Ultra-Fast Intraoperative IDH-Mutation Analysis Enables Rapid Stratification and Therapy Planning in Diffuse Gliomas
Abstract
1. Introduction
2. Results
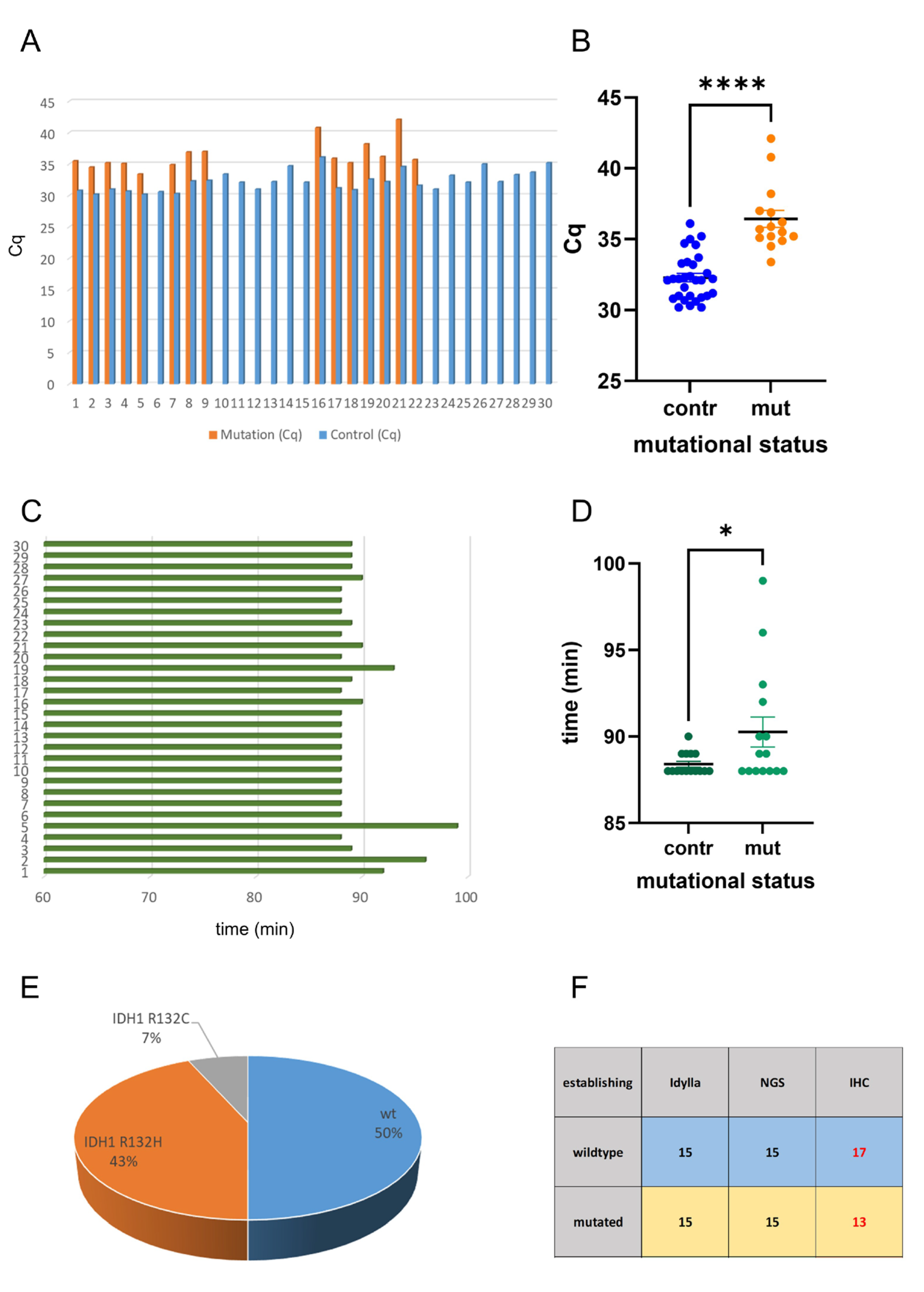
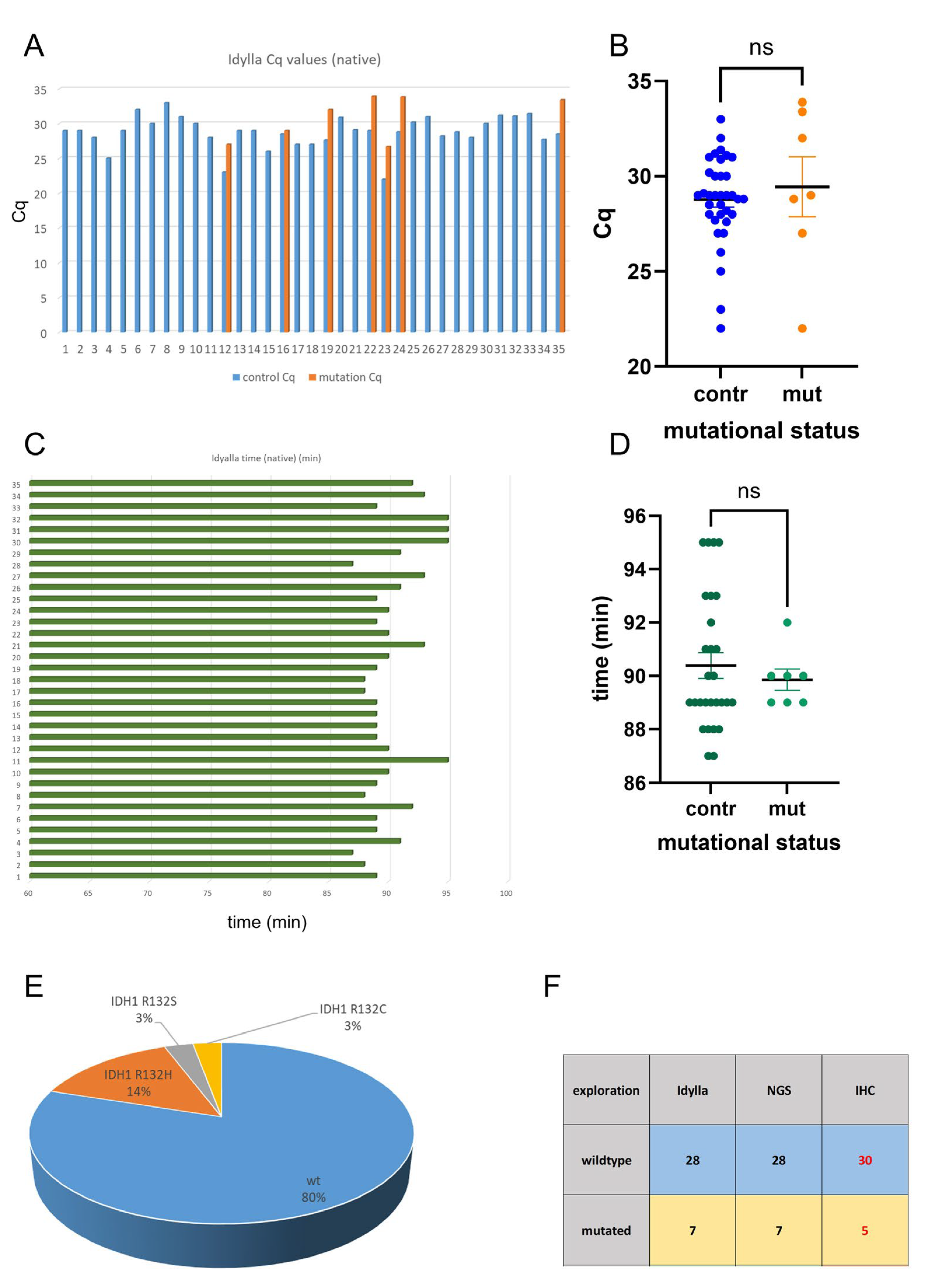
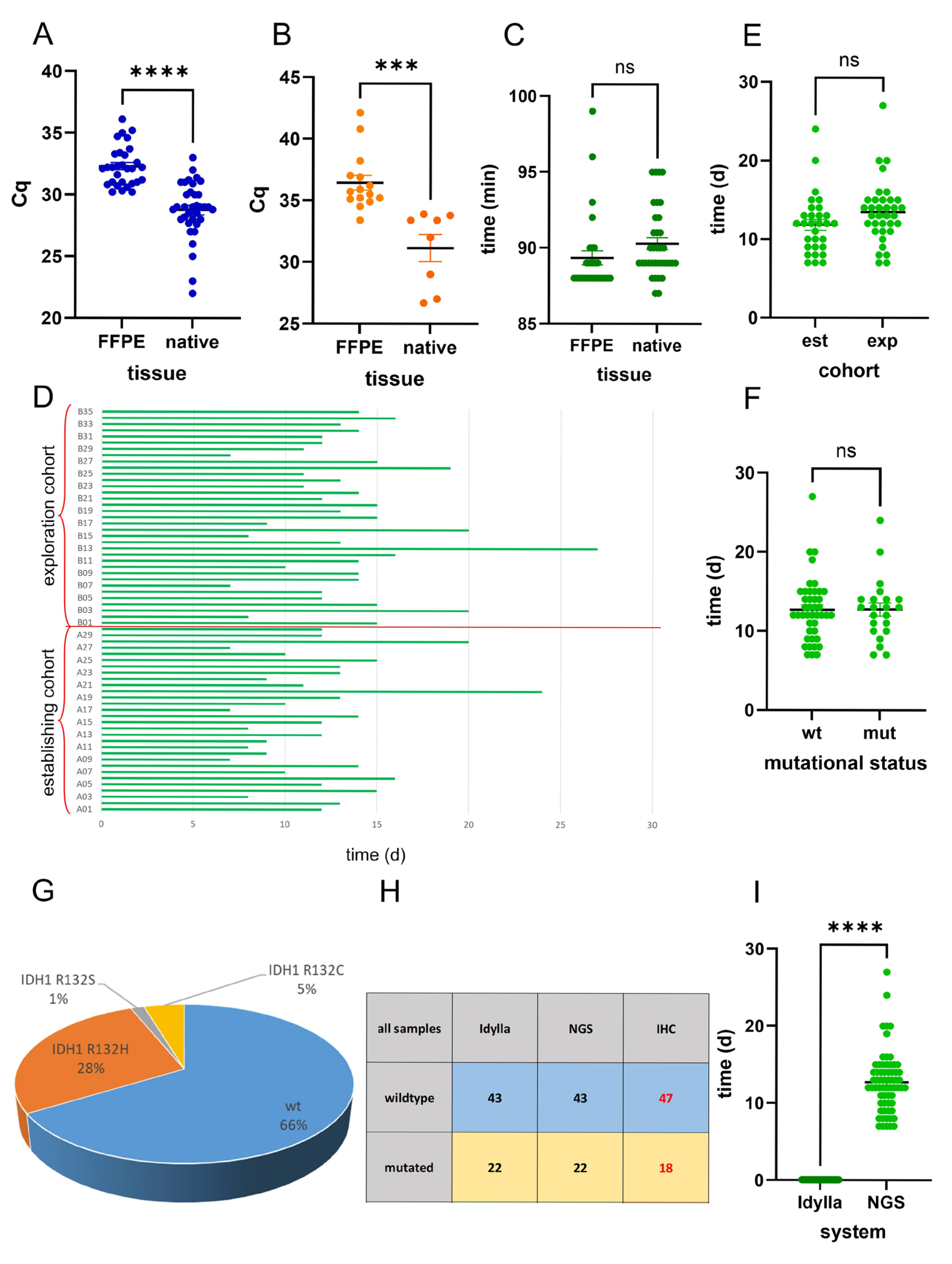
2.1. Idylla IDH Mutation Assay Delivers Accurate Results on FFPE Tissue
2.2. Analysis of Intraoperative Snap-Frozen and Fresh Samples Delivers Fast and Valid Results When Applying the Idylla IDH-Mutation Assay
2.3. Idylla IDH-Mutation Analysis Enables Ultra-Fast and Reliable IDH-Mutation Detection Compared with Routine NGS Analysis
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Molecular Genetic Characterization of Gliomas
4.3. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LGG | low-grade glioma |
| HGG | high-grade glioma |
| GBM | glioblastoma |
| IDH | isocitrate dehydrogenase |
| αKG | α-ketoglutarate |
| 2-HG | 2-hydroxyglutarate |
| LOH1p/19q | loss of heterozygosity of chromosome 1p and 19q |
| MGMT | O6-methylguanine-DNA methyltransferase |
| NGS | next-generation sequencing |
| ddPCR | digital droplet PCR |
| FFPE | formalin-fixed, paraffin-embedded |
| IHC | immunohistochemistry |
| BRAF | B-Raf proto-oncogene serine/threonine kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| NRAS | neuroblastoma RAS viral oncogene homolog |
| EGFR | epidermal growth factor receptor |
| dMMR | mismatch-repair deficiency |
| TERT | telomerase reverse transcriptase |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mallela, A.N.; Shi, D.D.; Tang, L.W.; Abou-Al-Shaar, H.; Gersey, Z.C.; Zhang, X.; McBrayer, S.K.; Abdullah, K.G. Isocitrate dehydrogenase mutations in gliomas: A review of current understanding and trials. Neuro-Oncol. Adv. 2023, 5, vdad053. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef]
- Lu, C.; Ward, P.S.; Kapoor, G.S.; Rohle, D.; Turcan, S.; Abdel-Wahab, O.; Edwards, C.R.; Khanin, R.; Figueroa, M.E.; Melnick, A.; et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012, 483, 474–478. [Google Scholar] [CrossRef]
- Sasaki, M.; Knobbe, C.B.; Munger, J.C.; Lind, E.F.; Brenner, D.; Brustle, A.; Harris, I.S.; Holmes, R.; Wakeham, A.; Haight, J.; et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 2012, 488, 656–659. [Google Scholar] [CrossRef]
- Baldewpersad Tewarie, N.M.; Burgers, I.A.; Dawood, Y.; den Boon, H.C.; den Brok, M.G.; Klunder, J.H.; Koopmans, K.B.; Rademaker, E.; van den Broek, H.B.; van den Bersselaar, S.M.; et al. NADP+ -dependent IDH1 R132 mutation and its relevance for glioma patient survival. Med. Hypotheses 2013, 80, 728–731. [Google Scholar] [CrossRef]
- Bleeker, F.E.; Atai, N.A.; Lamba, S.; Jonker, A.; Rijkeboer, D.; Bosch, K.S.; Tigchelaar, W.; Troost, D.; Vandertop, W.P.; Bardelli, A.; et al. The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010, 119, 487–494. [Google Scholar] [CrossRef]
- Sciuscio, D.; Diserens, A.C.; van Dommelen, K.; Martinet, D.; Jones, G.; Janzer, R.C.; Pollo, C.; Hamou, M.F.; Kaina, B.; Stupp, R.; et al. Extent and patterns of MGMT promoter methylation in glioblastoma- and respective glioblastoma-derived spheres. Clin. Cancer Res. 2011, 17, 255–266. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Neyns, B.; Goldbrunner, R.; Schlegel, U.; Clement, P.M.; Grabenbauer, G.G.; Ochsenbein, A.F.; Simon, M.; Dietrich, P.Y.; et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010, 28, 2712–2718. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef]
- Hegi, M.E.; Sciuscio, D.; Murat, A.; Levivier, M.; Stupp, R. Epigenetic deregulation of DNA repair and its potential for therapy. Clin. Cancer Res. 2009, 15, 5026–5031. [Google Scholar] [CrossRef]
- Kaina, B.; Christmann, M.; Naumann, S.; Roos, W.P. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 2007, 6, 1079–1099. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Lu, M.; Wen, P.Y.; Taylor, J.W.; Maher, E.A.; Arrillaga-Romany, I.; Peters, K.B.; Ellingson, B.M.; Rosenblum, M.K.; Chun, S.; et al. Vorasidenib and ivosidenib in IDH1-mutant low-grade glioma: A randomized, perioperative phase 1 trial. Nat. Med. 2023, 29, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Mellinghoff, I.K.; van den Bent, M.J.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023, 389, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, H.G.; Weller, M. The Role of Molecular Diagnostics in the Management of Patients with Gliomas. Curr. Treat. Options Oncol. 2016, 17, 51. [Google Scholar] [CrossRef]
- Wolter, M.; Felsberg, J.; Malzkorn, B.; Kaulich, K.; Reifenberger, G. Droplet digital PCR-based analyses for robust, rapid, and sensitive molecular diagnostics of gliomas. Acta Neuropathol. Commun. 2022, 10, 42. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.Y.; Li, J.F.; Guo, C.C.; Chen, F.R.; Su, H.K.; Zhao, H.F.; Long, Y.K.; Shao, J.Y.; To, S.; et al. IDH1 mutation detection by droplet digital PCR in glioma. Oncotarget 2015, 6, 39651–39660. [Google Scholar] [CrossRef]
- Satomi, K.; Yoshida, A.; Matsushita, Y.; Sugino, H.; Fujimoto, K.; Honda-Kitahara, M.; Takahashi, M.; Ohno, M.; Miyakita, Y.; Narita, Y.; et al. Clinical application of a highly sensitive digital PCR assay to detect a small fraction of IDH1 R132H-mutant alleles in diffuse gliomas. Brain Tumor Pathol. 2022, 39, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Zacher, A.; Kaulich, K.; Stepanow, S.; Wolter, M.; Kohrer, K.; Felsberg, J.; Malzkorn, B.; Reifenberger, G. Molecular Diagnostics of Gliomas Using Next Generation Sequencing of a Glioma-Tailored Gene Panel. Brain Pathol. 2017, 27, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Claes, B.; Huang, H.J.; Falchook, G.S.; Devogelaere, B.; Kockx, M.; Bempt, I.V.; Reijans, M.; Naing, A.; Fu, S.; et al. BRAF mutation testing with a rapid, fully integrated molecular diagnostics system. Oncotarget 2015, 6, 26886–26894. [Google Scholar] [CrossRef]
- Zekri, J.; Baghdadi, M.A.; Alardati, H.; Khallaf, H.; Kabanja, J.H. Evaluation of the Idylla KRAS and NRAS mutation test in colorectal cancer tissue. Exp. Mol. Pathol. 2019, 110, 104270. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Garcia, M.; Weynand, B.; Gomez-Izquierdo, L.; Hernandez, M.J.; Blanco, A.M.; Varela, M.; Matias-Guiu, X.; Nadal, E.; Marquez-Lobo, B.; Alarcao, A.; et al. Clinical performance evaluation of the Idylla EGFR Mutation Test on formalin-fixed paraffin-embedded tissue of non-small cell lung cancer. BMC Cancer 2020, 20, 275. [Google Scholar] [CrossRef]
- Mendiola, M.; Heredia-Soto, V.; Ruz-Caracuel, I.; Baillo, A.; Ramon-Patino, J.L.; Berjon, A.; Escudero, F.J.; Pelaez-Garcia, A.; Hernandez, A.; Feliu, J.; et al. Performance of the Idylla microsatellite instability test in endometrial cancer. Mol. Cell. Probes 2024, 77, 101976. [Google Scholar] [CrossRef]
- Zwaenepoel, K.; Holmgaard Duelund, J.; De Winne, K.; Maes, V.; Weyn, C.; Lambin, S.; Dendooven, R.; Broeckx, G.; Steiniche, T.; Pauwels, P. Clinical Performance of the Idylla MSI Test for a Rapid Assessment of the DNA Microsatellite Status in Human Colorectal Cancer. J. Mol. Diagn. 2020, 22, 386–395. [Google Scholar] [CrossRef]
- Farmkiss, L.; Hopkins, I.; Jones, M. Idylla microsatellite instability assay versus mismatch repair immunohistochemistry: A retrospective comparison in gastric adenocarcinoma. J. Clin. Pathol. 2021, 74, 604–607. [Google Scholar] [CrossRef]
- Kraus, T.F.J.; Machegger, L.; Poppe, J.; Zellinger, B.; Dovjak, E.; Schlicker, H.U.; Schwartz, C.; Ladisich, B.; Spendel, M.; Kral, M.; et al. Diffuse midline glioma of the cervical spinal cord with H3 K27M genotype phenotypically mimicking anaplastic ganglioglioma: A case report and review of the literature. Brain Tumor Pathol. 2020, 37, 89–94. [Google Scholar] [CrossRef]
- Kraus, T.F.J.; Poppe, J.; Machegger, L.; Zellinger, B.; Dovjak, E.; Schlicker, H.U.; Schwartz, C.; Ladisich, B.; Spendel, M.; Al-Schameri, A.R.; et al. Genotypical glioblastoma of the frontal lobe mimicking ganglioglioma: A case report and review of the literature. Clin. Case Rep. 2021, 9, e04544. [Google Scholar] [CrossRef]
- Kraus, T.F.J.; Schwartz, C.; Machegger, L.; Zellinger, B.; Holzl, D.; Schlicker, H.U.; Poppe, J.; Ladisich, B.; Spendel, M.; Kral, M.; et al. A patient with two gliomas with independent oligodendroglioma and glioblastoma biology proved by DNA-methylation profiling: A case report and review of the literature. Brain Tumor Pathol. 2022, 39, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.F.; Greiner, A.; Steinmaurer, M.; Dietinger, V.; Guibourt, V.; Kretzschmar, H.A. Genetic Characterization of Ten-Eleven-Translocation Methylcytosine Dioxygenase Alterations in Human Glioma. J. Cancer 2015, 6, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.F.; Kolck, G.; Greiner, A.; Schierl, K.; Guibourt, V.; Kretzschmar, H.A. Loss of 5-hydroxymethylcytosine and intratumoral heterogeneity as an epigenomic hallmark of glioblastoma. Tumor Biol. 2015, 36, 8439–8446. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.F.; Guibourt, V.; Kretzschmar, H.A. 5-Hydroxymethylcytosine, the “Sixth Base”, during brain development and ageing. J. Neural Transm. 2014, 122, 1035–1043. [Google Scholar] [CrossRef]
- Wesseling, P.; Capper, D. WHO 2016 Classification of gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef]
- Hartmann, C.; Meyer, J.; Balss, J.; Capper, D.; Mueller, W.; Christians, A.; Felsberg, J.; Wolter, M.; Mawrin, C.; Wick, W.; et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: A study of 1,010 diffuse gliomas. Acta Neuropathol. 2009, 118, 469–474. [Google Scholar] [CrossRef]
- Reuss, D.E.; Sahm, F.; Schrimpf, D.; Wiestler, B.; Capper, D.; Koelsche, C.; Schweizer, L.; Korshunov, A.; Jones, D.T.; Hovestadt, V.; et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015, 129, 133–146. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Figrarella-Branger, D.; Fuller, G.N.; Giannini, C.; Holland, E.C.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Komori, T.; et al. cIMPACT-NOW update 5: Recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020, 139, 603–608. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. cIMPACT-NOW update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef]
- Cairncross, G.; Wang, M.; Shaw, E.; Jenkins, R.; Brachman, D.; Buckner, J.; Fink, K.; Souhami, L.; Laperriere, N.; Curran, W.; et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: Long-term results of RTOG 9402. J. Clin. Oncol. 2013, 31, 337–343. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Capper, D.; Zentgraf, H.; Balss, J.; Hartmann, C.; von Deimling, A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009, 118, 599–601. [Google Scholar] [CrossRef]
- Bertero, L.; Mangherini, L.; Ricci, A.A.; Cassoni, P.; Sahm, F. Molecular neuropathology: An essential and evolving toolbox for the diagnosis and clinical management of central nervous system tumors. Virchows Arch. 2024, 484, 181–194. [Google Scholar] [CrossRef]
- Solomon, J.P.; Munoz-Zuluaga, C.; Slocum, C.; Dillard, A.; Cong, L.; Wang, J.; Lindeman, N.; Kluk, M.; Liechty, B.; Pisapia, D.; et al. Evaluation of the rapid Idylla IDH1-2 mutation assay in FFPE glioma samples. Diagn. Pathol. 2024, 19, 70. [Google Scholar] [CrossRef]
- Koontz, D.; Baecher, K.; Kobrynski, L.; Nikolova, S.; Gallagher, M. A pyrosequencing-based assay for the rapid detection of the 22q11.2 deletion in DNA from buccal and dried blood spot samples. J. Mol. Diagn. 2014, 16, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Setty, P.; Hammes, J.; Rothamel, T.; Vladimirova, V.; Kramm, C.M.; Pietsch, T.; Waha, A. A pyrosequencing-based assay for the rapid detection of IDH1 mutations in clinical samples. J. Mol. Diagn. 2010, 12, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Shahi, M.; Pringle, S.; Morris, M.; Garcia, D.M.; Quinones-Hinojosa, A.; Cooks, R.G. Detection of IDH mutation in glioma by desorption electrospray ionization (DESI) tandem mass spectrometry. Sci. Rep. 2024, 14, 26865. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Zhang, W.; Brown, H.; Wu, J.; Fang, X.; Shahi, M.; Chen, R.; Zhang, H.; Jiao, B.; Wang, N.; et al. Rapid detection of IDH mutations in gliomas by intraoperative mass spectrometry. Proc. Natl. Acad. Sci. USA 2024, 121, e2318843121. [Google Scholar] [CrossRef]
- Noble Anbunesan, S.; Alfonso-Garcia, A.; Zhou, X.; Bec, J.; Lee, H.S.; Jin, L.W.; Bloch, O.; Marcu, L. Intraoperative detection of IDH-mutant glioma using fluorescence lifetime imaging. J. Biophotonics 2023, 16, e202200291. [Google Scholar] [CrossRef]
- Mimosa, M.L.; Al-Ameri, W.; Simpson, J.T.; Nakhla, M.; Boissinot, K.; Munoz, D.G.; Das, S.; Feilotter, H.; Fattouh, R.; Saleeb, R.M. A Novel Approach to Detect IDH Point Mutations in Gliomas Using Nanopore Sequencing: Test Validation for the Clinical Laboratory. J. Mol. Diagn. 2023, 25, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Shih, H.A.; Andronesi, O.C.; Cahill, D.P. Isocitrate dehydrogenase-mutant glioma: Evolving clinical and therapeutic implications. Cancer 2017, 123, 4535–4546. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.D.; Tsai, A.C.; Abdullah, K.G.; McBrayer, S.K.; Shi, D.D. Treatment of IDH-mutant glioma in the INDIGO era. NPJ Precis. Oncol. 2024, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Lally, A.R.; Ghosh, S.R.; Pecorari, I.L.; Reynolds, J.; Ledet, A.; Begley, S.; Diaz, E.J.; Zhu, E.; Joseph, K.; McGeehan, K.; et al. Do the benefits of IDH mutations in high-grade glioma persist beyond the first recurrence? A multi-institutional retrospective analysis. J. Neuro-Oncol. 2025, 174, 167–175. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Mair, M.J.; Spiro, Z.; Abdel Malak, C.; El-Heliebi, A.; Eckert, F.; Furtner, J.; Konig, F.; Leibetseder, A.; Nowosielski, M.; et al. Personalized targeted glioblastoma therapies by ex vivo drug screening: Study protocol of the Advanced brain Tumor TheRApy Clinical Trial (ATTRACT). Neuro-Oncol. Adv. 2025, 7, vdaf056. [Google Scholar] [CrossRef]
- Wu, J.; Thust, S.C.; Wastling, S.J.; Abdalla, G.; Benenati, M.; Maynard, J.A.; Brandner, S.; Carrasco, F.P.; Barkhof, F. Automated Diffusion Analysis for Non-Invasive Prediction of IDH Genotype in WHO Grade 2-3 Gliomas. Am. J. Neuroradiol. 2025. [Google Scholar] [CrossRef]
- Park, Y.W.; Han, K.; Jang, G.; Cho, M.; Kim, S.B.; Kim, H.; Shin, N.Y.; Chang, J.H.; Kim, S.H.; Lee, S.K.; et al. Tumor oxygenation imaging biomarkers using dynamic susceptibility contrast imaging for prediction of IDH mutation status in adult-type diffuse gliomas. Eur. Radiol. 2025. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Xu, H.; Wang, M.; Weng, S.; Pei, D.; Chen, S.; Wang, W.; Yan, J.; Cui, L.; et al. Multimodal fusion of radio-pathology and proteogenomics identify integrated glioma subtypes with prognostic and therapeutic opportunities. Nat. Commun. 2025, 16, 3510. [Google Scholar] [CrossRef]
- Chouleur, T.; Etchegaray, C.; Villain, L.; Lesur, A.; Ferte, T.; Rossi, M.; Andrique, L.; Simoncini, C.; Giacobbi, A.S.; Gambaretti, M.; et al. A strategy for multimodal integration of transcriptomics, proteomics, and radiomics data for the prediction of recurrence in patients with IDH-mutant gliomas. Int. J. Cancer 2025, 157, 573–587. [Google Scholar] [CrossRef]
- Mohamed Sajer, R.; Pendem, S.; Kadavigere, R.; Priyanka; Nayak, S.S.; Nayak, K.; Pires, T.; Chandran, M.O.; Abhijith, S.; Raghu, V. Applications of MR Finger printing derived T1 and T2 values in Adult brain: A Systematic review. F1000Research 2025, 14, 54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, T.F.J.; Alinger-Scharinger, B.; Langwieder, C.K.; Mol, A.; Aleksic, T.; van Merkestijn, B.; Schlicker, H.U.; Spendel, M.; Pöppe, J.; Schwartz, C.; et al. Ultra-Fast Intraoperative IDH-Mutation Analysis Enables Rapid Stratification and Therapy Planning in Diffuse Gliomas. Int. J. Mol. Sci. 2025, 26, 9639. https://doi.org/10.3390/ijms26199639
Kraus TFJ, Alinger-Scharinger B, Langwieder CK, Mol A, Aleksic T, van Merkestijn B, Schlicker HU, Spendel M, Pöppe J, Schwartz C, et al. Ultra-Fast Intraoperative IDH-Mutation Analysis Enables Rapid Stratification and Therapy Planning in Diffuse Gliomas. International Journal of Molecular Sciences. 2025; 26(19):9639. https://doi.org/10.3390/ijms26199639
Chicago/Turabian StyleKraus, Theo F. J., Beate Alinger-Scharinger, Celina K. Langwieder, Anna Mol, Tereza Aleksic, Brain van Merkestijn, Hans U. Schlicker, Mathias Spendel, Johannes Pöppe, Christoph Schwartz, and et al. 2025. "Ultra-Fast Intraoperative IDH-Mutation Analysis Enables Rapid Stratification and Therapy Planning in Diffuse Gliomas" International Journal of Molecular Sciences 26, no. 19: 9639. https://doi.org/10.3390/ijms26199639
APA StyleKraus, T. F. J., Alinger-Scharinger, B., Langwieder, C. K., Mol, A., Aleksic, T., van Merkestijn, B., Schlicker, H. U., Spendel, M., Pöppe, J., Schwartz, C., Griessenauer, C. J., & Sotlar, K. (2025). Ultra-Fast Intraoperative IDH-Mutation Analysis Enables Rapid Stratification and Therapy Planning in Diffuse Gliomas. International Journal of Molecular Sciences, 26(19), 9639. https://doi.org/10.3390/ijms26199639




