Mechanosensing of Shear Stress and Uterine Spiral Artery Remodeling by Invasive Trophoblasts in Early Pregnancy
Abstract
1. Introduction
2. Shear Stress
3. Shear Stress Sensing
3.1. Mechanoreceptors of the Vascular Lining
- Ion channels directly activated by mechanical stimuli;
- Structures linked to the cell membrane;
- Cytoskeletal elements;
- Junctional proteins;
- Other proteins.
3.1.1. Mechanosensitive Ion Channels (MSICs)
- The structure of the channel includes the incorporation of a pore-forming subunit for rapid ion conduction.
- It is assumed that an isolated (purified) channel placed in an artificial, cell-free lipid bilayer will open in response to tension exerted on the bilayer.
- Directed mutagenesis within key domains of a given channel, affecting pore selectivity or conductivity, should modify mechanosensitivity.
- Enforcing the expression of a given channel in a nonmechanosensory cell is expected to result in mechanosensitivity.
- Expression of both the gene and protein of a given ion channel in a potentially mechanosensitive cell should be confirmed.
- Elimination (knockout) of a specific gene encoding the protein of a given channel makes it possible to establish that the channel not only functions in developmental processes but is also not a downstream signaling partner of another mechanosensor. The use of dominant-negative suppression of a given channel with a mutated ion channel subunit might be an improved option, considering that genetic deletion can perturb the formation of normal signaling complexes.
- The epithelial sodium channel/degenerin (ENaC/DEG) family;
- Transient receptor potential (TRP) channels;
- Two-pore domain potassium (K2P) channels;
- PIEZO channels.
- –
- TRPC (canonical);
- –
- TRPM (melastatin);
- –
- TRPV (vanilloid);
- –
- TRPA (ankyrin);
- –
- TRPP (polycystin);
- –
- TRPML (mucolipin).
- –
- TWIK channels (two-pore domain in a weak inward rectifying K+ channels);
- –
- TREK/TRAAK channels (TWIK-related K+ channels/TWIK-related arachidonic acid-activated K+ channels);
- –
- TASK channels (TWIK-related acid-sensitive K+ channels);
- –
- TALK channels (TWIK-related alkaline-activated K+ channels);
- –
- THIK channels (TWIK-related halothane-inhibited K+ channels);
- –
- TRESK channels (TWIK-related spinal cord K+ channels).
3.1.2. Mechanosensitive G-Protein Coupled Receptors (GPCRs)
3.1.3. Receptor Tyrosine Kinases (RTKs)
3.1.4. Mechanotransduction and Whole-Cell Mechanosensing
4. Shear Stress and Uterine Spiral Artery Remodeling by enEVT Cell Invasion
4.1. PIEZO1 Signaling in Trophoblast Fusion and Spiral Artery Remodeling
4.2. Calveolae as Mechanosensors and Mechanotransducers During EVT Migration and Differentiation
4.3. Trophoblast Plugs and Mechanosensing
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-AP | 4-aminopyridine |
| 7TM | seven transmembrane |
| aGPCRs | adhesion class of G protein-coupled receptors |
| Akt | protein kinase B (also known as PKB) |
| AQP3 | aquaporin 3, a membrane transporter of water and glycerol |
| ARD | ankyrin repeats domains in TRPV4 channel |
| ASIC | acid-sensing ion channel |
| AT1R | angiotensin II type 1 receptor |
| AVPR1A | vasopressin V1a receptor |
| B2R | bradykinin receptor B2 |
| CaM | calmodulin |
| CaPLSase | Ca2+-activated phospholipid scramblase |
| Cav | caveola |
| cGMP | cyclic guanosine monophosphate |
| CS | chondroitin sulfate |
| CSK | cytoskeleton |
| CTB | cytotrophoblast |
| D1BR | dopamine receptor D5 |
| EC | endothelial cell |
| ECL1–ECL3 | three extracellular loops 1–3 |
| ECCJ | endothelial cell–cell junctions |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor (also known as ErbB) |
| ENaC/DEG | epithelial sodium channel/degenerin family |
| eNOS | endothelial nitric oxide synthase |
| enEVT | endovascular extravillous trophoblast (enEVT) |
| ER | endoplasmic reticulum |
| ERKs | extracellular signal-regulated kinases |
| ETB | endothelin B receptor |
| EVT | extravillous trophoblast |
| FAK | focal adhesion kinase |
| FAs | focal adhesions |
| FASL | Fas ligand (also known as CD95L or Apo-1L), a type-II transmembrane protein in the tumor necrosis factor (TNF) superfamily |
| FGR | fetal growth restriction |
| GAGs | glycosaminoglycans |
| GDP | guanosine diphosphate |
| GJ | gap junction |
| GLX | glycocalyx |
| GPCR | G protein-coupled receptor |
| GPR68 | G protein-coupled receptor 68 (also known as OGR1) |
| GTP | guanosine triphosphate |
| H1R | histamine H1 receptor |
| HS | heparan sulfate |
| HSPGs | heparan sulfate proteoglycans |
| hTSCs | human trophoblast stem cells |
| HUGO | Human Genome Organization |
| ICAM-1 | endothelial intercellular adhesion molecule-1 |
| ICL1–ICL3 | three intracellular loops 1–3 |
| IEL | internal elastic lamina |
| IGF2BP1 | insulin-like growth factor 2 mRNA-binding protein 1 |
| IKCa | intermediate conductance Ca2+-activated K+ channels |
| ILK | integrin-linked kinase |
| IP3 | inositol trisphosphate |
| IP3R2 | inositol 1,4,5-trisphosphate receptor type 2 (also known as ITPR2) |
| IUGR | intrauterine growth retardation |
| IUPHAR | International Union of Basic and Clinical Pharmacology |
| K2P | two-pore domain potassium channels |
| LINC | complex—linker of nucleoskeleton and cytoskeleton complex |
| MAPKs | mitogen-activated protein kinases |
| METTL14 | protein methyltransferase-like 14 |
| MSICs | mechanosensitive ion channels |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NTF/CTF | complex—N-terminal fragment/C-terminal fragment complex of the adhesion class of G protein-coupled receptor |
| NO | nitric oxide |
| OS-9 | lectin protein binding domain in TRPV4 channel |
| P1, P2 | the two-pore domain K+ channels |
| PaO2 | partial pressure of oxygen |
| PECAM-1 | platelet endothelial adhesion molecule-1 |
| PI3K | phosphatidylinositol 3-kinase |
| PKB | protein kinase B (also known as Akt) |
| PLC | phospholipase C |
| PRD | proline-rich domain in TRPV4 channel |
| ProGs | proteoglycans |
| pS | picosiemens |
| qRT-PCR | quantitative reverse transcription—polymerase chain reaction analysis |
| RTKs | receptor tyrosine kinases |
| S1PR1 | sphingosine-1-phosphate receptor 1 |
| sAC | soluble adenylyl cyclase |
| sGC | soluble guanylate cyclase |
| SI | International System of Units |
| STB | syncytiotrophoblast |
| SynT-2 STB | syncytiotrophoblast layer 2 cells |
| TALK | TWIK-related alkaline activated K+ channel |
| TASK | TWIK-related acid-sensitive K+ channels |
| TEA | tetraethylammonium |
| THIK | TWIK-related halothane inhibited K+ channel |
| TKs | non-receptor tyrosine kinases |
| TM | second transmembrane helix (transmembrane domain) |
| TMEM16F | transmembrane protein 16 F |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand (also known as Apo2L) |
| TREK/TRAAK | TWIK-related K+ channels/TWIK-related arachidonic acid activated K+ channel |
| TRESK | TWIK-related spinal cord K+ channel |
| TRP | transient receptor potential channels |
| TRPA | transient receptor potential channel—ankyrin subfamily |
| TRPC | transient receptor potential channel—canonical subfamily |
| TRPM | transient receptor potential channel—melastatin subfamily |
| TRPML | transient receptor potential channel—mucolipin subfamily |
| TRPP | transient receptor potential channel—polycystin subfamily |
| TRPV | transient receptor potential channel—vanilloid subfamily |
| TSCs | trophoblast stem cells |
| TWIK | two-pore domain in a weak inward rectifying K+ channel |
| UtMVECs | uterine microvascular endothelial cells |
| VE-cadherin | vascular endothelial cadherin (also known as CDH5) |
| VEGFR2, VEGFR3 | vascular endothelial growth factor receptors 2 and 3 |
| VSMC | vascular smooth muscle cells |
References
- Pollock, J.D.; Murray, I.V.; Bordes, S.J.; Makaryus, A.N. Physiology, Cardiovascular Hemodynamics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK470310/ (accessed on 10 August 2025).
- Roux, E.; Bougaran, P.; Dufourcq, P.; Couffinhal, T. Fluid Shear Stress Sensing by the Endothelial Layer. Front. Physiol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Bertani, F.; Di Francesco, D.; Corrado, M.D.; Talmon, M.; Fresu, L.G.; Boccafoschi, F. Paracrine Shear-Stress-Dependent Signaling from Endothelial Cells Affects Downstream Endothelial Function and Inflammation. Int. J. Mol. Sci. 2021, 22, 13300. [Google Scholar] [CrossRef]
- Miyazaki, T.; Honda, K.; Ohata, H. Requirement of Ca2+ influx- and phosphatidylinositol 3-kinase-mediated m-calpain activity for shear stress-induced endothelial cell polarity. Am. J. Physiol. Cell Physiol. 2007, 293, C1216–C1225. [Google Scholar] [CrossRef] [PubMed]
- Kutikhin, A.G.; Sinitsky, M.Y.; Yuzhalin, A.E.; Velikanova, E.A. Shear stress: An essential driver of endothelial progenitor cells. J. Mol. Cell. Cardiol. 2018, 118, 46–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qian, J.-Y.; Cheng, H.; Li, X.-M. Effects of shear stress on differentiation of stem cells into endothelial cells. World J. Stem Cells 2021, 13, 894–913. [Google Scholar] [CrossRef]
- Tian, G.-E.; Zhou, J.-T.; Liu, X.-J.; Huang, Y.-C. Mechanoresponse of stem cells for vascular repair. World J. Stem Cells 2019, 11, 1104–1114. [Google Scholar] [CrossRef]
- Dimmeler, S.; Assmus, B.; Hermann, C.; Haendeler, J.; Zeiher, A.M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: Involvement in suppression of apoptosis. Circ. Res. 1998, 83, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Philip, N.M.; Murray, S.T.; Yun, X.; Croglio, M.P.; Suresh, K.; Damarla, M.; Shimoda, L.A.; Kolb, T.M. Physiological shear stress suppresses apoptosis in human pulmonary microvascular endothelial cells. Physiol. Rep. 2025, 13, e70269. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Jia, L.; Yu, H.; Wang, Z.; Wei, F.; Jiang, A. Shear stress leads to the dysfunction of endothelial cells through the Cav-1-mediated KLF2/eNOS/ERK signaling pathway under physiological conditions. Open Life Sci. 2023, 18, 20220587. [Google Scholar] [CrossRef]
- Xia, T.; Yu, J.; Du, M.; Chen, X.; Wang, C.; Li, R. Vascular endothelial cell injury: Causes, molecular mechanisms, and treatments. Medcomm 2025, 6, e70057. [Google Scholar] [CrossRef]
- Thosar, S.S.; Johnson, B.D.; Johnston, J.D.; Wallace, J.P. Sitting and endothelial dysfunction: The role of shear stress. Med. Sci. Monit. 2012, 18, RA173–RA180. [Google Scholar] [CrossRef]
- Restaino, R.M.; Walsh, L.K.; Morishima, T.; Vranish, J.R.; Martinez-Lemus, L.A.; Fadel, P.J.; Padilla, J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H648–H653. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chung, J.; Kim, K.H.; An, S.H.; Kim, M.; Park, J.; Kwon, K. Fluid shear stress regulates the expression of Lectin-like oxidized low density lipoprotein receptor-1 via KLF2-AP-1 pathway depending on its intensity and pattern in endothelial cells. Atherosclerosis 2018, 270, 76–88. [Google Scholar] [CrossRef]
- Cheng, H.; Zhong, W.; Wang, L.; Zhang, Q.; Ma, X.; Wang, Y.; Wang, S.; He, C.; Wei, Q.; Fu, C. Effects of shear stress on vascular endothelial functions in atherosclerosis and potential therapeutic approaches. Biomed. Pharmacother. 2023, 158, 114198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yu, Y.; Chen, R.; Liu, X.; Hu, Y.; Ma, Z.; Gao, L.; Jian, W.; Wang, L. Wall shear stress and its role in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1083547. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, H.J.; Ghayesh, M.H.; Zander, A.C.; Psaltis, P.J. On the nonlinear relationship between wall shear stress topology and multi-directionality in coronary atherosclerosis. Comput. Methods Programs Biomed. 2023, 231, 107418. [Google Scholar] [CrossRef]
- Chalkias, A. Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 17522. [Google Scholar] [CrossRef]
- Staarmann, B.; Smith, M.; Prestigiacomo, C.J. Shear stress and aneurysms: A review. Neurosurg. Focus 2019, 47, E2. [Google Scholar] [CrossRef]
- Lee, T.C.; Moulvi, A.; James, J.L.; Clark, A.R. Multi-scale Modelling of Shear Stress on the Syncytiotrophoblast: Could Maternal Blood Flow Impact Placental Function Across Gestation? Ann. Biomed. Eng. 2023, 51, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, J.-S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Wang, J.; Xia, F.; Zhou, Y.; Wei, X.; Zhuang, Y.; Huang, Y. Association Between Endometrial/Subendometrial Vasculature and Embryo Transfer Outcome: A Meta-analysis and Subgroup Analysis. J. Ultrasound Med. 2018, 37, 149–163. [Google Scholar] [CrossRef]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of Vascular Endothelial Growth Factor (VEGF) in Human Embryo Implantation: Clinical Implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Huppertz, B.; Gauster, M.; Orendi, K.; König, J.; Moser, G. Oxygen as modulator of trophoblast invasion. Am. J. Anat. 2009, 215, 14–20. [Google Scholar] [CrossRef]
- James, J.L.; Saghian, R.; Perwick, R.; Clark, A.R. Trophoblast plugs: Impact on utero-placental haemodynamics and spiral artery remodelling. Hum. Reprod. 2018, 33, 1430–1441. [Google Scholar] [CrossRef]
- Varberg, K.M.; Soares, M.J. Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling. Placenta 2021, 113, 48–56. [Google Scholar] [CrossRef]
- Allerkamp, H.H.; Clark, A.R.; Lee, T.C.; Morgan, T.K.; Burton, G.J.; James, J.L. Something old, something new: Digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation. Hum. Reprod. 2021, 36, 571–586. [Google Scholar] [CrossRef]
- Morley, L.C.; Beech, D.J.; Walker, J.J.; Simpson, N.A.B. Emerging concepts of shear stress in placental development and function. Mol. Hum. Reprod. 2019, 25, 329–339. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Whitley, G.S.; Cartwright, J.E. Shear stress and spiral artery remodelling: The effects of low shear stress on trophoblast-induced endothelial cell apoptosis. Cardiovasc. Res. 2011, 90, 130–139. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Cartwright, J.E.; Whitley, G.S.; Greenhill, D.R.; Hoppe, A. The regulation of trophoblast migration across endothelial cells by low shear stress: Consequences for vascular remodelling in pregnancy. Cardiovasc. Res. 2012, 93, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Khankin, E.V.; Ko, N.L.; Mandalà, M.; Karumanchi, S.A.; Osol, G. Normalization of wall shear stress as a physiological mechanism for regulating maternal uterine artery expansive remodeling during pregnancy. FASEB BioAdv. 2021, 3, 702–708. [Google Scholar] [CrossRef]
- Cheng, C.K.; Wang, N.; Wang, L.; Huang, Y. Biophysical and Biochemical Roles of Shear Stress on Endothelium: A Revisit and New Insights. Circ. Res. 2025, 136, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.-S.; Chien, S. Shear stress-initiated signaling and its regulation of endothelial function. Arter. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef]
- Nigro, P.; Abe, J.-I.; Berk, B.C. Flow shear stress and atherosclerosis: A matter of site specificity. Antioxid. Redox Signal. 2011, 15, 1405–1414. [Google Scholar] [CrossRef]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 16–26. [Google Scholar] [CrossRef]
- Tinken, T.M.; Thijssen, D.H.; Hopkins, N.; Black, M.A.; Dawson, E.A.; Minson, C.T.; Newcomer, S.C.; Laughlin, M.H.; Cable, N.T.; Green, D.J. Impact of shear rate modulation on vascular function in humans. Hypertension 2009, 54, 278–285. [Google Scholar] [CrossRef]
- Panteleev, M.A.; Korin, N.; Reesink, K.D.; Bark, D.L.; Cosemans, J.M.E.M.; Gardiner, E.E.; Mangin, P.H. Wall shear rates in human and mouse arteries: Standardization of hemodynamics for in vitro blood flow assays: Communication from the ISTH SSC subcommittee on biorheology. J. Thromb. Haemost. 2021, 19, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Mobaseri, A.; Kumar, S.; Cheng, X. Maximum spreading of impacting shear-thinning and shear-thickening drops. Proc. Natl. Acad. Sci. USA 2025, 122, e2500163122. [Google Scholar] [CrossRef]
- Chhabra, R.P. Non-Newtonian Fluids: An Introduction. In Rheology of Complex Fluids; Krishnan, J., Deshpande, A., Kumar, P., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood Rheology and Hemodynamics. Semin. Thromb. Hemost. 2024, 50, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Berg, N.; Fuchs, L.; Wittberg, L.P. Assessment of Rheological Models Applied to Blood Flow in Human Thoracic Aorta. Bioengineering 2023, 10, 1240. [Google Scholar] [CrossRef]
- Yin, Z.; Armour, C.; Kandail, H.; O’Regan, D.P.; Bahrami, T.; Mirsadraee, S.; Pirola, S.; Xu, X.Y. The impact of coronary outflow and non-Newtonian fluid property on aortic valve haemodynamics. Biomech. Model. Mechanobiol. 2025, 24, 1401–1416. [Google Scholar] [CrossRef]
- Mehri, R.; Mavriplis, C.; Fenech, M. Red blood cell aggregates and their effect on non-Newtonian blood viscosity at low hematocrit in a two-fluid low shear rate microfluidic system. PLoS ONE 2018, 13, e0199911. [Google Scholar] [CrossRef]
- Knüppel, F.; Malchow, S.; Sun, A.; Hussong, J.; Hartmann, A.; Wurm, F.-H.; Torner, B. Viscosity Modeling for Blood and Blood Analog Fluids in Narrow Gap and High Reynolds Numbers Flows. Micromachines 2024, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, M. Mathematical modelling and analysis of thermoregulation effects on blood viscosity under magnetic effects and thermal radiation in a permeable stretching capillary. J. Therm. Biol. 2023, 111, 103398. [Google Scholar] [CrossRef]
- Del Giudice, F.; Barnes, C. Rapid Temperature-Dependent Rheological Measurements of Non-Newtonian Solutions Using a Machine-Learning Aided Microfluidic Rheometer. Anal. Chem. 2022, 94, 3617–3628. [Google Scholar] [CrossRef] [PubMed]
- Caglar, S.E.; Karakoc, Y.; Tanoglu, A.; Demirtunc, R.; Tanrikulu, S.; Kilickaya, H.; Ercan, M. Investigation of hemorheology in patients with hyperthyroidism via blood viscosity, erythrocyte deformability and aggregation. Thyroid. Res. 2025, 18, 11. [Google Scholar] [CrossRef]
- Farina, A.; Rosso, F.; Fasano, A. A continuum mechanics model for the Fåhræus-Lindqvist effect. J. Biol. Phys. 2021, 47, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Innes, B.A.; Robson, S.C.; Lash, G.E. Transient loss of endothelial cells in human spiral artery remodelling during early pregnancy: Challenging the dogma. Placenta 2020, 101, 230–233. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Placentation in the Human and Higher Primates. Adv. Anat. Embryol. Cell Biol. 2021, 234, 223–254. [Google Scholar] [CrossRef]
- Stangret, A.; Skoda, M.; Wnuk, A.; Pyzlak, M.; Szukiewicz, D. Mild anemia during pregnancy upregulates placental vascularity development. Med. Hypotheses 2017, 102, 37–40. [Google Scholar] [CrossRef]
- Delforce, S.J.; Wang, Y.; Van-Aalst, M.E.; de Meaultsart, C.C.; Morris, B.J.; Broughton-Pipkin, F.; Roberts, C.T.; Lumbers, E.R.; Pringle, K.G. Effect of oxygen on the expression of renin–angiotensin system components in a human trophoblast cell line. Placenta 2016, 37, 1–6. [Google Scholar] [CrossRef]
- Rodesch, F.; Simon, P.; Donner, C.; Jauniaux, E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 1992, 80, 283–285. [Google Scholar]
- Soghomonians, A.; Barakat, A.I.; Thirkill, T.L.; Blankenship, T.N.; Douglas, G.C. Effect of shear stress on migration and integrin expression in macaque trophoblast cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2002, 1589, 233–246. [Google Scholar] [CrossRef]
- Roberts, V.H.J.; Morgan, T.K.; Bednarek, P.; Morita, M.; Burton, G.J.; Lo, J.O.; Frias, A.E. Early first trimester uteroplacental flow and the progressive disintegration of spiral artery plugs: New insights from contrast-enhanced ultrasound and tissue histopathology. Hum. Reprod. 2017, 32, 2382–2393. [Google Scholar] [CrossRef]
- Mierke, C.T. Mechanosensory entities and functionality of endothelial cells. Front. Cell Dev. Biol. 2024, 12, 1446452. [Google Scholar] [CrossRef]
- Inohaya, A.; Chigusa, Y.; Takakura, M.; Io, S.; Kim, M.-A.; Matsuzaka, Y.; Yasuda, E.; Ueda, Y.; Kawamura, Y.; Takamatsu, S.; et al. Shear stress in the intervillous space promotes syncytial formation of iPS cells-derived trophoblasts. Biol. Reprod. 2024, 110, 300–309. [Google Scholar] [CrossRef]
- Brugger, B.A.; Neuper, L.; Guettler, J.; Forstner, D.; Wernitznig, S.; Kummer, D.; Lyssy, F.; Feichtinger, J.; Krappinger, J.; El-Heliebi, A.; et al. Fluid shear stress induces a shift from glycolytic to amino acid pathway in human trophoblasts. Cell Biosci. 2023, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Allerkamp, H.H.; Leighton, S.; Pole, T.; Clark, A.R.; James, J.L. Synergistic regulation of uterine radial artery adaptation to pregnancy by paracrine and hemodynamic factors. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H790–H805. [Google Scholar] [CrossRef]
- James, B.D.; Allen, J.B. Vascular Endothelial Cell Behavior in Complex Mechanical Microenvironments. ACS Biomater. Sci. Eng. 2018, 4, 3818–3842. [Google Scholar] [CrossRef] [PubMed]
- Baratchi, S.; Khoshmanesh, K.; Woodman, O.L.; Potocnik, S.; Peter, K.; McIntyre, P. Molecular Sensors of Blood Flow in Endothelial Cells. Trends Mol. Med. 2017, 23, 850–868. [Google Scholar] [CrossRef] [PubMed]
- Varberg, K.M.; Dominguez, E.M.; Koseva, B.; Varberg, J.M.; McNally, R.P.; Moreno-Irusta, A.; Wesley, E.R.; Iqbal, K.; Cheung, W.A.; Schwendinger-Schreck, C.; et al. Extravillous trophoblast cell lineage development is associated with active remodeling of the chromatin landscape. Nat. Commun. 2023, 14, 4826. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ando, J. New molecular mechanisms for cardiovascular disease:blood flow sensing mechanism in vascular endothelial cells. J. Pharmacol. Sci. 2011, 116, 323–331. [Google Scholar] [CrossRef]
- Kamiya, A.; Bukhari, R.; Togawa, T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull. Math. Biol. 1984, 46, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Givens, C.; Tzima, E. Endothelial Mechanosignaling: Does One Sensor Fit All? Antioxidants Redox Signal. 2016, 25, 373–388. [Google Scholar] [CrossRef]
- Fels, B.; Kusche-Vihrog, K. It takes more than two to tango: Mechanosignaling of the endothelial surface. Pflugers Arch. Eur. J. Physiol. 2020, 472, 419–433. [Google Scholar] [CrossRef]
- Otero-Sobrino, Á.; Blanco-Carlón, P.; Navarro-Aguadero, M.Á.; Gallardo, M.; Martínez-López, J.; Velasco-Estévez, M. Mechanosensitive Ion Channels: Their Physiological Importance and Potential Key Role in Cancer. Int. J. Mol. Sci. 2023, 24, 13710. [Google Scholar] [CrossRef]
- Townson, J.; Progida, C. The emerging roles of the endoplasmic reticulum in mechanosensing and mechanotransduction. J. Cell Sci. 2025, 138, JCS263503. [Google Scholar] [CrossRef]
- Sala, S.; Caillier, A.; Oakes, P.W. Principles and regulation of mechanosensing. J. Cell Sci. 2024, 137, jcs261338. [Google Scholar] [CrossRef]
- Arnadóttir, J.; Chalfie, M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 2010, 39, 111–137. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I.; Hanukoglu, A. Epithelial sodium channel (ENaC) family: Phylogeny, structure–function, tissue distribution, and associated inherited diseases. Gene 2016, 579, 95–132. [Google Scholar] [CrossRef]
- Kaulich, E.; Grundy, L.J.; Schafer, W.R.; Walker, D.S. The diverse functions of the DEG/ENaC family: Linking genetic and physiological insights. J. Physiol. 2023, 601, 1521–1542. [Google Scholar] [CrossRef]
- Kashlan, O.B.; Kleyman, T.R. ENaC structure and function in the wake of a resolved structure of a family member. Am. J. Physiol. Renal Physiol. 2011, 301, F684–F696. [Google Scholar] [CrossRef]
- Noreng, S.; Bharadwaj, A.; Posert, R.; Yoshioka, C.; Baconguis, I. Structure of the human epithelial sodium channel by cryo-electron microscopy. eLife 2018, 7, e39340. [Google Scholar] [CrossRef] [PubMed]
- Noreng, S.; Posert, R.; Bharadwaj, A.; Houser, A.; Baconguis, I. Molecular principles of assembly, activation, and inhibition in epithelial sodium channel. eLife 2020, 9, e59038. [Google Scholar] [CrossRef] [PubMed]
- Houser, A.; Baconguis, I. Structural insights into subunit-dependent functional regulation in epithelial sodium channels. Structure 2025, 33, 349–362.e4. [Google Scholar] [CrossRef]
- Kusche-Vihrog, K.; Jeggle, P.; Oberleithner, H. The role of ENaC in vascular endothelium. Pflugers Arch. Eur. J. Physiol. 2014, 466, 851–859. [Google Scholar] [CrossRef]
- Paudel, P.; van Hout, I.; Bunton, R.W.; Parry, D.J.; Coffey, S.; McDonald, F.J.; Fronius, M. Epithelial Sodium Channel δ Subunit Is Expressed in Human Arteries and Has Potential Association With Hypertension. Hypertension 2022, 79, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Johnson, N.M.; Coca, C.; Helms, M.N. Angiotensin II regulates δ-ENaC in human umbilical vein endothelial cells. Microvasc. Res. 2018, 116, 26–33. [Google Scholar] [CrossRef]
- Ahmad, T.; Ertuglu, L.A.; Masenga, S.K.; Kleyman, T.R.; Kirabo, A. The epithelial sodium channel in inflammation and blood pressure modulation. Front. Cardiovasc. Med. 2023, 10, 1130148. [Google Scholar] [CrossRef]
- Pitzer, A.L.; Van Beusecum, J.P.; Kleyman, T.R.; Kirabo, A. ENaC in Salt-Sensitive Hypertension: Kidney and Beyond. Curr. Hypertens. Rep. 2020, 22, 69. [Google Scholar] [CrossRef]
- Mutchler, S.M.; Kirabo, A.; Kleyman, T.R. Epithelial Sodium Channel and Salt-Sensitive Hypertension. Hypertension 2021, 77, 759–767. [Google Scholar] [CrossRef]
- Montell, C. The TRP Superfamily of Cation Channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef]
- Plant, T.D. TRPs in mechanosensing and volume regulation. Handb. Exp. Pharmacol. 2014, 223, 743–766. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [CrossRef]
- Koide, T.; Giles, W.R.; Kondo, R.; Imaizumi, Y.; Yamamura, H.; Suzuki, Y. Ca2+ microdomain-based excitation-transcription coupling in cardiac myocytes and vascular smooth muscle cells. Inflamm. Regen. 2025, 45, 19. [Google Scholar] [CrossRef]
- Dai, C.; Khalil, R.A. Calcium Signaling Dynamics in Vascular Cells and Their Dysregulation in Vascular Disease. Biomolecules 2025, 15, 892. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-O.; Yao, X. TRP channels in vascular endothelial cells. Adv. Exp. Med. Biol. 2011, 704, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Brayden, J.E. Transient Receptor Potential Channels in the Vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef]
- Negri, S.; Faris, P.; Berra-Romani, R.; Guerra, G.; Moccia, F. Endothelial transient receptor potential channels and vascular remodeling: Extracellular Ca2+ entry for angiogenesis, arteriogenesis and vasculogenesis. Front. Physiol. 2020, 10, 1618. [Google Scholar] [CrossRef]
- Negri, S.; Faris, P.; Rosti, V.; Antognazza, M.R.; Lodola, F.; Moccia, F. Endothelial TRPV1 as an emerging molecular target to promote therapeutic angiogenesis. Cells 2020, 9, 1341. [Google Scholar] [CrossRef]
- García-Sanz, N.; Fernández-Carvajal, A.; Morenilla-Palao, C.; Planells-Cases, R.; Fajardo-Sánchez, E.; Fernández-Ballester, G.; Ferrer-Montiel, A. Identification of a Tetramerization Domain in the C Terminus of the Vanilloid Receptor. J. Neurosci. 2004, 24, 5307–5314. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, N.; Albrecht, N.; Harteneck, C.; Schultz, G.; Schaefer, M. Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 2005, 118 Pt 5, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Yang, H.; Reinach, P.S. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum. Genom. 2011, 5, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Montell, C.; Schultz, G.; Julius, D. International Union of Pharmacology. XLIII. Compendium of Voltage-Gated Ion Channels: Transient Receptor Potential Channels. Pharmacol. Rev. 2003, 55, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ko, J.; Myeong, J.; Kwak, M.; Hong, C.; So, I. TRPC1 as a negative regulator for TRPC4 and TRPC5 channels. Pflugers Arch. Eur. J. Physiol. 2019, 471, 1045–1053. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef]
- Numaga-Tomita, T.; Nishida, M. TRPC Channels in Cardiac Plasticity. Cells 2020, 9, 454. [Google Scholar] [CrossRef]
- Elzamzamy, O.M.; Penner, R.; Hazlehurst, L.A. The Role of TRPC1 in Modulating Cancer Progression. Cells 2020, 9, 388. [Google Scholar] [CrossRef]
- Louis, M.; Zanou, N.; Van Schoor, M.; Gailly, P. TRPC1 regulates skeletal myoblast migration and differentiation. J. Cell Sci. 2008, 121 Pt 23, 3951–3959. [Google Scholar] [CrossRef]
- Löf, C.; Viitanen, T.; Sukumaran, P.; Törnquist, K. TRPC2: Of mice but not men. Adv. Exp. Med. Biol. 2011, 704, 125–134. [Google Scholar] [CrossRef]
- Yildirim, E.; Birnbaumer, L. TRPC2: Molecular biology and functional importance. Handb. Exp. Pharmacol. 2007, 179, 53–75. [Google Scholar] [CrossRef]
- Sierra-Valdez, F.; Azumaya, C.M.; Romero, L.O.; Nakagawa, T.; Cordero-Morales, J.F. Structure–function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating. J. Biol. Chem. 2018, 293, 16102–16114. [Google Scholar] [CrossRef]
- Tang, Q.; Guo, W.; Zheng, L.; Wu, J.-X.; Liu, M.; Zhou, X.; Zhang, X.; Chen, L. Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res. 2018, 28, 746–755. [Google Scholar] [CrossRef]
- Patel, R.; Waltz, L.; Toogood, G.; Li, W.; Xu, J. The Role of Transient Receptor Potential Canonical 3 (TRPC3) in Wound Healing. J. Cell. Physiol. 2025, 240, e70065. [Google Scholar] [CrossRef]
- Thilo, F.; Scholze, A.; Liu, D.Y.; Zidek, W.; Tepel, M. Association of transient receptor potential canonical type 3 (TRPC3) channel transcripts with proinflammatory cytokines. Arch. Biochem. Biophys. 2008, 471, 57–62. [Google Scholar] [CrossRef]
- Zeng, W.; Ji, Y.; Zhang, H.; Chen, L.; Du, L.; Guo, R. Downregulation of TRPC4 and TRPC5 Inhibits Smooth Muscle Cell Proliferation without Affecting Endothelial Cell Proliferation. Genet. Res. 2021, 2021, 2949986. [Google Scholar] [CrossRef]
- Cornman, R.S. Molecular evolution of TRPC4 regulatory sequences supports a role in mammalian thermoregulatory adaptation. PeerJ 2025, 13, e19697. [Google Scholar] [CrossRef]
- Ptakova, A.; Vlachova, V. Thermosensing ability of TRPC5: Current knowledge and unsettled questions. J. Physiol. Sci. 2024, 74, 50. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Hua, D.; He, D.; Wang, L.; Zhang, P.; Wang, J.; Cai, Y.; Gao, C.; Zhang, X.; et al. Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc. Natl. Acad. Sci. USA 2014, 111, 6389–6394. [Google Scholar] [CrossRef] [PubMed]
- Corteling, R.L.; Li, S.; Giddings, J.; Westwick, J.; Poll, C.; Hall, I.P. Expression of Transient Receptor Potential C6 and Related Transient Receptor Potential Family Members in Human Airway Smooth Muscle and Lung Tissue. Am. J. Respir. Cell Mol. Biol. 2004, 30, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Staruschenko, A.; Spires, D.; Palygin, O. Role of TRPC6 in Progression of Diabetic Kidney Disease. Curr. Hypertens. Rep. 2019, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- ‘t Hart, D.C.; van der Vlag, J.; Nijenhuis, T. A Putative Role for TRPC6 in Immune-Mediated Kidney Injury. Int. J. Mol. Sci. 2023, 24, 16419. [Google Scholar] [CrossRef]
- Zhang, X.; Spinelli, A.M.; Masiello, T.; Trebak, M. Transient Receptor Potential Canonical 7 (TRPC7), a Calcium (Ca(2+)) Permeable Non-selective Cation Channel. Adv. Exp. Med. Biol. 2016, 898, 251–264. [Google Scholar] [CrossRef]
- Hsu, W.; Tsai, M.; Wu, C.; Liang, J.; Lu, J.; Kahle, J.S.; Yu, H.; Yen, C.; Yen, C.; Hsieh, Y.; et al. Nociceptive transient receptor potential canonical 7 (TRPC7) mediates aging-associated tumorigenesis induced by ultraviolet B. Aging Cell 2020, 19, e13075. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Tanaka, H.; Ueda, Y.; Oyama, J.-I.; Sugano, M.; Sumimoto, H.; Mori, Y.; Makino, N. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol. Cell. Biochem. 2007, 294, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Su, Y.-C.; Jiang, K.-Y.; Ito, T.; Li, T.-W.; Kaku-Ito, Y.; Cheng, S.-T.; Chen, L.-T.; Hwang, D.-Y.; Shen, C.-H. TRPM1 promotes tumor progression in acral melanoma by activating the Ca2+/CaMKIIδ/AKT pathway. J. Adv. Res. 2022, 43, 45–57. [Google Scholar] [CrossRef]
- Darszon, A.; Sánchez-Cárdenas, C.; Orta, G.; Sánchez-Tusie, A.A.; Beltrán, C.; López-González, I.; Granados-González, G.; Treviño, C.L. Are TRP channels involved in sperm development and function? Cell Tissue Res. 2012, 349, 749–764. [Google Scholar] [CrossRef]
- Faouzi, M.; Penner, R. TRPM2. Handb. Exp. Pharmacol. 2014, 222, 403–426. [Google Scholar] [CrossRef]
- Xia, S.; Wang, L.; Fu, T.; Wu, H. Mechanism of TRPM2 channel gating revealed by cryo-EM. FEBS J. 2019, 286, 3333–3339. [Google Scholar] [CrossRef]
- Hua, J.; Feng, X.; Pan, T.; Zhu, Q.-J.; Xu, L.-X.; Ding, X.; Li, J.-Q.; Sun, B. Knocking down TRPM2 expression reduces cell injury and NLRP3 inflammasome activation in PC12 cells subjected to oxygen-glucose deprivation. Neural Regen. Res. 2020, 15, 2154–2161. [Google Scholar] [CrossRef]
- Cheung, J.Y.; Miller, B.A. Transient Receptor Potential-Melastatin Channel Family Member 2: Friend or Foe. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 308–329. [Google Scholar] [PubMed]
- Thiel, G.; Rubil, S.; Lesch, A.; Guethlein, L.A.; Rössler, O.G. Transient receptor potential TRPM3 channels: Pharmacology, signaling, and biological functions. Pharmacol. Res. 2017, 124, 92–99. [Google Scholar] [CrossRef]
- Vangeel, L.; Benoit, M.; Miron, Y.; Miller, P.E.; De Clercq, K.; Chaltin, P.; Verfaillie, C.; Vriens, J.; Voets, T. Functional expression and pharmacological modulation of TRPM3 in human sensory neurons. Br. J. Pharmacol. 2020, 177, 2683–2695. [Google Scholar] [CrossRef]
- Turgambayeva, A.; Duisekova, S.; Tashenova, G.; Tulebayeva, A.; Kapanova, G.; Akhenbekova, A.; Farooqi, A.A. Role of TRP channels in carcinogenesis and metastasis: Pathophysiology and regulation by non-coding RNAs. Non-Coding RNA Res. 2023, 9, 359–366. [Google Scholar] [CrossRef]
- Hu, Y.; Cang, J.; Hiraishi, K.; Fujita, T.; Inoue, R. The Role of TRPM4 in Cardiac Electrophysiology and Arrhythmogenesis. Int. J. Mol. Sci. 2023, 24, 11798. [Google Scholar] [CrossRef]
- Yu, F.; Hubrack, S.; Raynaud, C.M.; Elmi, A.; Mackeh, R.; Agrebi, N.; Thareja, G.; Belkadi, A.; Al Saloos, H.; Ahmed, A.A.; et al. Loss of the TRPM4 channel in humans causes immune dysregulation with defective monocyte migration. J. Allergy Clin. Immunol. 2024, 154, 792–806. [Google Scholar] [CrossRef]
- Barbet, G.; Demion, M.; Moura, I.C.; Serafini, N.; Léger, T.; Vrtovsnik, F.; Monteiro, R.C.; Guinamard, R.; Kinet, J.-P.; Launay, P. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat. Immunol. 2008, 9, 1148–1156. [Google Scholar] [CrossRef]
- Borgström, A.; Peinelt, C.; Stokłosa, P. TRPM4 in Cancer—A New Potential Drug Target. Biomolecules 2021, 11, 229. [Google Scholar] [CrossRef]
- Banik, D.D.; Martin, L.E.; Freichel, M.; Torregrossa, A.-M.; Medler, K.F. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc. Natl. Acad. Sci. USA 2018, 115, E772–E781. [Google Scholar] [CrossRef]
- Vennekens, R.; Mesuere, M.; Philippaert, K. TRPM5 in the battle against diabetes and obesity. Acta Physiol. 2018, 222, e12949. [Google Scholar] [CrossRef] [PubMed]
- Richter, P.; Andersen, G.; Kahlenberg, K.; Mueller, A.U.; Pirkwieser, P.; Boger, V.; Somoza, V. Sodium-Permeable Ion Channels TRPM4 and TRPM5 are Functional in Human Gastric Parietal Cells in Culture and Modulate the Cellular Response to Bitter-Tasting Food Constituents. J. Agric. Food Chem. 2024, 72, 4906–4917. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Okumura, R.; Ono, C.; Okuzaki, D.; Kawai, T.; Okochi, Y.; Tanimura, N.; Murakami, M.; Kayama, H.; Umemoto, E.; et al. TRPM5 Negatively Regulates Calcium-Dependent Responses in Lipopolysaccharide-Stimulated B Lymphocytes. Cell Rep. 2020, 31, 107755. [Google Scholar] [CrossRef]
- Guinamard, R.; Sallé, L.; Simard, C. The non-selective monovalent cationic channels TRPM4 and TRPM5. Adv. Exp. Med. Biol. 2011, 704, 147–171. [Google Scholar] [CrossRef]
- Chubanov, V.; Gudermann, T.; Schlingmann, K.P. Essential role for TRPM6 in epithelial magnesium transport and body magnesium homeostasis. Pflugers Arch. Eur. J. Physiol. 2005, 451, 228–234. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7—Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 813–821. [Google Scholar] [CrossRef]
- Andriulė, I.; Pangonytė, D.; Almanaitytė, M.; Patamsytė, V.; Kuprytė, M.; Karčiauskas, D.; Mubagwa, K.; Mačianskienė, R. Evidence for the expression of TRPM6 and TRPM7 in cardiomyocytes from all four chamber walls of the human heart. Sci. Rep. 2021, 11, 15445. [Google Scholar] [CrossRef]
- Gwanyanya, A.; Andriulė, I.; Istrate, B.M.; Easmin, F.; Mubagwa, K.; Mačianskienė, R. Modulation of the Cardiac Myocyte Action Potential by the Magnesium-Sensitive TRPM6 and TRPM7-like Current. Int. J. Mol. Sci. 2021, 22, 8744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Sun, H.-Y.; Chen, K.-H.; Du, X.-L.; Liu, B.; Cheng, L.-C.; Li, X.; Jin, M.-W.; Li, G.-R. Evidence for functional expression of TRPM7 channels in human atrial myocytes. Basic Res. Cardiol. 2012, 107, 282. [Google Scholar] [CrossRef] [PubMed]
- Asrar, S.; Aarts, M. TRPM7, the cytoskeleton and neuronal death. Channels 2013, 7, 6–16. [Google Scholar] [CrossRef]
- Turlova, E.; Wong, R.; Xu, B.; Li, F.; Du, L.; Habbous, S.; Horgen, F.D.; Fleig, A.; Feng, Z.-P.; Sun, H.-S. TRPM7 Mediates Neuronal Cell Death Upstream of Calcium/Calmodulin-Dependent Protein Kinase II and Calcineurin Mechanism in Neonatal Hypoxic-Ischemic Brain Injury. Transl. Stroke Res. 2021, 12, 164–184. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Xiong, Z.-G.; Ueki, T. The TRPM7 Channel in the Nervous and Cardiovascular Systems. Curr. Protein Pept. Sci. 2020, 21, 985–992. [Google Scholar] [CrossRef]
- Qi, Y.; Gong, H.; Shen, Z.; Wu, L.; Xu, Z.; Shi, N.; Lin, K.; Tian, M.; Xu, Z.; Li, X.; et al. TRPM8 and TRPA1 ideal targets for treating cold-induced pain. Eur. J. Med. Chem. 2025, 282, 117043. [Google Scholar] [CrossRef]
- Ma, S.; Yu, H.; Zhao, Z.; Luo, Z.; Chen, J.; Ni, Y.; Jin, R.; Ma, L.; Wang, P.; Zhu, Z.; et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J. Mol. Cell Biol. 2012, 4, 88–96. [Google Scholar] [CrossRef]
- Moraes, M.N.; de Assis, L.V.M.; Henriques, F.D.S.; Batista, M.L., Jr.; Güler, A.D.; Castrucci, A.M.L. Cold-sensing TRPM8 channel participates in circadian control of the brown adipose tissue. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Luo, Y.; Zhang, F.; Tang, S.; Zhu, T. Involvement of TRP Channels in Adipocyte Thermogenesis: An Update. Front. Cell Dev. Biol. 2021, 9, 686173. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Terzo, S.; Lentini, L.; Marchesa, P.; Mulè, F. TRPM8 Channel Activation Reduces the Spontaneous Contractions in Human Distal Colon. Int. J. Mol. Sci. 2020, 21, 5403. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Urban, L.; Nagy, I. TRPV1 Function in Health and Disease. Curr. Pharm. Biotechnol. 2011, 12, 130–144. [Google Scholar] [CrossRef]
- Aneiros, E.; Cao, L.; Papakosta, M.; Stevens, E.B.; Phillips, S.; Grimm, C. The biophysical and molecular basis of TRPV1 proton gating. EMBO J. 2011, 30, 994–1002. [Google Scholar] [CrossRef]
- Xu, G.; Winston, J.H.; Shenoy, M.; Yin, H.; Pendyala, S.; Pasricha, P.J. Transient Receptor Potential Vanilloid 1 Mediates Hyperalgesia and Is Up-Regulated in Rats With Chronic Pancreatitis. Gastroenterology 2007, 133, 1282–1292. [Google Scholar] [CrossRef]
- Shuba, Y.M. Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions. Front. Cell. Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef]
- Li, F.; Wang, F. TRPV1 in Pain and Itch. Adv. Exp. Med. Biol. 2021, 1349, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K. Physiological significance of TRPV2 as a mechanosensor, thermosensor and lipid sensor. J. Physiol. Sci. 2016, 66, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.C.; Leffler, A. TRPV2: A universal regulator in cellular physiology with a yet poorly defined thermosensitivity. J. Physiol. Sci. 2024, 74, 42–48. [Google Scholar] [CrossRef]
- Liu, B.; Qin, F. Use Dependence of Heat Sensitivity of Vanilloid Receptor TRPV2. Biophys. J. 2016, 110, 1523–1537. [Google Scholar] [CrossRef]
- Li, C.; Zhao, M.; Liu, X.; Li, Y.; Xu, B.; Zhou, L.; Sun, X.; Sun, W.; Kang, N.; Ji, Z.; et al. Ion channel TRPV2 is critical in enhancing B cell activation and function. J. Exp. Med. 2024, 221, e20221042. [Google Scholar] [CrossRef]
- Lei, J.; Tominaga, M. TRPV3 in skin thermosensation and temperature responses. J. Physiol. Sci. 2025, 75, 100005. [Google Scholar] [CrossRef]
- Martin, L.S.; Josset-Lamaugarny, A.; El Jammal, T.; Ducreux, S.; Chevalier, F.P.; Fromy, B. Aging is associated with impaired triggering of TRPV3-mediated cutaneous vasodilation: A crucial process for local heat exposure. GeroScience 2024, 46, 3567–3580. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.W.; Sullivan, M.N.; Pritchard, H.A.T.; Robinson, J.J.; Earley, S. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H2031–H2041. [Google Scholar] [CrossRef]
- Fromy, B.; Josset-Lamaugarny, A.; Aimond, G.; Pagnon-Minot, A.; Marics, I.; Tattersall, G.J.; Moqrich, A.; Sigaudo-Roussel, D. Disruption of TRPV3 Impairs Heat-Evoked Vasodilation and Thermoregulation: A Critical Role of CGRP. J. Investig. Dermatol. 2018, 138, 688–696. [Google Scholar] [CrossRef]
- Hartmannsgruber, V.; Heyken, W.-T.; Kacik, M.; Kaistha, A.; Grgic, I.; Harteneck, C.; Liedtke, W.; Hoyer, J.; Köhler, R. Arterial Response to Shear Stress Critically Depends on Endothelial TRPV4 Expression. PLoS ONE 2007, 2, e827. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-Evoked Activation of the Ion Channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Baratchi, S.; Knoerzer, M.; Khoshmanesh, K.; Mitchell, A.; McIntyre, P. Shear Stress Regulates TRPV4 Channel Clustering and Translocation from Adherens Junctions to the Basal Membrane. Sci. Rep. 2017, 7, 15942. [Google Scholar] [CrossRef]
- O’Neil, R.G.; Heller, S. The mechanosensitive nature of TRPV channels. Pflugers Arch. Eur. J. Physiol. 2005, 451, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Moore, C. The role of TRPV4 channels in cutaneous epithelia. Curr. Top. Membr. 2022, 89, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Orsini, E.M.; Roychowdhury, S.; Gangadhariah, M.; Cross, E.; Abraham, S.; Reinhardt, A.; Grund, M.E.; Zhou, J.Y.; Stuehr, O.; Pant, B.; et al. TRPV4 Regulates the Macrophage Metabolic Response to Limit Sepsis-induced Lung Injury. Am. J. Respir. Cell Mol. Biol. 2024, 70, 457–467. [Google Scholar] [CrossRef]
- Fukuda, N.; Toriuchi, K.; Mimoto, R.; Aoki, H.; Kakita, H.; Suzuki, Y.; Takeshita, S.; Tamura, T.; Yamamura, H.; Inoue, Y.; et al. Hypothermia Attenuates Neurotoxic Microglial Activation via TRPV4. Neurochem. Res. 2024, 49, 800–813. [Google Scholar] [CrossRef]
- Mamenko, M.; Zaika, O.; Boukelmoune, N.; O’Neil, R.G.; Pochynyuk, O. Deciphering physiological role of the mechanosensitive TRPV4 channel in the distal nephron. Am. J. Physiol. Renal Physiol. 2015, 308, F275–F286. [Google Scholar] [CrossRef]
- Sánchez, J.C.; Rivera, R.A.; Muñoz, L.V. TRPV4 Channels in Human White Adipocytes: Electrophysiological Characterization and Regulation by Insulin. J. Cell. Physiol. 2016, 231, 954–963. [Google Scholar] [CrossRef]
- Fluck, E.C.; Yazici, A.T.; Rohacs, T.; Moiseenkova-Bell, V.Y. Structural basis of TRPV5 regulation by physiological and pathophysiological modulators. Cell Rep. 2022, 39, 110737. [Google Scholar] [CrossRef]
- de Groot, T.; Lee, K.; Langeslag, M.; Xi, Q.; Jalink, K.; Bindels, R.J.; Hoenderop, J.G. Parathyroid Hormone Activates TRPV5 via PKA-Dependent Phosphorylation. J. Am. Soc. Nephrol. 2009, 20, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Khattar, V.; Wang, L.; Peng, J.-B. Calcium selective channel TRPV6: Structure, function, and implications in health and disease. Gene 2022, 817, 146192. [Google Scholar] [CrossRef] [PubMed]
- Bächinger, D.; Egli, H.; Goosmann, M.M.; Naldi, A.M.; Eckhard, A.H. Immunolocalization of calcium sensing and transport proteins in the murine endolymphatic sac indicates calciostatic functions within the inner ear. Cell Tissue Res. 2019, 378, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Lieben, L.; Benn, B.; Ajibade, D.; Stockmans, I.; Moermans, K.; Hediger, M.; Peng, J.; Christakos, S.; Bouillon, R.; Carmeliet, G. Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone 2010, 47, 301–308. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Nielsen, T.A.; Eriksen, M.A.; Gazerani, P.; Andersen, H.H. Psychophysical and vasomotor evidence for interdependency of TRPA1 and TRPV1-evoked nociceptive responses in human skin: An experimental study. Pain 2018, 159, 1989–2001. [Google Scholar] [CrossRef]
- Tominaga, M.; Iwata, M. TRPA1 and thermosensitivity. J. Physiol. Sci. 2025, 75, 100010. [Google Scholar] [CrossRef] [PubMed]
- Thakore, P.; Alvarado, M.G.; Ali, S.; Mughal, A.; Pires, P.W.; Yamasaki, E.; Pritchard, H.A.; Isakson, B.E.; Tran, C.H.T.; Earley, S. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. eLife 2021, 10, e63040. [Google Scholar] [CrossRef]
- Du, J.; Fu, J.; Xia, X.-M.; Shen, B. The functions of TRPP2 in the vascular system. Acta Pharmacol. Sin. 2016, 37, 13–18. [Google Scholar] [CrossRef]
- Sharif-Naeini, R.; Folgering, J.H.; Bichet, D.; Duprat, F.; Lauritzen, I.; Arhatte, M.; Jodar, M.; Dedman, A.; Chatelain, F.C.; Schulte, U.; et al. Polycystin-1 and -2 Dosage Regulates Pressure Sensing. Cell 2009, 139, 587–596. [Google Scholar] [CrossRef]
- Gao, Z.; Joseph, E.; Ruden, D.M.; Lu, X. Drosophila Pkd2 Is Haploid-insufficient for Mediating Optimal Smooth Muscle Contractility. J. Biol. Chem. 2004, 279, 14225–14231. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hussein, S.; Yang, J.; Huang, J.; Zhang, F.; Hernandez-Anzaldo, S.; Fernandez-Patron, C.; Cao, Y.; Zeng, H.; Tang, J.; et al. A novel PKD2L1 C-terminal domain critical for trimerization and channel function. Sci. Rep. 2015, 5, 9460. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cui, Y.; Wei, X.; Gao, P.; Zhang, H.; Wei, X.; Li, Q.; Sun, F.; Yan, Z.; Zheng, H.; et al. Deficiency of PKD2L1 (TRPP3) Exacerbates Pathological Cardiac Hypertrophy by Augmenting NCX1-Mediated Mitochondrial Calcium Overload. Cell Rep. 2018, 24, 1639–1652. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, J.; Beauchamp, E.; Cai, R.; Hussein, S.; Hofmann, L.; Li, Q.; Flockerzi, V.; Berthiaume, L.G.; Tang, J.; et al. Regulation of TRPP3 Channel Function by N-terminal Domain Palmitoylation and Phosphorylation. J. Biol. Chem. 2016, 291, 25678–25691. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Weng, Z.; Xu, Q.; Zhou, C.; Tang, J.; Chen, X.-Z. Inhibition of TRPP3 by calmodulin through Ca2+/calmodulin-dependent protein kinase II. Cell Insight 2023, 2, 100088. [Google Scholar] [CrossRef]
- Walsh, S.; Izquierdo-Serra, M.; Acosta, S.; Edo, A.; Lloret, M.; Moret, R.; Bosch, E.; Oliva, B.; Bertranpetit, J.; Fernández-Fernández, J.M. Adaptive selection drives TRPP3 loss-of-function in an Ethiopian population. Sci. Rep. 2020, 10, 20999. [Google Scholar] [CrossRef]
- Xiao, Y.; Lv, X.; Cao, G.; Bian, G.; Duan, J.; Ai, J.; Sun, H.; Li, Q.; Yang, Q.; Chen, T.; et al. Overexpression of Trpp5 contributes to cell proliferation and apoptosis probably through involving calcium homeostasis. Mol. Cell. Biochem. 2010, 339, 155–161. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Lv, X.-Y.; Wang, Y.-D.; Hu, Z.-G.; Sun, H.; Tan, R.-Z.; Liu, Y.-H.; Bian, G.-H.; Xiao, Y.; et al. Expression of Pkd2l2 in Testis Is Implicated in Spermatogenesis. Biol. Pharm. Bull. 2008, 31, 1496–1500. [Google Scholar] [CrossRef]
- Schmiege, P.; Fine, M.; Blobel, G.; Li, X. Human TRPML1 channel structures in open and closed conformations. Nature 2017, 550, 366–370. [Google Scholar] [CrossRef]
- Spix, B.; Chao, Y.-K.; Abrahamian, C.; Chen, C.-C.; Grimm, C. TRPML Cation Channels in Inflammation and Immunity. Front. Immunol. 2020, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Wong, C.-O.; Zhu, M.X. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 2015, 58, 48–56. [Google Scholar] [CrossRef]
- Samie, M.A.; Grimm, C.; Evans, J.A.; Curcio-Morelli, C.; Heller, S.; Slaugenhaupt, S.A.; Cuajungco, M.P. The tissue-specific expression of TRPML2 (MCOLN-2) gene is influenced by the presence of TRPML1. Pflugers Arch. Eur. J. Physiol. 2009, 459, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, J.M.; Blomberg, K.E.M.; Wennborg, A.; Smith, C.E. Differential expression and molecular characterisation of Lmo7, Myo1e, Sash1, and Mcoln2 genes in Btk-defective B-cells. Cell. Immunol. 2005, 235, 46–55. [Google Scholar] [CrossRef]
- Song, Y.; Dayalu, R.; Matthews, S.A.; Scharenberg, A.M. TRPML cation channels regulate the specialized lysosomal compartment of vertebrate B-lymphocytes. Eur. J. Cell Biol. 2006, 85, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, F.; Belyantseva, I.A.; Kim, H.J.; Vogt, T.F.; Kachar, B.; Noben-Trauth, K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc. Natl. Acad. Sci. USA 2002, 99, 14994–14999. [Google Scholar] [CrossRef]
- Grimm, C.; Cuajungco, M.P.; van Aken, A.F.J.; Schnee, M.; Jörs, S.; Kros, C.J.; Ricci, A.J.; Heller, S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc. Natl. Acad. Sci. USA 2007, 104, 19583–19588. [Google Scholar] [CrossRef]
- Nagata, K.; Zheng, L.; Madathany, T.; Castiglioni, A.J.; Bartles, J.R.; García-Añoveros, J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 353–358. [Google Scholar] [CrossRef]
- Atiba-Davies, M.; Noben-Trauth, K. TRPML3 and hearing loss in the varitint-waddler mouse. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2007, 1772, 1028–1031. [Google Scholar] [CrossRef]
- Tytti, K.; Sanna, K.; Carla, G.; Jonatan, P.; Kaisa, R.; Sari, T. Mechanosensitive TRPV4 channel guides maturation and organization of the bilayered mammary epithelium. Sci. Rep. 2024, 14, 6774. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kresge, C.; Boggs, K.; Scott, J.; Feranchak, A. Mechanosensor transient receptor potential vanilloid member 4 (TRPV4) regulates mouse cholangiocyte secretion and bile formation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G277–G287. [Google Scholar] [CrossRef]
- Michalick, L.; Kuebler, W.M. TRPV4—A Missing Link Between Mechanosensation and Immunity. Front. Immunol. 2020, 11, 413. [Google Scholar] [CrossRef]
- Darby, W.; Grace, M.; Baratchi, S.; McIntyre, P. Modulation of TRPV4 by diverse mechanisms. Int. J. Biochem. Cell Biol. 2016, 78, 217–228. [Google Scholar] [CrossRef]
- Allerkamp, H.H.; Bondarenko, A.I.; Tawfik, I.; Kamali-Simsek, N.; Mercnik, M.H.; Madreiter-Sokolowski, C.T.; Wadsack, C. In vitro examination of Piezo1-TRPV4 dynamics: Implications for placental endothelial function in normal and preeclamptic pregnancies. Am. J. Physiol.-Cell Physiol. 2025, 328, C227–C244. [Google Scholar] [CrossRef]
- Coutiño, B.C.; Mayor, R. Mechanosensitive ion channels in cell migration. Cells Dev. 2021, 166, 203683. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.; Benítez-Angeles, M.; Hernández-Vega, A.M.; Rosenbaum, T. Recent advances on the structure and the function relationships of the TRPV4 ion channel. Channels 2024, 18, 2313323. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Anishkin, A.; Xie, Y.; Drachuk, K.; Nishijma, Y.; Fang, J.; Koukouritaki, S.B.; Wilcox, D.A.; Zhang, D.X. Phosphorylation of distal C-terminal residues promotes TRPV4 channel activation in response to arachidonic acid. J. Biol. Chem. 2025, 301, 108260. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Nilius, B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 2007, 428, 183–207. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Korsunsky, A.; Yazdani, M.; Chen, J. Targeting TRP channels: Recent advances in structure, ligand binding, and molecular mechanisms. Front. Mol. Neurosci. 2024, 16, 1334370. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.K.; Kim, M.; Van Horn, W.D. Structural and Evolutionary Insights Point to Allosteric Regulation of TRP Ion Channels. Acc. Chem. Res. 2019, 52, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Hill-Eubanks, D.C.; Gonzales, A.L.; Sonkusare, S.K.; Nelson, M.T. Vascular TRP Channels: Performing Under Pressure and Going with the Flow. Physiology 2014, 29, 343–360. [Google Scholar] [CrossRef]
- Cox, C.D.; Poole, K.; Martinac, B. Re-evaluating TRP channel mechanosensitivity. Trends Biochem. Sci. 2024, 49, 693–702. [Google Scholar] [CrossRef]
- Zhang, Z.; Kindrat, A.N.; Sharif-Naeini, R.; Bourque, C.W. Actin Filaments Mediate Mechanical Gating during Osmosensory Transduction in Rat Supraoptic Nucleus Neurons. J. Neurosci. 2007, 27, 4008–4013. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Schmidt, H.; Hucho, F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 2007, 274, 760–772. [Google Scholar] [CrossRef]
- Feliciangeli, S.; Chatelain, F.C.; Bichet, D.; Lesage, F. The family of K2P channels: Salient structural and functional properties. J. Physiol. 2015, 593, 2587–2603. [Google Scholar] [CrossRef]
- Decher, N.; Rinné, S.; Bedoya, M.; Gonzalez, W.; Kiper, A.K. Molecular Pharmacology of K2P Potassium Channels. Cell. Physiol. Biochem. 2021, 55 (Suppl. S3), 87–107. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; del Mármol, J.; MacKinnon, R. Mechanistic basis for low threshold mechanosensitivity in voltage-dependent K + channels. Proc. Natl. Acad. Sci. USA 2012, 109, 10352–10357. [Google Scholar] [CrossRef]
- Morris, C.E.; Prikryl, E.A.; Joós, B. Mechanosensitive Gating of Kv Channels. PLoS ONE 2015, 10, e0118335. [Google Scholar] [CrossRef]
- Brohawn, S.G. How ion channels sense mechanical force: Insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann. N. Y. Acad. Sci. 2015, 1352, 20–32. [Google Scholar] [CrossRef] [PubMed]
- del Mármol, J.; Rietmeijer, R.A.; Brohawn, S.G. Studying Mechanosensitivity of Two-Pore Domain K+ Channels in Cellular and Reconstituted Proteoliposome Membranes. Methods Mol. Biol. 2018, 1684, 129–150. [Google Scholar] [CrossRef]
- Sorum, B.; Docter, T.; Panico, V.; Rietmeijer, R.A.; Brohawn, S.G. Tension activation of mechanosensitive two-pore domain K+ channels TRAAK, TREK-1, and TREK-2. Nat. Commun. 2024, 15, 3142. [Google Scholar] [CrossRef]
- Wiedmann, F.; Frey, N.; Schmidt, C. Two-Pore-Domain Potassium (K2P-) Channels: Cardiac Expression Patterns and Disease-Specific Remodelling Processes. Cells 2021, 10, 2914. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.; Terrenoire, C.; Romey, G.; Lazdunski, M. Human TREK2, a 2P Domain Mechano-sensitive K+Channel with Multiple Regulations by Polyunsaturated Fatty Acids, Lysophospholipids, and Gs, Gi, and Gq Protein-coupled Receptors. J. Biol. Chem. 2000, 275, 28398–28405. [Google Scholar] [CrossRef]
- Sandoz, G.; Douguet, D.; Chatelain, F.; Lazdunski, M.; Lesage, F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc. Natl. Acad. Sci. USA 2009, 106, 14628–14633. [Google Scholar] [CrossRef] [PubMed]
- Noël, J.; Sandoz, G.; Lesage, F. Molecular regulations governing TREK and TRAAK channel functions. Channels 2011, 5, 402–409. [Google Scholar] [CrossRef]
- Lengyel, M.; Enyedi, P.; Czirják, G. Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels. Int. J. Mol. Sci. 2021, 22, 9062. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Honoré, E. Sensing pressure with ion channels. Trends Neurosci. 2012, 35, 477–486. [Google Scholar] [CrossRef]
- Berrout, J.; Jin, M.; Mamenko, M.; Zaika, O.; Pochynyuk, O.; O’Neil, R.G. Function of Transient Receptor Potential Cation Channel Subfamily V Member 4 (TRPV4) as a Mechanical Transducer in Flow-sensitive Segments of Renal Collecting Duct System. J. Biol. Chem. 2012, 287, 8782–8791. [Google Scholar] [CrossRef]
- Nilius, B.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T. TRPV4 calcium entry channel: A paradigm for gating diversity. Am. J. Physiol. Cell Physiol. 2004, 286, C195–C205. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Zhang, M.; Liu, W.; Deng, T.; Zhao, Q.; Li, Y.; Lei, J.; Li, X.; Xiao, B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019, 573, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, S.; Liu, H.; Ru, K.; Jia, Y.; Wu, Z.; Liang, S.; Khan, Z.; Chen, Z.; Qian, A.; et al. Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone. Int. J. Mol. Sci. 2021, 22, 6429. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Y.; Ning, X.; Li, H.; Li, Q.; Wu, J. The functional effects of Piezo channels in mesenchymal stem cells. Stem Cell Res. Ther. 2023, 14, 222. [Google Scholar] [CrossRef]
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Structure of the mechanically activated ion channel Piezo1. Nature 2018, 554, 481–486. Erratum in Nature 2022, 607, E10. [Google Scholar] [CrossRef]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Albarrán-Juárez, J.; Iring, A.; Wang, S.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 2018, 215, 2655–2672. [Google Scholar] [CrossRef]
- Vasileva, V.; Chubinskiy-Nadezhdin, V. Regulation of PIEZO1 channels by lipids and the structural components of extracellular matrix/cell cytoskeleton. J. Cell. Physiol. 2023, 238, 918–930. [Google Scholar] [CrossRef]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef]
- Wang, S.; Iring, A.; Strilic, B.; Juárez, J.A.; Kaur, H.; Troidl, K.; Tonack, S.; Burbiel, J.C.; Müller, C.E.; Fleming, I.; et al. P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J. Clin. Investig. 2015, 125, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, A.; Gilbert, G.; Pele, T.; Deweirdt, J.; Henrion, D.; Baudrimont, I.; Campagnac, M.; Marthan, R.; Guibert, C.; Ducret, T.; et al. Stretch-activated Piezo1 Channel in Endothelial Cells Relaxes Mouse Intrapulmonary Arteries. Am. J. Respir. Cell Mol. Biol. 2019, 60, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Nunez, D.S.; Malik, A.B.; Lee, Q.; Ahn, S.J.; Coctecon-Murillo, A.; Lazarko, D.; Levitan, I.; Mehta, D.; Komarova, Y.A. Piezo1 induces endothelial responses to shear stress via soluble adenylyl Cyclase-IP3R2 circuit. iScience 2023, 26, 106661, Erratum in iScience 2023, 27, 108633. [Google Scholar] [CrossRef]
- Lefkowitz, R.; Kobilka, B. Royal Swedish Academy of Sciences. The Nobel Prize in Chemistry 2012. Retrieved 10 October 2012. Available online: https://www.nobelprize.org/prizes/chemistry/2012/summary/ (accessed on 15 August 2025).
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G protein-coupled receptors (GPCRs): Advances in structures, mechanisms and drug discovery. Signal Transduct. Target. Ther. 2024, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Anderson, P.J.; Rajagopal, S.; Lefkowitz, R.J.; Rockman, H.A. G Protein-Coupled Receptors: A Century of Research and Discovery. Circ. Res. 2024, 135, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Vithani, N.; Todd, T.D.; Singh, S.; Trent, T.; Blumer, K.J.; Bowman, G.R. G Protein Activation Occurs via a Largely Universal Mechanism. J. Phys. Chem. B 2024, 128, 3554–3562. [Google Scholar] [CrossRef]
- Afzal, M.S. G proteins: Binary switches in health and disease. Cent. Eur. J. Immunol. 2020, 45, 364–367. [Google Scholar] [CrossRef]
- Kamato, D.; Thach, L.; Bernard, R.; Chan, V.; Zheng, W.; Kaur, H.; Brimble, M.; Osman, N.; Little, P.J. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Gα/q,11. Front. Cardiovasc. Med. 2015, 2, 14. [Google Scholar] [CrossRef]
- Szukiewicz, D. Potential Therapeutic Exploitation of G Protein-Coupled Receptor 120 (GPR120/FFAR4) Signaling in Obesity-Related Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 2501. [Google Scholar] [CrossRef]
- Zou, Y.; Akazawa, H.; Qin, Y.; Sano, M.; Takano, H.; Minamino, T.; Makita, N.; Iwanaga, K.; Zhu, W.; Kudoh, S.; et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat. Cell Biol. 2004, 6, 499–506. [Google Scholar] [CrossRef]
- Cui, Y.; Kassmann, M.; Nickel, S.; Zhang, C.; Alenina, N.; Anistan, Y.M.; Schleifenbaum, J.; Bader, M.; Welsh, D.G.; Huang, Y.; et al. Myogenic Vasoconstriction Requires Canonical G q/11 Signaling of the Angiotensin II Type 1 Receptor. J. Am. Heart Assoc. 2022, 11, e022070. [Google Scholar] [CrossRef]
- Yasuda, N.; Miura, S.; Akazawa, H.; Tanaka, T.; Qin, Y.; Kiya, Y.; Imaizumi, S.; Fujino, M.; Ito, K.; Zou, Y.; et al. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 2008, 9, 179–186. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, M.; Wang, M.; Li, X. Flow-mediated vasodilation through mechanosensitive G protein-coupled receptors in endothelial cells. Trends Cardiovasc. Med. 2022, 32, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, M.M.Y.; Storch, U.; Gudermann, T. Mechanosensitive Gq/11 Protein–Coupled Receptors Mediate Myogenic Vasoconstriction. Microcirculation 2016, 23, 621–625. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, J.; Xu, X.S. Mechanosensitive GPCRs and ion channels in shear stress sensing. Curr. Opin. Cell Biol. 2023, 84, 102216. [Google Scholar] [CrossRef]
- Xu, J.; Mathur, J.; Vessières, E.; Hammack, S.; Nonomura, K.; Favre, J.; Grimaud, L.; Petrus, M.; Francisco, A.; Li, J.; et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 2018, 173, 762–775.e16. [Google Scholar] [CrossRef]
- Holm, C.; Nguyen, S.N.; Mensah, S.A. Shear Stress-Dependent Modulation of Endothelin B Receptor: The Role of Endothelial Glycocalyx Heparan Sulfate. Cells 2025, 14, 1088. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, M.M.Y.; Storch, U.; Meibers, S.; Nurwakagari, P.; Breit, A.; Essin, K.; Gollasch, M.; Gudermann, T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008, 27, 3092–3103. [Google Scholar] [CrossRef] [PubMed]
- Chachisvilis, M.; Zhang, Y.-L.; Frangos, J.A. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15463–15468. [Google Scholar] [CrossRef]
- Jung, B.; Obinata, H.; Galvani, S.; Mendelson, K.; Ding, B.-S.; Skoura, A.; Kinzel, B.; Brinkmann, V.; Rafii, S.; Evans, T.; et al. Flow-Regulated Endothelial S1P Receptor-1 Signaling Sustains Vascular Development. Dev. Cell 2012, 23, 600–610. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Nauli, S.M. Dopamine Receptor Type 5 in the Primary Cilia Has Dual Chemo- and Mechano-Sensory Roles. Hypertension 2011, 58, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Erdogmus, S.; Storch, U.; Danner, L.; Becker, J.; Winter, M.; Ziegler, N.; Wirth, A.; Offermanns, S.; Hoffmann, C.; Gudermann, T.; et al. Helix 8 is the essential structural motif of mechanosensitive GPCRs. Nat. Commun. 2019, 10, 5784. [Google Scholar] [CrossRef]
- Schneider, J.-C.; El Kebir, D.; Chéreau, C.; Lanone, S.; Huang, X.-L.; Roessingh, A.S.D.B.; Mercier, J.-C.; Dall’Ava-Santucci, J.; Dinh-Xuan, A.T. Involvement of Ca2+/calmodulin-dependent protein kinase II in endothelial NO production and endothelium-dependent relaxation. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2311–H2319. [Google Scholar] [CrossRef]
- Morgan, R.K.; Anderson, G.R.; Araç, D.; Aust, G.; Balenga, N.; Boucard, A.; Bridges, J.P.; Engel, F.B.; Formstone, C.J.; Glitsch, M.D.; et al. The expanding functional roles and signaling mechanisms of adhesion G protein–coupled receptors. Ann. N. Y. Acad. Sci. 2019, 1456, 5–25. [Google Scholar] [CrossRef]
- Seufert, F.; Pérez-Hernández, G.; Pándy-Szekeres, G.; Guixà-González, R.; Langenhan, T.; Gloriam, D.E.; Hildebrand, P.W. Generic residue numbering of the GAIN domain of adhesion GPCRs. Nat. Commun. 2025, 16, 246. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.; Yan, J.; Song, G. Mechanical force induced activation of adhesion G protein–coupled receptor. Mechanobiol. Med. 2024, 2, 100078. [Google Scholar] [CrossRef]
- Lin, H.-H.; Ng, K.-F.; Chen, T.-C.; Tseng, W.-Y. Ligands and Beyond: Mechanosensitive Adhesion GPCRs. Pharmaceuticals 2022, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Fedorchak, G.R.; Kaminski, A.; Lammerding, J. Cellular mechanosensing: Getting to the nucleus of it all. Prog. Biophys. Mol. Biol. 2014, 115, 76–92. [Google Scholar] [CrossRef]
- Maurer, M.; Lammerding, J. The Driving Force: Nuclear Mechanotransduction in Cellular Function, Fate, and Disease. Annu. Rev. Biomed. Eng. 2019, 21, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Janota, C.S.; Calero-Cuenca, F.J.; Gomes, E.R. The role of the cell nucleus in mechanotransduction. Curr. Opin. Cell Biol. 2020, 63, 204–211. [Google Scholar] [CrossRef]
- Bhanumathy, K.K.; Balagopal, A.; Vizeacoumar, F.S.; Vizeacoumar, F.J.; Freywald, A.; Giambra, V. Protein Tyrosine Kinases: Their Roles and Their Targeting in Leukemia. Cancers 2021, 13, 184. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Miller, W.T. Receptor tyrosine kinases: Mechanisms of activation and signaling. Curr. Opin. Cell Biol. 2007, 19, 117–123. [Google Scholar] [CrossRef]
- Ségaliny, A.I.; Tellez-Gabriel, M.; Heymann, M.-F.; Heymann, D. Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers. J. Bone Oncol. 2015, 4, 1–12. [Google Scholar] [CrossRef]
- Database Page Citation: Receptor Tyrosine Kinases (RTKs). IUPHAR/BPS Guide to Pharmacology. Available online: http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=304 (accessed on 20 August 2025).
- Yang, B.; Lieu, Z.Z.; Wolfenson, H.; Hameed, F.M.; Bershadsky, A.D.; Sheetz, M.P. Mechanosensing Controlled Directly by Tyrosine Kinases. Nano Lett. 2016, 16, 5951–5961. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sharma, A.; Patne, K.; Tabasum, S.; Suryavanshi, J.; Rawat, L.; Machaalani, M.; Eid, M.; Singh, R.P.; Choueiri, T.K.; et al. AXL signaling in cancer: From molecular insights to targeted therapies. Signal Transduct. Target. Ther. 2025, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Kamizaki, K.; Minami, Y.; Nishita, M. Role of the Ror family receptors in Wnt5a signaling. Vitr. Cell. Dev. Biol.—Anim. 2024, 60, 489–501. [Google Scholar] [CrossRef]
- Tomuleasa, C.; Tigu, A.-B.; Munteanu, R.; Moldovan, C.-S.; Kegyes, D.; Onaciu, A.; Gulei, D.; Ghiaur, G.; Einsele, H.; Croce, C.M. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal Transduct. Target. Ther. 2024, 9, 201. [Google Scholar] [CrossRef]
- Critchley, W.R.; Pellet-Many, C.; Ringham-Terry, B.; Harrison, M.A.; Zachary, I.C.; Ponnambalam, S. Receptor Tyrosine Kinase Ubiquitination and De-Ubiquitination in Signal Transduction and Receptor Trafficking. Cells 2018, 7, 22. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Tzanakakis, P.; Spyridaki, I.; Pérez, S.; Nikitovic, D. Extracellular Matrix Components and Mechanosensing Pathways in Health and Disease. Biomolecules 2024, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Swoger, M.; Saldanha, R.; Schwarz, J.; Patteson, A.E. Reorganizing chromatin by cellular deformation. Curr. Opin. Cell Biol. 2024, 90, 102408. [Google Scholar] [CrossRef]
- Kohn, J.C.; Zhou, D.W.; Bordeleau, F.; Zhou, A.L.; Mason, B.N.; Mitchell, M.J.; King, M.R.; Reinhart-King, C.A. Cooperative Effects of Matrix Stiffness and Fluid Shear Stress on Endothelial Cell Behavior. Biophys. J. 2015, 108, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Uray, I.P.; Uray, K. Mechanotransduction at the Plasma Membrane-Cytoskeleton Interface. Int. J. Mol. Sci. 2021, 22, 11566. [Google Scholar] [CrossRef]
- Lee, W. The Cytoskeleton and Its Binding Proteins as Mechanosensors, Transducers, and Functional Regulators of Cells. Int. J. Mol. Sci. 2023, 25, 172. [Google Scholar] [CrossRef]
- Lim, C.-G.; Jang, J.; Kim, C. Cellular machinery for sensing mechanical force. BMB Rep. 2018, 51, 623–629. [Google Scholar] [CrossRef]
- Kim, J. Unconventional Mechanics of Lipid Membranes: A Potential Role for Mechanotransduction of Hair Cell Stereocilia. Biophys. J. 2015, 108, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Gianoli, F.; Risler, T.; Kozlov, A.S. Lipid bilayer mediates ion-channel cooperativity in a model of hair-cell mechanotransduction. Proc. Natl. Acad. Sci. USA 2017, 114, E11010–E11019. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ang, J.W.; Zhong, X.; Wong, D.C.P.; T, T.; Yow, I.; Lee, C.J.M.; Foo, R.S.-Y.; Kanchanawong, P.; Low, B.C. Coordination of Focal Adhesion Nanoarchitecture and Dynamics in Mechanosensing for Cardiomyoblast Differentiation. ACS Appl. Mater. Interfaces 2025, 17, 4463–4479. [Google Scholar] [CrossRef]
- Stutchbury, B.; Atherton, P.; Tsang, R.; Wang, D.-Y.; Ballestrem, C. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J. Cell Sci. 2017, 130, 1612–1624. [Google Scholar] [CrossRef]
- Zhu, L.; Plow, E.F.; Qin, J. Initiation of focal adhesion assembly by talin and kindlin: A dynamic view. Protein Sci. 2021, 30, 531–542. [Google Scholar] [CrossRef]
- Katoh, K. Signal Transduction Mechanisms of Focal Adhesions: Src and FAK-Mediated Cell Response. Front. Biosci. 2024, 29, 392. [Google Scholar] [CrossRef]
- Jin, L.; Qin, Y.; Zhao, Y.; Zhou, X.; Zeng, Y. Endothelial cytoskeleton in mechanotransduction and vascular diseases. J. Biomech. 2025, 182, 112579. [Google Scholar] [CrossRef]
- Power, G.; Ferreira-Santos, L.; Martinez-Lemus, L.A.; Padilla, J. Integrating molecular and cellular components of endothelial shear stress mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, H989–H1003. [Google Scholar] [CrossRef]
- Huveneers, S.; Phng, L.-K. Endothelial cell mechanics and dynamics in angiogenesis. Curr. Opin. Cell Biol. 2024, 91, 102441. [Google Scholar] [CrossRef] [PubMed]
- Mylvaganam, S.; Plumb, J.; Yusuf, B.; Li, R.; Lu, C.-Y.; Robinson, L.A.; Freeman, S.A.; Grinstein, S. The spectrin cytoskeleton integrates endothelial mechanoresponses. Nat. Cell Biol. 2022, 24, 1226–1238. [Google Scholar] [CrossRef]
- Tsata, V.; Beis, D. In Full Force. Mechanotransduction and Morphogenesis during Homeostasis and Tissue Regeneration. J. Cardiovasc. Dev. Dis. 2020, 7, 40. [Google Scholar] [CrossRef]
- Mohapatra, S.; Wegmann, S. Biomolecular condensation involving the cytoskeleton. Brain Res. Bull. 2023, 194, 105–117. [Google Scholar] [CrossRef]
- Lim, X.R.; Harraz, O.F. Mechanosensing by Vascular Endothelium. Annu. Rev. Physiol. 2024, 86, 71–97. [Google Scholar] [CrossRef]
- Stanton, A.E.; Tong, X.; Yang, F. Extracellular matrix type modulates mechanotransduction of stem cells. Acta Biomater. 2019, 96, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, Q.; Zhang, Q.; Wang, N.; Lv, W.; Han, D. Extracellular Matrix Stiffness-Induced Mechanotransduction of Capillarized Liver Sinusoidal Endothelial Cells. Pharmaceuticals 2024, 17, 644. [Google Scholar] [CrossRef]
- Tiskratok, W.; Chuinsiri, N.; Limraksasin, P.; Kyawsoewin, M.; Jitprasertwong, P. Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation. Polymers 2025, 17, 822. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.H.; Murphy, H.A.; George, E.M. The glycocalyx: A central regulator of vascular function. Am. J. Physiol. Integr. Comp. Physiol. 2021, 320, R508–R518. [Google Scholar] [CrossRef]
- Askari, H.; Sadeghinejad, M.; Fancher, I.S. Mechanotransduction and the endothelial glycocalyx: Interactions with membrane and cytoskeletal proteins to transduce force. Curr. Top. Membr. 2023, 91, 43–60. [Google Scholar] [CrossRef]
- Hofmann-Kiefer, K.F.; Chappell, D.; Knabl, J.; Frank, H.G.; Martinoff, N.; Conzen, P.; Becker, B.F.; Rehm, M. Placental Syncytiotrophoblast Maintains a Specific Type of Glycocalyx at the Fetomaternal Border: The Glycocalyx at the Fetomaternal Interface in Healthy Women and Patients With HELLP Syndrome. Reprod. Sci. 2013, 20, 1237–1245. [Google Scholar] [CrossRef]
- Fabre-Gray, A.C.; Down, C.J.; Neal, C.R.; Foster, R.R.; Satchell, S.C.; Bills, V.L. Imaging the placental glycocalyx with transmission electron microscopy. Placenta 2018, 74, 59–61. [Google Scholar] [CrossRef]
- Heyer-Chauhan, N.; Ovbude, I.J.; Hills, A.A.; Sullivan, M.H.; Hills, F.A. Placental syndecan-1 and sulphated glycosaminoglycans are decreased in preeclampsia. J. Perinat. Med. 2014, 42, 329–338. [Google Scholar] [CrossRef]
- Szabo, S.; Xu, Y.; Romero, R.; Fule, T.; Karaszi, K.; Bhatti, G.; Varkonyi, T.; Varkonyi, I.; Krenacs, T.; Dong, Z.; et al. Changes of placental syndecan-1 expression in preeclampsia and HELLP syndrome. Virchows Arch. 2013, 463, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Divino, L.F.; Poulsen, C.G.; Curovic, V.R.; Pedersen, O.B.; Tofte, N.; Frimodt-Møller, M.; Hansen, T.W.; Hvas, A.-M.; Rossing, P. Endothelial dysfunction markers syndecan-1 and thrombomodulin are associated with higher albuminuria levels in type 2 diabetes with no history of clinical cardiovascular disease. J. Diabetes Its Complicat. 2024, 38, 108879. [Google Scholar] [CrossRef]
- Martineau, L.C.; Gardiner, P.F. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J. Appl. Physiol. 2001, 91, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.K.; Wang, N.; Leckband, D.E. Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. J. Cell Sci. 2015, 128, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, A. Mechanotransduction: VE-cadherin lets it flow. Nat. Rev. Mol. Cell Biol. 2015, 16, 268. [Google Scholar] [CrossRef]
- Nan, W.; He, Y.; Wang, S.; Zhang, Y. Molecular mechanism of VE-cadherin in regulating endothelial cell behaviour during angiogenesis. Front. Physiol. 2023, 14, 1234104. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Masuda, M.; Kusano, K.-I.; Fujiwara, K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: Is it a mechanoresponsive molecule? J. Cell Biol. 2002, 158, 773–785. [Google Scholar] [CrossRef]
- Miller, B.; Sewell-Loftin, M.K. Mechanoregulation of Vascular Endothelial Growth Factor Receptor 2 in Angiogenesis. Front. Cardiovasc. Med. 2022, 8, 804934. [Google Scholar] [CrossRef]
- Graham, D.M.; Burridge, K. Mechanotransduction and nuclear function. Curr. Opin. Cell Biol. 2016, 40, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Guilluy, C.; Osborne, L.D.; Van Landeghem, L.; Sharek, L.; Superfine, R.; Garcia-Mata, R.; Burridge, K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 2014, 16, 376–381. [Google Scholar] [CrossRef]
- Piechowski, J. Plausibility of trophoblastic-like regulation of cancer tissue. Cancer Manag. Res. 2019, 11, 5033–5046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shan, K.Z.; Liang, P.; Lowry, A.J.; Feng, L.; Yang, H. PIEZO1 drives trophoblast fusion and placental development. Nat. Commun. 2025, 16, 6895. [Google Scholar] [CrossRef]
- Cao, J.; Li, H.; Tang, H.; Gu, X.; Wang, Y.; Guan, D.; Du, J.; Fan, Y. Stiff Extracellular Matrix Promotes Invasive Behaviors of Trophoblast Cells. Bioengineering 2023, 10, 384. [Google Scholar] [CrossRef]
- Dietrich, B.; Haider, S.; Meinhardt, G.; Pollheimer, J.; Knöfler, M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell. Mol. Life Sci. 2022, 79, 292. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef]
- Xie, B.; Wu, H.; Li, J.; Lv, X.; Zhou, Y.; Yu, Q.; Cui, S.; Zeng, L.; Li, J.; Huang, X.; et al. Mechanical forces on trophoblast motility and its potential role in spiral artery remodeling during pregnancy. Placenta 2022, 123, 46–53. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Straszewski-Chavez, S.L.; Guller, S.; Mor, G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol. Hum. Reprod. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Ashton, S.V.; Whitley, G.S.J.; Dash, P.R.; Wareing, M.; Crocker, I.P.; Baker, P.N.; Cartwright, J.E. Uterine Spiral Artery Remodeling Involves Endothelial Apoptosis Induced by Extravillous Trophoblasts Through Fas/FasL Interactions. Arter. Thromb. Vasc. Biol. 2005, 25, 102–108. [Google Scholar] [CrossRef]
- Cao, T.C.; Thirkill, T.L.; Wells, M.; Barakat, A.I.; Douglas, G.C. ORIGINAL ARTICLE: Trophoblasts and Shear Stress Induce an Asymmetric Distribution of ICAM-1 in Uterine Endothelial Cells. Am. J. Reprod. Immunol. 2008, 59, 167–181. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Hyun, J. Mechanosensitive ion channels in apoptosis and ferroptosis: Focusing on the role of Piezo1. BMB Rep. 2023, 56, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front. Physiol. 2018, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Chakraborty, S.; Islam, S.; Ain, R. Trans-differentiation of trophoblast stem cells: Implications in placental biology. Life Sci. Alliance 2022, 6, e202201583. [Google Scholar] [CrossRef]
- Godeau, A.L.; Seriola, A.; Tchaicheeyan, O.; Casals, M.; Denkova, D.; Aroca, E.; Massafret, O.; Parra, A.; Demestre, M.; Ferrer-Vaquer, A.; et al. Traction force and mechanosensitivity mediate species-specific implantation patterns in human and mouse embryos. Sci. Adv. 2025, 11, eadr5199. [Google Scholar] [CrossRef]
- Liu, L.; Tang, L.; Chen, S.; Zheng, L.; Ma, X. Decoding the molecular pathways governing trophoblast migration and placental development; a literature review. Front. Endocrinol. 2024, 15, 1486608. [Google Scholar] [CrossRef]
- Gauster, M.; Moser, G.; Wernitznig, S.; Kupper, N.; Huppertz, B. Early human trophoblast development: From morphology to function. Cell. Mol. Life Sci. 2022, 79, 345. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fan, Y.; Deng, X.; Li, N.; Guan, Z. Effect of flow-induced shear stress on migration of human trophoblast cells. Clin. Biomech. 2008, 23 (Suppl. S1), S112–S117. [Google Scholar] [CrossRef] [PubMed]
- Morley, L.C.; Shi, J.; Gaunt, H.J.; Hyman, A.J.; Webster, P.J.; Williams, C.; Forbes, K.; Walker, J.J.; Simpson, N.A.B.; Beech, D.J. Piezo1 channels are mechanosensors in human fetoplacental endothelial cells. Mol. Hum. Reprod. 2018, 24, 510–520. [Google Scholar] [CrossRef]
- Lapehn, S.; Nair, S.; Firsick, E.J.; MacDonald, J.; Thoreson, C.; Litch, J.A.; Bush, N.R.; Kadam, L.; Girard, S.; Myatt, L.; et al. A transcriptomic comparison of in vitro models of the human placenta. Placenta 2024, 159, 52–61. [Google Scholar] [CrossRef]
- Douguet, D.; Patel, A.; Xu, A.; Vanhoutte, P.M.; Honoré, E. Piezo Ion Channels in Cardiovascular Mechanobiology. Trends Pharmacol. Sci. 2019, 40, 956–970. [Google Scholar] [CrossRef]
- Shah, V.; Patel, S.; Shah, J. Emerging role of Piezo ion channels in cardiovascular development. Dev. Dyn. 2022, 251, 276–286. [Google Scholar] [CrossRef]
- Zong, B.; Yu, F.; Zhang, X.; Pang, Y.; Zhao, W.; Sun, P.; Li, L. Mechanosensitive Piezo1 channel in physiology and pathophysiology of the central nervous system. Ageing Res. Rev. 2023, 90, 102026. [Google Scholar] [CrossRef]
- Xiao, B. Mechanisms of mechanotransduction and physiological roles of PIEZO channels. Nat. Rev. Mol. Cell Biol. 2024, 25, 886–903. [Google Scholar] [CrossRef] [PubMed]
- Parameshwar, P.K.; Li, C.; Arnauts, K.; Jiang, J.; Rostami, S.; Campbell, B.E.; Lu, H.; Rosenzweig, D.H.; Vaillancourt, C.; Moraes, C. Directed biomechanical compressive forces enhance fusion efficiency in model placental trophoblast cultures. Sci. Rep. 2024, 14, 11312. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.-H.; Wang, Y.-X.; Liu, T.-H. A comprehensive review of human trophoblast fusion models: Recent developments and challenges. Cell Death Discov. 2023, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, Y.; Yu, X.; Shao, X.; Wang, Y.-L. Molecular regulation and functional benefits of trophoblast syncytialization in optimizing maternal-fetal nutrient allocation. Placenta 2025, S0143-4004(25)00208-5. [Google Scholar] [CrossRef]
- Zhang, Y.; Le, T.; Grabau, R.; Mohseni, Z.; Kim, H.; Natale, D.R.; Feng, L.; Pan, H.; Yang, H. TMEM16F phospholipid scramblase mediates trophoblast fusion and placental development. Sci. Adv. 2020, 6, eaba0310. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.M.; Chernomordik, L.V. Flagging fusion: Phosphatidylserine signaling in cell–cell fusion. J. Biol. Chem. 2021, 296, 100411. [Google Scholar] [CrossRef]
- Zhu, D.; Gong, X.; Miao, L.; Fang, J.; Zhang, J. Efficient Induction of Syncytiotrophoblast Layer II Cells from Trophoblast Stem Cells by Canonical Wnt Signaling Activation. Stem Cell Rep. 2017, 9, 2034–2049. [Google Scholar] [CrossRef]
- Shan, K.Z.; Le, T.; Liang, P.; Dong, P.; Lowry, A.J.; Kremmyda, P.; Claesson-Welsh, L.; Yang, H. TMEM16F scramblase regulates angiogenesis via endothelial intracellular signaling. J. Cell Sci. 2024, 137, jcs261566. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, P.; Yang, L.; Shan, K.Z.; Feng, L.; Chen, Y.; Liedtke, W.; Coyne, C.B.; Yang, H. Functional coupling between TRPV4 channel and TMEM16F modulates human trophoblast fusion. eLife 2022, 11, e78840. [Google Scholar] [CrossRef]
- Parameshwar, P.K.; Vaillancourt, C.; Moraes, C. Engineering placental trophoblast fusion: A potential role for biomechanics in syncytialization. Placenta 2024, 157, 50–54. [Google Scholar] [CrossRef]
- Kim, J.H.; Chen, E.H. The fusogenic synapse at a glance. J. Cell Sci. 2019, 132, jcs213124. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Hara, Y.; Okuda, M.; Itoh, K.; Nishioka, R.; Shiomi, A.; Nagao, K.; Mori, M.; Mori, Y.; Ikenouchi, J.; et al. Cell surface flip-flop of phosphatidylserine is critical for PIEZO1-mediated myotube formation. Nat. Commun. 2018, 9, 2049. [Google Scholar] [CrossRef] [PubMed]
- Morley, L.; Debant, M.; Walker, J.; Beech, D.; Simpson, N. Placental blood flow sensing and regulation in fetal growth restriction. Placenta 2021, 113, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Sagrillo-Fagundes, L.; Mok, S.; Vaillancourt, C.; Moraes, C. Mechanobiological regulation of placental trophoblast fusion and function through extracellular matrix rigidity. Sci. Rep. 2020, 10, 5837. [Google Scholar] [CrossRef] [PubMed]
- John, L.; Ko, N.L.; Gokin, A.; Gokina, N.; Mandalà, M.; Osol, G. The Piezo1 cation channel mediates uterine artery shear stress mechanotransduction and vasodilation during rat pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1019–H1026. [Google Scholar] [CrossRef]
- Ji, S.; Xin, H.; Li, Y.; Su, E.J. FMS-like tyrosine kinase 1 (FLT1) is a key regulator of fetoplacental endothelial cell migration and angiogenesis. Placenta 2018, 70, 7–14. [Google Scholar] [CrossRef]
- Del Pozo, M.A.; Lolo, F.-N.; Echarri, A. Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr. Opin. Cell Biol. 2021, 68, 113–123. [Google Scholar] [CrossRef]
- Stea, D.M.; D’Alessio, A. Caveolae: Metabolic Platforms at the Crossroads of Health and Disease. Int. J. Mol. Sci. 2025, 26, 2918. [Google Scholar] [CrossRef]
- Sotodosos-Alonso, L.; Pulgarín-Alfaro, M.; del Pozo, M.A. Caveolae Mechanotransduction at the Interface between Cytoskeleton and Extracellular Matrix. Cells 2023, 12, 942. [Google Scholar] [CrossRef]
- Cheng, J.P.; Mendoza-Topaz, C.; Howard, G.; Chadwick, J.; Shvets, E.; Cowburn, A.S.; Dunmore, B.J.; Crosby, A.; Morrell, N.W.; Nichols, B.J. Caveolae protect endothelial cells from membrane rupture during increased cardiac output. J. Cell Biol. 2015, 211, 53–61. [Google Scholar] [CrossRef]
- Drab, M.; Verkade, P.; Elger, M.; Kasper, M.; Lohn, M.; Lauterbach, B.; Menne, J.; Lindschau, C.; Mende, F.; Luft, F.C.; et al. Loss of Caveolae, Vascular Dysfunction, and Pulmonary Defects in Caveolin-1 Gene-Disrupted Mice. Science 2001, 293, 2449–2452. [Google Scholar] [CrossRef]
- Galbiati, F.; Engelman, J.A.; Volonte, D.; Zhang, X.L.; Minetti, C.; Li, M.; Hou, H.; Kneitz, B.; Edelmann, W.; Lisanti, M.P. Caveolin-3 Null Mice Show a Loss of Caveolae, Changes in the Microdomain Distribution of the Dystrophin-Glycoprotein Complex, and T-tubule Abnormalities. J. Biol. Chem. 2001, 276, 21425–21433. [Google Scholar] [CrossRef] [PubMed]
- Razani, B.; Wang, X.B.; Engelman, J.A.; Battista, M.; Lagaud, G.; Zhang, X.L.; Kneitz, B.; Hou, H.; Christ, G.J.; Edelmann, W.; et al. Caveolin-2-Deficient Mice Show Evidence of Severe Pulmonary Dysfunction without Disruption of Caveolae. Mol. Cell. Biol. 2002, 22, 2329–2344. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Smart, E.J. Caveolae structure and function. J. Cell. Mol. Med. 2008, 12, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Nong, Y.; Li, S.; Liu, W.; Zhang, X.; Fan, L.; Chen, Y.; Huang, Q.; Zhang, Q.; Liu, F. Aquaporin 3 promotes human extravillous trophoblast migration and invasion. Reprod. Biol. Endocrinol. 2021, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Reppetti, J.; Reca, A.; Seyahian, E.A.; Medina, Y.; Martínez, N.; Szpilbarg, N.; Damiano, A.E. Intact caveolae are required for proper extravillous trophoblast migration and differentiation. J. Cell. Physiol. 2020, 235, 3382–3392. [Google Scholar] [CrossRef]
- Nong, Y.; Zhai, Q.; Liu, W.; Wei, J.; Wang, Z.; Lv, X.; Li, Z.; Zhang, X.; Liu, F. AQP3 Influences Unexplained Recurrent Abortion by Regulating Trophoblast Cell Migration and Invasion via the METTL14/IGF2BP1/AQP3/PI3K/AKT Pathway. J. Cell. Mol. Med. 2025, 29, e70325. [Google Scholar] [CrossRef]
- Reppetti, J.; Medina, Y.; Farina, M.; Damiano, A.E.; Martínez, N.A. Hyperosmolarity Impairs Human Extravillous Trophoblast Differentiation by Caveolae Internalization. Front. Physiol. 2021, 12, 760163. [Google Scholar] [CrossRef]
- Saghian, R.; Bogle, G.; James, J.L.; Clark, A.R. Establishment of maternal blood supply to the placenta: Insights into plugging, unplugging and trophoblast behaviour from an agent-based model. Interface Focus 2019, 9, 20190019. [Google Scholar] [CrossRef]
- Weiss, G.; Sundl, M.; Glasner, A.; Huppertz, B.; Moser, G. The trophoblast plug during early pregnancy: A deeper insight. Histochem. Cell Biol. 2016, 146, 749–756. [Google Scholar] [CrossRef]
- Arutyunyan, A.; Roberts, K.; Troulé, K.; Wong, F.C.K.; Sheridan, M.A.; Kats, I.; Garcia-Alonso, L.; Velten, B.; Hoo, R.; Ruiz-Morales, E.R.; et al. Spatial multiomics map of trophoblast development in early pregnancy. Nature 2023, 616, 143–151. [Google Scholar] [CrossRef]
- LaValley, D.J.; Zanotelli, M.R.; Bordeleau, F.; Wang, W.; Schwager, S.C.; Reinhart-King, C.A. Matrix stiffness enhances VEGFR-2 internalization, signaling, and proliferation in endothelial cells. Converg. Sci. Phys. Oncol. 2017, 3, 044001. [Google Scholar] [CrossRef]
- Goldblatt, Z.E.; Cirka, H.A.; Billiar, K.L. Mechanical Regulation of Apoptosis in the Cardiovascular System. Ann. Biomed. Eng. 2021, 49, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, S.; Li, J.; Wang, L.; Zhang, Q.; Hou, L.; Yu, X.; Liu, Z.; Lv, T.; Shang, L. Low shear stress induces vascular endothelial cells apoptosis via miR-330 /SOD2 /HSP70 signaling pathway. Exp. Cell Res. 2025, 445, 114410. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Shreeve, N. Local immune recognition of trophoblast in early human pregnancy: Controversies and questions. Nat. Rev. Immunol. 2023, 23, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Helige, C.; Ahammer, H.; Moser, G.; Hammer, A.; Dohr, G.; Huppertz, B.; Sedlmayr, P. Distribution of decidual natural killer cells and macrophages in the neighbourhood of the trophoblast invasion front: A quantitative evaluation. Hum. Reprod. 2014, 29, 8–17. [Google Scholar] [CrossRef]
- Shihata, W.A.; Michell, D.L.; Andrews, K.L.; Chin-Dusting, J.P.F. Caveolae: A Role in Endothelial Inflammation and Mechanotransduction? Front. Physiol. 2016, 7, 628. [Google Scholar] [CrossRef]
- James, J.; Stone, P.; Chamley, L. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum. Reprod. Update 2006, 12, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Cinkornpumin, J.K.; Kwon, S.Y.; Prandstetter, A.-M.; Maxian, T.; Sirois, J.; Goldberg, J.; Zhang, J.; Saini, D.; Dasgupta, P.; Jeyarajah, M.J.; et al. Hypoxia and loss of GCM1 expression prevent differentiation and contact inhibition in human trophoblast stem cells. Stem Cell Rep. 2025, 20, 102481. [Google Scholar] [CrossRef] [PubMed]
- Atallah, A.; Sarda, M.; McCarey, C.; Massardier, J.; Huissoud, C. Endothelial Glycocalyx: The Missing Link Between Angiogenic Imbalance in Preeclampsia and Systemic Inflammation in HELLP Syndrome. Compr. Physiol. 2025, 15, e70032. [Google Scholar] [CrossRef]
- Prabaharan, E.; Armant, D.R.; Drewlo, S. Human placentation: Foundations and implications for reproductive endocrinology and infertility. Syst. Biol. Reprod. Med. 2025, 71, 279–306. [Google Scholar] [CrossRef]
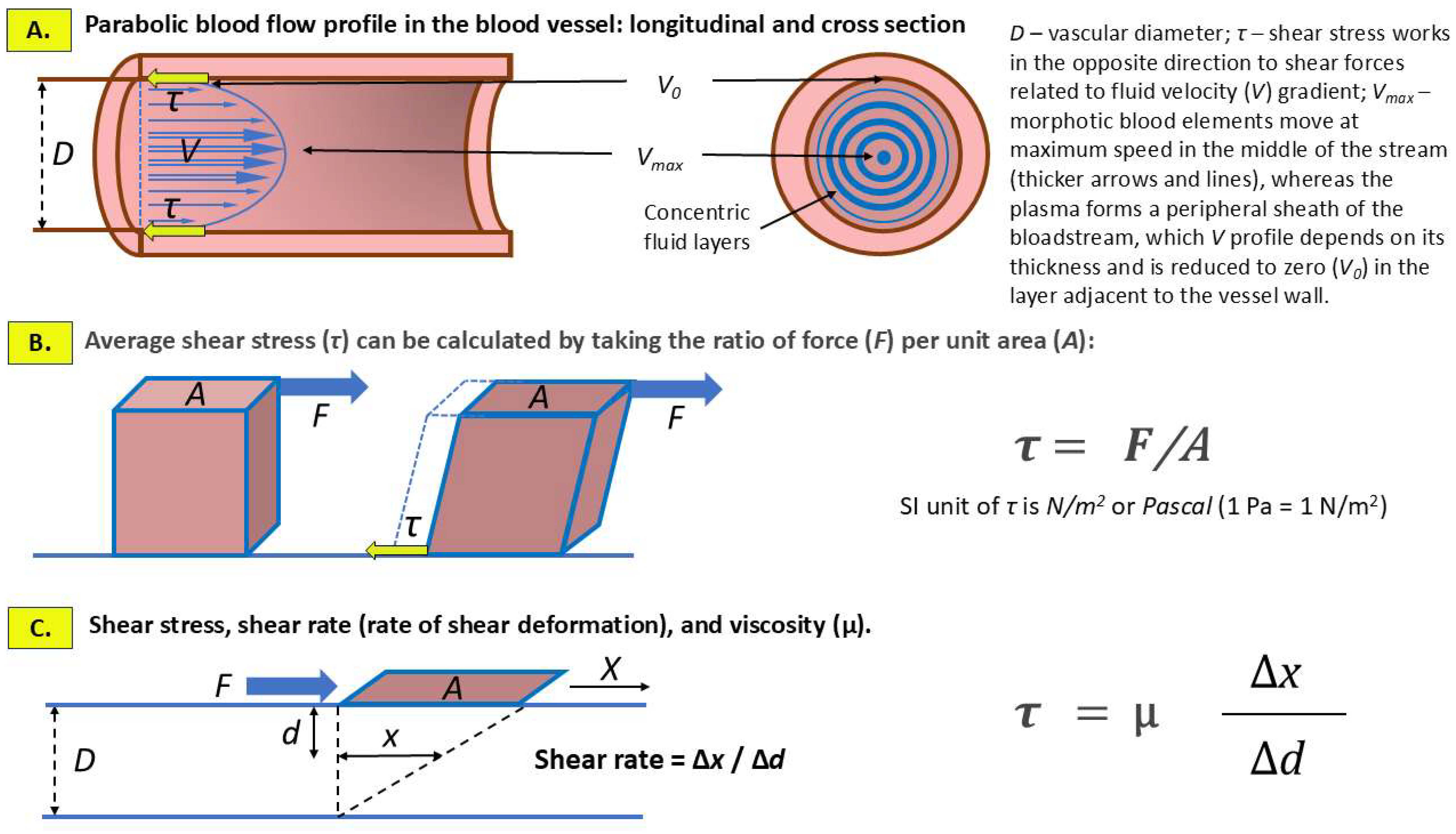
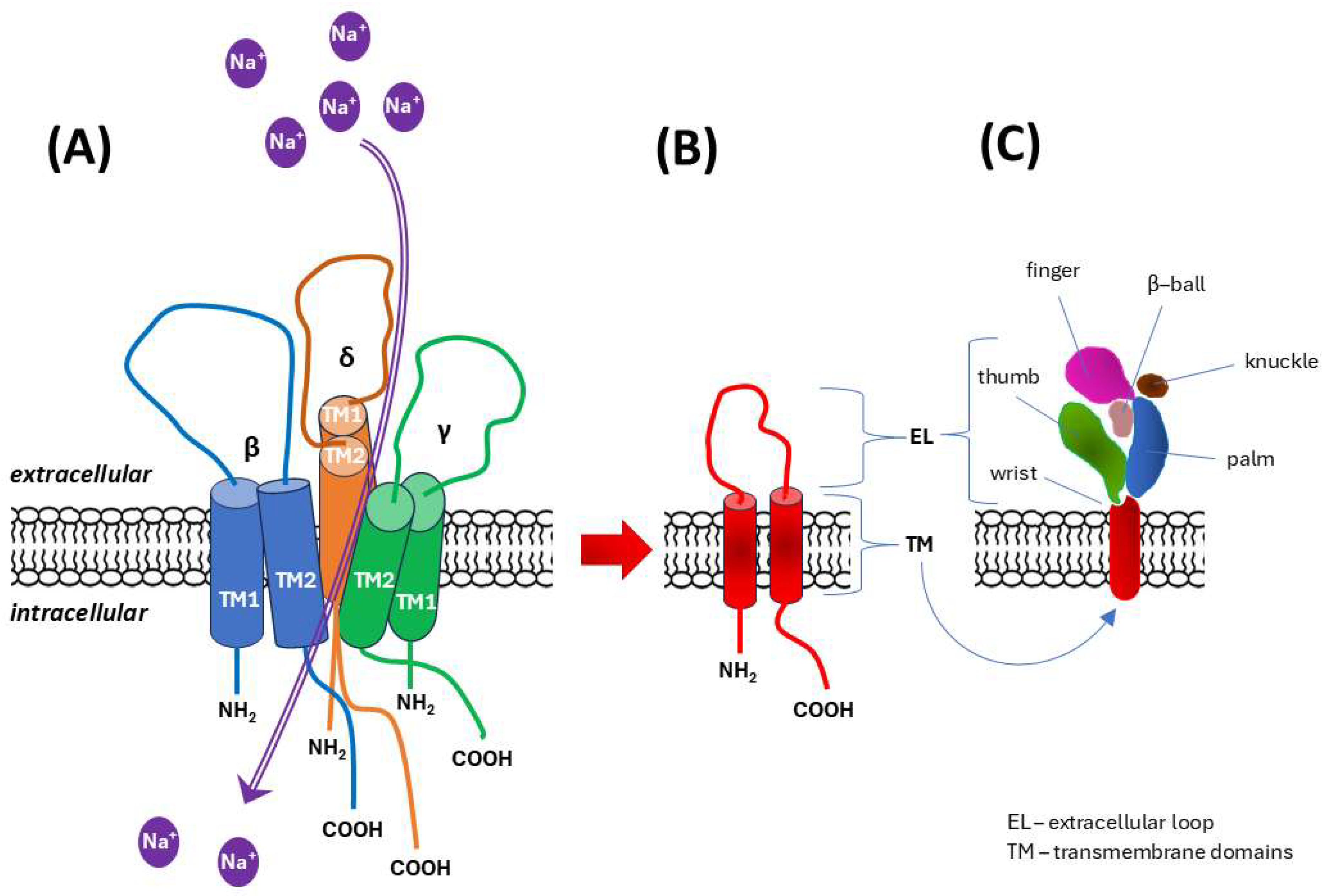
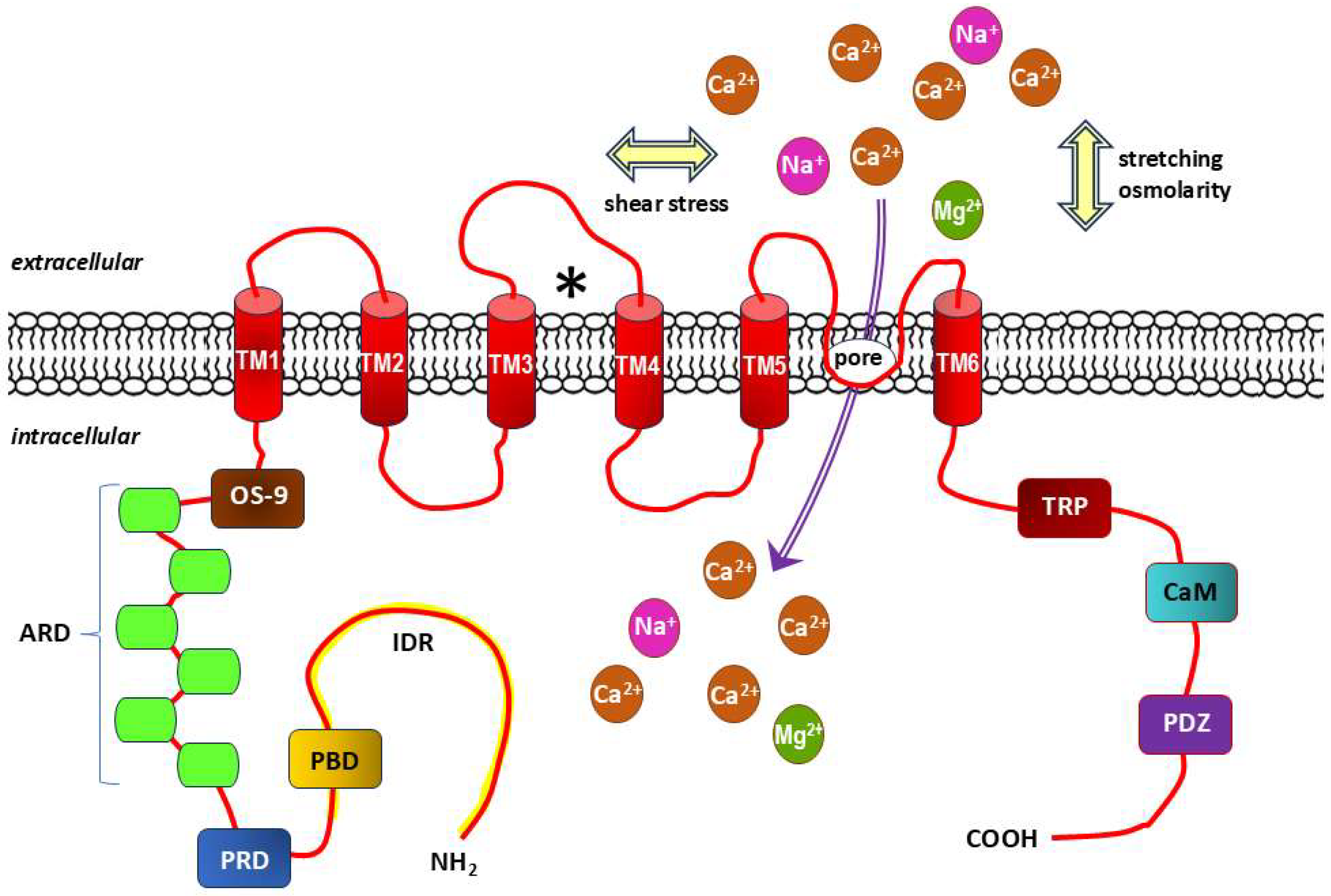
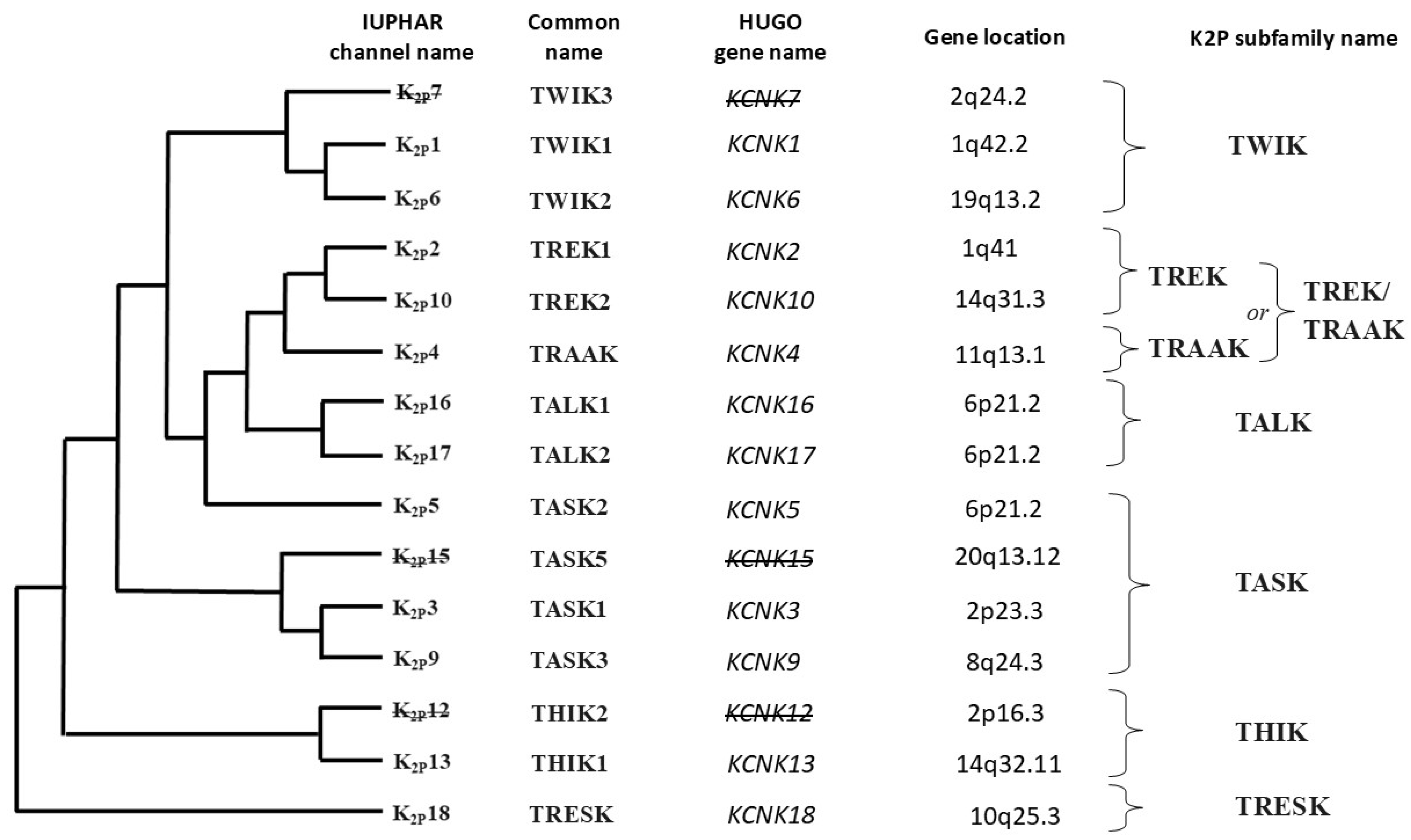
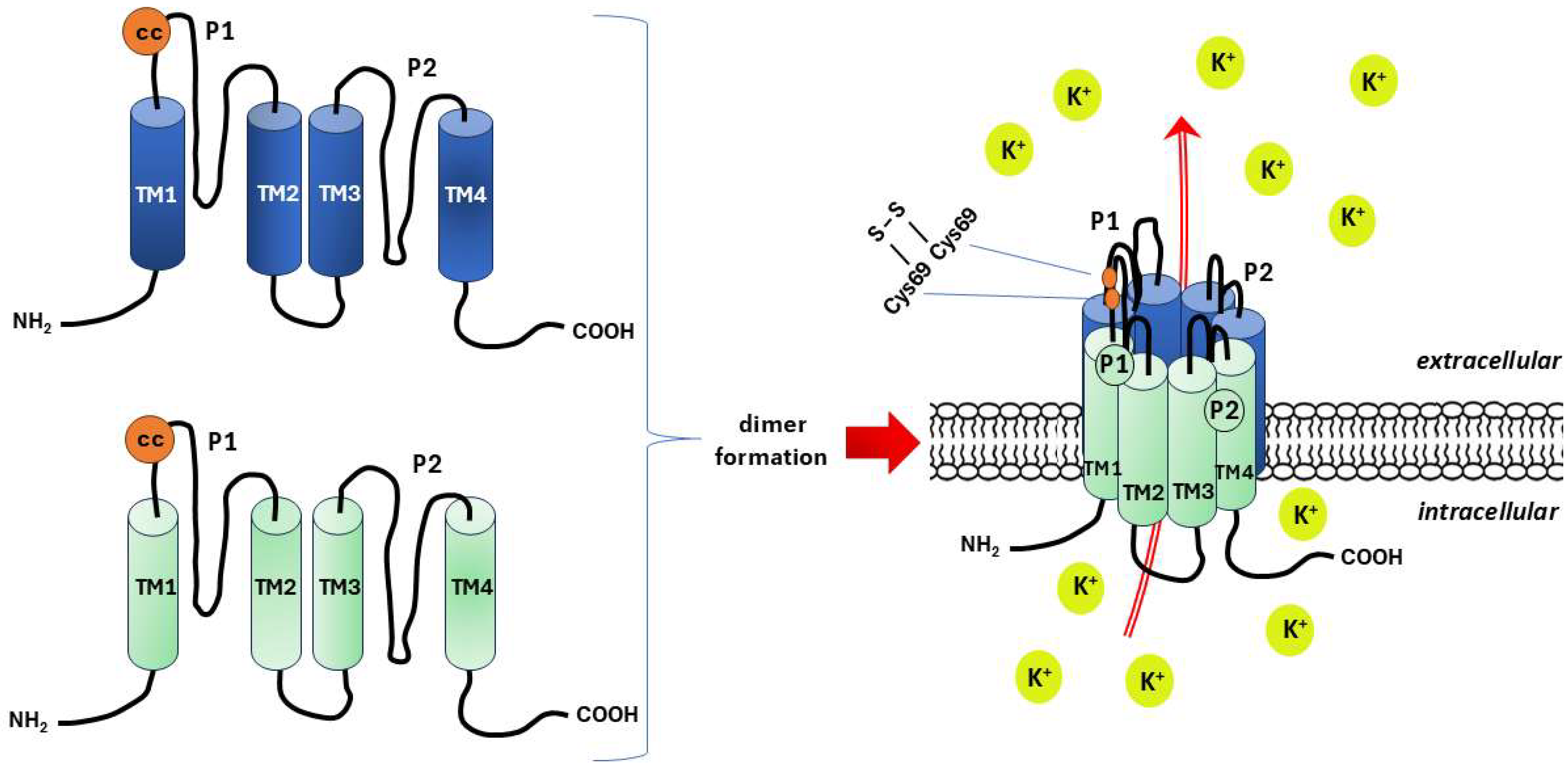

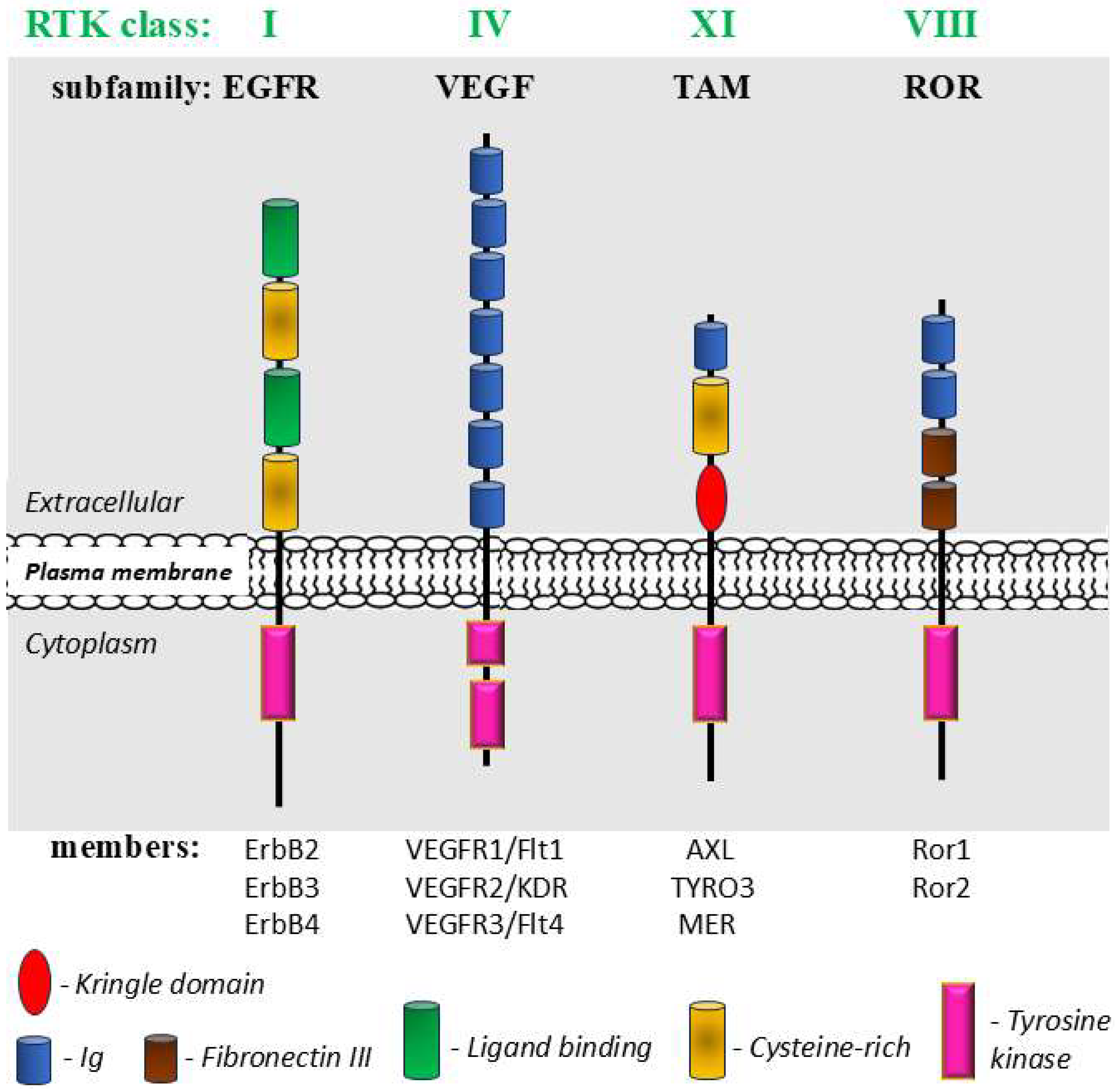
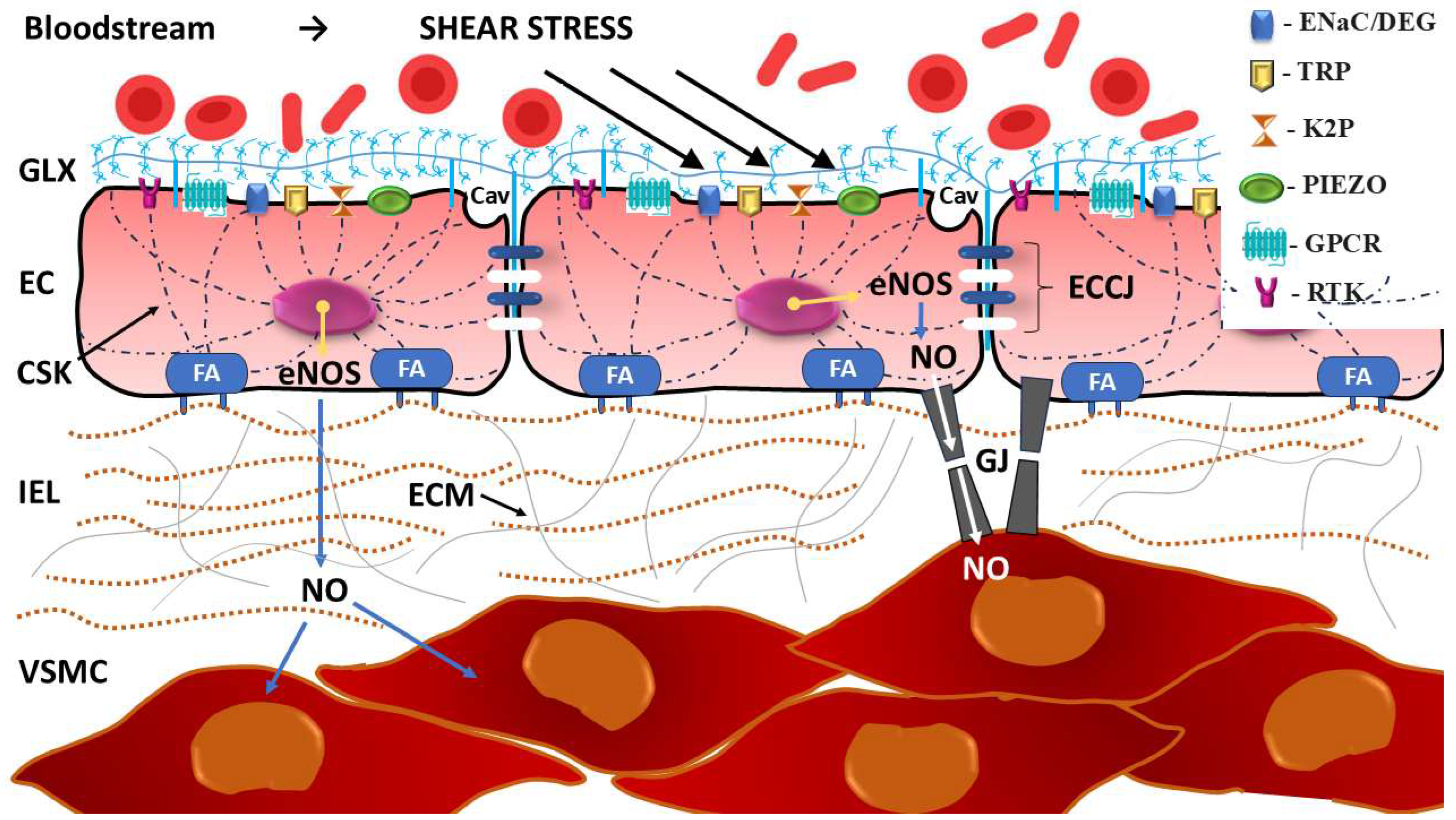
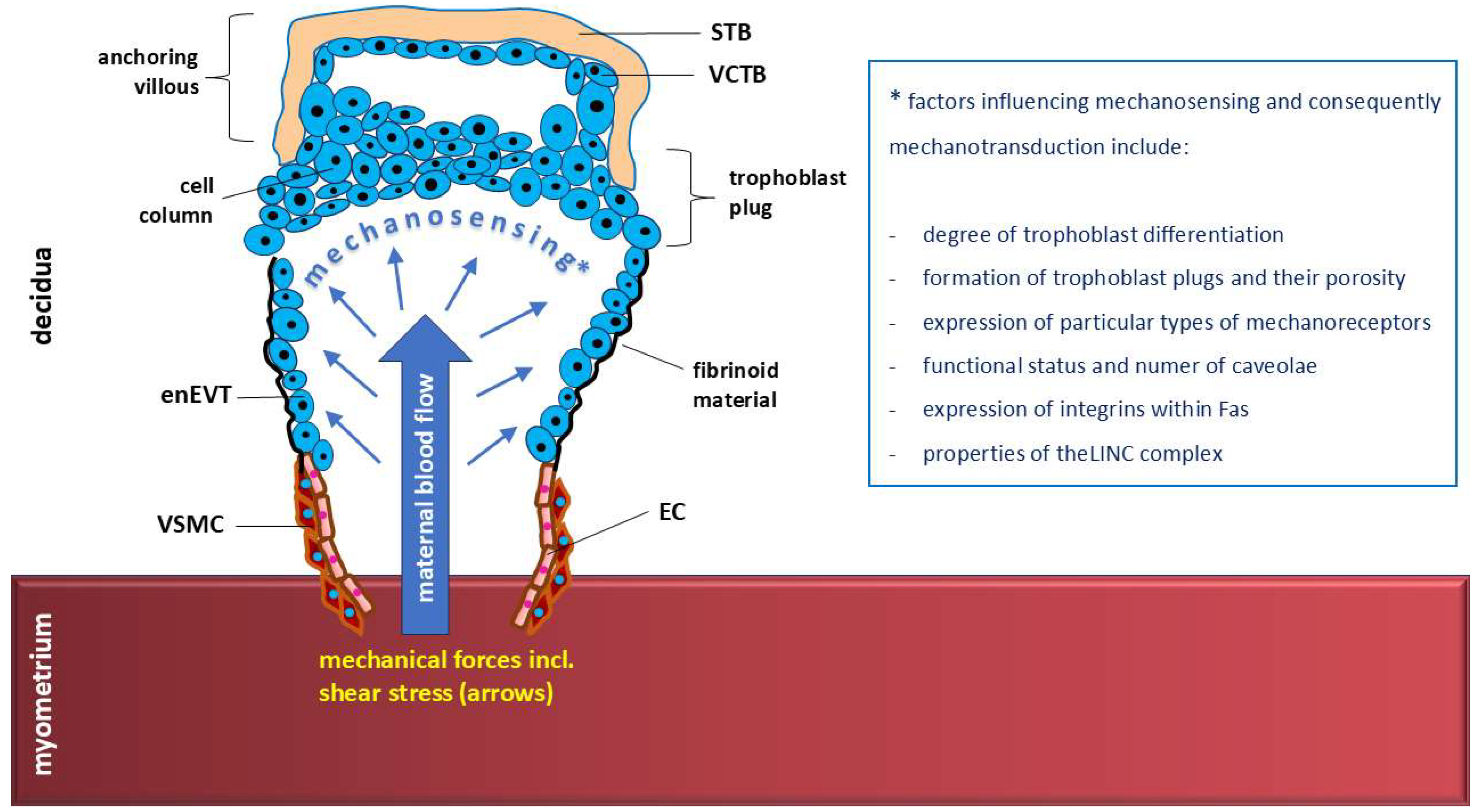
| TRP Subfamily | Members | Gene Location | Distribution (Cells, Tissues and Organs) | Main Functions | References |
|---|---|---|---|---|---|
| TRPC (canonical) | TRPC1 | 3q22–q24 | Widely and evenly distributed in vascular smooth muscle cells (VSMC), heart muscle, skeletal muscles, brain (pituitary gland, cerebellar hemisphere, frontal cortex, amygdalia, hippocampus, hypothalamus, substantia nigra, anterior cingulate cortex), testis, ovary, uterus, fallopian tube, tibial nerve, liver spleen, kidney, lungs | Vasoconstriction; mechanosensing; inhibition of tumor growth and metastasis; intensification of the immune response and proinflammatory effects; myoblast migration, fusion and differentiation; regulation of cardiac plasticity and promotion of cardiac hypertrophy | Kim et al., 2019 [97]; Zhang et al., 2023 [98]; Numaga-Tomita and Nishida 2020 [99]; Elzamzamy et al., 2020 [100]; Louis et al., 2008 [101] |
| TRPC2 | 7, 50.0 cM | In humans encoded by TRPC2 pseudogene—the respective protein is not expressed in humans. | No function in humans (expressed in mice: involvement in the regulation of thyroid function and olfactory sensations) | Löf, C. et al., 2011 [102]; Yildirim and Birnbaumer 2007 [103] | |
| TRPC3 | 4q27 | Endothelial cells, brain (mainly), heart, skeletal muscles, kidney, ovary, placenta, lungs, testis, prostate, small intestine, colon | Vasodilation; mechanosensing; regulation of vascular tone; cell growth and proliferation; wound healing and pathological hypertrophy; immune regulation | Sierra-Valdez et al., 2018 [104]; Tang, Q. et al., 2018 [105]; Patel et al., 2025 [106]; Thilo et al., 2008 [107] | |
| TRPC4 | 13q13.1–q13.2 | Vascular system (endothelial cells aorta, coronary artery), brain (pituitary gland, cerebellar hemisphere, frontal cortex, amygdalia, hippocampus, hypothalamus, substantia nigra, anterior cingulate cortex), liver, adrenal gland, retina, testis, placenta, kidney, sigmoid and transverse colon | Endothelial permeability; angiogenesis; vasodilation; neurotransmitter release and cell proliferation; thermoregulation (induction of nonshivering thermogenesis) | Kim et al., 2019 [97]; Zeng et al., 2021 [108]; Cornman 2025 [109] | |
| TRPC5 | Xq23 | Brain mainly (pituitary gland, cerebellum, frontal cortex, amygdalia, hippocampus, hypothalamus, substantia nigra, anterior cingulate cortex), liver | Cell proliferation; angiogenesis; endothelium-dependent vasoconstriction; regulation of blood pressure; thermosensing; promotion of extracellular vesicle formation | Kim et al., 2019 [97]; Zeng et al., 2021 [108]; Ptakova and Vlachova 2024 [110]; Ma et al., 2014 [111] | |
| TRPC6 | 11q21–q22 | Lungs, brain, myocytes, ovary, placenta | Mechanosensing; neuroprotective effects; development of glomerular injury and glomerular sclerosis; immune regulation | Tang et al., 2018 [105]; Corteling et al., 2004 [112]; Zhang et al., 2023 [98]; Staruschenko et al., 2019 [113]; ‘t Hart et al., 2023 [114] | |
| TRPC7 | 5q31.2 | Heart, lungs, spleen, brain, eye, testis | Nociceptive mechanosensing; single fertilization; may contribute to heart failure as an initiator linking angiotensin receptor (AT1) activation to myocardial apoptosis | Zhang et al., 2016 [115]; Zhang et al., 2023 [98]; Hsu et al., 2020 [116]; Satoh et al., 2007 [117] | |
| TRPM (melastatin) | TRPM1 | 15q13–q14 | Retina (dendrites of retinal ON-bipolar cells), skin (melanosomes of melanocytes), testis (cilia of early spermatids) | An essential role in the depolarizing photoresponse of retinal ON-bipolar cells; melanin synthesis; regulation of normal and malignant melanocyte behavior; promotion of the tumor progression and malignant transformation via activating the Ca2+/CaMKIIδ/AKT pathway in acral melanoma; sperm development | Guo et al., 2012 [118]; Hsieh et al., 2023 [119]; Darszon et al., 2012 [120] |
| TRPM2 | 21q22.3 | Wide variety of tissues including brain, spleen, lungs bone marrow, immune cells (macrophages), heart, vasculature (endothelial cells), endocrine cells | Biosensor of reactive oxygen species (ROS), which mediates some body’s responses to oxidative stress, including maintenance of mitochondrial function, immune response, insulin secretion, body temperature control and neuronal cell death | Faouzi and Penner 2014 [121]; Xia et al., 2019 [122]; Pan et al., 2020 [123]; Cheung and Miller 2017 [124] | |
| TRPM3 | 9q21.11 | Dorsal root ganglia, brain (e.g., pituitary), kidney, pancreas (the islet β-cells), eye, heart (cardiomyocytes). | The pain receptor (inflammatory pain sensation) and thermoreceptor (heat sensation) in somatosensory neurons, regulation of neurotransmitter release, maintaining glucose homeostasis through the regulation of insulin secretion by pancreatic β-cells, iris constriction, and promotion of tumor growth | Thiel et al., 2017 [125]; Vangeel L et al., 2020 [126]; Turgambayeva et al., 2023 [127] | |
| TRPM4 | 19q13.33 | Wide variety of cells/tissues including prostate, small intestine and colon, heart, kidney, testis, skin, pancreas, placenta, liver, thymus, spleen | Crucial role in regulating diverse cellular functions associated with intracellular Ca2+ homeostasis/dynamics, including electrical activity of cardiomyocytes by depolarizing the membrane, regulation of smooth muscle contraction and immune response (e.g., regulation of Ca2+ oscillations after T cell activation, regulation of dendritic cell migration); promotion of tumor growth | Hu et al., 2023 [128]; Yu et al., 2024 [129]; Barbet et al., 2008 [130]; Borgström et al., 2021 [131] | |
| TRPM5 | 11p15.5 | Intestine, liver, lungs, taste receptor cells, pancreas (the islet β-cells), olfactory and vomeronasal systems | Sweet, bitter and umami taste sensation (sensory transduction in taste cells); modulation of insulin secretion by pancreatic β-cells; immune response (e.g., negative regulation of LPS-induced proliferative and inflammatory response in B cells); constriction of cerebral arteries | Dutta et al., 2018 [132]; Vennekens et al., 2018 [133]; Richter et al., 2024 [134]; Sakaguchi et al., 2020 [135]; Guinamard et al., 2011 [136] | |
| TRPM6 | 9q21.13 | Kidney, small intestine, colon, heart (e.g., atrial fibroblasts and cardiomyocytes) | Magnesium uptake and homeostasis in kidney and intestine; regulation of cardiac function; | Chubanov et al., 2005 [137]; Schlingmann et al., 2007 [138]; Andriulė et al., 2021 [139]; Gwanyanya et al., 2021 [140] | |
| TRPM7 | 15q21 | Kidney, heart (e.g., atrial fibroblasts and cardiomyocytes), parathyroid gland (parathyroid glandular cells), bone, pituitary, adipose tissue | Magnesium and calcium homeostasis; regulation of cardiac function; cell viability (e.g., regulation of anoxic neuronal cell death); involvement in cytoskeletal organization, cell adhesion, cell migration and organogenesis. | Schlingmann et al., 2007 [138]; Zhang et al., 2012 [141]; Gwanyanya et al., 2021 [140]; Asrar and Aarts 2013 [142]; Turlova et al., 2021 [143]; Andriulė et al., 2021 [139]; Inoue et al., 2020 [144] | |
| TRPM8 | 2q37.2 | Nerve ganglia (e.g., dorsal root ganglia, trigeminal ganglia) containing sensory neurons innervating the skin, oral cavity, lungs, bladder and prostate; Moreover, to varying degrees within colon, adipose tissue (including brown adipose tissue), liver, pancreas | Cold sensation and response to cold (cold-induced thermogenesis); modulation of pain sensation (pain relief or pain intensification, depending on the context and location); intracellular Ca2+ homeostasis; regulation of gastrointestinal motility | Qi et al., 2025 [145]; Ma et al., 2012 [146]; Moraes et al., 2017 [147]; Sun et al., 2021 [148]; Amato et al., 2020 [149] | |
| TRPV (vanilloid) | TRPV1 | 17p13.3 | Sensory neurons, immune cells, liver, pancreas, muscle cells, adipocytes, bladder, testis | Neuronal depolarization; temperature sensing; mechanosensing; pH-sensing; mediation of inflammatory pain and hyperalgesia | White et al., 2011 [150]; Aneiros et al., 2011 [151]; Xu et al., 2007 [152]; Shuba 2021 [153]; Li and Wang 2021 [154] |
| TRPV2 | 17p11.2 | Pulmonary and vein endothelial cells; nerve ganglia (e.g., dorsal root ganglia), spinal cord, brain, spleen, intestine | Temperature sensing; mechanosensing; regulation of immune response by augmenting B cell activation and function | Shibasaki 2016 [155]; Fricke and Leffler 2024 [156]; Liu and Qin 2016 [157]; Li et al., 2024 [158] | |
| TRPV3 | 17p13.3 | Keratinocytes, corneal epithelial cells, nerve ganglia (e.g., dorsal root ganglia, trigeminal ganglia), spinal cord, brain, tongue, distal colon epithelium | Temperature sensing; vasoregulation | Lei and Tominaga 2025 [159]; Martin et al., 2024 [160]; Pires et al., 2015 [161]; Fromy et al., 2018 [162] | |
| TRPV4 | 12q24.11 | Wide variety of cells/tissues including endothelial cells, nerve ganglia (e.g., dorsal root ganglia), skin, kidney, urinary bladder, lung, spleen, testis, keratinocytes, heart, liver, connective tissue (including adipocytes), bone marrow | Mechanosensing; temperature sensing and thermoregulation; immune response by regulating macrophage phagocytosis and cytokine production; ensuring cellular osmotic homeostasis | Hartmannsgruber et al., 2007 [163]; Güler et al., 2002 [164]; Baratchi et al., 2017 [165]; O’Neil and Heller 2005 [166]; Moore 2022 [167]; Orsini et al., 2024 [168]; Fukuda et al., 2024 [169]; Mamenko et al., 2015 [170]; Sánchez et al., 2016 [171] | |
| TRPV5 | 7q35 | Kidney mainly (the distal convoluted tubules and connecting tubules), intestine, pancreas, placenta, brain | Modulation of calcium channel activity; calcium homeostasis by facilitating renal calcium reabsorption | Fluck et al., 2022 [172]; de Groot et al., 2009 [173] | |
| TRPV6 | 7q33–q34 | Small intestine, pancreas, placenta, prostate, epididymis, kidney, salivary glands | A vitamin D-regulated Ca2+—selective channel required for Ca2+ homeostasis; maintenance of bone homeostasis and skeletal integrity; required for mammalian male fertility and maternal-fetal transport of Ca2+ | Khattar et al., 2022 [174]; Bächinger et al., 2019 [175]; Lieben et al., 2010 [176] | |
| TRPA (ankyrin) | TRPA1 | 8q13 | Nerve ganglia (e.g., dorsal root ganglia, trigeminal ganglia), hair cells, ovary, spleen, testis, lung, heart, pancreas, liver, gastrointestinal tract, kidney, brain (including brain endothelial cells) | Mechanosensing; chemosensing (chemonociceptor); thermosensing; sensing of neuronal activity to regulate functional hyperemia and neurovascular coupling within the somatosensory cortex | Meents et al., 2019 [177]; Nielsen et al., 2018 [178]; Tominaga and Iwata 2025 [179]; Thakore et al., 2021 [180] |
| TRPP (polycystin) | TRPP2 | 4q21–q23 | Widely expressed in vascular endothelial and smooth muscle cells of all major vascular beds, kidney | Mechanosensing Ca2+ channel in endothelial cells; regulation of intracellular Ca2+ homeostasis; regulation of vascular smooth muscle cell function towards optimizing contractility | Du et al., 2016 [181]; Sharif-Naeini et al., 2009 [182]; Gao et al., 2004 [183] |
| TRPP3 | 10q24 | Endothelial cells, kidney, heart, liver, spleen, tongue, retina, testis, neurons | Ca2+ influx in response to receptor stimulation; regulating vascular tone and permeability; contributing to endothelial cell hyperpolarization and vasodilation; angiogenesis and vascular remodeling; sour taste perception; sour sensitivity | Clapham 2003 [184]; Zheng et al., 2015 [185]; Lu et al., 2018 [186]; Zheng et al., 2016 [187]; Liu et al., 2023 [188]; Walsh et al., 2020 [189] | |
| TRPP5 | 5q31 | Testis, heart, kidney | Ca2+ signaling; Ca2+ homeostasis in connection with cell proliferation or apoptosis; spermatogenesis; | Xiao et al., 2010 [190]; Chen et al., 2008 [191] | |
| TRPML (mucolipin) | TRPML1 | 19p13.2–p13.3 | Wide variety of tissues including brain, heart, skeletal muscle and endothelial cells | Ca2+ signaling and homeostasis of lysosomes (a lysosomal ion channel); endocytic and exocytic signaling events; immune response | Schmiege et al., 2017 [192]; Spix et al., 2020 [193]; Venkatachalam et al., 2015 [194] |
| TRPML2 | 1p22 | Lymphoid (A20 mature B lymphocyte, EL-4 T lymphocyte) and myeloid (5T33 myeloma) cell lines | B-lymphocyte development; endocytic and exocytic signaling events; immune response | Samie et al., 2009 [195]; Lindvall et al., 2005 [196]; Song et al., 2006 [197]; Spix et al., 2020 [193] | |
| TRPML3 | 1p22.3 | Melanosomes of melanocytes; hair cells | Endocytic and exocytic signaling events; cell depolarization; overload with Ca2+; depending on the location of the hair cells: reception of acoustic stimuli (cochlea) or sensory element of the balance organ (semicircular canals and vestibule), | Di Palma et al., 2002 [198]; Grimm et al., 2007 [199]; Nagata et al., 2008 [200]; Atiba-Davies and Noben-Trauth 2007 [201] |
| Mechanoreceptor Type | Expression Level in Trophoblasts/Placental Cells | ||||
|---|---|---|---|---|---|
| TSCs | EVTs | CTBs | STBs | FpEC | |
| PIEZO1 | ++ | +++ | ++ | ++ | +++ |
| TRPV4 | +++ | + | +++ | +++ | ++ |
| RTKs | +++ | ++ | +++ | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szukiewicz, D.; Trojanowski, S.; Wróbel, E.; Wojdasiewicz, P.; Szewczyk, G. Mechanosensing of Shear Stress and Uterine Spiral Artery Remodeling by Invasive Trophoblasts in Early Pregnancy. Int. J. Mol. Sci. 2025, 26, 9565. https://doi.org/10.3390/ijms26199565
Szukiewicz D, Trojanowski S, Wróbel E, Wojdasiewicz P, Szewczyk G. Mechanosensing of Shear Stress and Uterine Spiral Artery Remodeling by Invasive Trophoblasts in Early Pregnancy. International Journal of Molecular Sciences. 2025; 26(19):9565. https://doi.org/10.3390/ijms26199565
Chicago/Turabian StyleSzukiewicz, Dariusz, Seweryn Trojanowski, Edyta Wróbel, Piotr Wojdasiewicz, and Grzegorz Szewczyk. 2025. "Mechanosensing of Shear Stress and Uterine Spiral Artery Remodeling by Invasive Trophoblasts in Early Pregnancy" International Journal of Molecular Sciences 26, no. 19: 9565. https://doi.org/10.3390/ijms26199565
APA StyleSzukiewicz, D., Trojanowski, S., Wróbel, E., Wojdasiewicz, P., & Szewczyk, G. (2025). Mechanosensing of Shear Stress and Uterine Spiral Artery Remodeling by Invasive Trophoblasts in Early Pregnancy. International Journal of Molecular Sciences, 26(19), 9565. https://doi.org/10.3390/ijms26199565







