, N.; Takayanagi, H.; Konda, Y.; Toda, Y.; Harigaya, Y.; Iwai, Y.; Ōmura, S. Absolute Configuration of Staurosporine By X-Ray Analysis. Tetrahedron Lett. 1994, 35, 1251. [Google Scholar] [CrossRef]
- Fernández, A.; Maddipati, S. A Priori Inference of Cross Reactivity for Drug-Targeted Kinases. J. Med. Chem. 2006, 49, 3092. [Google Scholar] [CrossRef]
- Atwell, S.; Adams, J.M.; Badger, J.; Buchanan, M.D.; Feil, I.K.; Froning, K.J.; Gao, X.; Hendle, J.; Keegan, K.; Leon, B.C.; et al. A novel mode of Gleevec binding is revealed by the structure of spleen tyrosine kinase. J. Biol. Chem. 2004, 279, 55827. [Google Scholar] [CrossRef]
- Zhao, B.; Bower, M.J.; McDevitt, P.J.; Zhao, H.; Davis, S.T.; Johanson, K.O.; Green, S.M.; Concha, N.O.; Zhou, B.B. Structural basis for Chk1 inhibition by UCN-01. J. Biol. Chem. 2002, 277, 46609. [Google Scholar] [CrossRef]
- Cohen, P. Protein kinases—The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309. [Google Scholar] [CrossRef]
- Kolpaksidi, I.P.; Dmitrieva, M.V.; Yarosh, I.V.; Krasnyuk, I.I. Antitumor Drugs Based on Indolocarbazol Derivatives. Pharm. Pharmacol. 2021, 9, 252. [Google Scholar] [CrossRef]
- Omura, S.; Asami, Y.; Crump, A. Staurosporine: New lease of life for parent compound of today’s novel and highly successful anti-cancer drugs. J. Antibiot. 2018, 71, 688. [Google Scholar] [CrossRef]
- Hu, H. Recent discovery and development of selective protein kinase C inhibitors. Drug Discov. Today 1996, 1, 438. [Google Scholar] [CrossRef]
- Thomson, F.J.; Johnson, M.S.; Mitchell, R.; Wolbers, W.B.; Ison, A.J.; MacEwan, D.J. The differential effects of protein kinase C activators and inhibitors on rat anterior pituitary hormone release. Mol. Cell Endocrinol. 1993, 94, 223. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, U.T.; Burgess, G.M. Staurosporine, K-252 and UCN-01: Potent but nonspecific inhibitors of protein kinases. Trends Pharmacol. Sci. 1989, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Axtman, A.D.; Golden, J.E.; Kee, J.M.; Sirois, L.E.; Quiroz, R.V.; Stevens, M.C. Function through bio-inspired, synthesis-informed design: Step-economical syntheses of designed kinase inhibitors. Org. Chem. Front. 2014, 1, 1166. [Google Scholar] [CrossRef]
- Mukthavaram, R.; Jiang, P.; Saklecha, R.; Simberg, D.; Bharati, I.S.; Nomura, N.; Chao, Y.; Pastorino, S.; Pingle, S.C.; Fogal, V.; et al. High-efficiency liposomal encapsulation of a tyrosine kinase inhibitor leads to improved in vivo toxicity and tumor response profile. Int. J. Nanomed. 2013, 8, 3991. [Google Scholar] [CrossRef]
- Yoshida, S.; Anraku, Y. Characterization of staurosporine-sensitive mutants of Saccharomyces cerevisiae: Vacuolar functions affect staurosporine sensitivity. Mol. Gen. Genet. 2000, 263, 877. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Midostaurin (RYDAPT)—FDA-Approved Drugs. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/midostaurin (accessed on 17 September 2025).
- Sausville, E.A.; Lush, R.D.; Headlee, D.; Smith, A.C.; Figg, W.D.; Arbuck, S.G.; Senderowicz, A.M.; Fuse, E.; Tanii, H.; Kuwabara, T.; et al. Clinical pharmacology of UCN-01: Initial observations and comparison to preclinical models. Cancer Chemother. Pharmacol. 1998, 42, S54. [Google Scholar] [CrossRef]
- Knapper, S.; Burnett, A.K.; Littlewood, T.; Kell, W.J.; Agrawal, S.; Chopra, R.; Clark, R.; Levis, M.J.; Small, D. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood 2006, 108, 3262. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.K.; DiDone, L.; Ogu, D.; Schor, S.; Krysan, D.J. Identification, in vitro activity and mode of action of phosphoinositide-dependent-1 kinase inhibitors as antifungal molecules. ACS Chem. Biol. 2011, 6, 502. [Google Scholar] [CrossRef]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.H. Activators and Inhibitors of Protein Kinase C (PKC): Their Applications in Clinical Trials. Pharmaceutics 2021, 13, 1748. [Google Scholar] [CrossRef]
- Nakanishi, S.; Matsuda, Y.; Iwahashi, K.; Kase, H. K-252b, c and d, potent inhibitors of protein kinase C from microbial origin. J. Antibiot. 1986, 39, 1066. [Google Scholar] [CrossRef]
- Osada, H.; Takahashi, H.; Tsunoda, K.; Kusakabe, H.; Isono, K. A new inhibitor of protein kinase C, RK-286C (4′-demethylamino-4′-hydroxystaurosporine). I. Screening, taxonomy, fermentation and biological activity. J. Antibiot. 1990, 43, 163. [Google Scholar] [CrossRef]
- Sancelme, M.; Fabre, S.; Prudhomme, M. Antimicrobial activities of indolocarbazole and bis-indole protein kinase C inhibitors. J. Antibiot. 1994, 47, 792. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, R.; Fang, J.; Zhang, N.; Pu, F.; Lei, Z.; Ding, W.; Jiang, Y. Cytotoxic and Antifungal Staurosporine Derivatives from Marine-Derived Actinomycete Streptomyces sp. ZS-A121. Chem. Biodivers. 2024, 21, e202301712. [Google Scholar] [CrossRef] [PubMed]
- Makino, M.; Sugimoto, H.; Shiro, Y.; Asamizu, S.; Onaka, H.; Nagano, S. Crystal structures and catalytic mechanism of cytochrome P450 StaP that produces the indolocarbazole skeleton. Proc. Natl. Acad. Sci. USA 2007, 104, 11591. [Google Scholar] [CrossRef]
- Onaka, H.; Taniguchi, S.; Igarashi, Y.; Furumai, T. Cloning of the staurosporine biosynthetic gene cluster from Streptomyces sp. TP-A0274 and its heterologous expression in Streptomyces lividans. J. Antibiot. 2002, 55, 1063. [Google Scholar] [CrossRef]
- Onaka, H.; Asamizu, S.; Igarashi, Y.; Yoshida, R.; Furumai, T. Cytochrome P450 homolog is responsible for C-N bond formation between aglycone and deoxysugar in the staurosporine biosynthesis of Streptomyces sp. TP-A0274. Biosci. Biotechnol. Biochem. 2005, 69, 1753. [Google Scholar] [CrossRef]
- Salas, A.P.; Zhu, L.; Sanchez, C.; Brana, A.F.; Rohr, J.; Mendez, C.; Salas, J.A. Deciphering the late steps in the biosynthesis of the anti-tumour indolocarbazole staurosporine: Sugar donor substrate flexibility of the StaG glycosyltransferase. Mol. Microbiol. 2005, 58, 17. [Google Scholar] [CrossRef]
- Eui Il, H.; Bong Sik, Y.; Sang Han, L.; Soo Kie, K.; Se Jin, L. 7-Oxostaurosporine Selectively Inhibits the Mycelial Form of Candida albicans. J. Microbiol. Biotechnol. 2004, 14, 1067. [Google Scholar]
- Mojicevic, M.; D’Agostino, P.M.; Pavic, A.; Vojnovic, S.; Senthamaraikannan, R.; Vasiljevic, B.; Gulder, T.A.M.; Nikodinovic-Runic, J. Streptomyces sp. BV410 isolate from chamomile rhizosphere soil efficiently produces staurosporine with antifungal and antiangiogenic properties. Microbiologyopen 2020, 9, e986. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Koshino, H.; Kudo, T.; Onose, R.; Isono, K. A new inhibitor of protein kinase C, RK-1409 (7-oxostaurosporine). I. Taxonomy and biological activity. J. Antibiot. 1992, 45, 189. [Google Scholar] [CrossRef]
- Bonjouklian, R.; Smitka, T.A.; Doolin, L.E.; Molloy, R.M.; Debono, M.; Shaffer, S.A.; Moore, R.E.; Stewart, J.B.; Patterson, G.M.L. Tjipanazoles, new antifungal agents from the blue-green alga Tolypothrix tjipanasensis. Tetrahedron 1991, 47, 7739. [Google Scholar] [CrossRef]
- Li, X.; Huang, P.; Wang, Q.; Xiao, L.; Liu, M.; Bolla, K.; Zhang, B.; Zheng, L.; Gan, B.; Liu, X.; et al. Staurosporine from the endophytic Streptomyces sp. strain CNS-42 acts as a potential biocontrol agent and growth elicitor in cucumber. Antonie Van. Leeuwenhoek 2014, 106, 515. [Google Scholar] [CrossRef]
- Islam, M.T.; von Tiedemann, A.; Laatsch, H. Protein kinase C is likely to be involved in zoosporogenesis and maintenance of flagellar motility in the peronosporomycete zoospores. Mol. Plant Microbe Interact. 2011, 24, 938. [Google Scholar] [CrossRef] [PubMed]
- Magae, Y.; Magae, J. Effect of staurosporine on growth and hyphal morphology of Pleurotus ostreatus. J. Gen. Microbiol. 1993, 139, 161. [Google Scholar] [CrossRef][Green Version]
- Yoshida, S.; Ikeda, E.; Uno, I.; Mitsuzawa, H. Characterization of a staurosporine- and temperature-sensitive mutant, stt1, of Saccharomyces cerevisiae: STT1 is allelic to PKC1. Mol. Gen. Genet. 1992, 231, 337. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Chen, C.Y.; Levin, D.E. Saccharomyces cerevisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J. Biol. Chem. 1994, 269, 16829. [Google Scholar] [CrossRef]
- Abe, K.; Yoshida, M.; Usui, T.; Horinouchi, S.; Beppu, T. Highly synchronous culture of fibroblasts from G2 block caused by staurosporine, a potent inhibitor of protein kinases. Exp. Cell Res. 1991, 192, 122. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Yang, X.; Yang, M.; Xu, W.; Li, Y.; Tao, L. Staurosporine shows insecticidal activity against Mythimna separata Walker (Lepidoptera: Noctuidae) potentially via induction of apoptosis. Pestic. Biochem. Physiol. 2016, 128, 37. [Google Scholar] [CrossRef]
- Borah, S.; Shivarathri, R.; Kaur, R. The Rho1 GTPase-activating protein CgBem2 is required for survival of azole stress in Candida glabrata. J. Biol. Chem. 2011, 286, 34311. [Google Scholar] [CrossRef] [PubMed]
- Markovich, S.; Yekutiel, A.; Shalit, I.; Shadkchan, Y.; Osherov, N. Genomic approach to identification of mutations affecting caspofungin susceptibility in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2004, 48, 3871. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Uppuluri, P.; Zaas, A.K.; Collins, C.; Senn, H.; Perfect, J.R.; Heitman, J.; Cowen, L.E. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr. Biol. CB 2009, 19, 621. [Google Scholar] [CrossRef]
- Xie, J.L.; Grahl, N.; Sless, T.; Leach, M.D.; Kim, S.H.; Hogan, D.A.; Robbins, N.; Cowen, L.E. Signaling through Lrg1, Rho1 and Pkc1 Governs Candida albicans Morphogenesis in Response to Diverse Cues. PLoS Genet. 2016, 12, e1006405. [Google Scholar] [CrossRef]
- Macias-Paz, I.U.; Perez-Hernandez, S.; Tavera-Tapia, A.; Luna-Arias, J.P.; Guerra-Cardenas, J.E.; Reyna-Beltran, E. Candida albicans the main opportunistic pathogenic fungus in humans. Rev. Argent. Microbiol. 2023, 55, 189. [Google Scholar] [CrossRef]
- Bianchi, D.E.; Turian, G. Lipid Content Of Conidia Of Neurospora Crassa. Nature 1967, 214, 1344. [Google Scholar] [CrossRef]
- Kushwaha, S.C.; Kates, M.; Kramer, J.K.G.; Subden, R.E. Lipid-Composition Of Neurospora-Crassa. Lipids 1976, 11, 778. [Google Scholar] [CrossRef]
- Qin, X.Y.; Lv, J.H.; Cui, J.; Fang, X.; Zhang, Y. Curcumin protects against staurosporine toxicity in rat neurons. Neurosci. Bull. 2012, 28, 606. [Google Scholar] [CrossRef]
- Burugula, B.; Ganesh, B.S.; Chintala, S.K. Curcumin attenuates staurosporine-mediated death of retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4263. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Khan, N.; Shreaz, S.; Bhatia, R.; Ahmad, S.I.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Anticandidal activity of curcumin and methyl cinnamaldehyde. Fitoterapia 2012, 83, 434. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, J.; Rao, P.; Poojara, L.; Goswami, D.; Acharya, D.; Patel, S.K.; Rawal, R.M. Unravelling the antifungal mode of action of curcumin by potential inhibition of CYP51B: A computational study validated in vitro on mucormycosis agent, Rhizopus oryzae. Arch. Biochem. Biophys. 2021, 712, 109048. [Google Scholar] [CrossRef] [PubMed]
- Alsamman, K.; El-Masry, O.S. Staurosporine alleviates cisplatin chemoresistance in human cancer cell models by suppressing the induction of SQSTM1/p62. Oncol. Rep. 2018, 40, 2157. [Google Scholar] [CrossRef]
- Gayler, K.M.; Kong, K.; Reisenauer, K.; Taube, J.H.; Wood, J.L. Staurosporine Analogs Via C-H Borylation. ACS Med. Chem. Lett. 2020, 11, 2441. [Google Scholar] [CrossRef]
- Goncalves, A.P.; Cordeiro, J.M.; Monteiro, J.; Munoz, A.; Correia-de-Sa, P.; Read, N.D.; Videira, A. Activation of a TRP-like channel and intracellular Ca2+ dynamics during phospholipase-C-mediated cell death. J. Cell Sci. 2014, 127, 3817. [Google Scholar] [CrossRef]
- Gonçalves, A.P.; Cordeiro, J.M.; Monteiro, J.; Lucchi, C.; Correia-de-Sá, P.; Videira, A. Involvement of mitochondrial proteins in calcium signaling and cell death induced by staurosporine in Neurospora crassa. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 1064. [Google Scholar] [CrossRef][Green Version]
- Belmokhtar, C.A.; Hillion, J.; Segal-Bendirdjian, E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 2001, 20, 3354. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Kowbel, D.; Nueda, M.J.; Palma-Guerrero, J.; Glass, N.L.; Lopez-Llorca, L.V. Neurospora crassa transcriptomics reveals oxidative stress and plasma membrane homeostasis biology genes as key targets in response to chitosan. Mol. Biosyst. 2016, 12, 391. [Google Scholar] [CrossRef]
- Robinson, C.A.; Denison, C.; Burkenstock, A.; Nutter, C.; Gordon, D.M. Cellular conditions that modulate the fungicidal activity of occidiofungin. J. Appl. Microbiol. 2017, 123, 380. [Google Scholar] [CrossRef]
- Goncalves, A.P.; Videira, A. Mitochondrial type II NAD(P)H dehydrogenases in fungal cell death. Microb. Cell 2015, 2, 68. [Google Scholar] [CrossRef]
- Modok, S.; Heyward, C.; Callaghan, R. P-glycoprotein retains function when reconstituted into a sphingolipid- and cholesterol-rich environment. J. Lipid Res. 2004, 45, 1910. [Google Scholar] [CrossRef]
- Goodchild, J.A.; Curtis, B.N.; Pan, Y.; Jiang, Y.; Jiao, F.; Gladfelter, A.S.; Scheuring, S. Septin higher-order structure on yeast membranes in vitro. Nat. Commun. 2025, 16, 5055. [Google Scholar] [CrossRef]
- Mela, A.; Momany, M. Septins coordinate cell wall integrity and lipid metabolism in a sphingolipid-dependent process. J. Cell Sci. 2022, 135, jcs258336. [Google Scholar] [CrossRef]
- Bento-Oliveira, A.; Starosta, R.; de Almeida, R.F.M. Interaction of the antifungal ketoconazole and its diphenylphosphine derivatives with lipid bilayers: Insights into their antifungal action. Arch. Biochem. Biophys. 2024, 753, 109919. [Google Scholar] [CrossRef] [PubMed]
- Bento-Oliveira, A.; Starosta, R.; de Almeida, R.F.M. Membrane insertion properties of ketoconazole derivatives: Elucidating the path to fight drug resistant fungal infections. J. Mol. Liq. 2025, 425, 127195. [Google Scholar] [CrossRef]
- de Almeida, R.F.M.; Santos, F.C.; Marycz, K.; Alicka, M.; Krasowska, A.; Suchodolski, J.; Panek, J.J.; Jezierska, A.; Starosta, R. New diphenylphosphane derivatives of ketoconazole are promising antifungal agents. Sci. Rep. 2019, 9, 16214. [Google Scholar] [CrossRef] [PubMed]
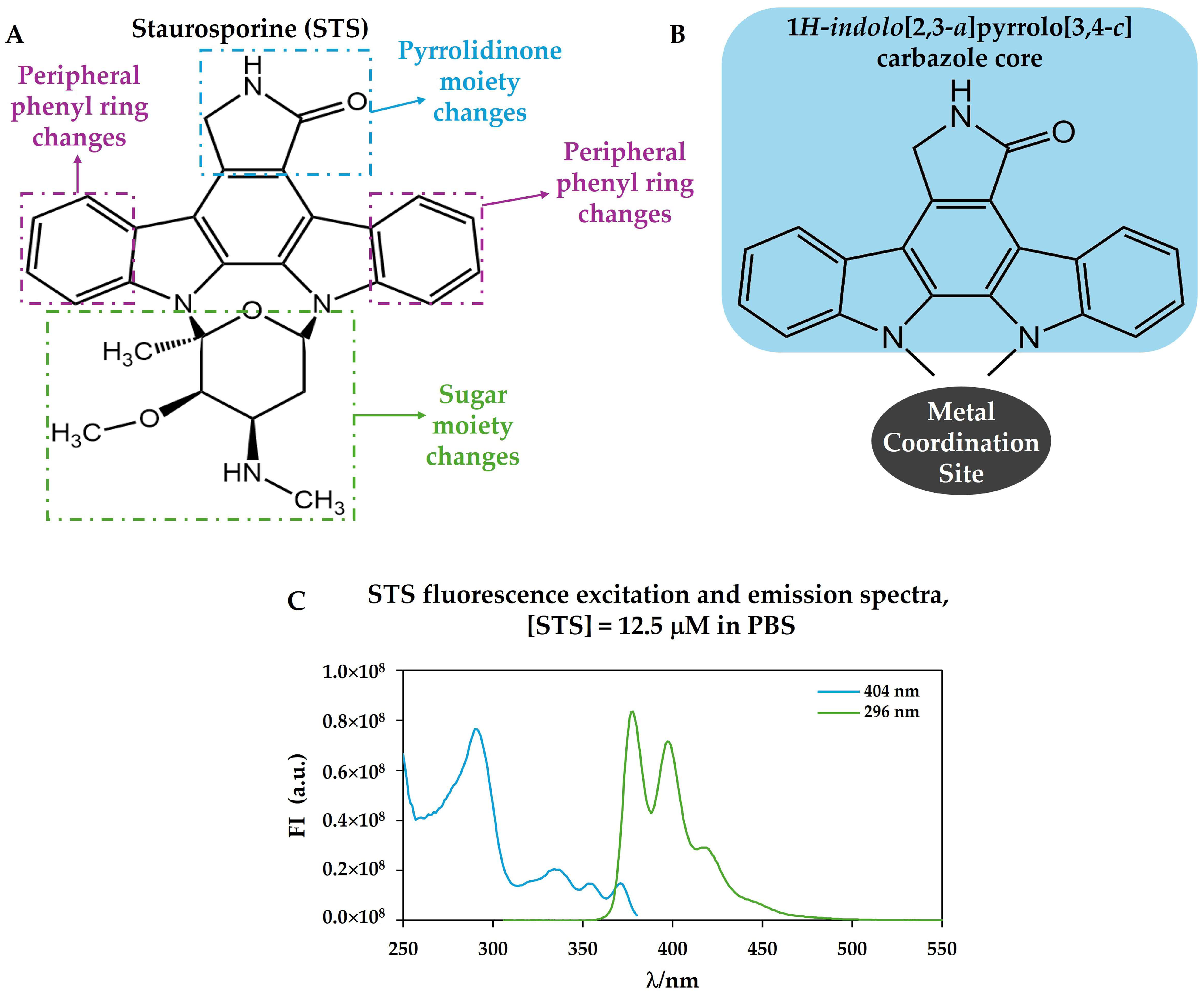
| Name | Biological Source | Protein Kinase | IC50 (nM) | References |
|---|---|---|---|---|
| Staurosporine (AM-2282) | Streptomyces staurosporeus | PKC PKA | 2.7 8.2 | [15,23,45] |
| UCN-01 (7-hydroxystaurosporine) | Streptomyces sp. N-126 | PKC PKA | 4.1 42 | [45,46,69] |
| UCN-02 (7-epi-hydroxystaurosporine) | PKC PKA | 62 250 | [45,46,70] | |
| K-252a | Nocardiopsis sp. K-252 | PKC | 32.9 | [23] |
| K-252d | Nocardiopsis sp. K-290 | PKC | 337 | [71] |
| RK-286c | Streptomyces sp. RK-286 | PKC | 3000 | [28,72] |
| Compound Name | Biological Source | Antifungal Activity | MIC (μg/mL) | Conventional Dilution Method | References |
|---|---|---|---|---|---|
| Staurosporine (AM-2282) | Streptomyces staurosporeus | C. albicans | 6.25 | Petri dish | [14] |
| C. pseudotropicalis | 3.13 | ||||
| Saccharomyces sake | 3.13 | ||||
| A. niger | 25 | ||||
| A. brevipus | 3.13 | ||||
| A. fumigatus | 12.5 | ||||
| Trichophyton rubrum | 6.25 | ||||
| Trichophyton mentagrophytes | 25 | ||||
| C. neoformans | 50 | ||||
| Sclerotinia cinerea | 0.78 | ||||
| P. oryzae | 0.78 | ||||
| Pleorotus ostreatus | 4.48 | Petri dish | [85] | ||
| Protoplasts | >3.03 | ||||
| N. crassa | ~6 | Petri dish | [31] | ||
| STS | S. roseoflavus LS-A24 | P. capsici | 1 | 24-well microtiter dish | [16] |
| R. solani | 1 | ||||
| Colletotrichum orbiculare | 1 | ||||
| Botrytis cinerea | 50 | ||||
| Cladosporium cucumerinum | 1 | ||||
| Didymella bryoniae | 10 | ||||
| F. oxysporum f.sp. lycopersici | 50 | ||||
| S. cerevisiae | 1 | ||||
| Bacillus subtilis ssp. subtilis | 10 | ||||
| X. vesicatoria | 50 | ||||
| STS | Streptomyces sp. B5136 | Plasmopara viticola (zoospores) | 0.02 | Petri dish | [84] |
| RK-1409 (7-oxostaurosporine) | 0.19 | ||||
| Candida krusei | 3.1 | [79] | |||
| Candida tropicalis | 50 | ||||
| Candida lusitaniae | 12.5 | ||||
| STS | Streptomyces sp. BV410 | C. albicans | 0.098 | Petri dish | [80] |
| C. krusei | 0.39 | ||||
| Candida parapsilosis | 0.098 | ||||
| Candida glabrata | 0.024 | ||||
| Holyrine A | Streptomyces sp. ZS-A121 | C. albicans | 12.5 | Petri dish | [74] |
| K-252d | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.C.; Marquês, J.T.; Santos, E.N.; de Almeida, R.F.M. Staurosporine as an Antifungal Agent. Int. J. Mol. Sci. 2025, 26, 9683. https://doi.org/10.3390/ijms26199683
Santos FC, Marquês JT, Santos EN, de Almeida RFM. Staurosporine as an Antifungal Agent. International Journal of Molecular Sciences. 2025; 26(19):9683. https://doi.org/10.3390/ijms26199683
Chicago/Turabian StyleSantos, Filipa C., Joaquim T. Marquês, Eva N. Santos, and Rodrigo F. M. de Almeida. 2025. "Staurosporine as an Antifungal Agent" International Journal of Molecular Sciences 26, no. 19: 9683. https://doi.org/10.3390/ijms26199683
APA StyleSantos, F. C., Marquês, J. T., Santos, E. N., & de Almeida, R. F. M. (2025). Staurosporine as an Antifungal Agent. International Journal of Molecular Sciences, 26(19), 9683. https://doi.org/10.3390/ijms26199683







