Synaptic Pathology in Traumatic Brain Injury and Therapeutic Insights
Abstract
1. Introduction
2. Post-TBI Synaptic Pathology—Mechanistic Insights into Chronology of Post-TBI Synaptic Changes
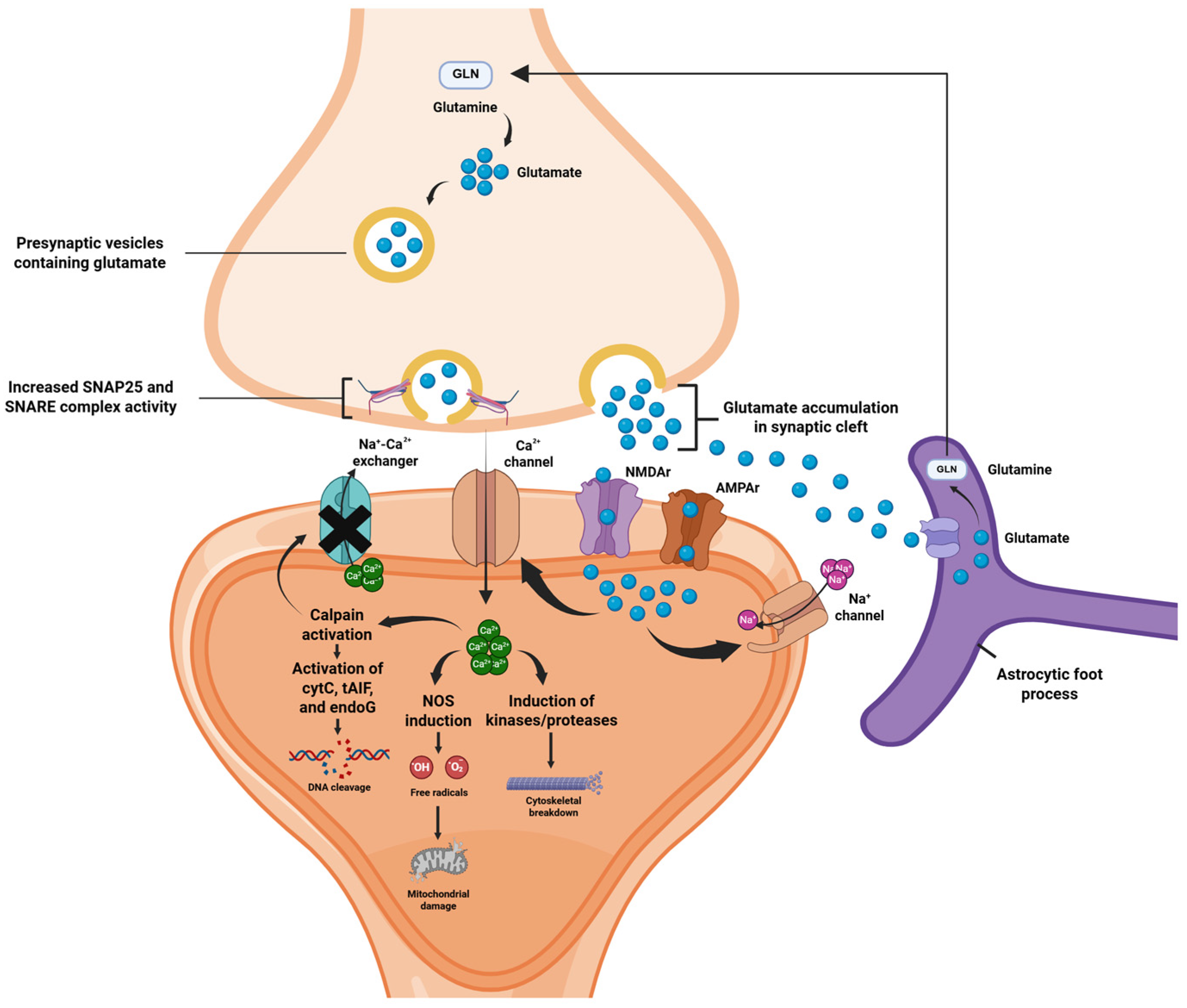
2.1. Acute Phase: Excitotoxicity
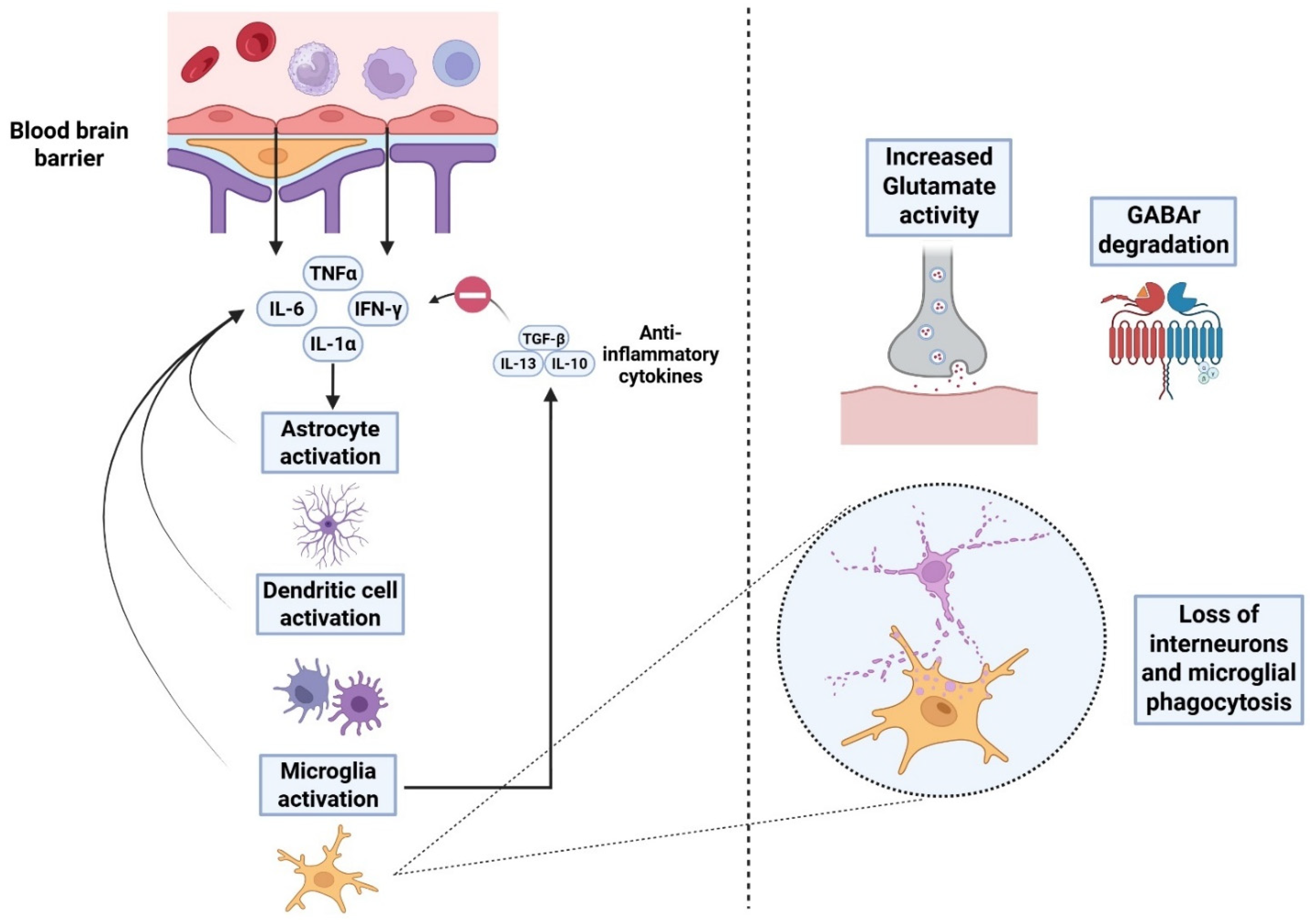
2.2. Subacute Phase: Neuroinflammation
2.3. Chronic Phase: Network Remodeling
3. Synaptic Impairment and Psychiatric Comorbidities
4. Synaptic Pathology in TBI—Therapeutic Implications
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lennon, M.J.; Brooker, H.; Creese, B.; Thayanandan, T.; Rigney, G.; Aarsland, D.; Hampshire, A.; Ballard, C.; Corbett, A.; Raymont, V. Lifetime Traumatic Brain Injury and Cognitive Domain Deficits in Late Life: The PROTECT-TBI Cohort Study. J. Neurotrauma 2023, 40, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.M.; Rose, N.B.; Temkin, N.R.; Barber, J.K.; Manley, G.T.; McCrea, M.A.; Nelson, L.D.; Badjatia, N.; Gopinath, S.; Keene, C.D.; et al. Profiles of Cognitive Functioning at 6 Months After Traumatic Brain Injury Among Patients in Level I Trauma Centers: A TRACK-TBI Study. JAMA Netw. Open 2023, 6, e2349118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Carson, A.; Dams-O’Connor, K. Psychiatric sequelae of traumatic brain injury—future directions in research. Nat. Rev. Neurol. 2023, 19, 556–571. [Google Scholar] [CrossRef]
- DeCuypere, M.; Klimo, P. Spectrum of Traumatic Brain Injury from Mild to Severe. Surg. Clin. N. Am. 2012, 92, 939–957. [Google Scholar] [CrossRef]
- Perry, D.C.; Sturm, V.E.; Peterson, M.J.; Pieper, C.F.; Bullock, T.; Boeve, B.F.; Miller, B.L.; Guskiewicz, K.M.; Berger, M.S.; Kramer, J.H.; et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: A meta-analysis. J. Neurosurg. 2016, 124, 511–526. [Google Scholar] [CrossRef]
- Orr, T.J.; Lesha, E.; Kramer, A.H.; Cecia, A.; Dugan, J.E.; Schwartz, B.; Einhaus, S.L. Traumatic Brain Injury: A Comprehensive Review of Biomechanics and Molecular Pathophysiology. World Neurosurg. 2024, 185, 74–88. [Google Scholar] [CrossRef]
- Thapa, K.; Khan, H.; Singh, T.G.; Kaur, A. Traumatic Brain Injury: Mechanistic Insight on Pathophysiology and Potential Therapeutic Targets. J. Mol. Neurosci. 2021, 71, 1725–1742. [Google Scholar] [CrossRef]
- Wakade, C.; Sukumari-Ramesh, S.; Laird, M.D.; Dhandapani, K.M.; Vender, J.R. Delayed reduction in hippocampal postsynaptic density protein-95 expression temporally correlates with cognitive dysfunction following controlled cortical impact in mice. J. Neurosurg. 2010, 113, 1195–1201. [Google Scholar] [CrossRef]
- Su, E.; Bell, M. Diffuse axonal injury. Transl. Res. Trauma. Brain Inj. 2016, 57, 41. [Google Scholar]
- Management of Concussion/mTBI Working Group. VA/DoD Clinical Practice Guideline for the Management of Concussion-Mild Traumatic Brain Injury. J. Rehabil. Res Dev. 2009, 46, CP1-68. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A. Oxidative Stress in Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 13000. [Google Scholar] [CrossRef]
- Zedde, M.; Piazza, F.; Pascarella, R. Traumatic Brain Injury and Chronic Traumatic Encephalopathy: Not Only Trigger for Neurodegeneration but Also for Cerebral Amyloid Angiopathy? Biomedicines 2025, 13, 881. [Google Scholar] [CrossRef]
- Karlander, M.; Ljungqvist, J.; Zelano, J. Post-traumatic epilepsy in adults: A nationwide register-based study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 617–621. [Google Scholar] [CrossRef]
- Pease, M.; Gonzalez-Martinez, J.; Puccio, A.; Nwachuku, E.; Castellano, J.F.; Okonkwo, D.O.; Elmer, J. Risk Factors and Incidence of Epilepsy after Severe Traumatic Brain Injury. Ann. Neurol. 2022, 92, 663–669. [Google Scholar] [CrossRef]
- Ritter, A.C.; Wagner, A.K.; Fabio, A.; Pugh, M.J.; Walker, W.C.; Szaflarski, J.P.; Zafonte, R.D.; Brown, A.W.; Hammond, F.M.; Bushnik, T.; et al. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: A Traumatic Brain Injury Model Systems Study. Epilepsia 2016, 57, 1968–1977. [Google Scholar] [CrossRef]
- Gao, X.; Deng, P.; Xu, Z.C.; Chen, J. Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS ONE 2011, 6, e24566. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp. Neurol. 2011, 70, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Little, D.M.; Kraus, M.F.; Joseph, J.; Geary, E.K.; Susmaras, T.; Zhou, X.J.; Pliskin, N.; Gorelick, P.B. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology 2010, 74, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Jamjoom, A.A.B.; Rhodes, J.; Andrews, P.J.D.; Grant, S.G.N. The synapse in traumatic brain injury. Brain 2021, 144, 18–31. [Google Scholar] [CrossRef]
- Sun, C.; Qi, L.; Cheng, Y.; Zhao, Y.; Gu, C. Immediate induction of varicosities by transverse compression but not uniaxial stretch in axon mechanosensation. Acta Neuropathol. Commun. 2022, 10, 7. [Google Scholar] [CrossRef]
- Witkowski, E.D.; Gao, Y.; Gavsyuk, A.F.; Maor, I.; DeWalt, G.J.; Eldred, W.D.; Mizrahi, A.; Davison, I.G. Rapid Changes in Synaptic Strength After Mild Traumatic Brain Injury. Front. Cell. Neurosci. 2019, 13, 166. [Google Scholar] [CrossRef]
- Chahin, M.; Mutschler, J.; Dzhuleva, S.P.; Dieterle, C.; Jimenez, L.R.; Bhattarai, S.R.; Van Steenbergen, V.; Bareyre, F.M. Repetitive concussions promote microglia-mediated engulfment of presynaptic excitatory input associated with cognitive dysfunction. Commun. Biol. 2025, 8, 335. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Remodeling dendritic spines for treatment of traumatic brain injury. Neural Regen. Res. 2019, 14, 1477–1480. [Google Scholar] [CrossRef]
- Eyolfson, E.; Suesser, K.R.B.; Henry, H.; Bonilla-Del Río, I.; Grandes, P.; Mychasiuk, R.; Christie, B.R. The effect of traumatic brain injury on learning and memory: A synaptic focus. Neuroscientist 2025, 31, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Weston, N.M.; Rolfe, A.T.; Freelin, A.H.; Reeves, T.M.; Sun, D. Traumatic brain injury modifies synaptic plasticity in newly-generated granule cells of the adult hippocampus. Exp. Neurol. 2021, 336, 113527. [Google Scholar] [CrossRef] [PubMed]
- Carron, S.F.; Alwis, D.S.; Rajan, R. Traumatic Brain Injury and Neuronal Functionality Changes in Sensory Cortex. Front. Syst. Neurosci. 2016, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA imbalance following traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 27. [Google Scholar] [CrossRef]
- Kang, Y.; Jamison, K.; Jaywant, A.; Dams-O’Connor, K.; Kim, N.; Karakatsanis, N.A.; Butler, T.; Schiff, N.D.; Kuceyeski, A.; Shah, S.A. Longitudinal alterations in gamma-aminobutyric acid (GABAA) receptor availability over ∼ 1 year following traumatic brain injury. Brain Commun. 2022, 4, fcac159. [Google Scholar] [CrossRef]
- Schwarzbach, E.; Bonislawski, D.P.; Xiong, G.; Cohen, A.S. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus 2006, 16, 541–550. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Kuo, T.-T.; Yi-Kung Huang, E.; Hoffer, B.J.; Chou, Y.-C.; Chiang, Y.-H.; Ma, H.-I.; Miller, J.P. Profound deficits in hippocampal synaptic plasticity after traumatic brain injury and seizure is ameliorated by prophylactic levetiracetam. Oncotarget 2018, 9, 11515. [Google Scholar] [CrossRef]
- Timofeev, I.; Bazhenov, M.; Avramescu, S.; Nita, D.A. Posttraumatic epilepsy: The roles of synaptic plasticity. Neuroscientist 2010, 16, 19–27. [Google Scholar] [CrossRef]
- Huang, J.; Xu, F.; Yang, L.; Tuolihong, L.; Wang, X.; Du, Z.; Zhang, Y.; Yin, X.; Li, Y.; Lu, K.; et al. Involvement of the GABAergic system in PTSD and its therapeutic significance. Front. Mol. Neurosci. 2023, 16, 1052288, Erratum in Front. Mol. Neurosci. 2023, 16, 1158825. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Rein, B. Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: Pathophysiological implications. Mol. Psychiatry 2022, 27, 445–465. [Google Scholar] [CrossRef]
- Sloley, S.S.; Main, B.S.; Winston, C.N.; Harvey, A.C.; Kaganovich, A.; Korthas, H.T.; Caccavano, A.P.; Zapple, D.N.; Wu, J.-y.; Partridge, J.G.; et al. High-frequency head impact causes chronic synaptic adaptation and long-term cognitive impairment in mice. Nat. Commun. 2021, 12, 2613. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Qiu, J.; Alcon, S.; Hashim, J.; Rotenberg, A.; Sun, Y.; Meehan, W.P., 3rd; Mannix, R. Memantine improves outcomes after repetitive traumatic brain injury. Behav. Brain Res. 2018, 340, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Olde Heuvel, F.; Li, Z.; Riedel, D.; Halbgebauer, S.; Oeckl, P.; Mayer, B.; Gotzman, N.; Shultz, S.; Semple, B.; Tumani, H.; et al. Dynamics of synaptic damage in severe traumatic brain injury revealed by cerebrospinal fluid SNAP-25 and VILIP-1. J. Neurol. Neurosurg. Psychiatry 2024, 95, 1158–1167. [Google Scholar] [CrossRef]
- Bigler, E.D. Traumatic brain injury, neuroimaging, and neurodegeneration. Front. Hum. Neurosci. 2013, 7, 395. [Google Scholar] [CrossRef]
- MacLean, M.A.; Muradov, J.H.; Greene, R.; Van Hameren, G.; Clarke, D.B.; Dreier, J.P.; Okonkwo, D.O.; Friedman, A. Memantine inhibits cortical spreading depolarization and improves neurovascular function following repetitive traumatic brain injury. Sci. Adv. 2023, 9, eadj2417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hameed, M.Q.; Hsieh, T.H.; Morales-Quezada, L.; Lee, H.H.C.; Damar, U.; MacMullin, P.C.; Hensch, T.K.; Rotenberg, A. Ceftriaxone Treatment Preserves Cortical Inhibitory Interneuron Function via Transient Salvage of GLT-1 in a Rat Traumatic Brain Injury Model. Cereb. Cortex 2019, 29, 4506–4518. [Google Scholar] [CrossRef]
- Vigil, F.A.; Belchior, H.; Bugay, V.; Bazaldua, I.I.; Stoja, A.; Dantas, D.C.; Chun, S.H.; Farmer, A.; Bozdemir, E.; Holstein, D.M.; et al. Acute Treatment with the M-Channel (Kv7, KCNQ) Opener Retigabine Reduces the Long-Term Effects of Repetitive Blast Traumatic Brain Injuries. Neurotherapeutics 2023, 20, 853–869. [Google Scholar] [CrossRef]
- Balleste, A.F.; Sangadi, A.; Titus, D.J.; Johnstone, T.; Hogenkamp, D.; Gee, K.W.; Atkins, C.M. Enhancing cognitive recovery in chronic traumatic brain injury through simultaneous allosteric modulation of α7 nicotinic acetylcholine and α5 GABA(A) receptors. Exp. Neurol. 2024, 379, 114879. [Google Scholar] [CrossRef]
- Titus, D.J.; Johnstone, T.; Johnson, N.H.; London, S.H.; Chapalamadugu, M.; Hogenkamp, D.; Gee, K.W.; Atkins, C.M. Positive allosteric modulation of the α7 nicotinic acetylcholine receptor as a treatment for cognitive deficits after traumatic brain injury. PLoS ONE 2019, 14, e0223180. [Google Scholar] [CrossRef]
- Zhu, B.; Eom, J.; Hunt, R.F. Transplanted interneurons improve memory precision after traumatic brain injury. Nat. Commun. 2019, 10, 5156. [Google Scholar] [CrossRef]
- Singh, R.; Ambasta, S.; Bais, P.S.; Azim, A.; Kumar, S.; Upreti, B.; Singh, S.; Mishra, P. Role of Gabapentin in Traumatic Brain Injury: A Prospective Comparative Study. Indian. J. Crit. Care Med. 2024, 28, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ou, F.Y.; Ning, Y.L.; Yang, N.; Chen, X.; Peng, Y.; Zhao, Y.; Li, P.; Zhou, Y.G.; Liu, Y. A 5-HT(6)R agonist alleviates cognitive dysfunction after traumatic brain injury in rats by increasing BDNF expression. Behav. Brain Res. 2022, 433, 113997. [Google Scholar] [CrossRef] [PubMed]
- Whitney, K.; Nikulina, E.; Rahman, S.N.; Alexis, A.; Bergold, P.J. Delayed dosing of minocycline plus N-acetylcysteine reduces neurodegeneration in distal brain regions and restores spatial memory after experimental traumatic brain injury. Exp. Neurol. 2021, 345, 113816. [Google Scholar] [CrossRef] [PubMed]
- Celorrio, M.; Rhodes, J.; Shumilov, K.; Moritz, J.; Xiao, S.; Anabayan, I.; Sauerbeck, A.; Kummer, T.; Friess, S. Recombinant human erythropoietin induces neuroprotection, activates MAPK/CREB pathway, and rescues fear memory after traumatic brain injury with delayed hypoxemia in mice. Brain Res. 2022, 1795, 148074. [Google Scholar] [CrossRef]
- Baudo, G.; Flinn, H.; Holcomb, M.; Tiwari, A.; Soriano, S.; Taraballi, F.; Godin, B.; Zinger, A.; Villapol, S. Sex-dependent improvement in traumatic brain injury outcomes after liposomal delivery of dexamethasone in mice. Bioeng. Transl. Med. 2024, 9, e10647. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A.; Datta, D. An overview of preclinical models of traumatic brain injury (TBI): Relevance to pathophysiological mechanisms. Front. Cell Neurosci. 2024, 18, 1371213. [Google Scholar] [CrossRef]
- Hoffe, B.; Holahan, M.R. Hyperacute Excitotoxic Mechanisms and Synaptic Dysfunction Involved in Traumatic Brain Injury. Front. Mol. Neurosci. 2022, 15, 831825. [Google Scholar] [CrossRef]
- Baracaldo-Santamaría, D.; Ariza-Salamanca, D.F.; Corrales-Hernández, M.G.; Pachón-Londoño, M.J.; Hernandez-Duarte, I.; Calderon-Ospina, C.A. Revisiting Excitotoxicity in Traumatic Brain Injury: From Bench to Bedside. Pharmaceutics 2022, 14, 152. [Google Scholar] [CrossRef]
- VanItallie, T.B. Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE). Metabolism 2019, 100, 153943. [Google Scholar] [CrossRef]
- Iwata, A.; Stys, P.K.; Wolf, J.A.; Chen, X.H.; Taylor, A.G.; Meaney, D.F.; Smith, D.H. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J. Neurosci. 2004, 24, 4605–4613. [Google Scholar] [CrossRef]
- Wolf, J.A.; Stys, P.K.; Lusardi, T.; Meaney, D.; Smith, D.H. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 2001, 21, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.F.; Morganti-Kosmann, M.C.; Lenzlinger, P.M.; Stocker, R.; Kempski, O.S.; Kossmann, T. Glutamate and taurine are increased in ventricular cerebrospinal fluid of severely brain-injured patients. J. Neurotrauma 1999, 16, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Stefani, M.; Modkovski, R.; Hansel, G.; Zimmer, E.; Kopczynski de Carvalho, A.; Muller, A.; Ryzewski Strogulski, N.; Rodolphi, M.; Carteri, R.; Schmidt, A.; et al. Elevated glutamate and lactate predict brain death after severe head trauma. Ann. Clin. Transl. Neurol. 2017, 4, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, Y.V.; Ji, S.G.; Yin, H.Z.; Weiss, J.H. Differential Vulnerability of CA1 versus CA3 Pyramidal Neurons After Ischemia: Possible Relationship to Sources of Zn2+ Accumulation and Its Entry into and Prolonged Effects on Mitochondria. J. Neurosci. 2017, 37, 726–737. [Google Scholar] [CrossRef]
- Kramer, D.R.; Fujii, T.; Ohiorhenuan, I.; Liu, C.Y. Cortical spreading depolarization: Pathophysiology, implications, and future directions. J. Clin. Neurosci. 2016, 24, 22–27. [Google Scholar] [CrossRef]
- Costa, C.; Tozzi, A.; Rainero, I.; Cupini, L.M.; Calabresi, P.; Ayata, C.; Sarchielli, P. Cortical spreading depression as a target for anti-migraine agents. J. Headache Pain. 2013, 14, 62. [Google Scholar] [CrossRef]
- Olatona, O.A.; Sterben, S.P.; Kansakar, S.B.S.; Symes, A.J.; Liaudanskaya, V. Mitochondria: The hidden engines of traumatic brain injury-driven neurodegeneration. Front. Cell. Neurosci. 2025, 19, 1570596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vespa, P.; Bergsneider, M.; Hattori, N.; Wu, H.M.; Huang, S.C.; Martin, N.A.; Glenn, T.C.; McArthur, D.L.; Hovda, D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005, 25, 763–774, Erratum in Chin. J. Traumatol. 2020, 28, 386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilmer, L.K.; Roberts, K.N.; Joy, K.; Sullivan, P.G.; Scheff, S.W. Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma 2009, 26, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, G.; Kong, R.H.; Zhang, L.M.; Zhang, J.N. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br. J. Pharmacol. 2012, 167, 699–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGinn, M.J.; Kelley, B.J.; Akinyi, L.; Oli, M.W.; Liu, M.C.; Hayes, R.L.; Wang, K.K.; Povlishock, J.T. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J. Neuropathol. Exp. Neurol. 2009, 68, 241–249. [Google Scholar] [CrossRef]
- Bell, J.D.; Park, E.; Ai, J.; Baker, A.J. PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ. 2009, 16, 1665–1680. [Google Scholar] [CrossRef]
- Bell, J.D.; Ai, J.; Chen, Y.; Baker, A.J. Mild in vitro trauma induces rapid Glur2 endocytosis, robustly augments calcium permeability and enhances susceptibility to secondary excitotoxic insult in cultured Purkinje cells. Brain 2007, 130, 2528–2542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujikawa, D.G. The Role of Excitotoxic Programmed Necrosis in Acute Brain Injury. Comput. Struct. Biotechnol. J. 2015, 13, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.N.; Chellappa, D.; Wilkins, T.; Barton, D.J.; Washington, P.M.; Loane, D.J.; Zapple, D.N.; Burns, M.P. Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation. J. Neurotrauma 2013, 30, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Welsh, C.A.; Barres, B.A.; Stevens, B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 2015, 18, 1539–1545. [Google Scholar] [CrossRef]
- Smith, D.H.; Chen, X.-H.; Pierce, J.E.S.; Wolf, J.A.; Trojanowski, J.Q.; Graham, D.I.; McIntosh, T.K. Progressive Atrophy and Neuron Death for One Year Following Brain Trauma in the Rat. J. Neurotrauma 1997, 14, 715–727. [Google Scholar] [CrossRef]
- He, L.; Zhang, R.; Yang, M.; Lu, M. The role of astrocyte in neuroinflammation in traumatic brain injury. Biochim. et. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 166992. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 2018, 21, 137–151. [Google Scholar] [CrossRef]
- Kumar, R.G.; Diamond, M.L.; Boles, J.A.; Berger, R.P.; Tisherman, S.A.; Kochanek, P.M.; Wagner, A.K. Acute CSF interleukin-6 trajectories after TBI: Associations with neuroinflammation, polytrauma, and outcome. Brain Behav. Immun. 2015, 45, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Hall, C.E.; Gupta, K.; Carpenter, K.L.H.; Chandran, S.; Hutchinson, P.J.; Patani, R.; Helmy, A. Elucidating Pro-Inflammatory Cytokine Responses after Traumatic Brain Injury in a Human Stem Cell Model. J. Neurotrauma 2018, 35, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of Pro- and Anti-Inflammatory Cytokines and Chemokines in the Pathophysiology of Traumatic Brain Injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Parga Becerra, A.; Logsdon, A.F.; Banks, W.A.; Ransom, C.B. Traumatic Brain Injury Broadly Affects GABAergic Signaling in Dentate Gyrus Granule Cells. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Nichols, J.; Bjorklund, G.R.; Newbern, J.; Anderson, T. Parvalbumin fast-spiking interneurons are selectively altered by paediatric traumatic brain injury. J. Physiol. 2018, 596, 1277–1293. [Google Scholar] [CrossRef]
- Park, K.; Biederer, T. Neuronal adhesion and synapse organization in recovery after brain injury. Future Neurol. 2013, 8, 555–567. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Hicks, R.R.; Baldwin, S.A.; Robinson, S.; Brackney, C. Synaptogenesis in the Hippocampal CA1 Field following Traumatic Brain Injury. J. Neurotrauma 2005, 22, 719–732. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Traumatic brain injury and amyloid-β pathology: A link to Alzheimer’s disease? Nat. Rev. Neurosci. 2010, 11, 361–370. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.H.; Liu, X.; Zhang, Z.; Gu, X.; Yu, S.P.; Keene, C.D.; Cheng, L.; Ye, K. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog. Neurobiol. 2020, 185, 101730. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, A.; Takeuchi, H.; Tanaka, F. Tau Pathology in Chronic Traumatic Encephalopathy and Alzheimer’s Disease: Similarities and Differences. Front. Neurol. 2019, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Grasset, L.; Glymour, M.M.; Yaffe, K.; Swift, S.L.; Gianattasio, K.Z.; Power, M.C.; Zeki Al Hazzouri, A. Association of traumatic brain injury with dementia and memory decline in older adults in the United States. Alzheimers Dement. 2020, 16, 853–861. [Google Scholar] [CrossRef]
- Jorge, R.E.; Arciniegas, D.B. Mood disorders after TBI. Psychiatr. Clin. N. Am. 2014, 37, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Nguyen, L.; Turner, R.C.; Logsdon, A.F.; Chen, Y.-W.; Smith, K.E.; Huber, J.D.; Matsumoto, R.; Rosen, C.L.; Tucker, E.S.; et al. Traumatic brain injury and epilepsy: Underlying mechanisms leading to seizure. Seizure 2015, 33, 13–23. [Google Scholar] [CrossRef]
- Klyce, D.W.; Perrin, P.B.; Fisher, L.B.; Hammond, F.M.; Juengst, S.B.; Bergquist, T.F.; Rabinowitz, A.R.; Wagner, A.K.; Bombardier, C.H.; Niemeier, J.P.; et al. Identifying group-based patterns of suicidal ideation over the first 10 years after moderate-to-severe TBI. J. Clin. Psychol. 2022, 78, 877–891. [Google Scholar] [CrossRef]
- Singh, R.; Mason, S.; Lecky, F.; Dawson, J. Comparison of early and late depression after TBI; (the SHEFBIT study). Brain Inj. 2019, 33, 584–591. [Google Scholar] [CrossRef]
- Ryttersgaard, T.O.; Riis, J.; Johnsen, S.P.; Mogensen, P.H.; Bjarkam, C.R. Depression and cognitive sequelae registered within the first year among young Danish TBI survivors. Scand. J. Psychol. 2020, 61, 663–670. [Google Scholar] [CrossRef]
- Bahader, G.A.; Naghavi, F.; Alotaibi, A.; Dehghan, A.; Swain, C.C.; Burkett, J.P.; Shah, Z.A. Neurobehavioral and inflammatory responses following traumatic brain injury in male and female mice. Behav. Brain Res. 2024, 456, 114711. [Google Scholar] [CrossRef]
- Pandey, D.K.; Yadav, S.K.; Mahesh, R.; Rajkumar, R. Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: A model of comorbid depression and anxiety? Behav. Brain Res. 2009, 205, 436–442. [Google Scholar] [CrossRef]
- Mahesh, R.; Pandey, D.K.; Katiyar, S.; Kukade, G.; Viyogi, S.; Rudra, A. Effect of anti-depressants on neuro-behavioural consequences following impact accelerated traumatic brain injury in rats. Indian. J. Exp. Biol. 2010, 48, 466–473. [Google Scholar] [PubMed]
- Medina, A.; Burke, S.; Thompson, R.C.; Bunney, W., Jr.; Myers, R.M.; Schatzberg, A.; Akil, H.; Watson, S.J. Glutamate transporters: A key piece in the glutamate puzzle of major depressive disorder. J. Psychiatr. Res. 2013, 47, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.S.; Holloway, A.L.; Hong-Routson, S.; Wainwright, M.S. Depression following traumatic brain injury in mice is associated with down-regulation of hippocampal astrocyte glutamate transporters by thrombin. J. Cereb. Blood Flow. Metab. 2019, 39, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, C.N.; Morganti, J.M.; Bachstetter, A.D. Depression following a traumatic brain injury: Uncovering cytokine dysregulation as a pathogenic mechanism. Neural Regen. Res. 2018, 13, 1693–1704. [Google Scholar] [CrossRef]
- Wang, J.; Gao, F.; Cui, S.; Yang, S.; Gao, F.; Wang, X.; Zhu, G. Utility of 7,8-dihydroxyflavone in preventing astrocytic and synaptic deficits in the hippocampus elicited by PTSD. Pharmacol. Res. 2022, 176, 106079. [Google Scholar] [CrossRef]
- He, M.; Wei, J.X.; Mao, M.; Zhao, G.Y.; Tang, J.J.; Feng, S.; Lu, X.M.; Wang, Y.T. Synaptic Plasticity in PTSD and associated Comorbidities: The Function and Mechanism for Diagnostics and Therapy. Curr. Pharm. Des. 2018, 24, 4051–4059. [Google Scholar] [CrossRef]
- Markicevic, M.; Mandino, F.; Toyonaga, T.; Cai, Z.; Fesharaki-Zadeh, A.; Shen, X.; Strittmatter, S.M.; Lake, E.M.R. Repetitive Mild Closed-Head Injury Induced Synapse Loss and Increased Local BOLD-fMRI Signal Homogeneity. J. Neurotrauma 2024, 41, 2528–2544. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A.; Miyauchi, J.T.; St Laurent-Arriot, K.; Tsirka, S.E.; Bergold, P.J. Increased Behavioral Deficits and Inflammation in a Mouse Model of Co-Morbid Traumatic Brain Injury and Post-Traumatic Stress Disorder. ASN Neuro 2020, 12, 1759091420979567. [Google Scholar] [CrossRef]
- Holmes, S.E.; Scheinost, D.; Finnema, S.J.; Naganawa, M.; Davis, M.T.; DellaGioia, N.; Nabulsi, N.; Matuskey, D.; Angarita, G.A.; Pietrzak, R.H.; et al. Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 2019, 10, 1529. [Google Scholar] [CrossRef]
- Cunningham, M.; Cho, J.H.; Leung, A.; Savvidis, G.; Ahn, S.; Moon, M.; Lee, P.K.; Han, J.J.; Azimi, N.; Kim, K.S.; et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 2014, 15, 559–573. [Google Scholar] [CrossRef]
- Spencer, H.F.; Boese, M.; Berman, R.Y.; Radford, K.D.; Choi, K.H. Effects of a Subanesthetic Ketamine Infusion on Inflammatory and Behavioral Outcomes after Closed Head Injury in Rats. Bioengineering 2023, 10, 941. [Google Scholar] [CrossRef]
- Boese, M.; Berman, R.; Spencer, H.; Rujan, O.; Metz, E.; Radford, K.; Choi, K. Effects of Subanesthetic Intravenous Ketamine Infusion on Stress Hormones and Synaptic Density in Rats with Mild Closed-Head Injury. Biomedicines 2025, 13, 787. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Villasana, L.; Schnell, E. Ketamine Alters Hippocampal Cell Proliferation and Improves Learning in Mice after Traumatic Brain Injury. Anesthesiology 2018, 129, 1. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Finnema, S.J.; Naganawa, M.; DellaGioia, N.; Holden, D.; Fowles, K.; Davis, M.; Ropchan, J.; Emory, P.; Ye, Y.; et al. Imaging the effect of ketamine on synaptic density (SV2A) in the living brain. Mol. Psychiatry 2022, 27, 2273–2281. [Google Scholar] [CrossRef]
- Jiang, D.G.; Jin, S.L.; Li, G.Y.; Li, Q.Q.; Li, Z.R.; Ma, H.X.; Zhuo, C.J.; Jiang, R.H.; Ye, M.J. Serotonin regulates brain-derived neurotrophic factor expression in select brain regions during acute psychological stress. Neural Regen. Res. 2016, 11, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Rychtyk, J.; Partyka, A.; Gdula-Argasińska, J.; Mysłowska, K.; Wilczyńska, N.; Jastrzębska-Więsek, M.; Wesołowska, A. 5-HT(6) receptor agonist and antagonist improve memory impairments and hippocampal BDNF signaling alterations induced by MK-801. Brain Res. 2019, 1722, 146375. [Google Scholar] [CrossRef]
- Mohamadpour, M.; Whitney, K.; Bergold, P.J. The Importance of Therapeutic Time Window in the Treatment of Traumatic Brain Injury. Front. Neurosci. 2019, 13, 7. [Google Scholar] [CrossRef]
- Bedi, S.S.; Aertker, B.M.; Liao, G.P.; Caplan, H.W.; Bhattarai, D.; Mandy, F.; Mandy, F.; Fernandez, L.G.; Zelnick, P.; Mitchell, M.B.; et al. Therapeutic time window of multipotent adult progenitor therapy after traumatic brain injury. J. Neuroinflammation 2018, 15, 84. [Google Scholar] [CrossRef]
| Study Authors | Participants | Experimental Group | Control Group | Main Intervention | Study Outcomes |
|---|---|---|---|---|---|
| [35] | Male rats, mean age of 8 weeks (n = 95) | rm-TBI (4 injuries in 4 days) treated with memantine, n = 29 | rm-TBI (4 injuries in 4 days and placebo treatment), n = 37 Sham injuries in 4 days, n = 30 | Memantine (NMDA antagonist) given within 1 h after the last mTBI (10 mg/kg) | Reduced tau phosphorylation and less glial cell activation; restored hippocampal LTP at 1 month; no major improvement noted in behavioral outcomes. |
| [38] | Male rats, mean age of 9 weeks (n = 31) | rm-TBI (4 injuries in 4 days) treated with memantine, n = 15 | rm-TBI (4 injuries in 4 days and placebo treatment), n = 16 | Memantine (10 mg/kg) after the first TBI and prior to the second, third and fourth impacts | Inhibited cortical spreading depolarizations and reduced post-depolarization oligemia; improved neurobehavioral scores. |
| [39] | Male rats, mean age of 12 weeks (n = 21) | Fluid percussion injury treated with ceftriaxone, n = 7 | Saline-sham, n = 7 Saline-TBI, n = 7 | Ceftriaxone (β-lactam antibiotic, 250 mg/kg), once daily for 7 days | Reduced post-TBI GLT-1 downregulation; preserved intracortical inhibition by electrophysiology and parvalbumin interneuron markers. |
| [40] | Male rats, mean age of 12 weeks (n = 23) | Repetitive blast injury with retigabine treatment, n = 12 | Blast group, n = 6 Sham group, n = 5 | Retigabine (M-channel opener), 1.2 mg/kg dose, administered 30 min after each blast exposure | Reduced duration of acute post-traumatic seizures; markedly decreased development of chronic epilepsy post-injury. |
| [41] | Adult male rats (n = 45) | Fluid percussion injury with 522-054 treatment | Sham-vehicle Sham-522-054 TBI-vehicle | 522-054 (α7-nAChr agonist + α5-GABA-A inhibitor) | Restored hippocampal LTP; improved fear memory and water-maze performance; no changes in hippocampal or cortical atrophy. |
| [42] | Male rats, mean age of 2–3 months (n = 94) | Fluid percussion injury with AVL-3288 treatment, n = 11 | Sham-vehicle, n =10 Sham-AVL-3288, n = 8 TBI-vehicle, n = 11 | A total of 8 treatments completed for AVL-3288 (0.3 mg/kg), a α7-nAChr agonist | Improved performance in water maze retention and working memory, better cue and contextual fear memory, reduction in hippocampal atrophy. |
| [43] | Adult male mice | Controlled cortical impact (CCI) with embryonic MGE transplant, n = 11 | Sham mice, n = 16 TBI only mice, n = 19 | Transplanted embryonic MGE-derived GABAergic interneurons | Grafted interneurons integrated and increased synaptic inhibition; improved spatial memory precision; reduced seizures. |
| [44] | RCT with TBI patients in ITU (n = 65), moderately decreased GCS (8–13) and severely decreased GCS (<8) | TBI patients receiving gabapentin, n = 30 | TBI patients receiving placebo, n = 30 | Administration of gabapentin, a GABA analog, 300 mg twice daily for 2 weeks | Significant improvements noted in Glasgow outcome scale and GCS measures up to 90 days after trauma. |
| [45] | Male rats, mean age of 6–7 weeks | Moderate CCI with administration of WAY-181187 | N/A | WAY-181187 (5-HT6R agonist), 3 mg/kg for 5 days | Improved Morris water maze performance at 1 and 4 weeks; increased hippocampal BDNF and improved dendritic spine density. |
| [46] | Male rats, mean age of 16–18 weeks | Closed-head injury with administration of minocycline/NAC, n = 5 | Sham injury-saline, n = 5 | Minocycline (22.5 mg/kg) plus NAC (75 mg/kg) 3–5 days after injury | Improved performance in Barnes maze and hippocampal neuroprotection, and decreased synaptic loss in TBI. |
| [47] | Male rats, mean age of 6–8 weeks | CCI with EPO administration, n = 15 | Sham-EPO, n = 15 CCI-vehicle, n = 15 | Up to 5000 U/kg EPO, total of 6–9 doses | Improved cued-fear memory response along with increased synaptic density in the hippocampus and amygdala. |
| [48] | Male and female rats, mean age of 12 weeks | CCI with left-sided moderate TBI, administered Lipo-Dex, n = 5 | Lipo alone, n = 5 | Up to 3 mg/kg of dexamethasone given 1 h post-CCI | Male mice experienced significantly greater reduction in lesion volume, astrogliosis, cytokine-mediated inflammation and microglia activation when compared to female mice. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuthalapati, P.; Holmes, S.E.; Altalib, H.H.; Fesharaki-Zadeh, A. Synaptic Pathology in Traumatic Brain Injury and Therapeutic Insights. Int. J. Mol. Sci. 2025, 26, 9604. https://doi.org/10.3390/ijms26199604
Nuthalapati P, Holmes SE, Altalib HH, Fesharaki-Zadeh A. Synaptic Pathology in Traumatic Brain Injury and Therapeutic Insights. International Journal of Molecular Sciences. 2025; 26(19):9604. https://doi.org/10.3390/ijms26199604
Chicago/Turabian StyleNuthalapati, Poojith, Sophie E. Holmes, Hamada H. Altalib, and Arman Fesharaki-Zadeh. 2025. "Synaptic Pathology in Traumatic Brain Injury and Therapeutic Insights" International Journal of Molecular Sciences 26, no. 19: 9604. https://doi.org/10.3390/ijms26199604
APA StyleNuthalapati, P., Holmes, S. E., Altalib, H. H., & Fesharaki-Zadeh, A. (2025). Synaptic Pathology in Traumatic Brain Injury and Therapeutic Insights. International Journal of Molecular Sciences, 26(19), 9604. https://doi.org/10.3390/ijms26199604






