A Comprehensive Review of Robinetin: Distribution, Biological Activity and Pharmacokinetic Parameters
Abstract
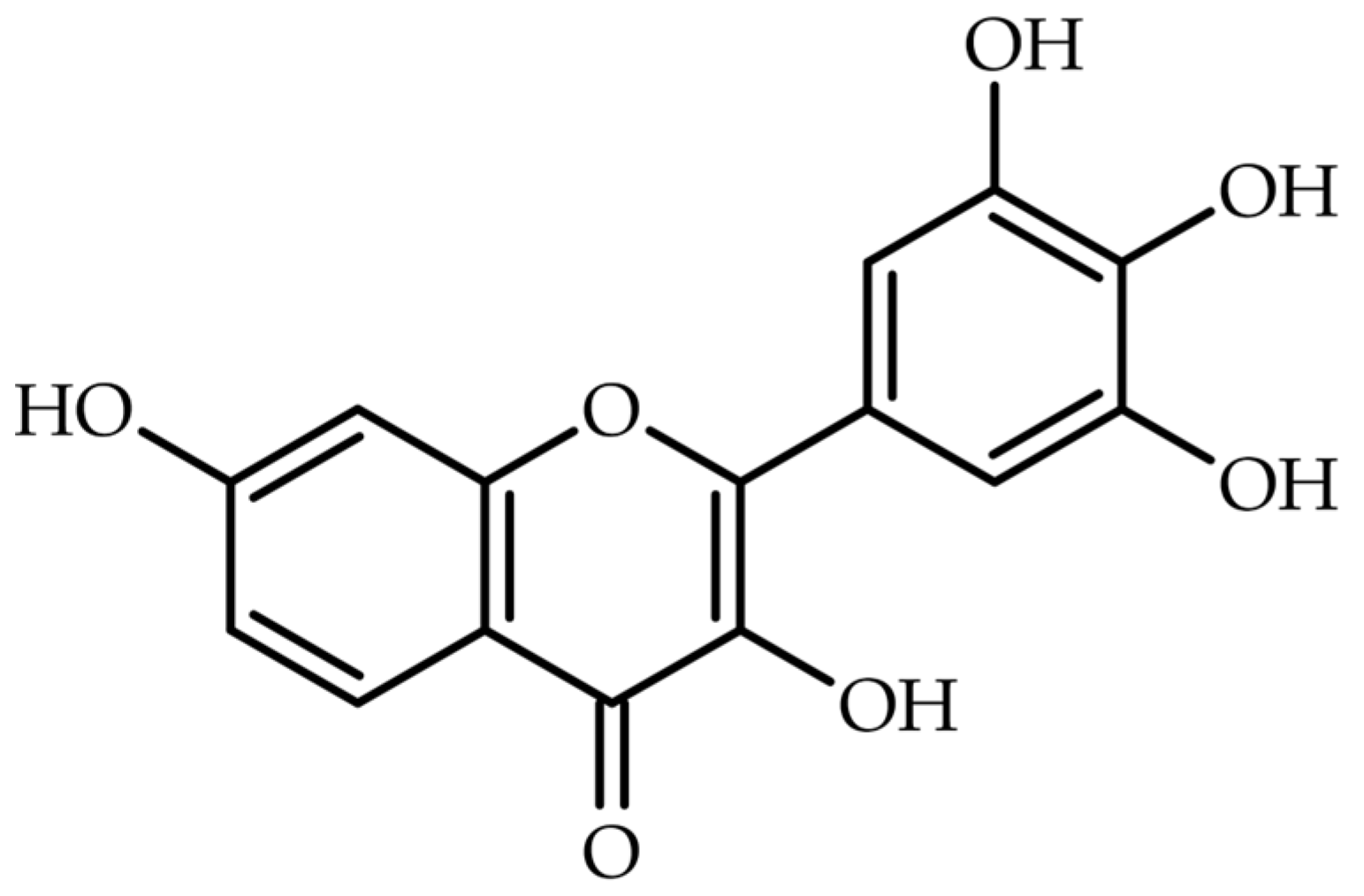
1. Introduction
2. Methodology
3. Natural Occurrence of Robinetin
4. Techniques for the Analysis of Robinetin in Plant Material
5. Biological Activities of Robinetin
5.1. Antiviral Activity
5.2. Antibacterial Activity
5.3. Antiparasitic Activity
5.4. Antioxidant Activity
5.5. Anticancer Activity
5.6. Anti-Mutagenic Activity
5.7. Anti-Necroptosis Activity
5.8. Enzyme Inhibition Activity
5.9. Activity in the Liver Diseases
6. ADMET of Robinetin
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PHF | polyhydroxylated flavonoids |
| HIV | human immunodeficiency virus |
| TG | triglyceride |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| HAT | histone acetyltransferase |
| SET-PT | single electron transfer followed by proton transfer |
| SPLET | sequential proton loss electron transfer |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NRU | neutral red uptake |
| DFT | density functional theory |
| Mpro | main protease |
| DMSO | dimethyl sulfoxide |
| HSA | human serum albumin |
| AD | Alzheimer disease |
| MeOH | methanol |
| HPLC | high-performance liquid chromatography |
| TLC | thin-layer chromatography |
| Et2O | diethyl ether |
| EtOAc | ethyl acetate |
| PhA | phosphoric acid |
| ACN | acetonitrile |
| NH4OAC | ammonium acetate |
| FA | aormic acid |
| MDR | multidrug-resistant |
| MNU | methylnitrosourea |
| MNNG | methyl-n-nltro-N-nitrosoguanidine |
| BaP | benzo-α-pyrene |
| 2-AA | 2-aminoanthracene |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| FRET | fluorescence resonance energy transfer |
| MIC | minimum inhibitory concertation |
| CDK1 | cyclin-dependent kinase 1 |
| BHP | tert-butyl hydroperoxide |
| CHP | cumene hydroperoxide |
| MEF | mouse embryonic fibroblasts |
| WD | western diet |
| OGTT | oral glucose tolerance test |
| ADMET | absorption, distribution, metabolism, excretion and toxicity |
References
- Li, C.; Dai, T.; Chen, J.; Chen, M.; Liang, R.; Liu, C.; Du, L.; McClements, D.J. Modification of flavonoids: Methods and influences on biological activities. Crit. Rev. Food Sci. Nutr. 2022, 63, 10637–10658. [Google Scholar] [CrossRef] [PubMed]
- Ayyanna, C.; Kuppusamy, S.; Kumar, P.P. An overview of pharmacological activities and beneficial effects of 3-hydroxyflavone. Macromol. Symp. 2024, 413, 2300078. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef]
- Walle, T. Absorption and metabolism of flavonoids. Free. Radic. Biol. Med. 2004, 36, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Lomozová, Z.; Hrubša, M.; Conte, P.F.; Papastefanaki, E.; Moravcová, M.; Catapano, M.C.; Proietti Silvestri, I.; Karlíčková, J.; Kučera, R.; Macáková, K.; et al. The effect of flavonoids on the reduction of cupric ions, the copper-driven fenton reaction and copper-triggered haemolysis. Food Chem. 2022, 394, 133461. [Google Scholar] [CrossRef]
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčková, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of iron and copper by quercetin b-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based fenton chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937. [Google Scholar] [CrossRef]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and flavonols’ antiradical structure– activity relationship—A quantum chemical study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Guharay, J.; Sengupta, P.K. Excited-state proton-transfer and dual fluorescence of robinetin in different environments. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 905–912. [Google Scholar] [CrossRef]
- Faisal Hayat, M.; Ur Rahman, A.; Tahir, A.; Batool, M.; Ahmed, Z.; Atique, U. Palliative potential of robinetin to avert polystyrene microplastics instigated pulmonary toxicity in rats. J. King Saud Univ. Sci. 2024, 36, 103348. [Google Scholar] [CrossRef]
- Li, H.; Sun, M.; Lei, F.; Liu, J.; Chen, X.; Li, Y.; Wang, Y.; Lu, J.; Yu, D.; Gao, Y.; et al. Methyl rosmarinate is an allosteric inhibitor of SARS-CoV-2 3 CL protease as a potential candidate against SARS-Cov-2 infection. Antivir. Res. 2024, 224, 105841. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, M.; Sladonja, B.; Šola, I.; Dudaš, S.; Bilić, J.; Famuyide, I.M.; McGaw, L.J.; Eloff, J.N.; Mikulic-Petkovsek, M.; Poljuha, D. Invasive alien species as a potential source of phytopharmaceuticals: Phenolic composition and antimicrobial and cytotoxic activity of Robinia pseudoacacia L. leaf and flower extracts. Plants 2023, 12, 2715. [Google Scholar] [CrossRef]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia Mearnsii De Wild. Bark: Tannin determination and biological activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Sakshi; Chauhan, M.; Dabas, A.; Arya, V. A Comprehensive insight into the phytochemical, pharmacological potential, and traditional medicinal uses of Albizia lebbeck (L.) Benth. eCAM 2022, 2022, 5359669. [Google Scholar] [CrossRef]
- Geronço, M.S.; Melo, R.C.; Mendes Barros, H.L.; Aquino, S.R.; Evangelista de Oliveira, F.d.C.; Islam, M.T.; do Ó Pessoa, C.; dos Santos Rizzo, M.; da Costa, M.P. Advances in the research of Adenanthera pavonina: From traditional use to intellectual property. J. Med. Plants Res. 2020, 14, 13–23. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Traditional uses, phytochemistry and pharmacology of the medicinal species of the genus Cordia (Boraginaceae). J. Pharm. Pharmacol. 2017, 69, 755–789. [Google Scholar] [CrossRef]
- Ahda, M.; Jaswir, I.; Khatib, A.; Ahmed, Q.U.; Syed Mohamad, S.N.A. A review on Cosmos caudatus as a potential medicinal plant based on pharmacognosy, phytochemistry, and pharmacological activities. Int. J. Food Prop. 2023, 26, 344–358. [Google Scholar] [CrossRef]
- Germanò, M.P.; Certo, G.; D’Angelo, V.; Sanogo, R.; Malafronte, N.; De Tommasi, N.; Rapisarda, A. Anti-angiogenic activity of Entada africana root. Nat. Prod. Res. 2015, 29, 1551–1556. [Google Scholar] [CrossRef]
- Koch, M.; Kehop, D.A.; Kinminja, B.; Sabak, M.; Wavimbukie, G.; Barrows, K.M.; Matainaho, T.K.; Barrows, L.R.; Rai, P.P. An ethnobotanical survey of medicinal plants used in the east sepik province of Papua New Guinea. J. Ethnobiol. Ethnomed. 2015, 11, 79. [Google Scholar] [CrossRef]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- Roux, D.G. Recent advances in the chemistry and chemical utilization of the natural condensed tannins. Phytochemistry 1972, 11, 1219–1230. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Afolayan, A.J. Pharmacological assessment of the medicinal potential of acacia mearnsii de wild: Antimicrobial and toxicity activities. Int. J. Mol. Sci. 2012, 13, 4255–4267. [Google Scholar] [CrossRef]
- Phoraksa, O.; Vongthip, W.; Juntranggoor, P.; Maiuthed, A.; Tuntipopipat, S.; Charoenkiatkul, S.; Tencomnao, T.; Muangnoi, C.; Sukprasansap, M. Bioavailable fraction from the edible leaf of Albizia lebbeck (L.) Benth. inhibits neurotoxicity in human microglial HMC3 cells and promotes lifespan in Caenorhabditis elegans. Int. J. Food Sci. Technol. 2025, 60, vvae067. [Google Scholar] [CrossRef]
- El-Shazly, M.; Mansour, M.; Elsayed, E.; Elgindi, M.; Singab, A.-N. Characterization of essential oils of the leaves and fruits of Adenanthera pavonina L. by GC/MS. APSU-ASU 2020, 4, 63–69. [Google Scholar] [CrossRef]
- Malan, J.C.S.; Young, D.A.; Steenkamp, J.A.; Ferreira, D. Oligomeric flavanoids. Part 2. The first profisetinidins with dihydroflavonol constituent units. J. Chem. Soc. 1988, 9, 2567–2572. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, W.; Alsallameh, S.M.S.; Albekairi, N.A.; Muhseen, Z.T.; Butch, C.J. Unraveling Cordia myxa’s anti-malarial potential: Integrative insights from network pharmacology, molecular modeling, and machine learning. BMC Infect. Dis. 2024, 24, 1180. [Google Scholar] [CrossRef] [PubMed]
- Sumarno, L.; Hendrawati, Y.T.; Ramadhan, I.A.; Yustinah; Hasyim, H.U.; Nugrahani, A.R.; Novika, H.R.G.; Setianto, W.B.; Nasruddin; Yohanes, H. Separation and characterization of kenikir leaf extract (Cosmos caudatus Kunt) in potential as a natural antioxidant. API Conf. Proc. 2025, 3166, 020013. [Google Scholar] [CrossRef]
- Dewi, R.T.; Ekapratiwi, Y.; Sundowo, A.; Ariani, N.; Yolanda, T.; Filaila, E. Bioconversion of quercetin glucosides from Dendrophthoe pentandra leaf using Aspergillus acueletus LS04-3. AIP Conf. Proc. 2019, 2175, 020048. [Google Scholar] [CrossRef]
- Hashem, F.A. Phenolic compounds of Erucaria microcarpa Boiss. and their effect as scavengers for singlet oxygen. J. Herbs. Spices Med. Plants 2006, 12, 27–41. [Google Scholar] [CrossRef]
- Jurd, L.; Manners, G.D. Isoflavene, isoflavan, and flavonoid constituents of Gliricidia speium. J. Agric. Food Chem. 1977, 25, 723–726. [Google Scholar] [CrossRef]
- Wafaey, A.A.; El-Hawary, S.S.; Kirollos, F.N.; Abdelhameed, M.F. An overview on Gliricidia sepium in the pharmaceutical aspect: A review article. Egypt. J. Chem. 2023, 66, 479–496. [Google Scholar] [CrossRef]
- Astuti, S.D.; Widyobroto, B.P.; Agus, A.; Yusiati, L.M. Identification of galactogogues in Gliricidia maculata. IOP Conf. Ser. Earth Environ. Sci. 2019, 387, 012119. [Google Scholar] [CrossRef]
- Wahman, R.; Moser, S.; Bieber, S.; Cruzeiro, C.; Schröder, P.; Gilg, A.; Lesske, F.; Letzel, T. Untargeted analysis of Lemna minor metabolites: Workflow and prioritization strategy comparing highly confident features between different mass spectrometers. Metabolites 2021, 11, 832. [Google Scholar] [CrossRef]
- Sosa, D.; Alves, F.M.; Prieto, M.A.; Pedrosa, M.C.; Heleno, S.A.; Barros, L.; Feliciano, M.; Carocho, M. Lemna Minor: Unlocking the value of this duckweed for the food and feed industry. Foods 2024, 13, 1435. [Google Scholar] [CrossRef]
- Hillis, W.E. Formation of robinetin crystals in vessels of Intsia species. IAWA J. 1996, 17, 405–419. [Google Scholar] [CrossRef]
- Angio, M.H.; Renjana, E.; Firdiana, E.R. Morphology characterization and phytochemical overview of the moluccan ironwood Intsia bijuga (Colebr.) kuntze, a living collection of purwodadi botanic garden, Indonesia. J. Threat. Taxa 2022, 14, 21853–21861. [Google Scholar] [CrossRef]
- Norscia, I.; Borgognini-Tarli, S.M. Ethnobotanical reputation of plant species from two forests of madagascar: A preliminary investigation. South Afr. J. Bot. 2006, 72, 656–660. [Google Scholar] [CrossRef]
- Sari, R.K.; Prayogo, Y.H.; Sari, R.A.L.; Asidah, N.; Rafi, M.; Wientarsih, I.; Darmawan, W. Intsia bijuga heartwood extract and its phytosome as tyrosinase inhibitor, antioxidant, and sun protector. Forests 2021, 12, 1792. [Google Scholar] [CrossRef]
- Hawthorne, B.J.; Morgan, J.W.W. The colouring matter from Millettia stuhlamannii. Chem. Ind. 1962, 33, 1504–1505. [Google Scholar]
- Hong, Z.; Xie, J.; Hu, H.; Bai, Y.; Hu, X.; Li, T.; Chen, J.; Sheng, J.; Tian, Y. Hypoglycemic effect of Moringa oleifera leaf extract and its mechanism prediction based on network pharmacology. J. Futur. Foods 2023, 3, 383–391. [Google Scholar] [CrossRef]
- Liu, R.; Liu, J.; Huang, Q.; Liu, S.; Jiang, Y. Moringa oleifera: A systematic review of its botany, traditional uses, phytochemistry, pharmacology and toxicity. J. Pharm. Pharmacol. 2022, 74, 296–320. [Google Scholar] [CrossRef]
- Hanaki, M.; Murakami, K.; Gunji, H.; Irie, K. Activity-differential search for amyloid-β aggregation inhibitors using LC-MS combined with principal component analysis. Bioorg. Med. Chem. Lett. 2022, 61, 128613. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Pinthong, D.; Hano, C. Flavonoids from Nelumbo nucifera Gaertn., a medicinal plant: Uses in traditional medicine, phytochemistry and pharmacological activities. Medicines 2018, 5, 127. [Google Scholar] [CrossRef]
- Pal, I.; Dey, P. A Review on lotus (Nelumbo nucifera) seed. Intern J. Scien Res. (IJSR) 2013, 14, 2319–7064. [Google Scholar]
- Charlesworth, E.H.; Robinson, R. 73. Anthoxanthins. Part XIII. Synthesis of a colouring matter of Robinia pseudoacacia. J. Chem. Soc. 1933, 0, 268–270. [Google Scholar] [CrossRef]
- Bostyn, S.; Destandau, E.; Charpentier, J.P.; Serrano, V.; Seigneuret, J.M.; Breton, C. optimization and kinetic modelling of robinetin and dihydrorobinetin extraction from Robinia pseudoacacia wood. Ind. Crop. Prod. 2018, 126, 22–30. [Google Scholar] [CrossRef]
- Sanz, M.; De Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; Hernández, M.T.; Estrella, I. Polyphenolic profile as a useful tool to identify the wood used in wine aging. Anal. Chim. Acta 2012, 732, 33–45. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Alvarez-Fernández, M.A.; Hornedo-Ortega, R.; Troncoso, A.M.; García-Parrilla, M.C. Phenolic composition of vinegars over an accelerated aging process using different wood species (acacia, cherry, chestnut, and oak): Effect of wood toasting. J. Agric. Food Chem. 2014, 62, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Fernández de Simón, B.; Esteruelas, E.; Muñoz, Á.M.; Cadahía, E.; Hernández, M.T.; Estrella, I.; Martinez, J. Polyphenols in red wine aged in acacia (Robinia pseudoacacia) and oak (Quercus petraea) wood barrels. Anal. Chim. Acta 2012, 732, 83–90. [Google Scholar] [CrossRef]
- Sánchez-Monedero, A.; Cañadas, R.; Martín-Sampedro, R.; Santos, J.I.; Eugenio, M.E.; Ibarra, D. A Sustainable valorization approach of kraft black liquor from Robinia pseudoacacia L.: Lignin precipitation followed by green solvents extraction of phenols from delignified liquor. J. Environ. Manag. 2025, 391, 126515. [Google Scholar] [CrossRef]
- Zarev, Y. Isolation and characterization of 3-O-caffeoyloleanolic acid from Robinia pseudoacacia stem bark. Pharmacia 2023, 70, 1209–1212. [Google Scholar] [CrossRef]
- Smith, A.L.; Campbell, C.L.; Walker, D.B.; Hanover, J.W. Extracts from black locust as wood preservatives: Extraction of decay resistance from black locust heartwood. Holzforschung 1989, 43, 293–296. [Google Scholar] [CrossRef]
- Tian, F.; McLaughlin, J.L. Bioactive flavonoids from the black locust tree, Robinia pseudoacacia. Pharm. Biol. 2000, 38, 229–234. [Google Scholar] [CrossRef]
- Destandau, E.; Charpentier, J.P.; Bostyn, S.; Zubrzycki, S.; Serrano, V.; Seigneuret, J.M.; Breton, C. Gram-scale purification of dihydrorobinetin from Robinia pseudoacacia L. wood by centrifugal partition chromatography. Separations 2016, 3, 23. [Google Scholar] [CrossRef]
- Sanz, M.; Fernández De Simón, B.; Esteruelas, E.; Muñoz, Á.M.; Cadahía, E.; Hernández, T.; Estrella, I.; Pinto, E. Effect of toasting intensity at cooperage on phenolic compounds in acacia (Robinia pseudoacacia) heartwood. J. Agric. Food Chem. 2011, 59, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Guran, R.; Zitka, O.; Visi-Rajczi, E.; Albert, L. Liquid chromatographic/mass spectrometric study on the role of beech (Fagus sylvatica L.) wood polyphenols in red heartwood formation. Forests 2022, 13, 10. [Google Scholar] [CrossRef]
- Hosseinihashemi, S.K.; Safdari, V.; Kanani, S. Comparative chemical composition of n-hexane and ethanol extractives from the heartwood of black locust. Asian J. Chem. 2013, 25, 929–933. [Google Scholar] [CrossRef]
- Madanat, H.M.; Al-Antary, T.M.; Abu Zarga, M. Identification and isolation of the insecticidal compounds from Robinia pseudoacacia L. (Fabaceae). Fresenius Environ. Bull 2018, 27, 1838–1849. [Google Scholar]
- Scheidemann, P.; Wetzel, A. Identification and characterization of flavonoids in the root exudate of Robinia pseudoacacia. Trees 1997, 11, 316–321. [Google Scholar] [CrossRef]
- Nasir, H.; Iqbal, Z.; Hiradate, S.; Fujii, Y. Allelopathic potential of Robinia pseudo-acacia L. J. Chem. Ecol. 2005, 31, 2179–2192. [Google Scholar] [CrossRef] [PubMed]
- Vek, V.; Poljanšek, I.; Oven, P. Efficiency of three conventional methods for extraction of dihydrorobinetin and robinetin from wood of black locust. Eur. J. Wood Wood Prod. 2019, 77, 891–901. [Google Scholar] [CrossRef]
- Yandamuri, N.; Nagabathula, S.; Kurra, S.; Batthula, S.; Allada, N.; Bandam, P. Comparative study of new trends in HPLC. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 52–57. [Google Scholar]
- Garcia, S.; Heinzen, H.; Martinez, R.; Moyna, P. Identification of flavonoids by TLC scanning analysis. Chromatographia 1993, 35, 430–434. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wu, T.; Zhou, X.; Shao, Y. Triggered excited-state intramolecular proton transfer fluorescence for selective triplex DNA recognition. Anal. Chem. 2015, 87, 11620–11624. [Google Scholar] [CrossRef]
- Vek, V.; Poljanšek, I.; Oven, P. Variability in content of hydrophilic extractives and individual phenolic compounds in black locust stem. Eur. J. Wood Wood Prod. 2020, 78, 501–511. [Google Scholar] [CrossRef]
- Pahari, B.P.; Chaudhuri, S.; Chakraborty, S.; Sengupta, P.K. Ground and excited state proton transfer of the bioactive plant flavonol robinetin in a protein environment: Spectroscopic and molecular modeling studies. J. Phys. Chem. B 2015, 119, 2533–2545. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Pahari, B.; Sengupta, B.; Sengupta, P.K. Binding of the bioflavonoid robinetin with model membranes and hemoglobin: Inhibition of lipid peroxidation and protein glycosylation. J. Photochem. Photobiol. B 2010, 98, 12–19. [Google Scholar] [CrossRef]
- Fesen, M.R.; Pommier, Y.; Leteurtre, F.; Hiroguchi, S.; Yung, J.; Kohn, K.W. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem. Pharmacol. 1994, 48, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 162750, 162750. [Google Scholar] [CrossRef]
- Mahmud, S.; Uddin, M.A.R.; Paul, G.K.; Shimu, M.S.S.; Islam, S.; Rahman, E.; Islam, A.; Islam, M.S.; Promi, M.M.; Emran, T.B.; et al. Virtual screening and molecular dynamics simulation study of plant-derived compounds to identify potential inhibitors of main protease from SARS-CoV-2. Brief. Bioinform. 2021, 22, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Krüger, N.; Kronenberger, T.; Xie, H.; Rocha, C.; Pöhlmann, S.; Su, H.; Xu, Y.; Laufer, S.A.; Pillaiyar, T. Discovery of polyphenolic natural products as SARS-CoV-2 Mpro inhibitors for COVID-19. Pharmaceuticals 2023, 16, 190. [Google Scholar] [CrossRef]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. [Google Scholar] [CrossRef]
- Banerjee, A.; Basu, K.; Sengupta, P.K. Effect of β-cyclodextrin nanocavity confinement on the photophysics of robinetin. J. Photochem. Photobiol. B 2007, 89, 88–97. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Menacer, R.; Rekkab, S.; Kabouche, Z. Fisetin and robinetin antiradical activity under solvent effect: Density functional theory study. J. Mol. Model. 2022, 28, 240. [Google Scholar] [CrossRef]
- Butkovic, V.; Klasinc, L.; Bors, W. Kinetic study of flavonoid reactions with stable radicals. J. Agric. Food Chem. 2004, 52, 2816–2820. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Szoka, Ł.; Surazynski, A.; Tomczyk, M. Anticancer effect of selected flavonoids on tongue squamous cell carcinoma (SCC-25): In vitro study. Acta Pol. Pharm. 2025, 82, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Jakimiuk, K.; Szoka, Ł.; Surażyński, A.; Tomczyk, M. Using flavonoid substitution status to predict anticancer effects in human melanoma cancers: An in vitro study. Cancers 2024, 16, 487. [Google Scholar] [CrossRef] [PubMed]
- Ogbodo, U.C.; Enejoh, O.A.; Okonkwo, C.H.; Gnanasekar, P.; Gachanja, P.W.; Osata, S.; Atanda, H.C.; Iwuchukwu, E.A.; Achilonu, I.; Awe, O.I. Computational identification of potential inhibitors targeting cdk1 in colorectal cancer. Front. Chem. 2023, 11, 1264808. [Google Scholar] [CrossRef]
- Chang, R.L.; Huang, M.T.; Wood, A.W.; Wong, C.Q.; Newmark, H.L.; Yagi, H.; Sayer, J.M.; Jerina, D.M.; Conney, A.H. Effect of ellagic acid and hydroxylated flavonoids on the tumorigenicity of benzo-γ-pyrene and (±)-7β, 8α-dihydroxy-9α, 10α-epoxy-7,8,9,10-Tetrahydrobenzo-α-pyrene on mouse skin and in the newborn mouse. Carcinogenesis 1985, 6, 1127–1133. [Google Scholar] [CrossRef]
- Ugocsai, K.; Varga, A.; Molnár, P.; Antus, S.; Molnár, J. Effects of selected flavonoids and carotenoids on drug accumulation and apoptosis induction in multidrug-resistant colon cancer cells expressing MDR1/LRP. In Vivo 2005, 19, 433–438. [Google Scholar]
- Richter, M.; Ebermann, R.; Marian, B. Quercetin-induced apoptosis in colorectal tumor cells: Possible role of EGF receptor signaling. Nutr. Cancer 1999, 34, 88–99. [Google Scholar] [CrossRef]
- Birt, D.F.; Walker, B.; Tibbels, M.G.; Bresnick, E. Anti-mutagenesis and anti-promotion by apigenin, robinectin and indole-3-carbinol. Carcinogenesis 1986, 7, 1617–1619. [Google Scholar] [CrossRef]
- Bhattacharya, R.K.; Firozi, P.F. Effect of plant flavonoids on microsome catalyzed reactions of aflatoxin B1, leading to activation and DNA adduct formation. Cancer Lett. 1988, 39, 85–91. [Google Scholar] [CrossRef]
- Xie, H.; Li, W.; Han, X.; Li, M.; Zhao, Q.; Xu, Y.; Su, H.; Meng, W. Identification of RIPK3 as a target of flavonoids for anti-necroptosis in vitro. Bioorg. Chem. 2025, 161, 108503. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Nazaruk, D.; Tomczyk, M. Acetylcholinesterase inhibitors: Structure-activity relationship and kinetic studies on selected flavonoids. Acta Pol. Pharm. 2022, 79, 835–840. [Google Scholar] [CrossRef]
- Van Zanden, J.J.; Wortelboer, H.M.; Bijlsma, S.; Punt, A.; Usta, M.; Bladeren, P.J.V.; Rietjens, I.M.C.M.; Cnubben, N.H.P. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem. Pharmacol. 2005, 69, 699–708. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, H.J.; Lee, J.; Hong, S.P.; Chung, M.Y.; Lee, Y.G.; Park, J.H.; Choi, H.K.; Hwang, J.T. Robinetin alleviates metabolic failure in liver through suppression of P300–CD38 axis. Biomol. Ther. 2024, 32, 214–223. [Google Scholar] [CrossRef]
- Añón, M.T.; Ubeda, A.; Alcaraz, M.J.H. Protective effects of phenolic compounds on CcL4-induced toxicity in isolated rat hepatocytes. Z Naturforsch. C 1992, 47, 275–279. [Google Scholar] [CrossRef]
- Manrique-de-la-Cuba, M.F.; Gamero-Begazo, P.; Valencia, D.E.; Barazorda-Ccahuana, H.L.; Gómez, B. Theoretical study of the antioxidant capacity of the flavonoids present in the Annona muricata (Soursop) leaves. J. Mol. Model. 2019, 25, 200. [Google Scholar] [CrossRef]
- Huguet, A.I.; Máñez, S.; Alcaraz, M.J. Superoxide scavenging properties of flavonoids in a non-enzymic system. Z Naturforsch. C 1990, 45, 19–24. [Google Scholar] [CrossRef]
- Takekoshi, S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014, 39, 116–121. [Google Scholar]
- Brown, J.P.; Dietrich, P.S. Mutagenicity of plant flavonols in the Salmonella/Mammalian microsome test. activation of flavonol glycosides by mixed glycosidases from rat cecal bacteria and other sources. Mutat. Res. Genet. Toxicol. Environ. Mutagen 1979, 66, 223–240. [Google Scholar] [CrossRef]
- Edenharder, R.; Grünhage, D. Free radical scavenging abilities of flavonoids as mechanism of protection against mutagenicity induced by tert-butyl hydroperoxide or cumene hydroperoxide in Salmonella typhimurium TA102. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2003, 540, 1–18. [Google Scholar] [CrossRef]
- Hodnick, W.F.; Duval, D.L.; Pardini, R.S. Inhibition of mitochondrial respiration and cyanide-stimulated generation of reactive oxygen species by selected flavonoids. Biochem. Pharmacol. 1994, 47, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, L.A.; Smith, G.E. Metabolism of myricetin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem. J. 1972, 130, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://biosig.lab.uq.edu.au/deeppk/prediction (accessed on 9 September 2025).
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of drugs—The current state of knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef] [PubMed]
| Plant | Family | Plant Part | Traditional Use | References |
|---|---|---|---|---|
| Acacia mearnsii | Fabaceae | bark | microbial infections | [15,23,24] |
| Albizia lebbeck | leaves | treatment of ulcers, night blindness, respiratory disorders, skin disorders, snake, bite, piles, leprosy gonorrhea, scorpion bite, cough, pharyngitis | [16,25] | |
| Adenanthera pavonina | seeds, leaves | treatment of boils and inflammations | [17,26] | |
| Burkea africana | bark | not found | [27] | |
| Cordia myxa | Boraginaceae | not given | treatment of wound, boils, tumors, gout and ulcer; blood purifier and febrifuge | [18,28] |
| Cosmos caudatus | Asteraceae | leaves | anti-diabetic, anti-hypertensive, anti-inflammatory, hepatoprotective, antimicrobial, blood circulation booster, bone strengthener, body-cooling agent and anti-aging agent | [19,29] |
| Dendrophthoe pentandra | Loranthaceae | leaves | immunological disorders and cancers | [30] |
| Entada africana | Fabaceae | root | treatment of inflammatory diseases, hepatitis, bronchitis and cough and wound-healing, diuretic, anti-gonococci and anti-syphilitic agent | [20] |
| Erucaria microcarpa | Brassicaceae | aerial parts | not found | [31] |
| Gliricidia sepium | Fabaceae | bark | anti-microbial, antibacterial, anti-inflammatory, thrombolytic, antisickling, wound healing agent | [32,33] |
| Gliricidia maculata | herbal galactogogue | [32,34] | ||
| Lemna minor | Araceae | leaves | human food | [35,36] |
| Intsia bijuga | Fabaceae | bark | rheumatism, dysentery, urinary tract infections, asthma, diabetes, ulcers, fractures | [21,37,38,39,40] |
| Millettia stuhlmannii | not found | [41] | ||
| Moringa oleifera | Moringaceae | leaves | paralysis, helminthiasis, sores and skin infections | [42,43] |
| Nelumbo nucifera | Nelumbonaceae | leaves | hyperlipidemia, hematemesis, metrorrhagia, fever treatment, release skin inflammatory symptoms | [44,45] |
| seeds | tissue inflammation, cancer, diuretics, skin diseases and as poison antidote | [44,46] | ||
| root | circulatory system disorders, diarrhea, insomnia, fever, body heat imbalance and gastritis | [44,45] | ||
| Robinia pseudoacacia | Fabaceae | bark | laxative, antispasmodic, diuretic agent | [22,47,48,49,50,51,52,53,54,55,56,57,58,59,60] |
| leaves | [61,62] |
| Extract | Column | Mobile Phase | Conditions | Ref. |
|---|---|---|---|---|
| Robinia pseudoacacia bark | ||||
| 50% MeOH | Hypersil ODS C18 | 0.1% PhA (A) MeOH; PhA 0.1% (B) | 100–95% A: 0–50 min 95–70% A: 50–85 min 70–0% A: 85–105 min | [66] |
| Et2O, EtOAc lyophilizates (in 50% MeOH) | [57] | |||
| Et2O, EtOAc | 100–85% A: 0–20 min 85–75% A: 20–30 min 75–50% A: 30–50 min 50–0% A: 50–70 min | |||
| 90% acetone | Thermo Accucore C18 | H2O + 0.1% FA (A); MeOH + 0.1% FA (B) | 5–95% of solvent B | [67] |
| Lemna minor leaves | ||||
| 50% MeOH | Poroshell 120 EC-C18; ZIC-HILIC | 10 mM NH4OAC in 9:1 (v/v) H2O–ACN (A); 10 mM NH4OAC in 1:9 (v/v) H2O–ACN (B) (RPLC); ACN (C); H2O (D) (HILIC) |
100–50% A: 7–13 min 50–0% A: 13–33 min 100% A: 33–58 min
100–60% A: 7–13 min 100% C: 13–53 min | [35] |
| Intsia bijuga bark | ||||
| EtOH | Accucore C18 column | H2O + 0.1% FA (A); ACN + 0.1% FA (B) | 5–25% B: 0–3 min 25–55%: 3–22.5 min 55–95%: 22.5–25 min 95% B: 25–28 min 5% B: 29–30 min | [40] |
| Experimental Model | Exposure/ Incubation | Concentration | Efficacy | Ref. |
|---|---|---|---|---|
| ANTIVIRAL | ||||
| HIV integrase catalytic assays | 1 h incubation | 4 µL of sample |
| [70,71,72] |
| SARS-CoV-2 virtual screening | - | - |
| [73] |
| SARS-CoV-2 Mpro inhibition (FRET) | - | 10 µM |
| [74] |
| ANTIBACTERIAL | ||||
| Proteus vulgaris, Staphylococcus aureus | 1 h incubation | not given |
| [71,75] |
| ANTIPARASITIC | ||||
| Leishmania donovani MHOM/ET/67/L82 | 72 h incubation | 30 to 0.041 µg/mL | IC50 = 5.9 µg/mL | [76,77] |
| Trypanosoma brucei rhodesiense SSTIB 900 | 72 h incubation | 90 to 0.123 µg/ml | IC50 = 5.3 µg/mL | [77] |
| Trypanosoma cruzi C2C4 | 96 h incubation | not given | IC50 > 30 µg/mL | |
| ANTIOXIDANT | ||||
| PM7 semiempirical method, HAT, SET-PT, SPLET | - | - | 4′-OH hydroxyl is the preferred active site, HAT mechanism is energetically the most favored pathway | [78] |
| DPPH assay, BDPA assay | not given | not given | Kinetic data: 1.4 × 102 kF/L mol−1 × s−1 in MeOH and 1:1 H2O/2-propanol (v/v) | [79] |
| ANTICANCER | ||||
| SCC-25 cell line | 24, 48 and 72 h incubation in MTT and NRU tests | 6.25–200 µM |
| [80] |
| C32 cell line |
| [81] | ||
| A375 cell line |
| |||
| inhibitory efficacies against CDK1 through molecular docking | - | - | stable within the binding pocket of the CDK1 protein | [82] |
| mice with skin tumors | 20 weeks | 2500 nmol | inhibited the number of tumors per mouse by 16–24% after 15–20 weeks of promotion with TPA | [83] |
| CCL-220.1 and CCL-222 cell lines (Rhodamine 123 accumulation) | 20 min followed by 10 min | 1 mg/mL (stock solution) | Fluorescence activity ratio (in CCL-222 MDR1/LRP-expressing cells):
| [84] |
| SW480 and T84 cell lines | 48 h incubation | not given | IC50 = 100 µM | [85] |
| ANTI-MUTAGENIC | ||||
| Salmonella typhimurium wit mutagenesis induced by MNU, MNNG, BaP, 2-AA | 48 h incubation | not given | % of inhibition:
| [86] |
| liver microsomes from rats (inhibition of aflatoxin B1) | 1 h incubation | not given | Microsome-mediated metabolic activation: 11.4% of control; DNA adduct formation of AFB1: 7.7% of control. | [87] |
| ANTI-NECROPTOSIS | ||||
| MEFs and HT-29 cells | 18–20 h incubation | 10 µM | IC50 = 9.1 µM | [88] |
| ENZYME INHIBITION | ||||
| acetylcholinesterase | 15 min incubation | 50 µL of sample | IC50 = 456.48 ± 2.57 µM | [89] |
| MRP1 and MRP2 | 0 and 45 min incubation | 1, 10, 20, 30, 40 and 50 µM of sample |
| [90] |
| LIVER DISEASES | ||||
| AML-12 hepatocytes, C57/BL6 mice |
| Wester diet (WD) with 0.025%, 0.05% of robinetin |
| [91] |
| liver cells from male Wistar rats | 10 min prior to toxic challenge | 2.4% | ALT activity: 2.16 IU/g wet cells | [92] |
| Property Name | Prediction Result | Predictive Confidence A |
|---|---|---|
| ABSORPTION | ||
| human oral bioavailability 20% | non-bioavailable | low |
| human oral bioavailability 50% | bioavailable | low |
| human intestinal absorption | absorbed | high |
| P-glycoprotein inhibitor | non-inhibitor | high |
| P-glycoprotein substrate | non-substrate | medium |
| DISTRIBUTION | ||
| Blood–Brain Barrier | non-penetrable | high |
| METABOLISM | ||
| CYP 1A2 inhibitor | inhibitor | high |
| CYP 1A2 substrate | substrate | high |
| CYP 3A4 inhibitor | non-inhibitor | medium |
| CYP 3A4 substrate | non-substrate | high |
| OATP1B1 | non-inhibitor | medium |
| EXCRETION | ||
| high-life of drug | <3 h | low |
| TOXICITY | ||
| AMES mutagenesis | safe | medium |
| eye irritation | toxic | low |
| carcinogenesis | safe | high |
| skin sensitization | toxic | medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakimiuk, K. A Comprehensive Review of Robinetin: Distribution, Biological Activity and Pharmacokinetic Parameters. Int. J. Mol. Sci. 2025, 26, 9546. https://doi.org/10.3390/ijms26199546
Jakimiuk K. A Comprehensive Review of Robinetin: Distribution, Biological Activity and Pharmacokinetic Parameters. International Journal of Molecular Sciences. 2025; 26(19):9546. https://doi.org/10.3390/ijms26199546
Chicago/Turabian StyleJakimiuk, Katarzyna. 2025. "A Comprehensive Review of Robinetin: Distribution, Biological Activity and Pharmacokinetic Parameters" International Journal of Molecular Sciences 26, no. 19: 9546. https://doi.org/10.3390/ijms26199546
APA StyleJakimiuk, K. (2025). A Comprehensive Review of Robinetin: Distribution, Biological Activity and Pharmacokinetic Parameters. International Journal of Molecular Sciences, 26(19), 9546. https://doi.org/10.3390/ijms26199546







