Utilization of Stem Cells in Medicine: A Narrative Review
Abstract
1. Introduction
2. Classification of Stem Cells
2.1. Totipotent Stem Cells
2.2. Pluripotent Stem Cells
2.3. Multipotent Stem Cells
2.4. Oligopotent Stem Cells
2.5. Unipotent Stem Cells
2.6. Induced Pluripotent Stem Cells
3. Therapeutic Applications of Stem Cells
3.1. Cardiovascular Diseases
3.2. Dermatology
3.2.1. Psoriasis
3.2.2. Atopic Dermatitis
3.2.3. Vitiligo
3.2.4. Epidermolysis Bullosa
3.2.5. Alopecia
3.2.6. Systemic Sclerosis
3.3. Plastic Surgery and Aesthetic Medicine
3.3.1. Wound Healing
3.3.2. Scars
3.3.3. Skin Rejuvenation
3.4. Infertility
3.4.1. Female Infertility
3.4.2. Male Infertility
3.5. Endocrinology
3.5.1. Diabetes Mellitus
3.5.2. Adrenal Insufficiency
3.5.3. Thyroid Dysfunction
3.6. Ophthalmology
3.6.1. Corneal Stem Cell Therapies (Limbal Stem Cell Deficiency)
3.6.2. Retinal Pigment Epithelium Disorders (Age-Related Macular Degeneration-AMD)
3.6.3. Inherited Photoreceptor Diseases (RP and Stargardt Disease)
3.7. Pulmonology
3.7.1. Chronic Obstructive Pulmonary Disease (COPD)
3.7.2. Idiopathic Pulmonary Fibrosis (IPF)
3.7.3. Acute Respiratory Distress Syndrome (ARDS)
3.7.4. Cystic Fibrosis (CF)
3.8. Nephrology
3.8.1. Chronic Kidney Disease (CKD)
3.8.2. Refractory Systemic Lupus Erythematosus (SLE)
3.8.3. Kidney Transplant
3.9. Stem Cell Applications in Neurology
3.10. Stem Cell Applications in Musculoskeletal Diseases
3.11. Stem Cell Applications in Hematology
3.12. Oncology
3.12.1. Induction of Tumor Cell Apoptosis
3.12.2. Stem Cell-Derived Exosomes as Anti-Tumor Agents
3.12.3. Immunomodulation of Tumor Microenvironment
3.12.4. Stem Cells as Drug Delivery Platforms
3.12.5. Tumor-Specific Applications and Translational Highlights
3.13. Gastrointestinal Diseases
3.13.1. Inflammatory Bowel Disease (IBD)
3.13.2. Liver Disease (Cirrhosis, ALF, NASH)
3.13.3. Short Bowel Syndrome/Intestinal Failure
3.13.4. Gastric Disorders
4. Ethical and Regulatory Considerations
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Moodley, Y. Introduction to Stem Cells and Regenerative Medicine. Respiration 2012, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Saini, A.; Kalsan, M.; Kumar, N.; Chandra, R. Describing the Stem Cell Potency: The Various Methods of Functional Assessment and In silico Diagnostics. Front. Cell Dev. Biol. 2016, 4, 134. [Google Scholar] [CrossRef]
- Mesfin, F.M.; Manohar, K.; Hunter, C.E.; Shelley, W.C.; Brokaw, J.P.; Liu, J.; Ma, M.; Markel, T.A. Stem cell derived therapies to preserve and repair the developing intestine. Semin. Perinatol. 2023, 47, 151727. [Google Scholar] [CrossRef]
- Blau, H.M.; Daley, G.Q. Stem Cells in the Treatment of Disease. N. Engl. J. Med. 2019, 380, 1748–1760, Erratum in N. Engl. J. Med. 2019, 381, 890. [Google Scholar] [CrossRef]
- Alvarez, C.V.; Garcia-Lavandeira, M.; Garcia-Rendueles, M.E.; Diaz-Rodriguez, E.; Garcia-Rendueles, A.R.; Perez-Romero, S.; Vila, T.V.; Rodrigues, J.S.; Lear, P.V.; Bravo, S.B. Defining stem cell types: Understanding the therapeutic potential of ESCs, ASCs, and iPS cells. J. Mol. Endocrinol. 2012, 49, R89–R111. [Google Scholar] [CrossRef]
- Wobus, A.M.; Boheler, K.R. Embryonic stem cells: Prospects for developmental biology and cell therapy. Physiol. Rev. 2005, 85, 635–678. [Google Scholar] [CrossRef]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef]
- Burt, R.K.; Loh, Y.; Pearce, W.; Beohar, N.; Barr, W.G.; Craig, R.; Wen, Y.; Rapp, J.A.; Kessler, J. Clinical Applications of Blood-Derived and Marrow-Derived Stem Cells for Nonmalignant Diseases. JAMA 2008, 299, 925–936. [Google Scholar] [CrossRef]
- Ali, F.; Taresh, S.; Al-Nuzaily, M.; Mok, P.L.; Ismail, A.; Ahmad, S. Stem cells differentiation and probing their therapeutic applications in hematological disorders: A critical review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4390–4400. [Google Scholar]
- Gurusamy, N.; Alsayari, A.; Rajasingh, S.; Rajasingh, J. Adult Stem Cells for Regenerative Therapy. Prog. Mol. Biol. Transl. Sci. 2018, 160, 1–22. [Google Scholar] [CrossRef]
- Pharoun, J.; Berro, J.; Sobh, J.; Abou-Younes, M.M.; Nasr, L.; Majed, A.; Khalil, A.; Joseph; Stephan; Faour, W.H. Mesenchymal stem cells biological and biotechnological advances: Implications for clinical applications. Eur. J. Pharmacol. 2024, 977, 176719. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 4290. [Google Scholar] [CrossRef] [PubMed]
- Yen, B.L.; Liu, K.J.; Sytwu, H.K.; Yen, M.L. Clinical implications of differential functional capacity between tissue-specific human mesenchymal stromal/stem cells. Febs J. 2023, 290, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef]
- Gao, S.; Li, D.; Wang, B.; Zhang, H.; Chen, L. Two promising approaches in the treatment of myocardial infarction: Stem cells and gene therapy. Front. Cardiovasc. Med. 2025, 12, 1540066. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Grigorian-Shamagian, L.; Liu, W.; Fereydooni, S.; Middleton, R.C.; Valle, J.; Cho, J.H.; Marbán, E. Cardiac and systemic rejuvenation after cardiosphere-derived cell therapy in senescent rats. Eur. Heart J. 2017, 38, 2957–2967. [Google Scholar] [CrossRef]
- Zhao, Z.A.; Han, X.; Lei, W.; Li, J.; Yang, Z.; Wu, J.; Yao, M.; Lu, X.A.; He, L.; Chen, Y.; et al. Lack of Cardiac Improvement After Cardiosphere-Derived Cell Transplantation in Aging Mouse Hearts. Circ. Res. 2018, 123, e21–e31. [Google Scholar] [CrossRef]
- Kasai-Brunswick, T.H.; Costa, A.R.; Barbosa, R.A.; Farjun, B.; Mesquita, F.C.; Silva Dos Santos, D.; Ramos, I.P.; Suhett, G.; Brasil, G.V.; Cunha, S.T.; et al. Cardiosphere-derived cells do not improve cardiac function in rats with cardiac failure. Stem Cell Res. Ther. 2017, 8, 36. [Google Scholar] [CrossRef]
- Makkar, R.R.; Kereiakes, D.J.; Aguirre, F.; Kowalchuk, G.; Chakravarty, T.; Malliaras, K.; Francis, G.S.; Povsic, T.J.; Schatz, R.; Traverse, J.H.; et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): A randomized, placebo-controlled, double-blinded trial. Eur. Heart J. 2020, 41, 3451–3458. [Google Scholar] [CrossRef]
- Chugh, A.R.; Beache, G.M.; Loughran, J.H.; Mewton, N.; Elmore, J.B.; Kajstura, J.; Pappas, P.; Tatooles, A.; Stoddard, M.F.; Lima, J.A.; et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The SCIPIO trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 2012, 126, S54–S64. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef]
- Ishigami, S.; Ohtsuki, S.; Eitoku, T.; Ousaka, D.; Kondo, M.; Kurita, Y.; Hirai, K.; Fukushima, Y.; Baba, K.; Goto, T.; et al. Intracoronary Cardiac Progenitor Cells in Single Ventricle Physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ. Res. 2017, 120, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Durrani, S.; Konoplyannikov, M.; Ashraf, M.; Haider, K.H. Skeletal myoblasts for cardiac repair. Regen. Med. 2010, 5, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Hagège, A.A.; Vilquin, J.T.; Desnos, M.; Abergel, E.; Pouzet, B.; Bel, A.; Sarateanu, S.; Scorsin, M.; Schwartz, K.; et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J. Am. Coll. Cardiol. 2003, 41, 1078–1083. [Google Scholar] [CrossRef]
- He, K.L.; Yi, G.H.; Sherman, W.; Zhou, H.; Zhang, G.P.; Gu, A.; Kao, R.; Haimes, H.B.; Harvey, J.; Roos, E.; et al. Autologous skeletal myoblast transplantation improved hemodynamics and left ventricular function in chronic heart failure dogs. J. Heart Lung Transplant. 2005, 24, 1940–1949. [Google Scholar] [CrossRef]
- Gavira, J.J.; Perez-Ilzarbe, M.; Abizanda, G.; García-Rodríguez, A.; Orbe, J.; Páramo, J.A.; Belzunce, M.; Rábago, G.; Barba, J.; Herreros, J.; et al. A comparison between percutaneous and surgical transplantation of autologous skeletal myoblasts in a swine model of chronic myocardial infarction. Cardiovasc. Res. 2006, 71, 744–753. [Google Scholar] [CrossRef][Green Version]
- Gavira, J.J.; Nasarre, E.; Abizanda, G.; Pérez-Ilzarbe, M.; de Martino-Rodriguez, A.; García de Jalón, J.A.; Mazo, M.; Macias, A.; García-Bolao, I.; Pelacho, B.; et al. Repeated implantation of skeletal myoblast in a swine model of chronic myocardial infarction. Eur. Heart J. 2010, 31, 1013–1021. [Google Scholar] [CrossRef]
- Hata, H.; Matsumiya, G.; Miyagawa, S.; Kondoh, H.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; Okano, T.; Matsuda, H.; Sawa, Y. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J. Thorac. Cardiovasc. Surg. 2006, 132, 918–924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Menasché, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.T.; Marolleau, J.P.; Seymour, B.; Larghero, J.; et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef]
- Correia, C.D.; Ferreira, A.; Fernandes, M.T.; Silva, B.M.; Esteves, F.; Leitão, H.S.; Bragança, J.; Calado, S.M. Human Stem Cells for Cardiac Disease Modeling and Preclinical and Clinical Applications-Are We on the Road to Success? Cells 2023, 12, 1727. [Google Scholar] [CrossRef]
- Singla, D.K.; Long, X.; Glass, C.; Singla, R.D.; Yan, B. Induced pluripotent stem (iPS) cells repair and regenerate infarcted myocardium. Mol. Pharm. 2011, 8, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, L.; Wei, Y.; Krishnamurthy, P.; Walcott, G.P.; Menasché, P.; Zhang, J. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci. Transl. Med. 2020, 12, eaay1318. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Z.; Dong, M. Cardiac repair in a murine model of myocardial infarction with human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2020, 11, 297. [Google Scholar] [CrossRef]
- Sun, X.; Wu, J.; Qiang, B.; Romagnuolo, R.; Gagliardi, M.; Keller, G.; Laflamme, M.A.; Li, R.K.; Nunes, S.S. Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci. Transl. Med. 2020, 12, eaax2992. [Google Scholar] [CrossRef]
- Kawamura, T.; Ito, Y.; Ito, E.; Takeda, M.; Mikami, T.; Taguchi, T.; Mochizuki-Oda, N.; Sasai, M.; Shimamoto, T.; Nitta, Y.; et al. Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: First three case reports. Front. Cardiovasc. Med. 2023, 10, 1182209. [Google Scholar] [CrossRef]
- Sugiura, T.; Shahannaz, D.C.; Ferrell, B.E. Current Status of Cardiac Regenerative Therapy Using Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2024, 25, 5772. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, W.; Zhao, P.; Zhang, J.; Lu, Y.; Zhu, Y.; Zhao, W.; Liu, Y.; Chen, Q.; Zhang, F. Down-Regulated Exosomal MicroRNA-221-3p Derived From Senescent Mesenchymal Stem Cells Impairs Heart Repair. Front. Cell Dev. Biol. 2020, 8, 263. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, C.; Zhang, G.; Tao, J. Endothelial progenitor cells in cardiovascular diseases. Aging Med. 2018, 1, 204–208. [Google Scholar] [CrossRef]
- Yu, H.; Lu, K.; Zhu, J.; Wang, J. Stem cell therapy for ischemic heart diseases. Br. Med. Bull. 2017, 121, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Stamm, C.; Kleine, H.D.; Choi, Y.H.; Dunkelmann, S.; Lauffs, J.A.; Lorenzen, B.; David, A.; Liebold, A.; Nienaber, C.; Zurakowski, D.; et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J. Thorac. Cardiovasc. Surg. 2007, 133, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Baharvand, H.; Ashtiani, S.K.; Soleimani, M.; Sadeghian, H.; Ardekani, J.M.; Mehrjerdi, N.Z.; Kouhkan, A.; Namiri, M.; Madani-Civi, M.; et al. Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Curr. Neurovasc Res. 2007, 4, 153–160. [Google Scholar] [CrossRef]
- Forcillo, J.; Stevens, L.M.; Mansour, S.; Prieto, I.; Salem, R.; Baron, C.; Roy, D.C.; Larose, E.; Masckauchan, D.; Noiseux, N. Implantation of CD133+ stem cells in patients undergoing coronary bypass surgery: IMPACT-CABG pilot trial. Can. J. Cardiol. 2013, 29, 441–447. [Google Scholar] [CrossRef]
- Erbs, S.; Linke, A.; Schächinger, V.; Assmus, B.; Thiele, H.; Diederich, K.W.; Hoffmann, C.; Dimmeler, S.; Tonn, T.; Hambrecht, R.; et al. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: The Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation 2007, 116, 366–374. [Google Scholar] [CrossRef]
- Yerebakan, C.; Kaminski, A.; Westphal, B.; Donndorf, P.; Glass, A.; Liebold, A.; Stamm, C.; Steinhoff, G. Impact of preoperative left ventricular function and time from infarction on the long-term benefits after intramyocardial CD133(+) bone marrow stem cell transplant. J. Thorac. Cardiovasc. Surg. 2011, 142, 1530–1539.e1533. [Google Scholar] [CrossRef][Green Version]
- Ahmadi, H.; Farahani, M.M.; Kouhkan, A.; Moazzami, K.; Fazeli, R.; Sadeghian, H.; Namiri, M.; Madani-Civi, M.; Baharvand, H.; Aghdami, N. Five-year follow-up of the local autologous transplantation of CD133+ enriched bone marrow cells in patients with myocardial infarction. Arch. Iran. Med. 2012, 15, 32–35. [Google Scholar][Green Version]
- Nasseri, B.A.; Ebell, W.; Dandel, M.; Kukucka, M.; Gebker, R.; Doltra, A.; Knosalla, C.; Choi, Y.H.; Hetzer, R.; Stamm, C. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: The Cardio133 trial. Eur. Heart J. 2014, 35, 1263–1274. [Google Scholar] [CrossRef]
- Steinhoff, G.; Nesteruk, J.; Wolfien, M.; Kundt, G.; Börgermann, J.; David, R.; Garbade, J.; Große, J.; Haverich, A.; Hennig, H.; et al. Cardiac Function Improvement and Bone Marrow Response—: Outcome Analysis of the Randomized PERFECT Phase III Clinical Trial of Intramyocardial CD133(+) Application After Myocardial Infarction. EBioMedicine 2017, 22, 208–224. [Google Scholar] [CrossRef]
- Tendera, M.; Wojakowski, W.; Ruzyłło, W.; Chojnowska, L.; Kepka, C.; Tracz, W.; Musiałek, P.; Piwowarska, W.; Nessler, J.; Buszman, P.; et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: Results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur. Heart J. 2009, 30, 1313–1321. [Google Scholar] [CrossRef]
- Miyagawa, S.; Domae, K.; Yoshikawa, Y.; Fukushima, S.; Nakamura, T.; Saito, A.; Sakata, Y.; Hamada, S.; Toda, K.; Pak, K.; et al. Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e003918. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2020, 22, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients with Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Mathiasen, A.B.; Mygind, N.D.; Kühl, J.T.; Jørgensen, E.; Helqvist, S.; Elberg, J.J.; Kofoed, K.F.; Vejlstrup, N.G.; Fischer-Nielsen, A.; et al. Adipose-Derived Stromal Cells for Treatment of Patients with Chronic Ischemic Heart Disease (MyStromalCell Trial): A Randomized Placebo-Controlled Study. Stem Cells Int. 2017, 2017, 5237063. [Google Scholar] [CrossRef] [PubMed]
- Aceves, J.L.; López, R.V.; Terán, P.M.; Escobedo, C.M.; Marroquín Muciño, M.A.; Castillo, G.G.; Estrada, M.M.; García, F.R.; Quiroz, G.D.; Montaño Estrada, L.F. Autologous CXCR4+ Hematopoietic Stem Cells Injected into the Scar Tissue of Chronic Myocardial Infarction Patients Normalizes Tissue Contractility and Perfusion. Arch. Med. Res. 2020, 51, 135–144. [Google Scholar] [CrossRef]
- Chen, W.; Ren, G.; Zuo, K.; Huang, X. Complete remission of both immunoglobulin light chain amyloidosis and psoriasis after autologous hematopoietic stem cell transplantation: A case report. Medicine 2018, 97, e13589. [Google Scholar] [CrossRef]
- Gardembas-Pain, M.; Ifrah, N.; Foussard, C.; Boasson, M.; Saint Andre, J.P.; Verret, J.L. Psoriasis after allogeneic bone marrow transplantation. Arch. Dermatol. 1990, 126, 1523. [Google Scholar] [CrossRef]
- Snowden, J.A.; Heaton, D.C. Development of psoriasis after syngeneic bone marrow transplant from psoriatic donor: Further evidence for adoptive autoimmunity. Br. J. Dermatol. 1997, 137, 130–132. [Google Scholar] [CrossRef]
- Naik, P.P. Stem cell therapy as a potential treatment option for psoriasis. An. Bras. Dermatol. 2022, 97, 471–477. [Google Scholar] [CrossRef]
- Kaffenberger, B.H.; Wong, H.K.; Jarjour, W.; Andritsos, L.A. Remission of psoriasis after allogeneic, but not autologous, hematopoietic stem-cell transplantation. J. Am. Acad. Dermatol. 2013, 68, 489–492. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, M.; Ma, K.; Li, H.; Ran, M.; Yang, S.; Yang, Y.; Fu, X.; Yang, S. Therapeutic effects of mesenchymal stem cells and their derivatives in common skin inflammatory diseases: Atopic dermatitis and psoriasis. Front. Immunol. 2023, 14, 1092668. [Google Scholar] [CrossRef]
- Chen, H.; Niu, J.W.; Ning, H.M.; Pan, X.; Li, X.B.; Li, Y.; Wang, D.H.; Hu, L.D.; Sheng, H.X.; Xu, M.; et al. Treatment of Psoriasis with Mesenchymal Stem Cells. Am. J. Med. 2016, 129, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, S.; Peng, C.; Zou, X.; Yang, C.; Mei, H.; Li, C.; Su, X.; Xiao, N.; Ouyang, Q.; et al. Human umbilical cord mesenchymal stem cells for psoriasis: A phase 1/2a, single-arm study. Signal Transduct. Target. Ther. 2022, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.H.; Zhu, W.; Lin, G.; Cheng, L.M.; Qin, Q.; Huang, Z.J.; Shi, Y.L.; Zhang, C.L.; Xu, J.H.; Yan, K.X.; et al. Expert Consensus on the Application of Stem Cells in Psoriasis Research and Clinical Trials. Aging Dis. 2024, 16, 1363. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, M.M.; Santiago, J.S.; Trinidad, C.V.; See, M.E.; Semon, K.R.; Fernandez, M.O., Jr.; Chung, F.S. Autologous Adipose-Derived Mesenchymal Stromal Cells for the Treatment of Psoriasis Vulgaris and Psoriatic Arthritis: A Case Report. Cell Transplant. 2016, 25, 2063–2069. [Google Scholar] [CrossRef]
- Bajouri, A.; Dayani, D.; Taj Sharghi, A.; Karimi, S.; Niknezhadi, M.; Moeinabadi Bidgoli, K.; Madani, H.; Abbasi Kakroodi, F.; Bolurieh, T.; Mardpour, S.; et al. Subcutaneous Injection of Allogeneic Adipose-Derived Mesenchymal Stromal Cells in Psoriasis Plaques: Clinical Trial Phase I. Cell J. 2023, 25, 363–371. [Google Scholar] [CrossRef]
- Yin, X.P.; Zhu, R.J.; Zhuang, C.; Wang, S.; Zhao, C.H.; Song, P. Immunoregulatory Effect of Adipose Mesenchymal Stem Cells on Peripheral Blood Lymphocytes in Psoriasis Vulgaris Patients. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2018, 40, 790–796. [Google Scholar] [CrossRef]
- Shin, T.H.; Lee, B.C.; Choi, S.W.; Shin, J.H.; Kang, I.; Lee, J.Y.; Kim, J.J.; Lee, H.K.; Jung, J.E.; Choi, Y.W.; et al. Human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis via regulation of B lymphocyte maturation. Oncotarget 2017, 8, 512–522. [Google Scholar] [CrossRef]
- Ra, J.C.; Kang, S.K.; Shin, I.S.; Park, H.G.; Joo, S.A.; Kim, J.G.; Kang, B.C.; Lee, Y.S.; Nakama, K.; Piao, M.; et al. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J. Transl. Med. 2011, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Campanati, A.; Caffarini, M.; Ganzetti, G.; Consales, V.; Lucarini, G.; Offidani, A.; Di Primio, R. T helper (Th)1, Th17 and Th2 imbalance in mesenchymal stem cells of adult patients with atopic dermatitis: At the origin of the problem. Br. J. Dermatol. 2017, 176, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.H.; Roh, K.H.; Jun, H.J.; Kang, K.S.; Kim, T.Y. Clinical Trial of Human Umbilical Cord Blood-Derived Stem Cells for the Treatment of Moderate-to-Severe Atopic Dermatitis: Phase I/IIa Studies. Stem Cells 2017, 35, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Yoo, H.S.; Zhang, Y.X.; Choi, M.S.; Lee, K.; Yi, T.G.; Song, S.U.; Jeon, M.S. Bone marrow-derived clonal mesenchymal stem cells inhibit ovalbumin-induced atopic dermatitis. Cell Death Dis. 2014, 5, e1345. [Google Scholar] [CrossRef]
- Shin, H.T.; Lee, S.H.; Yoon, H.S.; Heo, J.H.; Lee, S.B.; Byun, J.W.; Shin, J.; Cho, Y.K.; Chung, E.; Jeon, M.S.; et al. Long-term efficacy and safety of intravenous injection of clonal mesenchymal stem cells derived from bone marrow in five adults with moderate to severe atopic dermatitis. J. Dermatol. 2021, 48, 1236–1242. [Google Scholar] [CrossRef]
- Seo, H.M.; Lew, B.L.; Lee, Y.W.; Son, S.W.; Park, C.O.; Park, Y.L.; Baek, J.O.; Shin, M.K.; Kim, D.H.; Lee, D.H.; et al. Phase 1/2 trials of human bone marrow-derived clonal mesenchymal stem cells for treatment of adults with moderate to severe atopic dermatitis. J. Allergy Clin. Immunol. 2024, 154, 965–973. [Google Scholar] [CrossRef]
- Mohanty, S.; Kumar, A.; Dhawan, J.; Sreenivas, V.; Gupta, S. Noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Br. J. Dermatol. 2011, 164, 1241–1246. [Google Scholar] [CrossRef]
- Fisch, S.C.; Gimeno, M.L.; Phan, J.D.; Simerman, A.A.; Dumesic, D.A.; Perone, M.J.; Chazenbalk, G.D. Pluripotent nontumorigenic multilineage differentiating stress enduring cells (Muse cells): A seven-year retrospective. Stem Cell Res. Ther. 2017, 8, 227. [Google Scholar] [CrossRef]
- Lim, W.S.; Kim, C.H.; Kim, J.Y.; Do, B.R.; Kim, E.J.; Lee, A.Y. Adipose-derived stem cells improve efficacy of melanocyte transplantation in animal skin. Biomol. Ther. 2014, 22, 328–333. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, C.D.; Lee, J.H.; Lee, C.H.; Do, B.R.; Lee, A.Y. Co-culture of melanocytes with adipose-derived stem cells as a potential substitute for co-culture with keratinocytes. Acta Derm. Venereol. 2012, 92, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.N.; Zhang, Z.Q.; Wu, J.L.; Lin, F.Q.; Fu, L.F.; Wang, S.Q.; Guan, C.P.; Wang, H.L.; Xu, A. Dermal mesenchymal stem cells (DMSCs) inhibit skin-homing CD8+ T cell activity, a determining factor of vitiligo patients’ autologous melanocytes transplantation efficiency. PLoS ONE 2013, 8, e60254. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V.; Isaev, A.; Kudlay, D.; Manturova, N.; Ustugov, A.; Kopnin, P. Current Status of Biomedical Products for Gene and Cell Therapy of Recessive Dystrophic Epidermolysis Bullosa. Int. J. Mol. Sci. 2024, 25, 10270. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Stem cells from human hair follicles: First mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017, 4, 58. [Google Scholar] [CrossRef]
- Gentile, P. Autologous Cellular Method Using Micrografts of Human Adipose Tissue Derived Follicle Stem Cells in Androgenic Alopecia. Int. J. Mol. Sci. 2019, 20, 3446. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Cervelli, V.; Orlandi, A.; Garcovich, S. Autologous Micrografts from Scalp Tissue: Trichoscopic and Long-Term Clinical Evaluation in Male and Female Androgenetic Alopecia. Biomed. Res. Int. 2020, 2020, 7397162. [Google Scholar] [CrossRef]
- Fukuoka, H.; Suga, H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: Follow-up with trichograms. Eplasty 2015, 15, e10. [Google Scholar]
- Shin, H.; Ryu, H.H.; Kwon, O.; Park, B.-S.; Jo, S.J. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: A retrospective case series study. Int. J. Dermatol. 2015, 54, 730–735. [Google Scholar] [CrossRef]
- Li, Y.; Yan, B.; Wang, H.; Li, H.; Li, Q.; Zhao, D.; Chen, Y.; Zhang, Y.; Li, W.; Zhang, J.; et al. Hair regrowth in alopecia areata patients following Stem Cell Educator therapy. BMC Med. 2015, 13, 87. [Google Scholar] [CrossRef]
- Khandpur, S.; Gupta, S.; Gunaabalaji, D.R. Stem cell therapy in dermatology. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 753–767. [Google Scholar] [CrossRef]
- Maria, A.T.; Maumus, M.; Le Quellec, A.; Jorgensen, C.; Noël, D.; Guilpain, P. Adipose-Derived Mesenchymal Stem Cells in Autoimmune Disorders: State of the Art and Perspectives for Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2017, 52, 234–259. [Google Scholar] [CrossRef]
- Maria, A.T.; Toupet, K.; Bony, C.; Pirot, N.; Vozenin, M.C.; Petit, B.; Roger, P.; Batteux, F.; Le Quellec, A.; Jorgensen, C.; et al. Antifibrotic, Antioxidant, and Immunomodulatory Effects of Mesenchymal Stem Cells in HOCl-Induced Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 1013–1025. [Google Scholar] [CrossRef]
- Zhuang, X.; Hu, X.; Zhang, S.; Li, X.; Yuan, X.; Wu, Y. Mesenchymal Stem Cell-Based Therapy as a New Approach for the Treatment of Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2023, 64, 284–320. [Google Scholar] [CrossRef]
- Guiducci, S.; Porta, F.; Saccardi, R.; Guidi, S.; Ibba-Manneschi, L.; Manetti, M.; Mazzanti, B.; Dal Pozzo, S.; Milia, A.F.; Bellando-Randone, S.; et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: A case report. Ann. Intern. Med. 2010, 153, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Virzì, F.; Bianca, P.; Giammona, A.; Apuzzo, T.; Di Franco, S.; Mangiapane, L.R.; Colorito, M.L.; Catalano, D.; Scavo, E.; Nicotra, A.; et al. Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients. Stem Cell Res. Ther. 2017, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.G.; Ramos, D.G.; Ramos, C.G. Evaluation of treatment response to autologous transplantation of noncultured melanocyte/keratinocyte cell suspension in patients with stable vitiligo. An. Bras. Dermatol. 2017, 92, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Salam, O.; Metwally, G.; Abdelsalam, H.; Hassan, M.; Sosa, W. Comparison treatment of vitiligo by co-culture of melanocytes derived from hair follicle with adipose-derived stem cells with and without NB-UVB. Pigment. Disord. 2017, 4, 1000256. [Google Scholar] [CrossRef]
- Wagner, J.E.; Ishida-Yamamoto, A.; McGrath, J.A.; Hordinsky, M.; Keene, D.R.; Woodley, D.T.; Chen, M.; Riddle, M.J.; Osborn, M.J.; Lund, T.; et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N. Engl. J. Med. 2010, 363, 629–639, Erratum in N. Engl. J. Med. 2010, 363, 1383. [Google Scholar] [CrossRef]
- Petrof, G.; Lwin, S.M.; Martinez-Queipo, M.; Abdul-Wahab, A.; Tso, S.; Mellerio, J.E.; Slaper-Cortenbach, I.; Boelens, J.J.; Tolar, J.; Veys, P.; et al. Potential of Systemic Allogeneic Mesenchymal Stromal Cell Therapy for Children with Recessive Dystrophic Epidermolysis Bullosa. J. Investig. Dermatol. 2015, 135, 2319–2321. [Google Scholar] [CrossRef]
- Kiritsi, D.; Dieter, K.; Niebergall-Roth, E.; Fluhr, S.; Daniele, C.; Esterlechner, J.; Sadeghi, S.; Ballikaya, S.; Erdinger, L.; Schauer, F.; et al. Clinical trial of ABCB5+ mesenchymal stem cells for recessive dystrophic epidermolysis bullosa. JCI Insight 2021, 6, e151922. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, S.J.; Kim, S.E.; Kim, K.; Cho, B.; Roh, K.; Kim, S.C. Intravenous allogeneic umbilical cord blood-derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight 2021, 6, e143606. [Google Scholar] [CrossRef]
- Anderi, R.; Makdissy, N.; Azar, A.; Rizk, F.; Hamade, A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res. Ther. 2018, 9, 141. [Google Scholar] [CrossRef]
- Elmaadawi, I.H.; Mohamed, B.M.; Ibrahim, Z.A.S.; Abdou, S.M.; El Attar, Y.A.; Youssef, A.; Shamloula, M.M.; Taha, A.; Metwally, H.G.; El Afandy, M.M.; et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J. Dermatol. Treat. 2018, 29, 431–440. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y.; Cho, A.R.; Kim, Y.S. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Transl. Med. 2020, 9, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, M.J.; Lee, Y.J.; Lee, J.C.; Kim, J.H.; Kim, D.H.; Do, Y.H.; Choi, J.W.; Chung, S.I.; Do, B.R. Innovative method of alopecia treatment by autologous adipose-derived SVF. Stem Cell Res. Ther. 2021, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Oyama, Y.; Barr, W.G.; Statkute, L.; Corbridge, T.; Gonda, E.A.; Jovanovic, B.; Testori, A.; Burt, R.K. Autologous non-myeloablative hematopoietic stem cell transplantation in patients with systemic sclerosis. Bone Marrow Transplant. 2007, 40, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Granel, B.; Daumas, A.; Jouve, E.; Harlé, J.R.; Nguyen, P.S.; Chabannon, C.; Colavolpe, N.; Reynier, J.C.; Truillet, R.; Mallet, S.; et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: An open-label phase I trial. Ann. Rheum. Dis. 2015, 74, 2175–2182. [Google Scholar] [CrossRef]
- Gallicchio, V.S.; Doan, H.E.; Inayat, M.S. Stem Cell Use to Treat Dermatological Disorders. In Stem Cell Transplantation; Piccaluga, P.P., Visani, G., Khattab, S.S., Eds.; IntechOpen: Rijeka, Croatia, 2025. [Google Scholar]
- Czop, J.K.; Jałowska, M. Stem cells in plastic surgery and aesthetic medicine. Postep. Dermatol. Alergol. 2023, 40, 504–509. [Google Scholar] [CrossRef]
- Niebergall-Roth, E.; Frank, N.Y.; Ganss, C.; Frank, M.H.; Kluth, M.A. Skin-Derived ABCB5(+) Mesenchymal Stem Cells for High-Medical-Need Inflammatory Diseases: From Discovery to Entering Clinical Routine. Int. J. Mol. Sci. 2022, 24, 66. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pr. 2011, 92, 26–36. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Picardo, M. Therapeutic potential of adipose tissue-derivatives in modern dermatology. Exp. Dermatol. 2022, 31, 1837–1852. [Google Scholar] [CrossRef]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Liu, W.H.; Sun, J.; Hou, T.J.; Liu, Y.M.; Liu, H.R.; Luo, Y.H.; Zhao, N.N.; Tang, Y.; Deng, F.M. Mesenchymal stem cell-mediated suppression of hypertrophic scarring is p53 dependent in a rabbit ear model. Stem Cell Res. Ther. 2014, 5, 136. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, L.; Li, H.; Shi, H.; Luo, H.; Zhang, Y.; Yu, C.; Jin, Y. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J. Investig. Dermatol. 2014, 134, 2648–2657. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qiu, L.; Hu, W.; Deng, X.; Xu, H.; Cao, Y.; Xiao, Z.; Peng, L.; Johnson, S.; Alexey, L.; et al. Genetically-modified bone mesenchymal stem cells with TGF-β(3) improve wound healing and reduce scar tissue formation in a rabbit model. Exp. Cell Res. 2018, 367, 24–29. [Google Scholar] [CrossRef]
- Khaity, A.; Albakri, K.; Al-Dardery, N.M.; Yousef, Y.A.S.; Foppiani, J.A.; Lin, S.J. Adipose-Derived Stem Cell Therapy in Hypertrophic and Keloid Scars: A Systematic Review of Experimental Studies. Plast. Surg. 2025, 33, 318–328. [Google Scholar] [CrossRef]
- Almadori, A.; Butler, P.E. Scarring and Skin Fibrosis Reversal with Regenerative Surgery and Stem Cell Therapy. Cells 2024, 13, 443. [Google Scholar] [CrossRef]
- Lynn, J.V.; Ranganathan, K.; Luby, A.O.; Urlaub, K.M.; Donneys, A.; Nelson, N.S.; Buchman, S.R. Therapeutic Efficacy of Adipose-Derived Stem Cells Versus Bone Marrow Stromal Cells for Irradiated Mandibular Fracture Repair. Ann. Plast. Surg. 2022, 89, 459–464. [Google Scholar] [CrossRef]
- Park, C.S.; Park, J.H.; Kim, C.R.; Lee, J.H. Objective analysis of volume restoration in atrophic acne scars and skin pores: A split study using human stem cell-conditioned media. J. Dermatol. Treat. 2021, 32, 73–77. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef]
- Qian, H.; Shan, Y.; Gong, R.; Lin, D.; Zhang, M.; Wang, C.; Wang, L. Mechanism of action and therapeutic effects of oxidative stress and stem cell-based materials in skin aging: Current evidence and future perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1082403. [Google Scholar] [CrossRef] [PubMed]
- Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Maeda Takiya, C.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A.; Rigotti, G. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast. Reconstr. Surg. 2015, 135, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Rigotti, G.; Sbarbati, A.; Bernardi, P.; Benati, D.; Bizon Vieira Carias, R.; Maeda Takiya, C.; Borojevic, R. Photoaged Skin Therapy with Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2020, 145, 1037e–1049e. [Google Scholar] [CrossRef] [PubMed]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, E.G.; Kang, S.; Sung, J.H.; Chung, H.M.; Kim, D.H. Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: A randomized, controlled, blinded split-face study. Ann. Dermatol. 2014, 26, 584–591. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Pratiwi, F.D.; Herwanto, N.; Citrashanty, I.; Indramaya, D.M.; Murtiastutik, D.; Sukanto, H.; Rantam, F.A. The effects of amniotic membrane stem cell-conditioned medium on photoaging. J. Dermatol. Treat. 2019, 30, 478–482. [Google Scholar] [CrossRef]
- Zhou, B.R.; Zhang, T.; Bin Jameel, A.A.; Xu, Y.; Xu, Y.; Guo, S.L.; Wang, Y.; Permatasari, F.; Luo, D. The efficacy of conditioned media of adipose-derived stem cells combined with ablative carbon dioxide fractional resurfacing for atrophic acne scars and skin rejuvenation. J. Cosmet. Laser Ther. 2016, 18, 138–148. [Google Scholar] [CrossRef]
- Cucinella, G.; Gullo, G.; Catania, E.; Perino, A.; Billone, V.; Marinelli, S.; Napoletano, G.; Zaami, S. Stem Cells and Infertility: A Review of Clinical Applications and Legal Frameworks. J. Pers. Med. 2024, 14, 135. [Google Scholar] [CrossRef]
- Li, J.; Mao, Q.; He, J.; She, H.; Zhang, Z.; Yin, C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res. Ther. 2017, 8, 55. [Google Scholar] [CrossRef]
- Saha, S.; Roy, P.; Corbitt, C.; Kakar, S.S. Application of Stem Cell Therapy for Infertility. Cells 2021, 10, 1613. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Zhang, J.; Qin, W.; Chi, H.; Chen, J.; Yu, Z.; Chen, C. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014, 23, 1548–1557. [Google Scholar] [CrossRef]
- Manshadi, M.D.; Navid, S.; Hoshino, Y.; Daneshi, E.; Noory, P.; Abbasi, M. The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microsc. Res. Tech. 2019, 82, 635–642. [Google Scholar] [CrossRef]
- Jing, Z.; Qiong, Z.; Yonggang, W.; Yanping, L. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil. Steril. 2014, 101, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ju, B.; Pan, C.; Gu, Y.; Zhang, Y.; Sun, L.; Zhang, B.; Zhang, Y. Application of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Intrauterine Adhesions in Rats. Cell Physiol. Biochem. 2016, 39, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Domnina, A.; Novikova, P.; Obidina, J.; Fridlyanskaya, I.; Alekseenko, L.; Kozhukharova, I.; Lyublinskaya, O.; Zenin, V.; Nikolsky, N. Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium. Stem Cell Res. Ther. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, Y.O.; Silachev, D.N.; Nazarenko, T.A.; Birukova, A.M.; Vishnyakova, P.A.; Sukhikh, G.T. Stem-Cell-Derived Extracellular Vesicles: Unlocking New Possibilities for Treating Diminished Ovarian Reserve and Premature Ovarian Insufficiency. Life 2023, 13, 2247. [Google Scholar] [CrossRef]
- Xiao, G.Y.; Cheng, C.C.; Chiang, Y.S.; Cheng, W.T.; Liu, I.H.; Wu, S.C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016, 6, 23120. [Google Scholar] [CrossRef]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef]
- White, Y.A.; Woods, D.C.; Takai, Y.; Ishihara, O.; Seki, H.; Tilly, J.L. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012, 18, 413–421. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoshioka, N.; Takae, S.; Sugishita, Y.; Tamura, M.; Hashimoto, S.; Morimoto, Y.; Kawamura, K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum. Reprod. 2015, 30, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Yao, G.; Dong, F.; Bu, Z.; Cheng, Y.; Sato, Y.; Hu, L.; Zhang, Y.; Wang, J.; Dai, S.; et al. In Vitro Activation of Follicles and Fresh Tissue Auto-transplantation in Primary Ovarian Insufficiency Patients. J. Clin. Endocrinol. Metab. 2016, 101, 4405–4412. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Fujino, M.; Tong, G.; Zhang, Q.; Li, X.K.; Yan, H. Stem Cells as a Resource for Treatment of Infertility-related Diseases. Curr. Mol. Med. 2019, 19, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Hamazaki, N.; Hamada, N.; Nagamatsu, G.; Okamoto, I.; Ohta, H.; Nosaka, Y.; Ishikura, Y.; Kitajima, T.S.; Semba, Y.; et al. Generation of functional oocytes from male mice in vitro. Nature 2023, 615, 900–906. [Google Scholar] [CrossRef]
- Lee, Y.; Kang, E. Stem cells and reproduction. BMB Rep. 2019, 52, 482–489. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Subash, S.K.; Kumar, P.G. Spermatogonial stem cells: A story of self-renewal and differentiation. Front. Biosci. 2021, 26, 163–205. [Google Scholar] [CrossRef]
- Salem, M.; Khadivi, F.; Javanbakht, P.; Mojaverrostami, S.; Abbasi, M.; Feizollahi, N.; Abbasi, Y.; Heidarian, E.; Rezaei Yazdi, F. Advances of three-dimensional (3D) culture systems for in vitro spermatogenesis. Stem Cell Res. Ther. 2023, 14, 262. [Google Scholar] [CrossRef]
- Kita, K.; Watanabe, T.; Ohsaka, K.; Hayashi, H.; Kubota, Y.; Nagashima, Y.; Aoki, I.; Taniguchi, H.; Noce, T.; Inoue, K.; et al. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biol. Reprod. 2007, 76, 211–217. [Google Scholar] [CrossRef]
- Modanlou, M.; Mahdipour, M.; Mobarak, H. Effectiveness of stem cell therapy for male infertility restoration: A systematic review. J. Investig. Med. 2025, 73, 229–252. [Google Scholar] [CrossRef]
- von Rohden, E.; Jensen, C.F.S.; Andersen, C.Y.; Sønksen, J.; Fedder, J.; Thorup, J.; Ohl, D.A.; Fode, M.; Hoffmann, E.R.; Mamsen, L.S. Male fertility restoration: In vivo and in vitro stem cell-based strategies using cryopreserved testis tissue: A scoping review. Fertil. Steril. 2024, 122, 828–843. [Google Scholar] [CrossRef]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019, 363, 1314–1319. [Google Scholar] [CrossRef]
- Kumar, D.; Tanwar, R. World’s first: Stem cell therapy reverses diabetes. Stem Cell Res. Ther. 2024, 15, 487. [Google Scholar] [CrossRef]
- Carlsson, P.O.; Schwarcz, E.; Korsgren, O.; Le Blanc, K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015, 64, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, X.; Wang, Z.; Wang, F.; Wang, L.; Gao, H.; Chen, Y.; Zhao, W.; Jia, Z.; Yan, S.; et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr. J. 2013, 60, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, Z.; Xu, X.; Liao, L.; Chen, J.; Huang, L.; Wu, W.; Luo, F.; Wu, C.; Pugliese, A.; et al. Umbilical Cord Mesenchymal Stromal Cell with Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care 2016, 39, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Hoang, D.M.; Nguyen, K.T.; Bui, D.M.; Nguyen, H.T.; Le, H.T.A.; Hoang, V.T.; Bui, H.T.H.; Dam, P.T.M.; Hoang, X.T.A.; et al. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl. Med. 2021, 10, 1266–1278. [Google Scholar] [CrossRef]
- Ruiz-Babot, G.; Eceiza, A.; Abollo-Jiménez, F.; Malyukov, M.; Carlone, D.L.; Borges, K.; Da Costa, A.R.; Qarin, S.; Matsumoto, T.; Morizane, R.; et al. Generation of glucocorticoid-producing cells derived from human pluripotent stem cells. Cell Rep. Methods 2023, 3, 100627. [Google Scholar] [CrossRef]
- Ma, R.; Shi, R.; Morshed, S.A.; Latif, R.; Davies, T.F. Derivation and 97% Purification of Human Thyroid Cells From Dermal Fibroblasts. Front. Endocrinol. 2020, 11, 446. [Google Scholar] [CrossRef]
- Balkan, B.C.; Erbaş, O. Recent advances in stem cell-based therapeutics in ophthalmology. Demiroglu Sci. Univ. Florence Nightingale J. Med. 2023, 9, 175–182. [Google Scholar]
- Sotiropulos, K.; Kourkoutas, D.; Almaliotis, D.; Ploumidou, K.; Karampatakis, V. Ocular stem cells: A narrative review of current clinical trials. Int. J. Ophthalmol. 2022, 15, 1529–1537. [Google Scholar] [CrossRef]
- Chotikavanich, S.; Poriswanish, N.; Luangaram, A.; Numnoi, P.; Thamphithak, R.; Pinitpuwadol, W.; Uiprasertkul, M.; Chirapapaisan, C.; Sikarinkul, R.; Prabhasawat, P. Genetic analysis of allogenic donor cells after successful allo-limbal epithelial transplantation in simple and cultivated limbal epithelial transplantation procedures. Sci. Rep. 2023, 13, 4290. [Google Scholar] [CrossRef]
- Fasolo, A.; Pedrotti, E.; Passilongo, M.; Marchini, G.; Monterosso, C.; Zampini, R.; Bohm, E.; Birattari, F.; Franch, A.; Barbaro, V.; et al. Safety outcomes and long-term effectiveness of ex vivo autologous cultured limbal epithelial transplantation for limbal stem cell deficiency. Br. J. Ophthalmol. 2017, 101, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S.; Basu, S.; Vemuganti, G.K.; Sejpal, K.; Subramaniam, S.V.; Bandyopadhyay, S.; Krishnaiah, S.; Gaddipati, S.; Tiwari, S.; Balasubramanian, D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br. J. Ophthalmol. 2011, 95, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Miotti, G.; Parodi, P.C.; Zeppieri, M. Stem cell therapy in ocular pathologies in the past 20 years. World J. Stem Cells 2021, 13, 366–385. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Kaufman, A.R.; Yin, J.; Ayala, A.; Maguire, M.; Samarakoon, L.; Johns, L.K.; Parekh, M.; Li, S.; Gauthier, A.; et al. Cultivated autologous limbal epithelial cell (CALEC) transplantation for limbal tem cell deficiency: A phase I/II clinical trial of the first xenobiotic-free, serum-free, antibiotic-free manufacturing protocol developed in the US. Nat. Commun. 2025, 16, 1607. [Google Scholar] [CrossRef]
- Yin, J.; Jurkunas, U. Limbal Stem Cell Transplantation and Complications. Semin. Ophthalmol. 2018, 33, 134–141. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Shetty, R.; Ghosh, A. Corneal cell therapy: With iPSCs, it is no more a far-sight. Stem Cell Res. Ther. 2018, 9, 287. [Google Scholar] [CrossRef]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- McGill, T.J.; Stoddard, J.; Renner, L.M.; Messaoudi, I.; Bharti, K.; Mitalipov, S.; Lauer, A.; Wilson, D.J.; Neuringer, M. Allogeneic iPSC-Derived RPE Cell Graft Failure Following Transplantation Into the Subretinal Space in Nonhuman Primates. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef]
- Falkner-Radler, C.I.; Krebs, I.; Glittenberg, C.; Povazay, B.; Drexler, W.; Graf, A.; Binder, S. Human retinal pigment epithelium (RPE) transplantation: Outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br. J. Ophthalmol. 2011, 95, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef] [PubMed]
- Shadforth, A.M.A.; Suzuki, S.; Theodoropoulos, C.; Richardson, N.A.; Chirila, T.V.; Harkin, D.G. A Bruch’s membrane substitute fabricated from silk fibroin supports the function of retinal pigment epithelial cells in vitro. J. Tissue Eng. Regen. Med. 2017, 11, 1915–1924. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; Ingram, A.; Dang, W.; et al. One-Year Follow-Up in a Phase 1/2a Clinical Trial of an Allogeneic RPE Cell Bioengineered Implant for Advanced Dry Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef]
- An, W.; Zhang, W.; Qi, J.; Xu, W.; Long, Y.; Qin, H.; Yao, K. Mesenchymal stem cells and mesenchymal stem cell-derived exosomes: A promising strategy for treating retinal degenerative diseases. Mol. Med. 2025, 31, 75. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Liu, X.; Ghazaryan, E.; Li, Y.; Xie, J.; Su, G. Recent advances of stem cell therapy for retinitis pigmentosa. Int. J. Mol. Sci. 2014, 15, 14456–14474. [Google Scholar] [CrossRef]
- Moghadam Fard, A.; Mirshahi, R.; Naseripour, M.; Ghasemi Falavarjani, K. Stem Cell Therapy in Stargardt Disease: A Systematic Review. J. Ophthalmic Vis. Res. 2023, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, N.; Li, J.; Zhao, M.; Huang, L. Stem cell therapy for inherited retinal diseases: A systematic review and meta-analysis. Stem Cell Res. Ther. 2023, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Liovic, M. Industry updates from the field of stem cell research and regenerative medicine in September 2024. Regen. Med. 2025, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, S.S.; Lingam, G.; Kai, D.; Su, X.; Liu, Z. Advances in retinal pigment epithelial cell transplantation for retinal degenerative diseases. Stem Cell Res. Ther. 2024, 15, 390. [Google Scholar] [CrossRef]
- Chen, K.; Soleimani, M.; Koganti, R.; Cheraqpour, K.; Habeel, S.; Djalilian, A.R. Cell-based therapies for limbal stem cell deficiency: A literature review. Ann. Eye Sci. 2023, 8, 6. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, X.; Lin, Y.; Zeng, Y. Roles of airway basal stem cells in lung homeostasis and regenerative medicine. Respir. Res. 2022, 23, 122. [Google Scholar] [CrossRef]
- Le Thi Bich, P.; Nguyen Thi, H.; Dang Ngo Chau, H.; Phan Van, T.; Do, Q.; Dong Khac, H.; Le Van, D.; Nguyen Huy, L.; Mai Cong, K.; Ta Ba, T.; et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: A pilot clinical study. Stem Cell Res. Ther. 2020, 11, 60. [Google Scholar] [CrossRef]
- Río, C.; Jahn, A.K.; Martin-Medina, A.; Calvo Bota, A.M.; De Francisco Casado, M.T.; Pont Antona, P.J.; Gigirey Castro, O.; Carvajal Á, F.; Villena Portella, C.; Gómez Bellvert, C.; et al. Mesenchymal Stem Cells from COPD Patients Are Capable of Restoring Elastase-Induced Emphysema in a Murine Experimental Model. Int. J. Mol. Sci. 2023, 24, 5813. [Google Scholar] [CrossRef]
- Lai, S.; Guo, Z. Stem cell therapies for chronic obstructive pulmonary disease: Mesenchymal stem cells as a promising treatment option. Stem Cell Res. Ther. 2024, 15, 312. [Google Scholar] [CrossRef]
- Campo, A.; González-Ruiz, J.M.; Andreu, E.; Alcaide, A.B.; Ocón, M.M.; De-Torres, J.; Pueyo, J.; Cordovilla, R.; Villaron, E.; Sanchez-Guijo, F.; et al. Endobronchial autologous bone marrow-mesenchymal stromal cells in idiopathic pulmonary fibrosis: A phase I trial. ERJ Open Res. 2021, 7, 00773-2020. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Q.; Li, Z.; Huang, M.; Zhong, C.; Yu, R.; Jiang, R.; Dai, H.; Zhang, J.; Gu, X.; et al. Epithelial stem cells from human small bronchi offer a potential for therapy of idiopathic pulmonary fibrosis. eBioMedicine 2025, 112, 105538. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, L.; Liu, F.; Shen, J. Therapeutic Benefits of Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome: Potential Mechanisms and Challenges. J. Inflamm. Res. 2022, 15, 5235–5246. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Regmi, S.; Ganguly, A.; Pathak, S.; Primavera, R.; Chetty, S.; Wang, J.; Patel, S.; Thakor, A.S. Evaluating the therapeutic potential of different sources of mesenchymal stem cells in acute respiratory distress syndrome. Stem Cell Res. Ther. 2024, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Lazarus, H.M. Human mesenchymal stem cell therapy: Potential advances for reducing cystic fibrosis infection and organ inflammation. Best. Pract. Res. Clin. Haematol. 2025, 38, 101602. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.T.; Fletcher, D.; Episalla, N.; Auster, L.; Kaur, S.; Gwin, M.C.; Folz, M.; Velasquez, D.; Roy, V.; van Heeckeren, R.; et al. Mesenchymal Stem Cell Soluble Mediators and Cystic Fibrosis. J. Stem Cell Res. Ther. 2017, 7, 400. [Google Scholar] [CrossRef]
- Roesch, E.A.; Bonfield, T.L.; Lazarus, H.M.; Reese, J.; Hilliard, K.; Hilliard, J.; Khan, U.; Heltshe, S.; Gluvna, A.; Dasenbrook, E.; et al. A phase I study assessing the safety and tolerability of allogeneic mesenchymal stem cell infusion in adults with cystic fibrosis. J. Cyst. Fibros. 2023, 22, 407–413. [Google Scholar] [CrossRef]
- Makhlough, A.; Shekarchian, S.; Moghadasali, R.; Einollahi, B.; Dastgheib, M.; Janbabaee, G.; Hosseini, S.E.; Falah, N.; Abbasi, F.; Baharvand, H.; et al. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: A safety study with 18 months of follow-up. Cytotherapy 2018, 20, 660–669. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Chen, X.; Liu, Y.; Zhou, T. Mesenchymal stem cells: A new therapeutic tool for chronic kidney disease. Front. Cell Dev. Biol. 2022, 10, 910592. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Kinzhebay, A.; Kobayashi, S. Cell therapy in kidney diseases: Advancing treatments for renal regeneration. Front. Cell Dev. Biol. 2024, 12, 1505601. [Google Scholar] [CrossRef]
- Saad, A.; Dietz, A.B.; Herrmann, S.M.S.; Hickson, L.J.; Glockner, J.F.; McKusick, M.A.; Misra, S.; Bjarnason, H.; Armstrong, A.S.; Gastineau, D.A.; et al. Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. J. Am. Soc. Nephrol. 2017, 28, 2777–2785. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Hua, B.; Wang, H.; Lu, L.; Shi, S.; Hou, Y.; Zeng, X.; Gilkeson, G.S.; Sun, L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann. Rheum. Dis. 2010, 69, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Liang, J.; Li, X.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Lu, L.; Gilkeson, G.S.; et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013, 22, 2267–2277. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, P.; Guo, Y.; Lim, T.O. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann. Rheum. Dis. 2017, 76, 1436–1439. [Google Scholar] [CrossRef]
- Ranjbar, A.; Hassanzadeh, H.; Jahandoust, F.; Miri, R.; Bidkhori, H.R.; Monzavi, S.M.; Sanjar-Moussavi, N.; Matin, M.M.; Shariati-Sarabi, Z. Allogeneic adipose-derived mesenchymal stromal cell transplantation for refractory lupus nephritis: Results of a phase I clinical trial. Curr. Res. Transl. Med. 2022, 70, 103324. [Google Scholar] [CrossRef]
- Kamen, D.L.; Wallace, C.; Li, Z.; Wyatt, M.; Paulos, C.; Wei, C.; Wang, H.; Wolf, B.J.; Nietert, P.J.; Gilkeson, G. Safety, immunological effects and clinical response in a phase I trial of umbilical cord mesenchymal stromal cells in patients with treatment refractory SLE. Lupus Sci. Med. 2022, 9, e000704. [Google Scholar] [CrossRef]
- Tan, J.; Wu, W.; Xu, X.; Liao, L.; Zheng, F.; Messinger, S.; Sun, X.; Chen, J.; Yang, S.; Cai, J.; et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 2012, 307, 1169–1177. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, Z.; Han, F.; Zhao, M.; Cao, R.; Zhao, D.; Hong, L.; Na, N.; Li, H.; Miao, B.; et al. Allogeneic mesenchymal stem cells as induction therapy are safe and feasible in renal allografts: Pilot results of a multicenter randomized controlled trial. J. Transl. Med. 2018, 16, 52. [Google Scholar] [CrossRef]
- Burt, R.K.; Traynor, A.; Statkute, L.; Barr, W.G.; Rosa, R.; Schroeder, J.; Verda, L.; Krosnjar, N.; Quigley, K.; Yaung, K.; et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA 2006, 295, 527–535. [Google Scholar] [CrossRef]
- Sun, L.; Akiyama, K.; Zhang, H.; Yamaza, T.; Hou, Y.; Zhao, S.; Xu, T.; Le, A.; Shi, S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009, 27, 1421–1432. [Google Scholar] [CrossRef]
- Namiot, E.D.; Niemi, J.V.L.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Stem Cells in Clinical Trials on Neurological Disorders: Trends in Stem Cells Origins, Indications, and Status of the Clinical Trials. Int. J. Mol. Sci. 2022, 23, 11453. [Google Scholar] [CrossRef]
- Lo Furno, D.; Mannino, G.; Giuffrida, R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J. Cell Physiol. 2018, 233, 3982–3999. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Jones, D.T.; Singer, W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin. Proc. 2019, 94, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, H.; Zhu, M. Mesenchymal stem cells for regenerative medicine in central nervous system. Front. Neurosci. 2022, 16, 1068114. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Nemati, R.; Sadeghipour, Y.; Yaghoubi, Y.; Baghbani, R.; Javidi, K.; Zamani, M.; Hassanzadeh, A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: A promising frontier. Eur. J. Cell Biol. 2020, 99, 151097. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Pękała, M.; Serwin, N.; Gliźniewicz, M.; Grygorcewicz, B.; Michalczyk, A.; Heryć, R.; Budkowska, M.; Dołęgowska, B. The Use of Stem Cells as a Potential Treatment Method for Selected Neurodegenerative Diseases: Review. Cell Mol. Neurobiol. 2023, 43, 2643–2673. [Google Scholar] [CrossRef]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O.; et al. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients with Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Magota, H.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Ukai, R.; Kiyose, R.; Onodera, R.; Kocsis, J.D.; Honmou, O. Intravenous infusion of mesenchymal stem cells delays disease progression in the SOD1G93A transgenic amyotrophic lateral sclerosis rat model. Brain Res. 2021, 1757, 147296. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; Lindborg, S.R.; Goyal, N.A.; Miller, R.G.; Burford, M.J.; Berry, J.D.; Nicholson, K.A.; Mozaffar, T.; Katz, J.S.; Jenkins, L.J.; et al. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve 2022, 65, 291–302. [Google Scholar] [CrossRef]
- Bhatt, A.; Bhardwaj, H.; Srivastava, P. Mesenchymal stem cell therapy for Alzheimer’s disease: A novel therapeutic approach for neurodegenerative diseases. Neuroscience 2024, 555, 52–68. [Google Scholar] [CrossRef]
- Neves, A.F.; Camargo, C.; Premer, C.; Hare, J.M.; Baumel, B.S.; Pinto, M. Intravenous administration of mesenchymal stem cells reduces Tau phosphorylation and inflammation in the 3xTg-AD mouse model of Alzheimer’s disease. Exp. Neurol. 2021, 341, 113706. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Lee, S.; Kim, K.; Song, M.; Lee, J. Mesenchymal Stem Cell Therapy and Alzheimer’s Disease: Current Status and Future Perspectives. J. Alzheimers Dis. 2020, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Liu, D.D.; Shen, M.; Kevadiya, B.D.; Ganguly, A.; Primavera, R.; Chetty, S.; Yarani, R.; Thakor, A.S. Mesenchymal stromal cells for the treatment of Alzheimer’s disease: Strategies and limitations. Front. Mol. Neurosci. 2022, 15, 1011225. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, M.T.; Genchi, A.; Brittain, G.; de Silva, T.I.; Sharrack, B.; Snowden, J.A.; Alexander, T.; Greco, R.; Muraro, P.A. Immune Reconstitution Following Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis: A Review on Behalf of the EBMT Autoimmune Diseases Working Party. Front. Immunol. 2021, 12, 813957. [Google Scholar] [CrossRef] [PubMed]
- Mariottini, A.; De Matteis, E.; Cencioni, M.T.; Muraro, P.A. Haematopoietic Stem Cell Transplantation for the Treatment of Multiple Sclerosis: Recent Advances. Curr. Neurol. Neurosci. Rep. 2023, 23, 507–520. [Google Scholar] [CrossRef]
- Boffa, G.; Massacesi, L.; Inglese, M.; Mariottini, A.; Capobianco, M.; Moiola, L.; Amato, M.P.; Cottone, S.; Gualandi, F.; De Gobbi, M.; et al. Long-term Clinical Outcomes of Hematopoietic Stem Cell Transplantation in Multiple Sclerosis. Neurology 2021, 96, e1215–e1226. [Google Scholar] [CrossRef]
- Nawar, A.A.; Farid, A.M.; Wally, R.; Tharwat, E.K.; Sameh, A.; Elkaramany, Y.; Asla, M.M.; Kamel, W.A. Efficacy and safety of stem cell transplantation for multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2024, 14, 12545. [Google Scholar] [CrossRef]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A.; et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 3369. [Google Scholar] [CrossRef]
- Moon, H.; Kim, B.; Kwon, I.; Oh, Y. Challenges involved in cell therapy for Parkinson’s disease using human pluripotent stem cells. Front. Cell Dev. Biol. 2023, 11, 1288168. [Google Scholar] [CrossRef]
- Song, B.; Cha, Y.; Ko, S.; Jeon, J.; Lee, N.; Seo, H.; Park, K.J.; Lee, I.H.; Lopes, C.; Feitosa, M.; et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J. Clin. Investig. 2020, 130, 904–920. [Google Scholar] [CrossRef]
- Kim, S.W.; Woo, H.J.; Kim, E.H.; Kim, H.S.; Suh, H.N.; Kim, S.H.; Song, J.J.; Wulansari, N.; Kang, M.; Choi, S.Y.; et al. Neural stem cells derived from human midbrain organoids as a stable source for treating Parkinson’s disease: Midbrain organoid-NSCs (Og-NSC) as a stable source for PD treatment. Prog. Neurobiol. 2021, 204, 102086. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Wu, P.; Fang, X.; Poeggeler, B.; Sambamurti, K.; Wisniewski, T.; Perry, G. Stem Cell Interventions in Neurology: From Bench to Bedside. J. Alzheimers Dis. 2024, 101, S395–S416. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.C.; Liu, Y.Y.; Zhu, F.Q.; Xiong, M.J.; Hu, D.X.; Zhang, W.J. Therapeutic role of neural stem cells in neurological diseases. Front. Bioeng. Biotechnol. 2024, 12, 1329712. [Google Scholar] [CrossRef]
- Khandia, R.; Gurjar, P.; Romashchenko, V.; Al-Hussain, S.A.; Zaki, M.E. Recent advances in stem cell therapy: Efficacy, ethics, safety concerns, and future directions focusing on neurodegenerative disorders—A review. Int. J. Surg. 2024, 110, 6367–6381. [Google Scholar] [CrossRef]
- Isaković, J.; Šerer, K.; Barišić, B.; Mitrečić, D. Mesenchymal stem cell therapy for neurological disorders: The light or the dark side of the force? Front. Bioeng. Biotechnol. 2023, 11, 1139359. [Google Scholar] [CrossRef]
- Zayed, M.A.; Sultan, S.; Alsaab, H.O.; Yousof, S.M.; Alrefaei, G.I.; Alsubhi, N.H.; Alkarim, S.; Al Ghamdi, K.S.; Bagabir, S.A.; Jana, A.; et al. Stem-Cell-Based Therapy: The Celestial Weapon against Neurological Disorders. Cells 2022, 11, 3476. [Google Scholar] [CrossRef] [PubMed]
- Izrael, M.; Chebath, J.; Molakandov, K.; Revel, M. Clinical perspective on pluripotent stem cells derived cell therapies for the treatment of neurodegenerative diseases. Adv. Drug Deliv. Rev. 2025, 218, 115525. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; Zhang, J.; Zhang, H.; Gao, S.; Guo, X.; Lin, J.; Wu, H.; Hong, Y. Stem cells in central nervous system diseases: Promising therapeutic strategies. Exp. Neurol. 2023, 369, 114543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kuang, Q.; Xu, J.; Lin, Q.; Chi, H.; Yu, D. MSC-Based Cell Therapy in Neurological Diseases: A Concise Review of the Literature in Pre-Clinical and Clinical Research. Biomolecules 2024, 14, 538. [Google Scholar] [CrossRef]
- Liu, X.; Jia, X. Neuroprotection of Stem Cells Against Ischemic Brain Injury: From Bench to Clinic. Transl. Stroke Res. 2024, 15, 691–713. [Google Scholar] [CrossRef]
- Wei, M.; Yang, Z.; Li, S.; Le, W. Nanotherapeutic and Stem Cell Therapeutic Strategies in Neurodegenerative Diseases: A Promising Therapeutic Approach. Int. J. Nanomed. 2023, 18, 611–626. [Google Scholar] [CrossRef]
- Ford, E.; Pearlman, J.; Ruan, T.; Manion, J.; Waller, M.; Neely, G.G.; Caron, L. Human Pluripotent Stem Cells-Based Therapies for Neurodegenerative Diseases: Current Status and Challenges. Cells 2020, 9, 2517. [Google Scholar] [CrossRef]
- Kozlowska, U.; Klimczak, A.; Bednarowicz, K.A.; Zalewski, T.; Rozwadowska, N.; Chojnacka, K.; Jurga, S.; Barnea, E.R.; Kurpisz, M.K. Assessment of Immunological Potential of Glial Restricted Progenitor Graft In Vivo-Is Immunosuppression Mandatory? Cells 2021, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lin, J.; Zhao, K.; Jin, K.; He, Q.; Hu, Y.; Feng, G.; Cai, Y.; Xia, C.; Liu, H.; et al. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am. J. Sports Med. 2019, 47, 1722–1733. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Wang, S.; Xu, H.; Sun, H.; Fan, X. Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Medicine 2020, 99, e23343. [Google Scholar] [CrossRef]
- Ha, C.W.; Park, Y.B.; Kim, S.H.; Lee, H.J. Intra-articular Mesenchymal Stem Cells in Osteoarthritis of the Knee: A Systematic Review of Clinical Outcomes and Evidence of Cartilage Repair. Arthroscopy 2019, 35, 277–288.e272. [Google Scholar] [CrossRef]
- Whittle, S.L.; Johnston, R.V.; McDonald, S.; Worthley, D.; Campbell, T.M.; Cyril, S.; Bapna, T.; Zhang, J.; Buchbinder, R. Stem cell injections for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2025, 4, Cd013342. [Google Scholar] [CrossRef]
- Lee, B.W.; Lee, J.J.; Jung, J.Y.; Ju, J.H. Intra-Articular Injection of Human Bone Marrow-Derived Mesenchymal Stem Cells in Knee Osteoarthritis: A Randomized, Double-Blind, Controlled Trial. Cell Transplant. 2025, 34, 9636897241303275. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Wang, Y.; Liang, Q.; Mao, X. Preclinical and Clinical Amelioration of Bone Fractures with Mesenchymal Stromal Cells: A Systematic Review and Meta-Analysis. Cell Transplant. 2022, 31, 9636897211051743. [Google Scholar] [CrossRef] [PubMed]
- Meesuk, L.; Suwanprateeb, J.; Thammarakcharoen, F.; Tantrawatpan, C.; Kheolamai, P.; Palang, I.; Tantikanlayaporn, D.; Manochantr, S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci. Rep. 2022, 12, 19509. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Monahan, D.S.; Brulin, B.; Gallinetti, S.; Humbert, P.; Tringides, C.; Canal, C.; Ginebra, M.P.; Layrolle, P. Biomimetic versus sintered macroporous calcium phosphate scaffolds enhanced bone regeneration and human mesenchymal stromal cell engraftment in calvarial defects. Acta Biomater. 2021, 135, 689–704. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, J.; Chen, Y.; Lyu, K.; Long, L.; Wang, X.; Liu, T.; Li, S. Mesenchymal stem cells: An efficient cell therapy for tendon repair (Review). Int. J. Mol. Med. 2023, 52, 70. [Google Scholar] [CrossRef]
- Hooper, N.; Marathe, A.; Jain, N.B.; Jayaram, P. Cell-Based Therapies for Rotator Cuff Injuries: An Updated Review of the Literature. Int. J. Mol. Sci. 2024, 25, 3139. [Google Scholar] [CrossRef]
- Goldberg, A.J.; Masci, L.; O’Donnell, P.; Green, R.; Brooking, D.; Bassett, P.; Lowdell, M.W.; Smith, R.K.W. Autologous bone marrow derived mesenchymal stem cells are safe for the treatment of Achilles tendinopathy. Sci. Rep. 2024, 14, 11421. [Google Scholar] [CrossRef]
- Lamandé, S.R.; Ng, E.S.; Cameron, T.L.; Kung, L.H.W.; Sampurno, L.; Rowley, L.; Lilianty, J.; Patria, Y.N.; Stenta, T.; Hanssen, E.; et al. Modeling human skeletal development using human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2211510120. [Google Scholar] [CrossRef]
- Trompet, D.; Melis, S.; Chagin, A.S.; Maes, C. Skeletal stem and progenitor cells in bone development and repair. J. Bone Min. Res. 2024, 39, 633–654. [Google Scholar] [CrossRef]
- Jeffery, E.C.; Mann, T.L.A.; Pool, J.A.; Zhao, Z.; Morrison, S.J. Bone marrow and periosteal skeletal stem/progenitor cells make distinct contributions to bone maintenance and repair. Cell Stem Cell 2022, 29, 1547–1561.e1546. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, L.; Cao, Z.; Liu, X.; Pan, J. Periosteal Skeletal Stem Cells and Their Response to Bone Injury. Front. Cell Dev. Biol. 2022, 10, 812094. [Google Scholar] [CrossRef]
- Rong, L.; Zhang, L.; Yang, Z.; Xu, L. New insights into the properties, functions, and aging of skeletal stem cells. Osteoporos. Int. 2023, 34, 1311–1321. [Google Scholar] [CrossRef]
- Griffith, L.A.; Arnold, K.M.; Sengers, B.G.; Tare, R.S.; Houghton, F.D. A scaffold-free approach to cartilage tissue generation using human embryonic stem cells. Sci. Rep. 2021, 11, 18921. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Song, W.; Jiang, X.; Wang, Y.; Li, C.; Yu, W.; He, Y. Adipose-derived stem cell-based optimization strategies for musculoskeletal regeneration: Recent advances and perspectives. Stem Cell Res. Ther. 2024, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Juric, M.K.; Ghimire, S.; Ogonek, J.; Weissinger, E.M.; Holler, E.; van Rood, J.J.; Oudshoorn, M.; Dickinson, A.; Greinix, H.T. Milestones of Hematopoietic Stem Cell Transplantation—From First Human Studies to Current Developments. Front. Immunol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; de Lima, M.; Anand, S.; Bhatt, V.R.; Bookout, R.; Chen, G.; Couriel, D.; Di Stasi, A.; El-Jawahri, A.; Giralt, S.; et al. Hematopoietic Cell Transplantation, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2020, 18, 599–634. [Google Scholar] [CrossRef]
- Maurer, K.; Antin, J.H. The graft versus leukemia effect: Donor lymphocyte infusions and cellular therapy. Front. Immunol. 2024, 15, 1328858. [Google Scholar] [CrossRef]
- Loke, J.; Buka, R.; Craddock, C. Allogeneic stem cell transplantation for acute myeloid leukemia: Who, when, and how? Front. Immunol. 2021, 12, 659595. [Google Scholar] [CrossRef]
- Gomez-Arteaga, A.; Gyurkocza, B. Recent advances in allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Curr. Opin. Hematol. 2020, 27, 115–121. [Google Scholar] [CrossRef]
- Magee, G.; Ragon, B.K. Allogeneic hematopoietic cell transplantation in acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2023, 36, 101466. [Google Scholar] [CrossRef]
- Claude Gorin, N. Autologous stem cell transplantation versus alternative allogeneic donor transplants in adult acute leukemias. Semin. Hematol. 2016, 53, 103–110. [Google Scholar] [CrossRef]
- Takami, A. Hematopoietic stem cell transplantation for acute myeloid leukemia. Int. J. Hematol. 2018, 107, 513–518. [Google Scholar] [CrossRef]
- Algeri, M.; Merli, P.; Locatelli, F.; Pagliara, D. The Role of Allogeneic Hematopoietic Stem Cell Transplantation in Pediatric Leukemia. J. Clin. Med. 2021, 10, 3790. [Google Scholar] [CrossRef]
- Iftikhar, R.; Chaudhry, Q.U.N.; Anwer, F.; Neupane, K.; Rafae, A.; Mahmood, S.K.; Ghafoor, T.; Shahbaz, N.; Khan, M.A.; Khattak, T.A.; et al. Allogeneic hematopoietic stem cell transplantation in aplastic anemia: Current indications and transplant strategies. Blood Rev. 2021, 47, 100772. [Google Scholar] [CrossRef] [PubMed]
- Bernaudin, F.; Pondarré, C.; Galambrun, C.; Thuret, I. Allogeneic/Matched Related Transplantation for β-Thalassemia and Sickle Cell Anemia. Adv. Exp. Med. Biol. 2017, 1013, 89–122. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.M.; Fitzhugh, C.D.; Weitzel, R.P.; Link, M.E.; Coles, W.A.; Zhao, X.; Rodgers, G.P.; Powell, J.D.; Tisdale, J.F. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA 2014, 312, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. β-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef]
- Leonard, A.; Tisdale, J.; Abraham, A. Curative options for sickle cell disease: Haploidentical stem cell transplantation or gene therapy? Br. J. Haematol. 2020, 189, 408–423. [Google Scholar] [CrossRef]
- Kassim, A.A.; DeBaun, M.R. The range of haploidentical transplant protocols in sickle cell disease: All haplos are not created equally. Hematol. Am. Soc. Hematol. Educ. Program. 2023, 2023, 532–541. [Google Scholar] [CrossRef]
- Guilcher, G.M.T.; Truong, T.H.; Saraf, S.L.; Joseph, J.J.; Rondelli, D.; Hsieh, M.M. Curative therapies: Allogeneic hematopoietic cell transplantation from matched related donors using myeloablative, reduced intensity, and nonmyeloablative conditioning in sickle cell disease. Semin. Hematol. 2018, 55, 87–93. [Google Scholar] [CrossRef]
- Pratumkaew, P.; Issaragrisil, S.; Luanpitpong, S. Induced Pluripotent Stem Cells as a Tool for Modeling Hematologic Disorders and as a Potential Source for Cell-Based Therapies. Cells 2021, 10, 3250. [Google Scholar] [CrossRef]
- Dolatshad, H.; Tatwavedi, D.; Ahmed, D.; Tegethoff, J.F.; Boultwood, J.; Pellagatti, A. Application of induced pluripotent stem cell technology for the investigation of hematological disorders. Adv. Biol. Regul. 2019, 71, 19–33. [Google Scholar] [CrossRef]
- Wattanapanitch, M. Recent Updates on Induced Pluripotent Stem Cells in Hematological Disorders. Stem Cells Int. 2019, 2019, 5171032. [Google Scholar] [CrossRef]
- Georgomanoli, M.; Papapetrou, E.P. Modeling blood diseases with human induced pluripotent stem cells. Dis. Model. Mech. 2019, 12, dmm039321. [Google Scholar] [CrossRef]
- Ng, E.S.; Sarila, G.; Li, J.Y.; Edirisinghe, H.S.; Saxena, R.; Sun, S.; Bruveris, F.F.; Labonne, T.; Sleebs, N.; Maytum, A.; et al. Long-term engrafting multilineage hematopoietic cells differentiated from human induced pluripotent stem cells. Nat. Biotechnol. 2025, 43, 1274–1287. [Google Scholar] [CrossRef]
- Demirci, S.; Leonard, A.; Tisdale, J.F. Hematopoietic stem cells from pluripotent stem cells: Clinical potential, challenges, and future perspectives. Stem Cells Transl. Med. 2020, 9, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Piau, O.; Brunet-Manquat, M.; L’Homme, B.; Petit, L.; Birebent, B.; Linard, C.; Moeckes, L.; Zuliani, T.; Lapillonne, H.; Benderitter, M.; et al. Generation of transgene-free hematopoietic stem cells from human induced pluripotent stem cells. Cell Stem Cell 2023, 30, 1610–1623.e1617. [Google Scholar] [CrossRef] [PubMed]
- Elbadry, M.I.; Espinoza, J.L.; Nakao, S. Disease modeling of bone marrow failure syndromes using iPSC-derived hematopoietic stem progenitor cells. Exp. Hematol. 2019, 71, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Broxmeyer, H.E.; Lee, M.R. Generating autologous hematopoietic cells from human-induced pluripotent stem cells through ectopic expression of transcription factors. Curr. Opin. Hematol. 2017, 24, 283–288. [Google Scholar] [CrossRef]
- Locatelli, F.; Lang, P.; Wall, D.; Meisel, R.; Corbacioglu, S.; Li, A.M.; de la Fuente, J.; Shah, A.J.; Carpenter, B.; Kwiatkowski, J.L.; et al. Exagamglogene Autotemcel for Transfusion-Dependent β-Thalassemia. N. Engl. J. Med. 2024, 390, 1663–1676. [Google Scholar] [CrossRef]
- Frangoul, H.; Locatelli, F.; Sharma, A.; Bhatia, M.; Mapara, M.; Molinari, L.; Wall, D.; Liem, R.I.; Telfer, P.; Shah, A.J.; et al. Exagamglogene Autotemcel for Severe Sickle Cell Disease. N. Engl. J. Med. 2024, 390, 1649–1662. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Singh, A.K.; McGuirk, J.P. Allogeneic Stem Cell Transplantation: A Historical and Scientific Overview. Cancer Res. 2016, 76, 6445–6451. [Google Scholar] [CrossRef] [PubMed]
- Tokaz, M.C.; Baldomero, H.; Cowan, A.J.; Saber, W.; Greinix, H.; Koh, M.B.C.; Kröger, N.; Mohty, M.; Galeano, S.; Okamoto, S.; et al. An Analysis of the Worldwide Utilization of Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia. Transplant. Cell Ther. 2023, 29, 279.e1–279.e10. [Google Scholar] [CrossRef]
- Xu, L.P.; Lu, P.H.; Wu, D.P.; Huang, H.; Jiang, E.L.; Liu, D.H.; Cao, W.J.; Zhang, X.; Fu, Y.W.; Li, N.N.; et al. Hematopoietic stem cell transplantation activity in China 2022–2023. The proportions of peripheral blood for stem cell source continue to grow: A report from the Chinese Blood and Marrow Transplantation Registry Group. Bone Marrow Transplant. 2024, 59, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.; Bertaina, A.; Bonfim, C.; Cohen, S.; Prockop, S.; Purtill, D.; Russell, A.; Boelens, J.J.; Wynn, R.; Ruggeri, A.; et al. Curative therapy for hemoglobinopathies: An International Society for Cell & Gene Therapy Stem Cell Engineering Committee review comparing outcomes, accessibility and cost of ex vivo stem cell gene therapy versus allogeneic hematopoietic stem cell transplantation. Cytotherapy 2022, 24, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Arranz, L. The Hematology of Tomorrow Is Here-Preclinical Models Are Not: Cell Therapy for Hematological Malignancies. Cancers 2022, 14, 580. [Google Scholar] [CrossRef]
- De Becker, A.; Van Riet, I. Mesenchymal Stromal Cell Therapy in Hematology: From Laboratory to Clinic and Back Again. Stem Cells Dev. 2015, 24, 1713–1729. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Basak, G.W.; Chabannon, C.; Corbacioglu, S.; Duarte, R.; Kuball, J.; Lankester, A.; Montoto, S.; de Latour, R.P.; et al. The EBMT activity survey report 2017: A focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transplant. 2019, 54, 1575–1585. [Google Scholar] [CrossRef]
- Cheng, S.; Nethi, S.K.; Rathi, S.; Layek, B.; Prabha, S. Engineered Mesenchymal Stem Cells for Targeting Solid Tumors: Therapeutic Potential beyond Regenerative Therapy. J. Pharmacol. Exp. Ther. 2019, 370, 231–241. [Google Scholar] [CrossRef]
- Choi, S.A.; Hwang, S.K.; Wang, K.C.; Cho, B.K.; Phi, J.H.; Lee, J.Y.; Jung, H.W.; Lee, D.H.; Kim, S.K. Therapeutic efficacy and safety of TRAIL-producing human adipose tissue-derived mesenchymal stem cells against experimental brainstem glioma. Neuro Oncol. 2011, 13, 61–69. [Google Scholar] [CrossRef]
- Loebinger, M.R.; Eddaoudi, A.; Davies, D.; Janes, S.M. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009, 69, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Lyons, M.; Deedigan, L.; Harte, T.; Shaw, G.; Howard, L.; Barry, F.; O’Brien, T.; Zwacka, R. Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. J. Cell Mol. Med. 2008, 12, 2628–2643. [Google Scholar] [CrossRef] [PubMed]
- Balyasnikova, I.V.; Ferguson, S.D.; Han, Y.; Liu, F.; Lesniak, M.S. Therapeutic effect of neural stem cells expressing TRAIL and bortezomib in mice with glioma xenografts. Cancer Lett. 2011, 310, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Meng, H. Exploring the Potential of Stem Cells to Enhance Conventional Cancer Therapies. Theor. Nat. Sci. 2024, 63, 9–14. [Google Scholar] [CrossRef]
- Alnasser, S.M. Stem cell challenge in cancer progression, oncology and therapy. Gene 2022, 840, 146748. [Google Scholar] [CrossRef]
- Fakiruddin, K.S.; Ghazalli, N.; Lim, M.N.; Zakaria, Z.; Abdullah, S. Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour. Int. J. Mol. Sci. 2018, 19, 2188. [Google Scholar] [CrossRef]
- Attar, R.; Sajjad, F.; Qureshi, M.Z.; Tahir, F.; Hussain, E.; Fayyaz, S.; Farooqi, A.A. TRAIL based therapy: Overview of mesenchymal stem cell based delivery and miRNA controlled expression of TRAIL. Asian Pac. J. Cancer Prev. 2014, 15, 6495–6497. [Google Scholar] [CrossRef]
- Atsuta, I.; Liu, S.; Miura, Y.; Akiyama, K.; Chen, C.; An, Y.; Shi, S.; Chen, F.M. Mesenchymal stem cells inhibit multiple myeloma cells via the Fas/Fas ligand pathway. Stem Cell Res. Ther. 2013, 4, 111. [Google Scholar] [CrossRef]
- Davies, A.; Sage, B.; Kolluri, K.; Alrifai, D.; Graham, R.; Weil, B.; Rego, R.; Bain, O.; Patrick, P.S.; Champion, K. TACTICAL: A phase I/II trial to assess the safety and efficacy of MSCTRAIL in the treatment of metastatic lung adenocarcinoma. J. Clin. Oncol. 2019, 37 (Suppl. S15), TPS9116. [Google Scholar] [CrossRef]
- Davies, A.; Sage, E.; Kolluri, K.; Weil, B.; Rego, R.V.T.P.; Edwards, A.; Bain, O.; Santilli, G.; Fullen, D.; Champion, K.; et al. P256 Targeted stem cells expressing trail as a therapy for lung cancer tactical: A phase i/ii trial. Thorax 2017, 72, A222–A224. [Google Scholar] [CrossRef]
- von Einem, J.C.; Peter, S.; Günther, C.; Volk, H.D.; Grütz, G.; Salat, C.; Stoetzer, O.; Nelson, P.J.; Michl, M.; Modest, D.P.; et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells-TREAT-ME-1-a phase I, first in human, first in class trial. Oncotarget 2017, 8, 80156–80166. [Google Scholar] [CrossRef]
- Sharma, A. Role of stem cell derived exosomes in tumor biology. Int. J. Cancer 2018, 142, 1086–1092. [Google Scholar] [CrossRef]
- Ding, B.; Lou, W.; Fan, W.; Pan, J. Exosomal miR-374c-5p derived from mesenchymal stem cells suppresses epithelial-mesenchymal transition of hepatocellular carcinoma via the LIMK1-Wnt/β-catenin axis. Environ. Toxicol. 2023, 38, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xia, Z.; Deng, J. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles carrying miR-655-3p inhibit the development of esophageal cancer by regulating the expression of HIF-1α via a LMO4/HDAC2-dependent mechanism. Cell Biol. Toxicol. 2023, 39, 1319–1339. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xin, H.; Lu, L. Extracellular vesicle-encapsulated microRNA-424 exerts inhibitory function in ovarian cancer by targeting MYB. J. Transl. Med. 2021, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Li, Q.; Lin, L.; Xu, C.; Xue, Y.; Hu, M.; Shi, Y.; Wang, Y. Mesenchymal stromal cells equipped by IFNα empower T cells with potent anti-tumor immunity. Oncogene 2022, 41, 1866–1881. [Google Scholar] [CrossRef]
- Andreeff, M.; Marini, F.C.; Westin, S.N.; Coleman, R.L.; Thall, P.F.; Aljahdami, V.; Qazilbash, M.H.; Rezvani, K.; Timmons, M.; Heese, L. a phase I trial of mesenchymal stem cells transfected with a plasmid secreting interferon beta in advanced ovarian cancer. Cancer Res. 2018, 78, 75. [Google Scholar] [CrossRef]
- Yin, P.; Gui, L.; Wang, C.; Yan, J.; Liu, M.; Ji, L.; Wang, Y.; Ma, B.; Gao, W.Q. Targeted Delivery of CXCL9 and OX40L by Mesenchymal Stem Cells Elicits Potent Antitumor Immunity. Mol. Ther. 2020, 28, 2553–2563. [Google Scholar] [CrossRef]
- Hombach, A.A.; Geumann, U.; Günther, C.; Hermann, F.G.; Abken, H. IL7-IL12 Engineered Mesenchymal Stem Cells (MSCs) Improve A CAR T Cell Attack Against Colorectal Cancer Cells. Cells 2020, 9, 873. [Google Scholar] [CrossRef]
- Sadhukha, T.; O’Brien, T.D.; Prabha, S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J. Control Release 2014, 196, 243–251. [Google Scholar] [CrossRef]
- Merino, J.J.; Cabaña-Muñoz, M.E. Nanoparticles and Mesenchymal Stem Cell (MSC) Therapy for Cancer Treatment: Focus on Nanocarriers and a si-RNA CXCR4 Chemokine Blocker as Strategies for Tumor Eradication In Vitro and In Vivo. Micromachines 2023, 14, 2068. [Google Scholar] [CrossRef]
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells with Anti-neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sehgal, D.; Argenta, P.A.; Panyam, J.; Prabha, S. Nanoengineering of mesenchymal stem cells via surface modification for efficient cancer therapy. Adv. Ther. 2019, 2, 1900043. [Google Scholar] [CrossRef]
- Moku, G.; Layek, B.; Trautman, L.; Putnam, S.; Panyam, J.; Prabha, S. Improving payload capacity and anti-tumor efficacy of mesenchymal stem cells using TAT peptide functionalized polymeric nanoparticles. Cancers 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sadhukha, T.; Prabha, S. Glycoengineered mesenchymal stem cells as an enabling platform for two-step targeting of solid tumors. Biomaterials 2016, 88, 97–109. [Google Scholar] [CrossRef]
- Benmelouka, A.Y.; Munir, M.; Sayed, A.; Attia, M.S.; Ali, M.M.; Negida, A.; Alghamdi, B.S.; Kamal, M.A.; Barreto, G.E.; Ashraf, G.M.; et al. Neural Stem Cell-Based Therapies and Glioblastoma Management: Current Evidence and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2258. [Google Scholar] [CrossRef]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef]
- Lim, J.; Chang, D.-Y.; Sim, J.; Jung, J.-H.; Park, J.; Kim, Y.J.; Kim, D.; Hwang, S.; Kim, S.-S.; Cho, H.-Y.; et al. Ctni-30. Mesenchymal Stem Cell (Msc11fcd) Based Therapy Elicited a Favorable Response with Efficient 5-Fu Conversion in Patients with Recurrent Glioblastoma: An Open Label, Phase I/Iia Trial. Neuro Oncol. 2023, 25, v80. [Google Scholar]
- Jing, W.; Chen, Y.; Lu, L.; Hu, X.; Shao, C.; Zhang, Y.; Zhou, X.; Zhou, Y.; Wu, L.; Liu, R. Human umbilical cord blood–derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol. Cancer Ther. 2014, 13, 2127–2137. [Google Scholar] [CrossRef]
- Kidd, S.; Caldwell, L.; Dietrich, M.; Samudio, I.; Spaeth, E.L.; Watson, K.; Shi, Y.; Abbruzzese, J.; Konopleva, M.; Andreeff, M.; et al. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy 2010, 12, 615–625. [Google Scholar] [CrossRef]
- Timaner, M.; Letko-Khait, N.; Kotsofruk, R.; Benguigui, M.; Beyar-Katz, O.; Rachman-Tzemah, C.; Raviv, Z.; Bronshtein, T.; Machluf, M.; Shaked, Y. Therapy-Educated Mesenchymal Stem Cells Enrich for Tumor-Initiating Cells. Cancer Res. 2018, 78, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Dietz, A.B.; Dozois, E.J.; Fletcher, J.G.; Butler, G.W.; Radel, D.; Lightner, A.L.; Dave, M.; Friton, J.; Nair, A.; Camilleri, E.T.; et al. Autologous Mesenchymal Stem Cells, Applied in a Bioabsorbable Matrix, for Treatment of Perianal Fistulas in Patients with Crohn’s Disease. Gastroenterology 2017, 153, 59–62.e52. [Google Scholar] [CrossRef] [PubMed]
- García-Olmo, D.; García-Arranz, M.; Herreros, D.; Pascual, I.; Peiro, C.; Rodríguez-Montes, J.A. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis. Colon. Rectum 2005, 48, 1416–1423. [Google Scholar] [CrossRef]
- Knyazev, O.V.; Fadeeva, N.A.; Kagramanova, A.V.; Belyakov, N.I.; Orlova, N.V.; Lishchinskaya, A.A.; Konoplyannikov, A.G.; Parfenov, A.I. Stem Cell Therapy for Perianal Crohn’s Disease. Ter. Arkh 2018, 90, 60–66. [Google Scholar] [CrossRef]
- Lightner, A.L.; Otero-Pineiro, A.; Reese, J.; Ream, J.; Nachand, D.; Adams, A.C.; VanDenBossche, A.; Kurowski, J.A. A Phase I study of ex vivo expanded allogeneic bone marrow–derived mesenchymal stem cells for the treatment of pediatric perianal Fistulizing Crohn’s disease. Inflamm. Bowel Dis. 2023, 29, 1912–1919. [Google Scholar] [CrossRef]
- Dalal, J.; Gandy, K.; Domen, J. Role of mesenchymal stem cell therapy in Crohn’s disease. Pediatr. Res. 2012, 71, 445–451. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, G.; Zhang, L.; Qiao, C.; Di, A.; Gao, H.; Xu, H. Safety and therapeutic effect of mesenchymal stem cell infusion on moderate to severe ulcerative colitis. Exp. Ther. Med. 2016, 12, 2983–2989. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Q.; Wang, F. Mesenchymal stem cells for the treatment of ulcerative colitis: A systematic review and meta-analysis of experimental and clinical studies. Stem Cell Res. Ther. 2019, 10, 266. [Google Scholar] [CrossRef]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R., Jr.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144–1165. [Google Scholar] [CrossRef]
- Kharaziha, P.; Hellström, P.M.; Noorinayer, B.; Farzaneh, F.; Aghajani, K.; Jafari, F.; Telkabadi, M.; Atashi, A.; Honardoost, M.; Zali, M.R.; et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase I-II clinical trial. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; Alimoghaddam, K.; Mohyeddin-Bonab, M.; Bagheri, M.; Bashtar, M.; Ghanaati, H.; Baharvand, H.; Ghavamzadeh, A.; Malekzadeh, R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch. Iran. Med. 2007, 10, 459–466. [Google Scholar] [PubMed]
- Winkler, S.; Borkham-Kamphorst, E.; Stock, P.; Brückner, S.; Dollinger, M.; Weiskirchen, R.; Christ, B. Human mesenchymal stem cells towards non-alcoholic steatohepatitis in an immunodeficient mouse model. Exp. Cell Res. 2014, 326, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Wu, X.; Xu, X.; Niu, J. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: A systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res. Ther. 2022, 13, 204. [Google Scholar] [CrossRef]
- Shi, M.; Li, Y.Y.; Xu, R.N.; Meng, F.P.; Yu, S.J.; Fu, J.L.; Hu, J.H.; Li, J.X.; Wang, L.F.; Jin, L.; et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: A long-term follow-up analysis of the randomized controlled clinical trial. Hepatol. Int. 2021, 15, 1431–1441. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, N.; Huang, Y.; Zhang, Q.; Fang, Y.; Fu, J.; Yuan, Y.; Chen, L.; Chen, X.; Xu, Z.; et al. Multiple Dimensions of using Mesenchymal Stem Cells for Treating Liver Diseases: From Bench to Beside. Stem Cell Rev. Rep. 2023, 19, 2192–2224. [Google Scholar] [CrossRef]
- Martin, L.Y.; Ladd, M.R.; Werts, A.; Sodhi, C.P.; March, J.C.; Hackam, D.J. Tissue engineering for the treatment of short bowel syndrome in children. Pediatr. Res. 2018, 83, 249–257. [Google Scholar] [CrossRef]
- Finkbeiner, S.R.; Freeman, J.J.; Wieck, M.M.; El-Nachef, W.; Altheim, C.H.; Tsai, Y.H.; Huang, S.; Dyal, R.; White, E.S.; Grikscheit, T.C.; et al. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol. Open 2015, 4, 1462–1472. [Google Scholar] [CrossRef]
- Sémont, A.; Mouiseddine, M.; François, A.; Demarquay, C.; Mathieu, N.; Chapel, A.; Saché, A.; Thierry, D.; Laloi, P.; Gourmelon, P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010, 17, 952–961. [Google Scholar] [CrossRef]
- Gong, W.; Guo, M.; Han, Z.; Wang, Y.; Yang, P.; Xu, C.; Wang, Q.; Du, L.; Li, Q.; Zhao, H.; et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 2016, 7, e2387. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, X.; Wan, Z.; Liu, X.; Chen, F.; Zhang, J.; Leng, Y. Mesenchymal stem cells against intestinal ischemia-reperfusion injury: A systematic review and meta-analysis of preclinical studies. Stem Cell Res. Ther. 2022, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.N.; Dunn, J.C.; Stelzner, M.; Martín, M.G. Concise Review: The Potential Use of Intestinal Stem Cells to Treat Patients with Intestinal Failure. Stem Cells Transl. Med. 2017, 6, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Tsuji, S.; Tsujii, M.; Nishida, T.; Ishii, S.; Iijima, H.; Nakamura, T.; Eguchi, H.; Miyoshi, E.; Hayashi, N.; et al. Topical transplantation of mesenchymal stem cells accelerates gastric ulcer healing in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G778–G786. [Google Scholar] [CrossRef]
- El Kasaby, A.; Ghaly, M.; Abo Zeid, A.; Fayed, I. Therapeutic potential of adipose derived mesenchymal stem cells in regeneration of gastric ulcer in rats. J. Med. Histol. 2017, 1, 190–201. [Google Scholar] [CrossRef]
- Xia, X.; Chan, K.F.; Wong, G.T.Y.; Wang, P.; Liu, L.; Yeung, B.P.M.; Ng, E.K.W.; Lau, J.Y.W.; Chiu, P.W.Y. Mesenchymal stem cells promote healing of nonsteroidal anti-inflammatory drug-related peptic ulcer through paracrine actions in pigs. Sci. Transl. Med. 2019, 11, eaat7455. [Google Scholar] [CrossRef]
- Hassan, S.A.; Elghait, A.T.A.; Abdelqader, Z.S.; Meligy, F.Y. Therapeutic efficiency of adipose-derived mesenchymal stem cells in healing of experimentally induced gastric ulcers in rats. Anat. Cell Biol. 2021, 54, 361–374. [Google Scholar] [CrossRef]
- Kupcova Skalnikova, H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013, 95, 2196–2211. [Google Scholar] [CrossRef]
- Micci, M.A.; Kahrig, K.M.; Simmons, R.S.; Sarna, S.K.; Espejo-Navarro, M.R.; Pasricha, P.J. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology 2005, 129, 1817–1824. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Assen, L.S.; Jongsma, K.R.; Isasi, R.; Tryfonidou, M.A.; Bredenoord, A.L. Recognizing the ethical implications of stem cell research: A call for broadening the scope. Stem Cell Rep. 2021, 16, 1656–1661. [Google Scholar] [CrossRef]
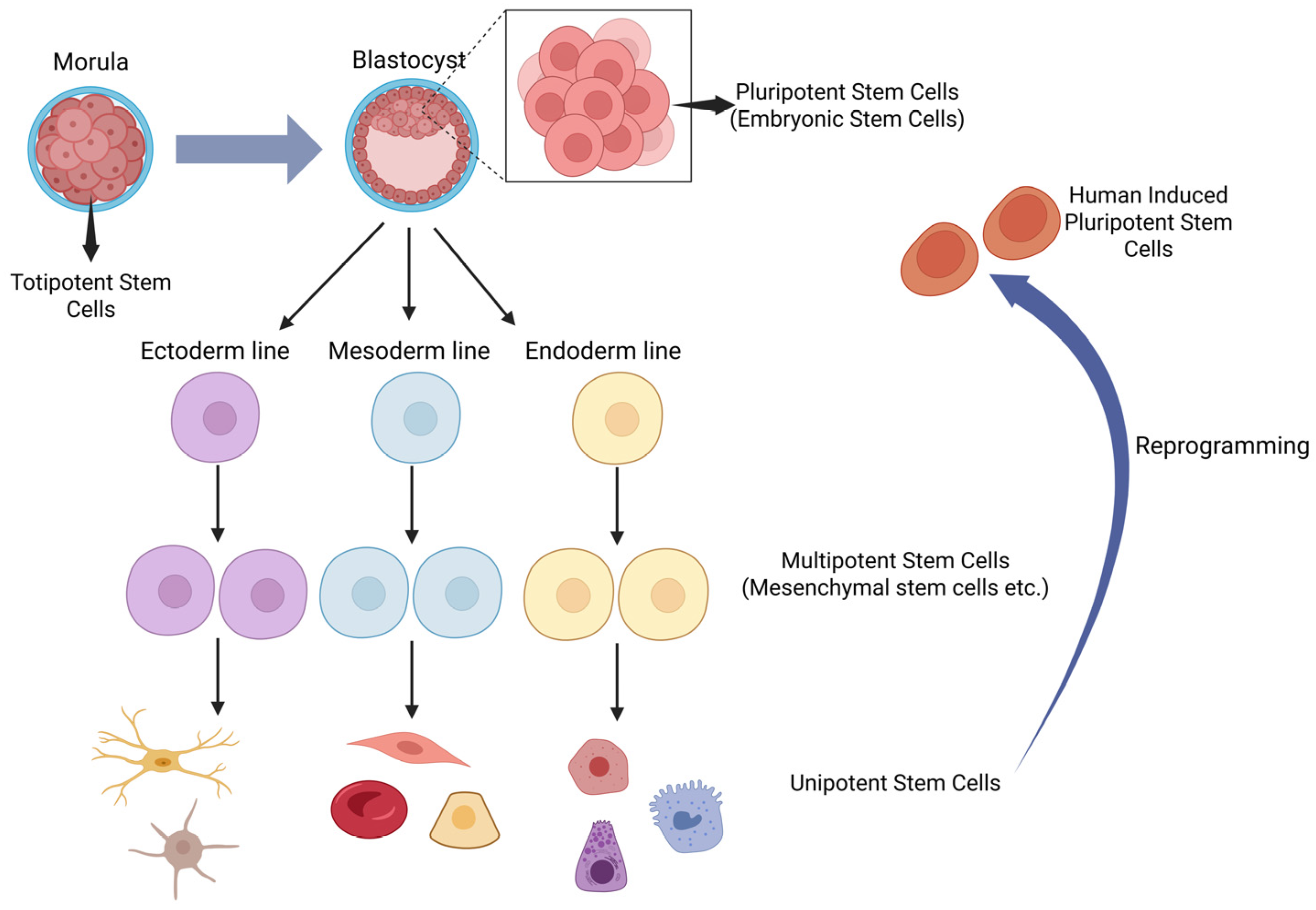
| Stem Cell Type | Differentiation Potential | Source(s) | Key Markers | Clinical Relevance/Applications | Limitations/Risks | References |
|---|---|---|---|---|---|---|
| Embryonic Stem Cells (ESCs) | Pluripotent (all somatic cell types) | Inner cell mass of blastocyst | OCT4, SOX2, NANOG | Regenerative medicine, disease modeling | Ethical concerns, teratoma risk, immunogenicity | [5,6,7] |
| Induced Pluripotent Stem Cells (iPSCs) | Pluripotent (all somatic cell types) | Reprogrammed adult somatic cells (e.g., skin, blood, urine) | OCT4, SOX2, NANOG | Disease modeling, drug screening, potential for autologous cell therapy | Tumorigenicity, genetic/epigenetic instability | [5,6,8] |
| Hematopoietic Stem Cells (HSCs) | Multipotent (all blood cell lineages) | Bone marrow, peripheral blood, umbilical cord blood | CD34+, CD133+ | Hematopoietic reconstitution (e.g., transplantation for leukemia, anemia) | Limited to blood lineages, graft-vs-host disease | [9,10,11] |
| Mesenchymal Stem Cells (MSCs) | Multipotent (bone, cartilage, fat, muscle) | Bone marrow, adipose tissue, umbilical cord, placenta, urine | CD73+, CD90+, CD105+ | Tissue repair, immunomodulation, autoimmune and degenerative disease therapy | Heterogeneity, variable efficacy, rare tumorigenicity | [10,12,13,14] |
| Stem Cell Type | Study | Outcomes |
|---|---|---|
| Allogeneic Cardiac Stem Cells (Progenitor Cells) | Makkar et al. [21] | There was no difference in wound size proportionate to baseline between the CDC and placebo groups. However, significant reductions in LV end-diastolic volume, LV end-systolic volume, and N-terminal pro b-type natriuretic peptide were observed in patients treated with CDC compared to placebo. There were no deaths related to treatment. The authors also noted that allogeneic therapy has an advantage over autologous therapy as it does not require myocardial biopsy and is readily available. |
| Autologus Cardiac Stem Cells | Makkar et al. [23] | Patients treated with CDCs showed reduced scar tissue, increased viable heart mass, and improved regional contractility and regional systolic wall thickening compared to controls. However, changes in end-diastolic volume, end-systolic volume, and LVEF during follow-up did not differ between the groups. No patients died during follow-up, nor did they develop cardiac tumors or MACE in either group. |
| Skeletal Myoblasts | Miyagawa et al. [54] | The majority of patients with ischemic cardiomyopathy showed significant symptomatic improvement. There was also a significant decrease in serum brain natriuretic peptide, pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress. However, the efficacy was not as good as that in patients with dilated cardiomyopathy. No major procedure-related complications were observed during follow-up. |
| Menasche et al. [33] | Myoblast transfer did not enhance regional or global LV function beyond what was observed in control patients. However, patients receiving the high-dose cell line showed a significant reduction in LV volumes compared to placebo. Myoblast-treated patients experienced a higher number of arrhythmias. | |
| BM-MSCs | Mathiasen et al. [55] | LVESV was significantly reduced in the MSC group compared to placebo. Significant improvements were also observed in LVEF, stroke volume, and myocardial mass. Additionally, the MSC group experienced a reduction in scar tissue and an improvement in quality of life scores, unlike the placebo group. Angina hospitalizations were significantly fewer in the MSC group at long-term follow-up. No adverse events were identified. |
| UC-MSCs | Bartolucci et al. [56] | The UC-MSC-treated group demonstrated significant improvements in left ventricular ejection fraction and quality of life. There were no differences compared to the control group in terms of mortality, heart failure hospitalizations, arrhythmias, or new malignancies. |
| ADSCs | Qayyum et al. [57] | Intramyocardial ADSC treatment was safe but did not improve exercise capacity compared to placebo. However, exercise capacity increased after treatment with ADSC compared to baseline but not in the placebo group. |
| HSCs | Aceves et al. [58] | Improvements were observed in left ventricular ejection fraction, stress ratio values, stress tests, and the number of affected segments of the left ventricle. After 6 months, ECG results were normal in all patients. |
| EPCs | Steinhoff et al. [52] | Although a reduction in scar size and nonviable tissue, along with an improvement in segmental myocardial perfusion, were observed, no significant difference in LVEF was detected after CD133+ SC injection compared to placebo. |
| hESCs | Menasche et al. [34] | All patients experienced symptomatic improvement, and systolic motion increased in treated segments. No serious treatment-related side effects occurred. hESCs can differentiate into cardiovascular progenitors after transplantation in patients with severe ischemic LV dysfunction. |
| iPSCs | Kawamura et al. [40] | Improvements were observed in heart failure symptoms, left ventricular contractility, and myocardial blood flow. No treatment-related adverse events occurred. |
| Disease/Condition | Stem Cell Therapy Utilized | Study | Outcomes | |
|---|---|---|---|---|
| Psoriasis | HSCT | Kaffenberger et al. [63] | Allogeneic transplantation has a longer remission period than autologous transplantation but has higher mortality rates. | |
| UC-MSCs | Cheng et al. [66] | In 47.1% of patients, there was at least a 40% improvement in the PASI score. Allogeneic UC-MSC treatment is safe and partially effective for patients with psoriasis, and Treg levels may serve as a biomarker to predict treatment efficacy. | ||
| ADMSCs | Bajouri et al. [69] | No significant side effects were observed during the 6-month follow-up, and the PASI score and appearance of lesions were reported to have decreased in most patients. | ||
| Atopic Dermatitis | UCB-MSCs | Kim et al. [74] | In the high-dose hUCB-MSC-treated group, 55% of patients showed a 50% decrease in EASI score. Serum IgE levels, blood eosinophil counts, and pruritus scores decreased significantly. No serious adverse events reported. | |
| BM-MSCs | Shin et al. [76] | Eighty percent of patients achieved EASI-50, with no serious side effects observed. MSC therapy may be a promising option for patients with moderate to severe atopic dermatitis, particularly those with elevated IL-17 levels. | ||
| Seo et al. [77] | A significantly higher EASI-50 rate was achieved compared to the control group, with no serious side effects. However, there were no significant differences in pruritus scores and quality of life index. | |||
| ADMSCs | Ra et al. [72] | Partial decrease in SCORAD scores of 4 patients and no serious side effects were observed. | ||
| Vitiligo | Cell Transplantation with Hair Follicle Origin | Mohanty et al. [78] | Nine of 14 patients achieved >75% repigmentation. The transplantation procedure is recommended for patients with stable vitiligo for at least 1 year. | |
| Combined Cell Transplantation with Epidermal and Hair Follicle Origin | Ramos et al. [96] | Of the 24 patients, 25% showed an excellent repigmentation rate and 50% showed a good repigmentation rate. The best results were in the face and neck lesions, with better responses observed in patients with segmental vitiligo than in those without segmental vitiligo. | ||
| ADSCs with Melanocyte Culture | Saleh et al. [97] | The study indicated that culturing stem cells derived from adipose tissue alongside melanocytes from hair follicles could be a safe and effective treatment for patients with stable localized vitiligo resistant to other therapies. | ||
| Epidermolysis Bullosa | BM-HSC | Wagner et al. [98] | Five of six recipients showed increased C7 deposition at the dermal-epidermal junction, although there was no normalization of connective fibers. One recipient died from graft rejection and infection. None of the patients had detectable anti-C7 antibodies. It is recommended that treatment be initiated with a cost–benefit analysis. | |
| BM-MSCs | Petrof et al. [99] | A regression in the Birmingham Epidermolysis BEBSS and general severity score was observed in all patients. No side effects necessitating treatment discontinuation were reported. | ||
| ABCB5+ DMSCs | Kiritsi et al. [100] | A 13.0% decrease in the Epidermolysis Bullosa Disease Activity and Scarring Index score and an 18.2% decrease in the Epidermolysis Bullosa Clinician-Researcher Results Scoring Tool were observed. Significant reductions in pruritus and pain scores were also noted. Hypersensitivity reactions occurred in 2 out of 16 patients, resolving after treatment discontinuation. | ||
| UCB-MSCs | Lee et al. [101] | Improvements were noted in the BEBSS, body surface area involvement, blister count, pain, pruritus reduction, and quality of life, with no serious treatment-related side effects reported. | ||
| Alopecia | AA | ADSCs | Anderi et al. [102] | All patients exhibited increased hair density and diameter, along with decreased pull-test results. |
| BM-MSCs | Elmaadawi et al. [103] | 40 patients (20 AA and 20 AGA) included in the study. All patients demonstrated significant improvement with no serious side effects observed. | ||
| UCB-MSCs (Stem Cell Educator Therapy) | Li et al. [89] | Treatment resulted in significant improvement in all patients, which persisted for 2 years, with no serious side effects observed. | ||
| AGA | Autologous HFSCs | Gentile et al. [84] | The average hair count and density increased from baseline values, with a reported 29% average increase in hair density in the treated area. | |
| AFSCs | Gentile et al. [85] | Patients exhibited improvements in hair density, compared to baseline values in the treated area. Scalp biopsy evaluations revealed an increase in the number of hair follicles. HD-AFSCs in micrografts may offer a safe and effective alternative treatment option for hair loss. | ||
| ADSCs | Tak et al. [104] | A greater increase in hair count obtained in the treatment group than in the control group. A significant improvement in hair diameter was also noted in the treatment group. | ||
| Kim et al. [105] | The increase in hair density on the SVF-treated side compared to the untreated side was statistically significant. However, while an increase in thickness was observed, it was not statistically significant. | |||
| BM-MSCs | Elmaadawi et al. [103] | - | ||
| SSc | HSCT | Oyama et al. [106] | A statistically significant improvement in the Modified Rodnan skin score was achieved. However, cardiac and renal functions remained stable. Overall and progression-free survival rates were 90% and 70%, respectively. The study showed that non-myeloablative autologous HSCT had similar success to the myeloablative regimen, with fewer side effects and no toxicity. | |
| ADSC | Granel et al. [107] | The study evaluated injection of autologous SVF cells into the hands of 12 patients with systemic sclerosis and resulted in significant improvements in hand disability and pain, Raynaud phenomenon, finger edema, and quality of life. No serious adverse events occurred during the procedure or follow-up. | ||
| Study | Stem Cell Type | Ophthalmic Disorder | Outcome |
|---|---|---|---|
| Fasolo et al. (2017) [165] | Cultured Limbal stem cells | Limbal Stem Cell Deficiency | One year after surgery, 41% of the 59 primary CLET procedures were successful, 39% partially successful and 20% failed. The most common ADRs recorded for the primary unsuccessful CLETs were ulcerative keratitis, melting/perforation, and epithelial defects/disepithelialisation. |
| Sangwan et al. (2011) [166] | Limbal epithelial cells | Limbal Stem Cell Deficiency | A completely epithelised, avascular and clinically stable corneal surface was seen in 142 of 200 (71%) eyes at a mean follow-up of 3 ± 1.6 (range: 1–7.6) years; improvement in visual acuity in 60.5% of eyes. |
| Rama et al. (2010) [167] | Limbal epithelial cells | Limbal Stem Cell Deficiency | Permanent restoration of a transparent, renewing corneal epithelium was attained in 76.6% of eyes. |
| Jurkunas et al. (2025) [169] | Limbal epithelial cells | Limbal Stem Cell Deficiency | Transplantation of CALEC constructs in patients with both mild and severe forms of LSCD achieved corneal surface restoration with limbal epithelial cells and improved clinical symptoms |
| Miotti et al. (2021) [168] | Limbal tissue autograft transplantation (LSCs), iPSCs, MSCs, BM-MSCs | Limbal Stem Cell Deficiency | Improved visual acuity, rapid surface healing, stable epithelial adhesion without recurrent erosion, arrest or regression of corneal neovascularization |
| LIu et al. (2024) [185] | hESC, iPSCs | Age related macular degeneration | Slower rates of disease progression |
| Sharma et al. (2019) [175] | iPSCs | Age related macular degeneration | RPE patches have been derived from patient-specific iPSCs and applied on a scaffold to support long-term cell viability and function |
| Chen et al. (2023) [186] | RPCs, MSCs, hESCs-RPE, iPSCs-RPE | Retinitis pigmentosa | Improvement in best corrected visual acuity |
| Chen et al. (2023) [186] | RPCs, MSCs, hESCs-RPE, iPSCs-RPE | Stargardt disease | Improvement in best corrected visual acuity |
| Moghadam Fard et al. (2023) [182] | hESCs, BM-MSCs, MSCs | Stargardt disease | Improvement in visual acuity |
| Study | Stem Cell Type | Pulmonary Disorder | Outcome |
|---|---|---|---|
| Le Thi Bich et al. (2020) [188] | UC-MSCs | Chronic Obstructive Pulmonary Disease | Therapy was well-tolerated with no infusion-related toxicities or serious adverse effects, and treated patients had significantly fewer acute exacerbations over 6 months. |
| Rio et al. (2023) [189] | AD-MSCs, UC-MSCs | Chronic Obstructive Pulmonary Disease | COPD AD-MSC were as efficient in reducing elastase-induced lung emphysema, UC-MSC reduced lung emphysema, equal therapeutic potential of AD-MSC from COPD and non-COPD subjects in the pre-clinical model |
| Wu et al. (2022) [187] | Autologous bronchial BSCs, | Chronic Obstructive Pulmonary Disease | Alleviation of symptoms and pulmonary function enhancement in patients receiving transplantation, procedure was safe and well-tolerated |
| Campo et al. (2021) [191] | BM-MSCs | Idiopathic Pulmonary Fibrosis | The treatment was well tolerated, though no significant improvements in pulmonary function were observed |
| Liu et al. (2025) [192] | Basal Cells | Idiopathic Pulmonary Fibrosis | Preclinical study utilizing autologous proximal airway basal cells demonstrated fibrotic repair and alveolar regeneration in bleomycin-injured mice |
| Matthay et al. (2018) [194] | BM-MSCs | Acute Respiratory Distress Syndrome | No improvement in outcomes, but the infusion was safe and well tolerated |
| Regmi et al. (2024) [195] | UC-MSCs, BM-MSCs, and AD-MSCs | Acute Respiratory Distress Syndrome | Umbilical cord MSCs showed superior efficacy to MSCs from bone marrow or adipose tissue. These mice had higher survival, improved lung histology, and less alveolar damage |
| Bonfield and Lazarus (2025) [196] | MSCs | Cystic Fibrosis | Allogeneic MSCs were safe and play a role in reducing infections and inflammation in CF patients |
| Roesch et al. (2023) [198] | BM-MSCs | Cystic Fibrosis | No dose-limiting toxicities, deaths or life-threatening adverse events were observed, no significant functional change observed |
| Sutton et al. (2017) [197] | MSCs | Cystic Fibrosis | MSCs secrete supernatants that are anti-inflammatory and anti-microbial and have potential in CF |
| Study | Stem Cell Type | Nephrology Disorder | Outcome |
|---|---|---|---|
| Maklough et al. (2018) [199] | BM-MSCs | Chronic Kidney Disease | Improved renal function and structure in preclinical models, safety and tolerability were established in patients with CKD |
| Salybekov et al. (2024) [201] | MSCs | Acute Kidney Injury and Chronic Kidney Disease | Clinical trials have shown that MSCs are safe and well-tolerated in both chronic and acute kidney disease, but efficacy results have been modest |
| Wang et al. (2022) [200] | MSCs | Chronic Kidney Disease | Preclinical trials have indicated that MSCs can slow progress of diabetes mellitus, decrease mesangial thickening and macrophage infiltration in mice models |
| Burt et al. (2006) [210] | HSCT | Refractory Systemic Lupus Erythematosus | Stabilization of renal function and significant improvements in SLEDAI score, ANA, anti-ds DNA, complement levels, and lung diffusing capacity were reported. Treatment-related mortality was 2%. Overall 5-year survival was 84%, and dis-ease-free survival 5 years post-HSCT was 50%. |
| Sun et al. (2009) [211] | BM-MSCs | Refractory Systemic Lupus Erythematosus | Four SLE patients resistant to immunosuppressive therapy were included in the study, and all treated patients achieved remission lasting 12–18 months. Improvements in disease activity, serological markers, and renal function were observed. |
| Deng et al. (2017) [205] | UC-MSCs | Refractory Systemic Lupus Erythematosus | hUC-MSC has no apparent additional effect over and above standard immunosuppression. |
| Kamen et al. (2022) [207] | UC-MSC | Refractory Systemic Lupus Erythematosus | UC-MSC infusions were safe and may have efficacy in lupus |
| Liang et al. (2025) [203] | MSCs | Refractory Systemic Lupus Erythematosus | Patients clinically improved with decreased in SLEDAI score and 24 h proteinuria, Anti-dsDNA levels decreased, improvement in glomerular filtration rate |
| Ranjbar et al. (2022) [206] | AD-MSCs | Refractory Systemic Lupus Erythematosus | AD-MSC transplantation was associated with favorable safety and was efficient in reducing urine protein excretion and disease activity, single dose may not be adequate for long term remission |
| Wang et al. (2013) [204] | MSCs | Refractory Systemic Lupus Erythematosus | 28–32.5% of patients in complete clinical remission in 1 year, rising to near 50% among those observed 4 years later |
| Sun et al. (2018) [209] | UC-MSCs | Kidney Transplant | UC-MSCs could achieve substantial reduction in the incidence of delayed graft function but there was no significant difference between MSC and non-MSC groups |
| Tan et al. (2012) [208] | MSCs | Kidney transplant | The use of autologous MSCs compared with anti-IL-2 receptor antibody induction therapy resulted in lower incidence of acute rejection, decreased risk of opportunistic infection, and better estimated renal function at 1 year |
| Study | Stem Cell Type | Neurological Disorder | Outcome |
|---|---|---|---|
| Cecerska-Heryć et al. (2023) [217] | MSCs, HSCs, iPSCs | ALS, HD, MS, PD | Slowed ALS progression, reduced Htt aggregation in HD, immune recalibration in MS, accurate PD modeling |
| Namiot et al. (2022) [212] | MSCs | Brain injuries, stroke, MS, brain tumors | Dominance of MSCs in trials, most trials in early phases |
| Pappolla et al. (2024) [233] | NSCs, MSCs | Stroke, MS, ALS, TBI, PD, AD | Translational difficulties, potential of stem cell-derived exosomes |
| Khandia et al. (2024) [235] | Various stem cells | AD, PD, ALS, HD, SCA, PSP | Improved synaptic plasticity, apoptosis inhibition, reduction in tau-phosphorylation and Aβ production in AD |
| Isaković et al. (2023) [236] | MSCs | PD, AD, ischemic stroke, glioblastoma, MS | Safety established, concerns about immunocompatibility and tumorigenicity |
| Zayed et al. (2022) [237] | Various stem cells | AD, PD, HD, MS, ALS | Tailoring stem cells to specific disease defects, mixed clinical trial results |
| Izrael et al. (2025) [238] | Pluripotent stem cells | SCI, PD, ALS, retinal diseases | Preclinical and clinical trial progress, regulatory approvals |
| Ying et al. (2023) [239] | Various stem cells | CNS diseases | Cell replacement, immunoregulation, neurotrophic effects |
| Zhang et al. (2024) [240] | MSCs | Congenital nervous system and neurodegenerative diseases | Potential in treating neurological diseases, preclinical and clinical evidence |
| Liu et al. (2024) [241] | Various stem cells | Ischemic brain injury | Promising preclinical and clinical results, various administration routes |
| Yang et al. (2024) [234] | NSCs | Neurological diseases | Neuroprotection, axonal regeneration, remyelination |
| Wei et al. (2023) [242] | Various stem cells | Neurodegenerative diseases | Combination with nanotechnology to enhance efficacy |
| Disease/Condition | Stem Cell Therapy Utilized | Outcomes |
|---|---|---|
| Acute Myeloid Leukemia (AML) [294,295] | Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) | Increased survival, potential cure, but with risks of graft-versus-host disease (GVHD) and relapse |
| Acute Lymphoblastic Leukemia (ALL) [296] | Allogeneic HSCT | Improved survival rates, potential cure, similar risks as AML |
| Thalassemia [297] | Allogeneic HSCT, Autologous HSCT with gene therapy | Curative potential, reduced transfusion dependency, risks include GVHD and graft rejection |
| Sickle Cell Disease (SCD) [297] | Allogeneic HSCT, Autologous HSCT with gene therapy | Curative potential, reduced vaso-occlusive crises, risks include GVHD and graft rejection |
| Graft-versus-Host Disease (GVHD) [298,299] | Mesenchymal Stem Cells (MSCs) | Reduction in GVHD severity, improved engraftment |
| Hematopoietic Support Post-Transplant [299] | MSCs | Enhanced hematopoiesis, reduced tissue toxicities |
| Non-Malignant Hematologic Disorders (e.g., Aplastic Anemia) [300] | Allogeneic HSCT | Potential cure, improved hematopoiesis, risks include GVHD and infections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail Mendi, B.; Hirani, R.; Sayegh, A.; Hassan, M.; Fleshner, L.; Farabi, B.; Atak, M.F.; Safai, B. Utilization of Stem Cells in Medicine: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 9659. https://doi.org/10.3390/ijms26199659
Ismail Mendi B, Hirani R, Sayegh A, Hassan M, Fleshner L, Farabi B, Atak MF, Safai B. Utilization of Stem Cells in Medicine: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(19):9659. https://doi.org/10.3390/ijms26199659
Chicago/Turabian StyleIsmail Mendi, Banu, Rahim Hirani, Alyssa Sayegh, Mariah Hassan, Lauren Fleshner, Banu Farabi, Mehmet Fatih Atak, and Bijan Safai. 2025. "Utilization of Stem Cells in Medicine: A Narrative Review" International Journal of Molecular Sciences 26, no. 19: 9659. https://doi.org/10.3390/ijms26199659
APA StyleIsmail Mendi, B., Hirani, R., Sayegh, A., Hassan, M., Fleshner, L., Farabi, B., Atak, M. F., & Safai, B. (2025). Utilization of Stem Cells in Medicine: A Narrative Review. International Journal of Molecular Sciences, 26(19), 9659. https://doi.org/10.3390/ijms26199659






