Analysis of Genomic and Transcriptomic Data Revealed Key Genes and Processes in the Development of Major Depressive Disorder
Abstract
1. Introduction
2. Results
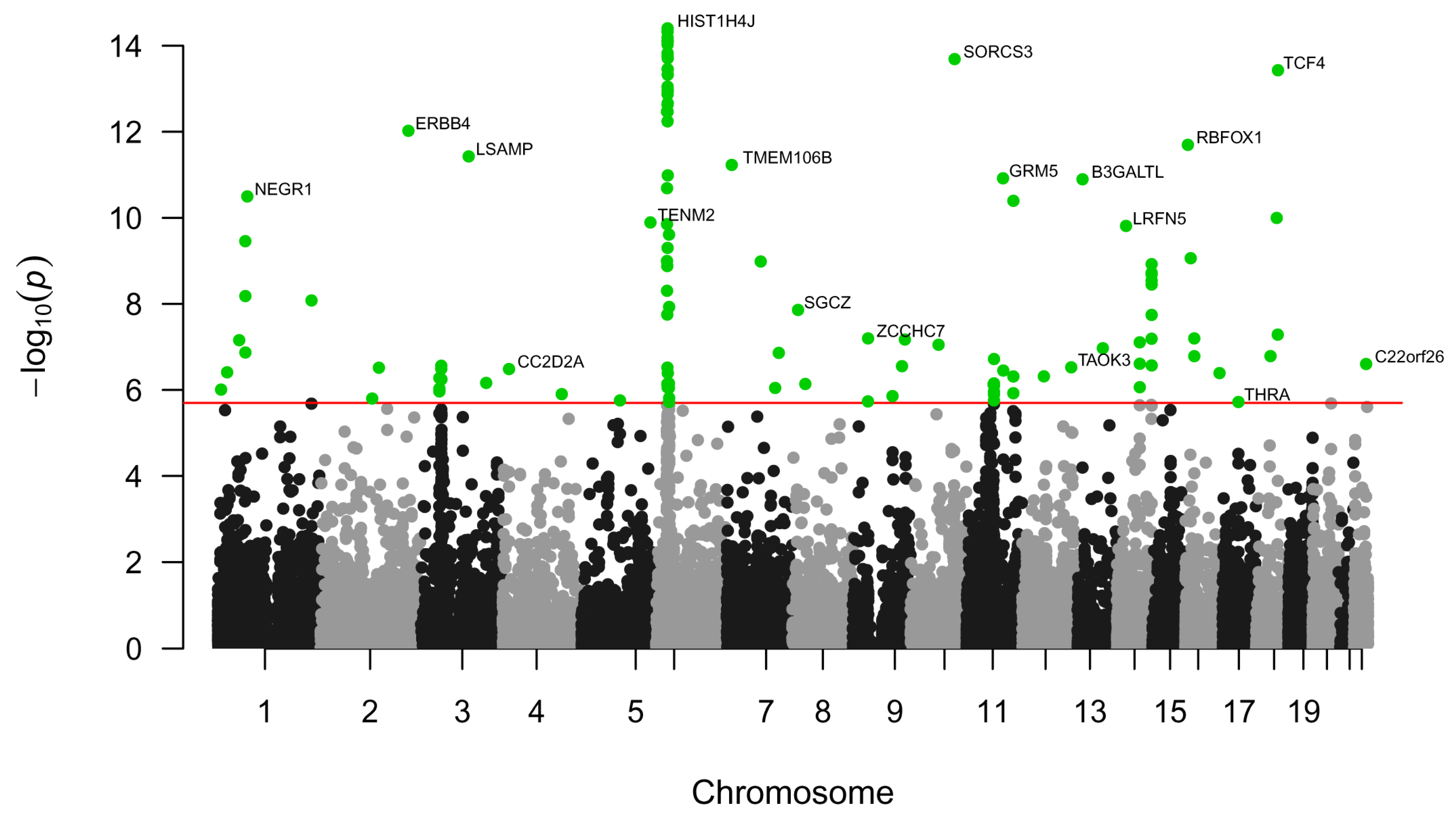
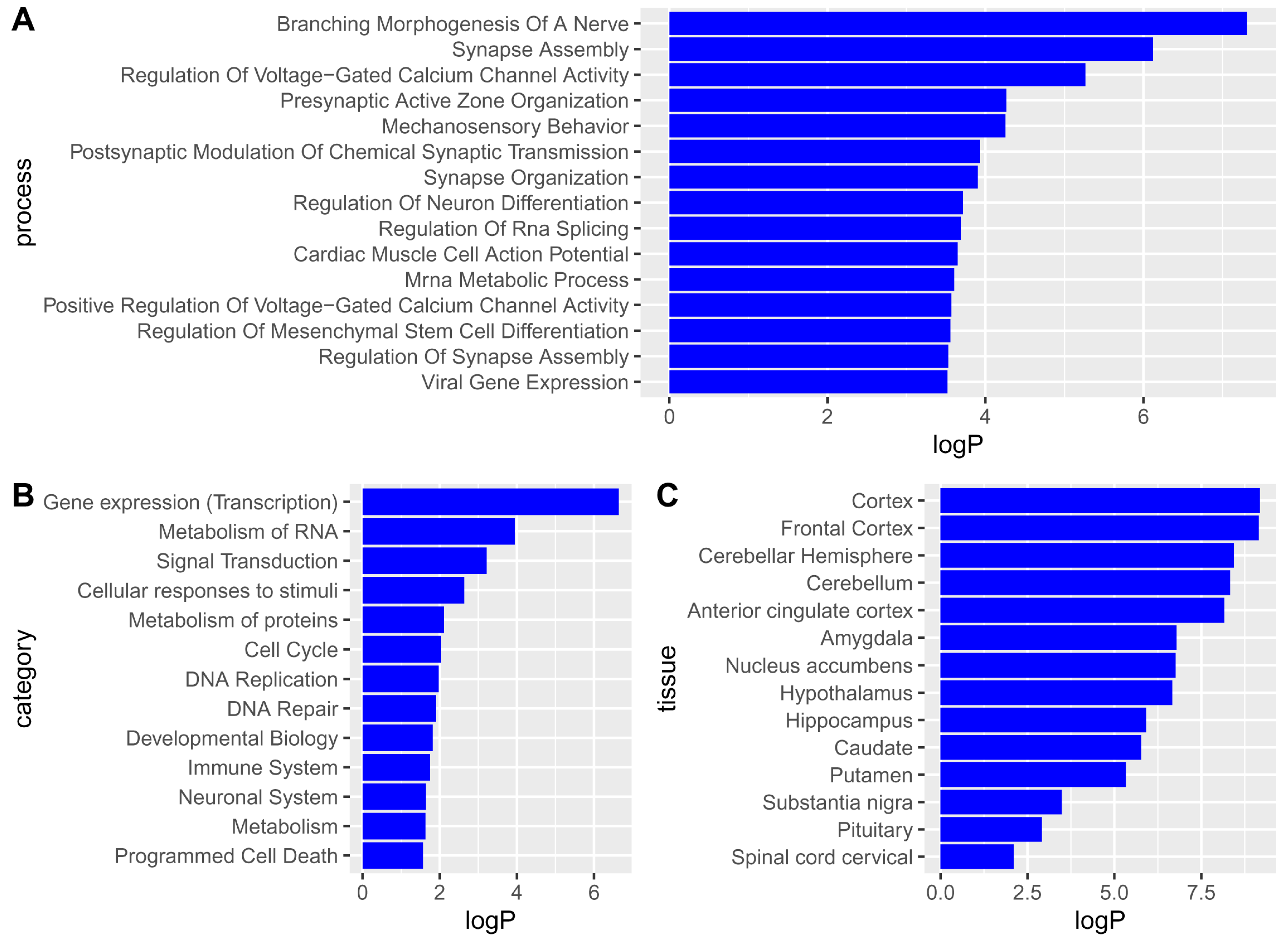
2.1. Single-Nucleotide Polymorphisms, Genes, Pathways, and Tissues Associated with Major Depressive Disorder
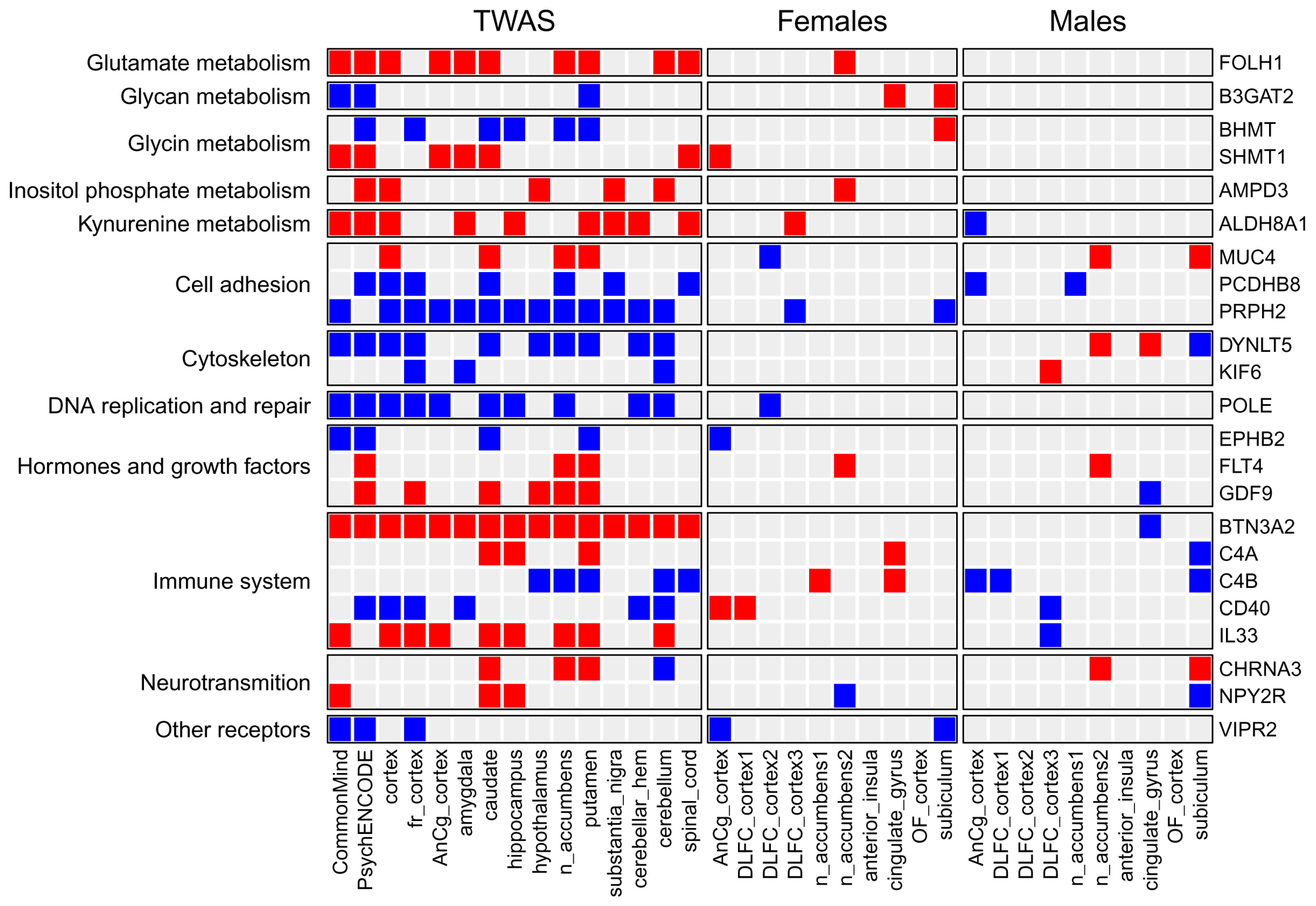
2.2. Overview of Results from Transcriptome-Wide Association Study
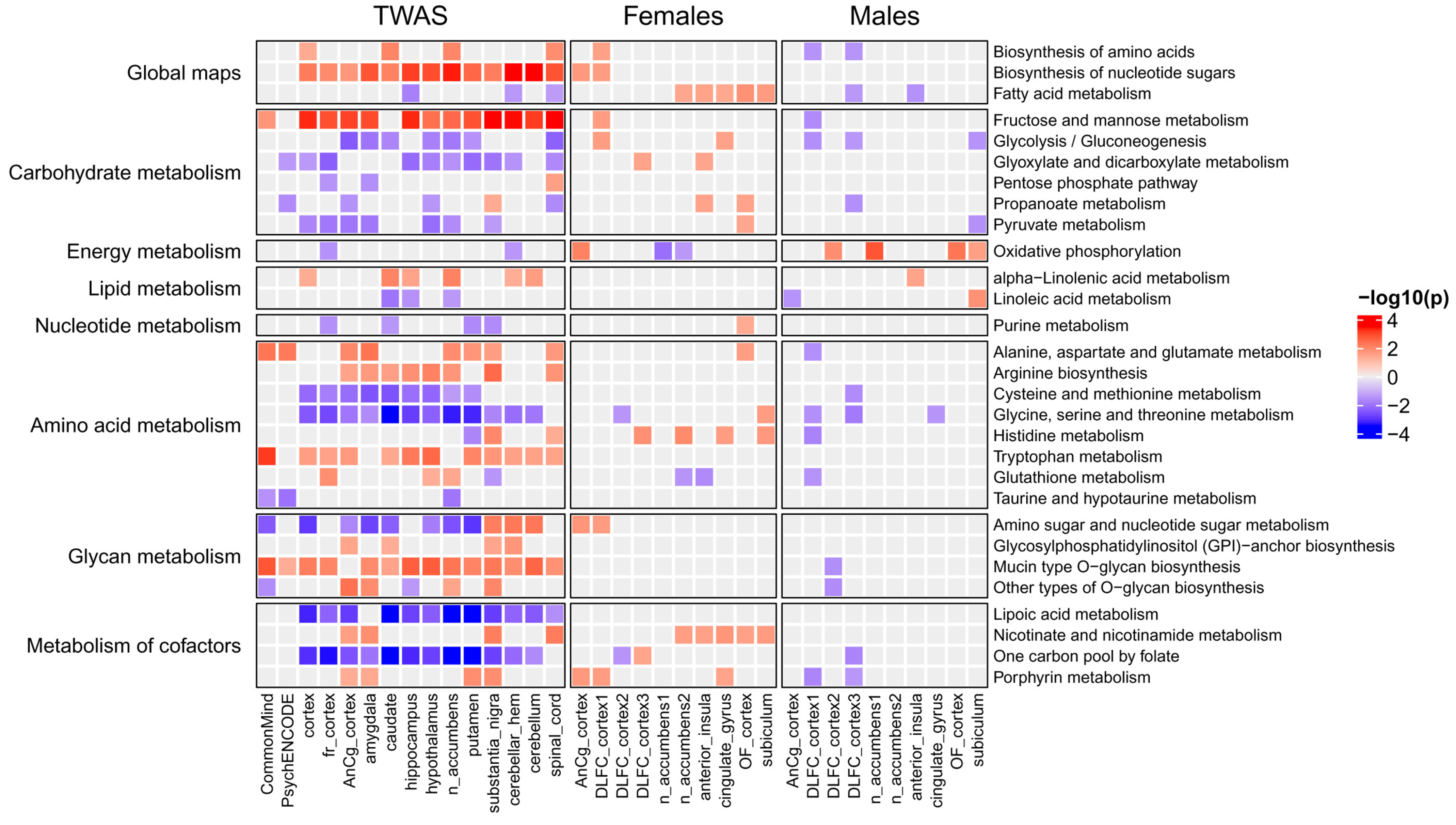
2.3. Metabolic Pathways Associated with Major Depressive Disorder
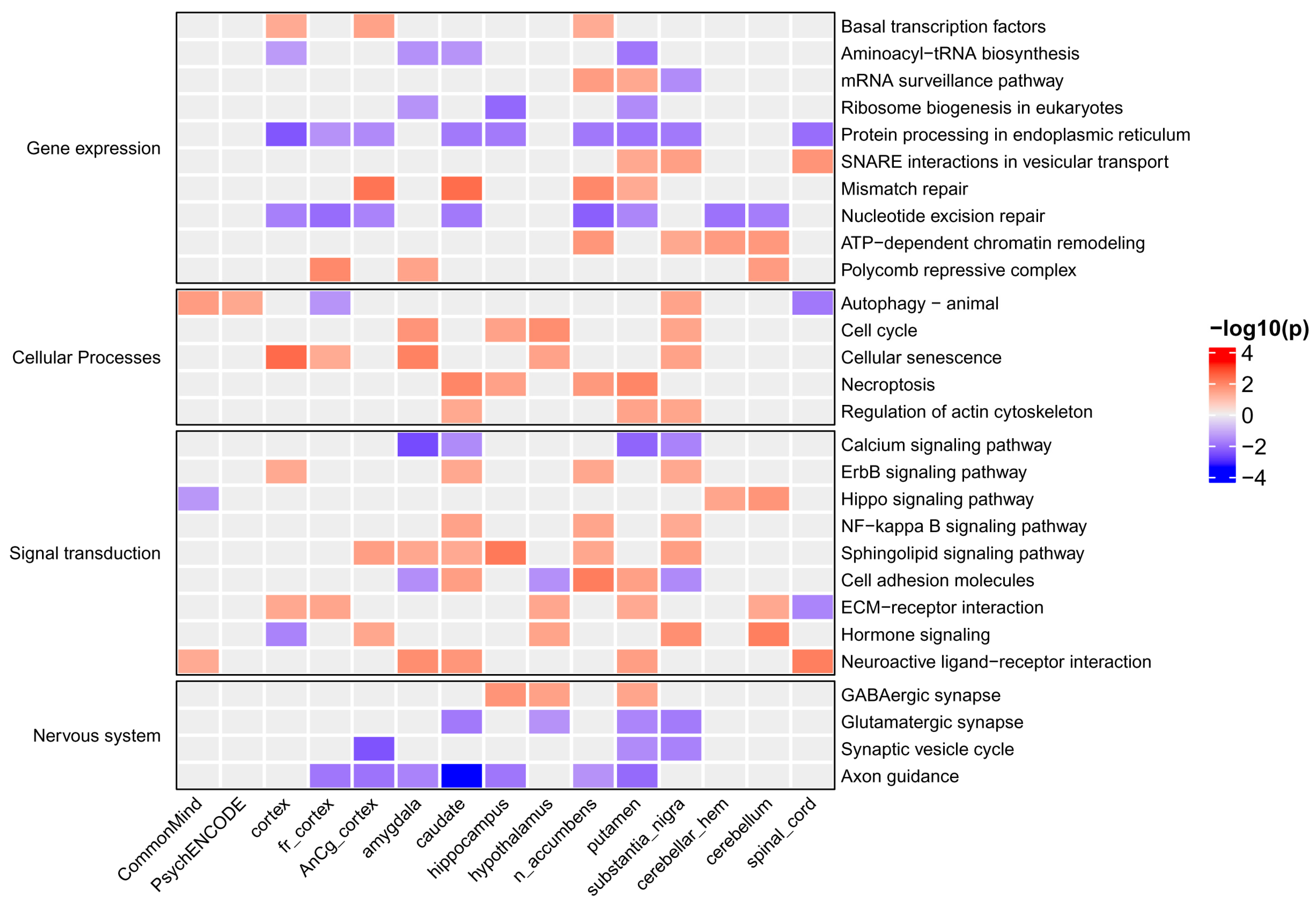
2.4. Signaling Pathways and Cellular Processes Associated with Major Depressive Disorder
2.5. Differentially Expressed Genes and Associated Pathways in Post-Mortem Brain Tissues Revealed by Transcriptomic Analysis
2.6. Identification of Master Regulators Responsible for the Observed Gene Expression Changes
3. Discussion
3.1. Single-Nucleotide Polymorphisms Associated with Major Depressive Disorder and Their Mapping to Genes, Pathways, and Tissues
3.2. Genes, Pathways, and Processes Identified by TWAS and Associated with Major Depressive Disorder
3.2.1. Genes and Processes Associated with Brain Metabolism
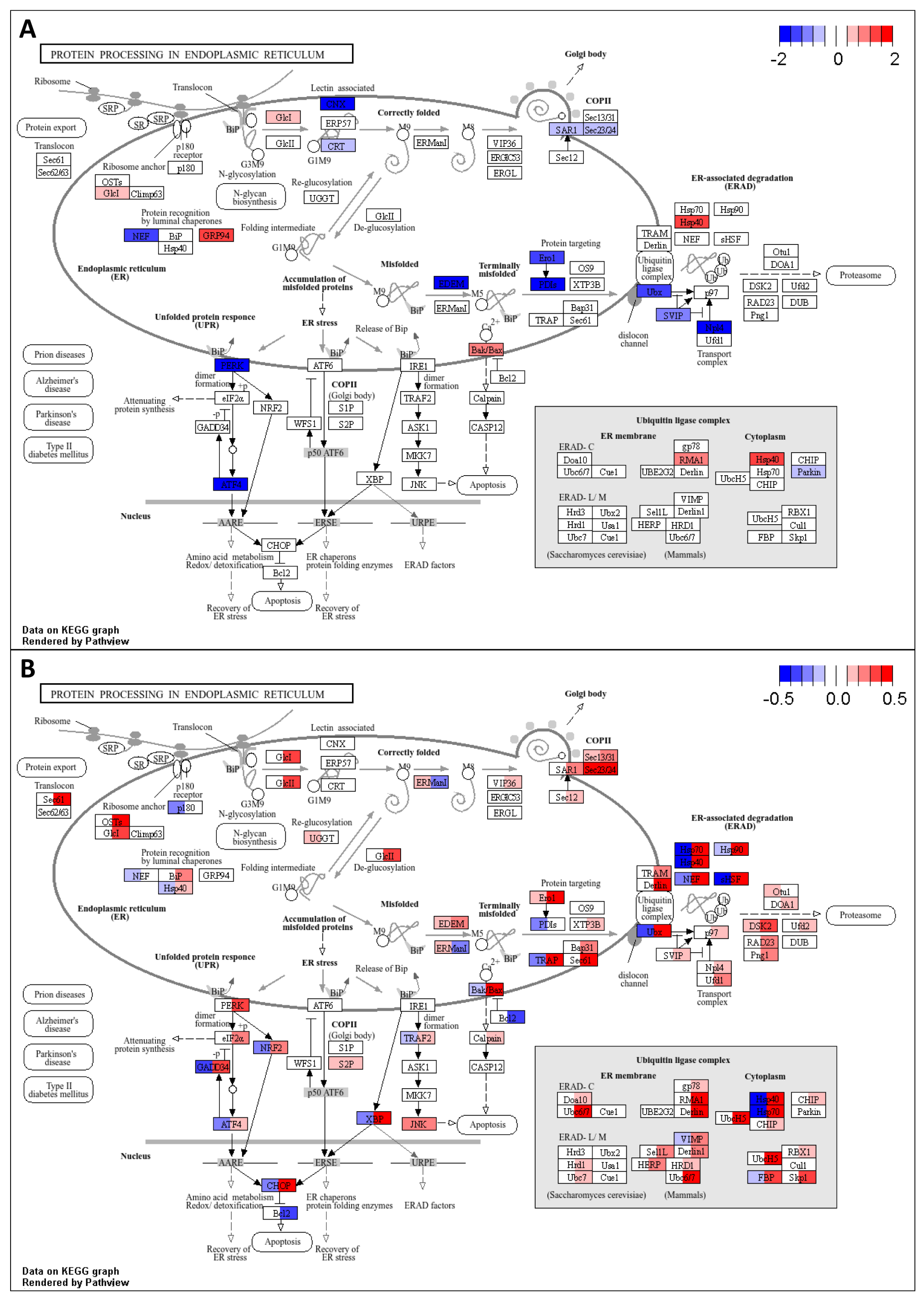
3.2.2. Genes and Processes Associated with Gene Expression and Cellular Functions
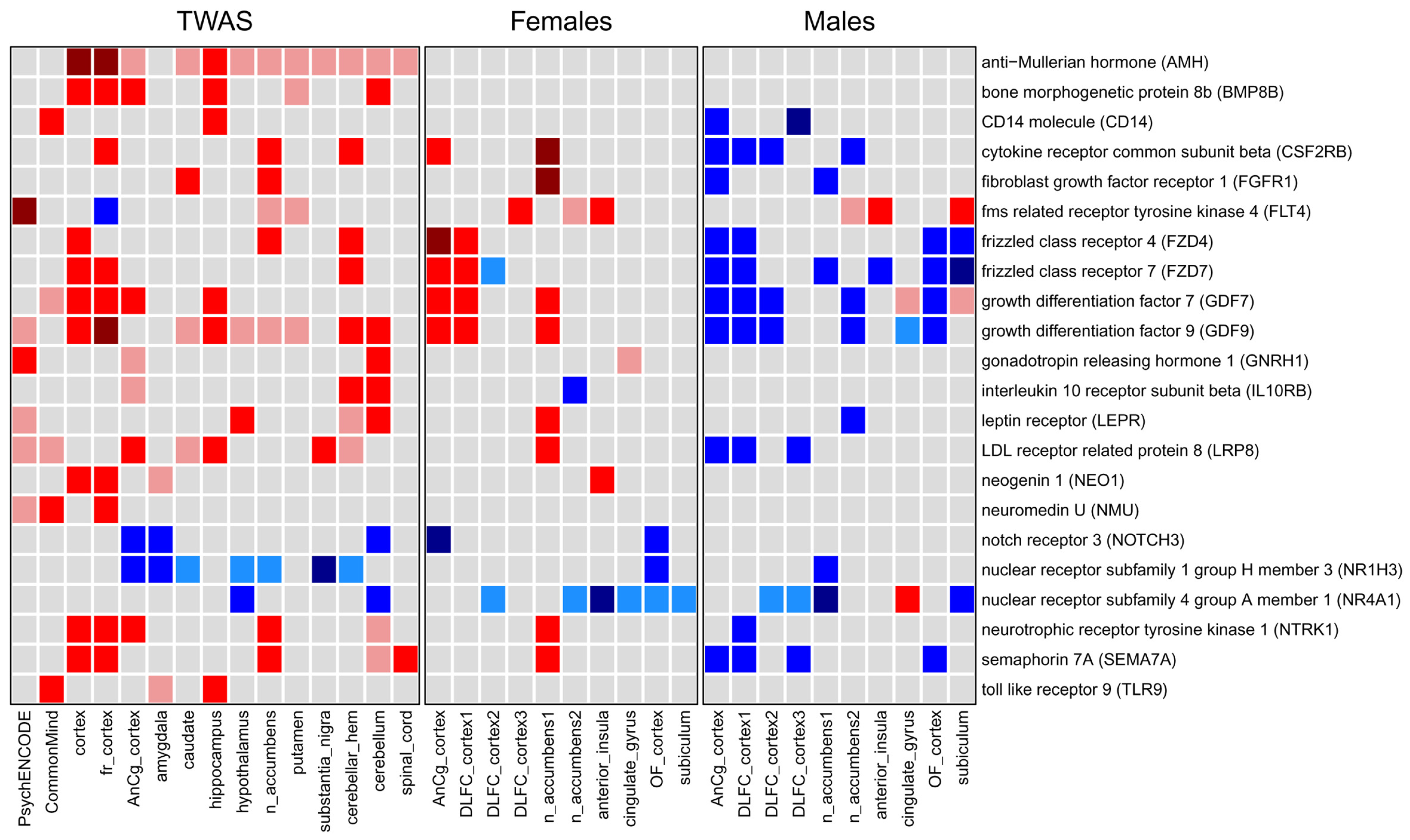
3.2.3. Genes Involved in Signaling Pathways
3.2.4. Genes Involved in Immune Response and Inflammation
3.3. The Role of Identified Genes and Processes in Other Diseases
3.4. Comparison of Genes and Pathways Obtained by TWAS with Genes and Pathways Identified by Differential Expression Analysis in Post-Mortem Brain Regions
3.5. Master Regulators Potentially Responsible for Observed Gene Expression Changes in Major Depressive Disorder
4. Materials and Methods
4.1. Mapping Single-Nucleotide Polymorphisms Associated with Major Depressive Disorder to Human Genes
4.2. Transcriptome-Wide Association Study
4.3. Identification of Differentially Expressed Genes and Pathways in Post-Mortem Brain Tissues
4.4. Identification of Master Regulators Based on TWAS Results and Transcriptomic Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major Depressive Disorder. Nat. Rev. Dis. Primers 2023, 9, 44. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- World Health Organization. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 10 July 2025).
- Caldiroli, A.; Capuzzi, E.; Tagliabue, I.; Capellazzi, M.; Marcatili, M.; Mucci, F.; Colmegna, F.; Clerici, M.; Buoli, M.; Dakanalis, A. Augmentative Pharmacological Strategies in Treatment-Resistant Major Depression: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 13070. [Google Scholar] [CrossRef]
- Shadrina, M.; Bondarenko, E.A.; Slominsky, P.A. Genetics Factors in Major Depression Disease. Front. Psychiatry 2018, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Wittenberg, G.M.; Bullmore, E.T.; Manji, H.K. Immune Targets for Therapeutic Development in Depression: Towards Precision Medicine. Nat. Rev. Drug Discov. 2022, 21, 224–244. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular Pathways of Major Depressive Disorder Converge on the Synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Mora, F.; Rodríguez-Jiménez, R.; Fernandez-Rojo, S.; et al. Nutrition, Epigenetics, and Major Depressive Disorder: Understanding the Connection. Front. Nutr. 2022, 9, 867150. [Google Scholar] [CrossRef]
- Patil, C.R.; Suryakant Gawli, C.; Bhatt, S. Targeting Inflammatory Pathways for Treatment of the Major Depressive Disorder. Drug Discov. Today 2023, 28, 103697. [Google Scholar] [CrossRef]
- Tsugiyama, L.E.; Moraes, R.C.M.; Moraes, Y.A.C.; Francis-Oliveira, J. Promising New Pharmacological Targets for Depression: The Search for Efficacy. Drug Discov. Today 2023, 28, 103804. [Google Scholar] [CrossRef]
- Dai, T.-T.; Wang, B.; Xiao, Z.-Y.; You, Y.; Tian, S.-W. Apelin-13 Upregulates BDNF Against Chronic Stress-Induced Depression-like Phenotypes by Ameliorating HPA Axis and Hippocampal Glucocorticoid Receptor Dysfunctions. Neuroscience 2018, 390, 151–159. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.-B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Maydych, V. The Interplay Between Stress, Inflammation, and Emotional Attention: Relevance for Depression. Front. Neurosci. 2019, 13, 384. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.-K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-Wide Association Study of Depression Phenotypes in UK Biobank Identifies Variants in Excitatory Synaptic Pathways. Nat. Commun. 2018, 9, 1470, Erratum in Nat. Commun. 2021, 12, 2012. [Google Scholar] [CrossRef]
- Hyde, C.L.; Nagle, M.W.; Tian, C.; Chen, X.; Paciga, S.A.; Wendland, J.R.; Tung, J.Y.; Hinds, D.A.; Perlis, R.H.; Winslow, A.R. Identification of 15 Genetic Loci Associated with Risk of Major Depression in Individuals of European Descent. Nat. Genet. 2016, 48, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Levey, D.F.; Stein, M.B.; Wendt, F.R.; Pathak, G.A.; Zhou, H.; Aslan, M.; Quaden, R.; Harrington, K.M.; Nuñez, Y.Z.; Overstreet, C.; et al. Bi-Ancestral Depression GWAS in the Million Veteran Program and Meta-Analysis in >1.2 Million Individuals Highlight New Therapeutic Directions. Nat. Neurosci. 2021, 24, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Navoly, G.; Giannakopoulou, O.; Levey, D.F.; Koller, D.; Pathak, G.A.; Koen, N.; Lin, K.; Adams, M.J.; Rentería, M.E.; et al. Multi-Ancestry Genome-Wide Association Study of Major Depression Aids Locus Discovery, Fine Mapping, Gene Prioritization and Causal Inference. Nat. Genet. 2024, 56, 222–233. [Google Scholar] [CrossRef]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-Wide Association Analyses Identify 44 Risk Variants and Refine the Genetic Architecture of Major Depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.-K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-Wide Meta-Analysis of Depression Identifies 102 Independent Variants and Highlights the Importance of the Prefrontal Brain Regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef]
- Dall’Aglio, L.; Lewis, C.M.; Pain, O. Delineating the Genetic Component of Gene Expression in Major Depression. Biol. Psychiatry 2021, 89, 627–636. [Google Scholar] [CrossRef]
- Fabbri, C.; Pain, O.; Hagenaars, S.P.; Lewis, C.M.; Serretti, A. Transcriptome-Wide Association Study of Treatment-Resistant Depression and Depression Subtypes for Drug Repurposing. Neuropsychopharmacology 2021, 46, 1821–1829. [Google Scholar] [CrossRef]
- Gaspar, H.A.; Gerring, Z.; Hübel, C.; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; Middeldorp, C.M.; Derks, E.M.; Breen, G. Using Genetic Drug-Target Networks to Develop New Drug Hypotheses for Major Depressive Disorder. Transl. Psychiatry 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, X.; Liu, J.; Li, H.; Li, M.; The 23andMe Research Team; Li, W.; Luo, X.-J. Transcriptome-Wide Association Study Identifies New Susceptibility Genes and Pathways for Depression. Transl. Psychiatry 2021, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Pain, O.; Hodgson, K.; Trubetskoy, V.; Ripke, S.; Marshe, V.S.; Adams, M.J.; Byrne, E.M.; Campos, A.I.; Carrillo-Roa, T.; Cattaneo, A.; et al. Identifying the Common Genetic Basis of Antidepressant Response. Biol. Psychiatry Glob. Open Sci. 2022, 2, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Su, Y.; O’Donnell, K.; Caron, J.; Meaney, M.; Meng, X.; Li, Y. Differential Interactions between Gene Expressions and Stressors across the Lifespan in Major Depressive Disorder. J. Affect. Disord. 2024, 362, 688–697. [Google Scholar] [CrossRef]
- Yu, S.; Lin, Y.; Yang, Y.; Jin, X.; Liao, B.; Lu, D.; Huang, J. Shared Genetic Effect of Kidney Function on Bipolar and Major Depressive Disorders: A Large-Scale Genome-Wide Cross-Trait Analysis. Hum. Genom. 2024, 18, 60. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Zhang, H.; Li, C.; Chen, Y.; Zhang, J.; Pan, C.; Cheng, S.; Yang, X.; Meng, P.; et al. The Genetic Structure of Pain in Depression Patients: A Genome-Wide Association Study and Proteome-Wide Association Study. J. Psychiatr. Res. 2022, 156, 547–556. [Google Scholar] [CrossRef]
- Zeng, L.; Fujita, M.; Gao, Z.; White, C.C.; Green, G.S.; Habib, N.; Menon, V.; Bennett, D.A.; Boyle, P.; Klein, H.-U.; et al. A Single-Nucleus Transcriptome-Wide Association Study Implicates Novel Genes in Depression Pathogenesis. Biol. Psychiatry 2024, 96, 34–43. [Google Scholar] [CrossRef]
- Chang, L.-C.; Jamain, S.; Lin, C.-W.; Rujescu, D.; Tseng, G.C.; Sibille, E. A Conserved BDNF, Glutamate- and GABA-Enriched Gene Module Related to Human Depression Identified by Coexpression Meta-Analysis and DNA Variant Genome-Wide Association Studies. PLoS ONE 2014, 9, e90980. [Google Scholar] [CrossRef]
- Hagenauer, M.H.; Schulmann, A.; Li, J.Z.; Vawter, M.P.; Walsh, D.M.; Thompson, R.C.; Turner, C.A.; Bunney, W.E.; Myers, R.M.; Barchas, J.D.; et al. Inference of Cell Type Content from Human Brain Transcriptomic Datasets Illuminates the Effects of Age, Manner of Death, Dissection, and Psychiatric Diagnosis. PLoS ONE 2018, 13, e0200003. [Google Scholar] [CrossRef]
- Labonté, B.; Engmann, O.; Purushothaman, I.; Menard, C.; Wang, J.; Tan, C.; Scarpa, J.R.; Moy, G.; Loh, Y.-H.E.; Cahill, M.; et al. Sex-Specific Transcriptional Signatures in Human Depression. Nat. Med. 2017, 23, 1102–1111, Erratum in Nat. Med. 2018, 24, 525. [Google Scholar] [CrossRef]
- Lanz, T.A.; Reinhart, V.; Sheehan, M.J.; Rizzo, S.J.S.; Bove, S.E.; James, L.C.; Volfson, D.; Lewis, D.A.; Kleiman, R.J. Postmortem Transcriptional Profiling Reveals Widespread Increase in Inflammation in Schizophrenia: A Comparison of Prefrontal Cortex, Striatum, and Hippocampus among Matched Tetrads of Controls with Subjects Diagnosed with Schizophrenia, Bipolar or Major Depressive Disorder. Transl. Psychiatry 2019, 9, 151. [Google Scholar] [CrossRef]
- Ma, Y.; Ming, Y.; Hou, Z.; Yu, Y.; Liu, J.; Wang, Z. Deciphering the Overlapping Immune Mechanism Between Depression and Breast Cancer. Int. J. Mol. Sci. 2025, 26, 5229. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Pessoni, A.M.; Marroquín-Rivera, A.; Parise, E.M.; Tamminga, C.A.; Turecki, G.; Nestler, E.J.; Chen, T.-H.; Labonté, B. Transcriptional Dissection of Symptomatic Profiles across the Brain of Men and Women with Depression. Nat. Commun. 2023, 14, 6835. [Google Scholar] [CrossRef] [PubMed]
- Pantazatos, S.P.; Huang, Y.-Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Mann, J.J. Whole-Transcriptome Brain Expression and Exon-Usage Profiling in Major Depression and Suicide: Evidence for Altered Glial, Endothelial and ATPase Activity. Mol. Psychiatry 2017, 22, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Ramaker, R.C.; Bowling, K.M.; Lasseigne, B.N.; Hagenauer, M.H.; Hardigan, A.A.; Davis, N.S.; Gertz, J.; Cartagena, P.M.; Walsh, D.M.; Vawter, M.P.; et al. Post-Mortem Molecular Profiling of Three Psychiatric Disorders. Genome Med. 2017, 9, 72. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Xie, Z.; Zeng, C.; Shu, K. Transcriptomics Evidence for Common Pathways in Human Major Depressive Disorder and Glioblastoma. Int. J. Mol. Sci. 2018, 19, 234. [Google Scholar] [CrossRef]
- Yao, P.-A.; Sun, H.-J.; Li, X.-Y. Identification of Key Genes in Late-Onset Major Depressive Disorder through a Co-Expression Network Module. Front. Genet. 2022, 13, 1048761. [Google Scholar] [CrossRef]
- Zeng, D.; He, S.; Ma, C.; Wen, Y.; Xie, Y.; Zhao, N.; Sun, X.; Wang, D.; Shen, Y.; Yu, Y.; et al. Co-Expression Network Analysis Revealed That the ATP5G1 Gene Is Associated With Major Depressive Disorder. Front. Genet. 2019, 10, 703. [Google Scholar] [CrossRef]
- Carvalho Silva, R.; Pisanu, C.; Maffioletti, E.; Menesello, V.; Bortolomasi, M.; Gennarelli, M.; Baune, B.T.; Squassina, A.; Minelli, A. Biological Markers of Sex-Based Differences in Major Depressive Disorder and in Antidepressant Response. Eur. Neuropsychopharmacol. 2023, 76, 89–107. [Google Scholar] [CrossRef]
- Seney, M.L.; Huo, Z.; Cahill, K.; French, L.; Puralewski, R.; Zhang, J.; Logan, R.W.; Tseng, G.; Lewis, D.A.; Sibille, E. Opposite Molecular Signatures of Depression in Men and Women. Biol. Psychiatry 2018, 84, 18–27. [Google Scholar] [CrossRef]
- Talishinsky, A.; Downar, J.; Vértes, P.E.; Seidlitz, J.; Dunlop, K.; Lynch, C.J.; Whalley, H.; McIntosh, A.; Vila-Rodriguez, F.; Daskalakis, Z.J.; et al. Regional Gene Expression Signatures Are Associated with Sex-Specific Functional Connectivity Changes in Depression. Nat. Commun. 2022, 13, 5692. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Taskesen, E.; Van Bochoven, A.; Posthuma, D. Functional Mapping and Annotation of Genetic Associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef]
- Barbeira, A.N.; Dickinson, S.P.; Bonazzola, R.; Zheng, J.; Wheeler, H.E.; Torres, J.M.; Torstenson, E.S.; Shah, K.P.; Garcia, T.; Edwards, T.L.; et al. Exploring the Phenotypic Consequences of Tissue Specific Gene Expression Variation Inferred from GWAS Summary Statistics. Nat. Commun. 2018, 9, 1825. [Google Scholar] [CrossRef]
- Pan, S.; Kang, H.; Liu, X.; Li, S.; Yang, P.; Wu, M.; Yuan, N.; Lin, S.; Zheng, Q.; Jia, P. COLOCdb: A comprehensive resource for multi-model colocalization of complex traits. Nucleic Acids Res. 2024, 52, D871–D881. [Google Scholar] [CrossRef]
- Giambartolomei, C.; Vukcevic, D.; Schadt, E.-E.; Franke, L.; Hingorani, A.-D.; Wallace, C.; Plagnol, V. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014, 10, e1004383. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.-R.; Powell, J.-E.; Montgomery, G.-W.; Goddard, M.-E.; Wray, N.R.; Visscher, P.-M.; et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef]
- Zuccoli, G.S.; Saia-Cereda, V.M.; Nascimento, J.M.; Martins-de-Souza, D. The Energy Metabolism Dysfunction in Psychiatric Disorders Postmortem Brains: Focus on Proteomic Evidence. Front. Neurosci. 2017, 11, 493. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Lan, X.; Cohen, D.; Zhang, Y.; Ravindran, A.V.; Yuan, S.; Zheng, P.; Coghill, D.; Yang, L.; et al. Polyunsaturated Fatty Acids Metabolism, Purine Metabolism and Inosine as Potential Independent Diagnostic Biomarkers for Major Depressive Disorder in Children and Adolescents. Mol. Psychiatry 2019, 24, 1478–1488. [Google Scholar] [CrossRef]
- Davyson, E.; Shen, X.; Gadd, D.A.; Bernabeu, E.; Hillary, R.F.; McCartney, D.L.; Adams, M.; Marioni, R.; McIntosh, A.M. Metabolomic Investigation of Major Depressive Disorder Identifies a Potentially Causal Association With Polyunsaturated Fatty Acids. Biol. Psychiatry 2023, 94, 630–639. [Google Scholar] [CrossRef]
- Walther, A.; Cannistraci, C.V.; Simons, K.; Durán, C.; Gerl, M.J.; Wehrli, S.; Kirschbaum, C. Lipidomics in Major Depressive Disorder. Front. Psychiatry 2018, 9, 459. [Google Scholar] [CrossRef]
- Zeng, L.; Lv, H.; Wang, X.; Xue, R.; Zhou, C.; Liu, X.; Yu, H. Causal Effects of Fatty Acids on Depression: Mendelian Randomization Study. Front. Nutr. 2022, 9, 1010476. [Google Scholar] [CrossRef]
- Müller, C.P.; Reichel, M.; Mühle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain Membrane Lipids in Major Depression and Anxiety Disorders. Biochim. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Chen, Z.; Yu, H.; Tian, Y.; He, Y.; Cheng, K.; Xie, P. Disturbances of Phosphatidylcholines Metabolism in Major Depressive Disorder. CNS Spectr. 2023, 28, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cho, J.-H.; Kim, H.; Eum, Y.-J.; Cheong, E.-N.; Choi, S.; Park, J.-H.; Tak, S.; Park, B.; Sohn, J.-H.; et al. Association Between Taurine Level in the Hippocampus and Major Depressive Disorder in Young Women: A Proton Magnetic Resonance Spectroscopy Study at 7T. Biol. Psychiatry 2024, 95, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-F.; Ren, S.; Tang, R.-Y.; Xu, C.; Zhou, J.-Q.; Lin, S.-M.; Feng, Y.; Yang, Q.-H.; Hu, J.-M.; Yang, J.-C. Antidepressant Effect of Taurine in Chronic Unpredictable Mild Stress-Induced Depressive Rats. Sci. Rep. 2017, 7, 4989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, R.; Fan, Z.; Luo, D.; Cai, G.; Li, X.; Han, J.; Zhuo, L.; Zhang, L.; Zhang, H.; et al. Taurine Alleviates Chronic Social Defeat Stress-Induced Depression by Protecting Cortical Neurons from Dendritic Spine Loss. Cell Mol. Neurobiol. 2023, 43, 827–840. [Google Scholar] [CrossRef]
- De Sousa, C.N.S.; Meneses, L.N.; Vasconcelos, G.S.; Da Silva Medeiros, I.; Silva, M.C.C.; Mouaffak, F.; Kebir, O.; Da Silva Leite, C.M.G.; Patrocinio, M.C.A.; Macedo, D.; et al. Neuroprotective Evidence of Alpha-Lipoic Acid and Desvenlafaxine on Memory Deficit in a Neuroendocrine Model of Depression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 803–817. [Google Scholar] [CrossRef]
- Soczynska, J.K.; Kennedy, S.H.; Chow, C.S.; Woldeyohannes, H.O.; Konarski, J.Z.; McIntyre, R.S. Acetyl-L-Carnitine and α-Lipoic Acid: Possible Neurotherapeutic Agents for Mood Disorders? Expert Opin. Investig. Drugs 2008, 17, 827–843. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Wang, W.-D.; He, S.; Yuan, T.-F.; Hu, J.; Peng, D.-H. Altered N-Linked Glycosylation in Depression: A Pre-Clinical Study. J. Affect. Disord. 2024, 359, 333–341. [Google Scholar] [CrossRef]
- Kucuker, M.U.; Ozerdem, A.; Ceylan, D.; Cabello-Arreola, A.; Ho, A.M.C.; Joseph, B.; Webb, L.M.; Croarkin, P.E.; Frye, M.A.; Veldic, M. The Role of Base Excision Repair in Major Depressive Disorder and Bipolar Disorder. J. Affect. Disord. 2022, 306, 288–300. [Google Scholar] [CrossRef]
- Raza, M.U.; Tufan, T.; Wang, Y.; Hill, C.; Zhu, M.-Y. DNA Damage in Major Psychiatric Diseases. Neurotox. Res. 2016, 30, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Glatt, S.J.; Cohen, O.S.; Faraone, S.V.; Tsuang, M.T. Dysfunctional Gene Splicing as a Potential Contributor to Neuropsychiatric Disorders. Am. J. Med. Genet. Part B 2011, 156, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic Regulation in Major Depression and Other Stress-Related Disorders: Molecular Mechanisms, Clinical Relevance and Therapeutic Potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Laguesse, S.; Ron, D. Protein Translation and Psychiatric Disorders. Neuroscientist 2020, 26, 21–42. [Google Scholar] [CrossRef]
- Sharma, V.; Swaminathan, K.; Shukla, R. The Ribosome Hypothesis: Decoding Mood Disorder Complexity. Int. J. Mol. Sci. 2024, 25, 2815. [Google Scholar] [CrossRef]
- Zhang, X.; Eladawi, M.A.; Ryan, W.G.; Fan, X.; Prevoznik, S.; Devale, T.; Ramnani, B.; Malathi, K.; Sibille, E.; Mccullumsmith, R.; et al. Ribosomal Dysregulation: A Conserved Pathophysiological Mechanism in Human Depression and Mouse Chronic Stress. Proc. Natl. Acad. Sci. Nexus 2023, 2, pgad299. [Google Scholar] [CrossRef]
- Kouba, B.R.; De Araujo Borba, L.; Borges De Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of Inflammatory Mechanisms in Major Depressive Disorder: From Etiology to Potential Pharmacological Targets. Cells 2024, 13, 423. [Google Scholar] [CrossRef]
- Patrício, P.; Mateus-Pinheiro, A.; Sousa, N.; Pinto, L. Re-Cycling Paradigms: Cell Cycle Regulation in Adult Hippocampal Neurogenesis and Implications for Depression. Mol. Neurobiol. 2013, 48, 84–96. [Google Scholar] [CrossRef]
- Bakhtiarzadeh, F.; Nahavandi, A.; Goudarzi, M.; Shirvalilou, S.; Rakhshan, K.; Niknazar, S. Axonal Transport Proteins and Depressive like Behavior, Following Chronic Unpredictable Mild Stress in Male Rat. Physiol. Behav. 2018, 194, 9–14. [Google Scholar] [CrossRef]
- Wong, G.T.-H.; Chang, R.C.-C.; Law, A.C.-K. A Breach in the Scaffold: The Possible Role of Cytoskeleton Dysfunction in the Pathogenesis of Major Depression. Ageing Res. Rev. 2013, 12, 67–75. [Google Scholar] [CrossRef]
- The Swedish Bipolar Study Group; Chang, H.; MooDS Bipolar Consortium; Hoshina, N.; Zhang, C.; Ma, Y.; Cao, H.; Wang, Y.; Wu, D.; Bergen, S.E.; et al. The Protocadherin 17 Gene Affects Cognition, Personality, Amygdala Structure and Function, Synapse Development and Risk of Major Mood Disorders. Mol. Psychiatry 2018, 23, 400–412. [Google Scholar] [CrossRef]
- Hofstra, B.M.; Kas, M.J.H.; Verbeek, D.S. Comprehensive Analysis of Genetic Risk Loci Uncovers Novel Candidate Genes and Pathways in the Comorbidity between Depression and Alzheimer’s Disease. Transl. Psychiatry 2024, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Bassani, S.; Passafaro, M. Right Place at the Right Time: How Changes in Protocadherins Affect Synaptic Connections Contributing to the Etiology of Neurodevelopmental Disorders. Cells 2020, 9, 2711. [Google Scholar] [CrossRef] [PubMed]
- Miozzo, F.; Murru, L.; Maiellano, G.; Di Iasio, I.; Zippo, A.G.; Zambrano Avendano, A.; Metodieva, V.D.; Riccardi, S.; D’Aliberti, D.; Spinelli, S.; et al. Disruption of the Autism-Associated Pcdh9 Gene Leads to Transcriptional Alterations, Synapse Overgrowth, and Defective Network Activity in the CA1. J. Neurosci. 2024, 44, e0491242024. [Google Scholar] [CrossRef] [PubMed]
- Halsted, C.H.; Wong, D.H.; Peerson, J.M.; Warden, C.H.; Refsum, H.; Smith, A.D.; Nygård, O.K.; Ueland, P.M.; Vollset, S.E.; Tell, G.S. Relations of Glutamate Carboxypeptidase II (GCPII) Polymorphisms to Folate and Homocysteine Concentrations and to Scores of Cognition, Anxiety, and Depression in a Homogeneous Norwegian Population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2007, 86, 514–521. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Jain, R. Glutamatergic Modulators for Major Depression from Theory to Clinical Use. CNS Drugs 2024, 38, 869–890. [Google Scholar] [CrossRef]
- Moriguchi, S.; Takamiya, A.; Noda, Y.; Horita, N.; Wada, M.; Tsugawa, S.; Plitman, E.; Sano, Y.; Tarumi, R.; ElSalhy, M.; et al. Glutamatergic Neurometabolite Levels in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Proton Magnetic Resonance Spectroscopy Studies. Mol. Psychiatry 2019, 24, 952–964. [Google Scholar] [CrossRef]
- Sarawagi, A.; Soni, N.D.; Patel, A.B. Glutamate and GABA Homeostasis and Neurometabolism in Major Depressive Disorder. Front. Psychiatry 2021, 12, 637863. [Google Scholar] [CrossRef]
- Du, Y.; Wei, J.; Zhang, Z.; Yang, X.; Wang, M.; Wang, Y.; Qi, X.; Zhao, L.; Tian, Y.; Guo, W.; et al. Plasma Metabolomics Profiling of Metabolic Pathways Affected by Major Depressive Disorder. Front. Psychiatry 2021, 12, 644555. [Google Scholar] [CrossRef] [PubMed]
- Henter, I.D.; De Sousa, R.T.; Gold, P.W.; Brunoni, A.R.; Zarate, C.A.; Machado-Vieira, R. Mood Therapeutics: Novel Pharmacological Approaches for Treating Depression. Expert. Rev. Clin. Pharmacol. 2017, 10, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Henter, I.D.; De Sousa, R.T.; Zarate, C.A. Glutamatergic Modulators in Depression. Harv. Rev. Psychiatry 2018, 26, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, L. Interactions Involving Glycine and Other Amino Acid Neurotransmitters: Focus on Transporter-Mediated Regulation of Release and Glycine–Glutamate Crosstalk. Biomedicines 2024, 12, 1518. [Google Scholar] [CrossRef]
- Schmidt, R.W.; Thompson, M.L. Glycinergic Signaling in the Human Nervous System: An Overview of Therapeutic Drug Targets and Clinical Effects. Ment. Health Clin. 2016, 6, 266–276. [Google Scholar] [CrossRef]
- McNamara, R.K.; Liu, Y. Reduced Expression of Fatty Acid Biosynthesis Genes in the Prefrontal Cortex of Patients with Major Depressive Disorder. J. Affect. Disord. 2011, 129, 359–363. [Google Scholar] [CrossRef]
- Connor, S.A.; Siddiqui, T.J. Synapse Organizers as Molecular Codes for Synaptic Plasticity. Trends Neurosci. 2023, 46, 971–985. [Google Scholar] [CrossRef]
- Geng, C.; Hao, G.; Yi, Q.; Guo, Y.; Chen, D.; Han, W.; Zhang, J.; Yang, M.; Jiang, P. The Impact of Dl-3-n-Butylphthalide on the Lipidomics of the Hippocampus in a Rat Model of Lipopolysaccharide-Induced Depression. Prostaglandins Other Lipid Mediat. 2020, 150, 106464. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Hearn, E.; Moulana, M.; Saleem, K.; Clark, A.; Holmes, M.; Wadhwa, K.; Kelly, I.; Stockmeier, C.A.; Rajkowska, G. Reduced Length of Nodes of Ranvier and Altered Proteoglycan Immunoreactivity in Prefrontal White Matter in Major Depressive Disorder and Chronically Stressed Rats. Sci. Rep. 2023, 13, 16419. [Google Scholar] [CrossRef]
- Ong, E.; Suzuki, M.; Belot, F.; Yeh, J.-C.; Franceschini, I.; Angata, K.; Hindsgaul, O.; Fukuda, M. Biosynthesis of HNK-1 Glycans on O-Linked Oligosaccharides Attached to the Neural Cell Adhesion Molecule (NCAM). J. Biol. Chem. 2002, 277, 18182–18190. [Google Scholar] [CrossRef]
- Senn, C. Mice Deficient for the HNK-1 Sulfotransferase Show Alterations in Synaptic Efficacy and Spatial Learning and Memory. Mol. Cell. Neurosci. 2002, 20, 712–729. [Google Scholar] [CrossRef]
- Syková, E.; Voříšek, I.; Mazel, T.; Antonova, T.; Schachner, M. Reduced Extracellular Space in the Brain of tenascin-R- and HNK-1-sulphotransferase Deficient Mice. Eur. J. Neurosci. 2005, 22, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Poli, J.; Gasser, S.M.; Papamichos-Chronakis, M. The INO80 Remodeller in Transcription, Replication and Repair. Phil. Trans. R. Soc. B 2017, 372, 20160290. [Google Scholar] [CrossRef] [PubMed]
- Sokpor, G.; Castro-Hernandez, R.; Rosenbusch, J.; Staiger, J.F.; Tuoc, T. ATP-Dependent Chromatin Remodeling During Cortical Neurogenesis. Front. Neurosci. 2018, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Colino-Sanguino, Y.; Clark, S.J.; Valdes-Mora, F. The H2A.Z-Nucleosome Code in Mammals: Emerging Functions. Trends Genet. 2022, 38, 273–289. [Google Scholar] [CrossRef]
- Lowden, C.; Boulet, A.; Boehler, N.A.; Seecharran, S.; Rios Garcia, J.; Lowe, N.J.; Liu, J.; Ong, J.L.K.; Wang, W.; Ma, L.; et al. Homeostatic Control of Nuclear-Encoded Mitochondrial Gene Expression by the Histone Variant H2A.Z Is Essential for Neuronal Survival. Cell Rep. 2021, 36, 109704. [Google Scholar] [CrossRef]
- Shen, T.; Ji, F.; Wang, Y.; Lei, X.; Zhang, D.; Jiao, J. Brain-Specific Deletion of Histone Variant H2A.z Results in Cortical Neurogenesis Defects and Neurodevelopmental Disorder. Nucleic Acids Res. 2018, 46, 2290–2307. [Google Scholar] [CrossRef]
- Zovkic, I.B.; Paulukaitis, B.S.; Day, J.J.; Etikala, D.M.; Sweatt, J.D. Histone H2A.Z Subunit Exchange Controls Consolidation of Recent and Remote Memory. Nature 2014, 515, 582–586. [Google Scholar] [CrossRef]
- Brahma, S.; Udugama, M.I.; Kim, J.; Hada, A.; Bhardwaj, S.K.; Hailu, S.G.; Lee, T.-H.; Bartholomew, B. INO80 Exchanges H2A.Z for H2A by Translocating on DNA Proximal to Histone Dimers. Nat. Commun. 2017, 8, 15616. [Google Scholar] [CrossRef]
- Wright, J.L.; Jiang, Y.; Nayar, S.G.; Li, H.; Richardson, W.D. The INO80 Chromatin Remodeling Complex Regulates Histone H2A.Z Mobility and the G1-S Transition in Oligodendrocyte Precursors. Glia 2025, 73, 1307–1323. [Google Scholar] [CrossRef]
- Rorbach, J.; Boesch, P.; Gammage, P.A.; Nicholls, T.J.J.; Pearce, S.F.; Patel, D.; Hauser, A.; Perocchi, F.; Minczuk, M. MRM2 and MRM3 Are Involved in Biogenesis of the Large Subunit of the Mitochondrial Ribosome. Mol. Biol. Cell 2014, 25, 2542–2555. [Google Scholar] [CrossRef]
- Baranova, A.; Liu, D.; Chandhoke, V.; Cao, H.; Zhang, F. Unraveling the Genetic Links between Depression and Type 2 Diabetes. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 137, 111258. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Jiang, D.; Harrison, M.; Zhou, Y.; Chen, G.; Mao, Y. An EJC Factor RBM8a Regulates Anxiety Behaviors. Curr. Mol. Med. 2013, 13, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.J.; Wang, Q.; Zheng, X.X.; Cheng, Y.; Zhang, Y. Involvement of SNARE Complex in the Hippocampus and Prefrontal Cortex of Offspring with Depression Induced by Prenatal Stress. J. Affect. Disord. 2018, 235, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Li, X.; Fu, M.; Gao, X.; Li, P.; Guo, W. NLRP3-Dependent Pyroptosis: A Candidate Therapeutic Target for Depression. Front. Cell. Neurosci. 2022, 16, 863426. [Google Scholar] [CrossRef]
- Ulbrich, P.; Khoshneviszadeh, M.; Jandke, S.; Schreiber, S.; Dityatev, A. Interplay between Perivascular and Perineuronal Extracellular Matrix Remodelling in Neurological and Psychiatric Diseases. Eur. J. Neurosci. 2021, 53, 3811–3830. [Google Scholar] [CrossRef]
- Golenbock, S.W.; Wise, L.A.; Lambert-Messerlian, G.M.; Eklund, E.E.; Harlow, B.L. Association between a History of Depression and Anti-Müllerian Hormone among Late-Reproductive Aged Women: The Harvard Study of Moods and Cycles. Womens Midlife Health 2020, 6, 9. [Google Scholar] [CrossRef]
- Valenti, M.A.; Farland, L.V.; Huang, K.; Liu, Y.; Beitel, S.C.; Jahnke, S.A.; Hollerbach, B.; Clair, C.C.S.; Gulotta, J.J.; Kolar, J.J.; et al. Evaluating the Effect of Depression, Anxiety, and Post-Traumatic Stress Disorder on Anti-Müllerian Hormone Levels Among Women Firefighters. J. Women’s Health 2024, 34, 354–361. [Google Scholar] [CrossRef]
- Meffre, D.; Shackleford, G.; Hichor, M.; Gorgievski, V.; Tzavara, E.T.; Trousson, A.; Ghoumari, A.M.; Deboux, C.; Nait Oumesmar, B.; Liere, P.; et al. Liver X Receptors Alpha and Beta Promote Myelination and Remyelination in the Cerebellum. Proc. Natl. Acad. Sci. USA 2015, 112, 7587–7592. [Google Scholar] [CrossRef]
- Mouzat, K.; Chudinova, A.; Polge, A.; Kantar, J.; Camu, W.; Raoul, C.; Lumbroso, S. Regulation of Brain Cholesterol: What Role Do Liver X Receptors Play in Neurodegenerative Diseases? Int. J. Mol. Sci. 2019, 20, 3858. [Google Scholar] [CrossRef]
- Wouters, E.; De Wit, N.M.; Vanmol, J.; Van Der Pol, S.M.A.; Van Het Hof, B.; Sommer, D.; Loix, M.; Geerts, D.; Gustafsson, J.A.; Steffensen, K.R.; et al. Liver X Receptor Alpha Is Important in Maintaining Blood-Brain Barrier Function. Front. Immunol. 2019, 10, 1811. [Google Scholar] [CrossRef]
- Li, L.-Y.; Guan, Y.-D.; Chen, X.-S.; Yang, J.-M.; Cheng, Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 2021, 11, 629266. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kitty, I.; Renata, K.; Qin, S.; Zhao, F.; Kim, W. DNA Damage and Its Role in Cancer Therapeutics. Int. J. Mol. Sci. 2023, 24, 4741. [Google Scholar] [CrossRef] [PubMed]
- Parreno, V.; Martinez, A.-M.; Cavalli, G. Mechanisms of Polycomb group protein function in cancer. Cell Res. 2022, 32, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qin, J.-J.; Voruganti, S.; Nag, S.; Zhou, J.; Zhang, R. Polycomb Group (PcG) Proteins and Human Cancers: Multifaceted Functions and Therapeutic Implications. Med. Res. Rev. 2015, 35, 1220–1267. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, B.; Wang, L.; Li, P.; Bennett, B.-D.; Snyder, R.; Garantziotis, S.; Fargo, D.-C.; Cox, A.-D.; Chen, L.; et al. INO80 is required for oncogenic transcription and tumor growth in non-small cell lung cancer. Oncogene 2017, 36, 1430–1439. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Zhang, S.; Bennett, B.-D.; He, F.; Zhang, Y.; Xiong, C.; Han, L.; Diao, L.; Li, P.; et al. INO80 governs superenhancer-mediated oncogenic transcription and tumor growth in melanoma. Genes Dev. 2016, 30, 1440–1453. [Google Scholar] [CrossRef]
- Yang, G.-J.; Zhu, M.-H.; Lu, X.-J.; Liu, Y.-J.; Lu, J.-F.; Leung, C.-H.; Ma, D.-L.; Chen, J. The emerging role of KDM5A in human cancer. J. Hematol. Oncol. 2021, 14, 30. [Google Scholar] [CrossRef]
- Nakhlband, A.; Farahzadi, R.; Saeedi, N.; Barzegar, H.; Montazersaheb, S.; Soofiyani, S.-R. Bidirectional Relations Between Anxiety, Depression, and Cancer: A Review. Curr. Drug Targets 2023, 24, 118–130. [Google Scholar] [CrossRef]
- Kang, H.-J.; Park, Y.; Yoo, K.-H.; Kim, K.-T.; Kim, E.-S.; Kim, J.-W.; Kim, S.-W.; Shin, I.-S.; Yoon, J.-S.; Kim, J.H.; et al. Sex Differences in the Genetic Architecture of Depression. Sci. Rep. 2020, 10, 9927. [Google Scholar] [CrossRef]
- Aw, E.; Zhang, Y.; Carroll, M. Microglial Responses to Peripheral Type 1 Interferon. J. Neuroinflamm. 2020, 17, 340. [Google Scholar] [CrossRef]
- Yilmaz, M.; Yalcin, E.; Presumey, J.; Aw, E.; Ma, M.; Whelan, C.W.; Stevens, B.; McCarroll, S.A.; Carroll, M.C. Overexpression of Schizophrenia Susceptibility Factor Human Complement C4A Promotes Excessive Synaptic Loss and Behavioral Changes in Mice. Nat. Neurosci. 2021, 24, 214–224. [Google Scholar] [CrossRef]
- Guo, A.; Wang, B.; Ding, J.; Zhao, L.; Wang, X.; Huang, C.; Guo, B. Serum Proteomic Analysis Uncovers Novel Serum Biomarkers for Depression. Front. Psychiatry 2024, 15, 1346151. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Filimonov, D.; Tarasova, O. A Computational Analysis of Transcriptional Profiles from CD8(+) T Lymphocytes Reveals Potential Mechanisms of HIV/AIDS Control and Progression. Comput. Struct. Biotechnol. J. 2021, 19, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.M.; Lagunin, A.A.; Tarasova, O.A. Analysis of Transcription Profiles for the Identification of Master Regulators as the Key Players in Glioblastoma. Comput. Struct. Biotechnol. J. 2024, 23, 3559–3574. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.M.; Tarasova, O.A.; Poroikov, V.V. Transcriptome-Based Analysis of Human Peripheral Blood Reveals Regulators of Immune Response in Different Viral Infections. Front. Immunol. 2023, 14, 1199482. [Google Scholar] [CrossRef]
- Parkash, J.; Messina, A.; Langlet, F.; Cimino, I.; Loyens, A.; Mazur, D.; Gallet, S.; Balland, E.; Malone, S.A.; Pralong, F.; et al. Semaphorin7A Regulates Neuroglial Plasticity in the Adult Hypothalamic Median Eminence. Nat. Commun. 2015, 6, 7385. [Google Scholar] [CrossRef]
- Jongbloets, B.C.; Lemstra, S.; Schellino, R.; Broekhoven, M.H.; Parkash, J.; Hellemons, A.J.C.G.M.; Mao, T.; Giacobini, P.; Van Praag, H.; De Marchis, S.; et al. Stage-Specific Functions of Semaphorin7A during Adult Hippocampal Neurogenesis Rely on Distinct Receptors. Nat. Commun. 2017, 8, 14666. [Google Scholar] [CrossRef]
- Uesaka, N.; Uchigashima, M.; Mikuni, T.; Nakazawa, T.; Nakao, H.; Hirai, H.; Aiba, A.; Watanabe, M.; Kano, M. Retrograde Semaphorin Signaling Regulates Synapse Elimination in the Developing Mouse Brain. Science 2014, 344, 1020–1023. [Google Scholar] [CrossRef]
- Gutiérrez-Franco, A.; Eixarch, H.; Costa, C.; Gil, V.; Castillo, M.; Calvo-Barreiro, L.; Montalban, X.; Del Río, J.A.; Espejo, C. Semaphorin 7A as a Potential Therapeutic Target for Multiple Sclerosis. Mol. Neurobiol. 2017, 54, 4820–4831. [Google Scholar] [CrossRef]
- Körner, A.; Bernard, A.; Fitzgerald, J.C.; Alarcon-Barrera, J.C.; Kostidis, S.; Kaussen, T.; Giera, M.; Mirakaj, V. Sema7A Is Crucial for Resolution of Severe Inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017527118. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, A.; Paulis, L.; Te Riet, J.; Vasaturo, A.; Reinieren-Beeren, I.; Van Der Schaaf, A.; Kuipers, A.J.; Schulte, L.P.; Jongbloets, B.C.; Pasterkamp, R.J.; et al. Semaphorin 7A Promotes Chemokine-Driven Dendritic Cell Migration. J. Immunol. 2016, 196, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ruiz, J.A.; Treviño-Alvarez, A.M.; Zambrano-Lucio, M.; Lozano Díaz, S.T.; Wang, N.; Biernacka, J.M.; Tye, S.J.; Cuellar-Barboza, A.B. The Wnt Signaling Pathway in Major Depressive Disorder: A Systematic Review of Human Studies. Psychiatry Res. 2024, 339, 115983. [Google Scholar] [CrossRef]
- Issler, O.; Nestler, E.-J. The molecular basis for sex differences in depression susceptibility. Curr. Opin. Behav. Sci. 2018, 23, 1–6. [Google Scholar] [CrossRef]
- Köhler, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of Anti-Inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. JAMA Psychiatry 2014, 71, 1381. [Google Scholar] [CrossRef]
- Paul, E.R.; Östman, L.; Heilig, M.; Mayberg, H.S.; Hamilton, J.P. Towards a Multilevel Model of Major Depression: Genes, Immuno-Metabolic Function, and Cortico-Striatal Signaling. Transl. Psychiatry 2023, 13, 171. [Google Scholar] [CrossRef]
- Chen, Z.; Boehnke, M.; Wen, X.; Mukherjee, B. Revisiting the Genome-Wide Significance Threshold for Common Variant GWAS. G3 Genes|Genomes|Genet. 2021, 11, jkaa056. [Google Scholar] [CrossRef]
- CommonMind Consortium; Huckins, L.M.; The Schizophrenia Working Group of the Psychiatric Genomics Consortium; iPSYCH-GEMS Schizophrenia Working Group; Dobbyn, A.; Ruderfer, D.M.; Hoffman, G.; Wang, W.; Pardiñas, A.F.; Rajagopal, V.M.; et al. Gene Expression Imputation across Multiple Brain Regions Provides Insights into Schizophrenia Risk. Nat. Genet. 2019, 51, 659–674. [Google Scholar] [CrossRef]
- Gandal, M.J.; Zhang, P.; Hadjimichael, E.; Walker, R.L.; Chen, C.; Liu, S.; Won, H.; Van Bakel, H.; Varghese, M.; Wang, Y.; et al. Transcriptome-Wide Isoform-Level Dysregulation in ASD, Schizophrenia, and Bipolar Disorder. Science 2018, 362, aat8127. [Google Scholar] [CrossRef]
- Zhu, S.; Qian, T.; Hoshida, Y.; Shen, Y.; Yu, J.; Hao, K. GIGSEA: Genotype Imputed Gene Set Enrichment Analysis Using GWAS Summary Level Data. Bioinformatics 2019, 35, 160–163. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Müller-Dott, S.; Tsirvouli, E.; Vazquez, M.; Ramirez Flores, R.O.; Badia-i-Mompel, P.; Fallegger, R.; Türei, D.; Lægreid, A.; Saez-Rodriguez, J. Expanding the Coverage of Regulons from High-Confidence Prior Knowledge for Accurate Estimation of Transcription Factor Activities. Nucleic Acids Res. 2023, 51, 10934–10949. [Google Scholar] [CrossRef]
- Alvarez, M.J.; Shen, Y.; Giorgi, F.M.; Lachmann, A.; Ding, B.B.; Ye, B.H.; Califano, A. Functional Characterization of Somatic Mutations in Cancer Using Network-Based Inference of Protein Activity. Nat. Genet. 2016, 48, 838–847. [Google Scholar] [CrossRef]
- Türei, D.; Korcsmáros, T.; Saez-Rodriguez, J. OmniPath: Guidelines and Gateway for Literature-Curated Signaling Pathway Resources. Nat. Methods 2016, 13, 966–967. [Google Scholar] [CrossRef]
- Bradley, G.; Barrett, S.J. CausalR: Extracting Mechanistic Sense from Genome Scale Data. Bioinformatics 2017, 33, 3670–3672. [Google Scholar] [CrossRef]


| Functional Category | Protein Name | Main Known Function |
|---|---|---|
| Fatty acid metabolism | Acyl-CoA synthetase family member 3 (ACSF3 *) ↓ | Intramitochondrial fatty acid synthesis |
| Fatty acid desaturase 1 (FADS1 *) ↓ | Synthesis of highly unsaturated fatty acids, phospholipids, and icosanoids | |
| Hydroxysteroid 17-beta dehydrogenase 8 (HSD17B8 *) ↓ | Fatty acid synthesis | |
| Sterol carrier protein 2 (SCP2) ↓ | Peroxisomal oxidation of branched-chain fatty acids | |
| Glutamate metabolism | Folate hydrolase 1 (FOLH1 *&) ↑ | Modulates excitatory neurotransmission through the hydrolysis of the neuropeptide, N-aceylaspartylglutamate (NAAG), thereby releasing glutamate |
| Gamma-glutamyl hydrolase (GGH *)↑ | Hydrolyzes the polyglutamate sidechains of pteroylpolyglutamates | |
| Glycan metabolism | ALG10 alpha-1,2-glucosyltransferase (ALG10) ↑ | N-Glycan biosynthesis |
| ALG10 alpha-1,2-glucosyltransferase B (ALG10B) ↓ | N-Glycan biosynthesis | |
| ALG3 alpha-1,3- mannosyltransferase (ALG3) ↑ | N-Glycan biosynthesis | |
| Beta-1,3-glucuronyltransferase 2 (B3GAT2) ↓ | Glycosaminoglycan biosynthesis | |
| Beta-1,3-glucuronyltransferase 3 (B3GAT3 *) ↑ | Glycosaminoglycan biosynthesis | |
| Beta 3-glucosyltransferase (B3GLCT *#) ↓ | Protein O-linked fucosylation | |
| Core 1 synthase, glycoprotein-N-acetylgalactosamine 3-beta-galactosyltransferase 1 (C1GALT1 *) ↑ | Protein O-linked glycosylation | |
| Carbohydrate sulfotransferase 10 (CHST10) ↓ | Proteoglycan biosynthesis | |
| Phosphatidylinositol glycan anchor biosynthesis class X (PIGX) ↑ | GPI-anchor biosynthesis | |
| Phosphatidylinositol glycan anchor biosynthesis class Z (PIGZ *) ↑ | GPI-anchor biosynthesis | |
| ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 1 (ST8SIA1) ↑ | Glycosphingolipid biosynthesis | |
| Uronyl 2-sulfotransferase (UST) ↓ | Glycosaminoglycan biosynthesis | |
| Glycin metabolism | Dihydrolipoamide dehydrogenase (DLD *) ↓ | Component of the glycine cleavage system, acetyl-CoA biosynthesis from pyruvate |
| Aminomethyltransferase (AMT *#&) ↓ | Component of the glycine cleavage system | |
| Betaine--homocysteine S-methyltransferase (BHMT *) ↓ | Converts betaine and homocysteine to dimethylglycine and methionine, irreversible oxidation of choline | |
| Dimethylglycine dehydrogenase (DMGDH) ↓ | Catalyzes the demethylation of N, N-dimethylglycine to sarcosine | |
| Serine hydroxymethyltransferase 1 (SHMT1 *) ↑ | Interconversion of serine and glycine | |
| Hypotaurine metabolism | 2-aminoethanethiol dioxygenase (ADO *&) ↓ | Hypotaurine synthesis |
| Inositol phosphate metabolism | Adenosine monophosphate deaminase 3 (AMPD3 *) ↑ | IMP biosynthesis from AMP |
| Inosine triphosphatase (ITPA *) ↓ | Hydrolyzes the inosine triphosphate (ITP), deoxyinosine triphosphate (dITP), and xanthosine triphosphate (XTP) to their monophosphate derivatives | |
| Kynurenine metabolism | Aldehyde dehydrogenase 8 family member A1 (ALDH8A1 *) ↑ | L-kynurenine catabolic process |
| Mitochondrial regulation | Inorganic pyrophosphatase 2 (PPA2 *) ↓ | Regulation of mitochondrial membrane potential and mitochondrial organization and function |
| Phospholipid metabolism | Arylsulfatase A (ARSA *) ↓ | Hydrolyzes cerebroside sulfate |
| Choline kinase beta (CHKB *) ↓ | Phosphatidylethanolamine and phosphatidylcholine biosynthesis | |
| Phospholipase A2 group IVB (PLA2G4B *) ↑ | Remodeling of membrane phospholipids | |
| Sphingomyelin phosphodiesterase 2 (SMPD2 *) ↑ | Hydrolysis of sphingomyelin to form ceramide and phosphocholine | |
| Prostaglandin metabolism | Prostaglandin E synthase 2 (PTGES2) ↑ | Conversion of prostaglandin H2 into the more stable prostaglandin E2 |
| Tricarboxylic acid cycle | Dihydrolipoamide S-succinyltransferase (DLST) ↑ | Conversion of 2-oxoglutarate to succinyl-CoA and CO2 |
| Process | Direction | Proteins (Genes) |
|---|---|---|
| DNA replication and repair | Increase | DEAD/H-box helicase 11 (DDX11 *), PIF1 5′-to-3′ DNA helicase (PIF1 *), Nibrin (NBN), RAD54 like (RAD54L), TIMELESS interacting protein (TIPIN *) |
| Decrease | DNA polymerase epsilon (POLE *), DNA polymerase iota (POLI *), DNA topoisomerase III alpha (TOP3A*), O-6-methylguanine-DNA methyltransferase (MGMT *), Nei-like DNA glycosylase 2 (NEIL2 *&), PWWP domain containing 3A, DNA repair factor (PWWP3A *), RecQ mediated genome instability 2 (RMI2 *), replication protein A3 (RPA3 *) | |
| mRNA processing | Increase | FAST kinase domains 5 (FASTKD5 *), mitochondrial poly(A) polymerase (MTPAP), exoribonuclease 1 (ERI1) |
| rRNA processing | Increase | mitochondrial rRNA methyltransferase 2 (MRM2 *) |
| Decrease | KRR1 small subunit processome component homolog (KRR1 *), WD repeat domain 55 (WDR55 *) | |
| tRNA processing | Increase | THUMP domain containing 3 (THUMPD3 *&), tRNA methyltransferase 61A (TRMT61A *&), tRNA phosphotransferase 1 (TRPT1 *), WD repeat domain 6 (WDR6 *) |
| mRNA splicing | Increase | CASC3 exon junction complex subunit (CASC3), RNA binding motif protein 8A (RBM8A), splicing factor 3b subunit 1 (SF3B1), gem nuclear organelle associated protein 7 (GEMIN7 *), arginine and serine rich coiled-coil 1 (RSRC1 *) |
| Decrease | SLU7 homolog, splicing factor (SLU7), small nuclear ribonucleoprotein U4/U6.U5 subunit 27 (SNRNP27), U2 small nuclear RNA auxiliary factor 1 like 4 (U2AF1L4 *), zinc finger matrin-type 2 (ZMAT2 *) | |
| Chromatin regulators | Increase | INO80 complex: actin-related protein 5 (ACTR5), INO80 complex subunits E and D (INO80E *, INO80D); Polycomb group proteins: L3MBTL histone methyl-lysine binding protein 2 (L3MBTL2 *), WD repeat domain 5B (WDR5B *); histones and modifiers: lysine methyltransferase 5A (KMT5A) |
| Decrease | Polycomb group proteins: Scm-like with four MBT domains 1 (SFMBT1); histones and modifiers: H1.2 linker histone (H1-2), H2A.Z variant histone 2 (H2AZ2), protein arginine methyltransferase 6 (PRMT6 *&), SET domain bifurcated histone lysine methyltransferase 2 (SETDB2) | |
| Ribosomal proteins | Increase | Mitochondrial ribosomal protein L34 (MRPL34 *), ribosomal protein L12 (RPL12 *), ribosomal protein L36a-like (RPL36AL *) |
| Translation | Increase | GTP binding elongation factor GUF1 (GUF1 *), HBS1-like translational GTPase (HBS1L *), La ribonucleoprotein 6, translational regulator (LARP6 *), histidyl-tRNA synthetase 1 (HARS1), valyl-tRNA synthetase 2, mitochondrial (VARS2 *#&) |
| Decrease | Mitochondrial translation release factor 1-like (MTRF1L *), histidyl-tRNA synthetase 2, mitochondrial (HARS2) | |
| Vesicular transport | Increase | BCL2 interacting protein 1 (BNIP1 *), sec1 family domain containing 1 (SCFD1), syntaxin-17 (STX17), Golgi SNAP receptor complex member 2 (GOSR2), vesicle-associated membrane protein 2 (VAMP2 *), dynamin 1 (DNM1), N-ethylmaleimide sensitive factor, vesicle-fusing ATPase (NSF) |
| Decrease | B cell receptor associated protein 29 (BCAP29), KDEL endoplasmic reticulum protein retention receptor 2 (KDELR2) | |
| Unfolded protein response | Increase | Activating transcription factor 6 beta (ATF6B *#&), glutamine rich 1 (QRICH1 *#) |
| Cell death | Increase | Gasdermins D and E (GSDMD *, GSDME *&) |
| Cell cycle | Increase | EMAP-like 3 (EML3 *), zwilch kinetochore protein (ZWILCH) |
| Decrease | Cell division cycle 25B (CDC25B), minichromosome maintenance complex component 6 (MCM6), checkpoint with forkhead and ring finger domains (CHFR *), HAUS augmin-like complex subunit 4 (HAUS4 *), small kinetochore-associated protein (KNSTRN *) | |
| Cytoskeleton | Increase | Kinesins: kinesin family member C2 (KIFC2), kinesin light chain 1 (KLC1); myosins: myosin XVA and XVB (MYO15A *, MYO15B); dyneins: dynein cytoplasmic 2 light intermediate chain 1 (DYNC2LI1) |
| Decrease | Actin regulators: actin-related protein 2/3 complex subunit 5-like protein (ARPC5L), diaphanous-related formin 3 (DIAPH3), vinculin (VCL); dyneins: dynein axonemal heavy chain 7 (DNAH7), dynein cytoplasmic 1 intermediate chain 2 (DYNC1I2), dynein light chain Tctex-type family member 5 (DYNLT5 *); kinesins: kinesin family member 6 (KIF6) | |
| Cell adhesion | Increase | Integrins: integrin binding sialoprotein (IBSP), integrin subunit alpha V (ITGAV *); protocadherins: protocadherin alpha 13 (PCDHA13), protocadherins beta 2, 3, 8, and 10 (PCDHB2 *, PCDHB3, PCDHB8 *, PCDHB10); FRAS1 related extracellular matrix 1 (FREM1 *), collagen type XXVIII alpha 1 chain (COL28A1 *), mucin 4, cell surface associated (MUC4), neuronal growth regulator 1 (NEGR1 *#&), neurotrimin (NTM *) |
| Decrease | Integrins: integrin subunit beta 6 (ITGB6); protocadherins: protocadherins alpha 7, 8, and 10 (PCDHA7 *, PCDHA8 *&, PCDHA10 *); cell adhesion molecule 2 (CADM2 *), neurocan (NCAN), peripherin 2 (PRPH2 *) |
| Functional Category | Protein Name | Main Known Function |
|---|---|---|
| Calcium signaling | Adenylate cyclase 3 (ADCY3) ↓ | cAMP synthesis |
| Mucolipin TRP cation channel 2 and 3 (MCOLN2, MCOLN3) ↓ | Ca2+-permeable cation channels | |
| Nitric oxide synthase 1 and 2 (NOS1, NOS2 *) ↑ | Produce nitric oxide (NO). In the brain, NO displays many properties of a neurotransmitter | |
| Ryanodine receptor 1 (RYR1) ↑ | Mediates the release of Ca2+ from the sarcoplasmic reticulum into the cytosol | |
| Hormones and growth factors | Anti-Mullerian hormone (AMH *) ↑ | Gonadal differentiation, ovarian follicle development |
| EPH receptor B2 (EPHB2 *&) ↓ | Functions in axon guidance during development. Dendritic spine development, formation of excitatory synapses | |
| Fms-related receptor tyrosine kinase 4 (FLT4) ↑ | Angiogenesis | |
| Growth differentiation factor 9 (GDF9 *) ↑ | Required for ovarian folliculogenesis | |
| Nuclear receptor subfamily 1 group H member 3 (NR1H3) ↓ | Regulates cholesterol metabolism and inflammation | |
| Wnt family member 3 (WNT3 *) ↑ | Ligand in canonical Wnt signaling pathway | |
| Immune system | Butyrophilin subfamily 3 member A1 (BTN3A1 *&) ↓ | Regulates T-cell responses and the release of cytokines |
| Butyrophilin subfamily 3 member A2 (BTN3A2 *#&) ↑ | Regulates T-cell responses and the release of cytokines | |
| Butyrophilin subfamily 3 member A3 (BTN3A3 *#&) ↓ | Regulates T-cell responses and the release of cytokines | |
| C1q and TNF related 4 (C1QTNF4 *&) ↑ | Regulation of the inflammatory network | |
| CD276 molecule (CD276) ↓ | Regulates T-cell responses and the release of cytokines | |
| CD40 molecule (CD40 *&) ↓ | Regulation of inflammatory response | |
| Complement C4A (C4A) ↑ | Component of the complement pathway. Mediator of local inflammatory processes | |
| Complement C4B (C4B *#&) ↓ | Component of the complement pathway. Mediator of local inflammatory processes | |
| Interleukin 11 receptor subunit alpha (IL11RA) ↑ | Receptor for interleukin 11. Controls the development of craniofacial bones and teeth | |
| Interleukin 21 receptor (IL21R) ↓ | Receptor for interleukin-21 | |
| Interleukin 33 (IL33 *) ↑ | Regulates functions of various immune cells: T-helper type 2, macrophages, microglia, and NK cells | |
| Macrophage stimulating 1 receptor (MST1R *) ↑ | Regulates cell survival, migration, and differentiation | |
| Neurotransmission | 5-hydroxytryptamine receptor 1D (HTR1D *) ↑ | Neurotransmitter receptors |
| Cholinergic receptor nicotinic alpha 3 subunit (CHRNA3) ↑ | Neurotransmitter receptors | |
| Cholinergic receptor nicotinic beta 1 subunit (CHRNB1) ↓ | Neurotransmitter receptors | |
| Dopamine receptor D2 (DRD2) ↓ | Neurotransmitter receptors | |
| Neuropeptide Y receptor Y2 (NPY2R *) ↑ | Receptor for neuropeptide Y and peptide YY | |
| Purinergic receptor P2X 2 (P2RX2) ↓ | Receptor activated by extracellular ATP, mediates synaptic transmission between neurons | |
| Sodium voltage-gated channel alpha subunit 9 (SCN9A) ↑ | Influx of Na+ ions provokes membrane depolarization | |
| Solute carrier family 1 member 6 (SLC1A6) ↓ | Uptake of L-glutamate. Potentially plays a role in terminating the postsynaptic action of glutamate | |
| Solute carrier family 1 member 7 (SLC1A7 *) ↓ | Uptake of L-glutamate. Potentially plays a role in terminating the postsynaptic action of glutamate | |
| Solute carrier family 12 member 5 (SLC12A5 *#&) ↑ | Potassium-chloride cotransport in GABA-A and glycine neurons | |
| Other receptors | G protein-coupled receptor 108 (GPR108 *) ↑ | Regulation of immune response |
| G protein-coupled receptor 27 (GPR27) ↑ | Orphan receptor | |
| LDL receptor-related protein 4 (LRP4 *) ↓ | Regulator of Wnt signaling, neuron differentiation | |
| LDL receptor-related protein 8 (LRP8) ↑ | Participates in brain development | |
| Oxoglutarate receptor 1 (OXGR1 *) ↑ | Inflammation regulation | |
| Vasoactive intestinal peptide receptor 2 (VIPR2) ↓ | Receptor activated by the neuropeptides |
| GEO Dataset | Brain Region | Sample Size (Male) (MDD/Control) | Sample Size (Female) (MDD/Control) | |logFC| > 0.5 and p-Value < 0.05 | |logFC| > 1 and Adjusted p-Value < 0.05 | ||
|---|---|---|---|---|---|---|---|
| N of DEGs (Male) | N of DEGs (Female) | N of DEGs (Male) | N of DEGs (Female) | ||||
| GSE80655 | Anterior cingulate gyrus | 18/21 | 6/3 | 70 | 416 | 0 | 0 |
| Dorsolateral prefrontal cortex | 17/21 | 6/3 | 57 | 451 | 0 | 33 | |
| Nucleus accumbens | 16/20 | 6/2 | 193 | 469 | 1 | 5 | |
| GSE102556 | Orbitofrontal cortex | 13/13 | 12/9 | 44 | 147 | 0 | 9 |
| Dorsolateral prefrontal cortex | 13/13 | 13/9 | 50 | 291 | 0 | 3 | |
| Cingulate gyrus 25 | 3/8 | 9/7 | 593 | 378 | 51 | 24 | |
| Anterior Insula | 13/13 | 13/9 | 77 | 144 | 0 | 13 | |
| Nucleus Accumbens | 13/13 | 13/9 | 148 | 331 | 9 | 3 | |
| Subiculum | 12/12 | 12/7 | 439 | 390 | 54 | 28 | |
| GSE101521 | Dorsolateral prefrontal cortex | 19/23 | 11/6 | 389 | 58 | 6 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, S.M.; Sukhachev, V.S.; Tarasova, O.A.; Lagunin, A.A.; Poroikov, V.V. Analysis of Genomic and Transcriptomic Data Revealed Key Genes and Processes in the Development of Major Depressive Disorder. Int. J. Mol. Sci. 2025, 26, 9557. https://doi.org/10.3390/ijms26199557
Ivanov SM, Sukhachev VS, Tarasova OA, Lagunin AA, Poroikov VV. Analysis of Genomic and Transcriptomic Data Revealed Key Genes and Processes in the Development of Major Depressive Disorder. International Journal of Molecular Sciences. 2025; 26(19):9557. https://doi.org/10.3390/ijms26199557
Chicago/Turabian StyleIvanov, Sergey M., Vladislav S. Sukhachev, Olga A. Tarasova, Alexey A. Lagunin, and Vladimir V. Poroikov. 2025. "Analysis of Genomic and Transcriptomic Data Revealed Key Genes and Processes in the Development of Major Depressive Disorder" International Journal of Molecular Sciences 26, no. 19: 9557. https://doi.org/10.3390/ijms26199557
APA StyleIvanov, S. M., Sukhachev, V. S., Tarasova, O. A., Lagunin, A. A., & Poroikov, V. V. (2025). Analysis of Genomic and Transcriptomic Data Revealed Key Genes and Processes in the Development of Major Depressive Disorder. International Journal of Molecular Sciences, 26(19), 9557. https://doi.org/10.3390/ijms26199557








