Tailored Reaction Conditions and Automated Radiolabeling of [177Lu]Lu-PSMA-ALB-56 in a 68Ga Setting: The Critical Impact of Antioxidant Concentrations
Abstract
1. Introduction
2. Results and Discussions
2.1. Reaction Conditions Study at Small Scale
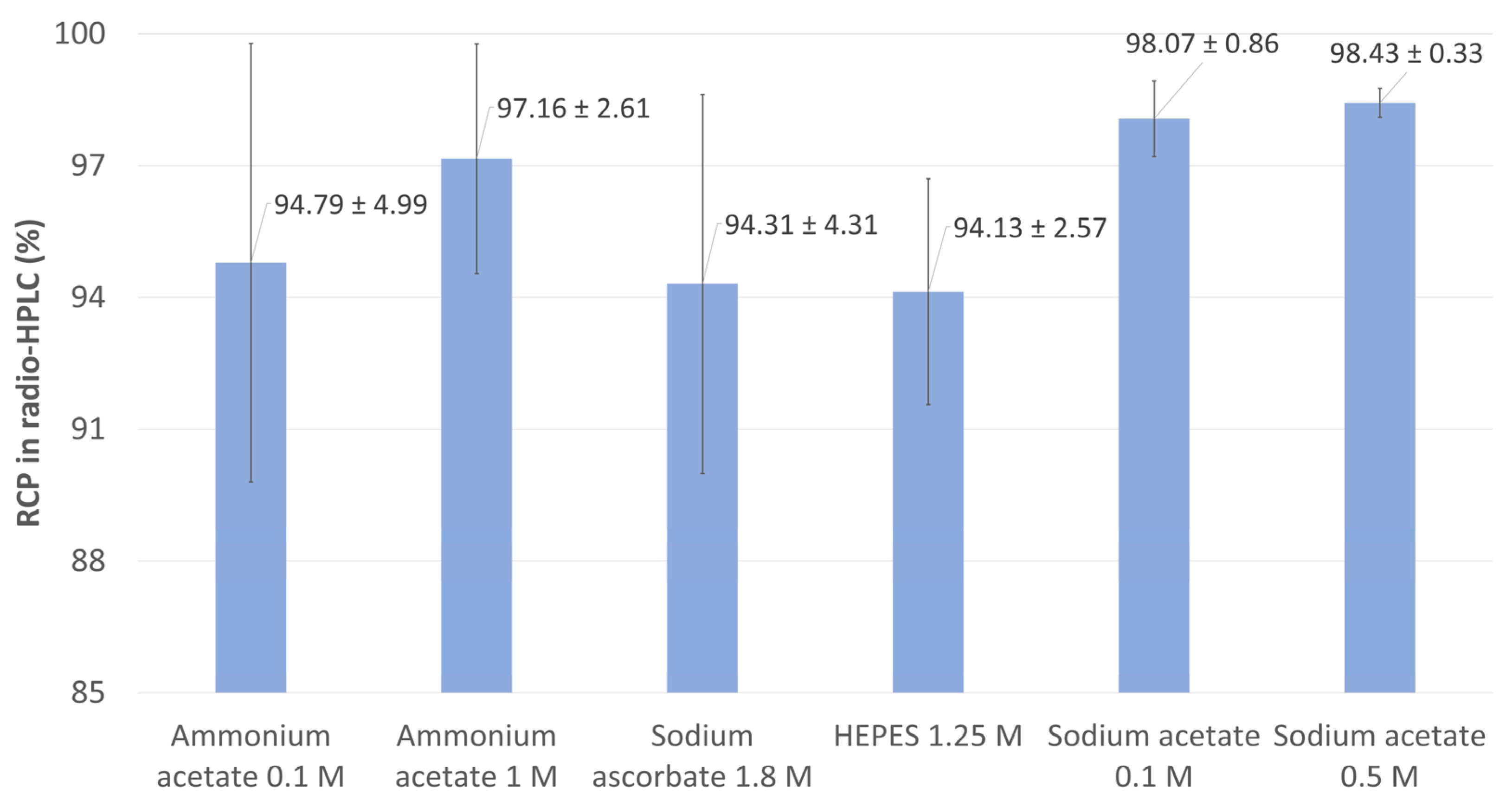
2.1.1. Influence of the Reaction Buffer
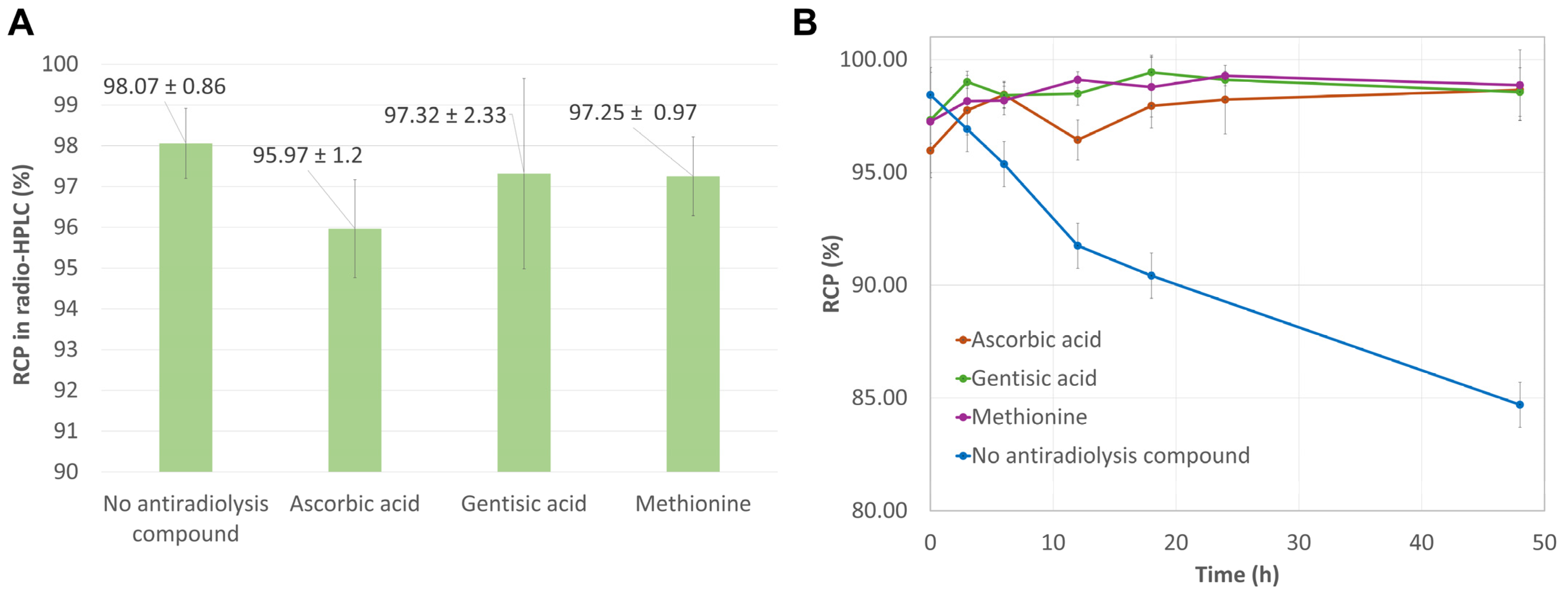
2.1.2. Influence of Antioxidant Agents
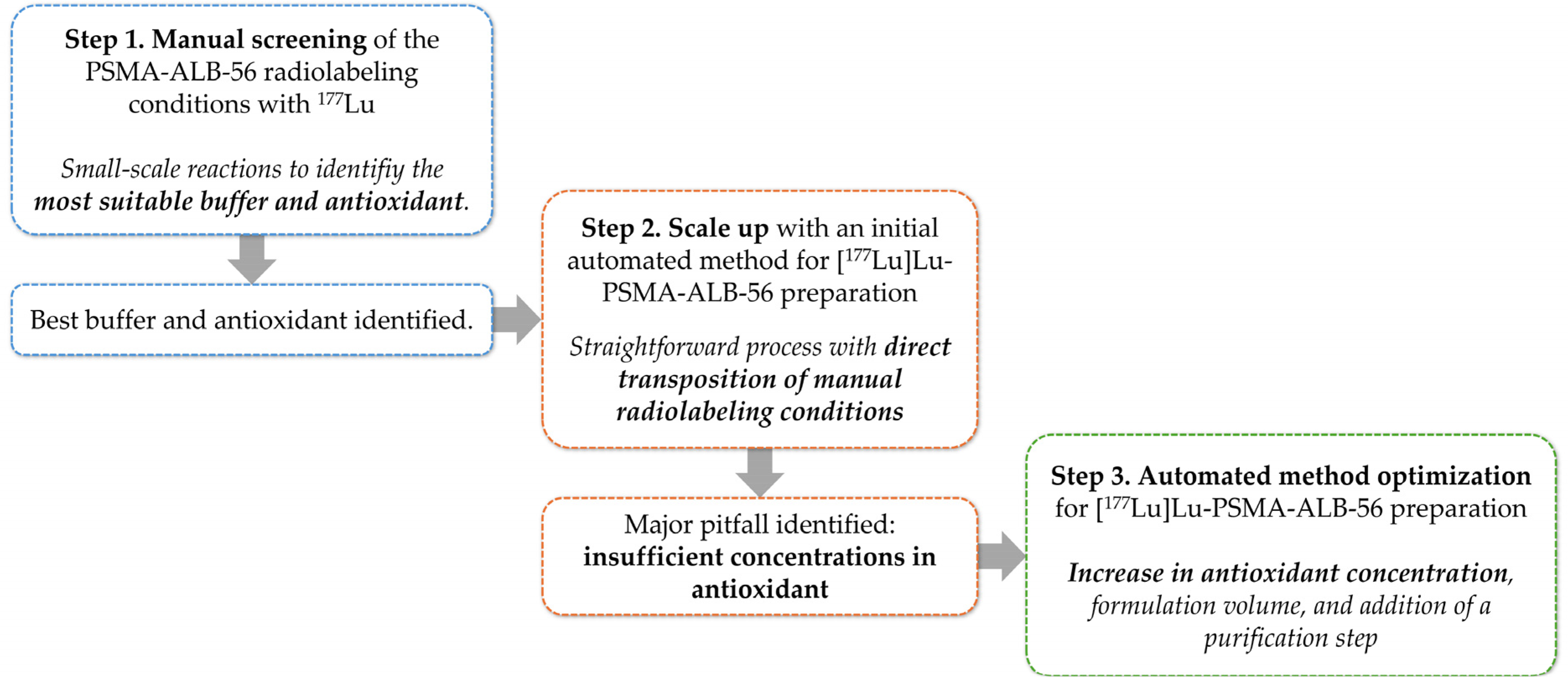
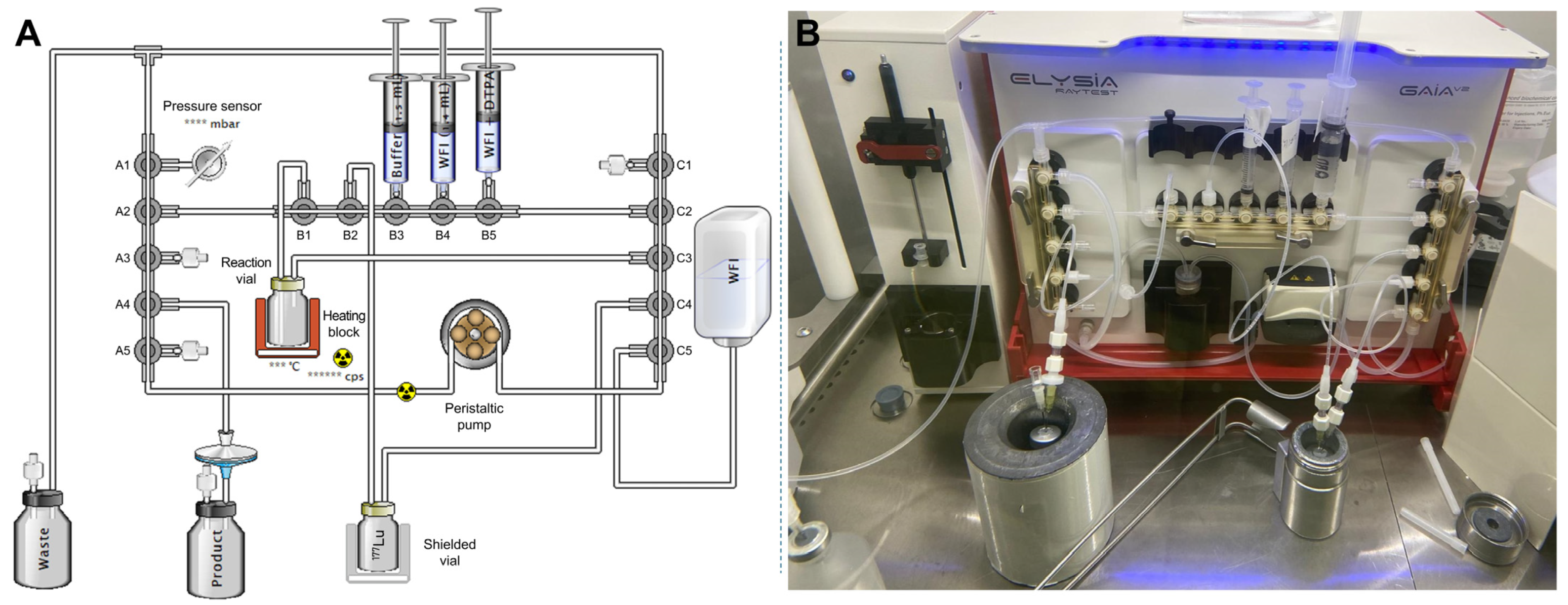
2.2. Design of the Initial Automated Radiolabeling Protocol and First Preparation of High-Activity Doses
2.2.1. Conception of the Automated Procedure
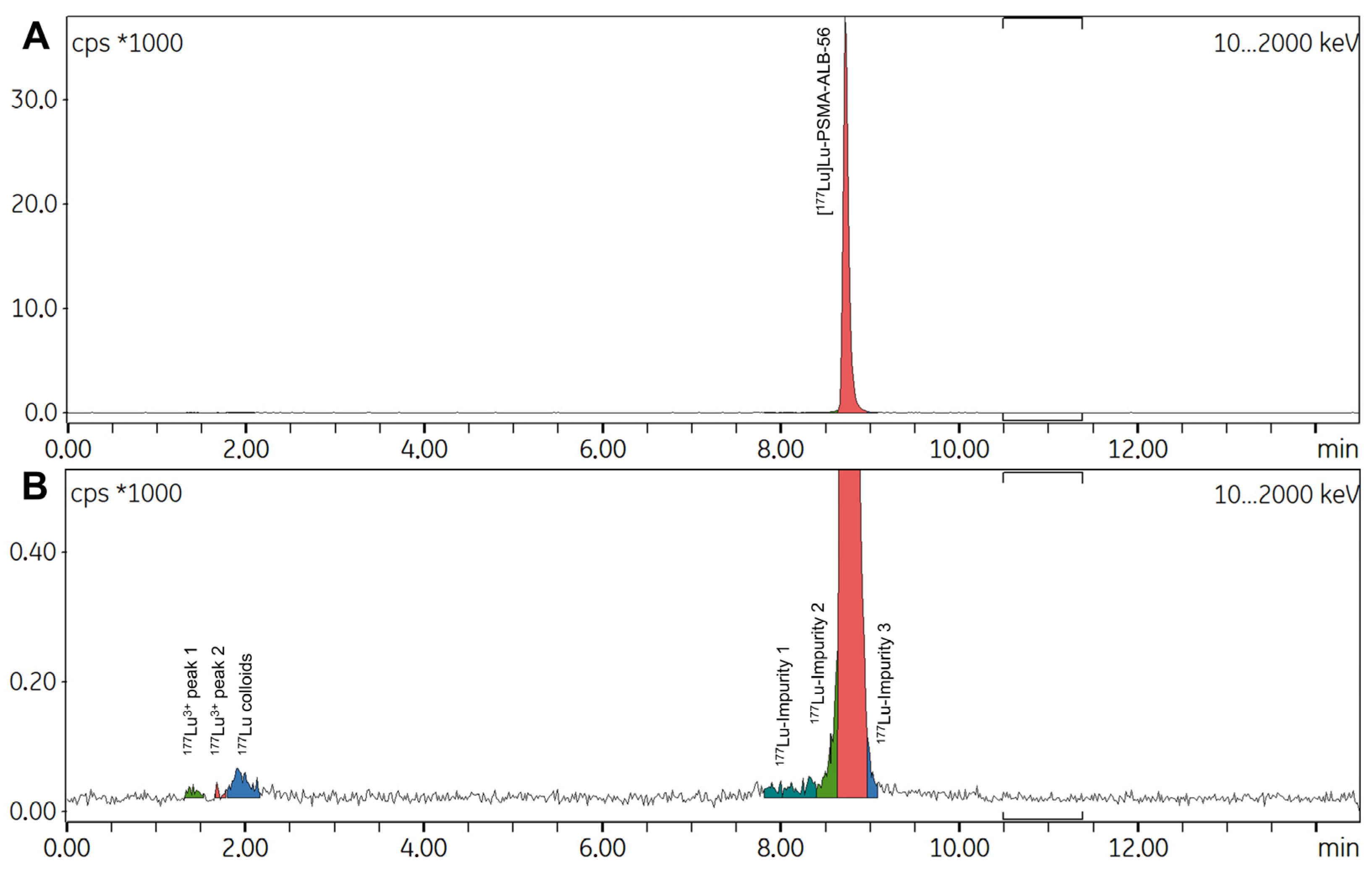
2.2.2. Production of Test Batches
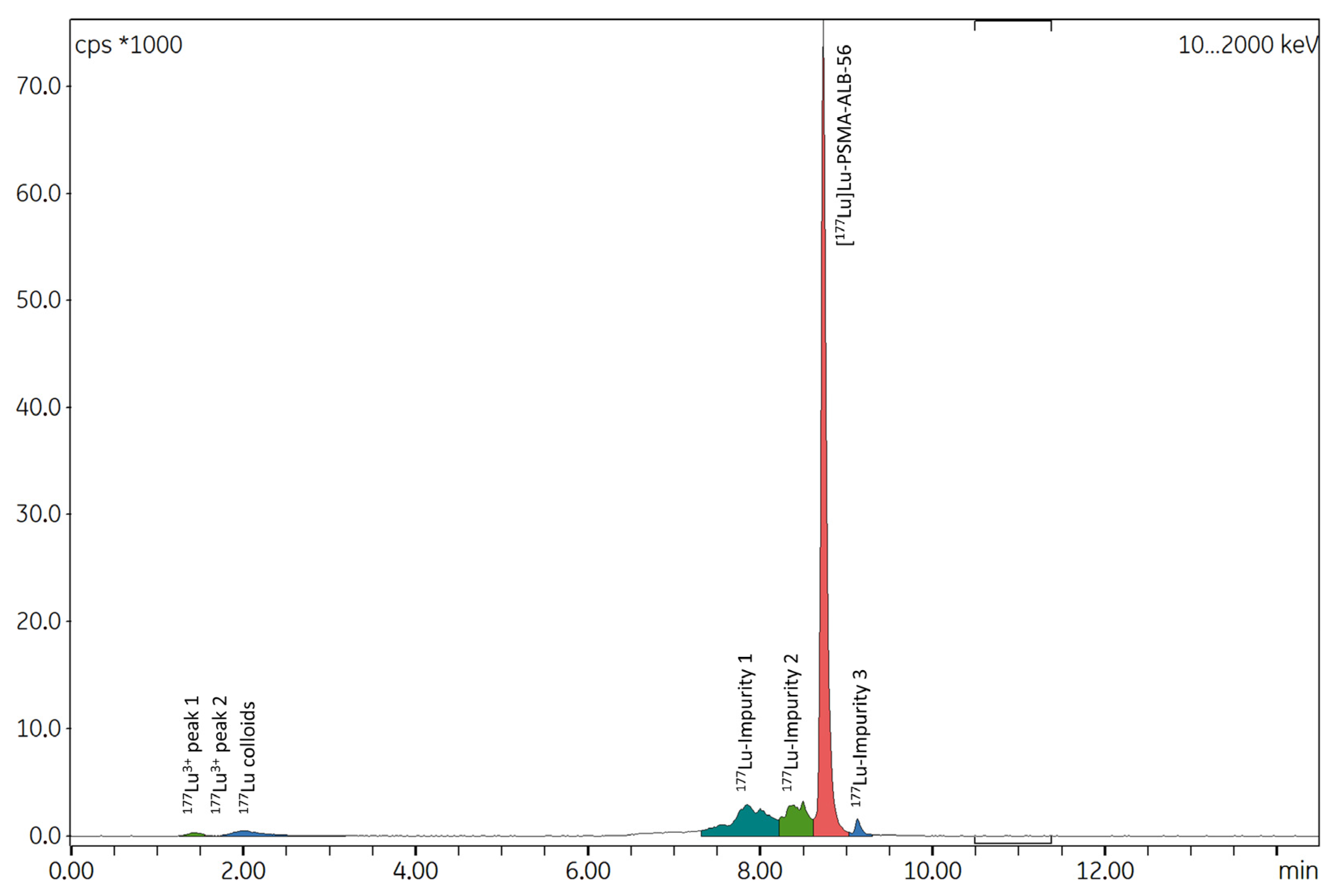
2.2.3. Possible Causes for the Formation of Radiolysis Byproducts
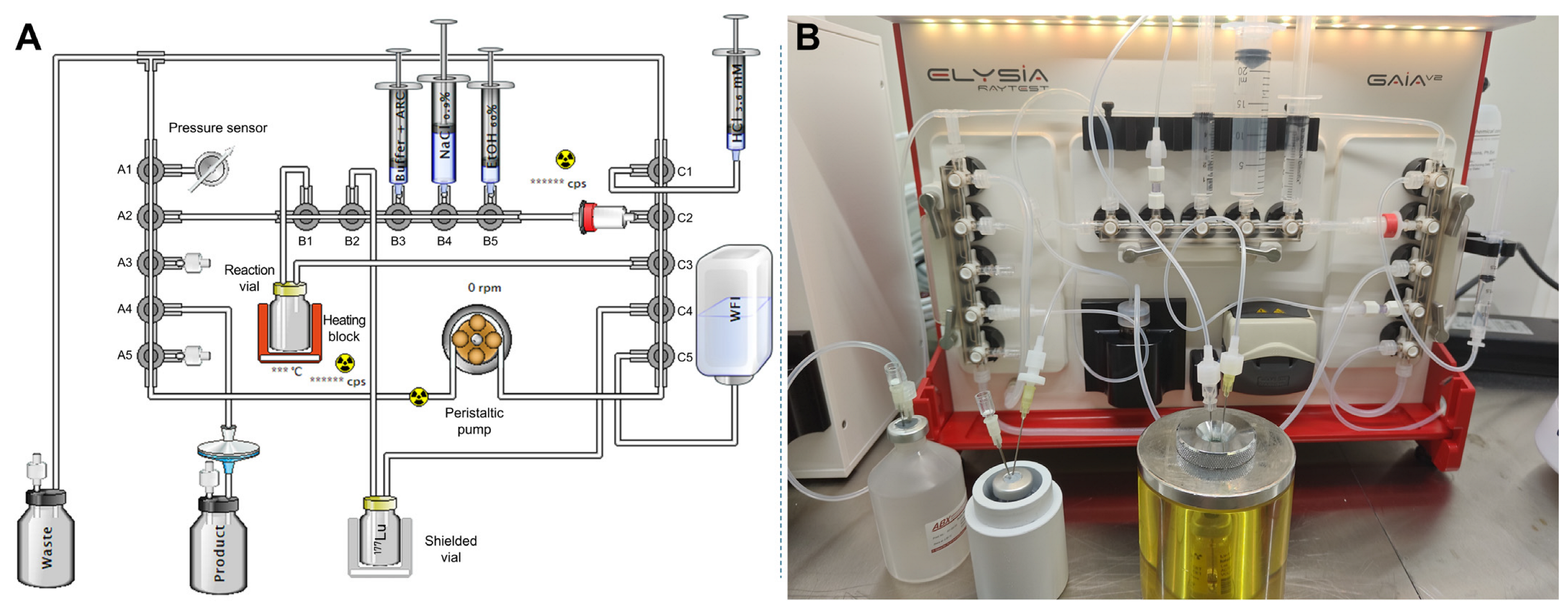
2.3. Design of the Optimized Automated Radiolabeling Protocol and Second Preparation of High-Activity Doses
2.3.1. Modifications to the Initial Automated Procedure
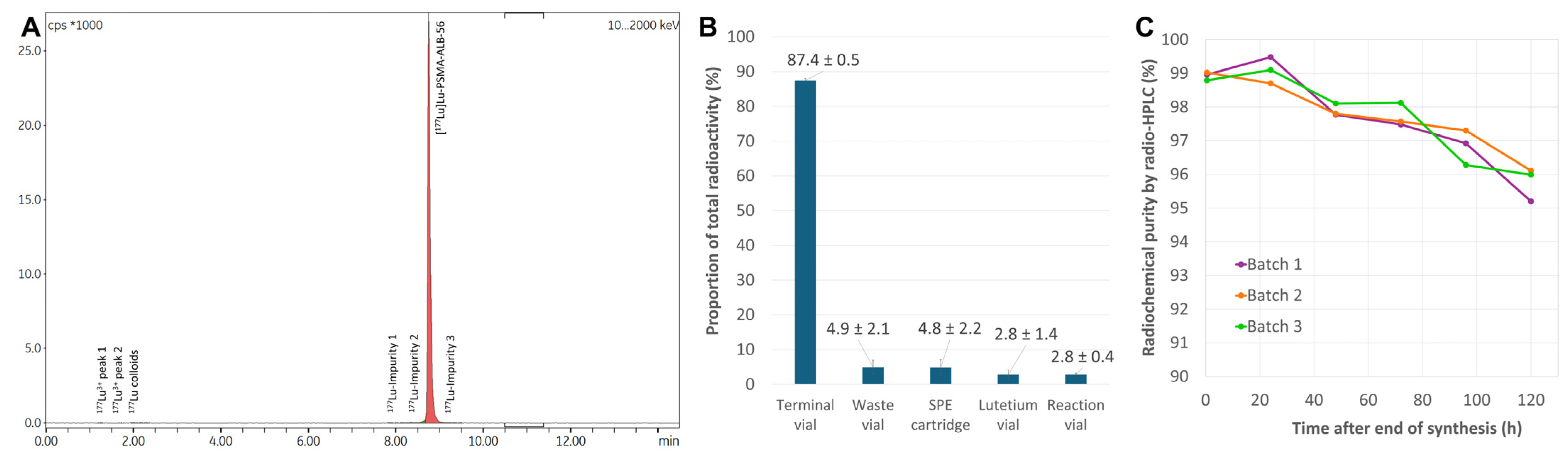
2.3.2. Production of Test Batches
3. Materials and Methods
3.1. Chemicals, Solvents and Equipment
General Information
3.2. Radiolabeling Conditions Study
3.3. Initial Automated Radiolabeling Protocol Without Terminal Purification
3.4. Optimized Automated Radiolabeling Protocol with Terminal Sold-Phase Extraction
3.5. Radio-HPLC Method for Radiochemical Purity Determination
3.6. Additional Quality Controls on Automated Synthesis Productions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lepareur, N.; Ramée, B.; Mougin-Degraef, M.; Bourgeois, M. Clinical Advances and Perspectives in Targeted Radionuclide Therapy. Pharmaceutics 2023, 15, 1733. [Google Scholar] [CrossRef]
- Pouget, J.-P.; Lozza, C.; Deshayes, E.; Boudousq, V.; Navarro-Teulon, I. Introduction to Radiobiology of Targeted Radionuclide Therapy. Front. Med. 2015, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves Lutathera for GEP NET Therapy. J. Nucl. Med. 2018, 59, 9N. [Google Scholar]
- Jang, A.; Kendi, A.T.; Sartor, O. Status of PSMA-Targeted Radioligand Therapy in Prostate Cancer: Current Data and Future Trials. Ther. Adv. Med. Oncol. 2023, 15, 17588359231157632. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves Pluvicto/Locametz for Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2022, 63, 13N. [Google Scholar]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Alati, S.; Singh, R.; Pomper, M.G.; Rowe, S.P.; Banerjee, S.R. Preclinical Development in Radiopharmaceutical Therapy for Prostate Cancer. Semin. Nucl. Med. 2023, 53, 663–686. [Google Scholar] [CrossRef]
- Lau, J.; Jacobson, O.; Niu, G.; Lin, K.-S.; Bénard, F.; Chen, X. Bench to Bedside: Albumin Binders for Improved Cancer Radioligand Therapies. Bioconjug. Chem. 2019, 30, 487–502. [Google Scholar] [CrossRef]
- Brandt, M.; Cardinale, J.; Giammei, C.; Guarrochena, X.; Happl, B.; Jouini, N.; Mindt, T.L. Mini-Review: Targeted Radiopharmaceuticals Incorporating Reversible, Low Molecular Weight Albumin Binders. Nucl. Med. Biol. 2019, 70, 46–52. [Google Scholar] [CrossRef]
- Kelly, J.M.; Amor-Coarasa, A.; Nikolopoulou, A.; Wüstemann, T.; Barelli, P.; Kim, D.; Williams, C.; Zheng, X.; Bi, C.; Hu, B.; et al. Dual-Target Binding Ligands with Modulated Pharmacokinetics for Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2017, 58, 1442–1449. [Google Scholar] [CrossRef]
- mbricht, C.A.; Benešová, M.; Schibli, R.; Müller, C. Preclinical Development of Novel PSMA-Targeting Radioligands: Modulation of Albumin-Binding Properties To Improve Prostate Cancer Therapy. Mol. Pharm. 2018, 15, 2297–2306. [Google Scholar] [CrossRef]
- Müller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA Conjugate with an Albumin-Binding Entity Enables the First Folic Acid-Targeted 177Lu-Radionuclide Tumor Therapy in Mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef]
- Farkas, R.; Siwowska, K.; Ametamey, S.M.; Schibli, R.; van der Meulen, N.P.; Müller, C. (64)Cu- and (68)Ga-Based PET Imaging of Folate Receptor-Positive Tumors: Development and Evaluation of an Albumin-Binding NODAGA-Folate. Mol. Pharm. 2016, 13, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Siwowska, K.; Haller, S.; Bortoli, F.; Benešová, M.; Groehn, V.; Bernhardt, P.; Schibli, R.; Müller, C. Preclinical Comparison of Albumin-Binding Radiofolates: Impact of Linker Entities on the in Vitro and in Vivo Properties. Mol. Pharm. 2017, 14, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, Y.; Zhang, J.; Zang, J.; Li, H.; Liu, Q.; Wang, J.; Jacobson, O.; Li, F.; Zhu, Z.; et al. Response to Single Low-Dose 177Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study. Theranostics 2018, 8, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Jacobson, O.; Cheng, Y.; Niu, G.; Li, F.; Bai, C.; Zhu, Z.; Chen, X. Safety, Pharmacokinetics, and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analog 177Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 1699–1705. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, Y.; Zang, J.; Sui, H.; Wang, H.; Jacobson, O.; Zhu, Z.; Chen, X. Dose Escalation of an Evans Blue-Modified Radiolabeled Somatostatin Analog 177Lu-DOTA-EB-TATE in the Treatment of Metastatic Neuroendocrine Tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 947–957. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Millo, C.; Cochran, C.; Jacobson, O.; Lu, H.; Wang, Z.; Kiesewetter, D.; Chen, X.; Vasko, V.; et al. 177Lu-DOTA-EB-TATE, a Radiolabeled Analogue of Somatostatin Receptor Type 2, for the Imaging and Treatment of Thyroid Cancer. Clin. Cancer Res. 2021, 27, 1399–1409. [Google Scholar] [CrossRef]
- Liu, Q.; Zang, J.; Sui, H.; Ren, J.; Guo, H.; Wang, H.; Wang, R.; Jacobson, O.; Zhang, J.; Cheng, Y.; et al. Peptide Receptor Radionuclide Therapy of Late-Stage Neuroendocrine Tumor Patients with Multiple Cycles of 177Lu-DOTA-EB-TATE. J. Nucl. Med. 2021, 62, 386–392. [Google Scholar] [CrossRef]
- Hänscheid, H.; Hartrampf, P.E.; Schirbel, A.; Buck, A.K.; Lapa, C. Intraindividual Comparison of [177Lu]Lu-DOTA-EB-TATE and [177Lu]Lu-DOTA-TOC. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2566–2572. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Q.; Wang, G.; Sui, H.; Wang, R.; Wang, J.; Zhang, J.; Zhu, Z.; Chen, X. Safety and Efficacy of Peptide Receptor Radionuclide Therapy with 177Lu-DOTA-EB-TATE in Patients with Metastatic Neuroendocrine Tumors. Theranostics 2022, 12, 6437–6445. [Google Scholar] [CrossRef]
- Njotu, F.N.; Ketchemen, J.P.; Tikum, A.F.; Babeker, H.; Gray, B.D.; Pak, K.Y.; Uppalapati, M.; Fonge, H. Efficacy of [67Cu]Cu-EB-TATE Theranostic Against Somatostatin Receptor Subtype-2-Positive Neuroendocrine Tumors. J. Nucl. Med. 2024, 65, 533–539. [Google Scholar] [CrossRef]
- Zang, J.; Fan, X.; Wang, H.; Liu, Q.; Wang, J.; Li, H.; Li, F.; Jacobson, O.; Niu, G.; Zhu, Z.; et al. First-in-Human Study of 177Lu-EB-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Liu, Q.; Sui, H.; Wang, R.; Jacobson, O.; Fan, X.; Zhu, Z.; Chen, X. 177Lu-EB-PSMA Radioligand Therapy with Escalating Doses in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, M.; Zang, J.; Jiang, Y.; Chen, X.; Zhu, Z.; Chen, X. A Pilot Study of 68Ga-PSMA-617 PET/CT Imaging and 177Lu-EB-PSMA-617 Radioligand Therapy in Patients with Adenoid Cystic Carcinoma. EJNMMI Res. 2022, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zang, J.; Jiang, Y.; Liu, Q.; Sui, H.; Wang, R.; Fan, X.; Zhang, J.; Zhu, Z.; Chen, X. A Single-Arm, Low-Dose, Prospective Study of 177Lu-EB-PSMA Radioligand Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2022, 64, 611–617. [Google Scholar] [CrossRef]
- Wen, X.; Xu, P.; Zeng, X.; Liu, J.; Du, C.; Zeng, X.; Cheng, X.; Wang, X.; Liang, Y.; Zhao, T.; et al. Development of [177Lu]Lu-LNC1003 for Radioligand Therapy of Prostate Cancer with a Moderate Level of PSMA Expression. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2846–2860. [Google Scholar] [CrossRef]
- Zang, J.; Wang, G.; Zhao, T.; Liu, H.; Lin, X.; Yang, Y.; Shao, Z.; Wang, C.; Chen, H.; Chen, Y.; et al. A Phase 1 Trial to Determine the Maximum Tolerated Dose and Patient-Specific Dosimetry of [177Lu]Lu-LNC1003 in Patients with Metastatic Castration-Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 871–882. [Google Scholar] [CrossRef]
- Deberle, L.M.; Benešová, M.; Umbricht, C.A.; Borgna, F.; Büchler, M.; Zhernosekov, K.; Schibli, R.; Müller, C. Development of a New Class of PSMA Radioligands Comprising Ibuprofen as an Albumin-Binding Entity. Theranostics 2020, 10, 1678–1693. [Google Scholar] [CrossRef]
- Deberle, L.M.; Tschan, V.J.; Borgna, F.; Sozzi-Guo, F.; Bernhardt, P.; Schibli, R.; Müller, C. Albumin-Binding PSMA Radioligands: Impact of Minimal Structural Changes on the Tissue Distribution Profile. Molecules 2020, 25, 2542. [Google Scholar] [CrossRef]
- Borgna, F.; Deberle, L.M.; Busslinger, S.D.; Tschan, V.J.; Walde, L.M.; Becker, A.E.; Schibli, R.; Müller, C. Preclinical Investigations to Explore the Difference between the Diastereomers [177Lu]Lu-SibuDAB and [177Lu]Lu-RibuDAB toward Prostate Cancer Therapy. Mol. Pharm. 2022, 19, 2105–2114. [Google Scholar] [CrossRef]
- Tschan, V.J.; Borgna, F.; Busslinger, S.D.; Stirn, M.; Rodriguez, J.M.M.; Bernhardt, P.; Schibli, R.; Müller, C. Preclinical Investigations Using [177Lu]Lu-Ibu-DAB-PSMA toward Its Clinical Translation for Radioligand Therapy of Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3639–3650. [Google Scholar] [CrossRef]
- Busslinger, S.D.; Tschan, V.J.; Richard, O.K.; Talip, Z.; Schibli, R.; Müller, C. [225Ac]Ac-SibuDAB for Targeted Alpha Therapy of Prostate Cancer: Preclinical Evaluation and Comparison with [225Ac]Ac-PSMA-617. Cancers 2022, 14, 5651. [Google Scholar] [CrossRef]
- Dumelin, C.E.; Trüssel, S.; Buller, F.; Trachsel, E.; Bootz, F.; Zhang, Y.; Mannocci, L.; Beck, S.C.; Drumea-Mirancea, M.; Seeliger, M.W.; et al. A Portable Albumin Binder from a DNA-Encoded Chemical Library. Angew. Chem. Int. Ed. Engl. 2008, 47, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Trüssel, S.; Dumelin, C.; Frey, K.; Villa, A.; Buller, F.; Neri, D. New Strategy for the Extension of the Serum Half-Life of Antibody Fragments. Bioconjug. Chem. 2009, 20, 2286–2292. [Google Scholar] [CrossRef]
- Benešová, M.; Umbricht, C.A.; Schibli, R.; Müller, C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C.; Schlyer, D.; Zhao, Y.; Kim, D.; Babich, J.W. Trifunctional PSMA-Targeting Constructs for Prostate Cancer with Unprecedented Localization to LNCaP Tumors. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-T.; Merkens, H.; Zhang, Z.; Uribe, C.F.; Lau, J.; Zhang, C.; Colpo, N.; Lin, K.-S.; Bénard, F. Enhancing Treatment Efficacy of 177Lu-PSMA-617 with the Conjugation of an Albumin-Binding Motif: Preclinical Dosimetry and Endoradiotherapy Studies. Mol. Pharm. 2018, 15, 5183–5191. [Google Scholar] [CrossRef]
- Ling, X.; Latoche, J.D.; Choy, C.J.; Kurland, B.F.; Laymon, C.M.; Wu, Y.; Salamacha, N.; Shen, D.; Geruntho, J.J.; Rigatti, L.H.; et al. Preclinical Dosimetry, Imaging, and Targeted Radionuclide Therapy Studies of Lu-177-Labeled Albumin-Binding, PSMA-Targeted CTT1403. Mol. Imaging Biol. 2020, 22, 274–284. [Google Scholar] [CrossRef]
- Boinapally, S.; Alati, S.; Jiang, Z.; Yan, Y.; Lisok, A.; Singh, R.; Lofland, G.; Minn, I.; Hobbs, R.F.; Pomper, M.G.; et al. Preclinical Evaluation of a New Series of Albumin-Binding 177Lu-Labeled PSMA-Based Low-Molecular-Weight Radiotherapeutics. Molecules 2023, 28, 6158. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Wang, G.; Wang, R.; Jin, W.; Zang, J.; Sui, H.; Jia, C.; Jiang, Y.; Hong, H.; et al. Comparison of Novel PSMA-Targeting [177Lu]Lu-P17-087 with Its Albumin Binding Derivative [177Lu]Lu-P17-088 in Metastatic Castration-Resistant Prostate Cancer Patients: A First-in-Human Study. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Kramer, V.; Fernández, R.; Lehnert, W.; Jiménez-Franco, L.D.; Soza-Ried, C.; Eppard, E.; Ceballos, M.; Meckel, M.; Benešová, M.; Umbricht, C.A.; et al. Biodistribution and Dosimetry of a Single Dose of Albumin-Binding Ligand [177Lu]Lu-PSMA-ALB-56 in Patients with mCRPC. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Borgna, F.; Deberle, L.M.; Cohrs, S.; Schibli, R.; Müller, C. Combined Application of Albumin-Binding [177Lu]Lu-PSMA-ALB-56 and Fast-Cleared PSMA Inhibitors: Optimization of the Pharmacokinetics. Mol. Pharm. 2020, 17, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- De Decker, M.; Turner, J.H. Automated Module Radiolabeling of Peptides and Antibodies with Gallium-68, Lutetium-177 and Iodine-131. Cancer Biother. Radiopharm. 2012, 27, 72–76. [Google Scholar] [CrossRef]
- Iori, M.; Capponi, P.C.; Rubagotti, S.; Esposizione, L.R.; Seemann, J.; Pitzschler, R.; Dreger, T.; Formisano, D.; Grassi, E.; Fioroni, F.; et al. Labelling of 90Y- and 177Lu-DOTA-Bioconjugates for Targeted Radionuclide Therapy: A Comparison among Manual, Semiautomated, and Fully Automated Synthesis. Contrast Media Mol. Imaging 2017, 2017, 8160134. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benešová, M.; Hasler, R.; Schibli, R.; Van Der Meulen, N.P.; Müller, C. Design and Preclinical Evaluation of an Albumin-Binding PSMA Ligand for 64Cu-Based PET Imaging. Mol. Pharm. 2018, 15, 5556–5564. [Google Scholar] [CrossRef]
- Fouillet, J.; Donzé, C.; Deshayes, E.; Santoro, L.; Rubira, L.; Fersing, C. “One Method to Label Them All”: A Single Fully Automated Protocol for GMP-Compliant 68Ga Radiolabeling of PSMA-11, Transposable to PSMA-I&T and PSMA-617. Curr. Radiopharm. 2024, 17, 285–301. [Google Scholar] [CrossRef]
- Breeman, W.A.P.; Jong, M.; Visser, T.J.; Erion, J.L.; Krenning, E.P. Optimising Conditions for Radiolabelling of DOTA-Peptides with 90Y, 111In and 177Lu at High Specific Activities. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 917–920. [Google Scholar] [CrossRef]
- Velikyan, I.; Beyer, G.J.; Långström, B. Microwave-Supported Preparation of 68Ga Bioconjugates with High Specific Radioactivity. Bioconjug. Chem. 2004, 15, 554–560. [Google Scholar] [CrossRef]
- Bauwens, M.; Chekol, R.; Vanbilloen, H.; Bormans, G.; Verbruggen, A. Optimal Buffer Choice of the Radiosynthesis of 68Ga–Dotatoc for Clinical Application. Nucl. Med. Commun. 2010, 31, 753–758. [Google Scholar] [CrossRef]
- Eppard, E.; Wuttke, M.; Nicodemus, P.L.; Rösch, F. Ethanol-Based Post-Processing of Generator-Derived 68Ga Toward Kit-Type Preparation of 68Ga-Radiopharmaceuticals. J. Nucl. Med. 2014, 55, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, S.; Nehring, T.; Pichler, V.; Cardinale, J.; Mitterhauser, M.; Hacker, M.; Wadsak, W. Development and Evaluation of a Rapid Analysis for HEPES Determination in 68Ga-Radiotracers. EJNMMI Res. 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Larenkov, A.; Mitrofanov, I.; Pavlenko, E.; Rakhimov, M. Radiolysis-Associated Decrease in Radiochemical Purity of 177Lu-Radiopharmaceuticals and Comparison of the Effectiveness of Selected Quenchers against This Process. Molecules 2023, 28, 1884. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ellars, C.E.; Edwards, D.S. Ascorbic Acid: Useful as a Buffer Agent and Radiolytic Stabilizer for Metalloradiopharmaceuticals. Bioconjug. Chem. 2003, 14, 1052–1056. [Google Scholar] [CrossRef]
- Schötzig, U.; Schrader, H.; Schönfeld, E.; Günther, E.; Klein, R. Standardisation and Decay Data of 177Lu and 188Re. Appl. Radiat. Isot. 2001, 55, 89–96. [Google Scholar] [CrossRef]
- Evans, C.H. The Interaction of Lanthanides with Amino Acids and Proteins. In Biochemistry of the Lanthanides; Springer: Boston, MA, USA, 1990; pp. 85–172. ISBN 978-1-4684-8750-3. [Google Scholar]
- Mardani-Ghahfarokhi, A.; Farhoosh, R. Antioxidant Activity and Mechanism of Inhibitory Action of Gentisic and α-Resorcylic Acids. Sci. Rep. 2020, 10, 19487. [Google Scholar] [CrossRef]
- Tofe, A.J.; Bevan, J.A.; Fawzi, M.B.; Francis, M.D.; Silberstein, E.B.; Alexander, G.A.; Gunderson, D.E.; Blair, K. Gentisic Acid: A New Stabilizer for Low Tin Skeletal Imaging Agents: Concise Communication. J. Nucl. Med. 1980, 21, 366–370. [Google Scholar]
- European Medicines Agency, Edotreotide (SOMAKIT TOC): EPAR—Product Information. Available online: https://www.ema.europa.eu/en/Documents/Product-Information/Somakit-Toc-Epar-Product-Information_en.pdf (accessed on 14 April 2025).
- U.S. Food and Drug Administration (FDA), NetSpot (Kit for the Preparation of Gallium Ga 68 Dotatate Injection): Product Information. Available online: https://www.accessdata.fda.Gov/Drugsatfda_docs/Label/2023/208547s027lbl.pdf (accessed on 14 April 2025).
- De Zanger, R.M.S.; Chan, H.S.; Breeman, W.A.P.; De Blois, E. Maintaining Radiochemical Purity of [177Lu]Lu-DOTA-PSMA-617 for PRRT by Reducing Radiolysis. J. Radioanal. Nucl. Chem. 2019, 321, 285–291. [Google Scholar] [CrossRef]
- Baum, R.P.; Schuchardt, C.; Singh, A.; Chantadisai, M.; Robiller, F.C.; Zhang, J.; Mueller, D.; Eismant, A.; Almaguel, F.; Zboralski, D.; et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using 177Lu-FAP-2286: First-in-Humans Results. J. Nucl. Med. 2022, 63, 415–423. [Google Scholar] [CrossRef]
- Haskali, M.B. Automated Preparation of Clinical Grade [68Ga]Ga-DOTA-CP04, a Cholecystokinin-2 Receptor Agonist, Using iPHASE MultiSyn Synthesis Platform. EJNMMI Radiopharm. Chem. 2019, 4, 23. [Google Scholar] [CrossRef]
- Breeman, W.A.P.; Fröberg, A.C.; De Blois, E.; Van Gameren, A.; Melis, M.; De Jong, M.; Maina, T.; Nock, B.A.; Erion, J.L.; Mäcke, H.R.; et al. Optimised Labeling, Preclinical and Initial Clinical Aspects of CCK-2 Receptor-Targeting with 3 Radiolabeled Peptides. Nucl. Med. Biol. 2008, 35, 839–849. [Google Scholar] [CrossRef]
- Chen, J.; Linder, K.E.; Cagnolini, A.; Metcalfe, E.; Raju, N.; Tweedle, M.F.; Swenson, R.E. Synthesis, Stabilization and Formulation of [177Lu]Lu-AMBA, a Systemic Radiotherapeutic Agent for Gastrin Releasing Peptide Receptor Positive Tumors. Appl. Radiat. Isot. 2008, 66, 497–505. [Google Scholar] [CrossRef]
- Trindade, V.; Balter, H. Oxidant and Antioxidant Effects of Gentisic Acid in a 177Lu-Labelled Methionine-Containing Minigastrin Analogue. Curr. Radiopharm. 2020, 13, 107–119. [Google Scholar] [CrossRef]
- Wichmann, C.W.; Ackermann, U.; Poniger, S.; Young, K.; Nguyen, B.; Chan, G.; Sachinidis, J.; Scott, A.M. Automated Radiosynthesis of [68Ga]Ga-PSMA-11 and [177Lu]Lu-PSMA-617 on the iPHASE MultiSyn Module for Clinical Applications. J. Labelled Comp. Radiopharm. 2021, 64, 140–146. [Google Scholar] [CrossRef]
- Sørensen, M.A.; Andersen, V.L.; Hendel, H.W.; Vriamont, C.; Warnier, C.; Masset, J.; Huynh, T.H.V. Automated Synthesis of 68Ga/177Lu-PSMA on the Trasis miniAllinOne. J. Labelled Comp. Radiopharm. 2020, 63, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.A.; Van Der Wansem, K.; Bernard, B.F.; Van Gameren, A.; Erion, J.L.; Visser, T.J.; Krenning, E.P.; De Jong, M. The Addition of DTPA to [177Lu-DOTA0,Tyr3]Octreotate Prior to Administration Reduces Rat Skeleton Uptake of Radioactivity. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, A.; Balzer, M.; Amthauer, H.; Brenner, W.; Spreckelmeyer, S. Optimization of 177Lu-Labelling of DOTA-TOC, PSMA-I&T and FAPI-46 for Clinical Application. EJNMMI Radiopharm. Chem. 2023, 8, 10. [Google Scholar] [CrossRef]
- De Blois, E.; Sze Chan, H.; Konijnenberg, M.; De Zanger, R.; AP Breeman, W. Effectiveness of Quenchers to Reduce Radiolysis of 111In- or 177Lu-Labelled Methionine-Containing Regulatory Peptides. Maintaining Radiochemical Purity as Measured by HPLC. Curr. Top. Med. Chem. 2013, 12, 2677–2685. [Google Scholar] [CrossRef]
- Mu, L.; Hesselmann, R.; Oezdemir, U.; Bertschi, L.; Blanc, A.; Dragic, M.; Löffler, D.; Smuda, C.; Johayem, A.; Schibli, R. Identification, Characterization and Suppression of Side-Products Formed during the Synthesis of High Dose 68Ga-DOTA-TATE. Appl. Radiat. Isot. 2013, 76, 63–69. [Google Scholar] [CrossRef]
- Di Iorio, V.; Boschi, S.; Cuni, C.; Monti, M.; Severi, S.; Paganelli, G.; Masini, C. Production and Quality Control of [177Lu]Lu-PSMA-I&T: Development of an Investigational Medicinal Product Dossier for Clinical Trials. Molecules 2022, 27, 4143. [Google Scholar] [CrossRef]
- Martin, S.; Tönnesmann, R.; Hierlmeier, I.; Maus, S.; Rosar, F.; Ruf, J.; Holland, J.P.; Ezziddin, S.; Bartholomä, M.D. Identification, Characterization, and Suppression of Side Products Formed during the Synthesis of [177Lu]Lu-PSMA-617. J. Med. Chem. 2021, 64, 4960–4971. [Google Scholar] [CrossRef]
- Chakraborty, S.; Vimalnath, K.V.; Chakravarty, R.; Sarma, H.D.; Dash, A. Multidose Formulation of Ready-to-Use 177Lu-PSMA-617 in a Centralized Radiopharmacy Set-Up. Appl. Radiat. Isot. 2018, 139, 91–97. [Google Scholar] [CrossRef]
- Eryilmaz, K.; Kilbas, B. Fully-Automated Synthesis of 177Lu Labelled FAPI Derivatives on the Module Modular Lab-Eazy. EJNMMI Radiopharm. Chem. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Aslani, A.; Snowdon, G.M.; Bailey, D.L.; Schembri, G.P.; Bailey, E.A.; Pavlakis, N.; Roach, P.J. Lutetium-177 DOTATATE Production with an Automated Radiopharmaceutical Synthesis System. Asia Ocean. J. Nucl. Med. Biol. 2015, 3, 107–115. [Google Scholar] [PubMed]
- Kraihammer, M.; Garnuszek, P.; Bauman, A.; Maurin, M.; Alejandre Lafont, M.; Haubner, R.; Von Guggenberg, E.; Gabriel, M.; Decristoforo, C. Improved Quality Control of [177Lu]Lu-PSMA I&T. EJNMMI Radiopharm. Chem. 2023, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Hooijman, E.L.; Ntihabose, C.M.; Reuvers, T.G.A.; Nonnekens, J.; Aalbersberg, E.A.; van de Merbel, J.R.J.P.; Huijmans, J.E.; Koolen, S.L.W.; Hendrikx, J.J.M.A.; de Blois, E. Radiolabeling and Quality Control of Therapeutic Radiopharmaceuticals: Optimization, Clinical Implementation and Comparison of Radio-TLC/HPLC Analysis, Demonstrated by [177Lu]Lu-PSMA. EJNMMI Radiopharm. Chem. 2022, 7, 29. [Google Scholar] [CrossRef]
- Greifenstein, L.; Gunkel, A.; Hoehne, A.; Osterkamp, F.; Smerling, C.; Landvogt, C.; Mueller, C.; Baum, R.P. 3BP-3940, a Highly Potent FAP-Targeting Peptide for Theranostics—Production, Validation and First in Human Experience with Ga-68 and Lu-177. iScience 2023, 26, 108541. [Google Scholar] [CrossRef]
- Guleria, M.; Amirdhanayagam, J.; Sarma, H.D.; Rallapeta, R.P.; Krishnamohan, V.S.; Nimmagadda, A.; Ravi, P.; Patri, S.; Kalawat, T.; Das, T. Preparation of 177Lu-PSMA-617 in Hospital Radiopharmacy: Convenient Formulation of a Clinical Dose Using a Single-Vial Freeze-Dried PSMA-617 Kit Developed In-House. BioMed Res. Int. 2021, 2021, 1555712. [Google Scholar] [CrossRef]
- Liu, S.; Edwards, D.S. Stabilization of90 Y-Labeled DOTA-Biomolecule Conjugates Using Gentisic Acid and Ascorbic Acid. Bioconjug. Chem. 2001, 12, 554–558. [Google Scholar] [CrossRef]
- Maus, S.; Blois, E.D.; Ament, S.J.; Schreckenberger, M.; Breeman, W.A.P. Aspects on Radiolabeling of 177Lu-DOTA-TATE: After C18 Purification Re-Addition of Ascorbic Acid Is Required to Maintain Radiochemical Purity. Int. J. Diagn. Imaging 2014, 1, 5. [Google Scholar] [CrossRef]
- Fukumura, T.; Nakao, R.; Yamaguchi, M.; Suzuki, K. Stability of 11C-Labeled PET Radiopharmaceuticals. Appl. Radiat. Isot. 2004, 61, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. General Chapter, Injections and Implanted Drug Products (Parenterals)—Product Quality Tests; United States Pharmacopeia: Rockville, MD, USA, 2024. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Healthcare (EDQM). Parenteral Preparations. In European Pharmacopoeia 11.8; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2025; Volume 0520, pp. 6880–6882. [Google Scholar]
- European Directorate for the Quality of Medicines & Healthcare (EDQM). Radiopharmaceutical Preparations. In European Pharmacopoeia 11.8; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2025; Volume 0125, pp. 6857–6860. [Google Scholar]
- Shin, D.; Ha, S.; O, J.H.; Rhew, S.A.; Yoon, C.E.; Kwon, H.J.; Moon, H.W.; Park, Y.H.; Park, S.Y.; Park, C.; et al. A Single Dose of Novel PSMA-Targeting Radiopharmaceutical Agent [177Lu]Ludotadipep for Patients with Metastatic Castration-Resistant Prostate Cancer: Phase I Clinical Trial. Cancers 2022, 14, 6225. [Google Scholar] [CrossRef]
- Ritt, P.; Fernández, R.; Soza-Ried, C.; Nicolai, H.; Amaral, H.; Krieger, K.; Mapanao, A.K.; Rotger, A.; Zhernosekov, K.; Schibli, R.; et al. Biodistribution and Dosimetry of [177Lu]Lu-SibuDAB in Patients with Metastatic Castration-Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2431–2443. [Google Scholar] [CrossRef]
- Guleria, M.; Das, T.; Amirdhanayagam, J.; Sarma, H.D.; Dash, A. Preparation of [177Lu]Lu-DOTA-Ahx-Lys40-Exendin-4 for Radiotherapy of Insulinoma: A Detailed Insight into the Radiochemical Intricacies. Nucl. Med. Biol. 2019, 78–79, 31–40. [Google Scholar] [CrossRef]
- Smith, C.J.; Gali, H.; Sieckman, G.L.; Hayes, D.L.; Owen, N.K.; Mazuru, D.G.; Volkert, W.A.; Hoffman, T.J. Radiochemical Investigations of 177Lu-DOTA-8-Aoc-BBN [7-14]NH2: An in Vitro/in Vivo Assessment of the Targeting Ability of This New Radiopharmaceutical for PC-3 Human Prostate Cancer Cells. Nucl. Med. Biol. 2003, 30, 101–109. [Google Scholar] [CrossRef]
- Das, T.; Chakraborty, S.; Banerjee, S.; Mukherjee, A.; Samuel, G.; Sarma, H.D.; Nair, C.K.K.; Kagiya, V.T.; Venkatesh, M. Preparation and Preliminary Biological Evaluation of a 177Lu Labeled Sanazole Derivative for Possible Use in Targeting Tumor Hypoxia. Bioorg. Med. Chem. 2004, 12, 6077–6084. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, T.; Sarma, H.D.; Dash, A. Preparation and Evaluation of 177Lu-Labeled Gemcitabine: An Effort Toward Developing Radiolabeled Chemotherapeutics for Targeted Therapy Applications. Cancer Biother. Radiopharm. 2017, 32, 239–246. [Google Scholar] [CrossRef]
- Ghosh, A.; Woolum, K.; Kothandaraman, S.; Tweedle, M.F.; Kumar, K. Stability Evaluation and Stabilization of a Gastrin-Releasing Peptide Receptor (GRPR) Targeting Imaging Pharmaceutical. Molecules 2019, 24, 2878. [Google Scholar] [CrossRef]
- Liu, S.; Edwards, D.S. Bifunctional Chelators for Therapeutic Lanthanide Radiopharmaceuticals. Bioconjug. Chem. 2001, 12, 7–34. [Google Scholar] [CrossRef]
- Marin, G.; Vanderlinden, B.; Karfis, I.; Guiot, T.; Wimana, Z.; Flamen, P.; Vandenberghe, S. Accuracy and Precision Assessment for Activity Quantification in Individualized Dosimetry of 177Lu-DOTATATE Therapy. EJNMMI Phys. 2017, 4, 7. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Healthcare (EDQM). Lutetium (177Lu) Solution for Radiolabelling. In European Pharmacopoeia 11.0; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2017; Volume 2798, pp. 1286–1287. [Google Scholar]
- European Directorate for the Quality of Medicines & Healthcare (EDQM). Table of Physical Characteristics of Radionucleides Mentioned in the European Pharmacopoeia. In European Pharmacopoeia 11.0; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2008; Volume 50700, pp. 781–787. [Google Scholar]
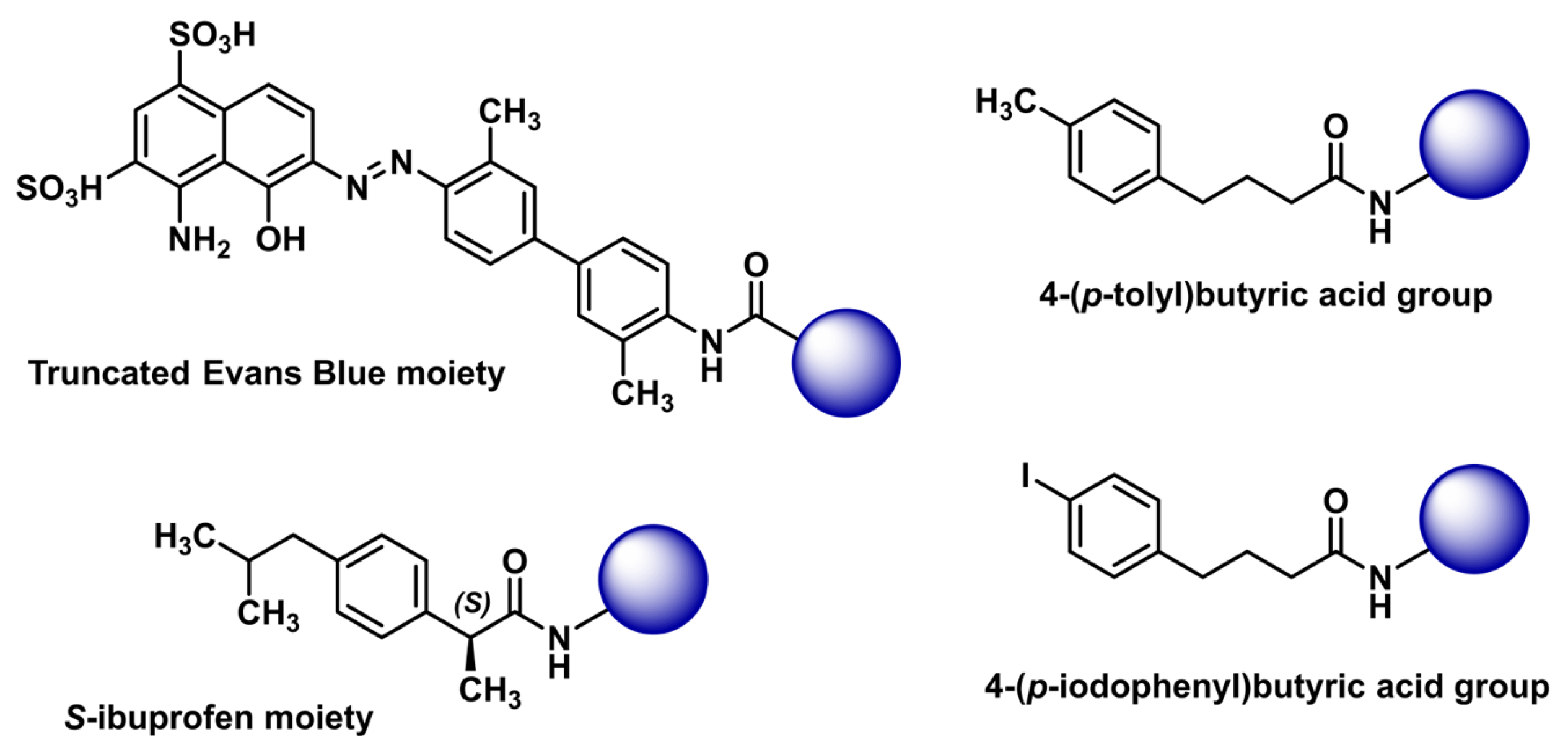
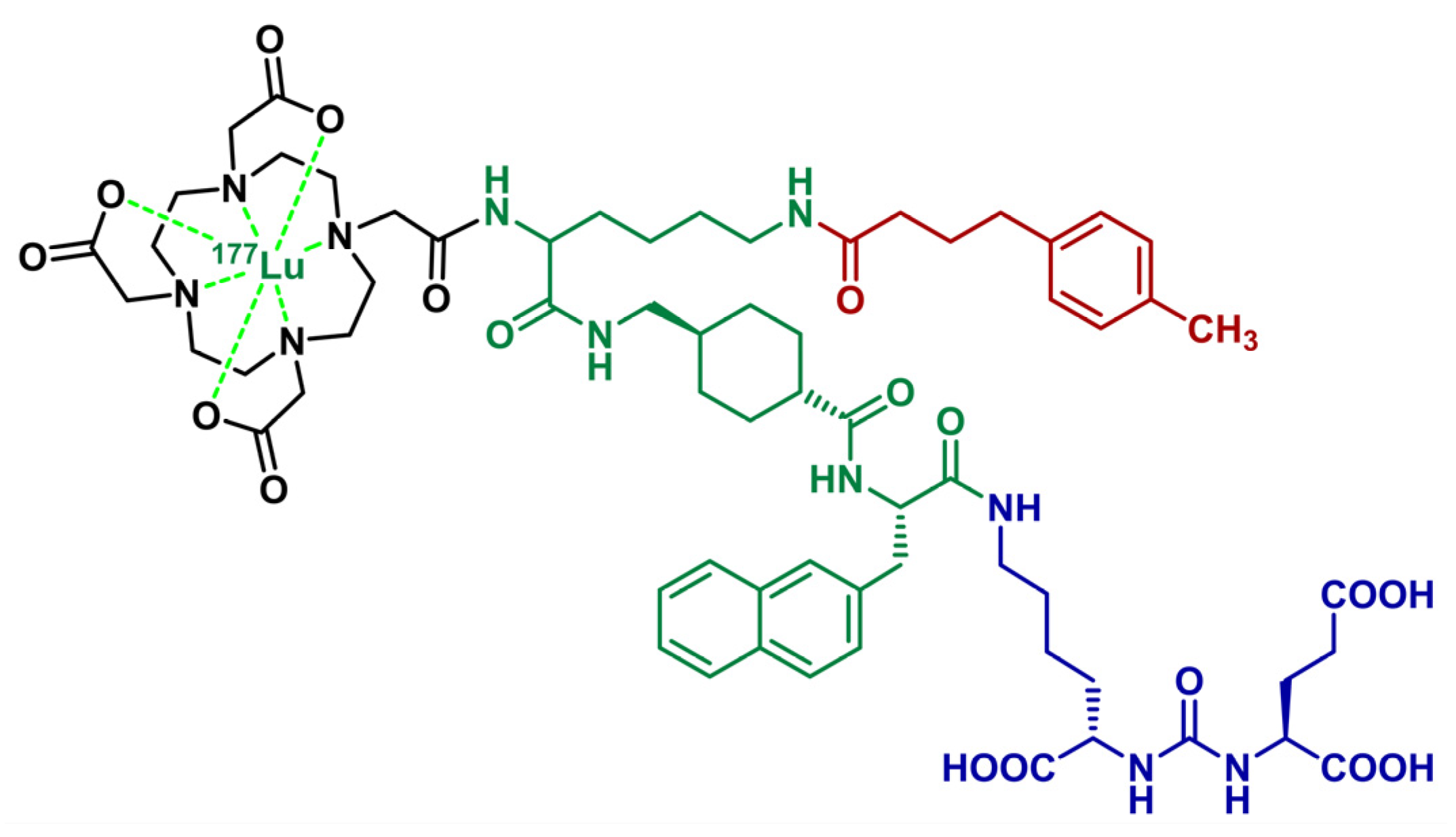
| Test | Batch 1 | Batch 2 | Batch 3 |
|---|---|---|---|
| Appearance | Clear, colorless solution | Clear, colorless solution | Clear, colorless solution |
| Identification | |||
| Energy of gamma photons (keV) | 113 and 208 | 113 and 208 | 113 and 208 |
| Half-life (days) | 6.86 ± 0.16 | 6.76 ± 0.10 | 7.00 ± 0.31 |
| pH | 4.5 | 4.5 | 4.5 |
| Radionuclidic purity | |||
| (177Lu) Lutetium (%) | 100 | 100 | 100 |
| γ-Emitting impurities | None identified | None identified | None identified |
| Radiochemical purity (HPLC) | |||
| [177Lu]Lu-PSMA-ALB-56 (%) | 98.96 | 99.02 | 98.79 |
| [177Lu]lutetium impurities (%) | 1.04 | 0.98 | 1.21 |
| Filter integrity test | >3500 mbar | >3500 mbar | >3500 mbar |
| Volume activity at EoS (MBq/mL) | 101.49 | 100.99 | 91.78 |
| Specific activity at EoS (MBq/µg) | 31.21 | 31.08 | 28.18 |
| Molar activity at EoS (GBq/µmol) | 41.53 | 41.35 | 37.49 |
| Ethanol amount (%, calculated) | ~8.9 | ~8.9 | ~8.9 |
| Ascorbic acid concentration (mg/mL, calculated) | ~3.6 | ~3.6 | ~3.6 |
| Radiochemical yield (%) (Based on RCP determined by HPLC) | 79.46 | 81.85 | 78.05 |
| Stability over 120 h (HPLC) | ≥95% | ≥96% | ≥96% |
| Buffer | Molarity | pH | Volume Set for Reaction | References |
|---|---|---|---|---|
| HEPES | 1.25 M | 4.5 | 160 µL | [91] |
| Ascorbate | 1.8 M | 4.5 | 160 µL | [75] |
| Ammonium acetate | 0.1 M | 4.5 | 160 µL | [92,93] |
| Ammonium acetate | 1 M | 4.5 | 160 µL | [94] |
| Sodium acetate | 0.1 M | 4.5 | 160 µL | [95] |
| Sodium acetate | 0.5 M | 4.5 | 160 µL | [11] |
| AR compound | Concentration (initial) | Volume added to reaction | References | |
| Ascorbic acid | 13 mg/mL | 10 µL | [54,65] | |
| Gentisic acid | 12 mg/mL | 10 µL | [96] | |
| Cysteine | 36 mg/mL | 10 µL | [53] | |
| Methionine | 30 mg/mL | 10 µL | [64] | |
| Initial Protocol Without SPE Purification | |
|---|---|
| 1 | Kit integrity test (pressurization > 1500 mbar and pressure reduction of no more than 400 mbar over 20 s). |
| 2 | Transfer of PSMA-ALB-56 solubilized in buffer to the 177Lu vial, then transfer back to the reaction vial. |
| 3 | Pre-heating of the reaction vial (60 °C). |
| 4 | Rinsing of the 177Lu vial with 1.4 mL HCl 3.6 mM, then transfer back to the reaction vial. |
| 5 | Tubing purge with filtered air. |
| 6 | Labeling at 95 °C during 15 min. |
| 7 | During labeling, rinsing and purge of manifold B. |
| 8 | End of radiolabeling: addition of 3 mL DTPA 1 mg/mL to the reaction medium. |
| 9 | Transfer of the reaction mixture from the reaction vial to the product vial |
| 10 | Reaction vial washing with 7 mL DTPA 1 mg/mL and transfer from the reaction vial to the product vial. |
| 11 | Product vial release and filter integrity testing |
| 12 | Closing all valves, end of synthesis |
| Optimized protocol with SPE purification | |
| 1 | Kit integrity test (pressurization > 1500 mbar and pressure reduction of no more than 400 mbar over 20 s). |
| 2 | Transfer of PSMA-ALB-56 solubilized in buffer to the 177Lu vial, then transfer back to the reaction vial. |
| 3 | Pre-heating of the reaction vial (60 °C). |
| 4 | Rinsing of the 177Lu vial with 1.0 mL WFI, then transfer back to the reaction vial. |
| 5 | Tubing purge with filtered air. |
| 6 | Labeling at 95 °C during 15 min. |
| 7 | Transfer of the reaction mixture from the reaction vial to the SPE cartridge |
| 8 | Washing of the reaction vial with 10 mL WFI and transfer from the reaction vial onto the SPE cartridge |
| 9 | SPE cartridge rinsing with WFI and purging of the tubing with filtered air |
| 10 | [177Lu]Lu-PSMA-ALB-56 elution to the terminal vial with alternating ethanol 60% (total 3 mL) and saline |
| 11 | Formulation of the final product with remaining NaCl 0.9% (total elution + formulation = 17.2 mL) |
| 12 | Product vial release and filter integrity testing |
| 13 | Closing all valves, end of synthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanney, J.; Rubira, L.; Torchio, J.; Fersing, C. Tailored Reaction Conditions and Automated Radiolabeling of [177Lu]Lu-PSMA-ALB-56 in a 68Ga Setting: The Critical Impact of Antioxidant Concentrations. Int. J. Mol. Sci. 2025, 26, 9642. https://doi.org/10.3390/ijms26199642
Vanney J, Rubira L, Torchio J, Fersing C. Tailored Reaction Conditions and Automated Radiolabeling of [177Lu]Lu-PSMA-ALB-56 in a 68Ga Setting: The Critical Impact of Antioxidant Concentrations. International Journal of Molecular Sciences. 2025; 26(19):9642. https://doi.org/10.3390/ijms26199642
Chicago/Turabian StyleVanney, Johanne, Léa Rubira, Jade Torchio, and Cyril Fersing. 2025. "Tailored Reaction Conditions and Automated Radiolabeling of [177Lu]Lu-PSMA-ALB-56 in a 68Ga Setting: The Critical Impact of Antioxidant Concentrations" International Journal of Molecular Sciences 26, no. 19: 9642. https://doi.org/10.3390/ijms26199642
APA StyleVanney, J., Rubira, L., Torchio, J., & Fersing, C. (2025). Tailored Reaction Conditions and Automated Radiolabeling of [177Lu]Lu-PSMA-ALB-56 in a 68Ga Setting: The Critical Impact of Antioxidant Concentrations. International Journal of Molecular Sciences, 26(19), 9642. https://doi.org/10.3390/ijms26199642







