From Bioinformatics Analysis to Recombinant Expression: Advancing Public Health with Taenia solium Proteins
Abstract
1. Introduction
2. Results
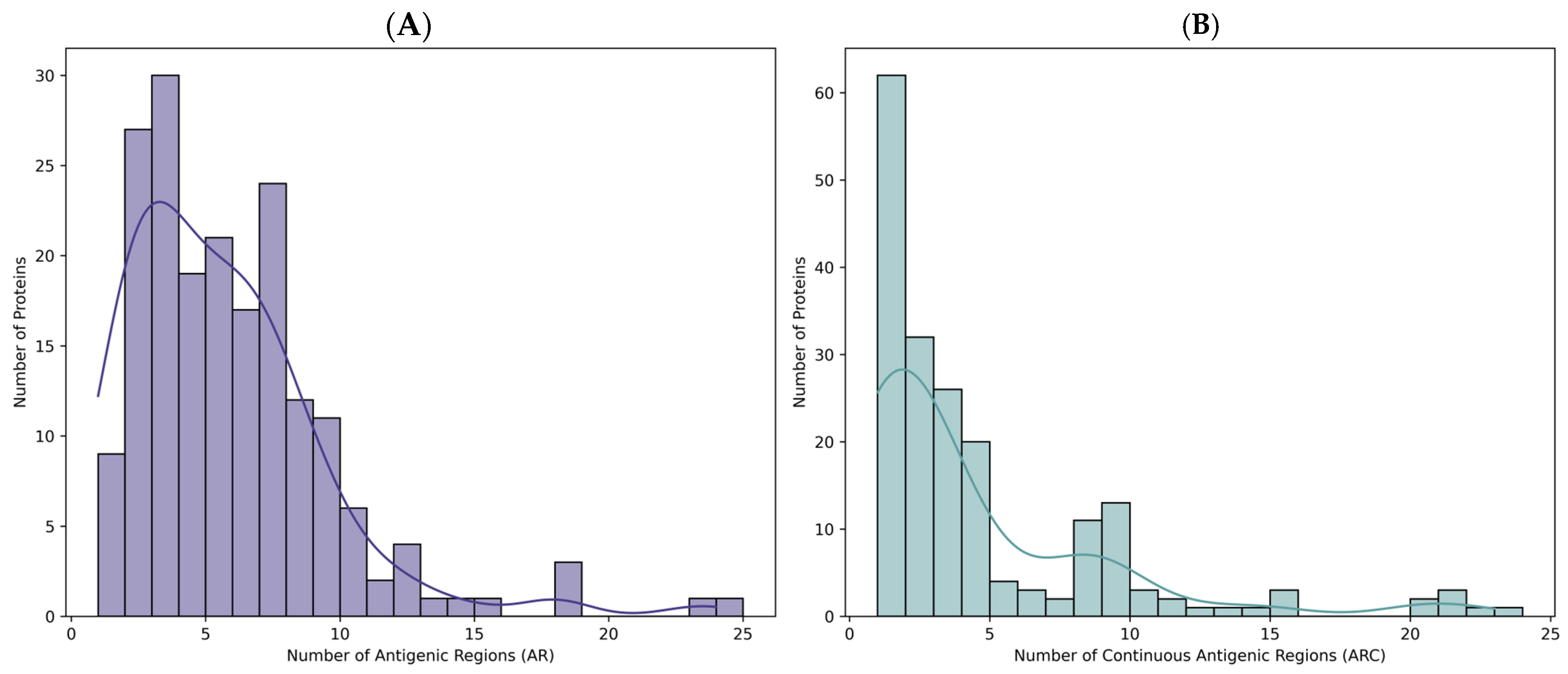
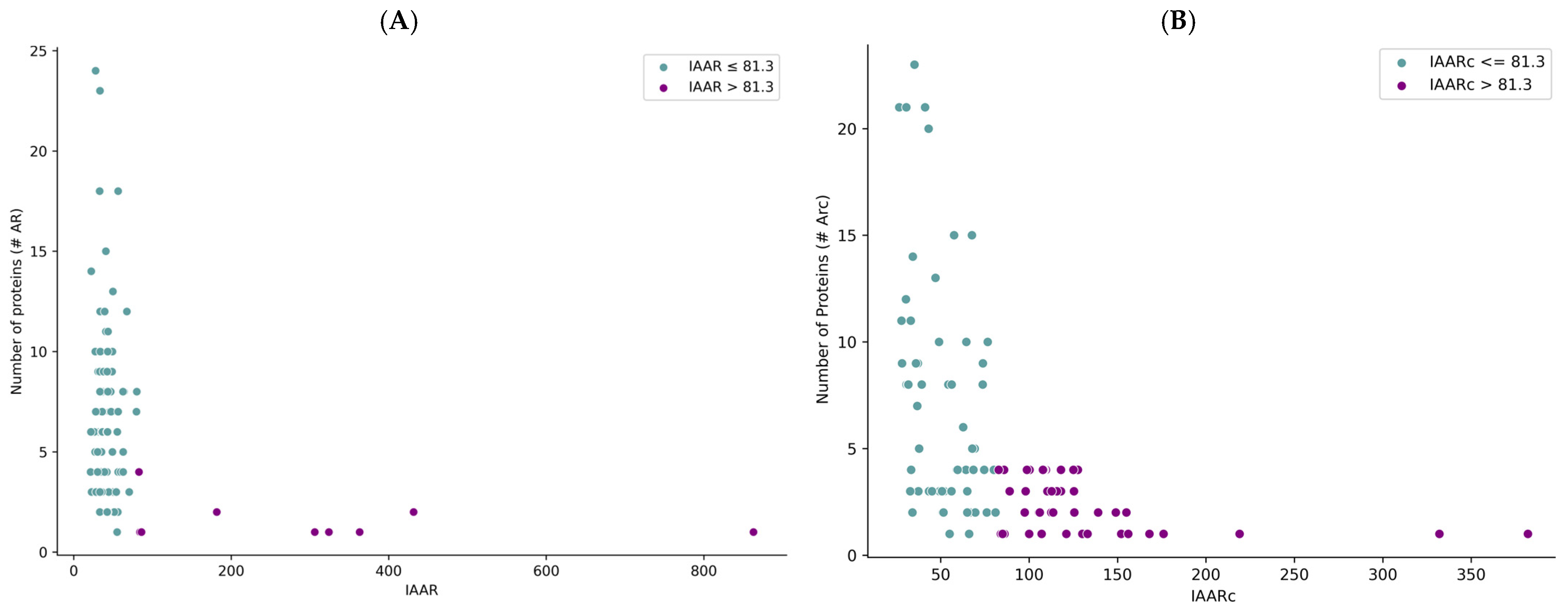
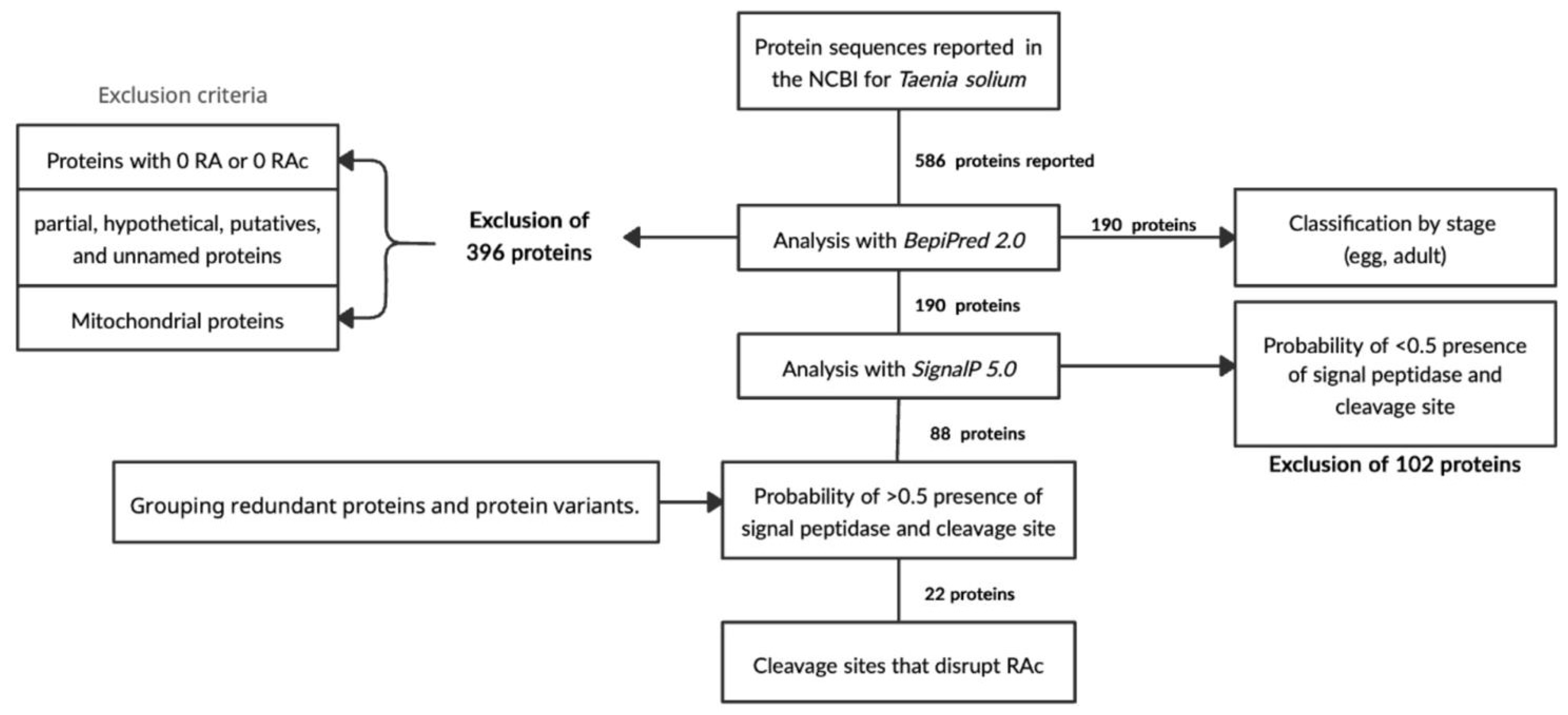
2.1. Selection of T. solium Sequences
2.2. Genetic Construction and Expression of T. solium Sequences
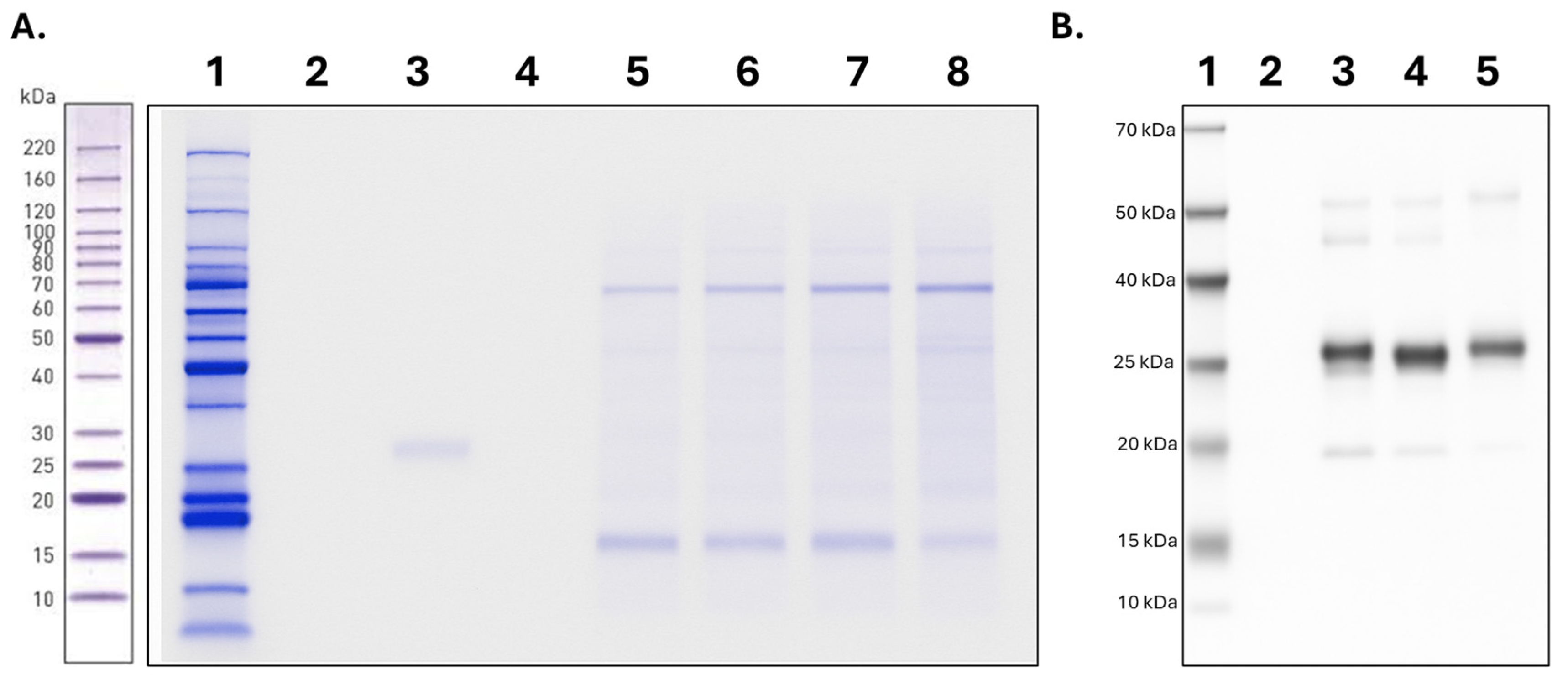
2.3. Purification of T. solium Proteins
3. Discussion
4. Materials and Methods
4.1. Taenia solium Protein Sequences
- Xp denotes the relative Abundance of Antigenic Regions in protein p,
- Lp the sequence length in protein p,
- Ap the number of antigenic regions in protein p.
4.2. Signal Peptide Prediction
4.3. Genetic Construction and Verification
4.4. Expression of Taenia solium Recombinant Proteins
4.5. Electrophoresis and Western Blot
4.6. Purification of Taenia solium Recombinant Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030, 1st ed.; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001035-2. [Google Scholar]
- Garcia, H.H.; O’Neal, S.E.; Noh, J.; Handali, S.; Cysticercosis Working Group in Peru. Laboratory Diagnosis of Neurocysticercosis (Taenia solium). J. Clin. Microbiol. 2018, 56, e00424-18. [Google Scholar] [CrossRef]
- Prodjinotho, U.F.; Lema, J.; Lacorcia, M.; Schmidt, V.; Vejzagic, N.; Sikasunge, C.; Ngowi, B.; Winkler, A.S.; Prazeres Da Costa, C. Host Immune Responses during Taenia solium Neurocysticercosis Infection and Treatment. PLoS Negl. Trop. Dis. 2020, 14, e0008005. [Google Scholar] [CrossRef] [PubMed]
- CDC About Human Tapeworm. Available online: https://www.cdc.gov/taeniasis/about/index.html (accessed on 2 April 2025).
- García, H.H.; Gonzalez, A.E.; Evans, C.A.; Gilman, R.H. Taenia solium Cysticercosis. Lancet 2003, 362, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud; Organización Panamericana de la Salud. Teniasis/Cisticercosis Por Taenia solium. Available online: https://www.paho.org/es/temas/teniasiscisticercosis-por-taenia-solium (accessed on 2 April 2025).
- Meza-Lucas, A.; Aguilar Rebolledo, F. Teniasis Humana Por Taenia solium. Rev. Mex. Patol. Clínica 2002, 49, 92–99. [Google Scholar]
- Consejo Directivo OMS. Plan de Acción Para La Eliminación de Las Enfermedades Infecciosas Desatendidas y Las Medidas Posteriores a La Eliminación 2016–2022; Organización Mundial de la Salud: Washington, DC, USA, 2016. [Google Scholar]
- Okello, A.; Thomas, L. Human Taeniasis: Current Insights into Prevention and Management Strategies in Endemic Countries. RMHP 2017, 10, 107–116. [Google Scholar] [CrossRef]
- Sarti, E. La Teniosis y Cisticercosis Por Taenia solium. Salud Publica Mex. 1997, 39, 225–231. [Google Scholar] [CrossRef]
- Mwape, K.E.; Gabriël, S. The Parasitological, Immunological, and Molecular Diagnosis of Human Taeniasis with Special Emphasis on Taenia solium Taeniasis. Curr. Trop. Med. Rep. 2014, 1, 173–180. [Google Scholar] [CrossRef][Green Version]
- Ferre, E. Teniasis/Cisticercosis: Avances En Diagnóstico Inmunológico y Molecular. Boletín Malariol. Salud Ambient. 2006, 46, 1–13. [Google Scholar][Green Version]
- Allan, J.C.; Wilkins, P.P.; Tsang, V.C.W.; Craig, P.S. Immunodiagnostic Tools for Taeniasis. Acta Trop. 2003, 87, 87–93. [Google Scholar] [CrossRef]
- Mwape, K.E.; Phiri, I.K.; Praet, N.; Muma, J.B.; Zulu, G.; Van Den Bossche, P.; De Deken, R.; Speybroeck, N.; Dorny, P.; Gabriël, S. Taenia solium Infections in a Rural Area of Eastern Zambia-A Community Based Study. PLoS Negl. Trop. Dis. 2012, 6, e1594. [Google Scholar] [CrossRef]
- Rajshekhar, V. Neurocysticercosis: Diagnostic Problems & Current Therapeutic Strategies. Indian J. Med. Res. 2016, 144, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Yanagida, T.; Wang, H.; Long, C.; Sako, Y.; Okamoto, M.; Wu, Y.; Giraudoux, P.; Raoul, F.; et al. Detection of Human Taeniases in Tibetan Endemic Areas, China. Parasitology 2013, 140, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Klarman, M.; Gaspard, A.N.; Dong, X.F.; LaBorde, R.; Noh, J.; Lee, Y.-M.; Rodriguez, S.; Gonzalez, A.E.; Garcia, H.H.; et al. Development and Evaluation of a Magnetic Immunochromatographic Test to Detect Taenia solium, Which Causes Taeniasis and Neurocysticercosis in Humans. Clin. Vaccine Immunol. 2010, 17, 631–637. [Google Scholar] [CrossRef]
- Allan, J.C.; Avila, G.; Noval, J.G.; Flisser, A.; Craig, P.S. Immunodiagnosis of Taeniasis by Coproantigen Detection. Parasitology 1990, 101, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Guezala, M.-C.; Rodriguez, S.; Zamora, H.; Garcia, H.H.; Gonzalez, A.E.; Tembo, A.; Allan, J.C.; Craig, P.S. Development of a Species-Specific Coproantigen ELISA for Human Taenia solium Taeniasis. Am. J. Trop. Med. Hyg. 2009, 81, 433–437. [Google Scholar] [CrossRef]
- Nava, G.; Laclette, J.P.; Bobes, R.; Carrero, J.C.; Reyes-Vivas, H.; Enriquez-Flores, S.; Mendoza-Hernández, G.; Plancarte, A. Cloning, Sequencing and Functional Expression of Cytosolic Malate Dehydrogenase from Taenia solium: Purification and Characterization of the Recombinant Enzyme. Exp. Parasitol. 2011, 128, 217–224. [Google Scholar] [CrossRef]
- Nava, G.; Maldonado, G.; Plancarte, A. Cloning, Expression, Purification, and Kinetic Characterization of Mitochondrial Thioredoxin (TsTrx2), Cytosolic Thioredoxin (TsTrx1), and Glutaredoxin (TsGrx1) from Taenia solium. Parasitol. Res. 2019, 118, 1785–1797. [Google Scholar] [CrossRef]
- Huang, J.; Li, B.; Dai, J.-L.; Zhang, A.-H. Prokaryotic expression and histological localization of the Taenia solium CDC37 gene. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2013, 31, 23–26. [Google Scholar]
- Gauci, C.; Jayashi, C.; Lightowlers, M.W. Vaccine Development against the Taenia solium Parasite: The Role of Recombinant Protein Expression in Escherichia coli. Bioengineered 2013, 4, 343–347. [Google Scholar] [CrossRef]
- Jiménez, L.; Castro-Nolasco, N.K.; Fleury, A.; Díaz-Camacho, S.P.; Ochoa-Sánchez, A.; Landa, A. Evaluation of Recombinant Glutathione Transferase 26 kDa, Thioredoxin-1, and Endophilin B1 of Taenia solium in the Diagnosis of Human Neurocysticercosis. Acta Trop. 2022, 227, 106294. [Google Scholar] [CrossRef]
- Hernández-González, A.; Noh, J.; Perteguer, M.J.; Gárate, T.; Handali, S. Comparison of T24H-His, GST-T24H and GST-Ts8B2 Recombinant Antigens in Western Blot, ELISA and Multiplex Bead-Based Assay for Diagnosis of Neurocysticercosis. Parasites Vectors 2017, 10, 237. [Google Scholar] [CrossRef]
- Chung, J.; Bahk, Y.Y.; Huh, S.; Kang, S.; Kong, Y.; Cho, S. A Recombinant 10-kDa Protein of Taenia solium Metacestodes Specific to Active Neurocysticercosis. J. Infect. Dis. 1999, 180, 1307–1315. [Google Scholar] [CrossRef]
- Ito, A.; Bøgh, H.O.; Lightowlers, M.W.; Mitchell, G.F.; Takami, T.; Kamiya, M.; Onitake, K.; Rickard, M.D. Vaccination against Taenia Taeniaeformis Infection in Rats Using a Recombinant Protein and Preliminary Analysis of the Induced Antibody Response. Mol. Biochem. Parasitol. 1991, 44, 43–49. [Google Scholar] [CrossRef]
- Gunyakti Kilinc, S.; Simsek, S.; Celik, F.; Kesik, H.K. Cloning, Expression and Serodiagnostic Potential of HSP70 of Taenia Multiceps in Sheep. Mol. Biochem. Parasitol. 2021, 245, 111397. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, D.; Gu, X.; Peng, X.; Yang, G. Evaluation of a Novel Dot-ELISA Assay Utilizing a Recombinant Protein for the Effective Diagnosis of Taenia pisiformis Larval Infections. Vet. Parasitol. 2014, 204, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, A.; Jafarihaghighi, F.; Kazemirad, E.; Sabzevar, S.S.; Tanipour, M.H.; Ardjmand, M. Recent Developments in Recombinant Proteins for Diagnosis of Human Fascioliasis. Acta Parasit. 2021, 66, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Boonroumkaew, P.; Sadaow, L.; Sanpool, O.; Rodpai, R.; Thanchomnang, T.; Phupiewkham, W.; Intapan, P.M.; Maleewong, W. Effectiveness of Strongyloides Recombinant IgG Immunoreactive Antigen in Detecting IgG and IgG4 Subclass Antibodies for Diagnosis of Human Strongyloidiasis Using Rapid Immunochromatographic Tests. Diagnostics 2020, 10, 615. [Google Scholar] [CrossRef]
- Laclette, J.P.; Bobes, R.J.; Carrero, J.C. La Era Posgenómica En El Estudio de Los Helmintos. Ciencia 2017, 68, 62–65. [Google Scholar]
- Nascimento Santos, L.; Carvalho Pacheco, L.G.; Silva Pinheiro, C.; Alcantara-Neves, N.M. Recombinant Proteins of Helminths with Immunoregulatory Properties and Their Possible Therapeutic Use. Acta Trop. 2017, 166, 202–211. [Google Scholar] [CrossRef]
- Gomez, S.; Adalid-Peralta, L.; Palafox-Fonseca, H.; Cantu-Robles, V.A.; Soberón, X.; Sciutto, E.; Fragoso, G.; Bobes, R.J.; Laclette, J.P.; Yauner, L.D.P.; et al. Genome Analysis of Excretory/Secretory Proteins in Taenia solium Reveals Their Abundance of Antigenic Regions (AAR). Sci. Rep. 2015, 5, 9683. [Google Scholar] [CrossRef]
- Wang, S.; Wei, W.; Cai, X. Genome-Wide Analysis of Excretory/Secretory Proteins in Echinococcus Multilocularis: Insights into Functional Characteristics of the Tapeworm Secretome. Parasites Vectors 2015, 8, 666. [Google Scholar] [CrossRef]
- Chang, V.T.; Crispin, M.; Aricescu, A.R.; Harvey, D.J.; Nettleship, J.E.; Fennelly, J.A.; Yu, C.; Boles, K.S.; Evans, E.J.; Stuart, D.I.; et al. Glycoprotein Structural Genomics: Solving the Glycosylation Problem. Structure 2007, 15, 267–273. [Google Scholar] [CrossRef]
- Mirzadeh, A.; Yoosefy, A.; Kazemirad, E.; Barati, Z.; Golkar, M.; Babaie, J.; Jafarihaghighi, F.; Valadkhani, Z. Evaluation of a Set of Refolded Recombinant Antigens for Serodiagnosis of Human Fascioliasis. PLoS ONE 2018, 13, e0203490. [Google Scholar] [CrossRef] [PubMed]
- Crosnier, C.; Wanaguru, M.; McDade, B.; Osier, F.H.; Marsh, K.; Rayner, J.C.; Wright, G.J. A Library of Functional Recombinant Cell-Surface and Secreted P. Falciparum Merozoite Proteins. Mol. Cell. Proteom. 2013, 12, 3976–3986. [Google Scholar] [CrossRef]
- Elton, C.M.; Rodriguez, M.; Ben Mamoun, C.; Lobo, C.A.; Wright, G.J. A Library of Recombinant Babesia Microti Cell Surface and Secreted Proteins for Diagnostics Discovery and Reverse Vaccinology. Int. J. Parasitol. 2019, 49, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lichty, J.J.; Malecki, J.L.; Agnew, H.D.; Michelson-Horowitz, D.J.; Tan, S. Comparison of Affinity Tags for Protein Purification. Protein Expr. Purif. 2005, 41, 98–105. [Google Scholar] [CrossRef] [PubMed]
- IBA Lifesciences. Comprehensive Comparison of the Strep-Tag® Technology with the His-Tag System; IBA Lifesciences: Göttingen, Germany, 2020. [Google Scholar]
- National Library of Medicine. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 8 April 2025).
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving Sequence-Based B-Cell Epitope Prediction Using Conformational Epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef]
- Department of Health Technology. BepiPred-2.0 Prediction of Potential Linear B-Cell Epitopes. Available online: https://services.healthtech.dtu.dk/services/BepiPred-2.0/ (accessed on 1 December 2023).
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Tyson, J. Bead-Free Long Fragment LSK109 Library Preparation V1; Snutch Lab, UBC: Vancouver, BC, Canada, 2019. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, J.; Guacaneme, M.C.J.; Ovalle-Bracho, C.; Trujillo, J.T.; Duque-Beltrán, S.; Arévalo, A.; Franco-Muñoz, C. From Bioinformatics Analysis to Recombinant Expression: Advancing Public Health with Taenia solium Proteins. Int. J. Mol. Sci. 2025, 26, 9585. https://doi.org/10.3390/ijms26199585
Muñoz J, Guacaneme MCJ, Ovalle-Bracho C, Trujillo JT, Duque-Beltrán S, Arévalo A, Franco-Muñoz C. From Bioinformatics Analysis to Recombinant Expression: Advancing Public Health with Taenia solium Proteins. International Journal of Molecular Sciences. 2025; 26(19):9585. https://doi.org/10.3390/ijms26199585
Chicago/Turabian StyleMuñoz, Juana, María Camila Jurado Guacaneme, Clemencia Ovalle-Bracho, Julián Trujillo Trujillo, Sofía Duque-Beltrán, Adriana Arévalo, and Carlos Franco-Muñoz. 2025. "From Bioinformatics Analysis to Recombinant Expression: Advancing Public Health with Taenia solium Proteins" International Journal of Molecular Sciences 26, no. 19: 9585. https://doi.org/10.3390/ijms26199585
APA StyleMuñoz, J., Guacaneme, M. C. J., Ovalle-Bracho, C., Trujillo, J. T., Duque-Beltrán, S., Arévalo, A., & Franco-Muñoz, C. (2025). From Bioinformatics Analysis to Recombinant Expression: Advancing Public Health with Taenia solium Proteins. International Journal of Molecular Sciences, 26(19), 9585. https://doi.org/10.3390/ijms26199585





