Transcriptomic Analysis Reveals Glycolysis and Gluconeogenesis Pathway Activation Underlying Growth Enhancement by Duck-Blood Protein Hydrolysate in Flowerhorn Cichlid Fish
Abstract
1. Introduction
2. Results
2.1. Growth Performance
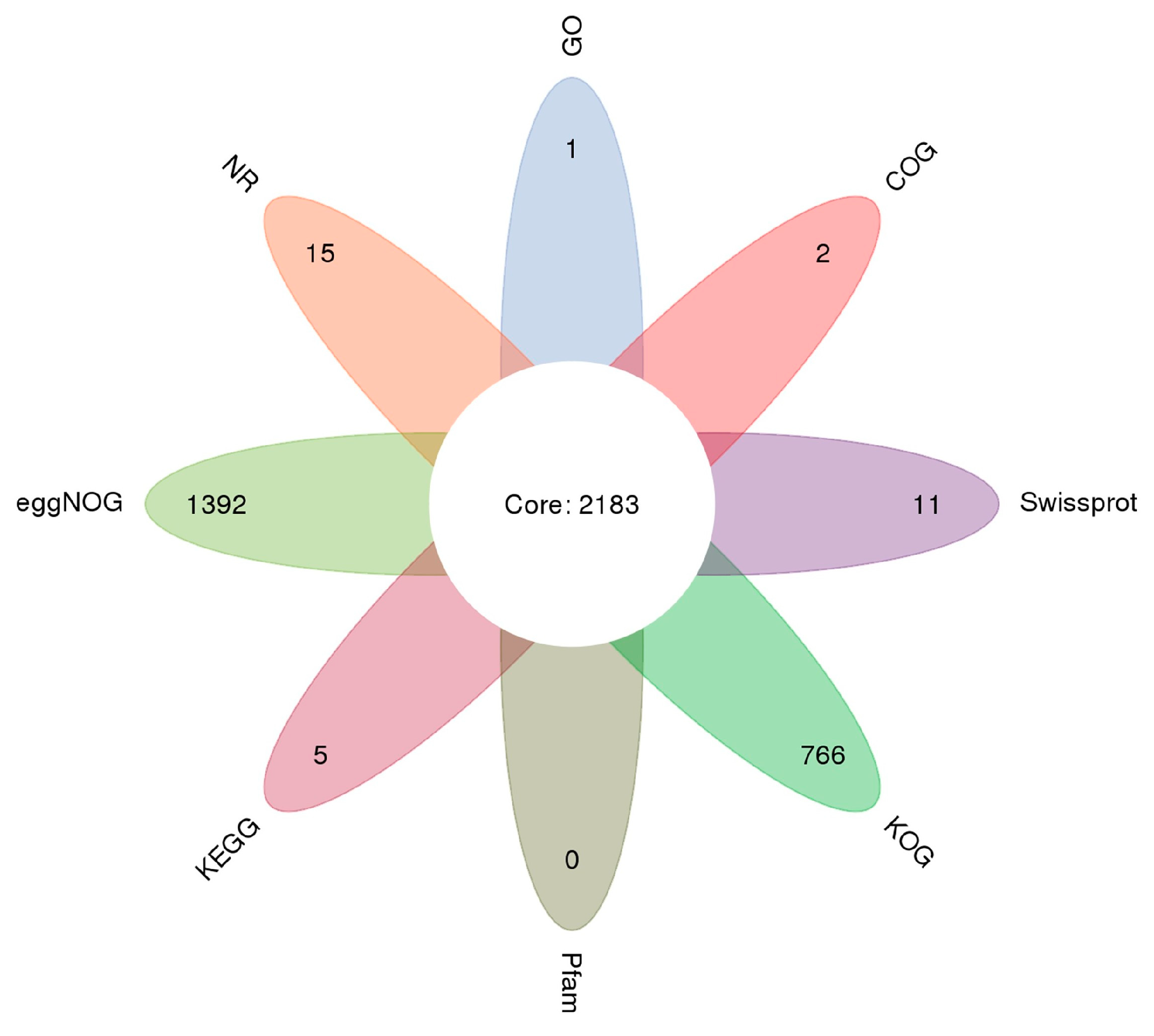
2.2. Sequencing and Annotation of Unigenes
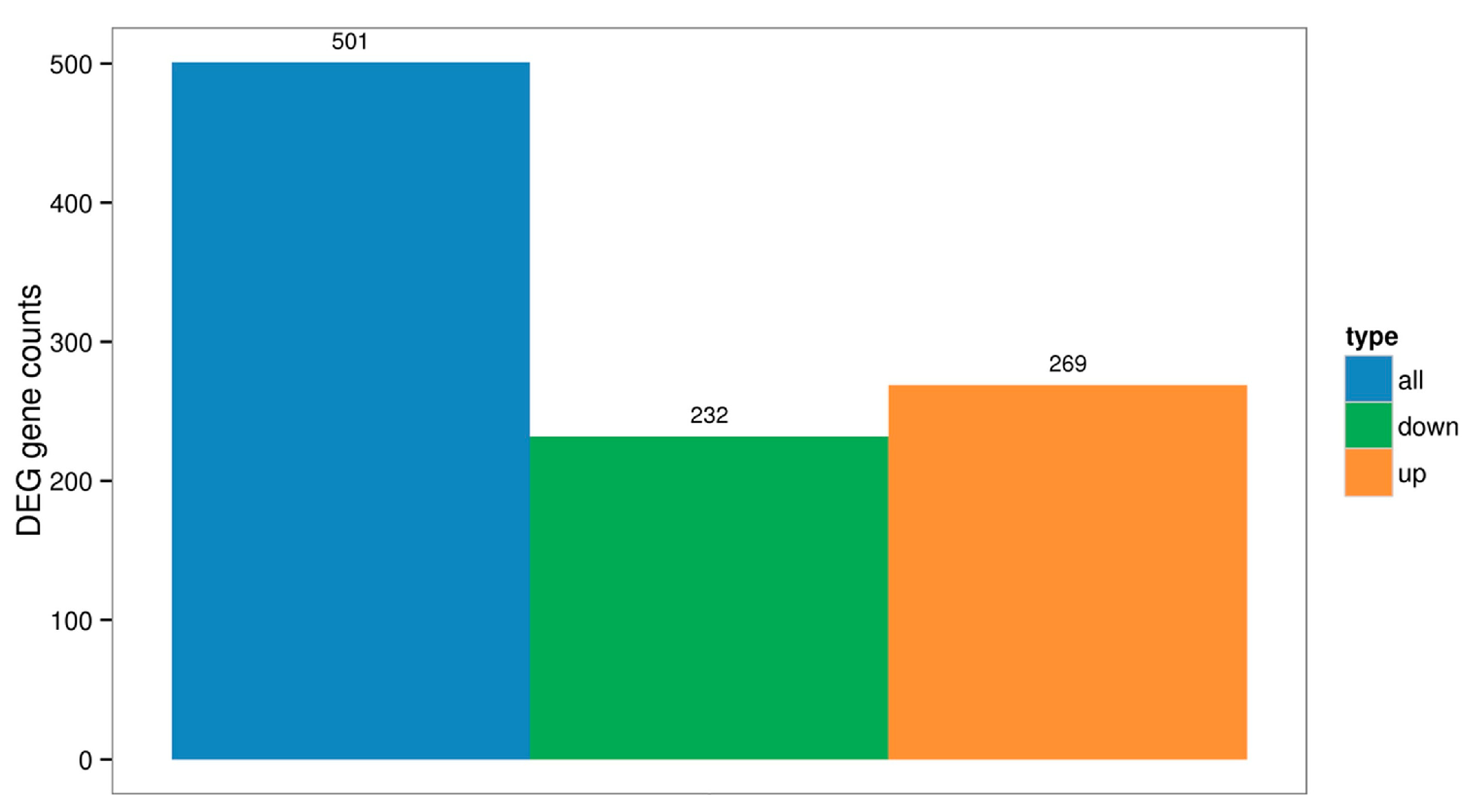
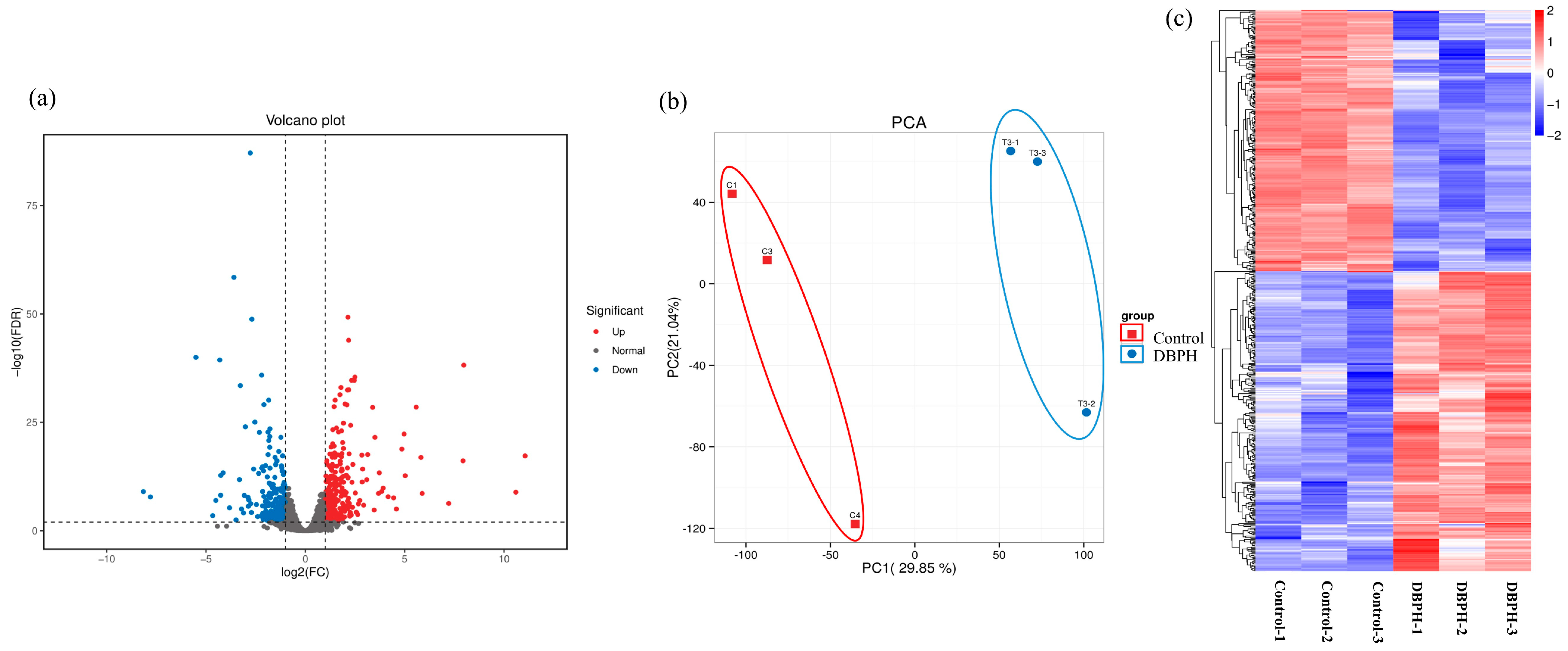
2.3. Differentially Expressed Genes
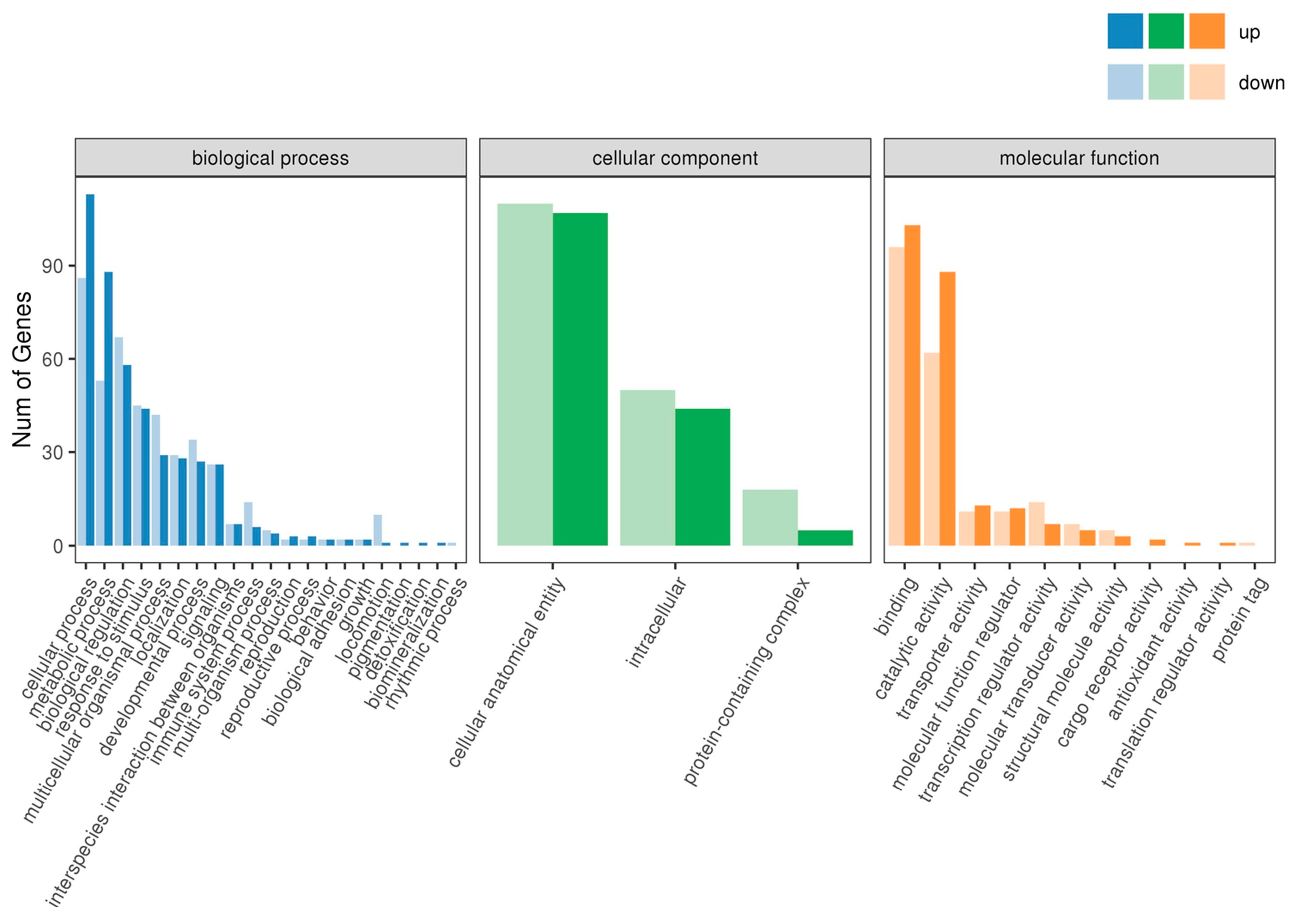
2.4. GO Enrichment Analysis of DEGs
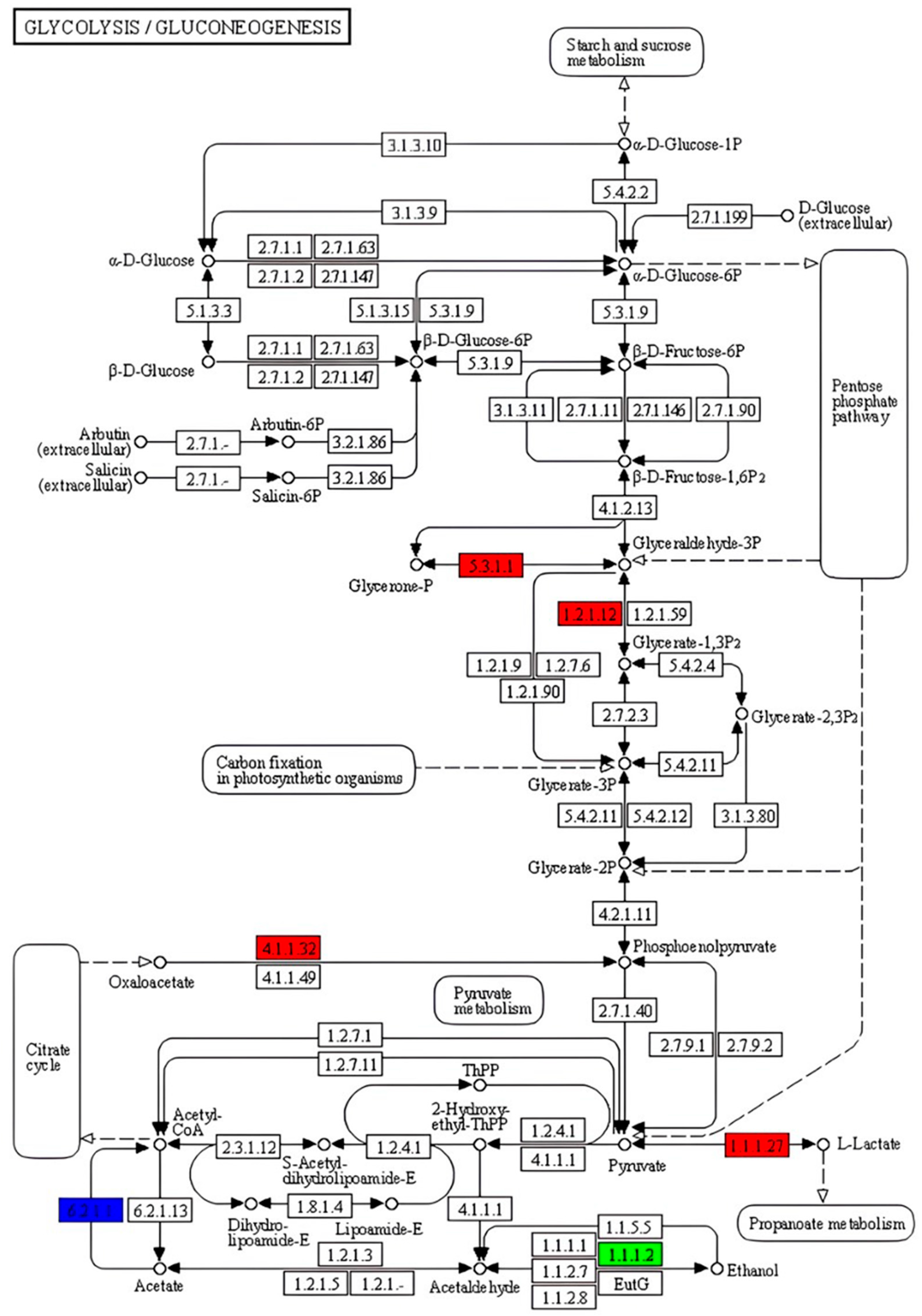
2.5. KEGG Enrichment Analysis of DEGs
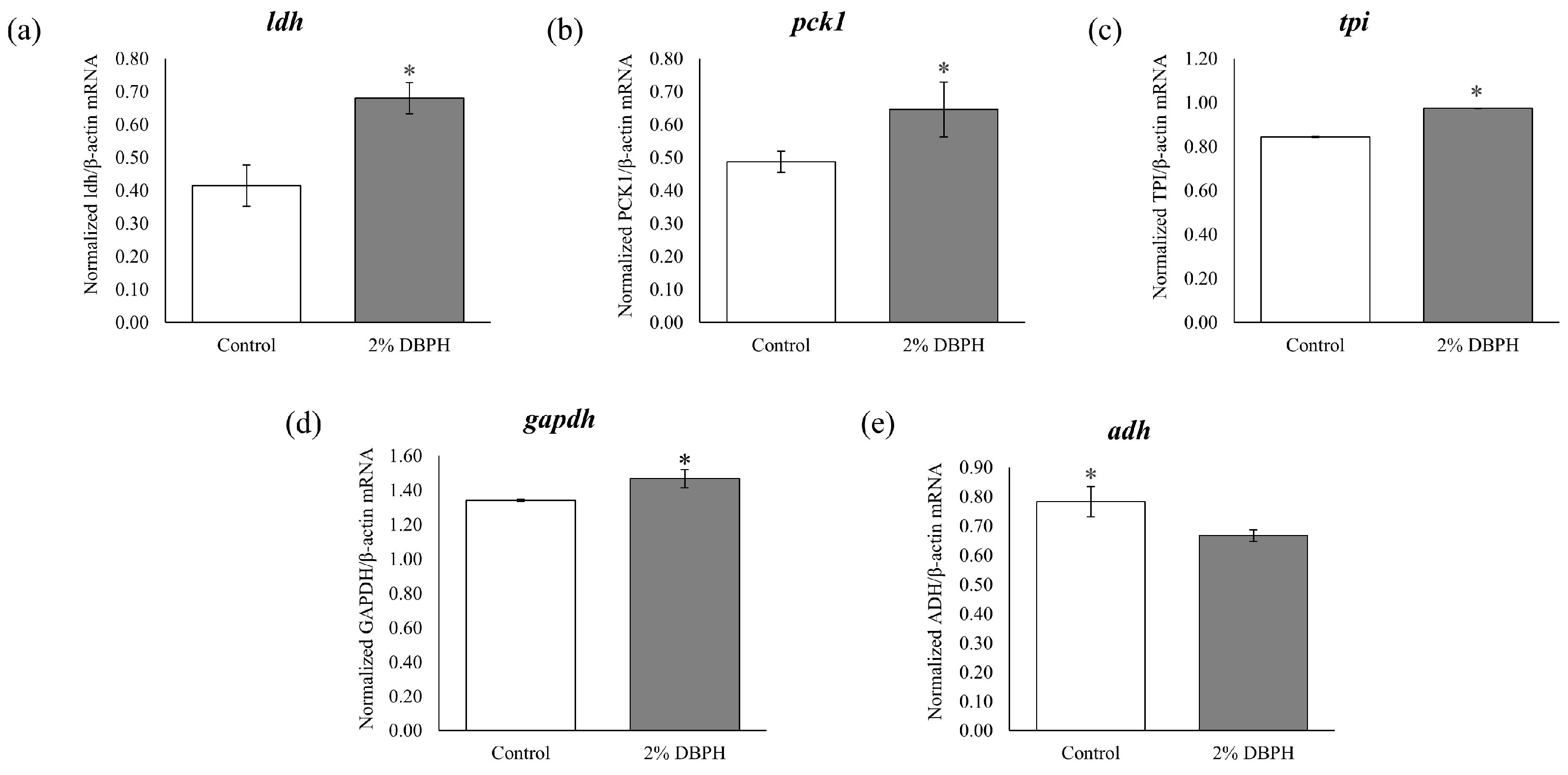
2.6. Validation of DEGs by qRT-PCR
2.7. Proximate Analysis of Muscle and Skin Color Measurement
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Diet Preparation
4.2. Assessment of Growth Performance
4.3. Total RNA Extraction
4.4. Library Preparation
4.5. Bioinformatics Analyses
4.6. Identification of DEGs and Functional Annotation
4.7. Validation of DEGs by qRT–PCR
4.8. Proximate Analysis
4.9. Assessment of Skin Coloration
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novák, J.; Kalous, L.; Patoka, J. Modern ornamental aquaculture in Europe: Early history of freshwater fish imports. Rev. Aquac. 2020, 12, 2042–2060. [Google Scholar] [CrossRef]
- Pountney, S.M. Survey indicates large proportion of fishkeeping hobbyists engaged in producing ornamental fish. Aquac. Rep. 2023, 29, 101503. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Maradonna, F.; Faheem, M.; Harikrishnan, R.; Devi, G.; Ringø, E.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Carnevali, O. Sustainable ornamental fish aquaculture: The implication of microbial feed additives. Animals 2023, 13, 1583. [Google Scholar] [CrossRef]
- Freshwater Ornamental Fish. Available online: https://oec.world/en/profile/hs/freshwater-ornamental-fish (accessed on 17 March 2025).
- Hilgers, L.; Herder, F.; Hadiaty, R.K.; Pfaender, J. Alien attack: Trophic interactions of flowerhorn cichlids with endemics of ancient Lake Matano (Sulawesi, Indonesia). Evol. Ecol. Res. 2018, 19, 561–574. [Google Scholar]
- Sari, D.W.K.; Achmad, H.; Rahman, H.; Bimasuci, H. Molecular identification of several morphologically distinct flowerhorn fish (family) using mitochondrial COI gene marker. J. Trop. Biodivers. Biotechnol. 2023, 8, 78459. [Google Scholar] [CrossRef]
- Sornsupharp, B.; Lomthaisong, K.; Dahms, H.; Sanoamuang, L. Effects of dried fairy shrimp Streptocephalus sirindhornae meal on pigmentation and carotenoid deposition in flowerhorn cichlid; Amphilophus citrinellus (Günther, 1864) × Cichlasoma trimaculatum (Günther, 1867). Aquac. Res. 2015, 46, 173–184. [Google Scholar] [CrossRef]
- Kaewda, J.; Sangsawad, P.; Boonanuntanasarn, S.; Manassila, P.; Boontawan, A.; Ketudat-Cairns, M.; Sriphuttha, C.; Nakharuthai, C. Effects of Probiotics Red Yeast Rhodotorula paludigena CM33 on Enhancing Color Pigmentation, Antioxidant Activity, Immune Response, Intestinal Microbiota, and Growth in a Commercial Ornamental Fish: Flowerhorn Fish. Aquac. Rep. 2025, 40, 102609. [Google Scholar] [CrossRef]
- Nico, L.; Beamish, W.H.; Musikasinthorn, P. Discovery of the invasive Mayan Cichlid fish “Cichlasoma” urophthalmus (Günther 1862) in Thailand, with comments on other introductions and potential impacts. Aquat. Invasions 2007, 2, 197–214. [Google Scholar] [CrossRef]
- Azimi, A.; Imanpoor, M.R.; Makeknejad, R.; Shokrollahi, S. Effects of natural (red bell pepper & tomato) and synthetic (astaxanthin & β-carotene) pigments on flower horn fish (Cichlasoma sp.) blood parameters. Int. J. Adv. Biol. Biom. Res. 2014, 2, 2761–2767. [Google Scholar]
- Joshi, O.; Chapagain, B.P.; Long, J.M.; York, B.; Taylor, A.T. Estimating the effects of fish quality and size on the economic value of fishing in Oklahoma streams and rivers: A revealed preference and contingent behavior approach. Fish. Res. 2021, 244, 106116. [Google Scholar] [CrossRef]
- Lim, L.C.; Dhert, P.; Sorgeloos, P. Recent developments in the application of live feeds in the freshwater ornamental fish culture. Aquaculture 2003, 227, 319–331. [Google Scholar] [CrossRef]
- Conceicao, L.E.C.; Yufera, M.; Makridis, P.; Morais, S.; Dinis, M.T. Live feeds for early stages of fish rearing. Rev. Aquac. 2010, 41, 613–640. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Kumar, B.D.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities, and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Bui, H.T.D.; Khosravi, S.; Fournier, V.; Herault, M.; Lee, K.J. Growth performance, feed utilization, innate immunity, and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquaculture 2014, 418–419, 11–16. [Google Scholar] [CrossRef]
- Robert, M.; Zatylny-Gaudin, C.; Fournier, V.; Corre, E.; Le Corguillé, G.; Bernay, B.; Henry, J. Molecular characterization of peptide fractions of a tilapia (Oreochromis niloticus) by-product hydrolysate and in vitro evaluation of antibacterial activity. Process Biochem. 2015, 50, 487–492. [Google Scholar] [CrossRef]
- Leduc, A.; Hervy, M.; Rangama, J.; Delépée, R.; Fournier, V.; Henry, J. Shrimp by-product hydrolysate induces intestinal myotropic activity in European seabass (Dicentrarchus labrax). Aquaculture 2018, 497, 380–388. [Google Scholar] [CrossRef]
- Sanches Alves, D.R.; de Oliveira, S.R.; Luczinski, T.G.; Guterres Paulo, I.P.; Boscolo, W.R.; Bittencourt, F.; Signor, A. Palatability of protein hydrolysates from industrial by-products for Nile tilapia juveniles. Animals 2019, 9, 311. [Google Scholar] [CrossRef]
- Velasco, C.; Resende, D.; Oliveira, B.; Canada, P.; Pereira, M.; Pereira, C.; Pintado, M.; Valente, L. Dietary inclusion of blood hydrolysates affects muscle growth in European seabass (Dicentrarchus labrax). Front. Mar. Sci. 2023, 10, 1193405. [Google Scholar] [CrossRef]
- Laosam, P.; Luasiri, P.; Nakharuthai, C.; Boonanuntanasarn, S.; Suwanangul, S.; Sarnthima, R.; Khammuang, S.; Sanachai, K.; Yongsawatdigul, J.; Rouabhia, M.; et al. Enzymatic hydrolysis of duck blood protein produces stable bioactive peptides: Pilot-scale production, identification, and stability during gastrointestinal and plasma digestion. Int. J. Biol. Macromol. 2024, 283, 137864, Erratum in Int. J. Biol. Macromol. 2025, 289, 138917. [Google Scholar] [CrossRef]
- Manassila, P.; Sangsawad, P.; Boonanuntanasarn, S.; Kaewda, J.; Boonchuen, P.; Limkul, S.; Nakharuthai, C. Effects of Low Molecular Weight Duck Blood Protein Hydrolysate as a Feed Additive on the Intestinal Microbiome, Antioxidant Activity, and Humoral Immune and Inflammatory Responses in Flowerhorn Fish. Aquac. Nutr. 2025, 2025, 9970984. [Google Scholar] [CrossRef]
- Paul, M.; Sardar, P.; Sahu, N.P.; Deo, A.D.; Varghese, T.; Shamna, N.; Jana, P.; Krishna, G. Effect of dietary protein level on growth and metabolism of GIFT juveniles reared in inland ground saline water of medium salinity. J. Appl. Aquacult. 2022, 35, 948–974. [Google Scholar] [CrossRef]
- Hermannsdottir, R.; Johannsdottir, J.; Smaradottir, H.; Sigurgisladottir, S.; Gudmundsdottir, B.; Bjornsdottir, R. Analysis of effects induced by a pollock protein hydrolysate on early development, innate immunity and the bacterial community structure of first feeding of Atlantic halibut (Hippoglossus hippoglossus L.) larvae. Fish Shellfish Immunol. 2009, 27, 595–602. [Google Scholar] [CrossRef]
- Siddik, M.A.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic fish protein hydrolysates in finfish aquaculture: A review. Rev. Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, D.; Li, J.; Zhang, Y.; Cui, Z.; Li, T.; Zheng, X.; Liu, H.; Wang, L.; Li, H. Assessment of fish protein hydrolysates in juvenile largemouth bass (Micropterus salmoides) diets: Effect on growth, intestinal antioxidant status, immunity, and microflora. Front. Nutr. 2022, 9, 816341. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.Q.; Ho, T.H.; Nan, F.H.; Liu, C.H.; Hu, Y.F.; Chong, C.M.; de Cruz, C.R.; Karim, M.; Liu, T.J.; Kuo, I.P.; et al. Assessment of fish protein hydrolysate as a substitute for fish meal in white shrimp diets: Impact on growth, immune response, and resistance against Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2024, 150, 109597. [Google Scholar] [CrossRef]
- Cai, Z.; Li, W.; Mai, K.; Xu, W.; Zhang, Y.; Ai, Q. Effects of dietary size-fractionated fish hydrolysates on growth, activities of digestive enzymes, and expression of protein metabolism genes in large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2015, 440, 40–47. [Google Scholar] [CrossRef]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.; Boirie, Y.; van Loon, L.J. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Turchini, G.M.; Xu, J.; Fang, Z.; Chen, N.; Xie, R.; Zhang, H.; Li, S. Functional properties of protein hydrolysates on growth, digestive enzyme activities, protein metabolism, and intestinal health of larval largemouth bass (Micropterus salmoides). Front. Immunol. 2022, 13, 913024. [Google Scholar] [CrossRef]
- Suma, A.Y.; Nandi, S.K.; Abdul Kari, Z.; Goh, K.W.; Wei, L.S.; Tahiluddin, A.B.; Seguin, P.; Herault, M.; Al Mamun, A.; Téllez-Isaías, G.; et al. Beneficial effects of graded levels of fish protein hydrolysate (FPH) on growth performance, blood biochemistry, liver and intestinal health, and disease resistance to Aeromonas hydrophila of Pabda (Ompok pabda) fingerling. Fishes 2023, 8, 147. [Google Scholar] [CrossRef]
- Ding, X.; Nie, X.; Yuan, C.; Jiang, L.; Ye, W.; Qian, L. Effects of dietary multienzyme complex supplementation on growth performance, digestive capacity, histomorphology, blood metabolites, and hepatic glycometabolism in snakehead (Channa argus). Animals 2022, 12, 380. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Y.; Guo, J.; Yang, H.; Chen, F.; Zhang, W.; Wu, W.; Xu, X.; Li, J. Glycolysis and gluconeogenesis are involved of glucose metabolism adaptation during fasting and re-feeding in black carp (Mylopharyngodon piceus). Aquac. Fish. 2004, 9, 226–233. [Google Scholar] [CrossRef]
- Panserat, S.; Kolditz, C.; Richard, N.; Plagnes-Juan, E.; Piumi, F.; Esquerré, D.; Médale, F.; Corraze, G.; Kaushik, S. Hepatic gene expression profiles in juvenile rainbow trout (Oncorhynchus mykiss) fed fishmeal or fish oil-free diets. Br. J. Nutr. 2008, 100, 953–967. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Jangprai, A.; Kumkhong, S.; Plagnes Juan, E.; Veron, V.; Burel, C. Marandel. L.; Panserat, S. Adaptation of Nile tilapia (Oreochromis niloticus) to different levels of dietary carbohydrates: New insights from a long-term nutritional study. Aquaculture 2018, 496, 58–65. [Google Scholar] [CrossRef]
- Wei, B.; Yang, Z.; Cheng, Y.; Wang, J.; Zhou, J. Effects of the complete replacement of fish oil with linseed oil on growth, fatty acid composition, and protein expression in the Chinese mitten crab (Eriocheir sinensis). Proteome Sci. 2018, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, K.; Prema, P.; Veeramani, V.; Kumar, K.R.; Nguyen, V.H.; Marraiki, N.; Zaghloul, N.S.; Balaji, P. Toxicity evaluation and oxidative stress response of fumaronitrile, a persistent organic pollutant (POP) of industrial wastewater on tilapia fish (Oreochromis mossambicus). Environ. Res. 2021, 204, 112030. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wu, J.; Zhang, Y.; Zhuang, W.; Liang, X.F. Role of phosphoenolpyruvate carboxykinase 1 (pck1) in mediating nutrient metabolism in zebrafish. Funct. Integr. Genom. 2023, 23, 67. [Google Scholar] [CrossRef]
- Dasmahapatra, A.K.; Doucet, H.L.; Bhattacharyya, C.; Carvan, M.J. Developmental expression of alcohol dehydrogenase (ADH3) in zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2001, 286, 1082–1086. [Google Scholar] [CrossRef]
- Reindl, K.M.; Sheridan, M.A. Peripheral Regulation of the Growth Hormone-Insulin-like Growth Factor System in Fish and Other Vertebrates. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 231–245. [Google Scholar] [CrossRef]
- Rutegwa, M.; Potužák, J.; Hejzlar, J.; Drozd, B. Carbon Metabolism and Nutrient Balance in a Hypereutrophic Semi-Intensive Fishpond. Knowl. Manag. Aquat. Ecosyst. 2019, 420, 49. [Google Scholar] [CrossRef]
- Olopade, O.A.; Taiwo, I.O.; Lamidi, A.A.; Awonaike, O.A. Proximate composition of Nile tilapia (Oreochromis niloticus) (Linnaeus, 1758) and tilapia hybrid (Red Tilapia) from Oyan Lake, Nigeria. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 19–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakharuthai, C.; Sreebun, S.; Kabpha, A.; Phuong, T.V.; Boonanuntanasarn, S. Characterization of ddx4 and dnd Homologs in Snakeskin Gourami (Trichopodus pectoralis) and Their Expression Levels during Larval Development and in Gonads of Males and Females. Animals 2022, 12, 3415. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]

| Parameters | Diets | |||
|---|---|---|---|---|
| Control | 0.5% DBPH | 1% DBPH | 2% DBPH | |
| FW (g) | 26.16 ± 0.53 a | 26.39 ± 0.47 a | 29.39 ± 0.24 b | 29.47 ± 0.32 b |
| FL (cm) | 8.38 ± 0.47 a | 9.70 ± 0.33 b | 10.93 ± 0.15 b | 10.87 ± 0.14 b |
| WG (g) | 22.92 ± 0.53 a | 23.15 ± 0.46 a | 26.15 ± 0.24 b | 26.23 ± 0.32 b |
| FCR | 3.03 ± 0.07 b | 2.97 ± 0.06 b | 2.74 ± 0.02 a | 2.67 ± 0.03 a |
| ADG (g day−1) | 0.25 ± 0.01 a | 0.26 ± 0.01 a | 0.29 ± 0.00 b | 0.29 ± 0.00 b |
| SGR (% day−1) | 0.25 ± 0.02 a | 0.26 ± 0.02 a | 0.29 ± 0.01 b | 0.29 ± 0.01 b |
| Treatment | Clean Reads | Clean Base | GC Content (%) | % ≥Q30 |
|---|---|---|---|---|
| Control1 | 20,334,724 | 6,092,042,946 | 49.72 | 94.40 |
| Control2 | 21,970,953 | 6,582,712,088 | 49.90 | 94.43 |
| Control3 | 22,642,113 | 6,783,876,573 | 50.04 | 94.23 |
| 2%DBPH1 | 21,972,991 | 6,583,108,021 | 49.64 | 94.65 |
| 2%DBPH2 | 22,239,091 | 6,662,959,823 | 49.76 | 94.67 |
| 2%DBPH3 | 23,773,994 | 7,121,481,778 | 49.56 | 94.86 |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | 0.5% DBPH | 1% DBPH | 2% DBPH | |
| L* | 37.96 ± 2.43 | 36.14 ± 2.07 | 41.67 ± 1.60 | 42.29 ± 1.01 |
| a* | 9.65 ± 1.01 | 9.79 ± 1.78 | 10.43 ± 0.18 | 11.73 ± 1.00 |
| b* | 10.37 ± 2.58 | 10.74 ± 3.41 | 9.99 ± 0.37 | 9.58 ± 2.05 |
| Crude protein (%) | 43.65 ± 1.26 | 45.22 ± 0.47 | 47.23 ± 1.30 | 47.64 ± 1.02 |
| Crude lipid (%) | 7.22 ± 0.52 | 5.20 ± 0.50 | 6.66 ± 0.52 | 5.90 ± 0.32 |
| Moisture (%) | 71.34 ± 2.30 | 72.34 ± 2.57 | 71.16 ± 2.30 | 72.54 ± 1.34 |
| Ash (%) | 13.08 ± 1.07 | 17.04 ± 1.29 | 14.29 ± 0.33 | 18.87 ± 1.15 |
| Other compounds (%) | 10.33 ± 0.50 | 8.49 ± 0.45 | 9.18 ± 0.44 | 7.58 ± 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manassila, P.; Sangsawad, P.; Boonanuntanasarn, S.; Kaewda, J.; Boonchuen, P.; Limkul, S.; Nakharuthai, C. Transcriptomic Analysis Reveals Glycolysis and Gluconeogenesis Pathway Activation Underlying Growth Enhancement by Duck-Blood Protein Hydrolysate in Flowerhorn Cichlid Fish. Int. J. Mol. Sci. 2025, 26, 9563. https://doi.org/10.3390/ijms26199563
Manassila P, Sangsawad P, Boonanuntanasarn S, Kaewda J, Boonchuen P, Limkul S, Nakharuthai C. Transcriptomic Analysis Reveals Glycolysis and Gluconeogenesis Pathway Activation Underlying Growth Enhancement by Duck-Blood Protein Hydrolysate in Flowerhorn Cichlid Fish. International Journal of Molecular Sciences. 2025; 26(19):9563. https://doi.org/10.3390/ijms26199563
Chicago/Turabian StyleManassila, Pimpisut, Papungkorn Sangsawad, Surintorn Boonanuntanasarn, Jirawadee Kaewda, Pakpoom Boonchuen, Sirawich Limkul, and Chatsirin Nakharuthai. 2025. "Transcriptomic Analysis Reveals Glycolysis and Gluconeogenesis Pathway Activation Underlying Growth Enhancement by Duck-Blood Protein Hydrolysate in Flowerhorn Cichlid Fish" International Journal of Molecular Sciences 26, no. 19: 9563. https://doi.org/10.3390/ijms26199563
APA StyleManassila, P., Sangsawad, P., Boonanuntanasarn, S., Kaewda, J., Boonchuen, P., Limkul, S., & Nakharuthai, C. (2025). Transcriptomic Analysis Reveals Glycolysis and Gluconeogenesis Pathway Activation Underlying Growth Enhancement by Duck-Blood Protein Hydrolysate in Flowerhorn Cichlid Fish. International Journal of Molecular Sciences, 26(19), 9563. https://doi.org/10.3390/ijms26199563







