Dynamics of Telomerase-Based PD-L1 Circulating Tumor Cells as a Longitudinal Biomarker for Treatment Response Prediction in Patients with Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
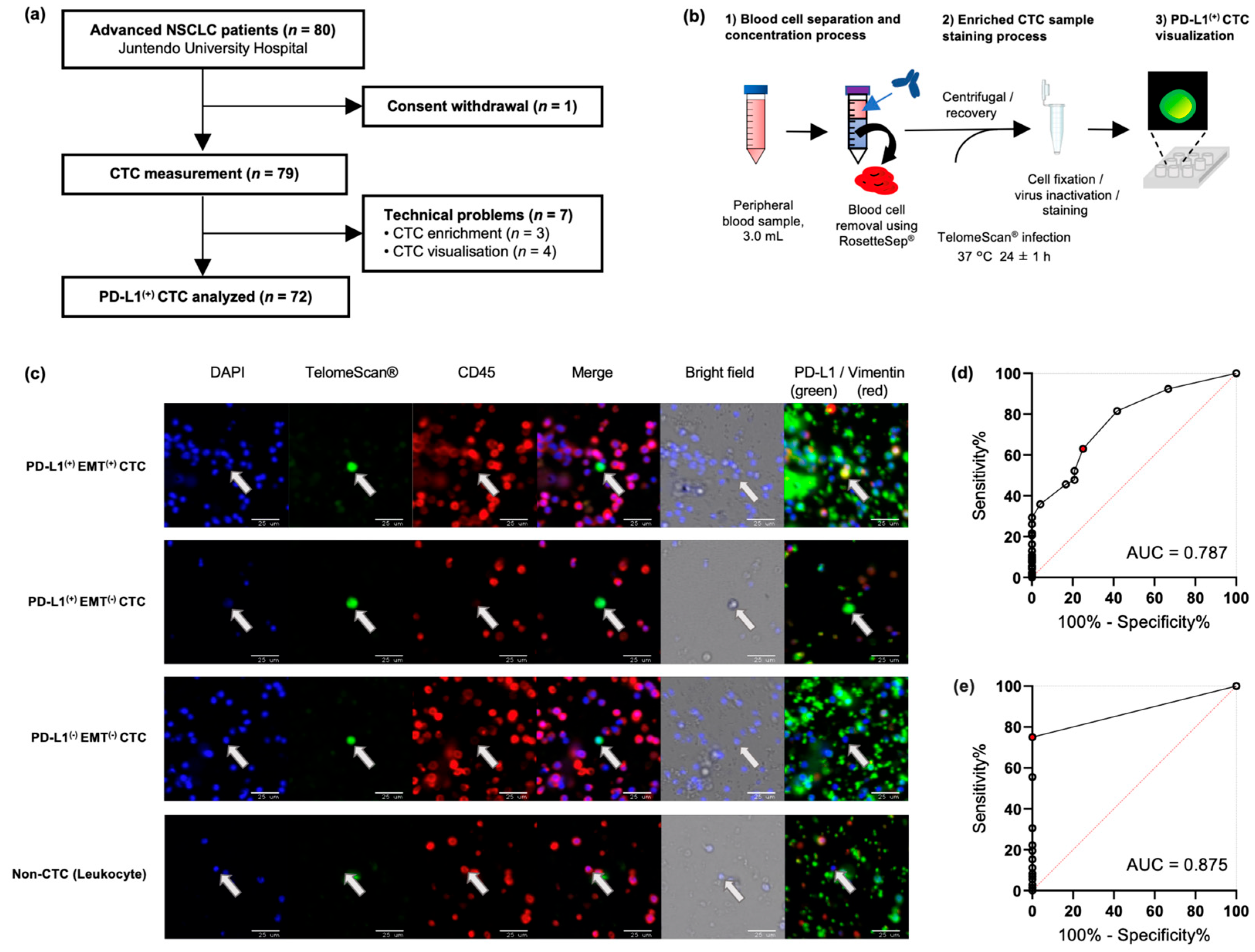
2.2. Highly Sensitive Detection of CTC Subtypes by TelomeScan®
2.3. CTC Detection and Association with Clinicopathological Subtypes
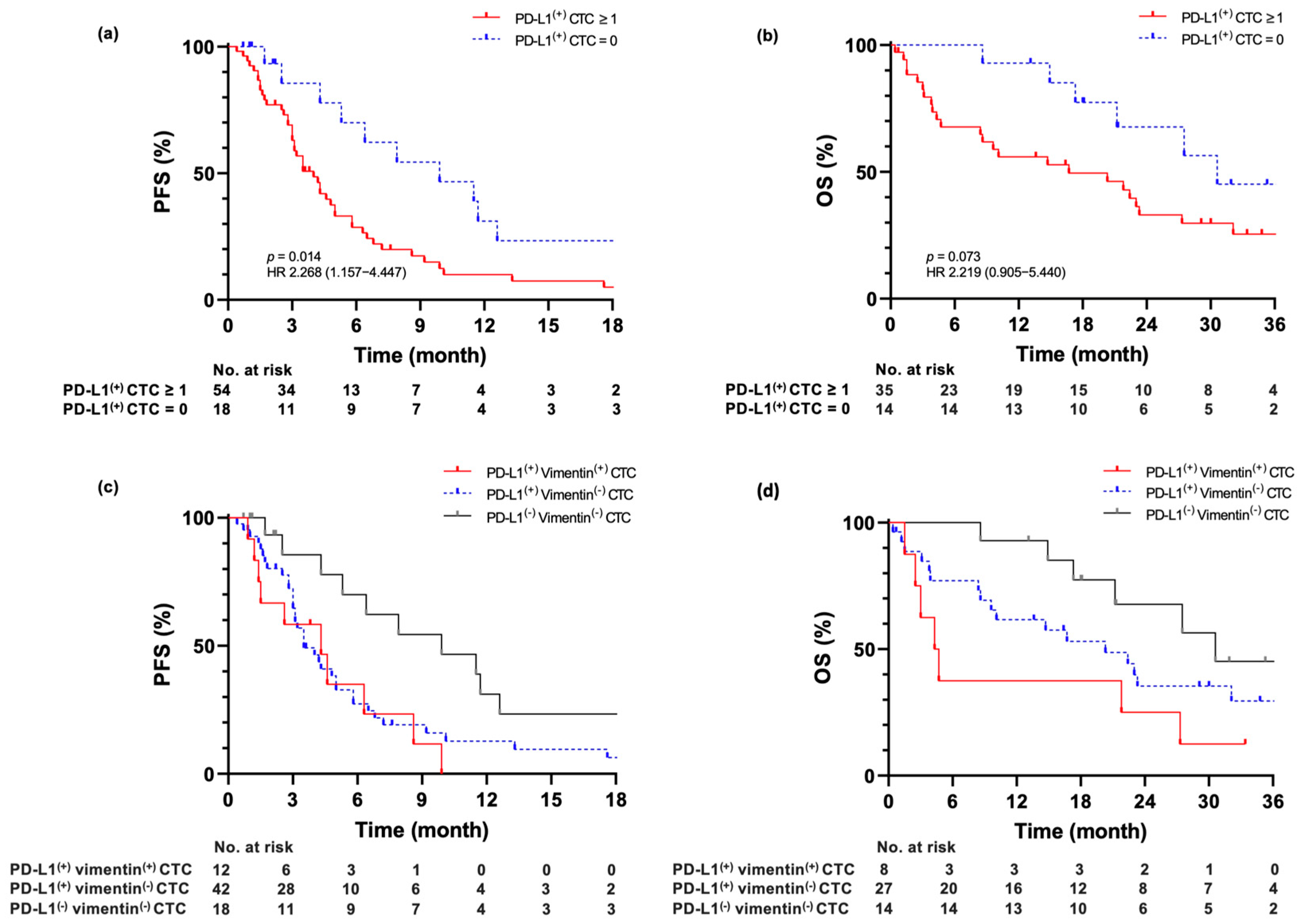
2.4. Association of Baseline PD-L1(+) CTC Measurements with Prognostic Outcome
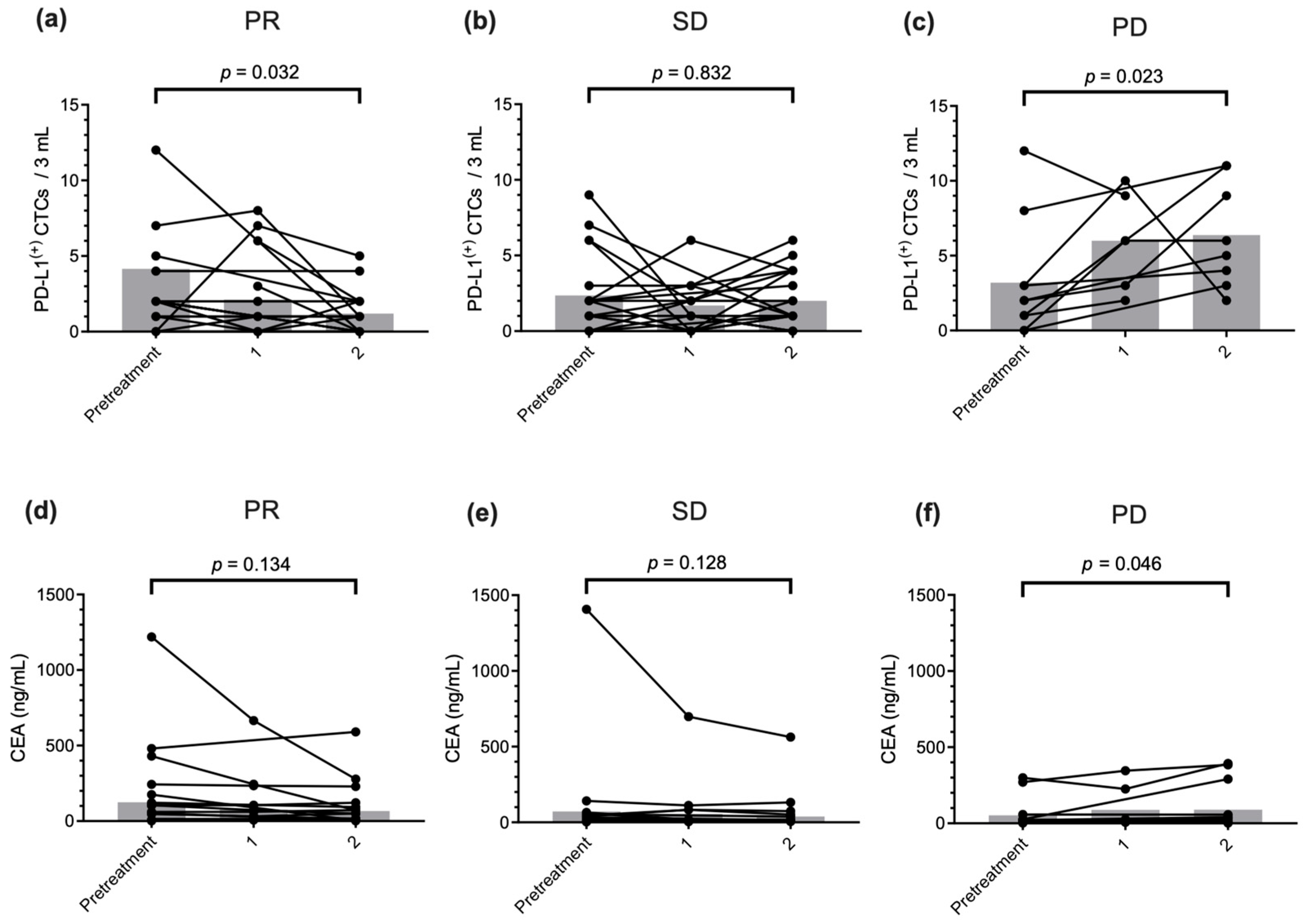
2.5. PD-L1(+) CTC Count Monitoring to Predict Therapeutic Response and Prognostic Outcome
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. TelomeScan®-Guided CTC Visualization
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alix-Panabières, C.; Riethdorf, S.; Pantel, K. Circulating tumor cells and bone marrow micrometastasis. Clin. Cancer Res. 2008, 14, 5013–5021. [Google Scholar] [CrossRef]
- Tanaka, F.; Yoneda, K.; Kondo, N.; Hashimoto, M.; Takuwa, T.; Matsumoto, S.; Okumura, Y.; Rahman, S.; Tsubota, N.; Tsujimura, T.; et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin. Cancer Res. 2009, 15, 6980–6986. [Google Scholar] [CrossRef]
- Rolle, A.; Günzel, R.; Pachmann, U.; Willen, B.; Höffken, K.; Pachmann, K. Increase in number of circulating disseminated epithelial cells after surgery for non-small cell lung cancer monitored by MAINTRAC(R) is a predictor for relapse: A preliminary report. World J. Surg. Oncol. 2005, 3, 18. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.A.; Caridà, G.; Ciliberto, D.; d’Apolito, M.; Pelaia, C.; Caracciolo, D.; Riillo, C.; Correale, P.; Galvano, A.; Russo, A.; et al. Efficacy and safety of first-line checkpoint inhibitors-based treatments for non-oncogene-addicted non-small-cell lung cancer: A systematic review and meta-analysis. ESMO Open 2022, 7, 100465. [Google Scholar] [CrossRef]
- Lin, E.P.Y.; Hsu, C.Y.; Berry, L.; Bunn, P.; Shyr, Y. Analysis of cancer survival associated with immune checkpoint inhibitors after statistical adjustment: A systematic review and meta-analyses. JAMA Netw. Open 2022, 5, e2227211. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Togo, S.; Katagiri, N.; Namba, Y.; Tulafu, M.; Nagahama, K.; Kadoya, K.; Takamochi, K.; Oh, S.; Suzuki, K.; Sakurai, F.; et al. Sensitive detection of viable circulating tumor cells using a novel conditionally telomerase-selective replicating adenovirus in non-small cell lung cancer patients. Oncotarget 2017, 8, 34884–34895. [Google Scholar] [CrossRef] [PubMed]
- Batth, I.S.; Dao, L.; Satelli, A.; Mitra, A.; Yi, S.; Noh, H.; Li, H.; Brownlee, Z.; Zhou, S.; Bond, J.; et al. Cell surface vimentin-positive circulating tumor cell-based relapse prediction in a long-term longitudinal study of postremission neuroblastoma patients. Int. J. Cancer 2020, 147, 3550–3559, Erratum in Int. J. Cancer 2021, 148, E6. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Saeki, H.; Nakashima, Y.; Ito, S.; Oki, E.; Morita, M.; Oda, Y.; Okano, S.; Maehara, Y. Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci. 2017, 108, 1119–1127. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhan, H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett. 2020, 468, 72–81. [Google Scholar] [CrossRef]
- Kim, S.J.; Masago, A.; Tamaki, Y.; Akazawa, K.; Tsukamoto, F.; Sato, J.; Ozawa, T.; Tsujino, Y.; Noguchi, S. A novel approach using telomerase-specific replication-selective adenovirus for detection of circulating tumor cells in breast cancer patients. Breast Cancer Res. Treat. 2011, 128, 765–773. [Google Scholar] [CrossRef]
- Igawa, S.; Gohda, K.; Fukui, T.; Ryuge, S.; Otani, S.; Masago, A.; Sato, J.; Murakami, K.; Maki, S.; Katono, K.; et al. Circulating tumor cells as a prognostic factor in patients with small cell lung cancer. Oncol. Lett. 2014, 7, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Inoue, H.; Kimura, S.; Ohmori, T.; Ishikawa, F.; Gohda, K.; Sato, J. Prognostic impact of the number of viable circulating cells with high telomerase activity in gastric cancer patients: A prospective study. Int. J. Oncol. 2014, 45, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Sato, J.; Tsujino, Y.; Yamaguchi, N.; Kimura, S.; Gohda, K.; Murakami, K.; Onimaru, M.; Ohmori, T.; Ishikawa, F.; et al. Long-term prognostic impact of circulating tumour cells in gastric cancer patients. World J. Gastroenterol. 2016, 22, 10232–10241. [Google Scholar] [CrossRef] [PubMed]
- Takakura, M.; Kyo, S.; Nakamura, M.; Maida, Y.; Mizumoto, Y.; Bono, Y.; Zhang, X.; Hashimoto, Y.; Urata, Y.; Fujiwara, T.; et al. Circulating tumour cells detected by a novel adenovirus-mediated system may be a potent therapeutic marker in gynaecological cancers. Br. J. Cancer 2012, 107, 448–454. [Google Scholar] [CrossRef]
- Goldkorn, A.; Ely, B.; Tangen, C.M.; Tai, Y.C.; Xu, T.; Li, H.; Twardowski, P.; Veldhuizen, P.J.V.; Agarwal, N.; Carducci, M.A.; et al. Circulating tumor cell telomerase activity as a prognostic marker for overall survival in SWOG 0421: A phase III metastatic castration resistant prostate cancer trial. Int. J. Cancer 2015, 136, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, Y.; Tazawa, H.; Tanabe, S.; Kanaya, N.; Noma, K.; Koujima, T.; Kashima, H.; Kato, T.; Kuroda, S.; Kikuchi, S.; et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur. J. Cancer 2021, 153, 98–108. [Google Scholar] [CrossRef]
- Heo, J.; Liang, D.; Kim, C.W.; Woo, H.Y.; Shih, L.; Su, H.; Lin, Z.; Yoo, S.Y.; Chang, S.; Urata, Y.; et al. Safety and dose escalation of the targeted oncolytic adenovirus OBP-301 for refractory advanced liver cancer: Phase I clinical trial. Mol. Ther. 2023, 31, 2077–2088. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.C.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef]
- Ito, H.; Inoue, H.; Sando, N.; Kimura, S.; Gohda, K.; Sato, J.; Murakami, K.; Ito, S.; Odaka, N.; Satodate, H.; et al. Prognostic impact of detecting viable circulating tumour cells in gastric cancer patients using a telomerase-specific viral agent: A prospective study. BMC Cancer 2012, 12, 346. [Google Scholar] [CrossRef]
- Spiliotaki, M.; Neophytou, C.M.; Vogazianos, P.; Stylianou, I.; Gregoriou, G.; Constantinou, A.I.; Deltas, C.; Charalambous, H. Dynamic monitoring of PD-L1 and Ki67 in circulating tumor cells of metastatic non-small cell lung cancer patients treated with pembrolizumab. Mol. Oncol. 2023, 17, 792–809. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.; Loges, S.; Pantel, K.; Wikman, H. Detection of circulating tumor cells in non-small cell lung cancer. Front. Oncol. 2015, 5, 207. [Google Scholar] [CrossRef]
- Krebs, M.G.; Hou, J.M.; Sloane, R.; Lancashire, L.; Priest, L.; Nonaka, D.; Ward, T.H.; Backen, A.; Clack, G.; Hughes, A.; et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 2012, 7, 306–315. [Google Scholar] [CrossRef]
- Sinoquet, L.; Jacot, W.; Gauthier, L.; Pouderoux, S.; Viala, M.; Cayrefourcq, L.; Quantin, X.; Alix-Panabières, C. Programmed cell death ligand 1-expressing circulating tumor cells: A new prognostic biomarker in non-small cell lung cancer. Clin. Chem. 2021, 67, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Janning, M.; Kobus, F.; Babayan, A.; Wikman, H.; Velthaus, J.L.; Bergmann, S.; Schatz, S.; Falk, M.; Berger, L.A.; Böttcher, L.M.; et al. Determination of PD-L1 expression in circulating tumor cells of NSCLC patients and correlation with response to PD-1/PD-L1 inhibitors. Cancers 2019, 11, 835. [Google Scholar] [CrossRef]
- Chiang, P.J.; Xu, T.; Cha, T.L.; Tsai, Y.T.; Liu, S.Y.; Wu, S.T.; Meng, E.; Tsao, C.W.; Kao, C.C.; Chen, C.L.; et al. Programmed cell death ligand 1 expression in circulating tumor cells as a predictor of treatment response in patients with urothelial carcinoma. Biology 2021, 10, 674. [Google Scholar] [CrossRef]
- Winograd, P.; Hou, S.; Court, C.M.; Lee, Y.T.; Chen, P.J.; Zhu, Y.; Sadeghi, S.; Finn, R.S.; Teng, P.C.; Wang, J.J.; et al. Hepatocellular carcinoma-circulating tumor cells expressing PD-L1 are prognostic and potentially associated with response to checkpoint inhibitors. Hepatol. Commun. 2020, 4, 1527–1540. [Google Scholar] [CrossRef]
- Ikeda, M.; Koh, Y.; Teraoka, S.; Sato, K.; Oyanagi, J.; Hayata, A.; Tokudome, N.; Akamatsu, H.; Ozawa, Y.; Endo, K.; et al. Longitudinal evaluation of PD-L1 expression on circulating tumor cells in non-small cell lung cancer patients treated with nivolumab. Cancers 2021, 13, 2290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Mo, W.; Lian, X.; Cao, D.; Cheng, H.; Wang, H. Detection of PD-L1 expression and epithelial-mesenchymal transition of circulating tumor cells in non-small cell lung cancer. Exp. Ther. Med. 2024, 28, 294. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhou, C.; Chen, S.; Ma, Q.; Xiao, H.; Chen, Q.; Zou, H.; Li, H.; Wang, Z.; Sun, Y.; et al. Prognosis value of circulating tumor cell PD-L1 and baseline characteristics in patients with NSCLC treated with immune checkpoint inhibitors plus platinum-containing drugs. Oncol. Lett. 2024, 27, 131. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yue, C.; Ji, S.; Zhao, C.; Jia, R.; Zhang, Y.; Liu, R.; Li, D.; Yu, Q.; Li, P.; et al. Assessment of PD-L1 expression on circulating tumor cells for predicting clinical outcomes in patients with cancer receiving PD-1/PD-L1 blockade therapies. Oncologist 2021, 26, e2227–e2238. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, X.; Li, J.; Tong, B.; Xu, Y.; Chen, M.; Liu, X.; Gao, X.; Shi, Y.; Zhao, J.; et al. Circulating tumor cells PD-L1 expression detection and correlation of therapeutic efficacy of immune checkpoint inhibition in advanced non-small-cell lung cancer. Thorac. Cancer 2023, 14, 470–478. [Google Scholar] [CrossRef]
- Salgia, R.; Harpole, D.; Herndon, J.E.; Pisick, E.; Elias, A.; Skarin, A.T. Role of serum tumor markers CA 125 and CEA in non-small cell lung cancer. Anticancer Res. 2001, 21, 1241–1246. [Google Scholar]
- Satoh, H.; Ishikawa, H.; Kamma, H.; Yamashita, Y.T.; Takahashi, H.; Ohtsuka, M.; Hasegawa, S. Serum sialyl lewis X-i antigen levels in non-small cell lung cancer: Correlation with distant metastasis and survival. Clin. Cancer Res. 1997, 3, 495–499. [Google Scholar]
- Ntzifa, A.; Strati, A.; Kallergi, G.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. Gene expression in circulating tumor cells reveals a dynamic role of EMT and PD-L1 during osimertinib treatment in NSCLC patients. Sci. Rep. 2021, 11, 2313. [Google Scholar] [CrossRef]
- Wang, X.; Ricciuti, B.; Alessi, J.V.; Nguyen, T.; Awad, M.M.; Lin, X.; Johnson, B.E.; Christiani, D.C. Smoking history as a potential predictor of immune checkpoint inhibitor efficacy in metastatic non-small cell lung cancer. J. Natl. Cancer Inst. 2021, 113, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Briere, D.M.; Li, S.; Calinisan, A.; Sudhakar, N.; Aranda, R.; Hargis, L.; Peng, D.H.; Deng, J.; Engstrom, L.D.; Hallin, J.; et al. The KRASG12C inhibitor MRTX849 reconditions the tumor immune microenvironment and sensitizes tumors to checkpoint inhibitor therapy. Mol. Cancer Ther. 2021, 20, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Liao, Y.C.; Ho, Y.S.; Chen, L.C.; Chang, H.W.; Cheng, T.C.; Liu, D.; Lee, W.R.; Shen, S.C.; Wu, C.H.; et al. The α9 nicotinic acetylcholine receptor mediates nicotine-induced PD-L1 expression and regulates melanoma cell proliferation and migration. Cancers 2019, 11, 1991. [Google Scholar] [CrossRef]
- Polioudaki, H.; Mala, A.; Gkimprixi, E.; Papadaki, M.A.; Chantziou, A.; Tzardi, M.; Mavroudis, D.; Agelaki, S.; Theodoropoulos, P.A. Epithelial/mesenchymal characteristics and PD-L1 co-expression in CTCs of metastatic breast cancer patients treated with eribulin: Correlation with clinical outcome. Cancers 2020, 12, 3735. [Google Scholar] [CrossRef]
- Manjunath, Y.; Upparahalli, S.V.; Avella, D.M.; Deroche, C.B.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Smith, C.J.; Li, G.; Kaifi, J.T. PD-L1 expression with epithelial mesenchymal transition of circulating tumor cells is associated with poor survival in curatively resected non-small cell lung cancer. Cancers 2019, 11, 806. [Google Scholar] [CrossRef]
- Satelli, A.; Batth, I.S.; Brownlee, Z.; Rojas, C.; Meng, Q.H.; Kopetz, S.; Li, S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci. Rep. 2016, 6, 28910. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLOS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kojima, T.; Hashimoto, Y.; Watanabe, Y.; Kagawa, S.; Uno, F.; Kuroda, S.; Tazawa, H.; Kyo, S.; Mizuguchi, H.; Urata, Y.; et al. A simple biological imaging system for detecting viable human circulating tumor cells. J. Clin. Investig. 2009, 119, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Shigeyasu, K.; Tazawa, H.; Hashimoto, Y.; Mori, Y.; Nishizaki, M.; Kishimoto, H.; Nagasaka, T.; Kuroda, S.; Urata, Y.; Goel, A.; et al. Fluorescence virus-guided capturing system of human colorectal circulating tumour cells for non-invasive companion diagnostics. Gut 2015, 64, 627–635. [Google Scholar] [CrossRef][Green Version]
| Patient Characteristics | PD-L1(+) CTC n = 54 | PD-L1(−) CTC n = 18 | P-Value | |
|---|---|---|---|---|
| Vimentin(+) n = 12 | Vimentin(−) n = 42 | |||
| Age, median (range), years | 69 (46–85) | 70 (40–88) | 70 (52–87) | 0.755 a |
| Sex | 0.052 b | |||
| Male | 11 | 27 | 16 | |
| Female | 1 | 15 | 2 | |
| Smoking history | 0.030 b | |||
| Heavy smoker (BI ≥ 400) | 12 † | 28 † | 15 | |
| Light/never smoked (BI < 400) | 0 † | 14 † | 3 | |
| Stage | 0.028 b | |||
| I, II, IIIA (Postoperative recurrence) | 5 | 5 † | 8 † | |
| IIIB | 1 | 3 | 2 | |
| IIIC | 0 | 0 | 1 | |
| IVA | 3 | 16 | 5 | |
| IVB | 3 | 18 † | 2 † | |
| Histology at diagnosis | 0.121 b | |||
| Adenocarcinoma | 8 | 32 | 8 | |
| Squamous cell carcinoma | 2 | 5 | 7 | |
| Others | 2 | 5 | 3 | |
| PD-L1 status | 0.937 b | |||
| TPS < 1% | 5 | 14 | 8 | |
| TPS 1–49% | 2 | 9 | 3 | |
| TPS ≥ 50% | 4 | 17 | 7 | |
| Unknown | 1 | 2 | 0 | |
| Line of therapy | 0.690 b | |||
| 1 | 8 | 27 | 14 | |
| 2 | 2 | 9 | 1 | |
| ≥3 | 2 | 6 | 3 | |
| Regimen | 0.080 b | |||
| ICI + chemotherapy | 2 | 20 | 7 | |
| ICI | 3 | 9 | 8 | |
| Chemotherapy | 7 | 13 | 3 | |
| Driver mutation | 0.953 b | |||
| EGFR | 1 | 5 | 1 | |
| ALK | 0 | 0 | 0 | |
| ROS-1 | 0 | 1 | 0 | |
| BRAF | 0 | 1 | 0 | |
| Negative/unknown | 11 | 35 | 17 | |
| RECIST (2 courses) | 0.716 b | |||
| PR | 6 | 12 | 6 | |
| SD | 2 | 16 | 7 | |
| PD | 3 | 8 | 2 | |
| Sensor | 1 | 6 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumiyoshi, I.; Togo, S.; Okabe, T.; Abe, K.; Watanabe, J.; Ochi, Y.; Hoshi, K.; Saiwaki, S.; Nojiri, S.; Fujimoto, Y.; et al. Dynamics of Telomerase-Based PD-L1 Circulating Tumor Cells as a Longitudinal Biomarker for Treatment Response Prediction in Patients with Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2025, 26, 9583. https://doi.org/10.3390/ijms26199583
Sumiyoshi I, Togo S, Okabe T, Abe K, Watanabe J, Ochi Y, Hoshi K, Saiwaki S, Nojiri S, Fujimoto Y, et al. Dynamics of Telomerase-Based PD-L1 Circulating Tumor Cells as a Longitudinal Biomarker for Treatment Response Prediction in Patients with Non-Small Cell Lung Cancer. International Journal of Molecular Sciences. 2025; 26(19):9583. https://doi.org/10.3390/ijms26199583
Chicago/Turabian StyleSumiyoshi, Issei, Shinsaku Togo, Takahiro Okabe, Kanae Abe, Junko Watanabe, Yusuke Ochi, Kazuaki Hoshi, Shoko Saiwaki, Shuko Nojiri, Yuichi Fujimoto, and et al. 2025. "Dynamics of Telomerase-Based PD-L1 Circulating Tumor Cells as a Longitudinal Biomarker for Treatment Response Prediction in Patients with Non-Small Cell Lung Cancer" International Journal of Molecular Sciences 26, no. 19: 9583. https://doi.org/10.3390/ijms26199583
APA StyleSumiyoshi, I., Togo, S., Okabe, T., Abe, K., Watanabe, J., Ochi, Y., Hoshi, K., Saiwaki, S., Nojiri, S., Fujimoto, Y., Namba, Y., Tabe, Y., Urata, Y., & Takahashi, K. (2025). Dynamics of Telomerase-Based PD-L1 Circulating Tumor Cells as a Longitudinal Biomarker for Treatment Response Prediction in Patients with Non-Small Cell Lung Cancer. International Journal of Molecular Sciences, 26(19), 9583. https://doi.org/10.3390/ijms26199583







