Gut Permeability and Microbiota in Parkinson’s Disease: Mechanistic Insights and Experimental Therapeutic Strategies
Abstract
1. Introduction
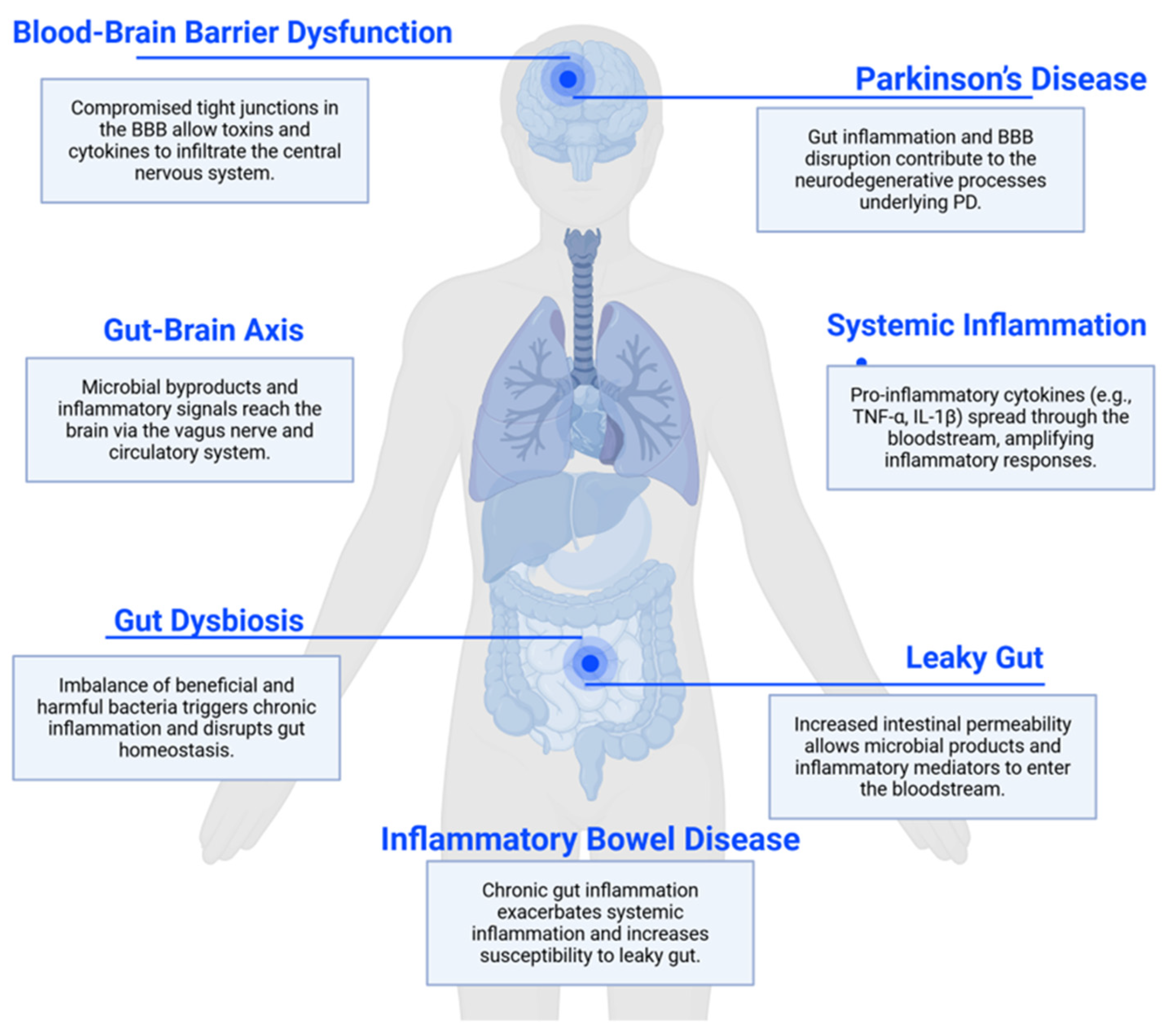
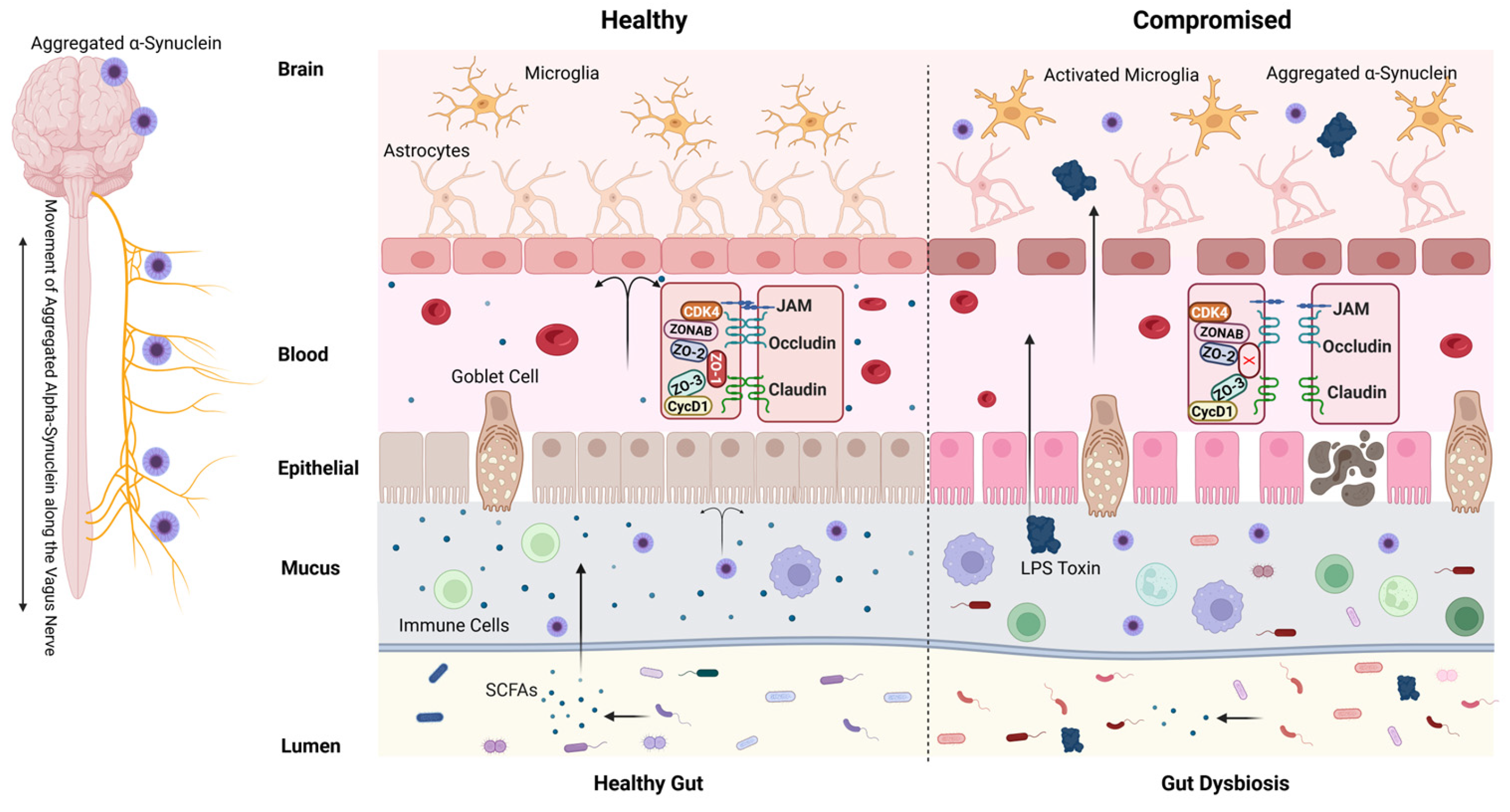
2. Intestinal Barrier and Gut Permeability
3. Disruptions in Intestinal Permeability and PD Risk
4. The Interplay Between Blood–Brain Barrier Integrity and Neurodegenerative Diseases
5. Leaky Gut and Gut Microbiota Dysbiosis
6. PD and Gut Microbiota Dysbiosis
7. Potential Therapeutic Approaches
7.1. Short-Chain Fatty Acids
7.2. Fecal Microbiota Transplantation
7.3. Induced Pluripotent Stem Cell (iPSC)-Based Therapy: A Personalized Regenerative Strategy
7.4. Microbiome Modulation: From Conventional Therapies to Precision Medicine
7.5. Genetic Stratification: Towards Precision Medicine in PD
7.6. Current and Emerging Therapies Targeting Gut Permeability
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| BBB | Blood–brain barrier |
| IBD | Inflammatory bowel disease |
| LPS | Lipopolysaccharides |
| SCFAs | Short-chain fatty acids |
| CNS | Central Nervous System |
References
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Park. Dis. 2018, 4, 21. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e627. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Chang, J.J.; Kulkarni, S.; Pasricha, T.S. Upper Gastrointestinal Mucosal Damage and Subsequent Risk of Parkinson Disease. JAMA Netw. Open 2024, 7, e2431949. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Mohan, M.; Chow, C.T.; Ryan, C.N.; Chan, L.S.; Dufour, J.; Aye, P.P.; Blanchard, J.; Moehs, C.P.; Sestak, K. Dietary Gluten-Induced Gut Dysbiosis Is Accompanied by Selective Upregulation of microRNAs with Intestinal Tight Junction and Bacteria-Binding Motifs in Rhesus Macaque Model of Celiac Disease. Nutrients 2016, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.R.; Lee, Y.R.; Kim, Y.S.; Park, H.Y. Diet-Induced Gut Dysbiosis and Leaky Gut Syndrome. J. Microbiol. Biotechnol. 2024, 34, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–4493. [Google Scholar] [CrossRef]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Gates, E.J.; Bernath, A.K.; Klegeris, A. Modifying the diet and gut microbiota to prevent and manage neurodegenerative diseases. Rev. Neurosci. 2022, 33, 767–787. [Google Scholar] [CrossRef]
- Hu, X.; Mao, C.; Fan, L.; Luo, H.; Hu, Z.; Zhang, S.; Yang, Z.; Zheng, H.; Sun, H.; Fan, Y.; et al. Modeling Parkinson’s Disease Using Induced Pluripotent Stem Cells. Stem Cells Int. 2020, 2020, 1061470. [Google Scholar] [CrossRef] [PubMed]
- Morizane, A. Cell therapy for Parkinson’s disease with induced pluripotent stem cells. Inflamm. Regen. 2023, 43, 16. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Grabacka, M.; Płonka, P.M.; Pierzchalska, M. The PPARα Regulation of the Gut Physiology in Regard to Interaction with Microbiota, Intestinal Immunity, Metabolism, and Permeability. Int. J. Mol. Sci. 2022, 23, 4156. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Kelly, L.P.; Carvey, P.M.; Keshavarzian, A.; Shannon, K.M.; Shaikh, M.; Bakay, R.A.; Kordower, J.H. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov. Disord. 2014, 29, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Carloni, S.; Rescigno, M. Unveiling the gut-brain axis: Structural and functional analogies between the gut and the choroid plexus vascular and immune barriers. Semin. Immunopathol. 2022, 44, 869–882. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Garabedian, E.M.; Roberts, L.J.; McNevin, M.S.; Gordon, J.I. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 1997, 272, 23729–23740. [Google Scholar] [CrossRef]
- Salzman, N.H. Paneth cell defensins and the regulation of the microbiome: Détente at mucosal surfaces. Gut Microbes 2010, 1, 401–406. [Google Scholar] [CrossRef]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Bjerke, K. Immunomorphological characteristics of human Peyer’s patches. Digestion 1990, 46 (Suppl. 2), 262–273. [Google Scholar] [CrossRef] [PubMed]
- Farstad, I.N.; Halstensen, T.S.; Fausa, O.; Brandtzaeg, P. Heterogeneity of M-cell-associated B and T cells in human Peyer’s patches. Immunology 1994, 83, 457–464. [Google Scholar] [PubMed]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology 2008, 135, 194–204.e193. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Z.; Zhao, X. Intestinal receptors for adhesive fimbriae of enterotoxigenic Escherichia coli (ETEC) K88 in swine—A review. Appl. Microbiol. Biotechnol. 2000, 54, 311–318. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public. Health 2021, 18, 2836. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann. N. Y Acad. Sci. 2000, 915, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef]
- Crawford, C.K.; Lopez Cervantes, V.; Quilici, M.L.; Armién, A.G.; Questa, M.; Matloob, M.S.; Huynh, L.D.; Beltran, A.; Karchemskiy, S.J.; Crakes, K.R.; et al. Inflammatory cytokines directly disrupt the bovine intestinal epithelial barrier. Sci. Rep. 2022, 12, 14578. [Google Scholar] [CrossRef]
- Aveleira, C.A.; Lin, C.M.; Abcouwer, S.F.; Ambrósio, A.F.; Antonetti, D.A. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 2010, 59, 2872–2882. [Google Scholar] [CrossRef]
- Ma, T.Y.; Boivin, M.A.; Ye, D.; Pedram, A.; Said, H.M. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G422–430. [Google Scholar] [CrossRef]
- Ye, D.; Ma, I.; Ma, T.Y. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G496–G504. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. Cellular and molecular mechanism of interleukin-1β modulation of Caco-2 intestinal epithelial tight junction barrier. J. Cell Mol. Med. 2011, 15, 970–982. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Vivinus-Nébot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Sun, J.; Shen, X.; Li, Y.; Guo, Z.; Zhu, W.; Zuo, L.; Zhao, J.; Gu, L.; Gong, J.; Li, J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients 2016, 8, 44. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef]
- Bonetti, L.; Horkova, V.; Grusdat, M.; Longworth, J.; Guerra, L.; Kurniawan, H.; Franchina, D.G.; Soriano-Baguet, L.; Binsfeld, C.; Verschueren, C.; et al. A Th17 cell-intrinsic glutathione/mitochondrial-IL-22 axis protects against intestinal inflammation. Cell Metab. 2024, 36, 1726–1744.e1710. [Google Scholar] [CrossRef]
- Lee, J.S.; Tato, C.M.; Joyce-Shaikh, B.; Gulen, M.F.; Cayatte, C.; Chen, Y.; Blumenschein, W.M.; Judo, M.; Ayanoglu, G.; McClanahan, T.K.; et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity 2015, 43, 727–738. [Google Scholar] [CrossRef]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef]
- Paisán-Ruíz, C.; Jain, S.; Evans, E.W.; Gilks, W.P.; Simón, J.; van der Brug, M.; López de Munain, A.; Aparicio, S.; Gil, A.M.; Khan, N.; et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 2004, 44, 595–600. [Google Scholar] [CrossRef]
- Cabezudo, D.; Tsafaras, G.; Van Acker, E.; Van den Haute, C.; Baekelandt, V. Mutant LRRK2 exacerbates immune response and neurodegeneration in a chronic model of experimental colitis. Acta Neuropathol. 2023, 146, 245–261. [Google Scholar] [CrossRef]
- Kars, M.E.; Wu, Y.; Stenson, P.D.; Cooper, D.N.; Burisch, J.; Peter, I.; Itan, Y. The landscape of rare genetic variation associated with inflammatory bowel disease and Parkinson’s disease comorbidity. Genome Med. 2024, 16, 66. [Google Scholar] [CrossRef]
- Herrick, M.K.; Tansey, M.G. Is LRRK2 the missing link between inflammatory bowel disease and Parkinson’s disease? NPJ Park. Dis. 2021, 7, 26. [Google Scholar] [CrossRef]
- Broadway, B.J.; Boneski, P.K.; Bredenberg, J.M.; Kolicheski, A.; Hou, X.; Soto-Beasley, A.I.; Ross, O.A.; Springer, W.; Fiesel, F.C. Systematic Functional Analysis of PINK1 and PRKN Coding Variants. Cells 2022, 11, 2426. [Google Scholar] [CrossRef]
- Sun, S.; Wang, H.; Ma, Q.; Li, N.; Cao, M.; Tam, K.Y.; Ying, Z. PRKN regulates inner mitochondrial membrane PHB2 during mitophagy. Autophagy Rep. 2023, 2, 2164643. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.M.; Silwal, P.; Jo, E.K. Inflammasome and Mitophagy Connection in Health and Disease. Int. J. Mol. Sci. 2020, 21, 4714. [Google Scholar] [CrossRef]
- Recinto, S.J.; Kazanova, A.; Liu, L.; Cordeiro, B.; Premachandran, S.; Bessaiah, H.; Allot, A.; Afanasiev, E.; Mukherjee, S.; Pei, J.; et al. PINK1 deficiency rewires early immune responses in a mouse model of Parkinson’s disease triggered by intestinal infection. NPJ Park. Dis. 2025, 11, 133. [Google Scholar] [CrossRef]
- Natah, S.S.; Mouihate, A.; Pittman, Q.J.; Sharkey, K.A. Disruption of the blood-brain barrier during TNBS colitis. Neurogastroenterol. Motil. 2005, 17, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Kim, S.J.; Howe, C.; Lee, S.; Her, J.Y.; Patel, M.; Kim, G.; Lee, J.; Im, E.; Rhee, S.H. Chronic Intestinal Inflammation Suppresses Brain Activity by Inducing Neuroinflammation in Mice. Am. J. Pathol. 2022, 192, 72–86. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, T.; Cheng, X.; Zhao, M.; Gong, S.H.; Zhao, Y.Q.; Wu, H.T.; Fan, M.; Zhu, L.L. Cortical Inflammation is Increased in a DSS-Induced Colitis Mouse Model. Neurosci. Bull. 2018, 34, 1058–1066. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158, Erratum in Sci. Transl. Med. 2014, 6, 266. [Google Scholar] [CrossRef]
- Kacimi, R.; Giffard, R.G.; Yenari, M.A. Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. J. Inflamm. 2011, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Teng, T.; Yin, B.; He, Y.; Jiang, Y.; Liu, X.; Yu, Y.; Li, X.; Zhou, X. Biomarkers of intestinal permeability and blood-brain barrier permeability in adolescents with major depressive disorder. J. Affect. Disord. 2023, 323, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Huang, Z.; Wong, L.W.; Su, Y.; Huang, X.; Wang, N.; Chen, H.; Yi, C. Blood-brain barrier integrity in the pathogenesis of Alzheimer’s disease. Front. Neuroendocrinol. 2020, 59, 100857. [Google Scholar] [CrossRef]
- Al-Bachari, S.; Naish, J.H.; Parker, G.J.M.; Emsley, H.C.A.; Parkes, L.M. Blood-Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020, 11, 593026. [Google Scholar] [CrossRef]
- Versele, R.; Sevin, E.; Gosselet, F.; Fenart, L.; Candela, P. TNF-α and IL-1β Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-β Peptide Efflux in a Human Blood-Brain Barrier Model. Int. J. Mol. Sci. 2022, 23, 235. [Google Scholar] [CrossRef] [PubMed]
- Akassoglou, K.; Adams, R.A.; Bauer, J.; Mercado, P.; Tseveleki, V.; Lassmann, H.; Probert, L.; Strickland, S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 6698–6703. [Google Scholar] [CrossRef]
- Hollville, E.; Romero, S.E.; Deshmukh, M. Apoptotic cell death regulation in neurons. Febs. J. 2019, 286, 3276–3298. [Google Scholar] [CrossRef]
- Andjelkovic, A.V.; Situ, M.; Citalan-Madrid, A.F.; Stamatovic, S.M.; Xiang, J.; Keep, R.F. Blood-Brain Barrier Dysfunction in Normal Aging and Neurodegeneration: Mechanisms, Impact, and Treatments. Stroke 2023, 54, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; van Boxel-Dezaire, A.H.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018, 507, 274–279. [Google Scholar] [CrossRef]
- Xu, R.; Tan, C.; He, Y.; Wu, Q.; Wang, H.; Yin, J. Dysbiosis of Gut Microbiota and Short-Chain Fatty Acids in Encephalitis: A Chinese Pilot Study. Front. Immunol. 2020, 11, 1994. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Velasco, A.I.; Clemente-Suárez, V.J. Impact of Peripheral Inflammation on Blood-Brain Barrier Dysfunction and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 2440. [Google Scholar] [CrossRef]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Sommer, F.; Nookaew, I.; Sommer, N.; Fogelstrand, P.; Bäckhed, F. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol. 2015, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Zhang, C.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Strain-specific effects of Akkermansia muciniphila on the regulation of intestinal barrier. Food Sci. Human. Wellness 2023, 12, 1526–1537. [Google Scholar] [CrossRef]
- Abdulqadir, R.; Engers, J.; Al-Sadi, R. Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives. Curr. Dev. Nutr. 2023, 7, 102026. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Holowacz, S.; Delannoy, J.; Lenoir, L.; Jacouton, E.; Gervason, S.; Meynier, M.; Boucard, A.-S.; Carvalho, F.A.; Barbut, F.; et al. Serpin-positive Bifidobacterium breve CNCM I-5644 improves intestinal permeability in two models of irritable bowel syndrome. Sci. Rep. 2022, 12, 19776. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Saelee, M.; Chaiyasut, K.; Kesika, P.; et al. Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial. Foods 2022, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol. Dis. 2012, 46, 559–564. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Engen, P.; Bonvegna, S.; Cilia, R. The gut microbiome in Parkinson’s disease: A culprit or a bystander? Prog. Brain Res. 2020, 252, 357–450. [Google Scholar] [CrossRef]
- Lu, Z.; Ding, L.; Lu, Q.; Chen, Y.H. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers 2013, 1, e24978. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. Npj Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Rahmani, E.; Garcia, E.; Jacobs, J.P. Gastrointestinal symptoms are predictive of trajectories of cognitive functioning in de novo Parkinson’s disease. Park. Relat. Disord. 2020, 72, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e1412. [Google Scholar] [CrossRef]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018. [Google Scholar]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Qu, Y.; Li, J.; Wang, D.; Mao, Z.; Xue, Z. Associations of diet-derived short chain fatty acids with Parkinson’s disease: A systematic review and meta-analysis. Nutr. Neurosci. 2025, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. (Lausanne) 2020, 11, 25. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Su, C.W.; Chen, C.Y.; Mao, T.; Chen, N.; Steudel, N.; Jiao, L.; Lan, J.; Fasano, A.; Walker, W.A.; Shi, H.N. Maternal helminth infection protects offspring from high-fat-diet-induced obesity through altered microbiota and SCFAs. Cell Mol. Immunol. 2023, 20, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Su, C.W.; Chen, C.Y.; Jiao, L.; Long, S.R.; Mao, T.; Ji, Q.; O’Donnell, S.; Stanton, C.; Zheng, S.; Walker, W.A.; et al. Helminth-Induced and Th2-Dependent Alterations of the Gut Microbiota Attenuate Obesity Caused by High-Fat Diet. Cell Mol. Gastroenterol. Hepatol. 2020, 10, 763–778. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef]
- Mariadason, J.M.; Catto-Smith, A.; Gibson, P.R. Modulation of distal colonic epithelial barrier function by dietary fibre in normal rats. Gut 1999, 44, 394–399. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Romano, S.; Heinzmann, S.S.; Duncan, A.; Traka, M.H.; Ng, D.; Segovia-Lizano, D.; Simon, M.C.; Narbad, A.; Wüllner, U.; et al. A prebiotic dietary pilot intervention restores faecal metabolites and may be neuroprotective in Parkinson’s Disease. NPJ Park. Dis. 2025, 11, 66. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Liu, S.; Du, J.; Hu, X.; Xiong, J.; Fang, R.; Chen, W.; Sun, J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017, 381, 176–181. [Google Scholar] [CrossRef]
- Paiva, I.; Pinho, R.; Pavlou, M.A.; Hennion, M.; Wales, P.; Schütz, A.L.; Rajput, A.; Szego, É.M.; Kerimoglu, C.; Gerhardt, E.; et al. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum. Mol. Genet. 2017, 26, 2231–2246. [Google Scholar] [CrossRef]
- Ostendorf, F.; Metzdorf, J.; Gold, R.; Haghikia, A.; Tönges, L. Propionic Acid and Fasudil as Treatment Against Rotenone Toxicity in an In Vitro Model of Parkinson’s Disease. Molecules 2020, 25, 2502. [Google Scholar] [CrossRef] [PubMed]
- Metzdorf, J.; Tönges, L. Short-chain fatty acids in the context of Parkinson’s disease. Neural Regen. Res. 2021, 16, 2015–2016. [Google Scholar] [CrossRef]
- Duan, W.X.; Wang, F.; Liu, J.Y.; Liu, C.F. Relationship Between Short-chain Fatty Acids and Parkinson’s Disease: A Review from Pathology to Clinic. Neurosci. Bull. 2024, 40, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, X.; Liu, C.; Zhang, M.; Zhang, X.; Ge, S.; Zhao, L. Neuroprotective effects of short-chain fatty acids in MPTP induced mice model of Parkinson’s disease. Exp. Gerontol. 2021, 150, 111376. [Google Scholar] [CrossRef] [PubMed]
- Boncuk Ulaş, S.; Güzey Aras, Y.; Irmak Gözükara, S.; Acar, T.; Acar, B.A. Correlates of Zonulin and Claudin-5, markers of intestinal and brain endothelial permeability, in Parkinson’s Disease: A pilot study. Park. Relat. Disord. 2023, 110, 105361. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Brain Small Chain Fatty Acid Metabolism in Parkinson Disease: Tributyrin Supplementation (BUTTER). Available online: https://clinicaltrials.gov/study/NCT05446168 (accessed on 29 August 2025).
- ClinicalTrials.gov. Prebiotics in the Parkinson′s Disease Microbiome. Available online: https://clinicaltrials.gov/study/NCT04512599 (accessed on 29 August 2025).
- Becker, A.; Schmartz, G.P.; Gröger, L.; Grammes, N.; Galata, V.; Philippeit, H.; Weiland, J.; Ludwig, N.; Meese, E.; Tierling, S.; et al. Effects of Resistant Starch on Symptoms, Fecal Markers, and Gut Microbiota in Parkinson’s Disease—The RESISTA-PD Trial. Genom. Proteom. Bioinform. 2022, 20, 274–287. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Bastiaanssen, T.F.S.; van de Wouw, M.; Moloney, G.M.; Gual-Grau, A.; Spichak, S.; Olavarría-Ramírez, L.; Fitzgerald, P.; Morillas, E.; et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 2021, 1, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liang, W.; Zhang, W.; Huang, Z.; Liang, H.; Liu, Q. Fecal microbiota transplantation repairs intestinal permeability and regulates the expression of 5-HT to influence alcohol-induced depression-like behaviors in C57BL/6J mice. Front. Microbiol. 2023, 14, 1241309. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Li, Y.; Zhao, H.; He, C.; Wang, Z.; Zhao, H. Fecal Microbiota Transplantation Protects the Intestinal Mucosal Barrier by Reconstructing the Gut Microbiota in a Murine Model of Sepsis. Front. Cell Infect. Microbiol. 2021, 11, 736204. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Ma, X.; Geng, S.; Jiang, X.; Li, Y.; Hu, L.; Li, J.; Wang, Y.; Han, X. Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury. mSystems 2018, 3, e00137-18. [Google Scholar] [CrossRef]
- Ma, S.; Wang, N.; Zhang, P.; Wu, W.; Fu, L. Fecal microbiota transplantation mitigates bone loss by improving gut microbiome composition and gut barrier function in aged rats. PeerJ 2021, 9, e12293. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, G.; Zhu, Q.; Wang, C.; Ruan, G.; Ying, S.; Qie, J.; Hu, X.; Xiao, Z.; Xu, F.; et al. Efficacy of fecal microbiota transplantation in patients with Parkinson’s disease: Clinical trial results from a randomized, placebo-controlled design. Gut Microbes 2023, 15, 2284247. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Barker, R.A.; Lao-Kaim, N.P.; Guzman, N.V.; Athauda, D.; Bjartmarz, H.; Björklund, A.; Church, A.; Cutting, E.; Daft, D.; Dayal, V.; et al. The TransEuro open-label trial of human fetal ventral mesencephalic transplantation in patients with moderate Parkinson’s disease. Nat. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Jeon, J.; Cha, Y.; Hong, Y.J.; Lee, I.-H.; Jang, H.; Ko, S.; Naumenko, S.; Kim, M.; Ryu, H.L.; Shrestha, Z.; et al. Pre-clinical safety and efficacy of human induced pluripotent stem cell-derived products for autologous cell therapy in Parkinson’s disease. Cell Stem Cell 2025, 32, 343–360.e347. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yoon, S.; Choi, J.; Kim, Y.J.; Lee, G. Stem Cell-Based Approaches in Parkinson’s Disease Research. Int. J. Stem Cells 2025, 18, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, M.; Bogetofte, H.; Lawson, T.; Jansson, J.; Smith, G.; Astradsson, A.; Moore, M.; Osborn, T.; Cooper, O.; Spealman, R.; et al. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells 2013, 31, 1548–1562. [Google Scholar] [CrossRef] [PubMed]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A.; et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 3369. [Google Scholar] [CrossRef]
- Clark, B.J.; Lelos, M.J.; Loring, J.F. Advancing Parkinson’s disease treatment: Cell replacement therapy with neurons derived from pluripotent stem cells. Stem Cells 2024, 42, 781–790. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef]
- Scott, K.P.; Antoine, J.M.; Midtvedt, T.; van Hemert, S. Manipulating the gut microbiota to maintain health and treat disease. Microb. Ecol. Health Dis. 2015, 26, 25877. [Google Scholar] [CrossRef]
- Rashed, R.; Valcheva, R.; Dieleman, L.A. Manipulation of Gut Microbiota as a Key Target for Crohn’s Disease. Front. Med. 2022, 9, 887044. [Google Scholar] [CrossRef]
- Mishima, Y.; Sartor, R.B. Manipulating resident microbiota to enhance regulatory immune function to treat inflammatory bowel diseases. J. Gastroenterol. 2020, 55, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Nagao-Kitamoto, H.; Kamada, N. Host-microbial Cross-talk in Inflammatory Bowel Disease. Immune Netw. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339.e324. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Desa, S.H.M.; Ibrahim, N.M. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef]
- Brödel, A.K.; Charpenay, L.H.; Galtier, M.; Fuche, F.J.; Terrasse, R.; Poquet, C.; Havránek, J.; Pignotti, S.; Krawczyk, A.; Arraou, M.; et al. In situ targeted base editing of bacteria in the mouse gut. Nature 2024, 632, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M.; Galbreath, A. Optimizing long-term therapy for Parkinson disease: Levodopa, dopamine agonists, and treatment-associated dyskinesia. Clin. Neuropharmacol. 2008, 31, 51–56. [Google Scholar] [CrossRef]
- Hey, G.; Nair, N.; Klann, E.; Gurrala, A.; Safarpour, D.; Mai, V.; Ramirez-Zamora, A.; Vedam-Mai, V. Therapies for Parkinson’s disease and the gut microbiome: Evidence for bidirectional connection. Front. Aging Neurosci. 2023, 15, 1151850. [Google Scholar] [CrossRef]
- Bandres-Ciga, S.; Diez-Fairen, M.; Kim, J.J.; Singleton, A.B. Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol. Dis. 2020, 137, 104782. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Pradas, E.; Martinez-Vicente, M. The Consequences of GBA Deficiency in the Autophagy-Lysosome System in Parkinson’s Disease Associated with GBA. Cells 2023, 12, 191. [Google Scholar] [CrossRef]
- Pellegrini, L.; Wetzel, A.; Grannó, S.; Heaton, G.; Harvey, K. Back to the tubule: Microtubule dynamics in Parkinson’s disease. Cell Mol. Life Sci. 2017, 74, 409–434. [Google Scholar] [CrossRef] [PubMed]
- Rudakou, U.; Yu, E.; Krohn, L.; Ruskey, J.A.; Asayesh, F.; Dauvilliers, Y.; Spiegelman, D.; Greenbaum, L.; Fahn, S.; Waters, C.H.; et al. Targeted sequencing of Parkinson’s disease loci genes highlights SYT11, FGF20 and other associations. Brain 2021, 144, 462–472. [Google Scholar] [CrossRef] [PubMed]
| Category | Subcategory | Treatment | Mechanism |
|---|---|---|---|
| Traditional PD Medications | Dopaminergic Medications | Levodopa/Carbidopa | Alleviates motor symptoms but may disrupt gut motility |
| Dopamine agonists (e.g., pramipexole, ropinirole) | Impact gut function through the enteric nervous system | ||
| Enzyme Inhibitors | MAO-B inhibitors (e.g., selegiline, rasagiline) | Potential neuroprotective effects extend to the enteric nervous system | |
| COMT inhibitors (e.g., entacapone) | May influence the gut metabolism of levodopa | ||
| Anticholinergics | Used for tremor control, can affect gut motility and potentially influence intestinal permeability | ||
| Gut-Specific Interventions | Microbiome-Targeted Interventions | Probiotics—Akkermansia muciniphila, Bifidobacterium | Enhances mucus production, reduces inflammation |
| Prebiotics—Galactooligosaccharides | Stimulates Bifidobacterium growth, improves barrier function | ||
| Fecal microbiota transplantation (FMT)—Healthy donor microbiota | Restores microbial diversity and reduces α-syn burden | ||
| SCFA-boosting diets | Restore microbial diversity and reinforce barrier function via GPR41/43 signaling | ||
| Anti-inflammatory Agents | To reduce gut inflammation and potentially improve barrier function | ||
| Immunomodulators | To regulate immune responses and mitigate chronic inflammation in the gut | ||
| Emerging Therapies | Mucus Layer Enhancers | Therapies targeting MUC2 production to strengthen the protective mucus barrier | |
| Tight Junction Modulators | Larazotide acetate | Inhibits zonulin and stabilizes tight junction integrity (e.g., targeting occludin, claudins, ZO-1) | |
| Personalized Microbiome Engineering | Phage-delivered gene editing | Tailored approaches to enhance specific beneficial bacterial populations, and enhance SCFA producers | |
| Neuroprotective Agents | Compounds that may benefit both gut and brain health |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Zhao, Y.; Fasano, A.; Su, C.-W. Gut Permeability and Microbiota in Parkinson’s Disease: Mechanistic Insights and Experimental Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 9593. https://doi.org/10.3390/ijms26199593
Liang Y, Zhao Y, Fasano A, Su C-W. Gut Permeability and Microbiota in Parkinson’s Disease: Mechanistic Insights and Experimental Therapeutic Strategies. International Journal of Molecular Sciences. 2025; 26(19):9593. https://doi.org/10.3390/ijms26199593
Chicago/Turabian StyleLiang, Yicheng, Yuhang Zhao, Alessio Fasano, and Chien-Wen Su. 2025. "Gut Permeability and Microbiota in Parkinson’s Disease: Mechanistic Insights and Experimental Therapeutic Strategies" International Journal of Molecular Sciences 26, no. 19: 9593. https://doi.org/10.3390/ijms26199593
APA StyleLiang, Y., Zhao, Y., Fasano, A., & Su, C.-W. (2025). Gut Permeability and Microbiota in Parkinson’s Disease: Mechanistic Insights and Experimental Therapeutic Strategies. International Journal of Molecular Sciences, 26(19), 9593. https://doi.org/10.3390/ijms26199593







