Role of Growth Factors in the Pathogenesis of Systemic-Sclerosis-Associated Fibrosis
Abstract
1. Introduction
2. Role of Growth Factors in SSc-Associated Tissue Fibrosis
2.1. Transforming Growth Factor Beta (TGF-β)
2.2. Receptor Activation and Intracellular TGF-β Pathways
2.3. TGF-β Pleiotropic Profibrotic Effects
2.4. Novel TGF-β-Regulatory Pathways
2.5. Connective Tissue Growth Factor (CTGF)
2.6. Platelet-Derived Growth Factor (PDGF)
2.7. Fibroblast Growth Factors (FGFs)
2.8. Vascular Endothelial Growth Factor (VEGF)
2.9. Insulin-like Growth Factors (IGFs)
3. Other Regulatory Pathways Involved in the SSc Fibrotic Process
3.1. PKC-Delta
3.2. P13-Kinase
3.3. Lysophosphatidic Acid
3.4. Caveolin-1-Mediated Regulation
3.5. Janus Kinases (JAK) and Signal Transducer and Activator of Transcription (STAT)
3.6. Peroxisome-Proliferator-Activated Receptors (PPAR)
4. Regulation by Wnt, Notch, and Hedgehog
4.1. Wnt Signaling
4.2. Hedgehog and Notch Signaling
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef]
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Primers 2015, 1, 15002. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni, M.G.; Piantoni, S.; Angeli, F.; Bertocchi, S.; Franceschini, F.; Airò, P. A Narrative Review of Pathogenetic and Histopathologic Aspects, Epidemiology, Classification Systems, and Disease Outcome Measures in Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2023, 64, 358–377. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.A.; Derk, C.T. Following the molecular pathways toward an understanding of the pathogenesis of Systemic Sclerosis. Ann. Int. Med. 2004, 140, 37–50. [Google Scholar] [CrossRef]
- Trojanowska, M.; Varga, J. Molecular pathways as novel therapeutic targets in systemic sclerosis. Curr. Opin. Rheumatol. 2007, 19, 568–573. [Google Scholar] [CrossRef]
- Varga, J.; Trojanowska, M.; Kuwana, M. Pathogenesis of systemic sclerosis: Recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2017, 2, 137–152. [Google Scholar] [CrossRef]
- Cutolo, M.; Soldano, S.; Smith, V. Pathophysiology of systemic sclerosis: Current understanding and new insights. Expert Rev. Clin. Immunol. 2019, 15, 753–764. [Google Scholar] [CrossRef]
- Truchetet, M.E.; Brembilla, N.C.; Chizzolini, C. Current Concepts on the Pathogenesis of Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2023, 64, 262–283. [Google Scholar] [CrossRef]
- Karasek, M.A. Does transformation of microvascular endothelial cells into myofibroblasts play a key role in the etiology and pathology of fibrotic disease? Med. Hypotheses 2007, 68, 650–655. [Google Scholar] [CrossRef]
- Krieg, T.; Abraham, D.; Lafyatis, R. Fibrosis in connective tissue disease: The role of the myofibroblast and fibroblast-epithelial cell interactions. Arthritis Res. Ther. 2007, 9 (Suppl. S2), S4. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Shigemitsu, H.; Kanangat, S. Cellular origins of fibroblasts: Possible implications for organ fibrosis in systemic sclerosis. Curr. Opin. Rheumatol. 2004, 16, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.A.; Trojanowska, M. Fibrosis in systemic sclerosis. Rheum. Dis. Clin. N. Am. 2008, 34, 115–143. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Bhattacharyya, S.; Tourtellotte, W.G.; Varga, J. Fibrosis in systemic sclerosis: Emerging concepts and implications for targeted therapy. Autoimmun. Rev. 2011, 10, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ebmeier, S.; Horsley, V. Origin of fibrosing cells in systemic sclerosis. Curr. Opin. Rheumatol. 2015, 27, 555–562. [Google Scholar] [CrossRef]
- Jimenez, S.A.; Piera-Velazquez, S. Cellular Transdifferentiation: A Crucial Mechanism of Fibrosis in Systemic Sclerosis. Curr. Rheumatol. Rev. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- McAnulty, R.J. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int. J. Biochem. Cell Biol. 2007, 39, 666–671. [Google Scholar] [CrossRef]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef]
- Kirk, T.Z.; Mark, M.E.; Chua, C.C.; Chua, B.H.; Mayes, M.D. Myofibroblasts from scleroderma skin synthesize elevated levels of collagen and tissue inhibitor of metalloproteinase (TIMP-1) with two forms of TIMP-1. J. Biol. Chem. 1995, 270, 3423–3428. [Google Scholar] [CrossRef]
- Abraham, D.J.; Eckes, B.; Rajkumar, V.; Krieg, T. New developments in fibroblast and myofibroblast biology: Implications for fibrosis and scleroderma. Curr. Rheumatol. Rep. 2007, 9, 136–143. [Google Scholar] [CrossRef]
- Gyftaki-Venieri, D.A.; Abraham, D.J.; Ponticos, M. Insights into myofibroblasts and their activation in scleroderma: Opportunities for therapy? Curr. Opin. Rheumatol. 2018, 30, 581–587. [Google Scholar] [CrossRef]
- Chadli, L.; Sotthewes, B.; Li, K.; Andersen, S.N.; Cahir-McFarland, E.; Cheung, M.; Cullen, P.; Dorjée, A.; de Vries-Bouwstra, J.-K.; Huizinga, T.W.J.; et al. Identification of regulators of the myofibroblast phenotype of primary dermal fibroblasts from early diffuse systemic sclerosis patients. Sci. Rep. 2019, 9, 4521. [Google Scholar] [CrossRef] [PubMed]
- Bellando-Randone, S.; Del Galdo, F.; Matucci-Cerinic, M. Insights into molecular and clinical characteristics of very early systemic sclerosis. Curr. Opin. Rheumatol. 2022, 34, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Györfi, A.-H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol. Asp. Med. 2023, 92, 101191. [Google Scholar] [CrossRef]
- Abraham, D.; Lescoat, A.; Stratton, R. Emerging diagnostic and therapeutic challenges for skin fibrosis in systemic sclerosis. Mol. Asp. Med. 2024, 96, 101252. [Google Scholar] [CrossRef]
- Jimenez, S.A.; Castro, S.V.; Piera-Velazquez, M. Role of Growth factors in the pathogenesis of tissue fibrosis in Systemic Sclerosis. Curr. Rheumatol. Rev. 2010, 6, 283–294. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Varga, J.; Whitfield, M.L. Transforming growth factor-beta in systemic sclerosis (scleroderma). Front. Biosci. (Schol. Ed) 2009, 1, 226–235. [Google Scholar] [CrossRef]
- Lafyatis, R. Transforming growth factor β—At the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef]
- Lodyga, M.; Hinz, B. TGF-β1-a truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Nieto, J.A.; Muñoz-Valle, J.F.; Baños-Hernández, C.J.; Navarro-Zarza, J.E.; Godínez-Rubí, J.M.; García-Arellano, S.; Ramírez-Dueñas, M.G.; Parra-Rojas, I.; Villanueva-Pérez, A.; Hernández-Bello, J. Transforming growth factor beta isoforms and TGF-βR1 and TGF-βR2 expression in systemic sclerosis patients. Clin. Exp. Med. 2023, 23, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Vander Heiden, J.A.; Gao, X.; Yin, J.; Uttarwar, S.; Liang, W.C.; Jia, G.; Yadav, R.; Huang, Z.; Mitra, M.; et al. Isoform-selective TGF-β3 inhibition for systemic sclerosis. Med 2024, 5, 132–147.e7. [Google Scholar] [CrossRef] [PubMed]
- Danielpour, D. Advances and Challenges in Targeting TGF-β Isoforms for Therapeutic Intervention of Cancer: A Mechanism-Based Perspective. Pharmaceuticals 2024, 17, 533. [Google Scholar] [CrossRef]
- Cheifetz, S.; Andres, J.L.; Massagué, J. The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J. Biol. Chem. 1988, 263, 16984–16991. [Google Scholar] [CrossRef]
- Rifkin, D.B. Latent transforming growth factor-beta (TGF-beta) binding proteins: Orchestrators of TGF-beta availability. J. Biol. Chem. 2005, 280, 7409–7412. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-β structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef]
- Verma, B.K.; Chatterjee, A.; Kondaiah, P.; Gundiah, N. Substrate Stiffness Modulates TGF-β Activation and ECM-Associated Gene Expression in Fibroblasts. Bioengineering 2023, 10, 998. [Google Scholar] [CrossRef]
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Rosendahl, A.; Sideras, P.; ten Dijke, P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002, 21, 1743–1753. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Lebrin, F.; Larsson, J.; Mummery, C.; Karlsson, S.; ten Dijke, P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol. Cell 2003, 12, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Zaheer, S. Multifaceted TGF-β signaling, a master regulator: From bench-to-bedside, intricacies, and complexities. Cell Biol. Int. 2024, 48, 87–127. [Google Scholar] [CrossRef]
- Budi, E.H.; Duan, D.; Derynck, R. Transforming Growth Factor-β Receptors and Smads: Regulatory Complexity and Functional Versatility. Trends Cell Biol. 2017, 27, 658–672. [Google Scholar] [CrossRef]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β receptors: In and beyond TGF-β signaling. Cell Signal. 2018, 52, 112–120. [Google Scholar] [CrossRef]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H.; et al. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef]
- Varga, J.; Rosenbloom, J.; Jimenez, S.A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem. J. 1987, 247, 597–604. [Google Scholar] [CrossRef]
- Varga, J. Scleroderma and Smads: Dysfunctional Smad family dynamics culminating in fibrosis. Arthrits Rheum. 2002, 46, 1703–1713. [Google Scholar] [CrossRef]
- Aashaq, S.; Batool, A.; Mir, S.A.; Beigh, M.A.; Andrabi, K.I.; Shah, Z.A. TGF-β signaling: A recap of SMAD-independent and SMAD-dependent pathways. J. Cell. Physiol. 2022, 237, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liao, H.; Cheng, M.; Shi, X.; Lin, X.; Feng, X.H.; Chen, Y.G. Smad7 Protein Interacts with Receptor-regulated Smads (R-Smads) to Inhibit Transforming Growth Factor-β (TGF-β)/Smad Signaling. J. Biol. Chem. 2016, 291, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Ungefroren, H. Autocrine TGF-β in Cancer: Review of the Literature and Caveats in Experimental Analysis. Int. J. Mol. Sci. 2021, 22, 977. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Wu, M.; Fang, F.; Tourtellotte, W.; Feghali-Bostwick, C.; Varga, J. Early growth response transcription factors: Key mediators of fibrosis and novel targets for anti-fibrotic therapy. Matrix Biol. 2011, 30, 235–242. [Google Scholar] [CrossRef]
- Shi-wen, X.; Parapuram, S.K.; Pala, D.; Chen, Y.; Carter, D.E.; Eastwood, M.; Denton, C.P.; Abraham, D.J.; Leask, A. Requirement of transforming growth factor beta-activated kinase 1 for transforming growth factor beta-induced alpha-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009, 60, 234–241. [Google Scholar] [CrossRef]
- Finnson, K.W.; Almadani, Y.; Philip, A. Non-canonical (non-SMAD2/3) TGF-β signaling in fibrosis: Mechanisms and targets. Semin. Cell Dev. Biol. 2020, 101, 115–122. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Jimenez, S.A. Molecular ablation of TGF-β signaling pathways by tyrosine kinase inhibition: The coming of a promising new era in the treatment of tissue fibrosis. Arthritis Rheum. 2008, 58, 2219–2224. [Google Scholar] [CrossRef]
- Li, Z.; Jimenez, S.A. Protein kinase Cδ and c-Abl kinase are required for transforming growth factor β induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum. 2011, 63, 2473–2483. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ono, J.; Masuoka, M.; Ohta, S.; Izuhara, K.; Ikezawa, Z.; Aihara, M.; Takahashi, K. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br. J. Dermatol. 2013, 168, 717–725. [Google Scholar] [CrossRef]
- De Luca, G.; Campochiaro, C.; Burastero, S.E.; Matucci-Cerinic, M.; Doglioni, C.; Dagna, L. Periostin expression in uninvolved skin as a potential biomarker for rapid cutaneous progression in systemic sclerosis patients: A preliminary explorative study. Front. Med. 2024, 10, 1214523. [Google Scholar] [CrossRef]
- Kanaoka, M.; Yamaguchi, Y.; Komitsu, N.; Feghali-Bostwick, C.A.; Ogawa, M.; Arima, K.; Izuhara, K.; Aihara, M. Pro-fibrotic phenotype of human skin fibroblasts induced by periostin via modulating TGF-β signaling. J. Dermatol. Sci. 2018, 90, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Nanri, Y.; Nunomura, S.; Honda, Y.; Takedomi, H.; Yamaguchi, Y.; Izuhara, K. A Positive Loop Formed by SOX11 and Periostin Upregulates TGF-β Signals Leading to Skin Fibrosis. J. Investig. Dermatol. 2023, 143, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Park, H.N.; Song, M.J.; Choi, Y.E.; Lee, D.H.; Chung, J.H.; Lee, S.-T. LRG1 Promotes ECM Integrity by Activating the TGF-β Signaling Pathway in Fibroblasts. Int. J. Mol. Sci. 2023, 24, 12445. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Fujimoto, M.; Serada, S.; Urushima, H.; Mishima, T.; Lee, H.; Ohkawara, T.; Kohno, N.; Hattori, N.; Yokoyama, A.; et al. Leucine-rich α-2 glycoprotein promotes lung fibrosis by modulating TGF-β signaling in fibroblasts. Physiol. Rep. 2017, 5, e13556. [Google Scholar] [CrossRef]
- Hong, Q.; Cai, H.; Zhang, L.; Li, Z.; Zhong, F.; Ni, Z.; Cai, G.; Chen, X.M.; He, J.C.; Lee, K. Modulation of transforming growth factor-β-induced kidney fibrosis by leucine-rich ⍺-2 glycoprotein-1. Kidney Int. 2022, 101, 299–314. [Google Scholar] [CrossRef]
- Bălănescu, P.; Bălănescu, E.; Băicuș, C.; Bălănescu, A. Circulatory cytokeratin 17, marginal zone B1 protein and leucine-rich α2-glycoprotein-1 as biomarkers for disease severity and fibrosis in systemic sclerosis patients. Biochem. Med. 2022, 32, 030707. [Google Scholar] [CrossRef]
- Bradham, D.M.; Igarashi, A.; Potter, R.L.; Grotendorst, G.R. Connective tissue growth factor: A cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J. Cell Biol. 1991, 114, 1285–1294. [Google Scholar] [CrossRef]
- Grotendorst, G.R. Connective tissue growth factor: A mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997, 8, 171–179. [Google Scholar] [CrossRef]
- Leask, A.; Denton, C.P.; Abraham, D.J. Insights into the molecular mechanism of chronic fibrosis: The role of connective tissue growth factor in scleroderma. J. Investig. Dermatol. 2004, 122, 1–6. [Google Scholar] [CrossRef]
- Sato, S.; Nagaoka, T.; Hasegawa, M.; Tamatani, T.; Nakanishi, T.; Takigawa, M.; Takehara, K. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: Association with extent of skin sclerosis and severity of pulmonary fibrosis. J. Rheumatol. 2000, 27, 149–154. [Google Scholar]
- Igarashi, A.; Nashiro, K.; Kikuchi, K.; Sato, S.; Ihn, H.; Grotendorst, G.R.; Takehara, K. Significant correlation between connective tissue growth factor gene expression and skin sclerosis in tissue sections from patients with systemic sclerosis. J. Investig. Dermatol. 1995, 105, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Bogatkevich, G.S.; Ludwicka-Bradley, A.; Singleton, C.B.; Bethard, J.R.; Silver, R.M. Proteomic analysis of CTGF-activated lung fibroblasts: Identification of IQGAP1 as a key player in lung fibroblast migration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L603–L611. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, N.; Chu, H.Y.; Yu, Y.; Zhang, Z.K.; Zhang, G.; Zhang, B.T. Connective Tissue Growth Factor: From Molecular Understandings to Drug Discovery. Front. Cell Dev. Biol. 2020, 8, 593269. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003, 81, 355–363. [Google Scholar] [CrossRef]
- Tejera-Muñoz, A.; Marquez-Exposito, L.; Tejedor-Santamaría, L.; Rayego-Mateos, S.; Orejudo, M.; Suarez-Álvarez, B.; López-Larrea, C.; Ruíz-Ortega, M.; Rodrigues-Díez, R.R. CCN2 Increases TGF-β Receptor Type II Expression in Vascular Smooth Muscle Cells: Essential Role of CCN2 in the TGF-β Pathway Regulation. Int. J. Mol. Sci. 2021, 23, 375. [Google Scholar] [CrossRef]
- Grotendorst, G.R.; Duncan, M.R. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005, 19, 729–738. [Google Scholar] [CrossRef]
- Zaykov, V.; Chaqour, B. The CCN2/CTGF interactome: An approach to understanding the versatility of CCN2/CTGF molecular activities. J. Cell Commun. Signal. 2021, 15, 567–580. [Google Scholar] [CrossRef]
- Trojanowska, M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology 2008, 47 (Suppl. S5), v2–v4. [Google Scholar] [CrossRef]
- Ludwicka, A.; Ohba, T.; Trojanowska, M.; Yamakage, A.; Strange, C.; Smith, E.A.; Leroy, E.C.; Sutherland, S.; Silver, R.M. Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J. Rheumatol. 1995, 22, 1876–1883. [Google Scholar]
- Antoniu, S.A. Targeting PDGF pathway in pulmonary arterial hypertension. Expert Opin. Ther. Targets 2012, 16, 1055–1063. [Google Scholar] [CrossRef]
- Baroni, S.S.; Santillo, M.; Bevilacqua, F.; Luchetti, M.; Spadoni, T.; Mancini, M.; Fraticelli, P.; Sambo, P.; Funaro, A.; Kazlauskas, A.; et al. Stimulatory Autoantibodies to the PDGF Receptor in Systemic Sclerosis. N. Engl. J. Med. 2006, 354, 2667–2676. [Google Scholar] [CrossRef]
- Gabrielli, A.; Svegliati, S.; Moroncini, G.; Luchetti, M.; Tonnini, C.; Avvedimento, E.V. Stimulatory autoantibodies to the PDGF receptor: A link to fibrosis in scleroderma and a pathway for novel therapeutic targets. Autoimmun. Rev. 2007, 7, 121–126. [Google Scholar] [CrossRef]
- Paolini, C.; Agarbati, S.; Benfaremo, D.; Mozzicafreddo, M.; Svegliati, S.; Moroncini, G. PDGF/PDGFR: A Possible Molecular Target in Scleroderma Fibrosis. Int. J. Mol. Sci. 2022, 23, 3904. [Google Scholar] [CrossRef] [PubMed]
- Gospodarowicz, D.; Neufeld, G.; Schweigerer, L. Fibroblast growth factor: Structural and biological properties. J. Cell. Physiol. Suppl. 1987, 133 (Suppl. S5), 15–26. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, Y.; Smith, E.A.; LeRoy, E.C.; Trojanowska, M. Basic fibroblast growth factor inhibits basal and transforming growth factor-beta induced collagen α 2(I) gene expression in scleroderma and normal fibroblasts. J. Rheumatol. 1997, 24, 90–95. [Google Scholar]
- Shimbori, C.; Bellaye, P.S.; Xia, J.; Gauldie, J.; Ask, K.; Ramos, C.; Becerril, C.; Pardo, A.; Selman, M.; Kolb, M. Fibroblast growth factor-1 attenuates TGF-β1-induced lung fibrosis. J. Pathol. 2016, 240, 197–210. [Google Scholar] [CrossRef]
- Lawrence, A.; Khanna, D.; Misra, R.; Aggarwal, A. Increased expression of basic fibroblast growth factor in skin of patients with systemic sclerosis. Dermatol. Online J. 2006, 12, 2. [Google Scholar] [CrossRef]
- Ferrara, N.; Henzel, W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989, 161, 851–858. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Abraham, J.A.; Schilling, J. Isolation and characterization of a vascular endothelial cell mitogen produced by pituitary-derived folliculo stellate cells. Proc. Natl. Acad. Sci. USA 1989, 86, 7311–7315. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, L.; Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 2023, 24, 816–834. [Google Scholar] [CrossRef]
- Choi, J.J.; Min, D.J.; Cho, M.L.; Min, S.Y.; Kim, S.J.; Lee, S.S.; Park, K.S.; Seo, Y.I.; Kim, W.U.; Park, S.H.; et al. Elevated vascular endothelial growth factor in systemic sclerosis. J. Rheumatol. 2003, 30, 1529–1533. [Google Scholar] [PubMed]
- Distler, O.; Distler, J.H.; Scheid, A.; Acker, T.; Hirth, A.; Rethage, J.; Michel, B.A.; Gay, R.E.; Müller-Ladner, U.; Matucci-Cerinic, M. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ. Res. 2004, 95, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Flower, V.A.; Barratt, S.L.; Ward, S.; Pauling, J.D. The Role of Vascular Endothelial Growth Factor in Systemic Sclerosis. Curr. Rheumatol. Rev. 2019, 15, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Palevsky, H.I. Pulmonary arterial hypertension related to connective tissue disease: A review. Rheum. Dis. Clin. N. Am. 2014, 40, 103–124. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Zakynthinos, E.; Kostikas, K.; Kiropoulos, T.; Koutsokera, A.; Ziogas, A.; Koutroumpas, A.; Sakkas, L.; Gourgoulianis, K.I.; Daniil, Z.D. Serum VEGF levels are related to the presence of pulmonary arterial hypertension in systemic sclerosis. BMC Pulm. Med. 2009, 9, 18. [Google Scholar] [CrossRef]
- McMahan, Z.; Schoenhoff, F.; Van Eyk, J.E.; Wigley, F.M.; Hummers, L.K. Biomarkers of pulmonary hypertension in patients with scleroderma: A case–control study. Arthritis Res. Ther. 2015, 17, 201. [Google Scholar] [CrossRef]
- Manetti, M.; Guiducci, S.; Romano, E.; Ceccarelli, C.; Bellando-Randone, S.; Conforti, M.L.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ. Res. 2011, 109, e14–e26. [Google Scholar] [CrossRef]
- Song, F.; Zhou, X.X.; Hu, Y.; Li, G.; Wang, Y. The Roles of Insulin-Like Growth Factor Binding Protein Family in Development and Diseases. Adv. Ther. 2021, 38, 885–903. [Google Scholar] [CrossRef]
- LeRoith, D.; Holly, J.M.P.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef]
- Hsu, E.; Feghali-Bostwick, C.A. Insulin-like growth factor-II is increased in systemic sclerosis-associated pulmonary fibrosis and contributes to the fibrotic process via Jun N-terminal kinase- and phosphatidylinositol-3 kinase-dependent pathways. Am. J. Pathol. 2008, 172, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.M.; Hsu, E.; Thomas, J.M.; Pilewski, J.M.; Feghali-Bostwick, C. Insulin-like growth factor (IGF)-II- mediated fibrosis in pathogenic lung conditions. PLoS ONE. 2019, 14, e0225422. [Google Scholar] [CrossRef] [PubMed]
- Waldrep, K.M.; Rodgers, J.I.; Garrett, S.M.; Wolf, B.J.; Feghali-Bostwick, C.A. The Role of SOX9 in IGF-II-Mediated Pulmonary Fibrosis. Int. J. Mol. Sci. 2023, 24, 11234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, L.; Song, B.; Cui, Z.; Chen, G.; Yu, Z.; Song, B. Insulin-like Growth Factor-2 (IGF-2) in Fibrosis. Biomolecules 2022, 12, 1557. [Google Scholar] [CrossRef]
- Jimenez, S.A.; Gaidarova, S.; Saitta, B.; Sandorfi, N.; Herrich, D.J.; Rosenbloom, J.C.; Kucich, U.; Abrams, W.R.; Rosenbloom, J. Role of protein kinase C-delta in the regulation of collagen gene expression in scleroderma fibroblasts. J. Clin. Investig. 2001, 108, 1395–1403. [Google Scholar] [CrossRef]
- Wermuth, P.J.; Addya, S.; Jimenez, S.A. Effect of protein kinase C delta (PKC-δ) inhibition on the transcriptome of normal and systemic sclerosis human dermal fibroblasts in vitro. PLoS ONE 2011, 6, e27110. [Google Scholar] [CrossRef]
- Zohlman, A.; Kamiya, K.; Kato, K.; Salvarezza, S.B.; Doty, S.B.; Wang, C.; Tsai, S.; Rodriguez-Boulan, E.; Kent, K.C.; Kato, K.; et al. Protein kinase C delta is necessary for the secretion of collagen type I from vascular smooth muscle cells. FASEB J. 2008, 22, 609. [Google Scholar] [CrossRef]
- Runyan, C.E.; Schnaper, H.W.; Poncelet, A.C. Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am. J. Physiol. Ren. Physiol. 2004, 285, F413–F422. [Google Scholar] [CrossRef]
- Jinnin, M.; Ihn, H.; Yamane, K.; Mimura, Y.; Asano, Y.; Tamaki, K. Alpha2(I) collagen gene regulation by protein kinase C signaling in human dermal fibroblasts. Nucleic Acids Res. 2005, 33, 1337–1351. [Google Scholar] [CrossRef]
- Mikelis, C.M.; Palmby, T.R.; Simaan, M.; Li, W.; Szabo, R.; Lyons, R.; Martin, D.; Yagi, H.; Fukuhara, S.; Chikumi, H.; et al. PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J. Biol. Chem. 2013, 288, 12232–12243. [Google Scholar] [CrossRef]
- Wuhanqimuge; Itakura, A.; Matsuki, Y.; Tanaka, M.; Arioka, M. Lysophosphatidylcholine enhances NGF-induced MAPK and Akt signals through the extracellular domain of TrkA in PC12 cells. FEBS Open Bio 2013, 3, 243–251. [Google Scholar] [CrossRef]
- Mendoza, F.A.; Jimenez, S.A. Serine/threonine kinase inhibition as antifibrotic therapy: Transforming growth factor-β and Rho kinase inhibitors. Rheumatology 2022, 61, 1354–1365. [Google Scholar] [CrossRef]
- Tokumura, A.; Carbone, L.D.; Yoshioka, Y.; Morishige, J.; Kikuchi, M.; Postlethwaite, A.; Watsky, M.A. Elevated serum levels of arachidonoyl-lysophosphatidic acid and sphingosine 1-phosphate in systemic sclerosis. Int. J. Med. Sci. 2009, 6, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ledein, L.; Léger, B.; Dees, C.; Beyer, C.; Distler, A.; Vettori, S.; Boukaiba, R.; Bidouard, J.P.; Schaefer, M.; Pernerstorfer, J.; et al. Translational engagement of lysophosphatidic acid receptor 1 in skin fibrosis: From dermal fibroblasts of patients with scleroderma to tight skin 1 mouse. Br. J. Pharmacol. 2020, 177, 4296–4309. [Google Scholar] [CrossRef] [PubMed]
- Castelino, F.V.; Bain, G.; Pace, A.; Black, K.; George, L.; Probst, C.K.; Goulet, L.; Lafyatis, R.; Tager, A.M. An Autotaxin/Lysophosphatidic Acid/Interleukin-6 Amplification Loop Drives Scleroderma Fibrosis. Arthritis Rheumatol. 2016, 68, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Distler, O.; Jagerschmidt, A.; Illiano, S.; Ledein, L.; Boitier, E.; Agueusop, I.; Denton, C.P.; Khanna, D. Lysophosphatidic acid receptor 1 antagonist SAR100842 for patients with diffuse cutaneous systemic sclerosis: A double-blind, randomized, eight-week placebo-controlled study followed by a sixteen-week open-label extension study. Arthritis Rheumatol. 2018, 70, 1634–1643. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P.; Furst, D.E.; Mayes, M.D.; Matucci-Cerinic, M.; Smith, V.; de Vries, D.; Ford, P.; Bauer, Y.; Randall, M.J.; et al. A 24-Week, Phase IIa, Randomized, Double-Blind, Placebo-Controlled Study of Ziritaxestat in Early Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol. 2023, 75, 1434–1444. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Denton, C.P.; Kolb, M.; Wijsenbeek-Lourens, M.S.; Emson, C.; Hudson, K.; Amatucci, A.J.; Distler, O.; Allanore, Y.; Khanna, D. Lysophosphatidic acid receptor 1 inhibition: A potential treatment target for pulmonary fibrosis. Eur. Respir. Rev. 2024, 33, 240015. [Google Scholar] [CrossRef]
- A Multicenter Trial to Evaluate the Efficacy, Safety, Tolerability and Pharmacokinetics of HZN-825 in Patients with Diffuse Cutaneous Systemic Sclerosis; ClinicalTrials.gov Identifier: NCT04781543 Accessed 8 September 2025; ClinicalTrials.gov: Bethesda, MD, USA, 2025.
- Williams, T.M.; Lisanti, M.P. The caveolin proteins. Genome Biol. 2004, 5, 214. [Google Scholar] [CrossRef]
- Pang, L.; Yang, S.; Dai, W.; Wu, S.; Kong, J. Role of caveolin-1 in human organ function and disease: Friend or foe? Carcinogenesis 2022, 43, 2–11. [Google Scholar] [CrossRef]
- Gvaramia, D.; Blaauboer, M.E.; Hanemaaijer, R.; Everts, V. Role of caveolin-1 in fibrotic diseases. Matrix Biol. 2013, 32, 307–315. [Google Scholar] [CrossRef]
- Di Guglielmo, G.M.; Le Roy, C.; Goodfellow, A.F.; Wrana, J.L. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 2003, 5, 410–421. [Google Scholar] [CrossRef]
- He, K.; Yan, X.; Li, N.; Dang, S.; Xu, L.; Zhao, B.; Li, Z.; Lv, Z.; Fang, X.; Zhang, Y.; et al. Internalization of the TGF-β type I receptor into caveolin-1 and EEA1 double-positive early endosomes. Cell Res. 2015, 25, 738–752. [Google Scholar] [CrossRef]
- Del Galdo, F.; Sotgia, F.; de Almeida, C.J.; Jasmin, J.F.; Musick, M.; Lisanti, M.P.; Jiménez, S.A. Decreased expression of caveolin 1 in patients with systemic sclerosis: Crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheumatol. 2008, 58, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Del Galdo, F.; Lisanti, M.P.; Jimenez, S.A. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr. Opin. Rheumatol. 2008, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Allanore, Y.; Saad, M.; Fatini, C.; Cohignac, V.; Guiducci, S.; Romano, E.; Airó, P.; Caramaschi, P.; Tinazzi, I.; et al. Evidence for caveolin-1 as a new susceptibility gene regulating tissue fibrosis in systemic sclerosis. Ann. Rheum. Dis. 2012, 71, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wermuth, P.J.; Benn, B.S.; Lisanti, M.P.; Jimenez, S.A. Caveolin-1 deficiency induces spontaneous endothelial-to-mesenchymal transition in murine pulmonary endothelial cells in vitro. Am. J. Pathol. 2013, 182, 325–331. [Google Scholar] [CrossRef]
- Tourkina, E.; Richard, M.; Gööz, P.; Bonner, M.; Pannu, J.; Harley, R.; Bernatchez, P.N.; Sessa, W.C.; Silver, R.M.; Hoffman, S. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L843–L861. [Google Scholar] [CrossRef]
- Reese, C.F.; Chinnakkannu, P.; Tourkina, E.; Hoffman, S.; Kuppuswamy, D. Multiple subregions within the caveolin-1 scaffolding domain inhibit fibrosis, microvascular leakage, and monocyte migration. PLoS ONE 2022, 17, e0264413. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Luo, F. The Role of JAK/STAT Pathway in Fibrotic Diseases: Molecular and Cellular Mechanisms. Biomolecules 2023, 13, 119. [Google Scholar] [CrossRef]
- Dees, C.; Tomcik, M.; Palumbo-Zerr, K.; Distler, A.; Beyer, C.; Lang, V.; Horn, A.; Zerr, P.; Zwerina, J.; Gelse, K.; et al. JAK-2 as a novel mediator of the pro-fibrotic effects of transforming growth factor β in systemic sclerosis. Arthritis Rheum. 2012, 64, 3006–3015. [Google Scholar] [CrossRef]
- Deverapalli, S.C.; Rosmarin, D. The use of JAK inhibitors in the treatment of progressive systemic sclerosis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e328. [Google Scholar] [CrossRef] [PubMed]
- Moriana, C.; Moulinet, T.; Jaussaud, R.; Decker, P. JAK inhibitors and systemic sclerosis: A systematic review of the literature. Autoimmun. Rev. 2022, 21, 103168. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Bothwell, A.L. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol. Cell 2012, 33, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Luo, S.; Zhan, Y.; Lu, Q. The roles of PPARγ and its agonists in autoimmune diseases: A comprehensive review. J. Autoimmun. 2020, 113, 102510. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bhattacharyya, S.; Lakos, G.; Chen, S.J.; Mori, Y.; Varga, J. Disruption of transforming growth factor beta signaling and pro-fibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2004, 50, 1305–1318. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Cheng, T.; Zheng, M.H.; Yi, C.G.; Pan, H.; Li, Z.J.; Chen, X.L.; Yu, Q.; Jiang, L.F.; Zhou, F.Y.; et al. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonist inhibits transforming growth factor-beta1 and matrix production in human dermal fibroblasts. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1209–1216. [Google Scholar] [CrossRef]
- Kapoor, M.; McCann, M.; Liu, S.; Huh, K.; Denton, C.P.; Abraham, D.J.; Leask, A. Loss of peroxisome proliferator-activated receptor gamma in mouse fibroblasts results in increased susceptibility to bleomycin-induced skin fibrosis. Arthritis Rheum. 2009, 60, 2822–2829. [Google Scholar] [CrossRef]
- Wei, J.; Ghosh, A.K.; Sargent, J.L.; Komura, K.; Wu, M.; Huang, Q.Q.; Jain, M.; Whitfield, M.L.; Feghali-Bostwick, C.; Varga, J. PPARγ downregulation by TGFß in fibroblast and impaired expression and function in systemic sclerosis: A novel mechanism for progressive fibrogenesis. PLoS ONE. 2010, 5, e13778. [Google Scholar] [CrossRef]
- Wei, J.; Bhattacharyya, S.; Varga, J. Peroxisome proliferator-activated receptor γ innate protection from excessive fibrogenesis and potential therapeutic target in systemic sclerosis. Curr. Opin. Rheumatol. 2010, 22, 671–676. [Google Scholar] [CrossRef]
- Ghosh, A.K. Pharmacological activation of PPAR-γ: A potential therapy for skin fibrosis. Int. J. Dermatol. 2021, 60, 376–383. [Google Scholar] [CrossRef]
- Beyer, C.; Distler, J.H. Morphogen pathways in systemic sclerosis. Curr. Rheumatol. Rep. 2013, 15, 299. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Dees, C.; Distler, J.H. Morphogen pathways as molecular targets for the treatment of fibrosis in systemic sclerosis. Arch. Dermatol. Res. 2013, 305, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Teves, M.E.; Varga, J. The dynamic organelle primary cilia: Emerging roles in organ fibrosis. Curr. Opin. Rheumatol. 2021, 33, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Distler, J.H. Canonical Wnt signaling in systemic sclerosis. Lab. Investig. 2016, 96, 151–155. [Google Scholar] [CrossRef]
- Griffin, M.F.; Huber, J.; Evan, F.J.; Quarto, N.; Longaker, M.T. The role of Wnt signaling in skin fibrosis. Med. Res. Rev. 2022, 42, 615–628. [Google Scholar] [CrossRef]
- Tinazzi, I.; Mulipa, P.; Colato, C.; Abignano, G.; Ballarin, A.; Biasi, D.; Emery, P.; Ross, R.L.; Del Galdo, F. SFRP4 Expression Is Linked to Immune-Driven Fibrotic Conditions, Correlates with Skin and Lung Fibrosis in SSc and a Potential EMT Biomarker. J. Clin. Med. 2021, 10, 5820. [Google Scholar] [CrossRef]
- Beyer, C.; Reichert, H.; Akan, H.; Mallano, T.; Schramm, A.; Dees, C.; Palumbo-Zerr, K.; Lin, N.Y.; Distler, A.; Gelse, K.; et al. Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Ann. Rheum. Dis. 2013, 72, 1255–1258. [Google Scholar] [CrossRef]
- Jarman, E.J.; Boulter, L. Targeting the Wnt signaling pathway: The challenge of reducing scarring without affecting repair. Expert Opin. Investig. Drugs 2020, 29, 179–190. [Google Scholar] [CrossRef]
- Castelino, F.V.; Varga, J. Emerging cellular and molecular targets in fibrosis: Implications for scleroderma pathogenesis and targeted therapy. Curr. Opin. Rheumatol. 2014, 26, 607–614. [Google Scholar] [CrossRef]
- Prince, E.; Marcetteau, J.; Thérond, P.P. Circulating Hedgehog: A fresh view of a classic morphogen. Development 2020, 147, dev186395. [Google Scholar] [CrossRef]
- Matusek, T.; Marcetteau, J.; Thérond, P.P. Functions of Wnt and Hedgehog-containing extracellular vesicles in development and disease. J. Cell Sci. 2020, 133, jcs209742. [Google Scholar] [CrossRef]
- Leask, A.; Naik, A.; Stratton, R.J. Back to the future: Targeting the extracellular matrix to treat systemic sclerosis. Nat. Rev. Rheumatol. 2023, 19, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Palumbo, K.; Cordazzo, C.; Dees, C.; Akhmetshina, A.; Tomcik, M.; Zerr, P.; Avouac, J.; Gusinde, J.; Zwerina, J.; et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 2012, 64, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T. The Hedgehog Signaling Pathway in Idiopathic Pulmonary Fibrosis: Resurrection Time. Int. J. Mol. Sci. 2021, 23, 171. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Cao, H.; Chen, L.; Hou, J.; Xiang, Z.; Hu, K.; Han, X. The hedgehog and Wnt/β-catenin system machinery mediate myofibroblast differentiation of LR-MSCs in pulmonary fibrogenesis. Cell Death Dis. 2018, 9, 639. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, G.F.; Liao, X.P.; Li, X.J.; Zhang, J.; Lin, H.; Chen, Z.; Zhang, X. Anti-fibrotic effects of pirfenidone by interference with the hedgehog signalling pathway in patients with systemic sclerosis-associated interstitial lung disease. Int. J. Rheum. Dis. 2018, 21, 477–486. [Google Scholar] [CrossRef]
- Lui, P.P.; Xu, J.Z.; Aziz, H.; Sen, M.; Ali, N. Jagged-1+ skin Tregs modulate cutaneous wound healing. Sci. Rep. 2024, 14, 20999. [Google Scholar] [CrossRef]
- Dees, C.; Tomcik, M.; Zerr, P.; Akhmetshina, A.; Horn, A.; Palumbo, K.; Beyer, C.; Zwerina, J.; Distler, O.; Schett, G.; et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann. Rheum. Dis. 2011, 70, 1304–1310. [Google Scholar] [CrossRef]
- Condorelli, A.G.; El Hachem, M.; Zambruno, G.; Nystrom, A.; Candi, E.; Castiglia, D. Notch-ing up knowledge on molecular mechanisms of skin fibrosis: Focus on the multifaceted Notch signalling pathway. J. Biomed. Sci. 2021, 28, 36. [Google Scholar] [CrossRef]
- Seguro Paula, F.; Delgado Alves, J. The role of the Notch pathway in the pathogenesis of systemic sclerosis: Clinical implications. Expert Rev. Clin. Immunol. 2021, 17, 1257–1267. [Google Scholar] [CrossRef]
- Zmorzyński, S.; Styk, W.; Filip, A.A.; Krasowska, D. The Significance of NOTCH Pathway in the Development of Fibrosis in Systemic Sclerosis. Ann. Dermatol. 2019, 31, 365–371. [Google Scholar] [CrossRef]
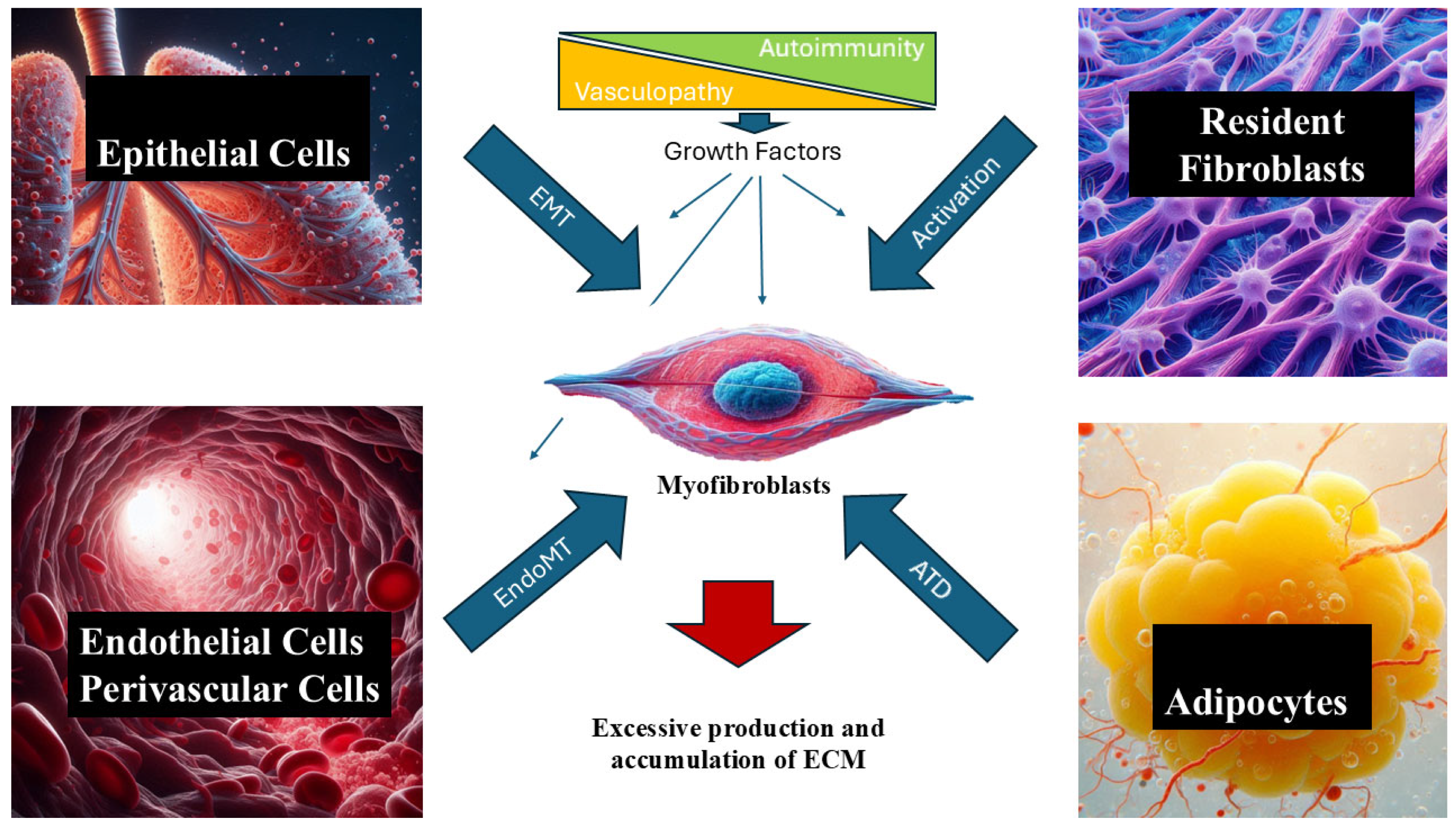
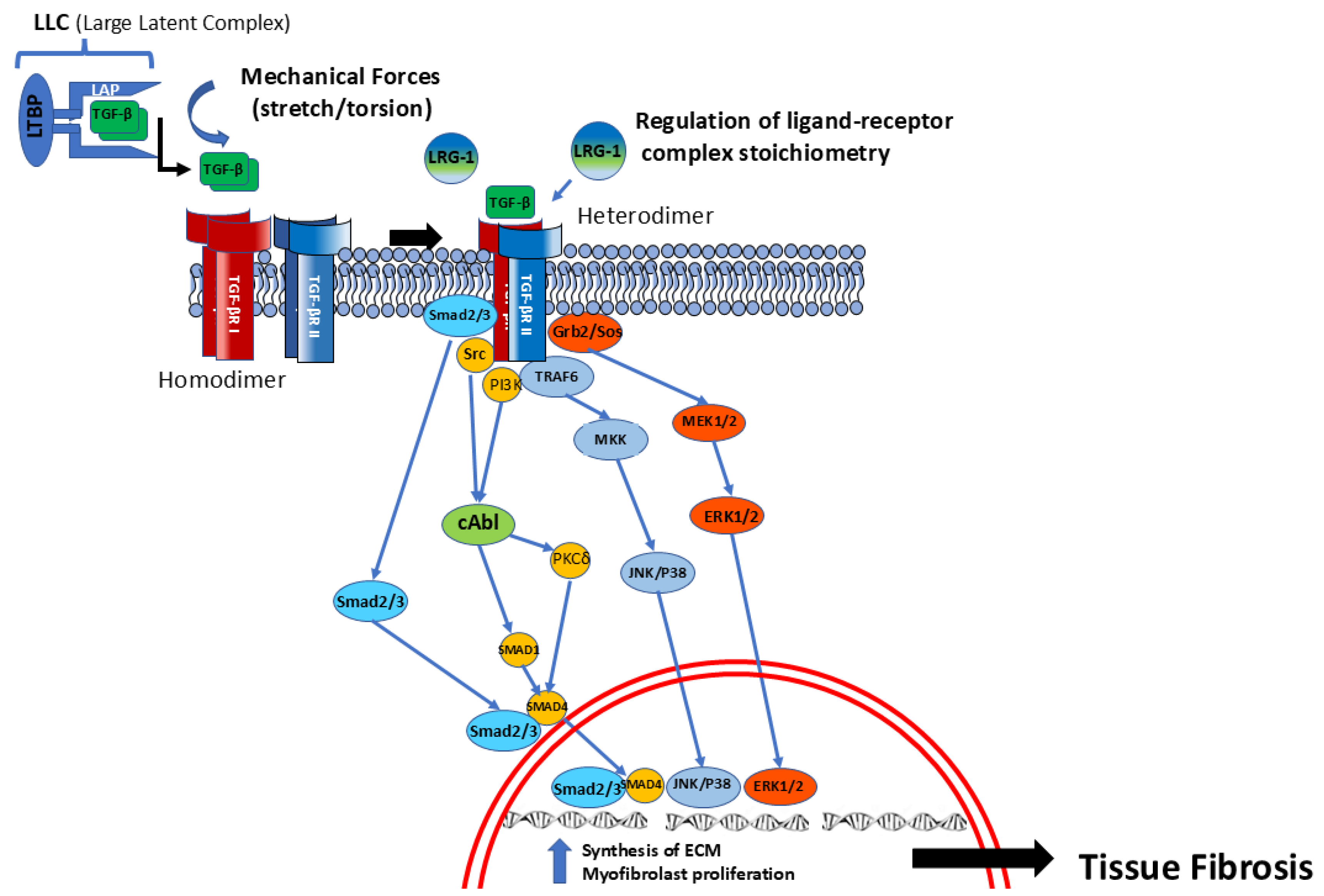
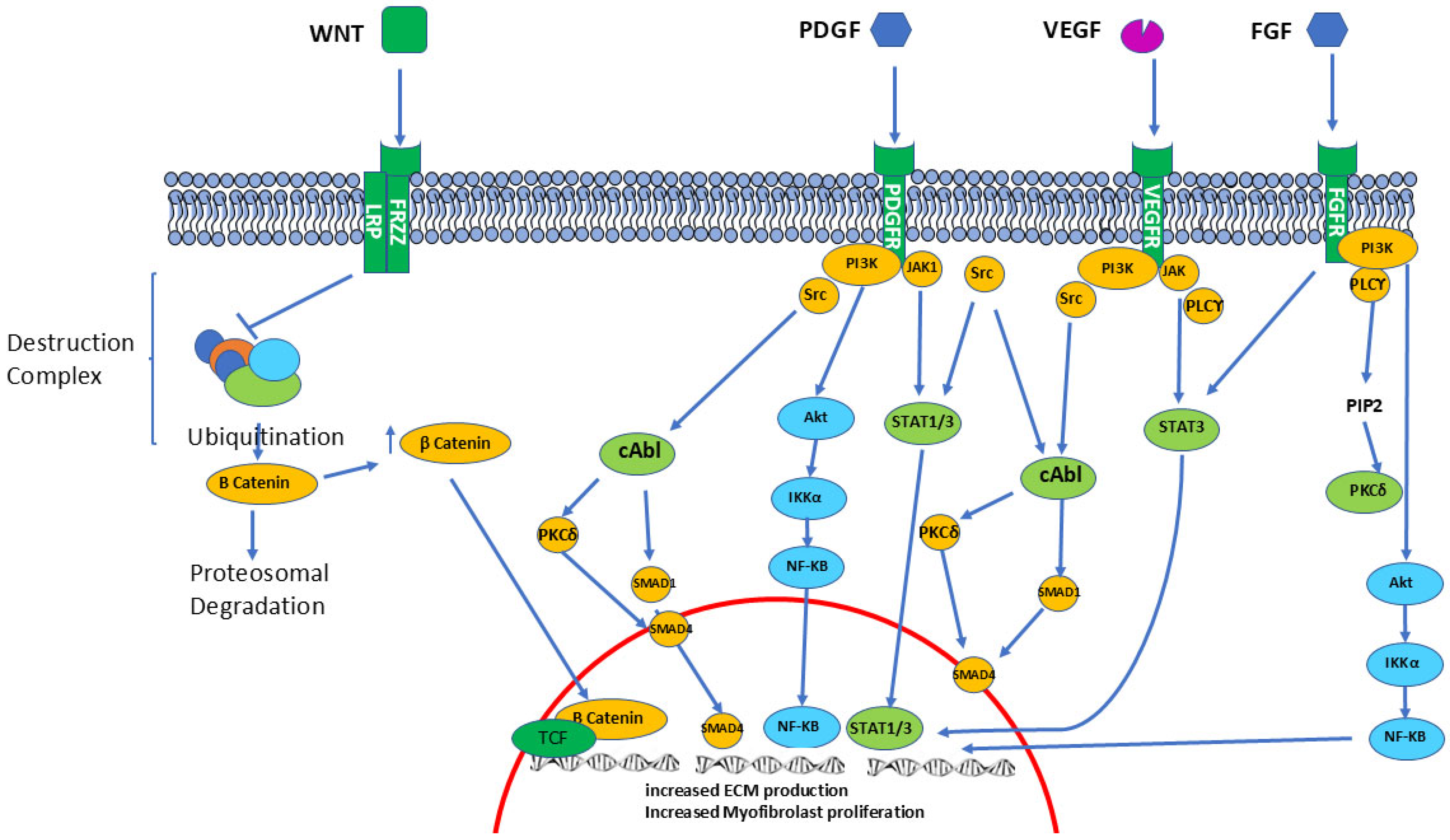
| Growth Factor(s) | Receptor(s) | Target Cell(s) | Effect in SSc | References |
|---|---|---|---|---|
| TGF-β (TGF-β1,2,3) | TβRI (ALK5 in fibroblasts, ALK1 in endothelial cells), TβRII, TβRIII, integrins (αvβ6) | Fibroblasts, endothelial cells, epithelial cells, adipocytes, vascular smooth muscle cells | Strongly pro-fibrotic: ↑ collagen I/III, TIMPs, ECM proteins; ↓ MMPs; induces fibroblast→myofibroblast transdifferentiation; EndoMT, EMT; angiogenesis (context-dependent) | [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,107,108,109,125,126,127,133,134,135,136,137,138,139,140] |
| CTGF (CCN2) | Interacts with TGF-βRII, integrins, EGFR, ECM proteins | Fibroblasts, endothelial cells, vascular smooth muscle cells | Mediates downstream TGF-β fibrogenic effects; fibroblast proliferation, myofibroblast differentiation, vascular changes | [67,68,69,70,71,72,73,74,75,76,77] |
| PDGF (A–D) | PDGFR-α, PDGFR-β (RTKs) | Fibroblasts, vascular smooth muscle cells | Potent mitogen; ↑ fibroblast and SMC proliferation; promotes pulmonary fibrosis and PAH; PDGFR-α autoantibodies activate fibroblasts → ROS, ERK pathway | [78,79,80,81,82,83] |
| FGFs (esp. FGF-2) | FGFRs (RTKs) | Fibroblasts, endothelial cells | Mitogenic, angiogenic; ↑ FGF-2 in SSc skin; context-dependent: pro-fibrotic or antifibrotic (FGF-1 inhibits TGF-β1 effects) | [84,85,86,87,88] |
| VEGF (VEGF-A, VEGF165, VEGF165b) | VEGFR-1, VEGFR-2 | Endothelial cells, fibroblasts | ↑ in SSc serum; correlates with fibrosis and capillary loss; VEGF165b isoform antiangiogenic → defective angiogenesis | [89,90,91,92,93,94,95,96,97,98] |
| IGFs (IGF1,IGF-2) | IGF-1R, regulated by IGFBPs | Fibroblasts, endothelial cells, skin & lung cells | ↑ IGF-1 and IGFBP-3 in SSc serum; IGF-2 promotes fibroblast activation via PI3K/JNK; ↑ collagen and FN | [99,100,101,102,103,104] |
| PKC-δ | Downstream of TGF-βR; intracellular kinase | Fibroblasts, endothelial cells, SMCs, mesangial cells | Modulates TGF-β/Smad signaling; ↑ collagen expression via phosphorylation cascades; higher in SSc fibroblasts | [105,106,107,108,109] |
| PI3K Pathway | PI3K receptors; interacts with endothelin receptor (ETA) | Fibroblasts, immune cells | ↑ PI3K activity in SSc platelets; regulates COL1A2; Rac/PI3K pathway promotes myofibroblast activation | [106,107,108,109] |
| Lysophosphatidic Acid (LPA) | LPA receptors (LPARs, GPCR family) | Fibroblasts, endothelial cells | Potent profibrotic mitogen; activates Rho/ROCK pathway → myofibroblast differentiation; amplification loop with IL-6 | [110,111,112,113,114,115,116,117,118,119] |
| Caveolin-1 | Regulates TGF-βR trafficking | Fibroblasts, endothelial cells | Caveolin-1 loss → uncontrolled TGF-β activation; ↓ Caveolin-1 induces EndoMT; restoration prevents fibrosis and PAH in models | [120,121,122,123,124,125,126,127,128,129,130] |
| JAK/STAT Pathway | JAK kinases, STAT proteins | Fibroblasts, immune cells | Amplifies TGF-β and cytokine (IL-4, IL-6, IL-13) effects; promotes fibroblast→myofibroblast transition | [131,132,133,134] |
| PPAR-γ | Nuclear receptor (PPAR family) | Fibroblasts, adipocytes | Normally antifibrotic; ↓ PPAR-γ in SSc → exaggerated fibrosis; agonists restore balance | [135,136,137,138,139,140,141,142] |
| Wnt Pathway | Frizzled receptors, LRP5/6 (canonical) | Fibroblasts, endothelial cells | TGF-β activates canonical Wnt → profibrotic; ↑ SFRP4 in SSc serum correlates with fibrosis severity | [147,148,149,150,151,152] |
| Hedgehog (Hh) | Patched (PTCH), Smoothened (SMO) | Fibroblasts | Overexpression induced by TGF-β, PDGF, Wnt; promotes fibroblast→myofibroblast differentiation, ↑ collagen | [157,158,159,160] |
| Notch | Notch receptors (Notch1–4), ligands (Jag-1, DLL) | Fibroblasts, T cells | Jag-1+ T cells activate Notch in dermal fibroblasts → myofibroblast transition; ↑ ECM production | [161,162,163,164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, F.A.; Piera-Velazquez, S.; Jimenez, S.A. Role of Growth Factors in the Pathogenesis of Systemic-Sclerosis-Associated Fibrosis. Int. J. Mol. Sci. 2025, 26, 9596. https://doi.org/10.3390/ijms26199596
Mendoza FA, Piera-Velazquez S, Jimenez SA. Role of Growth Factors in the Pathogenesis of Systemic-Sclerosis-Associated Fibrosis. International Journal of Molecular Sciences. 2025; 26(19):9596. https://doi.org/10.3390/ijms26199596
Chicago/Turabian StyleMendoza, Fabian A., Sonsoles Piera-Velazquez, and Sergio A. Jimenez. 2025. "Role of Growth Factors in the Pathogenesis of Systemic-Sclerosis-Associated Fibrosis" International Journal of Molecular Sciences 26, no. 19: 9596. https://doi.org/10.3390/ijms26199596
APA StyleMendoza, F. A., Piera-Velazquez, S., & Jimenez, S. A. (2025). Role of Growth Factors in the Pathogenesis of Systemic-Sclerosis-Associated Fibrosis. International Journal of Molecular Sciences, 26(19), 9596. https://doi.org/10.3390/ijms26199596





