Emerging Role of Tripartite Synaptic Transmission in the Pathomechanism of Autosomal-Dominant Sleep-Related Hypermotor Epilepsy
Abstract
1. Introduction
2. Clinical Features of ADSHE/SHE
3. Phenotypic Features of ADSHE Rodent Models and Validations
3.1. DEPDC5
3.2. CHRNB2-Mutant Models
3.3. CHRNA4-Mutant Models
3.3.1. S280F- and insL-Mutant Models
3.3.2. S284L-Mutant Models
4. Transmission Abnormalities in S284L-TG and S286L-TG
4.1. Transmission Abnormalities During the Interictal Stage, Including Wakefulness and SWS
4.2. Impact of Loss-of-Function of S286L-Mutant α4β2-nAChRs in S286L-TG
4.3. Transmission Abnormalities During ADSHE Seizures
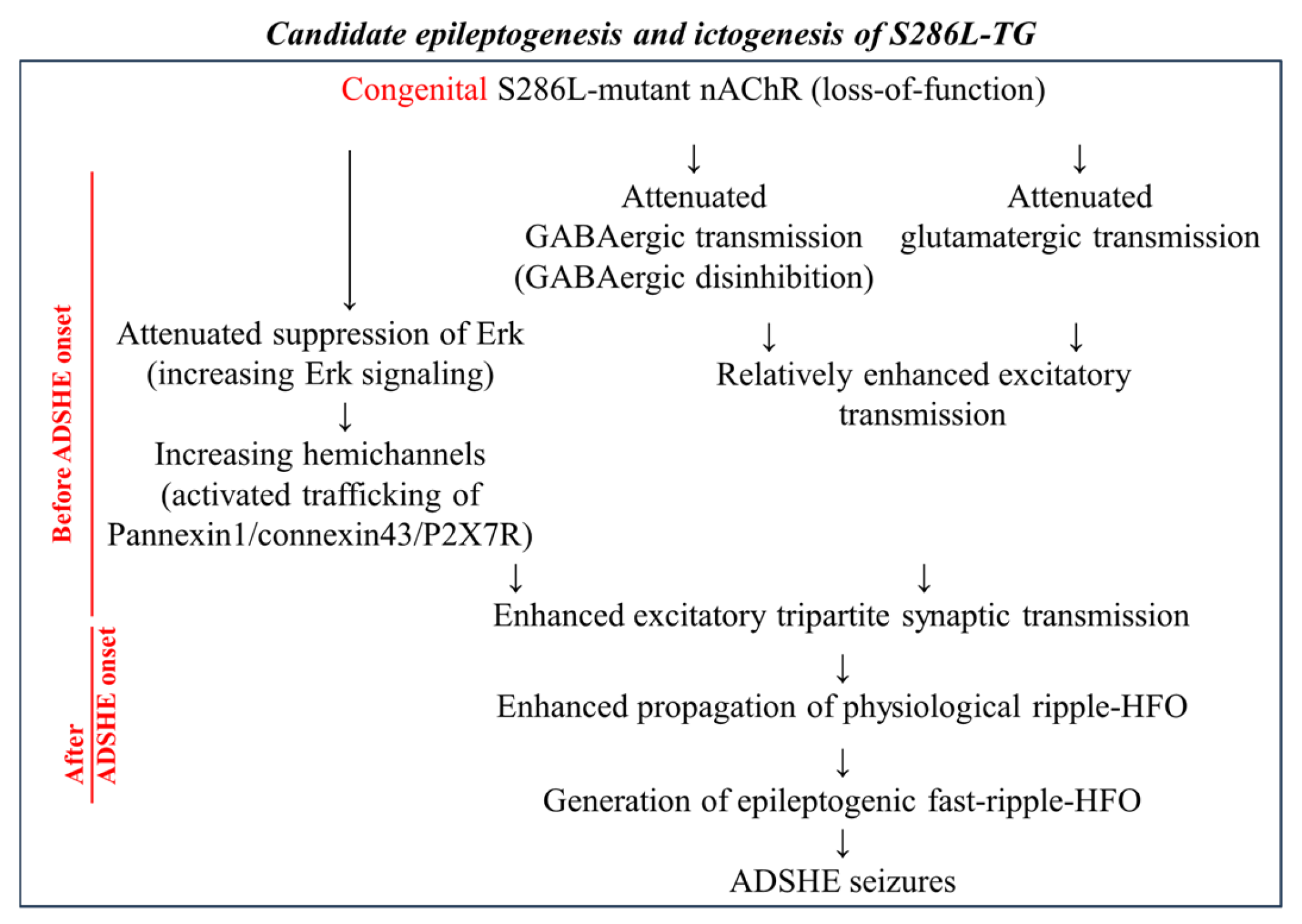
5. Astroglial Age-Dependent Epileptogenesis and Event-Related (Sleep-Related) Ictogenesis in S286L-TG
5.1. Impact of Upregulated Connexin43 in S286L-TG
5.2. Impact of Upregulated Pannexin1 in S286L-TG
5.3. Impact of High-Frequency Oscillation in S286L-TG
5.4. Impact of Upregulated P2X7R in S286L-TG
6. Potential Medication Targeting Tripartite Synaptic Transmission
7. Remaining Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| nAChRs | nicotinic acetylcholine receptors |
| ADSHE | autosomal-dominant sleep-related hypermotor epilepsy |
| DC | direct-current |
| ENW | episodic nocturnal wandering |
| HFO | high-frequency oscillation |
| M2C | secondary motor cortex |
| MDTN | mediodorsal thalamic nucleus |
| MoTN | motor thalamic nuclei |
| NPA | nocturnal paroxysmal arousal |
| NPD | nocturnal paroxysmal dystonia |
| non-REM | non-rapid eye movement |
| OFC | orbitofrontal cortex |
| PPN | pedunculopontine nucleus |
| RTN | reticular thalamic nucleus |
| SHE | sleep-related hypermotor epilepsy |
| SNr | substantia nigra pars reticulata |
| STN | subthalamic nucleus |
| SWS | slow-wave sleep |
References
- Provini, F.; Plazzi, G.; Tinuper, P.; Vandi, S.; Lugaresi, E.; Montagna, P. Nocturnal frontal lobe epilepsy: A clinical and polygraphic overview of 100 consecutive cases. Brain 1999, 122 Pt 6, 1017–1031. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Bhatia, K.P.; Lopes-Cendes, I.; Fish, D.R.; Marsden, C.D.; Andermann, E.; Andermann, F.; Desbiens, R.; Keene, D.; Cendes, F.; et al. Autosomal dominant nocturnal frontal lobe epilepsy. A distinctive clinical disorder. Brain 1995, 118 Pt 1, 61–73. [Google Scholar] [CrossRef]
- Riney, K.; Bogacz, A.; Somerville, E.; Hirsch, E.; Nabbout, R.; Scheffer, I.E.; Zuberi, S.M.; Alsaadi, T.; Jain, S.; French, J.; et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset at a variable age: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1443–1474. [Google Scholar] [CrossRef] [PubMed]
- Tinuper, P.; Bisulli, F.; Cross, J.H.; Hesdorffer, D.; Kahane, P.; Nobili, L.; Provini, F.; Scheffer, I.E.; Tassi, L.; Vignatelli, L.; et al. Definition and diagnostic criteria of sleep-related hypermotor epilepsy. Neurology 2016, 86, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.F.; Bhatia, K.P.; Fish, D.R.; Marsden, C.D.; Lopes-Cendes, I.; Andermann, F.; Andermann, E.; Desbiens, R.; Cendes, F.; et al. Autosomal dominant frontal epilepsy misdiagnosed as sleep disorder. Lancet 1994, 343, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Steinlein, O.K.; Mulley, J.C.; Propping, P.; Wallace, R.H.; Phillips, H.A.; Sutherland, G.R.; Scheffer, I.E.; Berkovic, S.F. A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 1995, 11, 201–203. [Google Scholar] [CrossRef]
- Steinlein, O.K.; Magnusson, A.; Stoodt, J.; Bertrand, S.; Weiland, S.; Berkovic, S.F.; Nakken, K.O.; Propping, P.; Bertrand, D. An insertion mutation of the CHRNA4 gene in a family with autosomal dominant nocturnal frontal lobe epilepsy. Hum. Mol. Genet. 1997, 6, 943–947. [Google Scholar] [CrossRef]
- Steinlein, O.K.; Stoodt, J.; Mulley, J.; Berkovic, S.; Scheffer, I.E.; Brodtkorb, E. Independent occurrence of the CHRNA4 Ser248Phe mutation in a Norwegian family with nocturnal frontal lobe epilepsy. Epilepsia 2000, 41, 529–535. [Google Scholar] [CrossRef]
- Hirose, S.; Iwata, H.; Akiyoshi, H.; Kobayashi, K.; Ito, M.; Wada, K.; Kaneko, S.; Mitsudome, A. A novel mutation of CHRNA4 responsible for autosomal dominant nocturnal frontal lobe epilepsy. Neurology 1999, 53, 1749–1753. [Google Scholar] [CrossRef]
- Ito, M.; Kobayashi, K.; Fujii, T.; Okuno, T.; Hirose, S.; Iwata, H.; Mitsudome, A.; Kaneko, S. Electroclinical picture of autosomal dominant nocturnal frontal lobe epilepsy in a Japanese family. Epilepsia 2000, 41, 52–58. [Google Scholar] [CrossRef]
- Cho, Y.-W.; Motamedi, G.K.; Laufenberg, I.; Sohn, S.-I.; Lim, J.-G.; Lee, H.; Yi, S.-D.; Lee, J.-H.; Kim, D.-K.; Reba, R.; et al. A Korean kindred with autosomal dominant nocturnal frontal lobe epilepsy and mental retardation. Arch. Neurol. 2003, 60, 1625–1632. [Google Scholar] [CrossRef]
- Magnusson, A.; Stordal, E.; Brodtkorb, E.; Steinlein, O. Schizophrenia, psychotic illness and other psychiatric symptoms in families with autosomal dominant nocturnal frontal lobe epilepsy caused by different mutations. Psychiatr. Genet. 2003, 13, 91–95. [Google Scholar] [CrossRef]
- McLellan, A.; Phillips, H.A.; Rittey, C.; Kirkpatrick, M.; Mulley, J.C.; Goudie, D.; Stephenson, J.B.P.; Tolmie, J.; Scheffer, I.E.; Berkovic, S.F.; et al. Phenotypic comparison of two Scottish families with mutations in different genes causing autosomal dominant nocturnal frontal lobe epilepsy. Epilepsia 2003, 44, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, T.; Kumada, T.; Saito, K.; Fujii, T. Autism in siblings with autosomal dominant nocturnal frontal lobe epilepsy. Brain Dev. 2013, 35, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.A.; Marini, C.; Scheffer, I.E.; Sutherland, G.R.; Mulley, J.C.; Berkovic, S.F. A de novo mutation in sporadic nocturnal frontal lobe epilepsy. Ann. Neurol. 2000, 48, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Rozycka, A.; Skorupska, E.; Kostyrko, A.; Trzeciak, W.H. Evidence for S284L mutation of the CHRNA4 in a white family with autosomal dominant nocturnal frontal lobe epilepsy. Epilepsia 2003, 44, 1113–1117. [Google Scholar] [CrossRef]
- Sansoni, V.; Nobili, L.; Proserpio, P.; Ferini-Strambi, L.; Combi, R. A de novo mutation in an Italian sporadic patient affected by nocturnal frontal lobe epilepsy. J. Sleep Res. 2012, 21, 352–353. [Google Scholar] [CrossRef]
- Bertrand, D.; Elmslie, F.; Hughes, E.; Trounce, J.; Sander, T.; Bertrand, S.; Steinlein, O.K. The CHRNB2 mutation I312M is associated with epilepsy and distinct memory deficits. Neurobiol. Dis. 2005, 20, 799–804. [Google Scholar] [CrossRef]
- Cho, Y.-W.; Yi, S.-D.; Lim, J.-G.; Kim, D.-K.; Motamedi, G.K. Autosomal dominant nocturnal frontal lobe epilepsy and mild memory impairment associated with CHRNB2 mutation I312M in the neuronal nicotinic acetylcholine receptor. Epilepsy Behav. 2008, 13, 361–365. [Google Scholar] [CrossRef]
- De Fusco, M.; Becchetti, A.; Patrignani, A.; Annesi, G.; Gambardella, A.; Quattrone, A.; Ballabio, A.; Wanke, E.; Casari, G. The nicotinic receptor β2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat. Genet. 2000, 26, 275–276. [Google Scholar] [CrossRef]
- Díaz-Otero, F.; Quesada, M.; Morales-Corraliza, J.; Martínez-Parra, C.; Gómez-Garre, P.; Serratosa, J.M. Autosomal dominant nocturnal frontal lobe epilepsy with a mutation in the CHRNB2 gene. Epilepsia 2008, 49, 516–520. [Google Scholar] [CrossRef]
- Gambardella, A.; Annesi, G.; De Fusco, M.; Patrignani, A.; Aguglia, U.; Annesi, F.; Pasqua, A.A.; Spadafora, P.; Oliveri, R.L.; Valentino, P.; et al. A new locus for autosomal dominant nocturnal frontal lobe epilepsy maps to chromosome 1. Neurology 2000, 55, 1467–1471. [Google Scholar] [CrossRef]
- Hoda, J.-C.; Gu, W.; Friedli, M.; Phillips, H.A.; Bertrand, S.; Antonarakis, S.E.; Goudie, D.; Roberts, R.; Scheffer, I.E.; Marini, C.; et al. Human nocturnal frontal lobe epilepsy: Pharmocogenomic profiles of pathogenic nicotinic acetylcholine receptor β-subunit mutations outside the ion channel pore. Mol. Pharmacol. 2008, 74, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Leniger, T.; Kananura, C.; Hufnagel, A.; Bertrand, S.; Bertrand, D.; Steinlein, O.K. A new Chrna4 mutation with low penetrance in nocturnal frontal lobe epilepsy. Epilepsia 2003, 44, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.A.; Favre, I.; Kirkpatrick, M.; Zuberi, S.M.; Goudie, D.; Heron, S.E.; Scheffer, I.E.; Sutherland, G.R.; Berkovic, S.F.; Bertrand, D.; et al. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am. J. Hum. Genet. 2001, 68, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Okada, M.; Kaneko, S.; Mitsudome, A. Are some idiopathic epilepsies disorders of ion channels?: A working hypothesis. Epilepsy Res. 2000, 41, 191–204. [Google Scholar] [CrossRef]
- Kaneko, S.; Okada, M.; Iwasa, H.; Yamakawa, K.; Hirose, S. Genetics of epilepsy: Current status and perspectives. Neurosci. Res. 2002, 44, 11–30. [Google Scholar] [CrossRef]
- Hirose, S.; Mitsudome, A.; Okada, M.; Kaneko, S.; Epilepsy Genetic Study Group, Japan. Genetics of idiopathic epilepsies. Epilepsia 2005, 46 (Suppl. S1), 38–43. [Google Scholar] [CrossRef]
- Combi, R.; Dalprà, L.; Ferini-Strambi, L.; Tenchini, M.L. Frontal lobe epilepsy and mutations of the corticotropin-releasing hormone gene. Ann. Neurol. 2005, 58, 899–904. [Google Scholar] [CrossRef]
- Sansoni, V.; Forcella, M.; Mozzi, A.; Fusi, P.; Ambrosini, R.; Ferini-Strambi, L.; Combi, R. Functional characterization of a CRH missense mutation identified in an ADNFLE family. PLoS ONE 2013, 8, e61306. [Google Scholar] [CrossRef]
- Heron, S.E.; Smith, K.R.; Bahlo, M.; Nobili, L.; Kahana, E.; Licchetta, L.; Oliver, K.L.; Mazarib, A.; Afawi, Z.; Korczyn, A.; et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 2012, 44, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Wang, C.; Zhuo, M.-Q.; Zhai, Q.-X.; Chen, Q.; Guo, Y.-X.; Zhang, Y.-X.; Gui, J.; Tang, Z.-H.; Zeng, X.-L. Exome sequencing identified a novel missense mutation c.464G>A (p.G155D) in Ca2+-binding protein 4 (CABP4) in a Chinese pedigree with autosomal dominant nocturnal frontal lobe epilepsy. Oncotarget 2017, 8, 78940–78947. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Picard, F.; Rudolf, G.; Noé, E.; Achaz, G.; Thomas, P.; Genton, P.; Mundwiller, E.; Wolff, M.; Marescaux, C.; et al. Mutations of DEPDC5 cause autosomal dominant focal epilepsies. Nat. Genet. 2013, 45, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, S.; Licchetta, L.; Tinuper, P.; Bisulli, F.; Pippucci, T. GATOR1 complex: The common genetic actor in focal epilepsies. J. Med. Genet. 2016, 53, 503–510. [Google Scholar] [CrossRef]
- Becchetti, A.; Grandi, L.C.; Colombo, G.; Meneghini, S.; Amadeo, A. Nicotinic Receptors in Sleep-Related Hypermotor Epilepsy: Pathophysiology and Pharmacology. Brain Sci. 2020, 10, 907. [Google Scholar] [CrossRef]
- Okada, M. Can rodent models elucidate the pathomechanisms of genetic epilepsy? Br. J. Pharmacol. 2022, 179, 1620–1639. [Google Scholar] [CrossRef]
- Jalaiei, A.; Asadi, M.R.; Daneshmandpour, Y.; Rezazadeh, M.; Ghafouri-Fard, S. Clinical, molecular, physiologic, and therapeutic feature of patients with CHRNA4 and CHRNB2 deficiency: A systematic review. J. Neurochem. 2025, 169, e16200. [Google Scholar] [CrossRef]
- Becchetti, A.; Grandi, L.C.; Cerina, M.; Amadeo, A. Nicotinic acetylcholine receptors and epilepsy. Pharmacol. Res. 2023, 189, 106698. [Google Scholar] [CrossRef]
- Bertrand, D.; Picard, F.; Le Hellard, S.; Weiland, S.; Favre, I.; Phillips, H.; Bertrand, S.; Berkovic, S.F.; Malafosse, A.; Mulley, J. How mutations in the nAChRs can cause ADNFLE epilepsy. Epilepsia 2002, 43 (Suppl. S5), 112–122. [Google Scholar] [CrossRef]
- Figl, A.; Viseshakul, N.; Shafaee, N.; Forsayeth, J.; Cohen, B.N. Two mutations linked to nocturnal frontal lobe epilepsy cause use-dependent potentiation of the nicotinic ACh response. J. Physiol. 1998, 513 Pt 3, 655–670. [Google Scholar] [CrossRef]
- Picard, F.; Bertrand, S.; Steinlein, O.K.; Bertrand, D. Mutated nicotinic receptors responsible for autosomal dominant nocturnal frontal lobe epilepsy are more sensitive to carbamazepine. Epilepsia 1999, 40, 1198–1209. [Google Scholar] [CrossRef]
- Rodrigues, S.; Ferreira, T.L. Muscimol injection into the substantia nigra but not globus pallidus affects prepulse inhibition and startle reflex. Neuropharmacology 2020, 162, 107796. [Google Scholar] [CrossRef]
- Rodrigues-Pinguet, N.; Jia, L.; Li, M.; Figl, A.; Klaassen, A.; Truong, A.; Lester, H.A.; Cohen, B.N. Five ADNFLE mutations reduce the Ca2+ dependence of the mammalian α4β2 acetylcholine response. J. Physiol. 2003, 550, 11–26. [Google Scholar] [CrossRef]
- Rodrigues-Pinguet, N.O.; Pinguet, T.J.; Figl, A.; Lester, H.A.; Cohen, B.N. Mutations linked to autosomal dominant nocturnal frontal lobe epilepsy affect allosteric Ca2+ activation of the α4β2 nicotinic acetylcholine receptor. Mol. Pharmacol. 2005, 68, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, A.; Glykys, J.; Maguire, J.; Labarca, C.; Mody, I.; Boulter, J. Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc. Natl. Acad. Sci. USA 2006, 103, 19152–19157. [Google Scholar] [CrossRef] [PubMed]
- Teper, Y.; Whyte, D.; Cahir, E.; Lester, H.A.; Grady, S.R.; Marks, M.J.; Cohen, B.N.; Fonck, C.; McClure-Begley, T.; McIntosh, J.M.; et al. Nicotine-induced dystonic arousal complex in a mouse line harboring a human autosomal-dominant nocturnal frontal lobe epilepsy mutation. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 10128–10142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Okada, M.; Yoshida, S.; Ueno, S.; Mori, F.; Takahara, T.; Saito, R.; Miura, Y.; Kishi, A.; Tomiyama, M.; et al. Rats harboring S284L Chrna4 mutation show attenuation of synaptic and extrasynaptic GABAergic transmission and exhibit the nocturnal frontal lobe epilepsy phenotype. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 12465–12476. [Google Scholar] [CrossRef]
- Gullo, F.; Manfredi, I.; Lecchi, M.; Casari, G.; Wanke, E.; Becchetti, A. Multi-electrode array study of neuronal cultures expressing nicotinic β2-V287L subunits, linked to autosomal dominant nocturnal frontal lobe epilepsy. An in vitro model of spontaneous epilepsy. Front. Neural Circuits 2014, 8, 87. [Google Scholar] [CrossRef]
- Manfredi, I.; Zani, A.D.; Rampoldi, L.; Pegorini, S.; Bernascone, I.; Moretti, M.; Gotti, C.; Croci, L.; Consalez, G.G.; Ferini-Strambi, L.; et al. Expression of mutant β2 nicotinic receptors during development is crucial for epileptogenesis. Hum. Mol. Genet. 2009, 18, 1075–1088. [Google Scholar] [CrossRef]
- O’Neill, H.C.; Laverty, D.C.; Patzlaff, N.E.; Cohen, B.N.; Fonck, C.; McKinney, S.; McIntosh, J.M.; Lindstrom, J.M.; Lester, H.A.; Grady, S.R.; et al. Mice expressing the ADNFLE valine 287 leucine mutation of the Β2 nicotinic acetylcholine receptor subunit display increased sensitivity to acute nicotine administration and altered presynaptic nicotinic receptor function. Pharmacol. Biochem. Behav. 2013, 103, 603–621. [Google Scholar] [CrossRef]
- Xu, J.; Cohen, B.N.; Zhu, Y.; Dziewczapolski, G.; Panda, S.; Lester, H.A.; Heinemann, S.F.; Contractor, A. Altered activity-rest patterns in mice with a human autosomal-dominant nocturnal frontal lobe epilepsy mutation in the β2 nicotinic receptor. Mol. Psychiatry 2011, 16, 1048–1061. [Google Scholar] [CrossRef]
- Shiba, Y.; Mori, F.; Yamada, J.; Migita, K.; Nikaido, Y.; Wakabayashi, K.; Kaneko, S.; Okada, M.; Hirose, S.; Ueno, S. Spontaneous epileptic seizures in transgenic rats harboring a human ADNFLE missense mutation in the β2-subunit of the nicotinic acetylcholine receptor. Neurosci. Res. 2015, 100, 46–54. [Google Scholar] [CrossRef][Green Version]
- Fukuyama, K.; Fukuzawa, M.; Shiroyama, T.; Okada, M. Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor. Br. J. Pharmacol. 2020, 177, 2143–2162. [Google Scholar] [CrossRef]
- Yamada, J.; Zhu, G.; Okada, M.; Hirose, S.; Yoshida, S.; Shiba, Y.; Migita, K.; Mori, F.; Sugawara, T.; Chen, L.; et al. A novel prophylactic effect of furosemide treatment on autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). Epilepsy Res. 2013, 107, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Fukuzawa, M.; Okada, M. Upregulated and hyperactivated thalamic connexin 43 plays important roles in pathomechanisms of cognitive impairment and seizure of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor. Pharmaceuticals 2020, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Fukuzawa, M.; Okubo, R.; Okada, M. Upregulated Connexin 43 Induced by Loss-of-Functional S284L-Mutant α4 Subunit of Nicotinic ACh Receptor Contributes to Pathomechanisms of Autosomal Dominant Sleep-Related Hypermotor Epilepsy. Pharmaceuticals 2020, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Fukuzawa, M.; Shiroyama, T.; Okada, M. Pathomechanism of nocturnal paroxysmal dystonia in autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor. Biomed. Pharmacother. 2020, 126, 110070. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. Age-dependent and sleep/seizure-induced pathomechanisms of autosomal dominant sleep-related hypermotor epilepsy. Int. J. Mol. Sci. 2020, 21, 8142. [Google Scholar] [CrossRef]
- Okada, M.; Oka, T.; Nakamoto, M.; Fukuyama, K.; Shiroyama, T. Astroglial Connexin43 as a Potential Target for a Mood Stabiliser. Int. J. Mol. Sci. 2021, 22, 339. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. High frequency oscillations play important roles in development of epileptogenesis/ictogenesis via activation of astroglial signallings. Biomed. Pharmacother. 2022, 149, 112846. [Google Scholar] [CrossRef]
- Fukuyama, K.; Motomura, E.; Okada, M. Age-Dependent Activation of Pannexin1 Function Contributes to the Development of Epileptogenesis in Autosomal Dominant Sleep-related Hypermotor Epilepsy Model Rats. Int. J. Mol. Sci. 2024, 25, 1619. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Motomura, E.; Okada, M. Age-Dependent Activation of Purinergic Transmission Contributes to the Development of Epileptogenesis in ADSHE Model Rats. Biomolecules 2024, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Okubo, R.; Motomura, E.; Okada, M. Subcortical Circuits Among Pedunculopontine Nucleus, Thalamus and Basal Ganglia Play Important Roles in Paroxysmal Arousal in Genetic Rat Models of Autosomal Dominant Sleep-Related Hypermotor Epilepsy. Int. J. Mol. Sci. 2025, 26, 5522. [Google Scholar] [CrossRef] [PubMed]
- Broughton, R.J. Sleep disorders: Disorders of arousal? Enuresis, somnambulism, and nightmares occur in confusional states of arousal, not in “dreaming sleep”. Science 1968, 159, 1070–1078. [Google Scholar] [CrossRef]
- Hirsch, E.; French, J.; Scheffer, I.E.; Bogacz, A.; Alsaadi, T.; Sperling, M.R.; Abdulla, F.; Zuberi, S.M.; Trinka, E.; Specchio, N.; et al. ILAE definition of the Idiopathic Generalized Epilepsy Syndromes: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1475–1499. [Google Scholar] [CrossRef]
- Zuberi, S.M.; Wirrell, E.; Yozawitz, E.; Wilmshurst, J.M.; Specchio, N.; Riney, K.; Pressler, R.; Auvin, S.; Samia, P.; Hirsch, E.; et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1349–1397. [Google Scholar] [CrossRef]
- Combi, R.; Dalprà, L.; Tenchini, M.L.; Ferini-Strambi, L. Autosomal dominant nocturnal frontal lobe epilepsy—A critical overview. J. Neurol. 2004, 251, 923–934. [Google Scholar] [CrossRef]
- Licchetta, L.; Poda, R.; Vignatelli, L.; Pippucci, T.; Zenesini, C.; Menghi, V.; Mostacci, B.; Baldassari, S.; Provini, F.; Tinuper, P.; et al. Profile of neuropsychological impairment in Sleep-related Hypermotor Epilepsy. Sleep Med. 2018, 48, 8–15. [Google Scholar] [CrossRef]
- Picard, F.; Pegna, A.J.; Arntsberg, V.; Lucas, N.; Kaczmarek, I.; Todica, O.; Chiriaco, C.; Seeck, M.; Brodtkorb, E. Neuropsychological disturbances in frontal lobe epilepsy due to mutated nicotinic receptors. Epilepsy Behav. 2009, 14, 354–359. [Google Scholar] [CrossRef]
- Combi, R.; Ferini-Strambi, L.; Tenchini, M.L. Compound heterozygosity with dominance in the Corticotropin Releasing Hormone (CRH) promoter in a case of nocturnal frontal lobe epilepsy. J. Sleep Res. 2008, 17, 361–362. [Google Scholar] [CrossRef]
- Blumenfeld, H. Impaired consciousness in epilepsy. Lancet Neurol. 2012, 11, 814–826. [Google Scholar] [CrossRef]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef]
- Bialer, M.; White, H.S. Key factors in the discovery and development of new antiepileptic drugs. Nat. Rev. Drug Discov. 2010, 9, 68–82. [Google Scholar] [CrossRef]
- Löscher, W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem. Res. 2017, 42, 1873–1888. [Google Scholar] [CrossRef]
- Chiron, C.; Dulac, O. The pharmacologic treatment of Dravet syndrome. Epilepsia 2011, 52 (Suppl. S2), 72–75. [Google Scholar] [CrossRef]
- Griffin, A.; Hamling, K.R.; Hong, S.; Anvar, M.; Lee, L.P.; Baraban, S.C. Preclinical Animal Models for Dravet Syndrome: Seizure Phenotypes, Comorbidities and Drug Screening. Front. Pharmacol. 2018, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Brun, L.; Viemari, J.-C.; Villard, L. Mouse models of Kcnq2 dysfunction. Epilepsia 2022, 63, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.C.; Shen, Y.; Hu, N.; Shen, W.; Narayanan, V.; Ramsey, K.; He, W.; Zou, L.; Macdonald, R.L. GABRG2 Variants Associated with Febrile Seizures. Biomolecules 2023, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Marsan, E.; Baulac, S. Review: Mechanistic target of rapamycin (mTOR) pathway, focal cortical dysplasia and epilepsy. Neuropathol. Appl. Neurobiol. 2018, 44, 6–17. [Google Scholar] [CrossRef]
- Okada, M.; Kaneko, S. Different Mechanisms Underlying the Antiepileptic and Antiparkinsonian Effects of Zonisamide. In Novel Treatment of Epilepsy; Foyaca-Sibat, H., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 23–36. [Google Scholar]
- Yamamura, S.; Hoshikawa, M.; Dai, K.; Saito, H.; Suzuki, N.; Niwa, O.; Okada, M. ONO-2506 inhibits spike-wave discharges in a genetic animal model without affecting traditional convulsive tests via gliotransmission regulation. Br. J. Pharmacol. 2013, 168, 1088–1100. [Google Scholar] [CrossRef]
- Boillot, M.; Baulac, S. Genetic models of focal epilepsies. J. Neurosci. Methods 2016, 260, 132–143. [Google Scholar] [CrossRef]
- Becchetti, A.; Aracri, P.; Meneghini, S.; Brusco, S.; Amadeo, A. The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy. Front. Physiol. 2015, 6, 22. [Google Scholar] [CrossRef]
- Okada, M.; Zhu, G.; Yoshida, S.; Kaneko, S. Validation criteria for genetic animal models of epilepsy. Epilepsy Seizure 2010, 3, 109–120. [Google Scholar] [CrossRef][Green Version]
- Picard, F.; Makrythanasis, P.; Navarro, V.; Ishida, S.; de Bellescize, J.; Ville, D.; Weckhuysen, S.; Fosselle, E.; Suls, A.; De Jonghe, P.; et al. DEPDC5 mutations in families presenting as autosomal dominant nocturnal frontal lobe epilepsy. Neurology 2014, 82, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Licchetta, L.; Pippucci, T.; Baldassari, S.; Minardi, R.; Provini, F.; Mostacci, B.; Plazzi, G.; Tinuper, P.; Bisulli, F.; Collaborative Group of Italian League Against Epilepsy Genetic Study Group on S.H.E. Sleep-related hypermotor epilepsy (SHE): Contribution of known genes in 103 patients. Seizure 2020, 74, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Z.; Liu, J.; Li, M.; Zhao, X.; Ye, J.; Wang, Y. Phenotypic and Genotypic Characterization of DEPDC5-Related Familial Focal Epilepsy: Case Series and Literature Review. Front. Neurol. 2021, 12, 641019. [Google Scholar] [CrossRef]
- Baldassari, S.; Picard, F.; Verbeek, N.E.; van Kempen, M.; Brilstra, E.H.; Lesca, G.; Conti, V.; Guerrini, R.; Bisulli, F.; Licchetta, L.; et al. The landscape of epilepsy-related GATOR1 variants. Genet. Med. 2019, 21, 398–408. [Google Scholar] [CrossRef]
- Bisulli, F.; Licchetta, L.; Baldassari, S.; Pippucci, T.; Tinuper, P. DEPDC5 mutations in epilepsy with auditory features. Epilepsia 2016, 57, 335. [Google Scholar] [CrossRef]
- D’Gama, A.M.; Woodworth, M.B.; Hossain, A.A.; Bizzotto, S.; Hatem, N.E.; LaCoursiere, C.M.; Najm, I.; Ying, Z.; Yang, E.; Barkovich, A.J.; et al. Somatic Mutations Activating the mTOR Pathway in Dorsal Telencephalic Progenitors Cause a Continuum of Cortical Dysplasias. Cell Rep. 2017, 21, 3754–3766. [Google Scholar] [CrossRef]
- Nobili, L.; Proserpio, P.; Combi, R.; Provini, F.; Plazzi, G.; Bisulli, F.; Tassi, L.; Tinuper, P. Nocturnal frontal lobe epilepsy. Curr. Neurol. Neurosci. Rep. 2014, 14, 424. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef]
- Hughes, J.; Dawson, R.; Tea, M.; McAninch, D.; Piltz, S.; Jackson, D.; Stewart, L.; Ricos, M.G.; Dibbens, L.M.; Harvey, N.L.; et al. Knockout of the epilepsy gene Depdc5 in mice causes severe embryonic dysmorphology with hyperactivity of mTORC1 signalling. Sci. Rep. 2017, 7, 12618. [Google Scholar] [CrossRef]
- Yuskaitis, C.J.; Jones, B.M.; Wolfson, R.L.; Super, C.E.; Dhamne, S.C.; Rotenberg, A.; Sabatini, D.M.; Sahin, M.; Poduri, A. A mouse model of DEPDC5-related epilepsy: Neuronal loss of Depdc5 causes dysplastic and ectopic neurons, increased mTOR signaling, and seizure susceptibility. Neurobiol. Dis. 2018, 111, 91–101. [Google Scholar] [CrossRef]
- Klofas, L.K.; Short, B.P.; Zhou, C.; Carson, R.P. Prevention of premature death and seizures in a Depdc5 mouse epilepsy model through inhibition of mTORC1. Hum. Mol. Genet. 2020, 29, 1365–1377. [Google Scholar] [CrossRef]
- Duga, S.; Asselta, R.; Bonati, M.T.; Malcovati, M.; Dalprà, L.; Oldani, A.; Zucconi, M.; Ferini-Strambi, L.; Tenchini, M.L. Mutational analysis of nicotinic acetylcholine receptor β2 subunit gene (CHRNB2) in a representative cohort of Italian probands affected by autosomal dominant nocturnal frontal lobe epilepsy. Epilepsia 2002, 43, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.; Weiland, S.; Berkovic, S.F.; Steinlein, O.K.; Bertrand, D. Properties of neuronal nicotinic acetylcholine receptor mutants from humans suffering from autosomal dominant nocturnal frontal lobe epilepsy. Br. J. Pharmacol. 1998, 125, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Nobili, L.; Sartori, I.; Terzaghi, M.; Tassi, L.; Mai, R.; Francione, S.; Cossu, M.; Cardinale, F.; Castana, L.; Lo Russo, G. Intracerebral recordings of minor motor events, paroxysmal arousals and major seizures in nocturnal frontal lobe epilepsy. Neurol. Sci. 2005, 26 (Suppl. S3), s215–s219. [Google Scholar] [CrossRef] [PubMed]
- Tinuper, P.; Provini, F.; Bisulli, F.; Vignatelli, L.; Plazzi, G.; Vetrugno, R.; Montagna, P.; Lugaresi, E. Movement disorders in sleep: Guidelines for differentiating epileptic from non-epileptic motor phenomena arising from sleep. Sleep Med. Rev. 2007, 11, 255–267. [Google Scholar] [CrossRef]
- Picard, F.; Bruel, D.; Servent, D.; Saba, W.; Fruchart-Gaillard, C.; Schöllhorn-Peyronneau, M.-A.; Roumenov, D.; Brodtkorb, E.; Zuberi, S.; Gambardella, A.; et al. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: A PET study. Brain 2006, 129, 2047–2060. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Houser, C.R.; Skinner, R.D.; Smith, W.; Woodward, D.J. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res. Bull. 1987, 18, 731–738. [Google Scholar] [CrossRef]
- Mena-Segovia, J.; Bolam, J.P. Rethinking the Pedunculopontine Nucleus: From Cellular Organization to Function. Neuron 2017, 94, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, T.L.; Stratton, P.G.; Coyne, T.J.; Cook, R.; Silberstein, P.; Silburn, P.A.; Windels, F.; Sah, P. Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat. Neurosci. 2014, 17, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.R.; Grant, S. Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci. Lett. 1990, 120, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Li, K.-Y.; Zheng, R.; Yan, Y.-Q.; Wang, Z.-X.; Chen, Y.; Liu, Y.; Tian, J.; Zhu, L.-Y.; Lou, H.-F.; et al. Cholinergic neurons in the pedunculopontine nucleus guide reversal learning by signaling the changing reward contingency. Cell Rep. 2022, 38, 110437. [Google Scholar] [CrossRef]
- Su, J.-H.; Hu, Y.-W.; Yang, Y.; Li, R.-Y.; Teng, F.; Li, L.-X.; Jin, L.-J. Dystonia and the pedunculopontine nucleus: Current evidences and potential mechanisms. Front. Neurol. 2022, 13, 1065163. [Google Scholar] [CrossRef]
- Zhao, P.; Jiang, T.; Wang, H.; Jia, X.; Li, A.; Gong, H.; Li, X. Upper brainstem cholinergic neurons project to ascending and descending circuits. BMC Biol. 2023, 21, 135. [Google Scholar] [CrossRef]
- Kroeger, D.; Ferrari, L.L.; Petit, G.; Mahoney, C.E.; Fuller, P.M.; Arrigoni, E.; Scammell, T.E. Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 1352–1366. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, C.; van Andel, J.; Bolam, J.P.; Mena-Segovia, J. Divergent motor projections from the pedunculopontine nucleus are differentially regulated in Parkinsonism. Brain Struct. Funct. 2014, 219, 1451–1462. [Google Scholar] [CrossRef]
- Ichinohe, N.; Teng, B.; Kitai, S.T. Morphological study of the tegmental pedunculopontine nucleus, substantia nigra and subthalamic nucleus, and their interconnections in rat organotypic culture. Anat. Embryol. 2000, 201, 435–453. [Google Scholar] [CrossRef]
- Beierlein, M. Synaptic mechanisms underlying cholinergic control of thalamic reticular nucleus neurons. J. Physiol. 2014, 592, 4137–4145. [Google Scholar] [CrossRef]
- Asanuma, C. Noradrenergic innervation of the thalamic reticular nucleus: A light and electron microscopic immunohistochemical study in rats. J. Comp. Neurol. 1992, 319, 299–311. [Google Scholar] [CrossRef]
- McElvain, L.E.; Chen, Y.; Moore, J.D.; Brigidi, G.S.; Bloodgood, B.L.; Lim, B.K.; Costa, R.M.; Kleinfeld, D. Specific populations of basal ganglia output neurons target distinct brain stem areas while collateralizing throughout the diencephalon. Neuron 2021, 109, 1721–1738.e4. [Google Scholar] [CrossRef]
- Roseberry, T.K.; Lee, A.M.; Lalive, A.L.; Wilbrecht, L.; Bonci, A.; Kreitzer, A.C. Cell-Type-Specific Control of Brainstem Locomotor Circuits by Basal Ganglia. Cell 2016, 164, 526–537. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Kawano, Y.; Shiroyama, T.; Ueda, Y. Memantine protects thalamocortical hyper-glutamatergic transmission induced by NMDA receptor antagonism via activation of system xc−. Pharmacol. Res. Perspect. 2019, 7, e00457. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, R.E.; Grundmann, K.; Pisani, A. New genetic insights highlight ‘old’ ideas on motor dysfunction in dystonia. Trends Neurosci. 2013, 36, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A.; Tokuno, H.; Takada, M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci. Res. 2002, 43, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Bédard, P.J.; Rouillard, C. Opioids and motor complications in Parkinson’s disease. Trends Pharmacol. Sci. 2006, 27, 512–517. [Google Scholar] [CrossRef]
- Dautan, D.; Kovács, A.; Bayasgalan, T.; Diaz-Acevedo, M.A.; Pal, B.; Mena-Segovia, J. Modulation of motor behavior by the mesencephalic locomotor region. Cell Rep. 2021, 36, 109594. [Google Scholar] [CrossRef]
- Huerta-Ocampo, I.; Dautan, D.; Gut, N.K.; Khan, B.; Mena-Segovia, J. Whole-brain mapping of monosynaptic inputs to midbrain cholinergic neurons. Sci. Rep. 2021, 11, 9055. [Google Scholar] [CrossRef]
- Vitale, F.; Capozzo, A.; Mazzone, P.; Scarnati, E. Neurophysiology of the pedunculopontine tegmental nucleus. Neurobiol. Dis. 2019, 128, 19–30. [Google Scholar] [CrossRef]
- Mena-Segovia, J.; Sims, H.M.; Magill, P.J.; Bolam, J.P. Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J. Physiol. 2008, 586, 2947–2960. [Google Scholar] [CrossRef]
- Kundishora, A.J.; Gummadavelli, A.; Ma, C.; Liu, M.; McCafferty, C.; Schiff, N.D.; Willie, J.T.; Gross, R.E.; Gerrard, J.; Blumenfeld, H. Restoring Conscious Arousal During Focal Limbic Seizures with Deep Brain Stimulation. Cereb. Cortex 2017, 27, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.; Nordberg, A. Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 2000, 61, 75–111. [Google Scholar] [CrossRef] [PubMed]
- Vallés, A.S.; Barrantes, F.J. Nicotinic Acetylcholine Receptor Dysfunction in Addiction and in Some Neurodegenerative and Neuropsychiatric Diseases. Cells 2023, 12, 2051. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ding, C.; Qi, G.; Feldmeyer, D. Cholinergic and Adenosinergic Modulation of Synaptic Release. Neuroscience 2021, 456, 114–130. [Google Scholar] [CrossRef]
- Goussakov, I.; Synowiec, S.; Aksenov, D.P.; Drobyshevsky, A. Occlusion of activity dependent synaptic plasticity by late hypoxic long term potentiation after neonatal intermittent hypoxia. Exp. Neurol. 2021, 337, 113575. [Google Scholar] [CrossRef]
- Yamamura, S.; Hamaguchi, T.; Ohoyama, K.; Sugiura, Y.; Suzuki, D.; Kanehara, S.; Nakagawa, M.; Motomura, E.; Matsumoto, T.; Tanii, H.; et al. Topiramate and zonisamide prevent paradoxical intoxication induced by carbamazepine and phenytoin. Epilepsy Res. 2009, 84, 172–186. [Google Scholar] [CrossRef]
- Panet, R.; Eliash, M.; Atlan, H. Na+/K+/Cl− cotransporter activates MAP-kinase cascade downstream to protein kinase C, and upstream to MEK. J. Cell. Physiol. 2006, 206, 578–585. [Google Scholar] [CrossRef]
- Okada, M.; Kawano, Y.; Fukuyama, K.; Motomura, E.; Shiroyama, T. Candidate Strategies for Development of a Rapid-Acting Antidepressant Class That Does Not Result in Neuropsychiatric Adverse Effects: Prevention of Ketamine-Induced Neuropsychiatric Adverse Reactions. Int. J. Mol. Sci. 2020, 21, 7951. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Murata, M. A Working Hypothesis Regarding Identical Pathomechanisms between Clinical Efficacy and Adverse Reaction of Clozapine via the Activation of Connexin43. Int. J. Mol. Sci. 2020, 21, 7019. [Google Scholar] [CrossRef]
- Mazaud, D.; Capano, A.; Rouach, N. The many ways astroglial connexins regulate neurotransmission and behavior. Glia 2021, 69, 2527–2545. [Google Scholar] [CrossRef]
- Giaume, C.; Naus, C.C.; Saez, J.C.; Leybaert, L. Glial Connexins and Pannexins in the Healthy and Diseased Brain. Physiol. Rev. 2021, 101, 93–145. [Google Scholar] [CrossRef] [PubMed]
- Willebrords, J.; Maes, M.; Crespo Yanguas, S.; Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther. 2017, 180, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Esseltine, J.L.; Laird, D.W. Next-Generation Connexin and Pannexin Cell Biology. Trends Cell Biol. 2016, 26, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Lapato, A.S.; Tiwari-Woodruff, S.K. Connexins and pannexins: At the junction of neuro-glial homeostasis & disease. J. Neurosci. Res. 2018, 96, 31–44. [Google Scholar] [CrossRef]
- Bedner, P.; Steinhäuser, C. Role of Impaired Astrocyte Gap Junction Coupling in Epileptogenesis. Cells 2023, 12, 1669. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girão, H. Role of connexin 43 in different forms of intercellular communication—Gap junctions, extracellular vesicles and tunnelling nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef]
- Bruzzone, R.; Hormuzdi, S.G.; Barbe, M.T.; Herb, A.; Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13644–13649. [Google Scholar] [CrossRef]
- Contreras, J.E.; Sáez, J.C.; Bukauskas, F.F.; Bennett, M.V.L. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef]
- Kar, R.; Batra, N.; Riquelme, M.A.; Jiang, J.X. Biological role of connexin intercellular channels and hemichannels. Arch. Biochem. Biophys. 2012, 524, 2–15. [Google Scholar] [CrossRef]
- Fasciani, I.; Temperán, A.; Pérez-Atencio, L.F.; Escudero, A.; Martínez-Montero, P.; Molano, J.; Gómez-Hernández, J.M.; Paino, C.L.; González-Nieto, D.; Barrio, L.C. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology 2013, 75, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, C.; Gassmann, O.; Pranskevich, J.N.; Boassa, D.; Smock, A.; Wang, J.; Dahl, G.; Steinem, C.; Sosinsky, G.E. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J. Biol. Chem. 2010, 285, 24420–24431. [Google Scholar] [CrossRef] [PubMed]
- Navis, K.E.; Fan, C.Y.; Trang, T.; Thompson, R.J.; Derksen, D.J. Pannexin 1 Channels as a Therapeutic Target: Structure, Inhibition, and Outlook. ACS Chem. Neurosci. 2020, 11, 2163–2172. [Google Scholar] [CrossRef]
- Shan, Y.; Ni, Y.; Gao, Z. Pannexin-1 Channel Regulates ATP Release in Epilepsy. Neurochem. Res. 2020, 45, 965–971. [Google Scholar] [CrossRef]
- Donahue, H.J.; Qu, R.W.; Genetos, D.C. Joint diseases: From connexins to gap junctions. Nat. Rev. Rheumatol. 2017, 14, 42–51. [Google Scholar] [CrossRef]
- Zijlmans, M.; Worrell, G.A.; Dümpelmann, M.; Stieglitz, T.; Barborica, A.; Heers, M.; Ikeda, A.; Usui, N.; Le Van Quyen, M. How to record high-frequency oscillations in epilepsy: A practical guideline. Epilepsia 2017, 58, 1305–1315. [Google Scholar] [CrossRef]
- Kanazawa, K.; Matsumoto, R.; Imamura, H.; Matsuhashi, M.; Kikuchi, T.; Kunieda, T.; Mikuni, N.; Miyamoto, S.; Takahashi, R.; Ikeda, A. Intracranially recorded ictal direct current shifts may precede high frequency oscillations in human epilepsy. Clin. Neurophysiol. 2015, 126, 47–59. [Google Scholar] [CrossRef]
- Inoue, T.; Inouchi, M.; Matsuhashi, M.; Matsumoto, R.; Hitomi, T.; Daifu-Kobayashi, M.; Kobayashi, K.; Nakatani, M.; Kanazawa, K.; Shimotake, A.; et al. Interictal slow and high-frequency oscillations: Is it an epileptic slow or red slow? J. Clin. Neurophysiol. 2019, 36, 166–170. [Google Scholar] [CrossRef]
- Foffani, G.; Uzcategui, Y.G.; Gal, B.; Menendez de la Prida, L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 2007, 55, 930–941. [Google Scholar] [CrossRef]
- Carmignoto, G.; Haydon, P.G. Astrocyte calcium signaling and epilepsy. Glia 2012, 60, 1227–1233. [Google Scholar] [CrossRef]
- Ikeda, A.; Takeyama, H.; Bernard, C.; Nakatani, M.; Shimotake, A.; Daifu, M.; Matsuhashi, M.; Kikuchi, T.; Kunieda, T.; Matsumoto, R.; et al. Active direct current (DC) shifts and “Red slow”: Two new concepts for seizure mechanisms and identification of the epileptogenic zone. Neurosci. Res. 2020, 156, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bruder, J.C.; Dümpelmann, M.; Piza, D.L.; Mader, M.; Schulze-Bonhage, A.; Jacobs-Le Van, J. Physiological Ripples Associated with Sleep Spindles Differ in Waveform Morphology from Epileptic Ripples. Int. J. Neural Syst. 2017, 27, 1750011. [Google Scholar] [CrossRef] [PubMed]
- Bruder, J.C.; Schmelzeisen, C.; Lachner-Piza, D.; Reinacher, P.; Schulze-Bonhage, A.; Jacobs, J. Physiological Ripples Associated with Sleep Spindles Can Be Identified in Patients with Refractory Epilepsy Beyond Mesio-Temporal Structures. Front. Neurol. 2021, 12, 612293. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Mishra, A.; Goel, R.K. PTZ kindling model for epileptogenesis, refractory epilepsy, and associated comorbidities: Relevance and reliability. Metab. Brain Dis. 2021, 36, 1573–1590. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, C.; Li, H.; Zhong, M.; Lin, J.; Hu, Y.; Chen, Z.; Gan, C.-L. Epileptic seizures induced by pentylenetetrazole kindling accelerate Alzheimer-like neuropathology in 5×FAD mice. Front. Pharmacol. 2024, 15, 1500105. [Google Scholar] [CrossRef]
- Imamura, H.; Matsumoto, R.; Inouchi, M.; Matsuhashi, M.; Mikuni, N.; Takahashi, R.; Ikeda, A. Ictal wideband ECoG: Direct comparison between ictal slow shifts and high frequency oscillations. Clin. Neurophysiol. 2011, 122, 1500–1504. [Google Scholar] [CrossRef]
- Pumain, R.; Menini, C.; Heinemann, U.; Louvel, J.; Silva-Barrat, C. Chemical synaptic transmission is not necessary for epileptic seizures to persist in the baboon Papio papio. Exp. Neurol. 1985, 89, 250–258. [Google Scholar] [CrossRef]
- Liu, B.; Ran, X.; Yi, Y.; Zhang, X.; Chen, H.; Hu, Y. Anticonvulsant Effect of Carbenoxolone on Chronic Epileptic Rats and Its Mechanism Related to Connexin and High-Frequency Oscillations. Front. Mol. Neurosci. 2022, 15, 870947. [Google Scholar] [CrossRef]
- Ran, X.; Xiang, J.; Song, P.-P.; Jiang, L.; Liu, B.-K.; Hu, Y. Effects of gap junctions blockers on fast ripples and connexin in rat hippocampi after status epilepticus. Epilepsy Res. 2018, 146, 28–35. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Chou, Y.C.; Sun, S.H. P2X7R-mediated Ca2+-independent d-serine release via pannexin-1 of the P2X7R-pannexin-1 complex in astrocytes. Glia 2015, 63, 877–893. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Appelt, K.; Grohmann, M.; Franke, H.; Nörenberg, W.; Illes, P. Increase of intracellular Ca2+ by P2X and P2Y receptor-subtypes in cultured cortical astroglia of the rat. Neuroscience 2009, 160, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kawata, Y.; Mizuno, K.; Wada, K.; Kondo, T.; Kaneko, S. Interaction between Ca2+, K+, carbamazepine and zonisamide on hippocampal extracellular glutamate monitored with a microdialysis electrode. Br. J. Pharmacol. 1998, 124, 1277–1285. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef]
- Song, J.; Ying, Y.; Wang, W.; Liu, X.; Xu, X.; Wei, X.; Ruan, X. The role of P2X7R/ERK signaling in dorsal root ganglia satellite glial cells in the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR). Brain Behav. Immun. 2018, 69, 180–189. [Google Scholar] [CrossRef]
- Gómez-Villafuertes, R.; García-Huerta, P.; Díaz-Hernández, J.I.; Miras-Portugal, M.T. PI3K/Akt signaling pathway triggers P2X7 receptor expression as a pro-survival factor of neuroblastoma cells under limiting growth conditions. Sci. Rep. 2015, 5, 18417. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Ueda, Y. Carbamazepine Attenuates Astroglial L-Glutamate Release Induced by Pro-Inflammatory Cytokines via Chronically Activation of Adenosine A2A Receptor. Int. J. Mol. Sci. 2019, 20, 3727. [Google Scholar] [CrossRef]
- Fukuyama, K.; Ueda, Y.; Okada, M. Effects of Carbamazepine, Lacosamide and Zonisamide on Gliotransmitter Release Associated with Activated Astroglial Hemichannels. Pharmaceuticals 2020, 13, 117. [Google Scholar] [CrossRef]
- Walrave, L.; Vinken, M.; Leybaert, L.; Smolders, I. Astrocytic Connexin43 Channels as Candidate Targets in Epilepsy Treatment. Biomolecules 2020, 10, 1578. [Google Scholar] [CrossRef]
- Zhang, L.; Zoidl, G.R.; Carlen, P.L. Connexins, Pannexins, and Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 5th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Vezzani, A., Delgado-Escueta, A.V., Eds.; Oxford University Press: New York, NY, USA, 2024; pp. 143–160. [Google Scholar]
- Abudara, V.; Bechberger, J.; Freitas-Andrade, M.; De Bock, M.; Wang, N.; Bultynck, G.; Naus, C.C.; Leybaert, L.; Giaume, C. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front. Cell. Neurosci. 2014, 8, 306. [Google Scholar] [CrossRef]
- De Bock, M.; De Smet, M.; Verwaerde, S.; Tahiri, H.; Schumacher, S.; Van Haver, V.; Witschas, K.; Steinhäuser, C.; Rouach, N.; Vandenbroucke, R.E.; et al. Targeting gliovascular connexins prevents inflammatory blood-brain barrier leakage and astrogliosis. JCI Insight 2022, 7, e135263. [Google Scholar] [CrossRef]
- Lissoni, A.; Tao, S.; Allewaert, R.; Witschas, K.; Leybaert, L. Cx43 Hemichannel and Panx1 Channel Modulation by Gap19 and 10Panx1 Peptides. Int. J. Mol. Sci. 2023, 24, 11612. [Google Scholar] [CrossRef] [PubMed]
- Garré, J.M.; Bukauskas, F.F.; Bennett, M.V.L. Single channel properties of pannexin-1 and connexin-43 hemichannels and P2X7 receptors in astrocytes cultured from rodent spinal cords. Glia 2022, 70, 2260–2275. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, C.; Mujica, P.; Illanes-González, J.; López, A.; Vargas, C.; Sáez, J.C.; González-Jamett, A.; Ardiles, Á.O. Probenecid, an Old Drug with Potential New Uses for Central Nervous System Disorders and Neuroinflammation. Biomedicines 2023, 11, 1516. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Wang, Q.; Ao, H.; Shoblock, J.R.; Lord, B.; Aluisio, L.; Fraser, I.; Nepomuceno, D.; Neff, R.A.; Welty, N.; et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br. J. Pharmacol. 2013, 170, 624–640. [Google Scholar] [CrossRef]
- Kesavan, J.; Wang, Y.; Dinkel, K.; Hamacher, M.; Prehn, J.H.M.; Henshall, D.C.; Engel, T. P2X7 receptor antagonism suppresses epileptiform-like activity in an inflammation-primed human iPSC-derived neuron model of drug-resistant epilepsy. Br. J. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Iffland, P.H., 2nd; Baybis, M.; Barnes, A.E.; Leventer, R.J.; Lockhart, P.J.; Crino, P.B. DEPDC5 and NPRL3 modulate cell size, filopodial outgrowth, and localization of mTOR in neural progenitor cells and neurons. Neurobiol. Dis. 2018, 114, 184–193. [Google Scholar] [CrossRef]
- Gautam, V.; Rawat, K.; Sandhu, A.; Kumari, P.; Singh, N.; Saha, L. An insight into crosstalk among multiple signaling pathways contributing to epileptogenesis. Eur. J. Pharmacol. 2021, 910, 174469. [Google Scholar] [CrossRef]
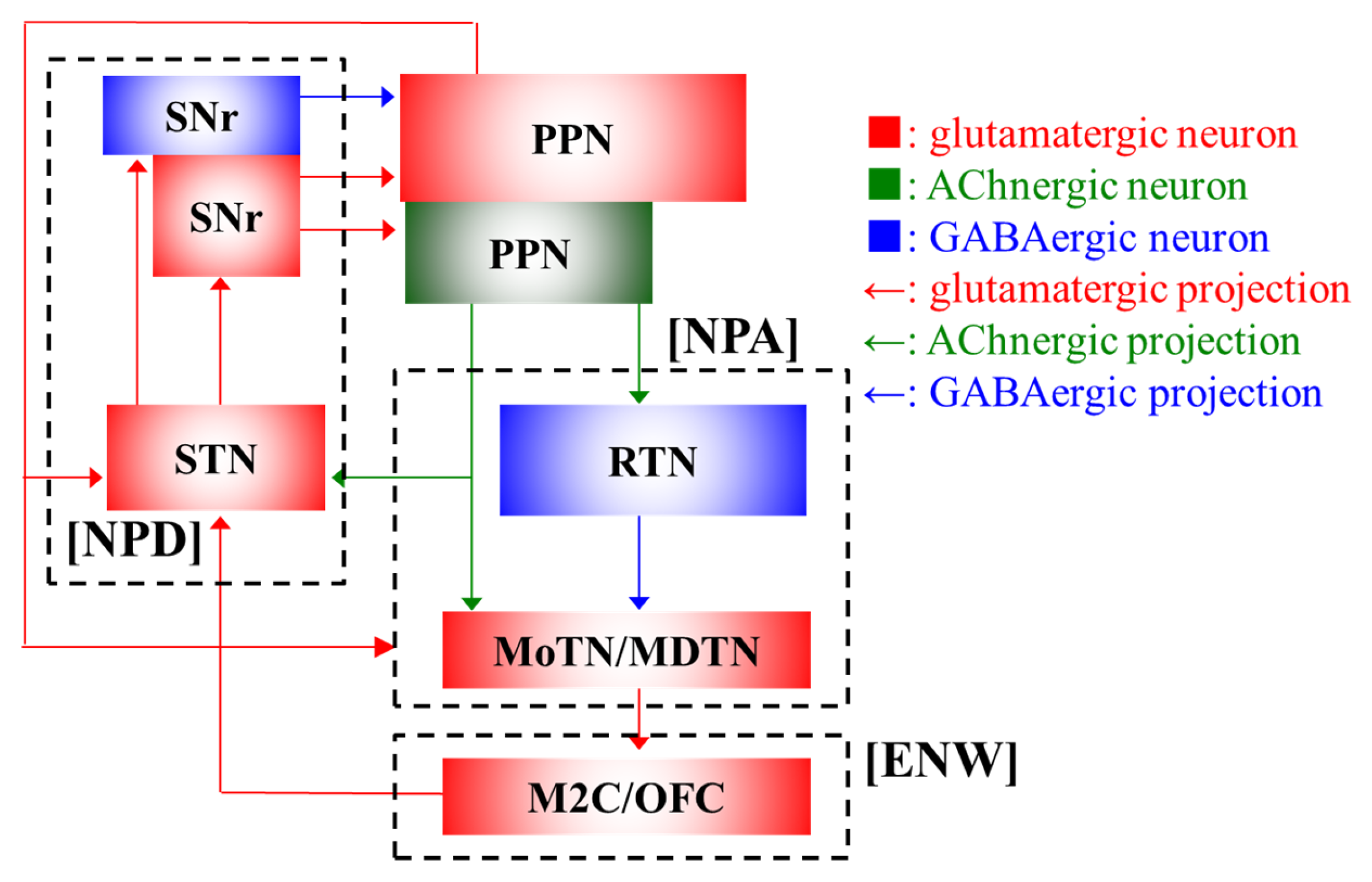
| Ach | GABA | L-Glutamate | ||||
|---|---|---|---|---|---|---|
| Wild | S286L-TG | Wild | S286L-TG | Wild | S286L-TG | |
| PPN | ↓ | ↓ | → | → | ↓ | → |
| RTN | ↓ | ↓ | ↓ | → | ↓ | → |
| MoTN/MDTN | ↓ | ↓ | ↓ | → | ↓ | → |
| M2C/OFC | ↓ | ↓ | → | → | ↓ | → |
| STN | ↓ | ↓ | → | → | ↓ | → |
| SNr | ↓ | → | ||||
| ACh | GABA | L-Glutamate | ||||
|---|---|---|---|---|---|---|
| Wakefulness | SWS | Wakefulness | SWS | Wakefulness | SWS | |
| PPN | → | → | → | → | ↑ | ↑ |
| RTN | → | → | → | → | ↑ | ↑ |
| MoTN/MDTN | → | → | → | → | ↑ | ↑ |
| M2C/OFC | → | → | → | → | ↑ | ↑ |
| STN | → | → | → | → | ↑ | ↑ |
| SNr | ↑ | ↑ | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oka, T.; Okubo, R.; Motomura, E.; Okada, M. Emerging Role of Tripartite Synaptic Transmission in the Pathomechanism of Autosomal-Dominant Sleep-Related Hypermotor Epilepsy. Int. J. Mol. Sci. 2025, 26, 9671. https://doi.org/10.3390/ijms26199671
Oka T, Okubo R, Motomura E, Okada M. Emerging Role of Tripartite Synaptic Transmission in the Pathomechanism of Autosomal-Dominant Sleep-Related Hypermotor Epilepsy. International Journal of Molecular Sciences. 2025; 26(19):9671. https://doi.org/10.3390/ijms26199671
Chicago/Turabian StyleOka, Tomoka, Ruri Okubo, Eishi Motomura, and Motohiro Okada. 2025. "Emerging Role of Tripartite Synaptic Transmission in the Pathomechanism of Autosomal-Dominant Sleep-Related Hypermotor Epilepsy" International Journal of Molecular Sciences 26, no. 19: 9671. https://doi.org/10.3390/ijms26199671
APA StyleOka, T., Okubo, R., Motomura, E., & Okada, M. (2025). Emerging Role of Tripartite Synaptic Transmission in the Pathomechanism of Autosomal-Dominant Sleep-Related Hypermotor Epilepsy. International Journal of Molecular Sciences, 26(19), 9671. https://doi.org/10.3390/ijms26199671






