Role of Nanobubble Cavitation in Triggering Drug Release from Boron-Nitride and Carbon Nanocapsules and Their Diffusion for Drug Delivery Applications: A Molecular Dynamics Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Results Verification
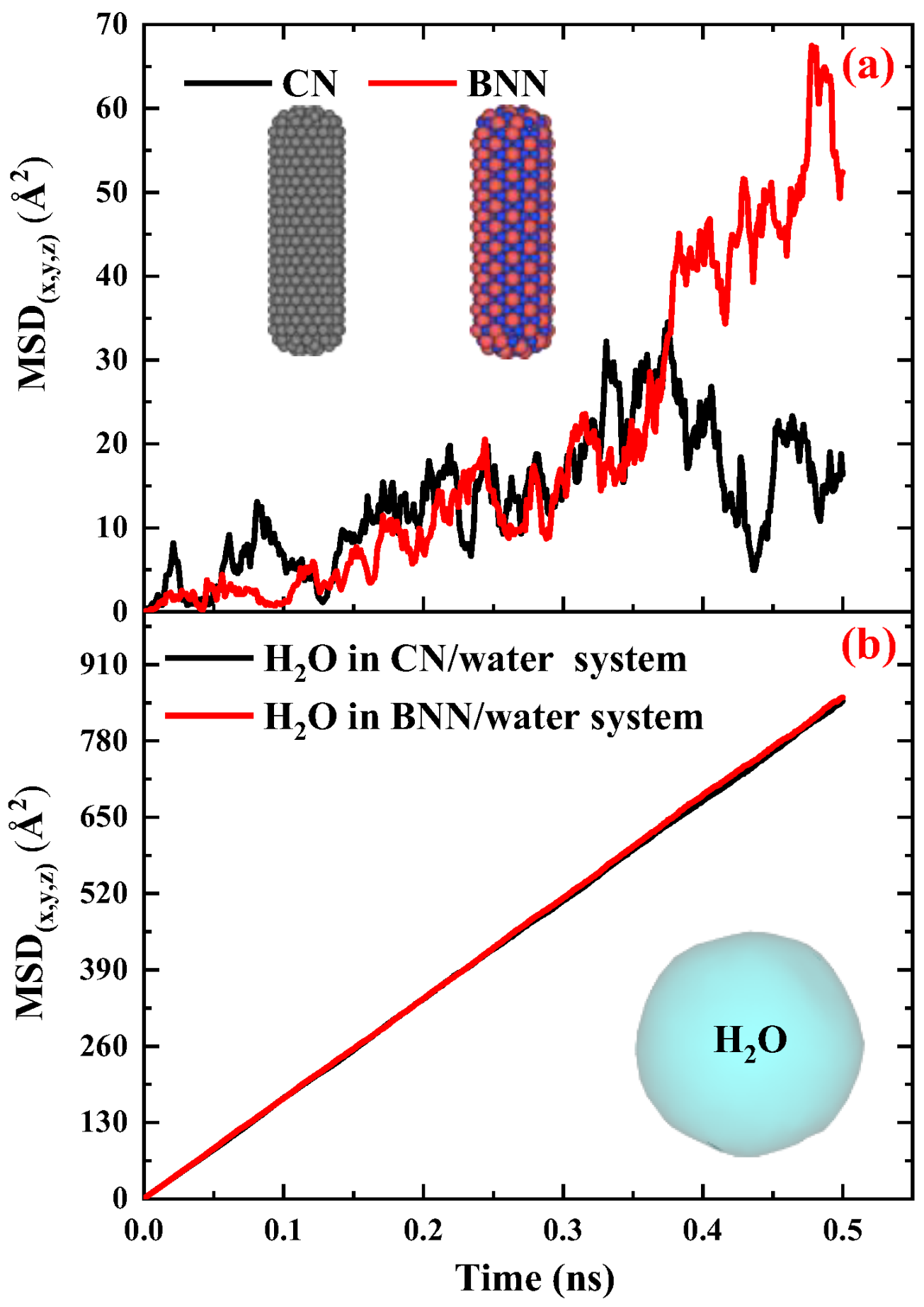
2.2. Nanocapsules Diffusivity as Drug Carrier Properties
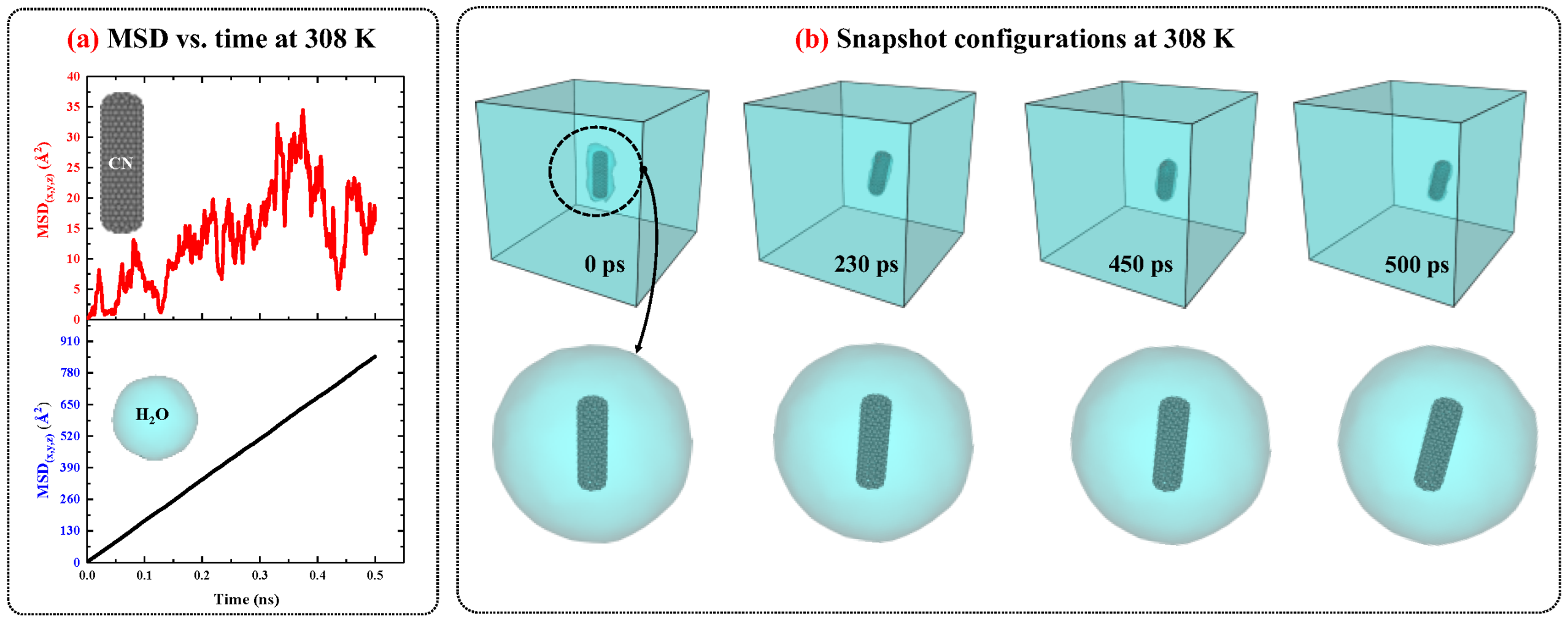
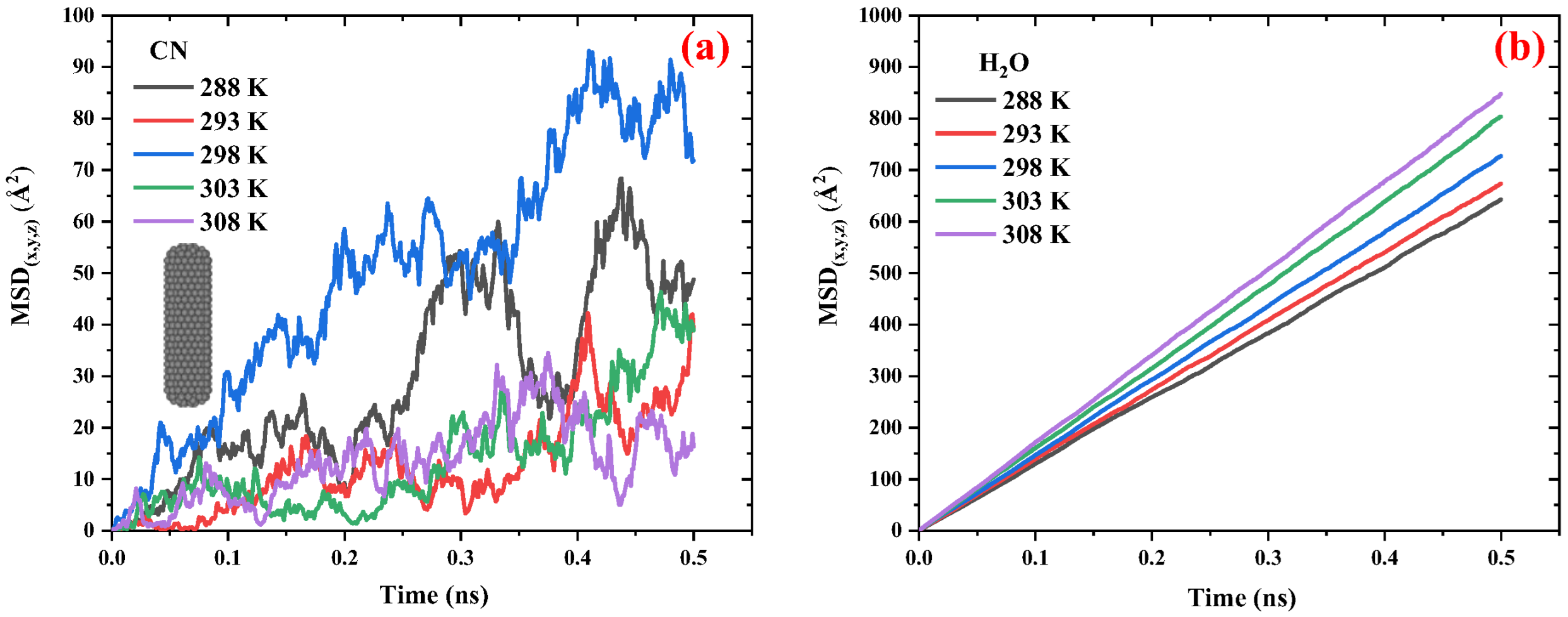
2.2.1. Carbon Nanocapsules/Water System
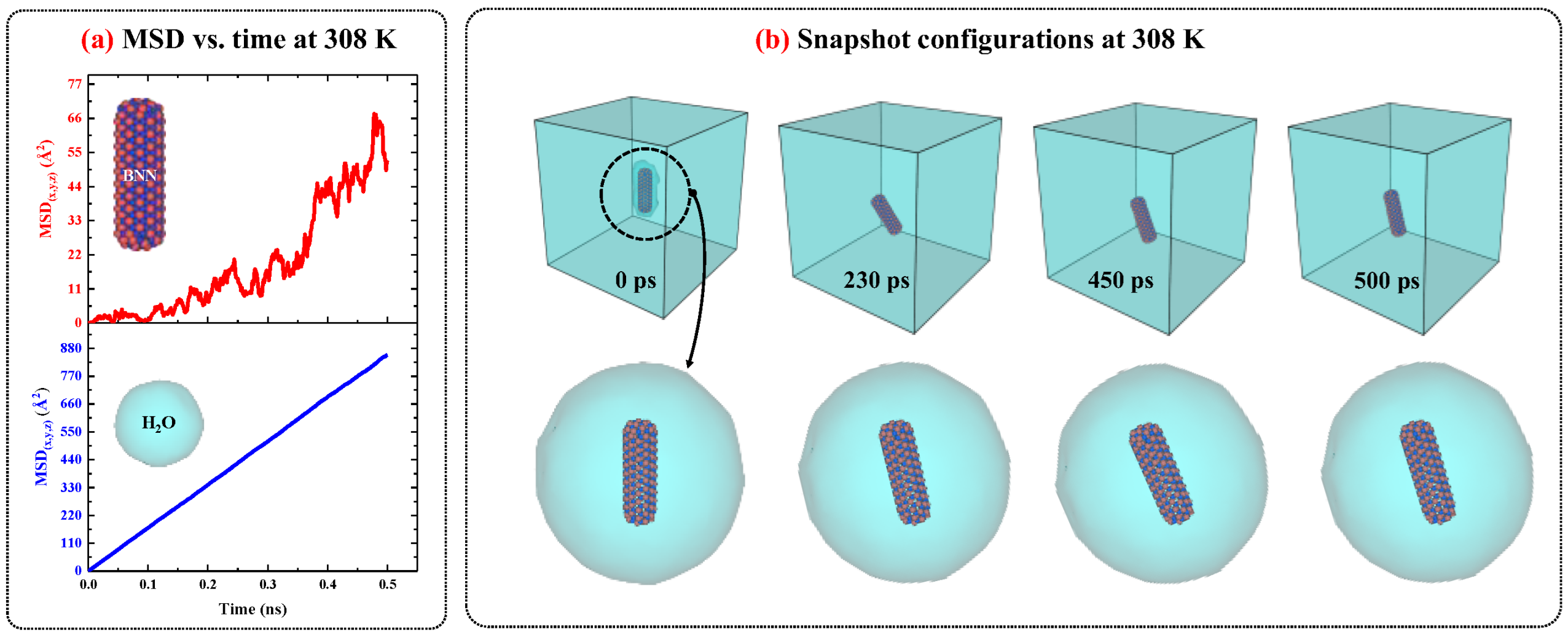
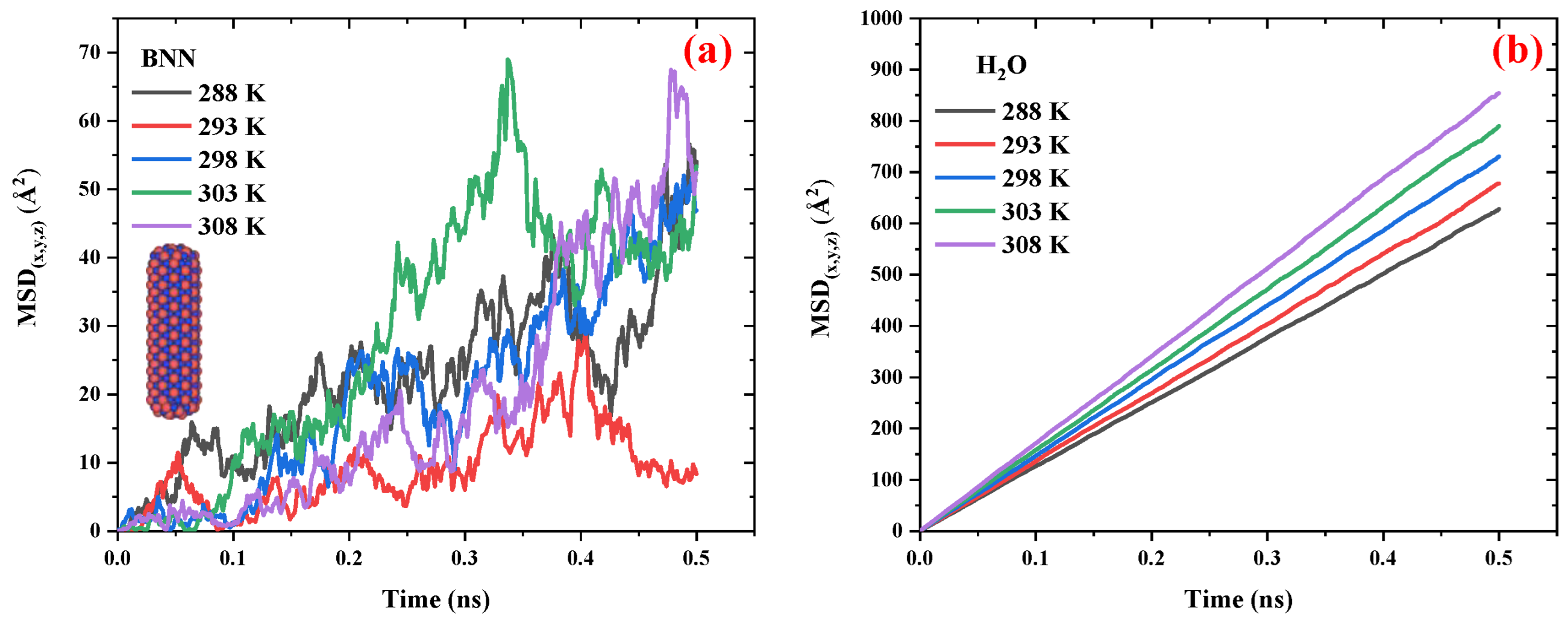
2.2.2. Boron-Nitride Nanocapsule/Water System
2.2.3. Comparison of the Diffusivity of Carbon and Boron-Nitride Nanocapsules
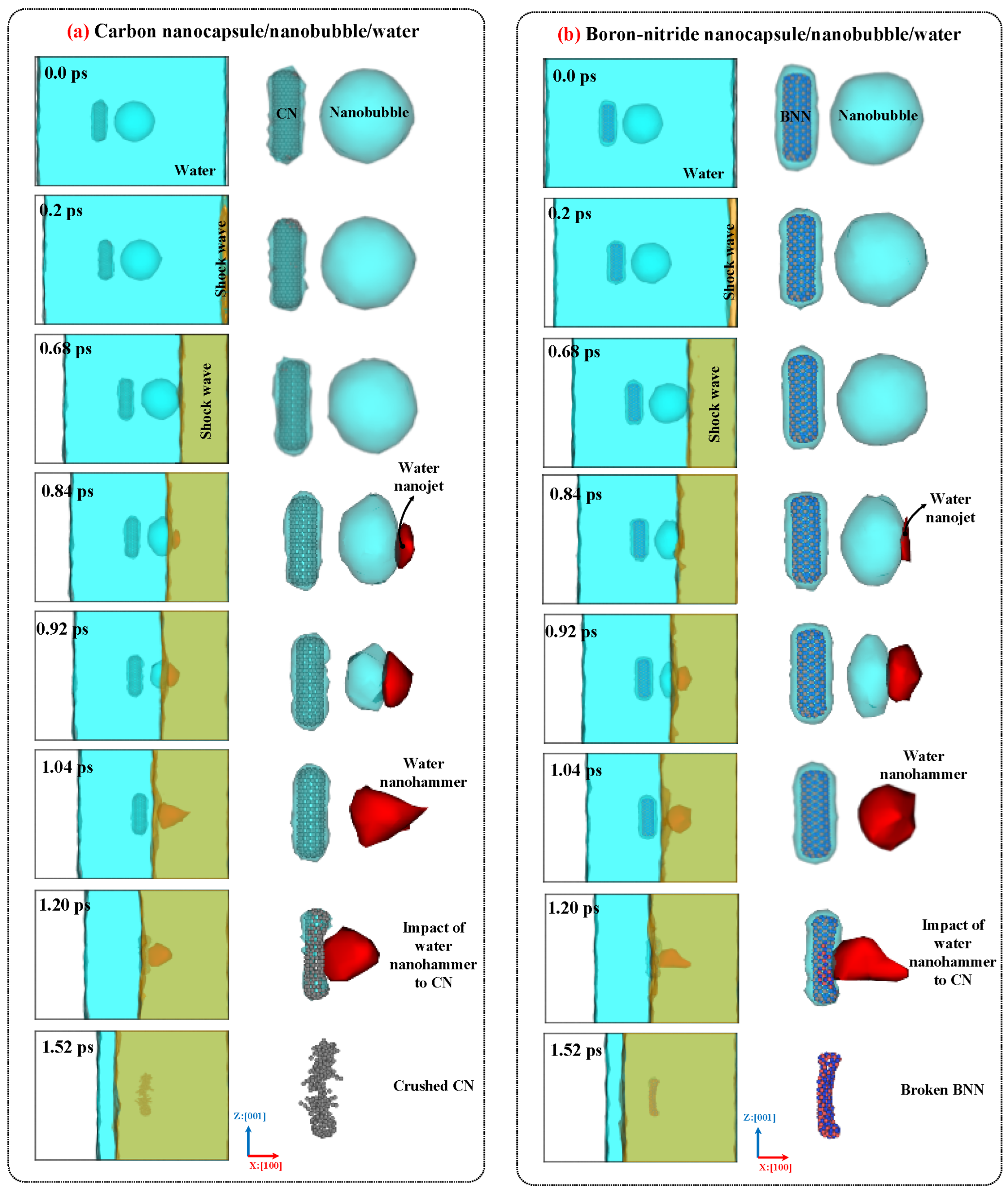
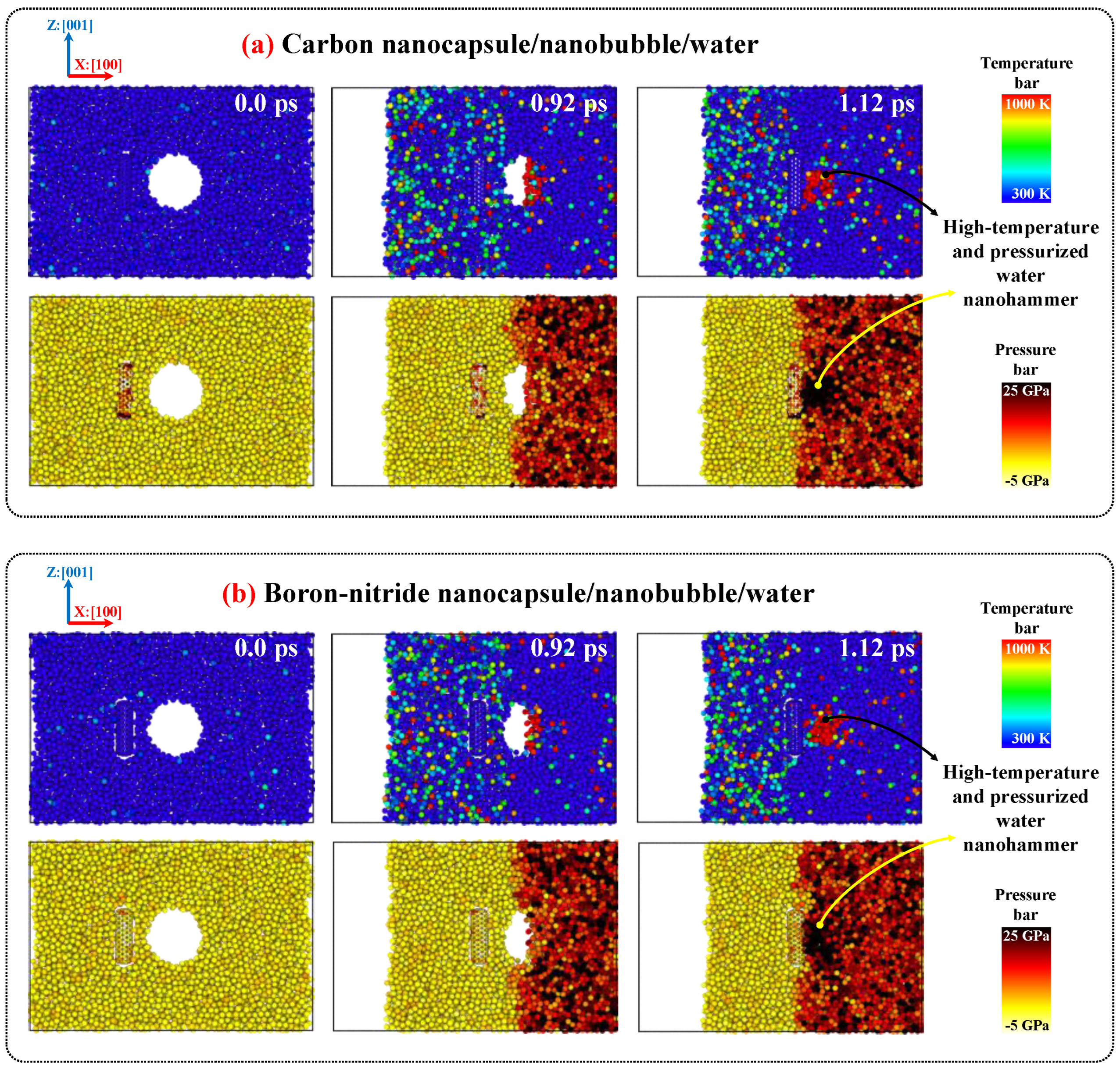
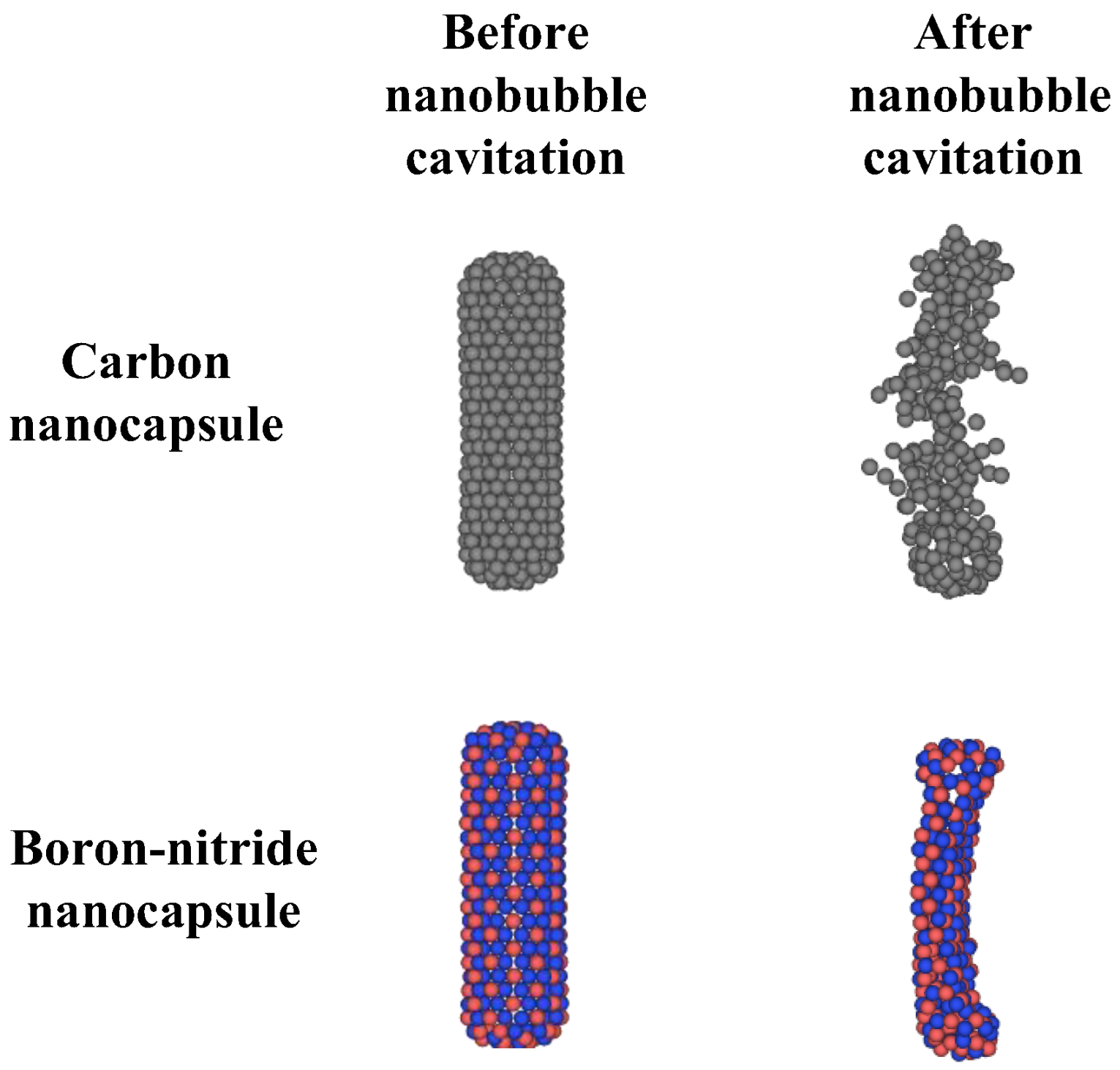
2.3. Nanobubble Cavitation as Releasing Agent
2.4. Outlook and Perspective
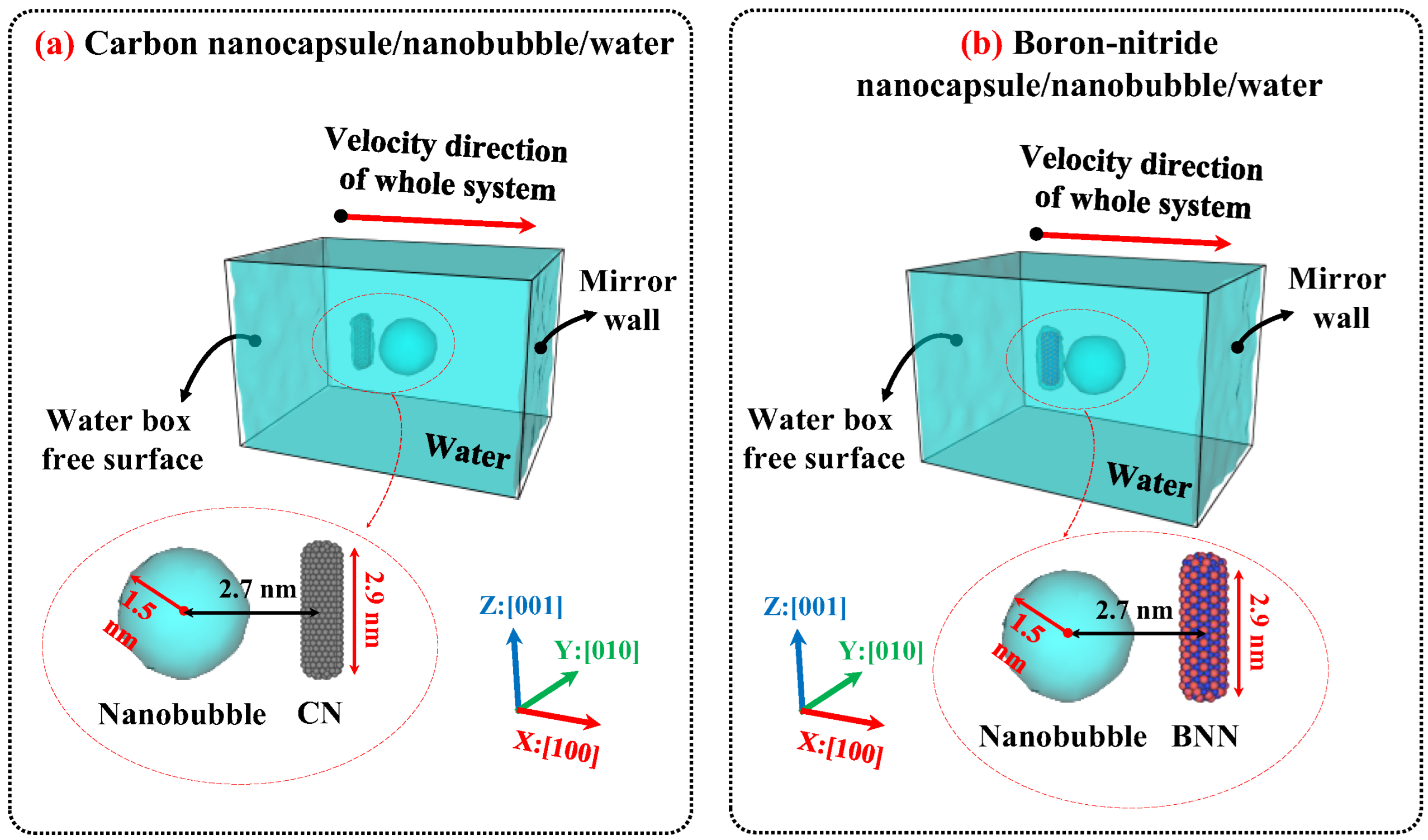
3. Materials and Methods
3.1. Configuration
3.2. Interatomic Potential
3.3. Simulation Algorithm
3.4. Formal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Gaidai, O.; Yan, P.; Xing, Y. Future world cancer death rate prediction. Sci. Rep. 2023, 13, 303. [Google Scholar] [CrossRef]
- Kakarla, A.B.; Kong, I. In Vitro and In Vivo Cytotoxicity of Boron Nitride Nanotubes: A Systematic Review. Nanomaterials 2022, 12, 2069. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, M.; Jorabchi, M.N.; Akbarzadeh, H.; Salemi, S.; Ebrahimi, R. Molecular dynamics simulation of anticancer drug delivery from carbon nanotube using metal nanowires. J. Comput. Chem. 2019, 40, 2179–2190. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Leroux, J.-C. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef]
- Arora, R.; Jain, C.P. Advances in niosome as a drug carrier: A review. Asian J. Pharm. 2007, 1, 29–39. [Google Scholar]
- Li, C.; Wang, Z.; Lei, H.; Zhang, D. Recent progress in nanotechnology-based drug carriers for resveratrol delivery. Drug Deliv. 2023, 30, 2174206. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Fang, Y.; Chen, L.; Wu, J. Therapeutic poly(amino acid)s as drug carriers for cancer therapy. Chin. Chem. Lett. 2023, 34, 107953. [Google Scholar] [CrossRef]
- Trucillo, P. Drug carriers: Classification, administration, release profiles, and industrial approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Shiva, Y.; Vadla, P.; Rodda, S.; Parimi, D.S.; Paila, B.; Dasari, V.V.; Suresh, A.K. Fundamentals of Nano-Based Drug Delivery Systems. In Emergence Sustainable Biomaterials Tackling Inflammatory Diseases; Springer: Singapore, 2025; pp. 131–152. [Google Scholar] [CrossRef]
- Saripilli, R.; Sharma, D.K. Nanotechnology-based drug delivery system for the diagnosis and treatment of ovarian cancer. Discov. Oncol. 2025, 16, 422. [Google Scholar] [CrossRef]
- Hilder, T.A.; Hill, J.M. Carbon nanotubes as drug delivery nanocapsules. Curr. Appl. Phys. 2008, 8, 258–261. [Google Scholar] [CrossRef]
- Mayer, C. Nanocapsules as Drug Delivery Systems. Int. J. Artif. Organs 2005, 28, 1163–1171. [Google Scholar] [CrossRef]
- Fan, C.-H.; Huang, E.; Lo, W.-C.; Yeh, C.-K. Ultrasound-cavitation-enhanced drug delivery via microbubble clustering induced by acoustic vortex tweezers. Ultrason. Sonochem. 2025, 114, 107273. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Mannaris, C.; Cabañas, M.V.; Carlisle, R.; Manzano, M.; Vallet-Regí, M.; Coussios, C.C. Ultrasound-mediated cavitation-enhanced extravasation of mesoporous silica nanoparticles for controlled-release drug delivery. Chem. Eng. J. 2018, 340, 2–8. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Al Refaai, K.A.; AlSawaftah, N.A.; Abuwatfa, W.; Husseini, G.A. Drug Release via Ultrasound-Activated Nanocarriers for Cancer Treatment: A Review. Pharmaceutics 2024, 16, 1383. [Google Scholar] [CrossRef]
- Harish, V.; Kumar, A.; Babu, M.R.; Leo, A.; Srivastav, S. Acoustic Cavitation-Based Drug Delivery. In Transdermal Applications of Minimally Invasive Drug Delivery Systems: Current Trends and Future Perspectives; Springer: Singapore, 2025; pp. 107–137. [Google Scholar]
- Chuang, C.-F.; Lin, C.-W.; Yeh, C.-K. Ultrasound-triggered drug release and cytotoxicity of microbubbles with diverse drug attributes. Ultrason. Sonochem. 2025, 112, 107182. [Google Scholar] [CrossRef] [PubMed]
- Faizi, A.; Kalantar, Z.; Hashemianzadeh, S.M. Drug delivery by SiC nanotubes as nanocarriers for anti-cancer drugs: Investigation of drug encapsulation and system stability using molecular dynamics simulation. Mater. Res. Express 2021, 8, 105012. [Google Scholar] [CrossRef]
- Shafiei, F.; Hashemianzadeh, S.M.; Bagheri, Y. Insight into the encapsulation of gemcitabine into boron- nitride nanotubes and gold cluster triggered release: A molecular dynamics simulation. J. Mol. Liq. 2019, 278, 201–212. [Google Scholar] [CrossRef]
- Xiao, H.; Shi, X.; Chen, X. Self-assembled nanocapsules in water: A molecular mechanistic study. Phys. Chem. Chem. Phys. 2017, 19, 20377–20382. [Google Scholar] [CrossRef]
- Dehaghani, M.Z.; Yousefi, F.; Seidi, F.; Sajadi, S.M.; Rabiee, N.; Habibzadeh, S.; Esmaeili, A.; Mashhadzadeh, A.H.; Spitas, C.; Mostafavi, E.; et al. Dynamics of Antimicrobial Peptide Encapsulation in Carbon Nanotubes: The Role of Hydroxylation. Int. J. Nanomed. 2022, 17, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Erdoğar, N.; Akkin, S.; Bilensoy, E. Nanocapsules for Drug Delivery: An Updated Review of the Last Decade. Recent Patents Drug Deliv. Formul. 2019, 12, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Jasim, S.A.; Al-Lami, M.S.; Shather, A.; Aldhalmi, A.K.; Taki, A.G.; Ahmed, B.A.; Al-Hamdani, M.M.; Saraswat, S.K. Nanostructures of boron nitride: A promising nanocarrier for anti-cancer drug delivery. Micro Nanostruct. 2024, 185, 207708. [Google Scholar] [CrossRef]
- Mortazavifar, A.; Raissi, H.; Akbari, A. DFT and MD investigations on the functionalized boron nitride nanotube as an effective drug delivery carrier for Carmustine anticancer drug. J. Mol. Liq. 2019, 276, 577–587. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Rady, A.S.S.M.; Sidhom, P.A.; Sayed, S.R.M.; Ibrahim, K.E.; Awad, A.M.; Shoeib, T.; Mohamed, L.A. A Comparative DFT Investigation of the Adsorption of Temozolomide Anticancer Drug over Beryllium Oxide and Boron Nitride Nanocarriers. ACS Omega 2024, 9, 25203–25214. [Google Scholar] [CrossRef] [PubMed]
- Dehaghani, M.Z.; Bagheri, B.; Yousefi, F.; Nasiriasayesh, A.; Mashhadzadeh, A.H.; Zarrintaj, P.; Rabiee, N.; Bagherzadeh, M.; Fierro, V.; Celzard, A.; et al. Boron nitride nanotube as an antimicrobial peptide carrier: A theoretical insight. Int. J. Nanomed. 2021, 16, 1837–1847. [Google Scholar] [CrossRef]
- Bibi, S.; Ur-Rehman, S.; Khalid, L.; Bhatti, I.A.; Bhatti, H.N.; Iqbal, J.; Bai, F.Q.; Zhang, H.-X. Investigation of the adsorption properties of gemcitabine anticancer drug with metal-doped boron nitride fullerenes as a drug-delivery carrier: A DFT study. RSC Adv. 2022, 12, 2873–2887. [Google Scholar] [CrossRef]
- Roosta, S.; Hashemianzadeh, S.M.; Ketabi, S. Encapsulation of cisplatin as an anti-cancer drug into boron-nitride and carbon nanotubes: Molecular simulation and free energy calculation. Mater. Sci. Eng. C 2016, 67, 98–103. [Google Scholar] [CrossRef]
- Cattaneo, M.; Guerriero, G.; Shakya, G.; Krattiger, L.A.; Paganella, L.G.; Narciso, M.L.; Supponen, O. Cyclic jetting enables microbubble-mediated drug delivery. Nat. Phys. 2025, 21, 590–598. [Google Scholar] [CrossRef]
- Zhou, M.; Wei, T.; Gu, L.; Yang, H.; Li, M.; Zhou, Y. Focal opening of the neuronal plasma membrane by shock-induced bubble collapse for drug delivery: A coarse-grained molecular dynamics simulation. Phys. Chem. Chem. Phys. 2022, 24, 29862–29869. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Bo, Z.; Yang, H.; Yang, J.; Shuai, X.; Yan, J.; Cen, K. Temperature dependence of ion diffusion coefficients in NaCl electrolyte confined within graphene nanochannels. Phys. Chem. Chem. Phys. 2017, 19, 7678–7688. [Google Scholar] [CrossRef]
- Holz, M.; Heil, S.R.; Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740–4742. [Google Scholar] [CrossRef]
- Rezaee, S.; Kadivar, E.; el Moctar, O. The role of sawtooth-shaped nano riblets on nanobubble dynamics and collapse-induced erosion near solid boundary. J. Mol. Liq. 2024, 405, 124947. [Google Scholar] [CrossRef]
- Geneva, I.I.; Cuzzo, B.; Fazili, T.; Javaid, W. Normal body temperature: A systematic review. Open Forum Infect. Dis. 2019, 6, ofz032. [Google Scholar] [CrossRef] [PubMed]
- Protsiv, M.; Ley, C.; Lankester, J.; Hastie, T.; Parsonnet, J. Decreasing human body temperature in the United States since the Industrial Revolution. eLife 2020, 9, e49555. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, N.; Rezaee, S.; Azizi, B. Mechanical properties and role of 2D alkynyl carbon monolayers in the progress of lithium-air batteries. J. Energy Storage 2023, 72, 108558. [Google Scholar] [CrossRef]
- Rezaee, S.; Kadivar, E.; el Moctar, O. Molecular Dynamics-Based Approach for Laser-Induced Cavitation Bubbles: Bridging Experimental and Hybrid Analytical–Computational Approaches. Langmuir 2025, 41, 19071–19087. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Kothawade, S.; Shende, P. Coordination bonded stimuli-responsive drug delivery system of chemical actives with metal in pharmaceutical applications. Coord. Chem. Rev. 2024, 510, 215851. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Z.; Zhang, H.; Onsori, S. Effect of metal atoms on the electronic properties of metal oxide nanoclusters for use in drug delivery applications: A density functional theory study. Mol. Phys. 2020, 118, e1692150. [Google Scholar] [CrossRef]
- Sauerwein, W.A.G.; Wittig, A.; Moss, R.; Nakagawa, Y. Neutron Capture Therapy: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, P.; Zhang, H.; Zhang, Z.; Ran, J. Exploring Targeted Delivery Systems for Boron Neutron Capture Therapy and Its Potential as a Promising Therapeutic Modality for Hepatocellular Carcinoma. Cancer Biother. Radiopharm. 2025; ahead of print. [Google Scholar] [CrossRef]
- Li, L.; Dai, K.; Li, J.; Shi, Y.; Zhang, Z.; Liu, T.; Xie, J.; Zhang, R.; Liu, Z. A Boron-10 nitride nanosheet for combinational boron neutron capture therapy and chemotherapy of tumor. Biomaterials 2021, 268, 120587. [Google Scholar] [CrossRef] [PubMed]
- Such, G.K.; Yan, Y.; Johnston, A.P.R.; Gunawan, S.T.; Caruso, F. Interfacing materials science and biology for drug carrier design. Adv. Mater. 2015, 27, 2278–2297. [Google Scholar] [CrossRef]
- Yang, P.; Quan, Z.; Hou, Z.; Li, C.; Kang, X.; Cheng, Z.; Lin, J. A magnetic, luminescent and mesoporous core–shell structured composite material as drug carrier. Biomaterials 2009, 30, 4786–4795. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal–organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef]
- Guo, Q.; Shen, X.-T.; Li, Y.-Y.; Xu, S.-Q. Carbon nanotubes-based drug delivery to cancer and brain. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2017, 37, 635–641. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef]
- Sesis, A.; Hodnett, M.; Memoli, G.; Wain, A.J.; Jurewicz, I.; Dalton, A.B.; Carey, J.D.; Hinds, G. Influence of acoustic cavitation on the controlled ultrasonic dispersion of carbon nanotubes. J. Phys. Chem. B 2013, 117, 15141–15150. [Google Scholar] [CrossRef]
- Hennrich, F.; Krupke, R.; Arnold, K.; Stütz, J.A.R.; Lebedkin, S.; Koch, T.; Schimmel, T.; Kappes, M.M. The mechanism of cavitation-induced scission of single-walled carbon nanotubes. J. Phys. Chem. B 2007, 111, 1932–1937. [Google Scholar] [CrossRef]
- Genchi, G.G.; Ciofani, G. Bioapplications of Boron Nitride Nanotubes. Nanomedicine 2015, 10, 3315–3319. [Google Scholar] [CrossRef]
- Ciofani, G.D.P. Potential applications of boron nitride nanotubes as drug delivery systems. Expert Opin. Drug Deliv. 2010, 7, 889–893. [Google Scholar] [CrossRef]
- Turhan, E.A.; Pazarçeviren, A.E.; Evis, Z.; Tezcaner, A. Properties and applications of boron nitride nanotubes. Nanotechnology 2022, 33, 242001. [Google Scholar] [CrossRef]
- Mohammed, S.M.; Paul, T.; John, D.; Zhang, C.; Agarwal, A. Understanding the role of ultrasonic cavitation assisted casting of boron nitride nanotube-reinforced aluminum matrix composite. J. Mater. Res. Technol. 2023, 25, 2405–2418. [Google Scholar] [CrossRef]
- Maselugbo, A.O.; Harrison, H.B.; Alston, J.R. Boron nitride nanotubes: A review of recent progress on purification methods and techniques. J. Mater. Res. 2022, 37, 4438–4458. [Google Scholar] [CrossRef]
- Zavadlav, J.; Arampatzis, G.; Koumoutsakos, P. Bayesian selection for coarse-grained models of liquid water. Sci. Rep. 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- O’cOnnor, T.C.; Andzelm, J.; Robbins, M.O. AIREBO-M: A reactive model for hydrocarbons at extreme pressures. J. Chem. Phys. 2015, 142, 024903. [Google Scholar] [CrossRef]
- Los, J.H.; Kroes, J.M.H.; Albe, K.; Gordillo, R.M.; Katsnelson, M.I.; Fasolino, A. Extended Tersoff potential for boron nitride: Energetics and elastic properties of pristine and defective h-BN. Phys. Rev. B 2017, 96, 184108. [Google Scholar] [CrossRef]
- Ertekin, N. Nano-electro-mechanical conduct of boron nitride nanotube as piezoelectric nanogenerators and nanoswitches. Smart Mater. Struct. 2024, 33, 025037. [Google Scholar] [CrossRef]
- Atabay, M.; Sardroodi, J.J.; Ebrahimzadeh, A.R. Adsorption and immobilisation of human insulin on graphene monoxide, silicon carbide and boron nitride nanosheets investigated by molecular dynamics simulation. Mol. Simul. 2017, 43, 298–311. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, X.; Dong, R.; Wang, H. The impact of low-velocity shock waves on the dynamic behaviour characteristics of nanobubbles. Phys. Chem. Chem. Phys. 2024, 26, 11945–11957. [Google Scholar] [CrossRef]
- Ertekin, N.; Rezaee, S. Lithium-Doped Barium Titanate as Advanced Cells of ReRAMs Technology. J. Electron. Mater. 2023, 52, 1575–1589. [Google Scholar] [CrossRef]

| Study | This Work | References | ||||
|---|---|---|---|---|---|---|
| System | Pure Water | CN/Water | BNN/Water | Pure Water a | Saline Water a | Saline Water b |
| DH2O (10−9 m2·s−1) | 2.22 | 2.33 | 2.50 | 2.29 | 2.01 | 2.03 |
| Temperature (K) | 288 | 293 | 298 | 303 | 308 |
|---|---|---|---|---|---|
| DCN (10−9 m2·s−1) | 0.11 | 0.08 | 0.12 | 0.10 | 0.09 |
| DH2O (10−9 m2·s−1) | 2.16 | 2.16 | 2.33 | 2.66 | 2.83 |
| Temperature (K) | 288 | 293 | 298 | 303 | 308 |
|---|---|---|---|---|---|
| DBNN (10−9 m2·s−1) | 0.11 | 0.10 | 0.11 | 0.11 | 0.11 |
| DH2O (10−9 m2·s−1) | 2.16 | 2.16 | 2.50 | 2.66 | 2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydarian, F.; Rajabi Moghadam, S.; Ghasemi, M.; Saniei, E.; Rezaee, S.; Kadivar, E.; el Moctar, O. Role of Nanobubble Cavitation in Triggering Drug Release from Boron-Nitride and Carbon Nanocapsules and Their Diffusion for Drug Delivery Applications: A Molecular Dynamics Study. Int. J. Mol. Sci. 2025, 26, 9582. https://doi.org/10.3390/ijms26199582
Heydarian F, Rajabi Moghadam S, Ghasemi M, Saniei E, Rezaee S, Kadivar E, el Moctar O. Role of Nanobubble Cavitation in Triggering Drug Release from Boron-Nitride and Carbon Nanocapsules and Their Diffusion for Drug Delivery Applications: A Molecular Dynamics Study. International Journal of Molecular Sciences. 2025; 26(19):9582. https://doi.org/10.3390/ijms26199582
Chicago/Turabian StyleHeydarian, Farshad, Sahar Rajabi Moghadam, Maryam Ghasemi, Elham Saniei, Sasan Rezaee, Ebrahim Kadivar, and Ould el Moctar. 2025. "Role of Nanobubble Cavitation in Triggering Drug Release from Boron-Nitride and Carbon Nanocapsules and Their Diffusion for Drug Delivery Applications: A Molecular Dynamics Study" International Journal of Molecular Sciences 26, no. 19: 9582. https://doi.org/10.3390/ijms26199582
APA StyleHeydarian, F., Rajabi Moghadam, S., Ghasemi, M., Saniei, E., Rezaee, S., Kadivar, E., & el Moctar, O. (2025). Role of Nanobubble Cavitation in Triggering Drug Release from Boron-Nitride and Carbon Nanocapsules and Their Diffusion for Drug Delivery Applications: A Molecular Dynamics Study. International Journal of Molecular Sciences, 26(19), 9582. https://doi.org/10.3390/ijms26199582








