Necrotizing Enterocolitis: What’s New and What’s Next?
Abstract
1. Introduction
2. Pathophysiology of NEC
2.1. Epidemiology and Risk Factors
2.2. Clinical Presentation and Diagnosis
2.3. Long-Term Implications
3. Current Treatment Options and Novel Approaches
3.1. Management of NEC
3.2. Clinical Trial Landscape in NEC
3.2.1. Drug Therapy
3.2.2. Non-Surgical Interventions
3.2.3. Dietary Supplements
3.2.4. Observational Studies
3.2.5. Antimicrobial Therapy
3.2.6. Surgical Interventions
3.2.7. Medical Devices
3.2.8. In Vitro Approaches
4. Diagnostic Biomarkers
4.1. Inflammatory Biomarkers
4.2. Fecal Biomarkers
4.3. Metabolomics and Metagenomics-Based Markers
4.4. Proteomics Biomarkers
4.5. Genomic and Transcriptomics-Based Markers
4.5.1. Genomic-Based Markers
4.5.2. Transcriptomics-Based Markers
4.6. Others
5. Experimental Models of Necrotizing Enterocolitis
5.1. In Vivo Models of NEC
5.1.1. Rodents
5.1.2. Pigs
5.2. In Vitro Models of NEC
5.2.1. Cell Lines
5.2.2. Organoids and Enteroids
6. Current Challenges and Future Directions
6.1. Many Directions, One Goal…
6.1.1. Diagnosis and Prognosis
6.1.2. Treatment and Management
6.1.3. Prevention
6.2. Future: Collaboration and Clinical Trials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrews, R.E.; Coe, K.L. Clinical Presentation and Multifactorial Pathogenesis of Necrotizing Enterocolitis in the Preterm Infant. Adv. Neonatal Care 2021, 21, 349–355. [Google Scholar] [CrossRef]
- Blum, L.; Vincent, D.; Boettcher, M.; Knopf, J. Immunological aspects of necrotizing enterocolitis models: A review. Front. Immunol. 2024, 15, 1434281. [Google Scholar] [CrossRef]
- De Bernardo, G.; Vecchione, C.; Langella, C.; Ziello, C.; Parisi, G.; Giordano, M.; Buonocore, G.; Perrone, S. Necrotizing Enterocolitis: A Current Understanding and Challenges for the Future. Curr. Pediatr. Rev. 2025, 21, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ginglen, J.G.; Butki, N. Necrotizing Enterocolitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Klinke, M.; Chaaban, H.; Boettcher, M. The role of neutrophil extracellular traps in necrotizing enterocolitis. Front. Pediatr. 2023, 11, 1121193. [Google Scholar] [CrossRef] [PubMed]
- Denning, N.L.; Prince, J.M. Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol. Med. 2018, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Wood, T.R.; Comstock, B.A.; Perez, K.; Gogcu, S.; Puia-Dumitrescu, M.; Berkelhamer, S.; Heagerty, P.J.; Consortium, P. Deaths in a Modern Cohort of Extremely Preterm Infants from the Preterm Erythropoietin Neuroprotection Trial. JAMA Netw. Open 2022, 5, e2146404. [Google Scholar] [CrossRef]
- Maayan-Metzger, A.; Itzchak, A.; Mazkereth, R.; Kuint, J. Necrotizing enterocolitis in full-term infants: Case-control study and review of the literature. J. Perinatol. 2004, 24, 494–499. [Google Scholar] [CrossRef]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595.e1591. [Google Scholar] [CrossRef]
- Hackam, D.J.; Afrazi, A.; Good, M.; Sodhi, C.P. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin. Dev. Immunol. 2013, 2013, 475415. [Google Scholar] [CrossRef]
- Nino, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [CrossRef]
- Samuels, N.; van de Graaf, R.A.; de Jonge, R.C.J.; Reiss, I.K.M.; Vermeulen, M.J. Risk factors for necrotizing enterocolitis in neonates: A systematic review of prognostic studies. BMC Pediatr. 2017, 17, 105. [Google Scholar] [CrossRef]
- Guthrie, S.O.; Gordon, P.V.; Thomas, V.; Thorp, J.A.; Peabody, J.; Clark, R.H. Necrotizing enterocolitis among neonates in the United States. J. Perinatol. 2003, 23, 278–285. [Google Scholar] [CrossRef]
- Fisher, J.G.; Bairdain, S.; Sparks, E.A.; Khan, F.A.; Archer, J.M.; Kenny, M.; Edwards, E.M.; Soll, R.F.; Modi, B.P.; Yeager, S.; et al. Serious congenital heart disease and necrotizing enterocolitis in very low birth weight neonates. J. Am. Coll. Surg. 2015, 220, 1018–1026.e1014. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kliegman, R.M. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin. N. Am. 1986, 33, 179–201. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Hu, X.; Liang, H.; Li, F.; Zhang, R.; Zhu, Y.; Zhu, X.; Xu, Y. Necrotizing enterocolitis: Current understanding of the prevention and management. Pediatr. Surg. Int. 2024, 40, 32. [Google Scholar] [CrossRef] [PubMed]
- Canvasser, J.; Patel, R.M.; Pryor, E.; Green, L.; Hintz, S.R.; Fagan, M.; Harrison, J.D. Long-term outcomes and life-impacts of necrotizing enterocolitis: A survey of survivors and parents. Semin. Perinatol. 2023, 47, 151696. [Google Scholar] [CrossRef] [PubMed]
- Hau, E.M.; Meyer, S.C.; Berger, S.; Goutaki, M.; Kordasz, M.; Kessler, U. Gastrointestinal sequelae after surgery for necrotising enterocolitis: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F265–F273. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.K.; Al Faqeeh, A.A.; Saeed, N.; Almas, T.; Khedro, T.; Niaz, M.A.; Kanawati, M.A.; Hussain, S.; Mohammad, H.; Alshaikh, L.; et al. Surgical Versus Medical Management of Necrotizing Enterocolitis with and Without Intestinal Perforation: A Retrospective Chart Review. Cureus 2021, 13, e15722. [Google Scholar] [CrossRef]
- Cai, X.; Liebe, H.L.; Golubkova, A.; Leiva, T.; Hunter, C.J. A Review of the Diagnosis and Treatment of Necrotizing Enterocolitis. Curr. Pediatr. Rev. 2023, 19, 285–295. [Google Scholar] [CrossRef]
- Colarelli, A.M.; Barbian, M.E.; Denning, P.W. Prevention Strategies and Management of Necrotizing Enterocolitis. Curr. Treat. Options Pediatr. 2024, 10, 126–146. [Google Scholar] [CrossRef]
- Alsohime, F.; Martin-Fernandez, M.; Temsah, M.H.; Alabdulhafid, M.; Le Voyer, T.; Alghamdi, M.; Qiu, X.; Alotaibi, N.; Alkahtani, A.; Buta, S.; et al. JAK Inhibitor Therapy in a Child with Inherited USP18 Deficiency. N. Engl. J. Med. 2020, 382, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.P.; Cutter, G.R.; Steinhorn, R.H.; Nelin, L.D.; Walsh, W.F.; Finer, N.N.; Abman, S.H. Noninvasive inhaled nitric oxide does not prevent bronchopulmonary dysplasia in premature newborns. J. Pediatr. 2014, 165, 1104–1108.e1101. [Google Scholar] [CrossRef] [PubMed]
- Colaizy, T.T.; Poindexter, B.B.; McDonald, S.A.; Bell, E.F.; Carlo, W.A.; Carlson, S.J.; DeMauro, S.B.; Kennedy, K.A.; Nelin, L.D.; Sanchez, P.J.; et al. Neurodevelopmental Outcomes of Extremely Preterm Infants Fed Donor Milk or Preterm Infant Formula: A Randomized Clinical Trial. JAMA 2024, 331, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Soderquist Kruth, S.; Persad, E.; Rakow, A. Probiotic Supplements Effect on Feeding Tolerance, Growth and Neonatal Morbidity in Extremely Preterm Infants: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1228. [Google Scholar] [CrossRef]
- Han, J.; Ren, Y.; Zhang, P.; Fang, C.; Yang, L.; Zhou, S.; Ji, Z. The effectiveness of treatment with probiotics in preventing necrotizing enterocolitis and related mortality: Results from an umbrella meta-analysis on meta-analyses of randomized controlled trials. BMC Gastroenterol. 2025, 25, 245. [Google Scholar] [CrossRef]
- Abdullahi, A.M.; Zhao, S.; Xu, Y. Efficacy of probiotic supplementation in preventing necrotizing enterocolitis in preterm infants: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2025, 38, 2485215. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, Q.; Zhang, F.; Ma, K.; Yan, X.; Chen, W.; Chen, X.; Li, S.; Han, S. Effect of probiotics on necrotizing enterocolitis in preterm infants: A network meta-analysis of randomized controlled trials. BMC Pediatr. 2025, 25, 237. [Google Scholar] [CrossRef]
- Mahboobipour, A.A.; Bitaraf, A.; Mohammadi, P.; Khosravifar, M.; Babaei, H.; Shahidolahi, A. Effects of synbiotics on necrotizing enterocolitis and full enteral feeding in very low birth weight infants: A double-blind, randomized controlled trial. Medicine 2024, 103, e39647. [Google Scholar] [CrossRef]
- Sowden, M.; van Weissenbruch, M.M.; Bulabula, A.N.H.; van Wyk, L.; Twisk, J.; van Niekerk, E. Effect of a Multi-Strain Probiotic on the Incidence and Severity of Necrotizing Enterocolitis and Feeding Intolerances in Preterm Neonates. Nutrients 2022, 14, 3305. [Google Scholar] [CrossRef]
- Sanusi, A.; Ye, Y.; Boggess, K.; Saade, G.; Longo, S.; Clark, E.; Esplin, S.; Cleary, K.; Wapner, R.; Owens, M.; et al. Timing of Adjunctive Azithromycin for Unscheduled Cesarean Delivery and Postdelivery Infection. Obstet. Gynecol. 2022, 139, 1043–1049. [Google Scholar] [CrossRef]
- Blakely, M.L.; Tyson, J.E.; Lally, K.P.; Hintz, S.R.; Eggleston, B.; Stevenson, D.K.; Besner, G.E.; Das, A.; Ohls, R.K.; Truog, W.E.; et al. Initial Laparotomy Versus Peritoneal Drainage in Extremely Low Birthweight Infants with Surgical Necrotizing Enterocolitis or Isolated Intestinal Perforation: A Multicenter Randomized Clinical Trial. Ann. Surg. 2021, 274, e370–e380. [Google Scholar] [CrossRef]
- Hansen, M.L.; Pellicer, A.; Hyttel-Sorensen, S.; Ergenekon, E.; Szczapa, T.; Hagmann, C.; Naulaers, G.; Mintzer, J.; Fumagalli, M.; Dimitriou, G.; et al. Cerebral Oximetry Monitoring in Extremely Preterm Infants. N. Engl. J. Med. 2023, 388, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, M.; Bhandari, V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: Clinical practice guidelines. Early Hum. Dev. 2017, 105, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.; Banerjee, J.; Eaton, S.; Aladangady, N. Biomarkers of gut injury in neonates—Where are we in predicting necrotising enterocolitis? Front. Pediatr. 2022, 10, 1048322. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C. Biomarkers of necrotising enterocolitis. Semin. Fetal Neonatal Med. 2014, 19, 33–38. [Google Scholar] [CrossRef]
- Leiva, T.; Lueschow, S.; Burge, K.; Devette, C.; McElroy, S.; Chaaban, H. Biomarkers of necrotizing enterocolitis in the era of machine learning and omics. Semin. Perinatol. 2023, 47, 151693. [Google Scholar] [CrossRef]
- Egozi, A.; Olaloye, O.; Werner, L.; Silva, T.; McCourt, B.; Pierce, R.W.; An, X.; Wang, F.; Chen, K.; Pober, J.S.; et al. Single-cell atlas of the human neonatal small intestine affected by necrotizing enterocolitis. PLoS Biol. 2023, 21, e3002124. [Google Scholar] [CrossRef]
- McElroy, S.J.; Lueschow, S.R. State of the art review on machine learning and artificial intelligence in the study of neonatal necrotizing enterocolitis. Front. Pediatr. 2023, 11, 1182597. [Google Scholar] [CrossRef]
- Cuestas, E.; Aguilera, B.; Cerutti, M.; Rizzotti, A. Sustained Neonatal Inflammation Is Associated with Poor Growth in Infants Born Very Preterm during the First Year of Life. J. Pediatr. 2019, 205, 91–97. [Google Scholar] [CrossRef]
- Arizaga-Ballesteros, V.; Alcorta-Garcia, M.R.; Lazaro-Martinez, L.C.; Amezquita-Gomez, J.M.; Alanis-Cajero, J.M.; Villela, L.; Castorena-Torres, F.; Lara-Diaz, V.J. Can sTREM-1 predict septic shock & death in late-onset neonatal sepsis? A pilot study. Int. J. Infect. Dis. 2015, 30, 27–32. [Google Scholar] [CrossRef][Green Version]
- Luo, J.; Li, H.P.; Xu, F.; Wu, B.Q.; Lin, H.C. Early diagnosis of necrotizing enterocolitis by plasma RELMbeta and thrombocytopenia in preterm infants: A pilot study. Pediatr. Neonatol. 2019, 60, 447–452. [Google Scholar] [CrossRef]
- Ma, F.; Li, S.; Gao, X.; Zhou, J.; Zhu, X.; Wang, D.; Cai, Y.; Li, F.; Yang, Q.; Gu, X.; et al. Interleukin-6-mediated CCR9+ interleukin-17-producing regulatory T cells polarization increases the severity of necrotizing enterocolitis. eBioMedicine 2019, 44, 71–85. [Google Scholar] [CrossRef]
- Chen, I.L.; Huang, H.C.; Ou-Yang, M.C.; Chen, F.S.; Chung, M.Y.; Chen, C.C. A novel method to detect bacterial infection in premature infants: Using a combination of inflammatory markers in blood and saliva. J. Microbiol. Immunol. Infect. 2020, 53, 892–899. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, L.; Li, B.; Li, J.; Chen, Y.; Richard, S.A.; Xu, Y.; Zhu, C. Screening inflammatory protein biomarkers on premature infants with necrotizing enterocolitis. Inflamm. Res. 2023, 72, 757–768. [Google Scholar] [CrossRef]
- Kumar, R.; Kausch, S.L.; Gummadi, A.K.S.; Fairchild, K.D.; Abhyankar, M.M.; Petri, W.A., Jr.; Sullivan, B.A. Inflammatory biomarkers and physiomarkers of late-onset sepsis and necrotizing enterocolitis in premature infants. Front. Pediatr. 2024, 12, 1337849. [Google Scholar] [CrossRef] [PubMed]
- Matyas, M.; Ilyes, T.; Valeanu, M.; Craciun, A.M.; Hasmasanu, M.; Grosu, N.; Zaharie, G. The predictive value of maternal and neonatal inflammatory biomarkers for necrotizing enterocolitis. Eur. J. Pediatr. 2025, 184, 316. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, X.Q.; Fu, M.R.; Mi, H.Y. Diagnostic accuracy of gasdermin D as a biomarker for necrotizing enterocolitis: A single-center diagnostic test study. Transl. Pediatr. 2024, 13, 2134–2143. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, B.C.; Christensen, R.D.; Yost, C.C.; Gordon, P.V.; Baer, V.L.; Schlaberg, R.; Lowe, J. Reference intervals for stool calprotectin in preterm neonates and their utility for the diagnosis of necrotizing enterocolitis. J. Perinatol. 2018, 38, 1379–1385. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, W.; Han, J.; Zhou, W.; Wu, H. Diagnostic value of fecal calprotectin in necrotizing enterocolitis: A meta-analysis. Early Hum. Dev. 2020, 151, 105170. [Google Scholar] [CrossRef] [PubMed]
- Carnazzo, V.; Redi, S.; Basile, V.; Natali, P.; Gulli, F.; Equitani, F.; Marino, M.; Basile, U. Calprotectin: Two sides of the same coin. Rheumatology 2024, 63, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Pergialiotis, V.; Konstantopoulos, P.; Karampetsou, N.; Koutaki, D.; Gkioka, E.; Perrea, D.N.; Papantoniou, N. Calprotectin levels in necrotizing enterocolitis: A systematic review of the literature. Inflamm. Res. 2016, 65, 847–852. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, G.; Brown, K.L.; Taylor, A.M. Faecal calprotectin concentrations in neonates with CHD: Pilot study. Cardiol. Young 2020, 30, 624–628. [Google Scholar] [CrossRef]
- Thibault, M.P.; Tremblay, E.; Horth, C.; Fournier-Morin, A.; Grynspan, D.; Babakissa, C.; Levy, E.; Ferretti, E.; Bertelle, V.; Beaulieu, J.F. Lipocalin-2 and calprotectin as stool biomarkers for predicting necrotizing enterocolitis in premature neonates. Pediatr. Res. 2022, 91, 129–136. [Google Scholar] [CrossRef]
- Dabritz, J.; Jenke, A.; Wirth, S.; Foell, D. Fecal phagocyte-specific S100A12 for diagnosing necrotizing enterocolitis. J. Pediatr. 2012, 161, 1059–1064. [Google Scholar] [CrossRef]
- Liu, X.C.; Li, L.Q.; Ling, K.R.; Guo, L.; Hu, X.Y.; Li, C. Fecal HBD-2 and Claudin-3 may be potential biomarkers to predict the deterioration of necrotizing enterocolitis: A prospective study. Front. Pediatr. 2022, 10, 1062798. [Google Scholar] [CrossRef]
- De Meij, T.G.; van der Schee, M.P.; Berkhout, D.J.; van de Velde, M.E.; Jansen, A.E.; Kramer, B.W.; van Weissenbruch, M.M.; van Kaam, A.H.; Andriessen, P.; van Goudoever, J.B.; et al. Early Detection of Necrotizing Enterocolitis by Fecal Volatile Organic Compounds Analysis. J. Pediatr. 2015, 167, 562–567.e561. [Google Scholar] [CrossRef]
- Probert, C.; Greenwood, R.; Mayor, A.; Hughes, D.; Aggio, R.; Jackson, R.E.; Simcox, L.; Barrow, H.; Garcia-Finana, M.; Ewer, A.K. Faecal volatile organic compounds in preterm babies at risk of necrotising enterocolitis: The DOVE study. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 474–479. [Google Scholar] [CrossRef]
- Garner, C.E.; Ewer, A.K.; Elasouad, K.; Power, F.; Greenwood, R.; Ratcliffe, N.M.; Costello Bde, L.; Probert, C.S. Analysis of faecal volatile organic compounds in preterm infants who develop necrotising enterocolitis: A pilot study. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 559–565. [Google Scholar] [CrossRef]
- Lin, C.; Lin, Y.; Zhang, H.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Intestinal ‘Infant-Type’ Bifidobacteria Mediate Immune System Development in the First 1000 Days of Life. Nutrients 2022, 14, 1498. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; de Los Reyes-Gavilan, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; Khalil, J.B.; Croce, O.; Saint-Faust, M.; Jacquot, A.; Million, M.; Azza, S.; Armstrong, N.; Henry, M.; et al. Clostridium butyricum Strains and Dysbiosis Linked to Necrotizing Enterocolitis in Preterm Neonates. Clin. Infect. Dis. 2015, 61, 1107–1115. [Google Scholar] [CrossRef]
- Gasparrini, A.J.; Wang, B.; Sun, X.; Kennedy, E.A.; Hernandez-Leyva, A.; Ndao, I.M.; Tarr, P.I.; Warner, B.B.; Dantas, G. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat. Microbiol. 2019, 4, 2285–2297. [Google Scholar] [CrossRef]
- Morrow, A.L.; Lagomarcino, A.J.; Schibler, K.R.; Taft, D.H.; Yu, Z.; Wang, B.; Altaye, M.; Wagner, M.; Gevers, D.; Ward, D.V.; et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Picaud, J.C.; De Magistris, A.; Mussap, M.; Corbu, S.; Dessi, A.; Noto, A.; Fanos, V.; Cesare Marincola, F. Urine NMR Metabolomics Profile of Preterm Infants With Necrotizing Enterocolitis Over the First Two Months of Life: A Pilot Longitudinal Case-Control Study. Front. Mol. Biosci. 2021, 8, 680159. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Nelson, A.; Treumann, A.; Skeath, T.; Cummings, S.P.; Embleton, N.D.; Berrington, J.E. Metabolomic and proteomic analysis of serum from preterm infants with necrotising entercolitis and late-onset sepsis. Pediatr. Res. 2016, 79, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.; Smith, D.P.; Nelson, A.; Abdulkadir, B.; Skeath, T.; Petrosino, J.F.; Perry, J.D.; Berrington, J.E.; et al. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 2016, 4, 67. [Google Scholar] [CrossRef]
- Wilcock, A.; Begley, P.; Stevens, A.; Whatmore, A.; Victor, S. The metabolomics of necrotising enterocolitis in preterm babies: An exploratory study. J. Matern. Fetal Neonatal Med. 2016, 29, 758–762. [Google Scholar] [CrossRef]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis. mSphere 2018, 3, e00104-18. [Google Scholar] [CrossRef]
- Rusconi, B.; Jiang, X.; Sidhu, R.; Ory, D.S.; Warner, B.B.; Tarr, P.I. Gut Sphingolipid Composition as a Prelude to Necrotizing Enterocolitis. Sci. Rep. 2018, 8, 10984. [Google Scholar] [CrossRef] [PubMed]
- Thomaidou, A.; Chatziioannou, A.C.; Deda, O.; Benaki, D.; Gika, H.; Mikros, E.; Agakidis, C.; Raikos, N.; Theodoridis, G.; Sarafidis, K. A pilot case-control study of urine metabolomics in preterm neonates with necrotizing enterocolitis. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2019, 1117, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.J.; Ye, C.; Chen, Y.; Zhang, D.; Li, T.; Ling, X.B.; Cohen, H.J.; Shaw, G.M.; Stevenson, D.K.; Chace, D.; et al. Progressive Metabolic Dysfunction and Nutritional Variability Precedes Necrotizing Enterocolitis. Nutrients 2020, 12, 1275. [Google Scholar] [CrossRef] [PubMed]
- Tarracchini, C.; Milani, C.; Longhi, G.; Fontana, F.; Mancabelli, L.; Pintus, R.; Lugli, G.A.; Alessandri, G.; Anzalone, R.; Viappiani, A.; et al. Unraveling the Microbiome of Necrotizing Enterocolitis: Insights in Novel Microbial and Metabolomic Biomarkers. Microbiol. Spectr. 2021, 9, e0117621. [Google Scholar] [CrossRef]
- Liu, X.C.; Du, T.T.; Gao, X.; Zhao, W.J.; Wang, Z.L.; He, Y.; Bao, L.; Li, L.Q. Gut microbiota and short-chain fatty acids may be new biomarkers for predicting neonatal necrotizing enterocolitis: A pilot study. Front. Microbiol. 2022, 13, 969656. [Google Scholar] [CrossRef]
- Thomaidou, A.; Deda, O.; Begou, O.; Lioupi, A.; Kontou, A.; Gika, H.; Agakidou, E.; Theodoridis, G.; Sarafidis, K. A Prospective, Case-Control Study of Serum Metabolomics in Neonates with Late-Onset Sepsis and Necrotizing Enterocolitis. J. Clin. Med. 2022, 11, 5270. [Google Scholar] [CrossRef]
- Moschino, L.; Verlato, G.; Duci, M.; Cavicchiolo, M.E.; Guiducci, S.; Stocchero, M.; Giordano, G.; Fascetti Leon, F.; Baraldi, E. The Metabolome and the Gut Microbiota for the Prediction of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Systematic Review. Nutrients 2022, 14, 3859. [Google Scholar] [CrossRef]
- Zenner, C.; Chalklen, L.; Adjei, H.; Dalby, M.J.; Mitra, S.; Cornwell, E.; Shaw, A.G.; Sim, K.; Kroll, J.S.; Hall, L.J. Noninvasive Fecal Cytokine and Microbiota Profiles Predict Commencement of Necrotizing Enterocolitis in a Proof-of-Concept Study. Gastro Hep Adv. 2023, 2, 666–675. [Google Scholar] [CrossRef]
- Moschino, L.; Verlato, G.; Stocchero, M.; Giordano, G.; Pirillo, P.; Meneghelli, M.; Guiducci, S.; Duci, M.; Fascetti Leon, F.; Baraldi, E. Metabolomic analysis to predict the onset and severity of necrotizing enterocolitis. BMC Gastroenterol. 2024, 24, 380. [Google Scholar] [CrossRef]
- Agakidou, E.; Agakidis, C.; Gika, H.; Sarafidis, K. Emerging Biomarkers for Prediction and Early Diagnosis of Necrotizing Enterocolitis in the Era of Metabolomics and Proteomics. Front. Pediatr. 2020, 8, 602255. [Google Scholar] [CrossRef]
- Chatziioannou, A.C.; Wolters, J.C.; Sarafidis, K.; Thomaidou, A.; Agakidis, C.; Govorukhina, N.; Kuivenhoven, J.A.; Bischoff, R.; Theodoridis, G. Targeted LC-MS/MS for the evaluation of proteomics biomarkers in the blood of neonates with necrotizing enterocolitis and late-onset sepsis. Anal. Bioanal. Chem. 2018, 410, 7163–7175. [Google Scholar] [CrossRef]
- Masi, A.C.; Embleton, N.D.; Lamb, C.A.; Young, G.; Granger, C.L.; Najera, J.; Smith, D.P.; Hoffman, K.L.; Petrosino, J.F.; Bode, L.; et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 2021, 70, 2273–2282. [Google Scholar] [CrossRef]
- Mackay, S.; Frazer, L.C.; Bailey, G.K.; Miller, C.M.; Gong, Q.; Dewitt, O.N.; Singh, D.K.; Good, M. Identification of serum biomarkers for necrotizing enterocolitis using aptamer-based proteomics. Front. Pediatr. 2023, 11, 1184940. [Google Scholar] [CrossRef]
- Wang, J.; Qu, M.; Qiu, A.; Yang, L.; Xu, H.; Yu, S.; Pan, Z. Quantitative Proteomic Analysis Identifying and Evaluating TRAF6 and IL-8 as Potential Diagnostic Biomarkers in Neonatal Patients with Necrotizing Enterocolitis. Mol. Biotechnol. 2025, 67, 1109–1121. [Google Scholar] [CrossRef]
- Good, M.; Chu, T.; Shaw, P.; McClain, L.; Chamberlain, A.; Castro, C.; Rimer, J.M.; Mihi, B.; Gong, Q.; Nolan, L.S.; et al. Global hypermethylation of intestinal epithelial cells is a hallmark feature of neonatal surgical necrotizing enterocolitis. Clin. Epigenetics 2020, 12, 190. [Google Scholar] [CrossRef]
- Good, M.; Chu, T.; Shaw, P.; Nolan, L.S.; McClain, L.; Chamberlain, A.; Castro, C.; Gong, Q.; Cooksey, K.; Linneman, L.; et al. Neonatal necrotizing enterocolitis-associated DNA methylation signatures in the colon are evident in stool samples of affected individuals. Epigenomics 2021, 13, 829–844. [Google Scholar] [CrossRef]
- Good, M.; Chu, T.; Shaw, P.; Nolan, L.S.; Wrobleski, J.; Castro, C.; Gong, Q.; DeWitt, O.; Finegold, D.N.; Peters, D. Selective hypermethylation is evident in small intestine samples from infants with necrotizing enterocolitis. Clin. Epigenet. 2022, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Frazer, L.C.; Yamaguchi, Y.; Singh, D.K.; Akopyants, N.S.; Good, M. DNA methylation in necrotizing enterocolitis. Expert Rev. Mol. Med. 2024, 26, e16. [Google Scholar] [CrossRef] [PubMed]
- Frazer, L.; Chu, T.; Shaw, P.; Boufford, C.; Naief, L.T.; Ednie, M.; Ritzert, L.; Green, C.P.; Good, M.; Peters, D. Detection of an intestinal cell DNA methylation signature in blood samples from neonates with necrotizing enterocolitis. Epigenomics 2025, 17, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.L.; Said, M.; Cappiello, C.D.; Gordish-Dressman, H.; Tatari-Calderone, Z.; Vukmanovic, S.; Rais-Bahrami, K.; Luban, N.L.; Devaney, J.M.; Sandler, A.D. Are Immune Modulating Single Nucleotide Polymorphisms Associated with Necrotizing Enterocolitis? Sci. Rep. 2015, 5, 18369. [Google Scholar] [CrossRef]
- Sampath, V.; Bhandari, V.; Berger, J.; Merchant, D.; Zhang, L.; Ladd, M.; Menden, H.; Garland, J.; Ambalavanan, N.; Mulrooney, N.; et al. A functional ATG16L1 (T300A) variant is associated with necrotizing enterocolitis in premature infants. Pediatr. Res. 2017, 81, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Banyasz, I.; Bokodi, G.; Vasarhelyi, B.; Treszl, A.; Derzbach, L.; Szabo, A.; Tulassay, T.; Vannay, A. Genetic polymorphisms for vascular endothelial growth factor in perinatal complications. Eur. Cytokine Netw. 2006, 17, 266–270. [Google Scholar] [PubMed]
- Moonen, R.M.; Huizing, M.J.; Gonzalez-Luis, G.E.; Cavallaro, G.; Mosca, F.; Villamor, E. Risk of Necrotizing Enterocolitis Associated with the Single Nucleotide Polymorphisms VEGF C-2578A, IL-18 C-607A, and IL-4 Receptor alpha-Chain A-1902G: A Validation Study in a Prospective Multicenter Cohort. Front. Pediatr. 2020, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ma, F.; Hao, H.; Dai, Y.; Liu, W.; Xiao, X.; Gao, P.; Li, S. Association of VEGFA polymorphisms with necrotizing enterocolitis in Chinese Han population. Pediatr. Neonatol. 2019, 60, 129–134. [Google Scholar] [CrossRef]
- Talavera, M.M.; Jin, Y.; Zmuda, E.J.; Frick, J.; Liu, Y.; McBride, K.L.; Nelin, L.D.; Trittmann, J.K. Single nucleotide polymorphisms in the dual specificity phosphatase genes and risk of necrotizing enterocolitis in premature infant. J. Neonatal Perinatal Med. 2020, 13, 373–380. [Google Scholar] [CrossRef]
- Tian, B.; Xu, X.; Li, L.; Tian, Y.; Liu, Y.; Mu, Y.; Lu, J.; Song, K.; Lv, J.; He, Q.; et al. Epigenetic Insights into Necrotizing Enterocolitis: Unraveling Methylation-Regulated Biomarkers. Inflammation 2025, 48, 236–253. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef]
- Conti, N.; Torricelli, M.; Voltolini, C.; Vannuccini, S.; Clifton, V.L.; Bloise, E.; Petraglia, F. Term histologic chorioamnionitis: A heterogeneous condition. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 188, 34–38. [Google Scholar] [CrossRef]
- Perkins, R.P.; Zhou, S.M.; Butler, C.; Skipper, B.J. Histologic chorioamnionitis in pregnancies of various gestational ages: Implications in preterm rupture of membranes. Obstet. Gynecol. 1987, 70, 856–860. [Google Scholar]
- Weitkamp, J.H.; Guthrie, S.O.; Wong, H.R.; Moldawer, L.L.; Baker, H.V.; Wynn, J.L. Histological chorioamnionitis shapes the neonatal transcriptomic immune response. Early Hum. Dev. 2016, 98, 1–6. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Rao, D.S.; Baltimore, D. microRNA regulation of inflammatory responses. Annu. Rev. Immunol. 2012, 30, 295–312. [Google Scholar] [CrossRef]
- De Oliveira, E.C.S.; Quaglio, A.E.V.; Grillo, T.G.; Di Stasi, L.C.; Sassaki, L.Y. MicroRNAs in inflammatory bowel disease: What do we know and what can we expect? World J. Gastroenterol. 2024, 30, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Lai, D.; Gao, J.; Wu, H.; Shi, B.; Ji, H.; Tou, J. The role and mechanisms of miRNA in neonatal necrotizing enterocolitis. Front. Pediatr. 2022, 10, 1053965. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.B. Systematic large-scale meta-analysis identifies miRNA-429/200a/b and miRNA-141/200c clusters as biomarkers for necrotizing enterocolitis in newborn. Biosci. Rep. 2019, 39, BSR20191503. [Google Scholar] [CrossRef]
- Galley, J.D.; Mar, P.; Wang, Y.; Han, R.; Rajab, A.; Besner, G.E. Urine-derived extracellular vesicle miRNAs as possible biomarkers for and mediators of necrotizing enterocolitis: A proof of concept study. J. Pediatr. Surg. 2021, 56, 1966–1975. [Google Scholar] [CrossRef]
- Agostini, S.; Mancuso, R.; Citterio, L.A.; Caputo, D.; Oreni, L.; Nuzzi, R.; Pasanisi, M.B.; Rovaris, M.; Clerici, M. Serum miR-34a-5p, miR-103a-3p, and miR-376a-3p as possible biomarkers of conversion from relapsing-remitting to secondary progressive multiple sclerosis. Neurobiol. Dis. 2024, 200, 106648. [Google Scholar] [CrossRef]
- Yin, X.; Wang, X.; Wang, S.; Xia, Y.; Chen, H.; Yin, L.; Hu, K. Screening for Regulatory Network of miRNA-Inflammation, Oxidative Stress and Prognosis-Related mRNA in Acute Myocardial Infarction: An in silico and Validation Study. Int. J. Gen. Med. 2022, 15, 1715–1731. [Google Scholar] [CrossRef]
- Ali, E.A.; Syed, A.; Khailova, L.; Iguidbashian, J.P.; Suarez-Pierre, A.; Klawitter, J.; Stone, M.; Mancuso, C.A.; Frank, B.S.; Davidson, J.A. Novel Biomarkers of Necrotizing Enterocolitis in Neonates Undergoing Congenital Heart Disease Surgery: A Pilot Cohort Study. J. Am. Heart Assoc. 2023, 12, e030712. [Google Scholar] [CrossRef]
- Ho, S.S.C.; Keenan, J.I.; Day, A.S. The Role of Gastrointestinal-Related Fatty Acid-Binding Proteins as Biomarkers in Gastrointestinal Diseases. Dig. Dis. Sci. 2020, 65, 376–390. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Lin, Z.; Fan, Y.; Bao, X.; Chen, X.; Zheng, A. I-FABP protein/mRNA and IL-6 as biomarkers of intestinal barrier dysfunction in neonates with necrotizing enterocolitis and SPF BALB/c mouse models. J. Int. Med. Res. 2024, 52, 3000605241254788. [Google Scholar] [CrossRef]
- Hoffsten, A.; Lilja, H.E.; Mobini-Far, H.; Sindelar, R.; Markasz, L. Paneth cell proteins DEFA6 and GUCA2A as tissue markers in necrotizing enterocolitis. Eur. J. Pediatr. 2023, 182, 2775–2784. [Google Scholar] [CrossRef]
- Chong, Q.; Wang, Z.; Guo, T.; Zhang, L.; Lu, L.; Cai, C.; Gong, X.; Lv, Z.; Sheng, Q. Gestational age-specific hematological features in preterm infants with necrotizing enterocolitis. Pediatr. Res. 2024, 95, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Sulistyo, A.; Rahman, A.; Biouss, G.; Antounians, L.; Zani, A. Animal models of necrotizing enterocolitis: Review of the literature and state of the art. Innov. Surg. Sci. 2018, 3, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.J.; McElroy, S.J.; Hunter, C.J. The science and necessity of using animal models in the study of necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Barlow, B.; Santulli, T.V. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery 1975, 77, 687–690. [Google Scholar]
- Zhang, X.; Zhang, Y.; He, Y.; Zhu, X.; Ai, Q.; Shi, Y. beta-glucan protects against necrotizing enterocolitis in mice by inhibiting intestinal inflammation, improving the gut barrier, and modulating gut microbiota. J. Transl. Med. 2023, 21, 14. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, J.; Fu, M.; He, Q.; Chen, J.; Chen, Y.; Zhang, R.; Zhong, W. A Neonatal BALB/c Mouse Model of Necrotizing Enterocolitis. J. Vis. Exp. 2021, 177, e63252. [Google Scholar] [CrossRef]
- Yang, C.; Feng, Z.; Deng, H.; Dai, L.; He, L.; Yin, L.; Zhao, J. CXCL1/CXCR2 is involved in white matter injury in neonatal rats via the gut-brain axis. BMC Neurosci. 2022, 23, 67. [Google Scholar] [CrossRef]
- Sha, C.; Van Brunt, T.; Kudria, J.; Schmidt, D.; Yurovsky, A.; Bandovic, J.; Giarrizzo, M.; Lin, J.; Tsirka, S.A.; Bialkowska, A.B.; et al. A graded neonatal mouse model of necrotizing enterocolitis demonstrates that mild enterocolitis is sufficient to activate microglia and increase cerebral cytokine expression. PLoS ONE 2025, 20, e0323626. [Google Scholar] [CrossRef]
- Sha, C.; Jin, Z.; Ku, S.Y.; Kogosov, A.S.; Yu, S.; Bergese, S.D.; Hsieh, H. Necrotizing Enterocolitis and Neurodevelopmental Impairments: Microbiome, Gut, and Brain Entanglements. Biomolecules 2024, 14, 1254. [Google Scholar] [CrossRef]
- Mihi, B.; Lanik, W.E.; Gong, Q.; Good, M. A Mouse Model of Necrotizing Enterocolitis. Methods Mol. Biol. 2021, 2321, 101–110. [Google Scholar] [CrossRef]
- Nino, D.F.; Zhou, Q.; Yamaguchi, Y.; Martin, L.Y.; Wang, S.; Fulton, W.B.; Jia, H.; Lu, P.; Prindle, T., Jr.; Zhang, F.; et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. 2018, 10, eaan0237. [Google Scholar] [CrossRef] [PubMed]
- Biouss, G.; Antounians, L.; Li, B.; O’Connell, J.S.; Seo, S.; Catania, V.D.; Guadagno, J.; Rahman, A.; Zani-Ruttenstock, E.; Svergun, N.; et al. Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J. Neuroinflamm. 2019, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.K.; Navarro, J.B.; Allen, J.M.; McCulloh, C.J.; Mashburn-Warren, L.; Wang, Y.; Varaljay, V.A.; Bailey, M.T.; Goodman, S.D.; Besner, G.E. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G408–G419. [Google Scholar] [CrossRef] [PubMed]
- Touloukian, R.J.; Posch, J.N.; Spencer, R. The pathogenesis of ischemic gastroenterocolitis of the neonate: Selective gut mucosal ischemia in asphyxiated neonatal piglets. J. Pediatr. Surg. 1972, 7, 194–205. [Google Scholar] [CrossRef]
- Sun, J.; Pan, X.; Christiansen, L.I.; Yuan, X.L.; Skovgaard, K.; Chatterton, D.E.W.; Kaalund, S.S.; Gao, F.; Sangild, P.T.; Pankratova, S. Necrotizing enterocolitis is associated with acute brain responses in preterm pigs. J. Neuroinflamm. 2018, 15, 180. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Nino, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The human milk oligosaccharides 2′-fucosyllactose and 6′-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef]
- Crissinger, K.D.; Burney, D.L.; Velasquez, O.R.; Gonzalez, E. An animal model of necrotizing enterocolitis induced by infant formula and ischemia in developing piglets. Gastroenterology 1994, 106, 1215–1222. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, D.; Zhang, N.; Xia, Z.; Wang, J.; Yang, H.; Guo, F.; Li, B. TNF-alpha induces autophagy through ERK1/2 pathway to regulate apoptosis in neonatal necrotizing enterocolitis model cells IEC-6. Cell Cycle 2018, 17, 1390–1402. [Google Scholar] [CrossRef]
- Gao, C.; Feng, Z.; Wang, L.; Fu, K.; Yang, Z.; Wang, S.; Yu, S. A comparative study on in vitro models of necrotizing enterocolitis induced by single and combined stimulation. Arab. J. Gastroenterol. 2025, 26, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, Z.; Lin, R.; Liu, Y.; Wu, X.; Puthiyakunnon, S.; Wang, Y.; Zhu, B.; Zhang, Q.; Bai, Y.; et al. Bacteroides fragilis Strain ZY-312 Defense against Cronobacter sakazakii-Induced Necrotizing Enterocolitis In Vitro and in a Neonatal Rat Model. mSystems 2019, 4, e00305-19. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.; Dymock, D.; Corfield, A.P.; Weaver, G.; Woodward, M.; Berry, M. Bacterial invasion of HT29-MTX-E12 monolayers: Effects of human breast milk. J. Pediatr. Surg. 2013, 48, 353–357; discussion 357–358. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.; Buonpane, C.; Sincavage, J.; Yuan, C.; Wood, D.R.; Hunter, C.J. Caveolin 1 is Associated with Upregulated Claudin 2 in Necrotizing Enterocolitis. Sci. Rep. 2019, 9, 4982. [Google Scholar] [CrossRef]
- Grothaus, J.S.; Ares, G.; Yuan, C.; Wood, D.R.; Hunter, C.J. Rho kinase inhibition maintains intestinal and vascular barrier function by upregulation of occludin in experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G514–G528. [Google Scholar] [CrossRef]
- Hoffmann, P.; Burmester, M.; Langeheine, M.; Brehm, R.; Empl, M.T.; Seeger, B.; Breves, G. Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells. PLoS ONE 2021, 16, e0257824. [Google Scholar] [CrossRef]
- Zito, A.; Wu, R.Y.; Li, B.; Botts, S.R.; Feizi, M.; Lee, D.; Lee, C.; Johnson-Henry, K.C.; Surette, M.G.; Sherman, P.M.; et al. Human milk oligosaccharides promote intestinal epithelium regeneration independent of the microbiota during necrotizing enterocolitis. Pediatr. Surg. Int. 2024, 40, 35. [Google Scholar] [CrossRef]
- Buonpane, C.; Ares, G.; Yuan, C.; Schlegel, C.; Liebe, H.; Hunter, C.J. Experimental Modeling of Necrotizing Enterocolitis in Human Infant Intestinal Enteroids. J. Investig. Surg. 2022, 35, 111–118. [Google Scholar] [CrossRef]
- Snyder, K.B.; Calkins, C.L.; Golubkova, A.; Leiva, T.; Schlegel, C.; Hunter, C.J. Despite Recovery from Necrotizing Enterocolitis Infants Retain a Hyperinflammatory Response to Injury. J. Inflamm. Res. 2024, 17, 331–341. [Google Scholar] [CrossRef]
- Frazer, L.C.; Yamaguchi, Y.; Jania, C.M.; Lanik, W.E.; Gong, Q.; Singh, D.K.; Mackay, S.; Akopyants, N.S.; Good, M. Microfluidic Model of Necrotizing Enterocolitis Incorporating Human Neonatal Intestinal Enteroids and a Dysbiotic Microbiome. J. Vis. Exp. 2023, e65605. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Albanese, C.T. Necrotizing Enterocolitis. Pediatr. Surg. 2006, 2, 1427–1452. [Google Scholar] [CrossRef]
- Faingold, R.; Daneman, A.; Tomlinson, G.; Babyn, P.S.; Manson, D.E.; Mohanta, A.; Moore, A.M.; Hellmann, J.; Smith, C.; Gerstle, T.; et al. Necrotizing enterocolitis: Assessment of bowel viability with color doppler US. Radiology 2005, 235, 587–594. [Google Scholar] [CrossRef]
- Van der Heide, M.; Hulscher, J.B.F.; Bos, A.F.; Kooi, E.M.W. Near-infrared spectroscopy as a diagnostic tool for necrotizing enterocolitis in preterm infants. Pediatr. Res. 2021, 90, 148–155. [Google Scholar] [CrossRef]
- Kooi, E.M.W.; Mintzer, J.P.; Rhee, C.J.; Ergenekon, E.; Schwarz, C.E.; Pichler, G.; de Boode, W.P.; Spectroscopy, E.S.I.G.N.-I. Neonatal somatic oxygenation and perfusion assessment using near-infrared spectroscopy: Part of the series on near-infrared spectroscopy by the European Society of Paediatric Research Special Interest Group “Near-Infrared Spectroscopy”. Pediatr. Res. 2024, 96, 1180–1194. [Google Scholar] [CrossRef]
- Chaaban, H.; Markel, T.A.; Canvasser, J.; Good, M. Biobanking for necrotizing enterocolitis: Needs and standards. J. Pediatr. Surg. 2020, 55, 1276–1279. [Google Scholar] [CrossRef]
- Sokou, R.; Mantzios, P.; Palioura, A.E.; Tsantes, A.G.; Lianou, A.; Piovani, D.; Tsante, K.A.; Lampropoulou, K.; Iacovidou, N.; Bonovas, S. Diagnostic and Prognostic Value of Hematological Parameters in Necrotizing Enterocolitis: A Systematic Review. J. Clin. Med. 2025, 14, 2530. [Google Scholar] [CrossRef]
- Bethell, G.S.; Hall, N.J.; Battersby, C.; Knight, M.; Darlington, A.S. Surgeons and neonatologists views about surgical decision-making in necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2025. [Google Scholar] [CrossRef]
- Itriago, E.; Trahan, K.F.; Massieu, L.A.; Garg, P.M.; Premkumar, M.H. Current Practices, Challenges, and Recommendations in Enteral Nutrition After Necrotizing Enterocolitis. Clin. Perinatol. 2023, 50, 683–698. [Google Scholar] [CrossRef]
- Neu, J.; Singh, R.; Demetrian, M.; Flores-Torres, J.; Hudak, M.; Zupancic, J.A.; Kronstrom, A.; Rastad, J.; Stromberg, S.; Thuresson, M.; et al. Clinical Characteristics of Necrotizing Enterocolitis Diagnosed by Independent Adjudication of Abdominal Radiographs, Laparotomy, or Autopsy in Preterm Infants in the “Connection Trial”. Am. J. Perinatol. 2025, 42, 268–280. [Google Scholar] [CrossRef]
- Feldman, K.; Noel-MacDonnell, J.R.; Pappas, L.B.; Romald, J.H.; Olson, S.L.; Oschman, A.; Cuna, A.C.; Sampath, V. Incidence of probiotic sepsis and morbidity risk in premature infants: A meta-analysis. Pediatr. Res. 2025. [Google Scholar] [CrossRef]
- Wang, Y.; Florez, I.D.; Morgan, R.L.; Foroutan, F.; Chang, Y.; Crandon, H.N.; Zeraatkar, D.; Bala, M.M.; Mao, R.Q.; Tao, B.; et al. Probiotics, Prebiotics, Lactoferrin, and Combination Products for Prevention of Mortality and Morbidity in Preterm Infants: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023, 177, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, B.; Committee on Fetus and Newborn. Use of Probiotics in Preterm Infants. Pediatrics 2021, 147, e2021051485. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Berni Canani, R.; Domellof, M.; Guarino, A.; Hojsak, I.; Indrio, F.; Lo Vecchio, A.; Mihatsch, W.A.; Mosca, A.; Orel, R.; et al. Probiotics for the Management of Pediatric Gastrointestinal Disorders: Position Paper of the ESPGHAN Special Interest Group on Gut Microbiota and Modifications. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, B.N.; Ting, J.; Lee, S.; Lemyre, B.; Wong, J.; Afifi, J.; Beltempo, M.; Shah, P.S.; Canadian Neonatal Network, I. Effectiveness and Risks of Probiotics in Preterm Infants. Pediatrics 2025, 155, e2024069102. [Google Scholar] [CrossRef]
- US FDA. Warning Regarding Use of Probiotics in Preterm Infants. Internet Document: 29 September 2023. Available online: https://www.fda.gov/media/172606/download?attachment (accessed on 23 September 2025).
- Robinson, J.R.; Kennedy, C.; van Arendonk, K.J.; Green, A.; Martin, C.R.; Blakely, M.L. Neurodevelopmental considerations in surgical necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 52–56. [Google Scholar] [CrossRef]
- Adams-Chapman, I. Necrotizing Enterocolitis and Neurodevelopmental Outcome. Clin. Perinatol. 2018, 45, 453–466. [Google Scholar] [CrossRef]
- Ganji, N.; Li, B.; Lee, C.; Pierro, A. Necrotizing enterocolitis: Recent advances in treatment with translational potential. Pediatr. Surg. Int. 2023, 39, 205. [Google Scholar] [CrossRef]
- Markel, T.A.; Martin, C.A.; Chaaban, H.; Canvasser, J.; Tanner, H.; Denchik, H.; Good, M. New directions in necrotizing enterocolitis with early-stage investigators. Pediatr. Res. 2020, 88, 35–40. [Google Scholar] [CrossRef]
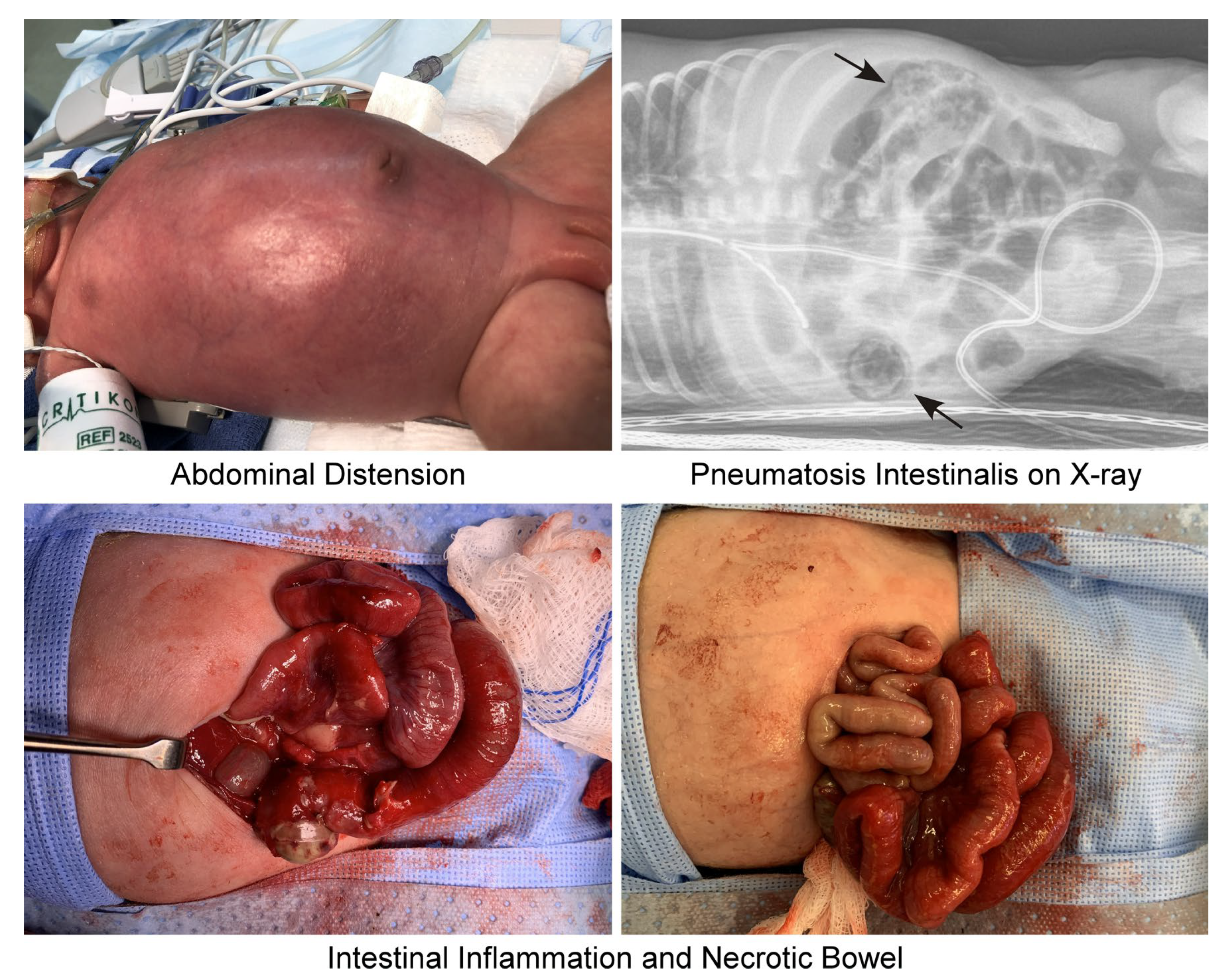
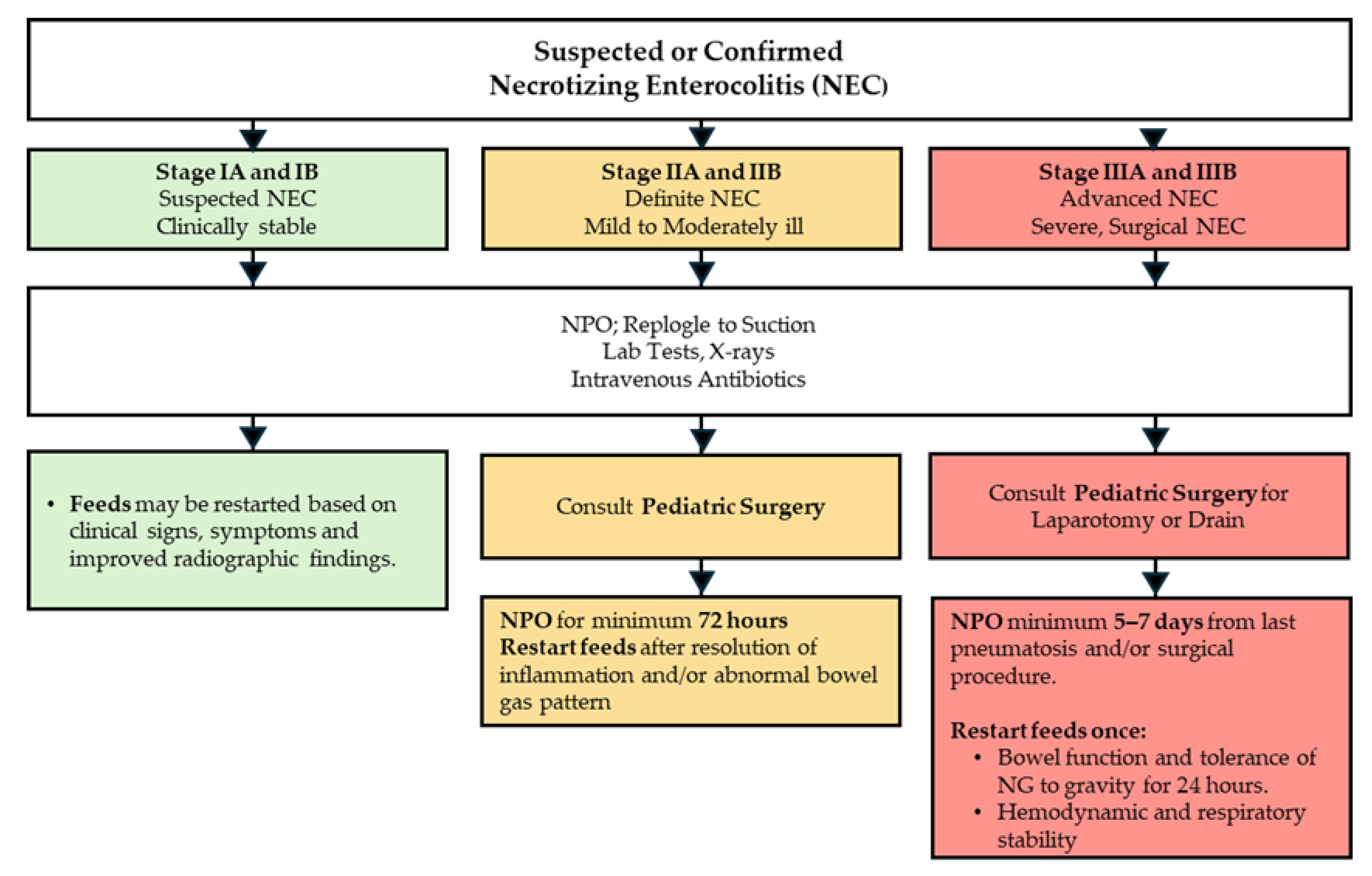
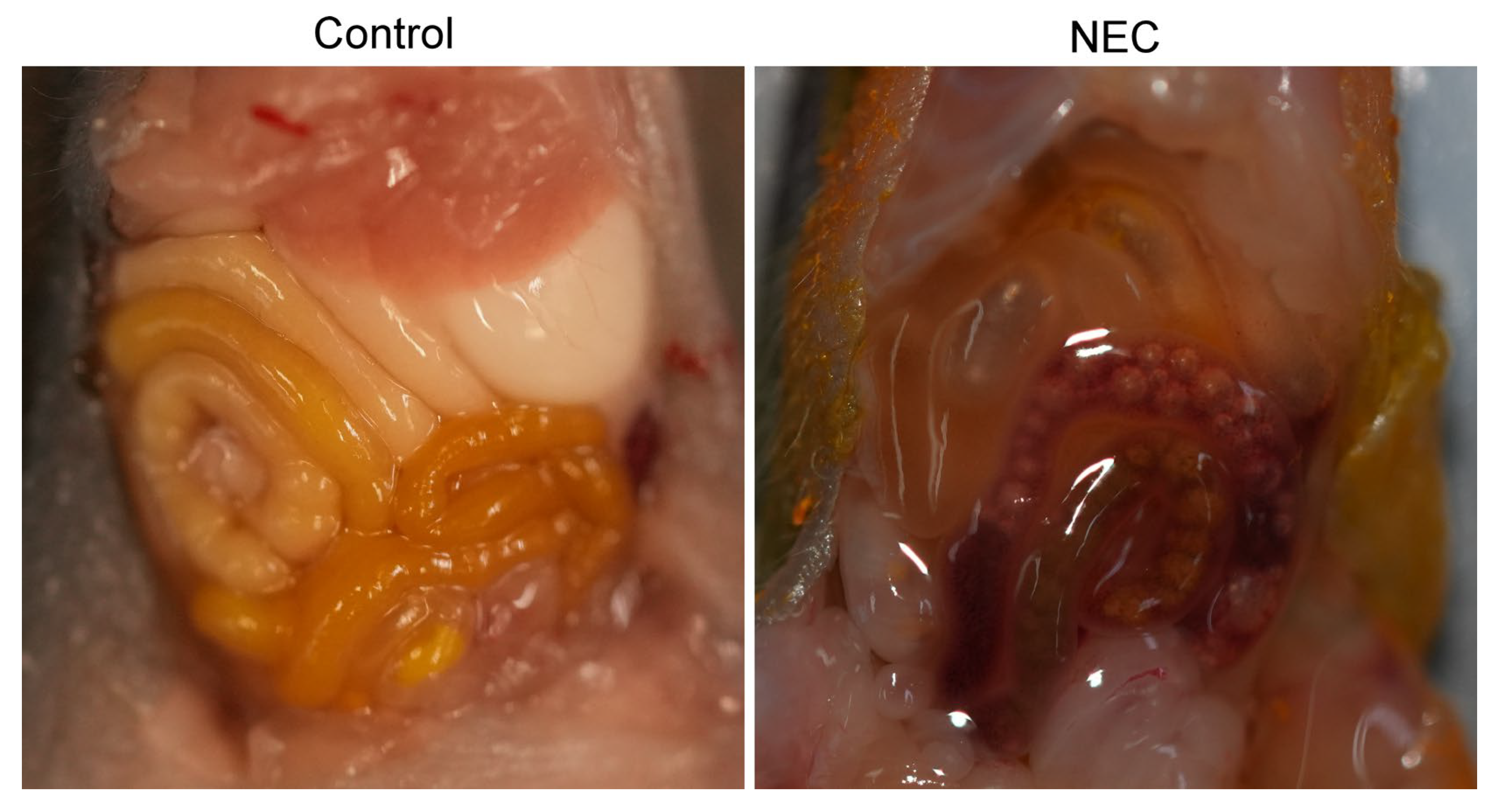
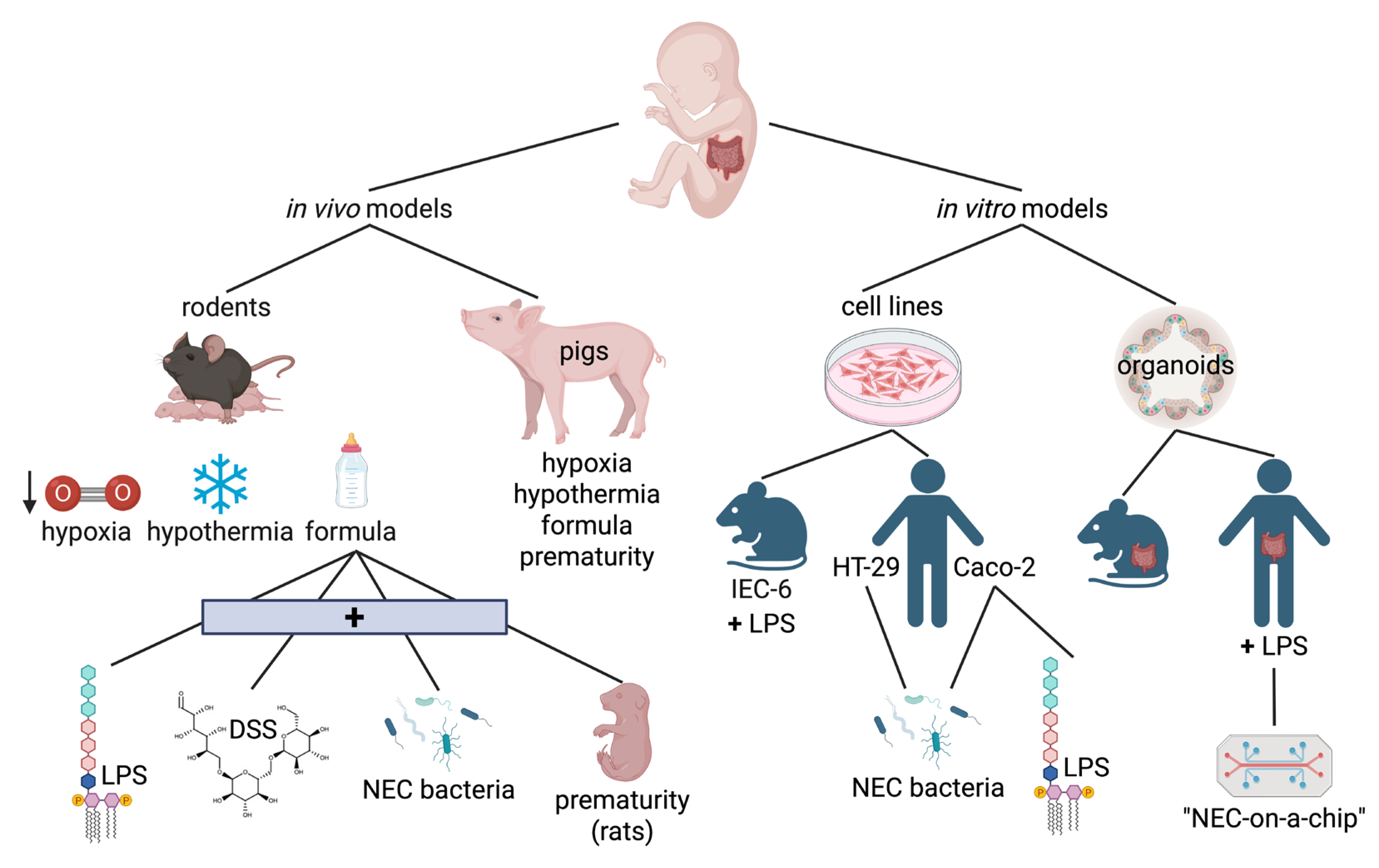
| STAGE | SYSTEMIC SIGNS | INTESTINAL SIGNS | RADIOLOGIC SIGNS |
|---|---|---|---|
| IA—SUSPECTED NEC | Temperature instability, apnea, bradycardia, lethargy | Elevated pre-gavage residuals, mild abdominal distention, emesis, guaiac-positive stool | Normal or intestinal dilation, mild ileus |
| IB—SUSPECTED NEC | Same as IA | Bright red blood from rectum | Same as IA |
| IIA—DEFINITE NEC, MILDLY ILL | Same as IA | Same as above, plus absent bowel sounds, +/− abdominal tenderness | Intestinal dilation, ileus, pneumatosis intestinalis |
| IIB—DEFINITE NEC, MODERATELY ILL | Same as IA, plus mild metabolic acidosis, mild thrombocytopenia | Same as above, plus absent bowel sounds, definite abdominal tenderness, +/− abdominal cellulitis or right lower quadrant mass | Same as IIA, plus portal vein gas, +/− ascites |
| IIIA—ADVANCED NEC, SEVERELY Ill, BOWEL INTACT | Same as IIB, plus hypotension, bradycardia, severe apnea, combined respiratory and metabolic acidosis, disseminated intravascular coagulation, neutropenia | Same as above, plus signs of generalized peritonitis, marked tenderness, and distention of abdomen | Same as IIB, plus definite ascites |
| IIIB—ADVANCED NEC, SEVERELY Ill, BOWEL PERFORATED | Same as IIIA | Same as IIIA | Same as IIB, plus pneumoperitoneum |
| NCT Identifier | Category (Type of Treatment) | NEC Outcome | Maternal, Paternal, or Infant-focused | Study Status | Study Results |
|---|---|---|---|---|---|
| NCT00252681 | Surgical | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT00059449 | Observational | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT01029353 | Surgical | NEC—PRIMARY OUTCOME | Infant | COMPLETED | YES |
| NCT00707785 | Drug Therapy | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT00005888 | Dietary Supplement | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT02741648 | Observational | NEC—PRIMARY OUTCOME | Infant | UNKNOWN | NO |
| NCT01106209 | In vitro study | NEC—PRIMARY OUTCOME | Infant | TERMINATED | YES |
| NCT01222585 | Antimicrobial Therapy | NEC—PRIMARY OUTCOME | Infant | COMPLETED | YES |
| NCT00747851 | In vitro study | NEC—PRIMARY OUTCOME | Infant | UNKNOWN | NO |
| NCT01223261 | Observational | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT02784821 | Antimicrobial Therapy | NEC—PRIMARY OUTCOME | Infant | COMPLETED | YES |
| NCT00392977 | Dietary Supplement | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT05535361 | Device | NEC—OTHER OUTCOME | Infant | RECRUITING | NO |
| NCT03174301 | Observational | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT00009646 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT01576003 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT01534481 | Dietary Supplement | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT00339235 | Observational | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | NO |
| NCT03115463 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT01473264 | Drug Therapy | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT00955487 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT01203475 | Observational | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT00840983 | Non-surgical | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT03997266 | Antimicrobial Therapy | NEC—PRIMARY OUTCOME | Infant | RECRUITING | NO |
| NCT00734539 | Antimicrobial Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT00114543 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT03169881 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | ACTIVE NOT RECRUITING | YES |
| NCT00005775 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT00809055 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT02451228 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | NO |
| NCT03456336 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | ACTIVE NOT RECRUITING | NO |
| NCT03728608 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT02299414 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | YES |
| NCT00615550 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | YES |
| NCT05676476 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | RECRUITING | NO |
| NCT04325308 | Dietary Supplement | NEC—SECONDARY OUTCOME | Infant | ACTIVE NOT RECRUITING | YES |
| NCT00005777 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | TERMINATED | NO |
| NCT00233324 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT01954056 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT05446272 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | RECRUITING | NO |
| NCT06153459 | Non-surgical | NEC—PRIMARY OUTCOME | Infant | RECRUITING | NO |
| NCT00874393 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT03292731 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | TERMINATED | YES |
| NCT01866982 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT06448780 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | RECRUITING | NO |
| NCT01863043 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT06605118 | Antimicrobial Therapy | NEC—SECONDARY OUTCOME | Maternal | RECRUITING | NO |
| NCT01378273 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT00135707 | Dietary Supplement | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | YES |
| NCT01035697 | Observational | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT00115934 | Surgical | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT01827358 | Antimicrobial Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT04519502 | Observational | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | NO |
| NCT00135902 | Dietary Supplement | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | NO |
| NCT03927833 | Device | NEC—SECONDARY OUTCOME | Infant | ACTIVE NOT RECRUITING | NO |
| NCT01235546 | Antimicrobial Therapy | NEC—PRIMARY OUTCOME | Maternal | COMPLETED | YES |
| NCT05459298 | Dietary Supplement | NEC—SECONDARY OUTCOME | Infant | RECRUITING | NO |
| NCT06245057 | Maternal Care | NEC—SECONDARY OUTCOME | Maternal | RECRUITING | NO |
| NCT01702805 | Non-surgical | NEC—SECONDARY OUTCOME | Infant | ACTIVE NOT RECRUITING | YES |
| NCT06679855 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | RECRUITING | NO |
| NCT06362798 | Maternal Care | NEC—SECONDARY OUTCOME | Maternal | RECRUITING | NO |
| NCT06676904 | Non-surgical | NEC—OTHER OUTCOME | Infant | RECRUITING | NO |
| NCT00388297 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | YES |
| NCT00439374 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | TERMINATED | YES |
| NCT05380401 | Dietary Supplement | NEC—OTHER OUTCOME | Infant | RECRUITING | NO |
| NCT00099164 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | NO |
| NCT01376778 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | COMPLETED | YES |
| NCT01857310 | Dietary Supplement | NEC—SECONDARY OUTCOME | Paternal | COMPLETED | YES |
| NCT02901626 | Device | NEC—SECONDARY OUTCOME | Maternal | TERMINATED | YES |
| NCT03944512 | Drug Therapy | NEC—SECONDARY OUTCOME | Maternal | TERMINATED | NO |
| NCT01954082 | Drug Therapy | NEC—OTHER OUTCOME | Infant | TERMINATED | YES |
| NCT01778634 | Antimicrobial Therapy | NEC—OTHER OUTCOME | Infant | COMPLETED | YES |
| NCT06878950 | Dietary Supplement | NEC—OTHER OUTCOME | Infant | RECRUITING | NO |
| NCT01353313 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | YES |
| NCT04413097 | Non-surgical | NEC—OTHER OUTCOME | Infant | ACTIVE NOT RECRUITING | NO |
| NCT06915428 | Maternal Care | NEC—SECONDARY OUTCOME | Maternal | NOT YET RECRUITING | NO |
| NCT06980025 | Drug Therapy | NEC—OTHER OUTCOME | Maternal | NOT YET RECRUITING | NO |
| NCT01203345 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | COMPLETED | NO |
| NCT06855108 | Drug Therapy | NEC—SECONDARY OUTCOME | Infant | NOT YET RECRUITING | NO |
| NCT00252681 | Surgical | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT00059449 | Observational | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT01029353 | Surgical | NEC—PRIMARY OUTCOME | Infant | COMPLETED | YES |
| NCT00707785 | Drug Therapy | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT00005888 | Dietary Supplement | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT02741648 | Observational | NEC—PRIMARY OUTCOME | Infant | UNKNOWN | NO |
| NCT01106209 | In vitro study | NEC—PRIMARY OUTCOME | Infant | TERMINATED | YES |
| NCT00747851 | In vitro study | NEC—PRIMARY OUTCOME | Infant | UNKNOWN | NO |
| NCT01223261 | Observational | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT02784821 | Antimicrobial Therapy | NEC—PRIMARY OUTCOME | Infant | COMPLETED | YES |
| NCT00392977 | Dietary Supplement | NEC—PRIMARY OUTCOME | Infant | COMPLETED | NO |
| NCT05535361 | Device | NEC—OTHER OUTCOME | Infant | RECRUITING | NO |
| Animal | Experimental NEC Protocol | Reference |
|---|---|---|
| Mouse | 3-day protocol starting with 10-day-old C57BL/6 mice; Gavage feed hyperosmolar formula/Esbilac puppy milk replacer every 4 h; Hypoxia (100% N2 for 90 s) 3 times per day; Hypothermia (4 °C for 10 min) 3 times per day | [119] |
| Mouse | 5-day protocol starting with 5-day-old BALB/c mice; Separate mice on night of Day 4 and fast overnight; Gavage with LPS 3 times per day and formula every 2 h (or overnight every 4 h); Hypoxia-reoxygenation-cold-shock cycle (5% O2 for 90 s, reoxygenate for 3 min, 4 °C for 15 min) 2 times per day | [120] |
| Mouse | 3-day protocol starting with 3-day-old C57BL/6 mice; Gavage feed Esbilac puppy milk replacer with DSS every 3 h—mice were randomly assigned to a DSS concentration group (0%, 0.25%, 1%, 2%, or 3%) | [122] |
| Mouse | 3-day protocol starting with 4-day-old C57BL/6 mice; Gavage feed NEC formula containing severe surgical NEC patient-derived enteric bacteria and LPS every 3 h; Hypoxia (95% N2 and 5% O2 for 10 min) 2 times per day | [124] |
| Mouse | 4-day protocol starting with 7-day-old C57BL/6 mice; Gavage feed formula/Esbilac puppy milk replacer with enteric bacteria from infant with NEC 5 times per day; Hypoxia (95% N2 and 5% O2 for 10 min) 2 times per day | [125] |
| Mouse | 4-day protocol starting with 5-day-old C57BL/6 mice; Gavage feed formula 3 times per day; Oral administration of LPS (4 mg/kg/day) with formula on days 1 and 2; Hypoxia (5% O2 for 10 min) 3 times per day | [126] |
| Rat | 3-day protocol starting with 1-day-old SD rats; Gavage with 3% dextran sodium sulfate (DSS) dissolved in normal saline every 6 h or 4 times per day | [121] |
| Rat | 4-day protocol starting with 20.5 days gestational age SD rats delivered prematurely via cesarean section; Gavage feed hypertonic, hypercaloric formula 5 times per day; Gavage 2 mg/kg LPS on the first day of life; Hypoxia (N2 for 90 s) and hypothermia (4 °C for 10 min) 3 times per day | [127] |
| Pig | 8-day protocol starting with 106 days gestational age pigs delivered prematurely via cesarean section; Gavage feed increasing doses of human donor milk and decreasing doses of parenteral nutrition across 8 days | [129] |
| Pig | 4-day protocol starting on 105–106 days gestational age pigs delivered prematurely via cesarean section; Gavage feed formula supplemented with enteric bacteria from an infant with surgical NEC every 3 h | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, C.; Sander, W.R.; Bass, K.; Hsieh, H.; Bialkowska, A.B. Necrotizing Enterocolitis: What’s New and What’s Next? Int. J. Mol. Sci. 2025, 26, 9660. https://doi.org/10.3390/ijms26199660
Sha C, Sander WR, Bass K, Hsieh H, Bialkowska AB. Necrotizing Enterocolitis: What’s New and What’s Next? International Journal of Molecular Sciences. 2025; 26(19):9660. https://doi.org/10.3390/ijms26199660
Chicago/Turabian StyleSha, Cuilee, William R. Sander, Kathryn Bass, Helen Hsieh, and Agnieszka B. Bialkowska. 2025. "Necrotizing Enterocolitis: What’s New and What’s Next?" International Journal of Molecular Sciences 26, no. 19: 9660. https://doi.org/10.3390/ijms26199660
APA StyleSha, C., Sander, W. R., Bass, K., Hsieh, H., & Bialkowska, A. B. (2025). Necrotizing Enterocolitis: What’s New and What’s Next? International Journal of Molecular Sciences, 26(19), 9660. https://doi.org/10.3390/ijms26199660








