Distinct B Cell Subsets Changes as Potential Biomarkers of Response to Biologic Therapy in Crohn’s Disease
Abstract
1. Introduction
2. Results
2.1. Disease Activity Markers in All Recruited Patients with CD upon Entry into Study
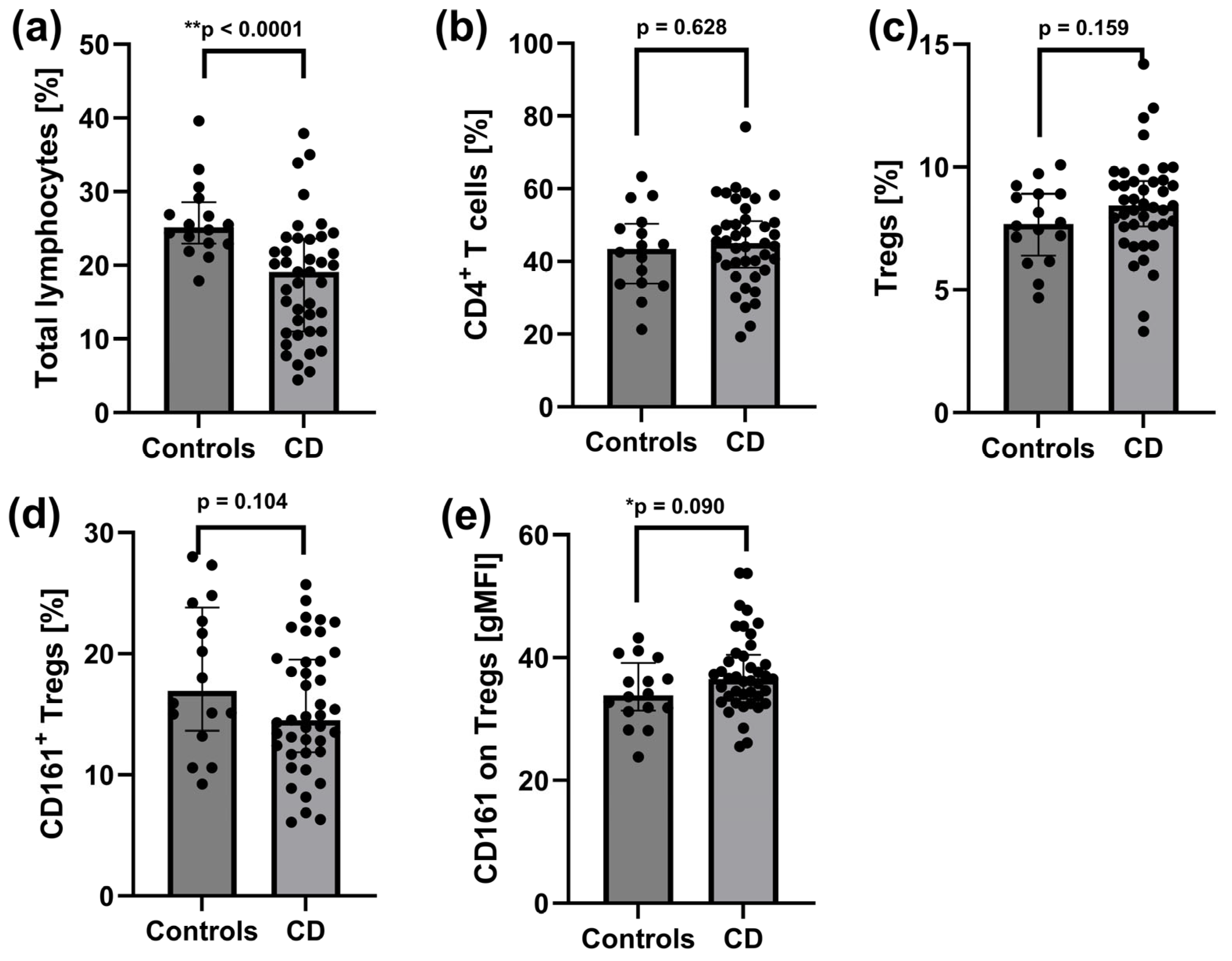
2.2. Reduced Pool of Total Lymphocytes and Increased CD161 Expression on Tregs in Peripheral Blood of Patients with Active CD
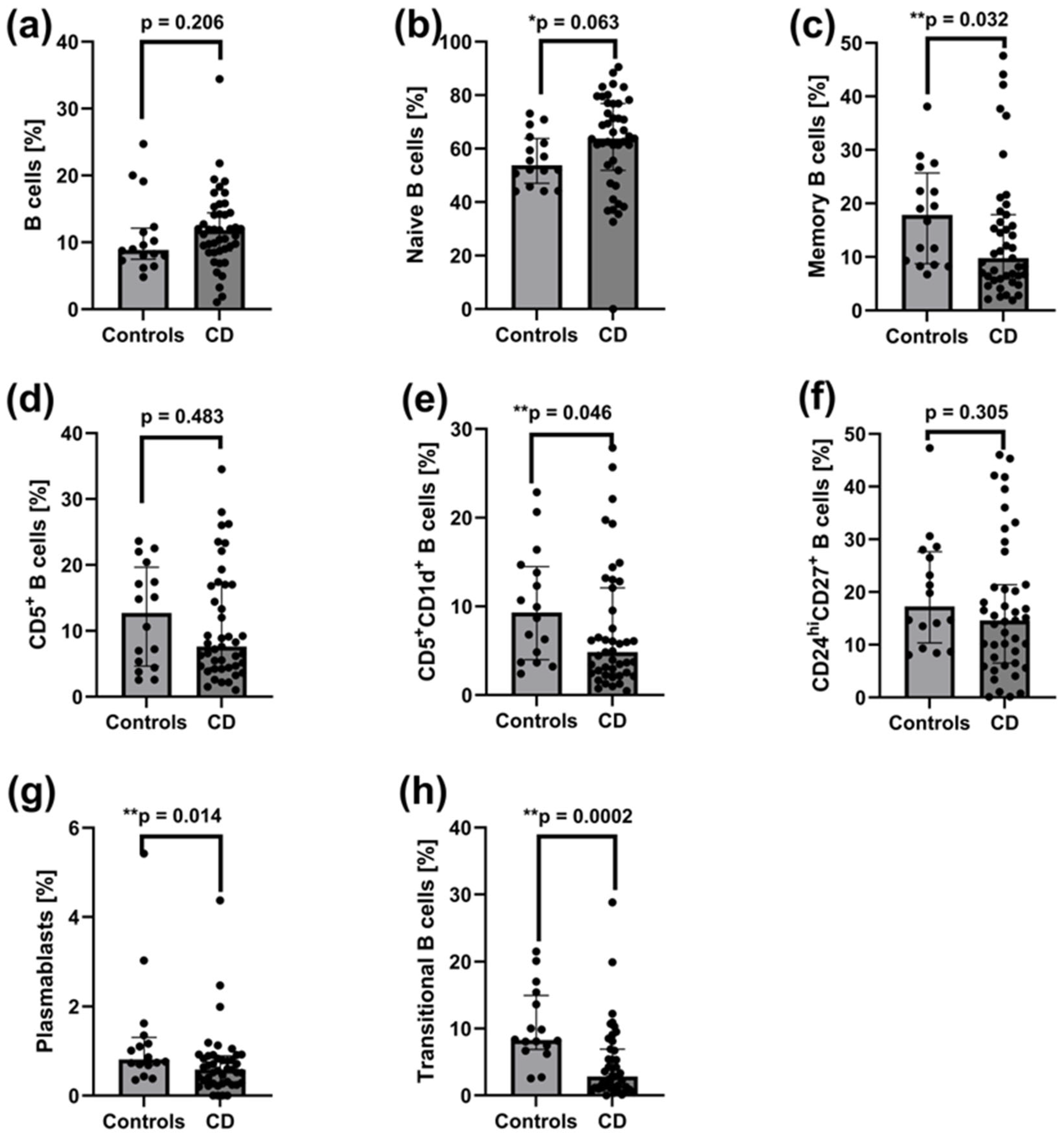
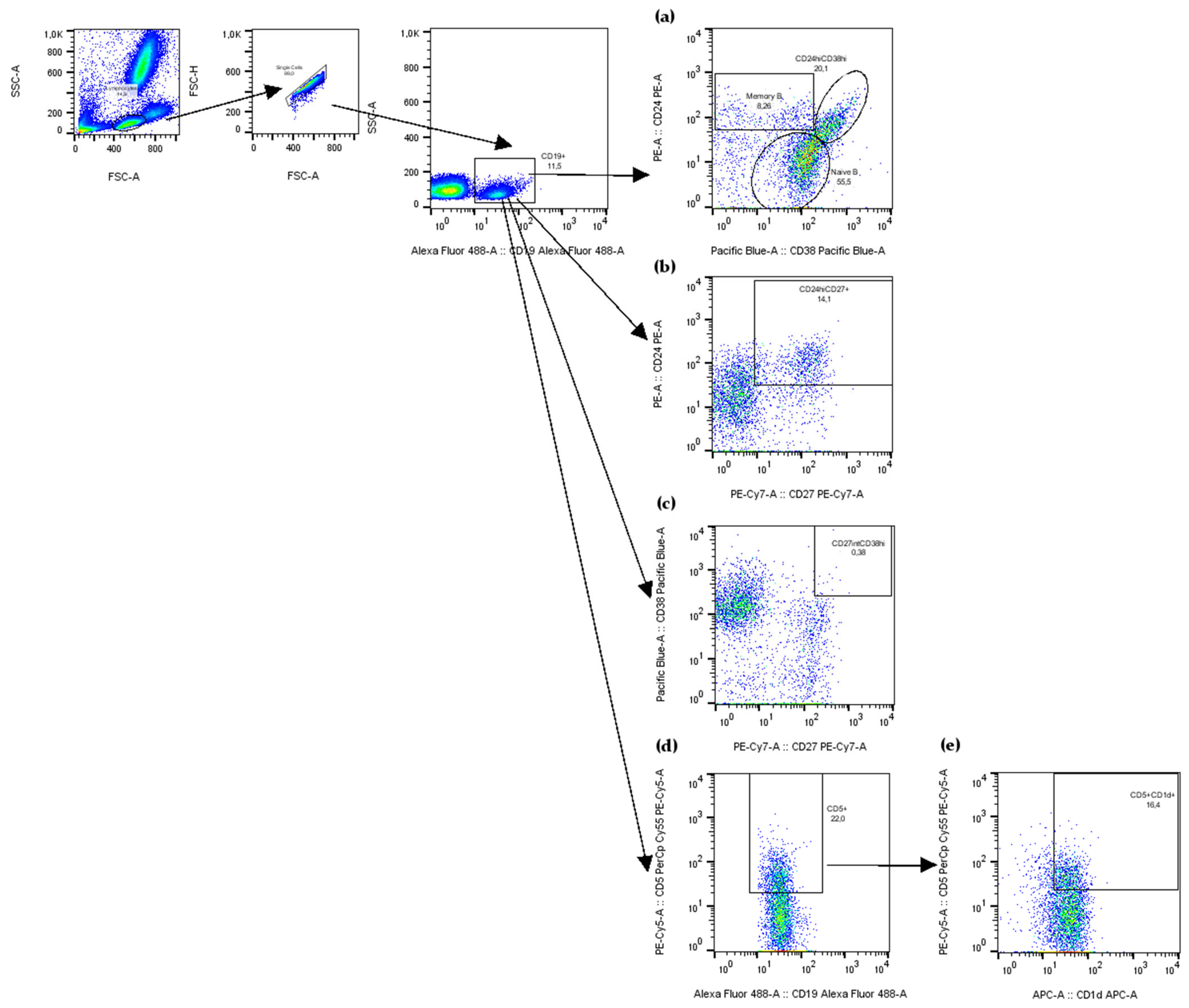
2.3. Decreased Frequencies of Memory B Cells, CD5+CD1d+ B Cells, CD27intCD38hi Plasmablasts, and CD24hiCD38hi Transitional B Cells in Peripheral Blood of Patients with Active CD
2.4. Association of T and B Cell Subset Frequencies with the Disease Activity Markers (i.e., Crohn’s Disease Activity Index, C-Reactive Protein, Faecal Calprotectin, and Platelet Count) in Patients with Active CD
2.5. Comparable Disease Activity Markers Between Pre-Treatment CD Patient Groups
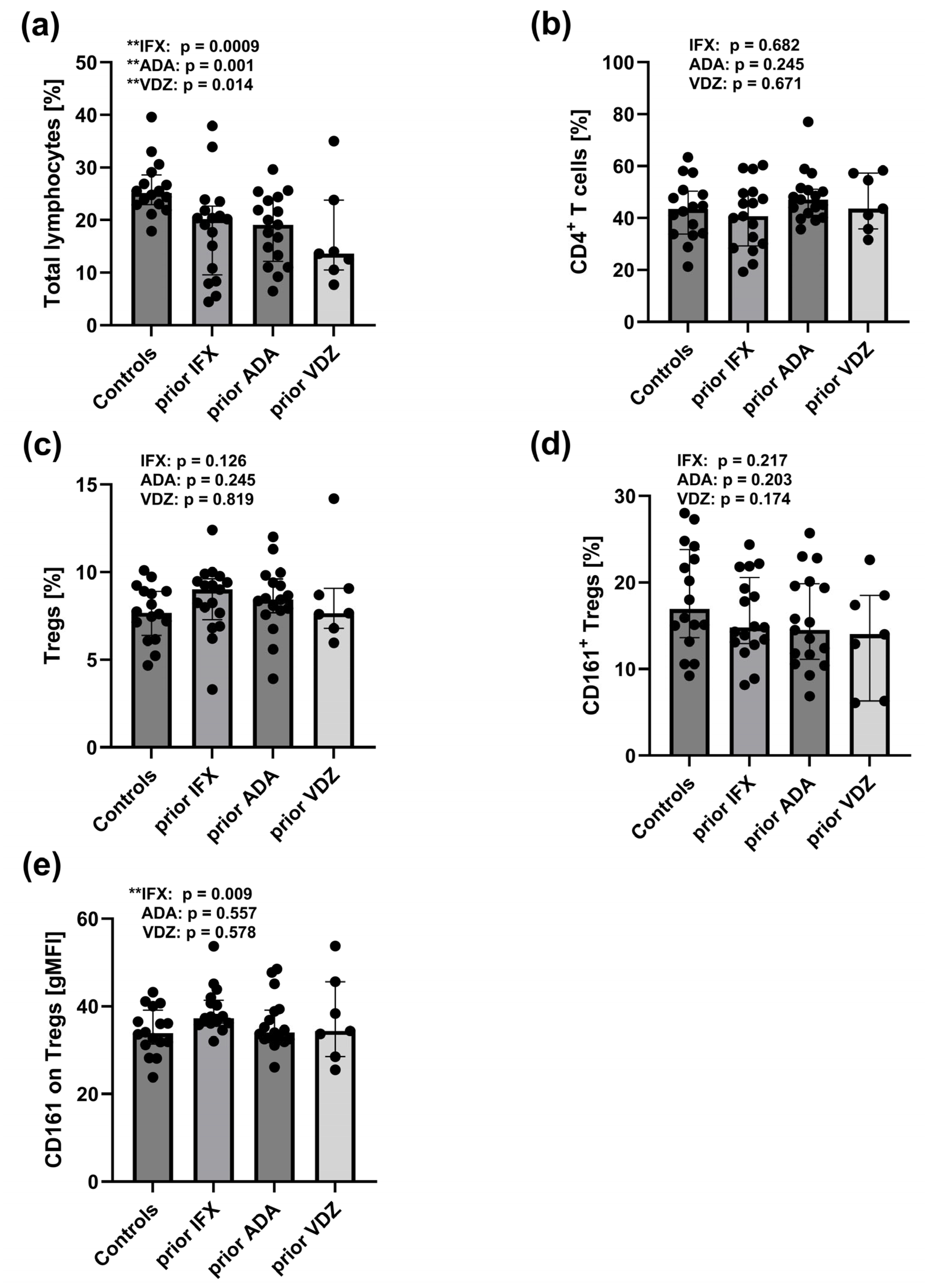
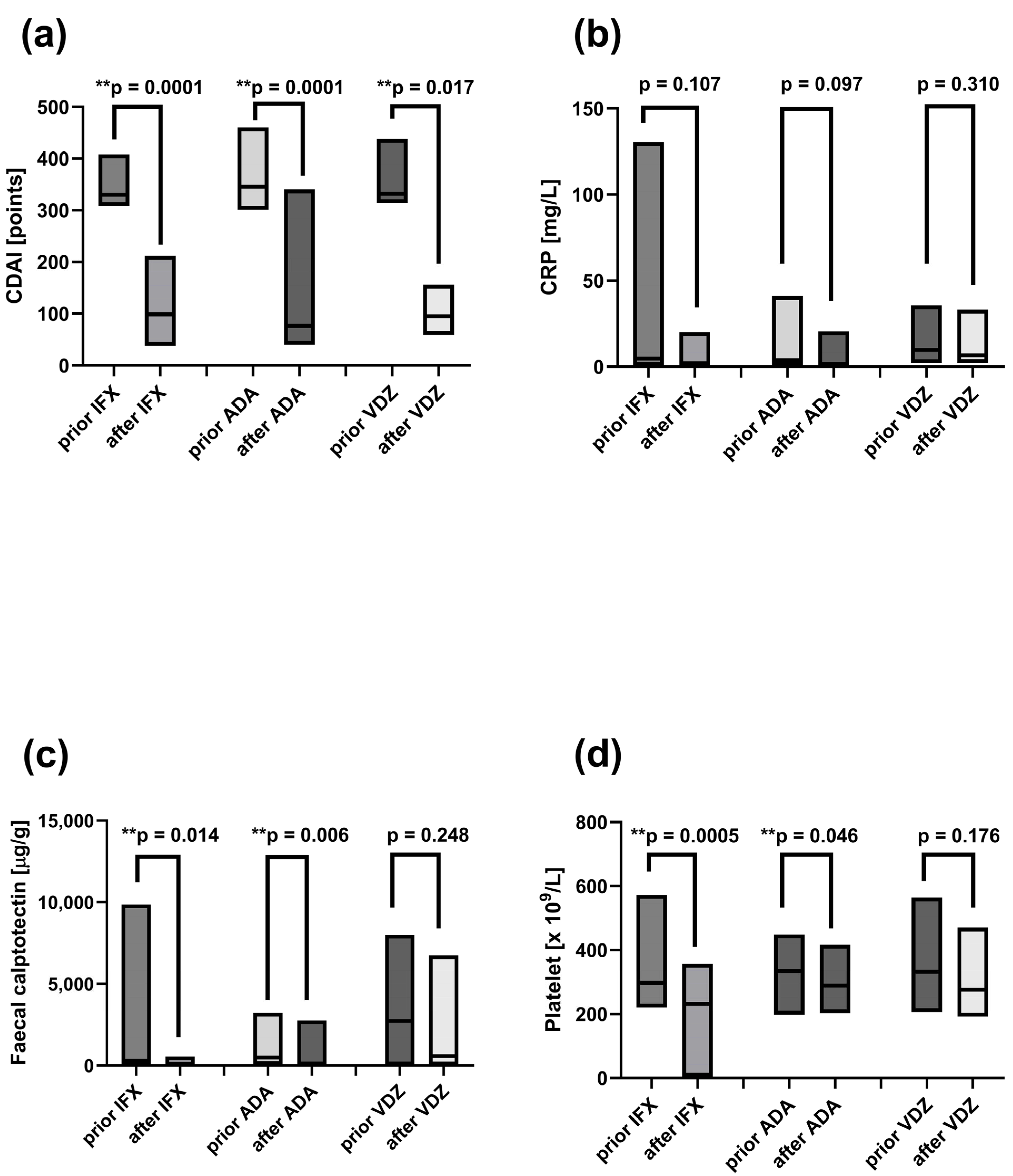
2.6. Reduced Total Lymphocyte Pool and Increased CD161 Expression on Tregs in Peripheral Blood of CD Patients Prior to IFX, ADA, and VDZ Treatment
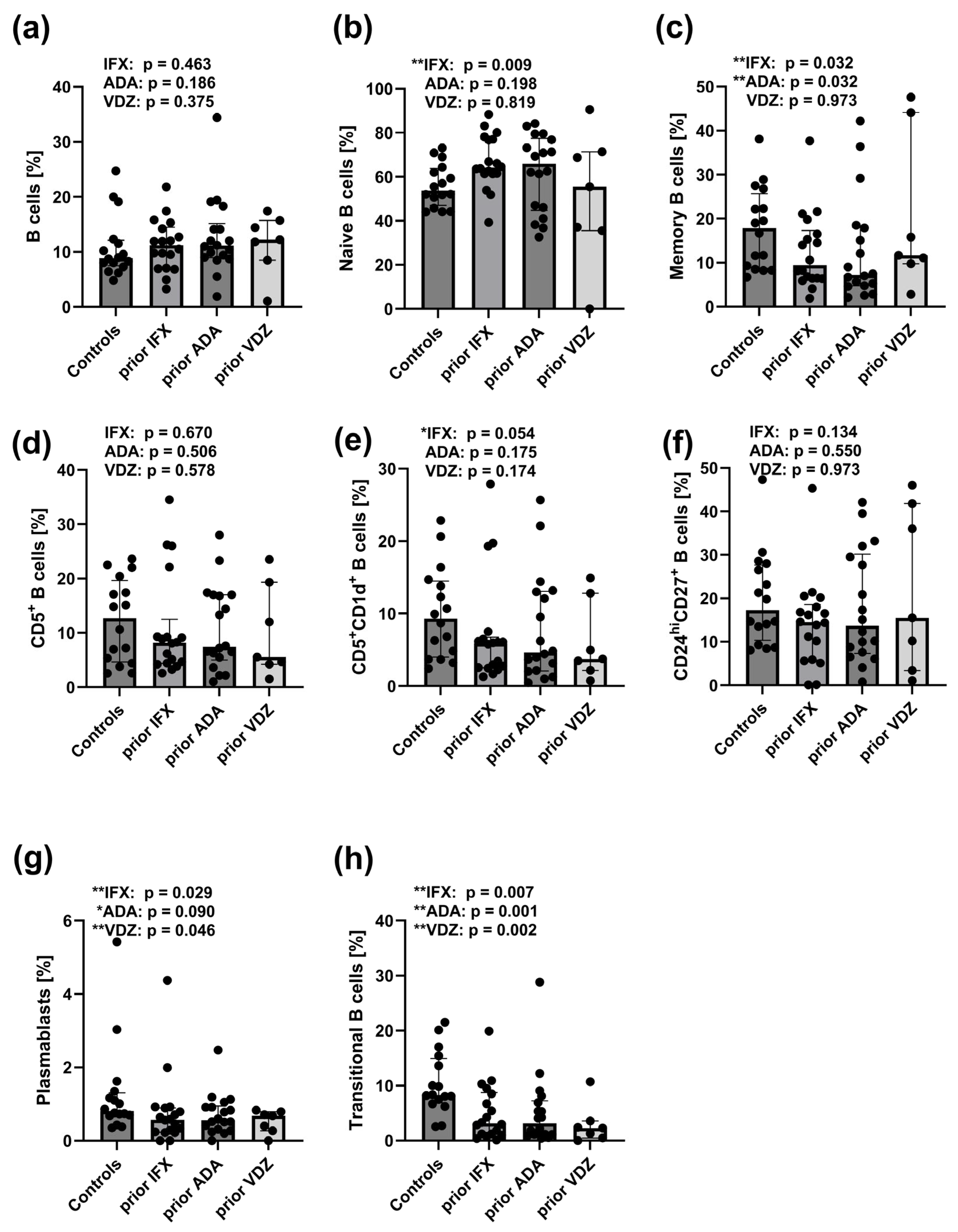
2.7. Reduced Frequencies of CD27intCD38hi and CD24hiCD38hi B Cell Subsets in Peripheral Blood of CD Patients Prior to IFX, ADA, and VDZ Treatment
2.8. Association of T and B Cell Subset Frequencies with the Disease Activity Markers (i.e., Crohn’s Disease Activity Index, C-Reactive Protein, Faecal Calprotectin and Platelet Count) in CD Patient Groups Before Biological Therapy
2.9. Decreased Disease Activity Markers Following Induction Therapy with ADA, IFX, and VDZ
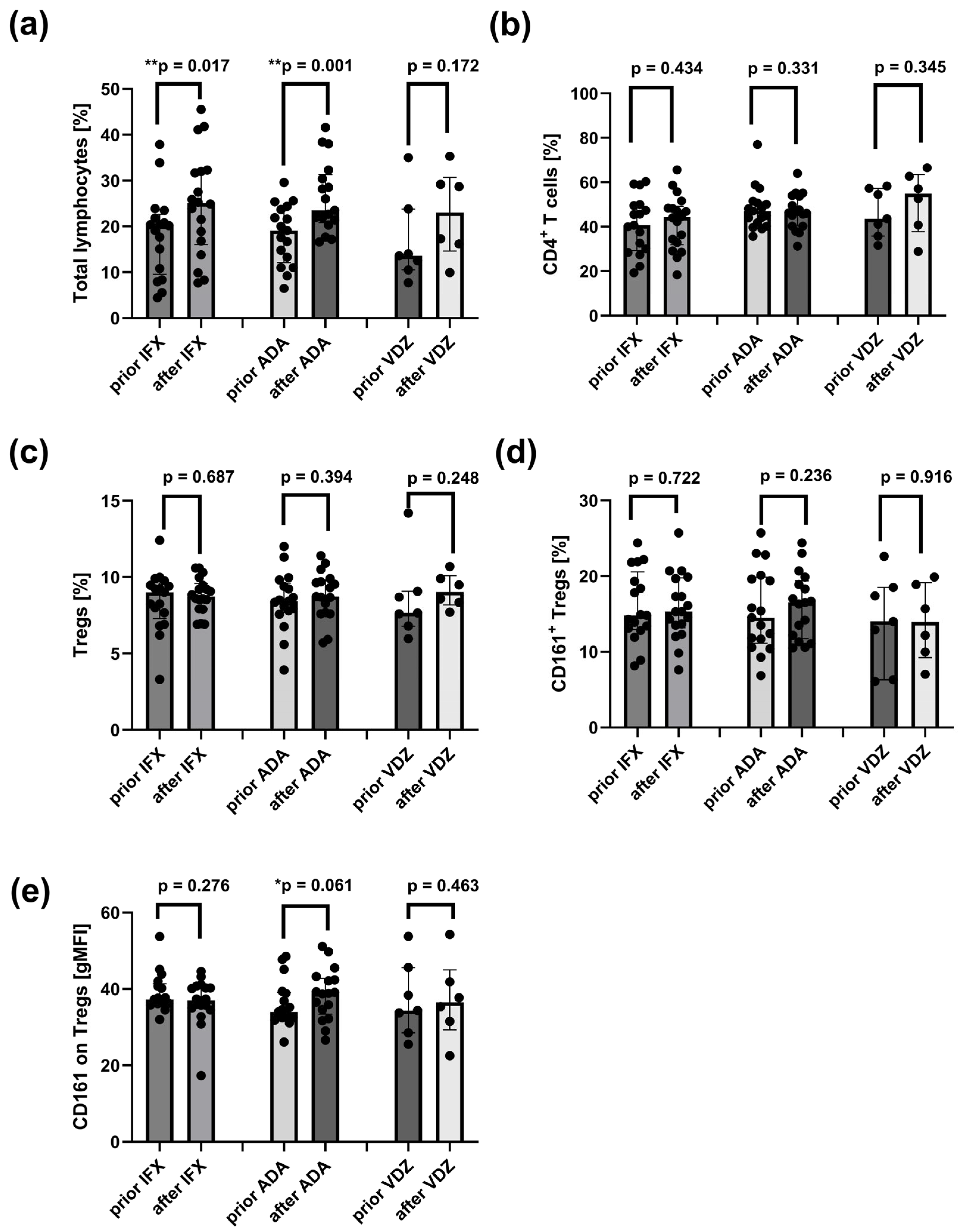
2.10. Increased Frequency of Peripheral Lymphocytes and CD161 Receptor on Tregs Following Induction Therapy with ADA and IFX
2.11. Reduced Frequencies of Mature Naïve B Cells and Increased Proportions of CD24hiCD27+ B Cells in CD Patients After Induction Therapy with ADA and IFX
2.12. Association of T and B Cell Subset Frequencies with the Disease Activity Markers (i.e.,Crohn’s Disease Activity Index, C-Reactive Protein, Faecal Calprotectin and Platelet Count) in CD Patient After Biological Therapy
3. Discussion
3.1. Altered B-Cell Subsets and Increased CD161 Expression on Tregs at Baseline
3.2. Therapy-Induced Changes in B-Cell Populations and CD161 Expression on Tregs
3.3. Study Limitations
4. Materials and Methods
4.1. Patients
4.2. Sample Collection
4.3. Flow Cytometry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | Adalimumab |
| Bregs | Regulatory B cells |
| CD | Crohn’s disease |
| CDAI | Crohn’s disease activity index |
| CRP | C-reactive protein |
| IFX | Infliximab |
| Tregs | Regulatory T cells |
| VDZ | Vedolizumab |
References
- Cockburn, E.; Kamal, S.; Chan, A.; Rao, V.; Liu, T.; Huang, J.Y.; Segal, J.P. Crohn’s Disease: An Update. Clin. Med. 2023, 23, 549–557. [Google Scholar] [CrossRef]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Hugot, J.-P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.-P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 Leucine-Rich Repeat Variants with Susceptibility to Crohn’s Disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Ammitzboell, M.; Nys, K.; Seidelin, J.B.; Nielsen, O.H. ATG16L1: A Multifunctional Susceptibility Factor in Crohn Disease. Autophagy 2015, 11, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.; Neurath, M.F.; Atreya, R. Role of the IL23/IL17 Pathway in Crohn’s Disease. Front. Immunol. 2021, 12, 622934. [Google Scholar] [CrossRef]
- Xu, W.-D.; Xie, Q.-B.; Zhao, Y.; Liu, Y. Association of Interleukin-23 Receptor Gene Polymorphisms with Susceptibility to Crohn’s Disease: A Meta-Analysis. Sci. Rep. 2015, 5, 18584. [Google Scholar] [CrossRef]
- Duerr, R.H.; Taylor, K.D.; Brant, S.R.; Rioux, J.D.; Silverberg, M.S.; Daly, M.J.; Steinhart, A.H.; Abraham, C.; Regueiro, M.; Griffiths, A.; et al. A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science 2006, 314, 1461–1463. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, B.; Zhang, H.; Fan, R.; Zhou, J.; Zhong, J. Association between STAT3 Gene Polymorphisms and Crohn’s Diseasesusceptibility: A Case–Control Study in a Chinese Han Population. Diagn. Pathol. 2014, 9, 104. [Google Scholar] [CrossRef]
- Tang, Y.; Tan, S.A.; Iqbal, A.; Li, J.; Glover, S.C. STAT3 Genotypic Variant Rs744166 and Increased Tyrosine Phosphorylation of STAT3 in IL-23 Responsive Innate Lymphoid Cells during Pathogenesis of Crohn’s Disease. J. Immunol. Res. 2019, 2019, 9406146. [Google Scholar] [CrossRef]
- Caparrós, E.; Wiest, R.; Scharl, M.; Rogler, G.; Gutiérrez Casbas, A.; Yilmaz, B.; Wawrzyniak, M.; Francés, R. Dysbiotic Microbiota Interactions in Crohn’s Disease. Gut Microbes 2021, 13, 1949096. [Google Scholar] [CrossRef]
- Chen, P.; Tang, X. Gut Microbiota as Regulators of Th17/Treg Balance in Patients with Myasthenia Gravis. Front. Immunol. 2021, 12, 803101. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Hatton, R.D. Interplay between the TH17 and TReg Cell Lineages: A (Co-)Evolutionary Perspective. Nat. Rev. Immunol. 2009, 9, 883–889. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current State of the Art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Gorfu, G.; Rivera-Nieves, J.; Ley, K. Role of β7 Integrins in Intestinal Lymphocyte Homing and Retention. CMM 2009, 9, 836–850. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Parker, C.M.; Cepek, K.L.; Russell, G.J.; Shaw, S.K.; Posnett, D.N.; Schwarting, R.; Brenner, M.B. A Family of Beta 7 Integrins on Human Mucosal Lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1924–1928. [Google Scholar] [CrossRef]
- Cepek, K.L.; Parker, C.M.; Madara, J.L.; Brenner, M.B. Integrin Alpha E Beta 7 Mediates Adhesion of T Lymphocytes to Epithelial Cells. J. Immunol. 1993, 150, 3459–3470. [Google Scholar] [CrossRef]
- Higgins, J.M.G.; Mandlebrot, D.A.; Shaw, S.K.; Russell, G.J.; Murphy, E.A.; Chen, Y.-T.; Nelson, W.J.; Parker, C.M.; Brenner, M.B. Direct and Regulated Interaction of Integrin αEβ7 with E-Cadherin. J. Cell Biol. 1998, 140, 197–210. [Google Scholar] [CrossRef]
- Leppkes, M.; Roulis, M.; Neurath, M.F.; Kollias, G.; Becker, C. Pleiotropic Functions of TNF-α in the Regulation of the Intestinal Epithelial Response to Inflammation. Int. Immunol. 2014, 26, 509–515. [Google Scholar] [CrossRef]
- Suenaert, P.; Bulteel, V.; Lemmens, L.; Noman, M.; Geypens, B.; Assche, G.V.; Geboes, K.; Ceuppens, J.L.; Rutgeerts, P. Anti-Tumor Necrosis Factor Treatment Restores the Gut Barrier in Crohn’s Disease. Am. J. Gastroenterol. 2002, 97, 2000–2004. [Google Scholar] [CrossRef]
- Noth, R.; Stüber, E.; Häsler, R.; Nikolaus, S.; Kühbacher, T.; Hampe, J.; Bewig, B.; Schreiber, S.; Arlt, A. Anti-TNF-α Antibodies Improve Intestinal Barrier Function in Crohn’s Disease. J. Crohn’s Colitis 2012, 6, 464–469. [Google Scholar] [CrossRef]
- Marini, M.; Bamias, G.; Rivera-Nieves, J.; Moskaluk, C.A.; Hoang, S.B.; Ross, W.G.; Pizarro, T.T.; Cominelli, F. TNF-Aneutralization Ameliorates the Severity of Murine Crohn’s-like Ileitis by Abrogation of Intestinal Epithelial Cell Apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 8366–8371. [Google Scholar] [CrossRef] [PubMed]
- Reinecker, H.-C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; MacDERMOTT, R.P.; Raedler, A. Enhand Secretion of Tumour Necrosis Factor-Alpha, IL-6, and IL-1β by Isolated Lamina Ropria Monouclear Cells from Patients with Ulcretive Cilitis and Crohn’s Disease. Clin. Exp. Immunol. 2008, 94, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Reimund, J.M.; Wittersheim, C.; Dumont, S.; Muller, C.D.; Kenney, J.S.; Baumann, R.; Poindron, P.; Duclos, B. Increased Production of Tumour Necrosis Factor-Alpha Interleukin-1 Beta, and Interleukin-6 by Morphologically Normal Intestinal Biopsies from Patients with Crohn’s Disease. Gut 1996, 39, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.; Billmeier, U.; Dieterich, W.; Rath, T.; Sonnewald, S.; Reid, S.; Hirschmann, S.; Hildner, K.; Waldner, M.J.; Mudter, J.; et al. Expansion of IL-23 Receptor Bearing TNFR2+ T Cells Is Associated with Molecular Resistance to Anti-TNF Therapy in Crohn’s Disease. Gut 2019, 68, 814–828. [Google Scholar] [CrossRef]
- Duvallet, E.; Semerano, L.; Assier, E.; Falgarone, G.; Boissier, M.-C. Interleukin-23: A Key Cytokine in Inflammatory Diseases. Ann. Med. 2011, 43, 503–511. [Google Scholar] [CrossRef]
- Yu, W.-L.; Hua, Z.-C. Efficacy and Prognosis of Adalimumab for Crohn’s Disease at Different Disease Locations: A Systematic Review and Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2036–2048. [Google Scholar] [CrossRef]
- Song, Y.-N.; Zheng, P.; Xiao, J.-H.; Lu, Z.-J. Efficacy and Safety of Adalimumab for the Crohn’s Disease: A Systematic Review and Meta-Analysis of Published Randomized Placebo-Controlled Trials. Eur. J. Clin. Pharmacol. 2014, 70, 907–914. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Panaccione, R.; Mallarkey, G. Efficacy and Safety of Adalimumab in Crohn’s Disease. Ther. Adv. Gastroenterol. 2008, 1, 43–50. [Google Scholar] [CrossRef]
- Abbass, M.; Cepek, J.; Parker, C.E.; Nguyen, T.M.; MacDonald, J.K.; Feagan, B.G.; Khanna, R.; Jairath, V. Adalimumab for Induction of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2019, 2019, CD012878. [Google Scholar] [CrossRef]
- Jiang, C.-Z.; Yu, W.-L. Clinical Efficacy of Infliximab in Patients with Crohn Disease in Different Locations of Disease Pathology: A Meta-Analysis. Clin. Investig. Med. 2021, 44, E27–E35. [Google Scholar] [CrossRef]
- González-Lama, Y.; López-San Román, A.; Marín-Jiménez, I.; Casis, B.; Vera, I.; Bermejo, F.; Lázaro Pérez-Calle, J.; Taxonera, C.; Martínez-Silva, F.; Menchén, L.; et al. Open-Label Infliximab Therapy in Crohn’s Disease: A Long-Term Multicenter Study of Efficacy, Safety and Predictors of Response. Gastroenterol. Hepatol. 2008, 31, 421–426. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, T.; Iida, M.; Motoya, S.; Haruma, K.; Suzuki, Y.; Kobayashi, K.; Ito, H.; Miyata, M.; Kusunoki, M.; Chiba, T.; et al. Therapeutic Efficacy of Infliximab on Patients with Short Duration of Crohn’s Disease: A Japanese Multicenter Survey. Dis. Colon Rectum 2008, 51, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Reynaert, C.; Su, A.L.; Sawh, S. Efficacy and Safety of Infliximab in Pediatric Crohn Disease: A Systematic Review and Meta-Analysis. Can. J. Hosp. Pharm. 2019, 72, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.-F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-J.; Bie, C.-Q.; Guo, L.-L.; Zhong, L.-X.; Tang, S.-H. Efficacy and Safety of Vedolizumab in the Treatment of Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Exp. Ther. Med. 2023, 25, 298. [Google Scholar] [CrossRef]
- Armuzzi, A.; Vermeire, S.; Chaparro, M.; Biedermann, P.; Brown, R.; McStravick, M.; Meyer, M.; Schreiber, S. Effectiveness and Treatment Persistence of Vedolizumab Compared to Anti-Tumour Necrosis Factor-α in Patients with Crohn’s Disease: A Systematic Literature Review and Meta-Analysis. UEG J. 2025, 13, 552–565. [Google Scholar] [CrossRef]
- Moćko, P.; Kawalec, P.; Smela-Lipińska, B.; Pilc, A. Effectiveness and Safety of Vedolizumab for Treatment of Crohn’s Disease: A Systematic Review and Meta-Analysis. Arch. Med. Sci. 2016, 12, 1088–1096. [Google Scholar] [CrossRef]
- Shi, Y.; He, W.; Zhong, M.; Yu, M. MIN Score Predicts Primary Response to Infliximab/Adalimumab and Vedolizumab Therapy in Patients with Inflammatory Bowel Diseases. Genomics 2021, 113, 1988–1998. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Novack, L.; Mao, R.; Guo, J.; Zhao, Y.; Sergienko, R.; Zhang, J.; Kobayashi, T.; Hibi, T.; Chowers, Y.; et al. Efficacy of Biologic Drugs in Short-Duration Versus Long-Duration Inflammatory Bowel Disease: A Systematic Review and an Individual-Patient Data Meta-Analysis of Randomized Controlled Trials. Gastroenterology 2022, 162, 482–494. [Google Scholar] [CrossRef]
- Peruhova, M.; Stoyanova, D.; Miteva, D.G.; Kitanova, M.; Mirchev, M.B.; Velikova, T. Genetic Factors That Predict Response and Failure of Biologic Therapy in Inflammatory Bowel Disease. World J. Exp. Med. 2025, 15, 97404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, Z.; Hao, J. Efficacy of Early Biologic Therapy versus Late/Conventional Therapy in Children and Adolescents with Crohn’s Disease: A Systematic Review and Meta-Analysis. Saudi J. Gastroenterol. 2023, 29, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Anjie, S.I.; Hanzel, J.; Gecse, K.B.; D’Haens, G.R.; Brandse, J.F. Anti-Drug Antibodies against Anti-TNF in Patients with Inflammatory Bowel Disease: An Evaluation of Possible Strategies. Scand. J. Gastroenterol. 2024, 59, 169–175. [Google Scholar] [CrossRef]
- Bots, S.J.; Parker, C.E.; Brandse, J.F.; Löwenberg, M.; Feagan, B.G.; Sandborn, W.J.; Jairath, V.; D’Haens, G.; Vande Casteele, N. Anti-Drug Antibody Formation Against Biologic Agents in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. BioDrugs 2021, 35, 715–733. [Google Scholar] [CrossRef]
- Samnani, S.; Wong, E.C.L.; Hamam, H.; Dulai, P.S.; Marshall, J.K.; Jairath, V.; Reinisch, W.; Narula, N. Outcomes of Patients with Prior Biologic Intolerance Are Better Than Those with Biologic Failure in Clinical Trials of Inflammatory Bowel Disease. J. Crohn’s Colitis 2025, 19, jjae151. [Google Scholar] [CrossRef] [PubMed]
- Laharie, D.; Salzmann, M.; Boubekeur, H.; Richy, F.; Amouretti, M.; Quinton, A.; Couzigou, P.; Lamouliatte, H.; Zerbib, F. Predictors of Response to Infliximab in Luminal Crohn’s Disease. Gastroentérol. Clin. Biol. 2005, 29, 145–149. [Google Scholar] [CrossRef]
- Matsuoka, K.; Hamada, S.; Shimizu, M.; Nanki, K.; Mizuno, S.; Kiyohara, H.; Arai, M.; Sugimoto, S.; Iwao, Y.; Ogata, H.; et al. Factors Predicting the Therapeutic Response to Infliximab during Maintenance Therapy in Japanese Patients with Crohn’s Disease. PLoS ONE 2018, 13, e0204632. [Google Scholar] [CrossRef]
- Parsi, M.A.; Achkar, J.; Richardson, S.; Katz, J.; Hammel, J.P.; Lashner, B.A.; Brzezinski, A. Predictors of Response to Infliximab in Patients with Crohn’s Disease. Gastroenterology 2002, 123, 707–713. [Google Scholar] [CrossRef]
- Orlando, A.; Colombo, E.; Kohn, A.; Biancone, L.; Rizzello, F.; Viscido, A.; Sostegni, R.; Benazzato, L.; Castiglione, F.; Papi, C.; et al. Infliximab in the Treatment of Crohn’s Disease: Predictors of Response in an Italian Multicentric Open Study. Dig. Liver Dis. 2005, 37, 577–583. [Google Scholar] [CrossRef]
- Xu, L.; Shen, J.; Zheng, Q. Development of a Clinical Model to Predict Secondary Non-Response of Infliximab Treatment in Crohn’s Disease. Int. J. Colorectal. Dis. 2020, 35, 2019–2026. [Google Scholar] [CrossRef]
- Mühl, L.; Becker, E.; Müller, T.M.; Atreya, R.; Atreya, I.; Neurath, M.F.; Zundler, S. Clinical Experiences and Predictors of Success of Treatment with Vedolizumab in IBD Patients: A Cohort Study. BMC Gastroenterol. 2021, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Barnes, E.L.; Stevens, B.; Storm, M.; Ananthakrishnan, A.; Yajnik, V.; Korzenik, J. Predictors of Clinical Response and Remission at 1 Year Among a Multicenter Cohort of Patients with Inflammatory Bowel Disease Treated with Vedolizumab. Dig. Dis. Sci. 2017, 62, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Barré, A.; Colombel, J.-F.; Ungaro, R. Review Article: Predictors of Response to Vedolizumab and Ustekinumab in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2018, 47, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Grad, S.; Farcas, R.A.; Dumitrascu, D.L.; Surdea-Blaga, T.; Ismaiel, A.; Popa, S. Predictors of Immunogenicity and Loss of Response to ANTI-TNFα Therapy in Crohn Disease—A Systematic Review. Am. J. Ther. 2025, 32, e262–e268. [Google Scholar] [CrossRef]
- Gorenjak, M.; Repnik, K.; Jezernik, G.; Jurgec, S.; Skok, P.; Potočnik, U. Genetic prediction profile for adalimumab response in Slovenian Crohn’s disease patients. Z Gastroenterol. 2019, 57, 1218–1225. [Google Scholar] [CrossRef]
- Genaro, L.M.; Carron, J.; De Castro, M.M.; Franceschini, A.P.M.D.F.; Lourenço, G.J.; Cruz, C.K.N.V.D.; Reis, G.F.S.R.; Pascoal, L.B.; Mello, J.D.C.; Pereira, I.M.; et al. Therapeutic Drug Monitoring and Immunogenetic Factors Associated with the Use of Adalimumab in Crohn’s Disease Patients. Int. J. Immunopathol. Pharmacol. 2025, 39, 1–21. [Google Scholar] [CrossRef]
- Jezernik, G.; Gorenjak, M.; Potočnik, U. MIF Variant Rs755622 Is Associated with Severe Crohn’s Disease and Better Response to Anti-TNF Adalimumab Therapy. Genes 2023, 14, 452. [Google Scholar] [CrossRef]
- Payen, E.; Neuraz, A.; Zenzeri, L.; Talbotec, C.; Abi Nader, E.; Chatenoud, L.; Chhun, S.; Goulet, O.; Ruemmele, F.M.; Pigneur, B. Adalimumab Therapy in Pediatric Crohn Disease: A 2-Year Follow-Up Comparing “Top-Down” and “Step-Up” Strategies. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 166–173. [Google Scholar] [CrossRef]
- Tursi, A.; Mocci, G.; Lorenzetti, R.; Allegretta, L.; Brandimarte, G.; Cassieri, C.; Colucci, R.; De Medici, A.; Faggiani, R.; Ferronato, A.; et al. Long-Term Real-Life Efficacy and Safety of Infliximab and Adalimumab in the Treatment of Inflammatory Bowel Diseases Outpatients. Eur. J. Gastroenterol. Hepatol. 2021, 33, 670–679. [Google Scholar] [CrossRef]
- Yang, H.-H.; Huang, Y.; Zhou, X.-C.; Wang, R.-N. Efficacy and Safety of Adalimumab in Comparison to Infliximab for Crohn’s Disease: A Systematic Review and Meta-Analysis. WJCC 2022, 10, 6091–6104. [Google Scholar] [CrossRef]
- Na, J.E.; Park, Y.E.; Park, J.; Kim, T.-O.; Lee, J.H.; Park, S.B.; Kim, S.; Lee, S.B.; Busan Ulsan Gyeongnam Intestinal Study Group Society (BIGS). Comparative Real-World Outcomes between Ustekinumab, Infliximab, and Adalimumab in Bio-Naïve and Bio-Experienced Crohn’s Disease Patients: A Retrospective Multicenter Study. BMC Gastroenterol. 2024, 24, 306. [Google Scholar] [CrossRef] [PubMed]
- Fanous, E.; Marshanski, T.; Tal, N.; Matar, M.; Weintraub, Y.; Shamir, R.; Shouval, D.S. Comparison of Clinical Outcomes in Pediatric Patients with Ileocolonic Crohn Disease Treated with Infliximab Versus Adalimumab. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Gils, A.; Rutgeerts, P.; Levesque, B.G.; Vermeire, S.; Sandborn, W.J.; Casteele, N.V. Role for Therapeutic Drug Monitoring During Induction Therapy with TNF Antagonists in IBD: Evolution in the Definition and Management of Primary Nonresponse. Inflamm. Bowel Dis. 2015, 21, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Bek, S.; Nielsen, J.V.; Bojesen, A.B.; Franke, A.; Bank, S.; Vogel, U.; Andersen, V. Systematic Review: Genetic Biomarkers Associated with anti-TNF Treatment Response in Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2016, 44, 554–567. [Google Scholar] [CrossRef]
- Boden, E.K.; Shows, D.M.; Chiorean, M.V.; Lord, J.D. Identification of Candidate Biomarkers Associated with Response to Vedolizumab in Inflammatory Bowel Disease. Dig. Dis. Sci. 2018, 63, 2419–2429. [Google Scholar] [CrossRef]
- Soendergaard, C.; Seidelin, J.B.; Steenholdt, C.; Nielsen, O.H. Putative Biomarkers of Vedolizumab Resistance and Underlying Inflammatory Pathways Involved in IBD. BMJ Open Gastroenterol. 2018, 5, e000208. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Biancheri, P.; Piconese, S.; Rosado, M.M.; Ardizzone, S.; Rovedatti, L.; Ubezio, C.; Massari, A.; Sampietro, G.M.; Foschi, D.; et al. Peripheral Regulatory T Cells and Serum Transforming Growth Factor-β: Relationship with Clinical Response to Infliximab in Crohn’s Disease. Inflamm. Bowel Dis. 2010, 16, 1891–1897. [Google Scholar] [CrossRef]
- Rosseto-Welter, E.A.; D’argenio-Garcia, L.; Blasco Tavares Pereira Lopes, F.; Zulim Carvalho, A.E.; Flaquer, F.; Severo-Lemos, V.; Viero Nora, C.C.; Steinwurz, F.; Pires Garcia Oliveria, L.; Aloia, T.; et al. Biologic Agents in Crohn’s Patients Reduce CD4+ T Cells Activation and Are Inversely Related to Treg Cells. Can. J. Gastroenterol. Hepatol. 2022, 2022, 1307159. [Google Scholar] [CrossRef]
- Abreu, M.T.; Davies, J.M.; Quintero, M.A.; Delmas, A.; Diaz, S.; Martinez, C.D.; Venables, T.; Reich, A.; Crynen, G.; Deshpande, A.R.; et al. Transcriptional Behavior of Regulatory T Cells Predicts IBD Patient Responses to Vedolizumab Therapy. Inflamm. Bowel Dis. 2022, 28, 1800–1812. [Google Scholar] [CrossRef]
- Carter, N.A.; Vasconcellos, R.; Rosser, E.C.; Tulone, C.; Muñoz-Suano, A.; Kamanaka, M.; Ehrenstein, M.R.; Flavell, R.A.; Mauri, C. Mice Lacking Endogenous IL-10–Producing Regulatory B Cells Develop Exacerbated Disease and Present with an Increased Frequency of Th1/Th17 but a Decrease in Regulatory T Cells. J. Immunol. 2011, 186, 5569–5579. [Google Scholar] [CrossRef]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B Cells Regulate Autoimmunity by Provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-X.; Yu, C.-R.; Dambuza, I.M.; Mahdi, R.M.; Dolinska, M.B.; Sergeev, Y.V.; Wingfield, P.T.; Kim, S.-H.; Egwuagu, C.E. Interleukin-35 Induces Regulatory B Cells That Suppress Autoimmune Disease. Nat. Med. 2014, 20, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Camarillo, G.; Furuzawa-Carballeda, J.; Yamamoto-Furusho, J.K. Interleukin 35 (IL-35) and IL-37: Intestinal and Peripheral Expression by T and B Regulatory Cells in Patients with Inflammatory Bowel Disease. Cytokine 2015, 75, 389–402. [Google Scholar] [CrossRef]
- Snir, A.; Kessel, A.; Haj, T.; Rosner, I.; Slobodin, G.; Toubi, E. Anti-IL-6 Receptor Antibody (Tocilizumab): A B Cell Targeting Therapy. Clin. Exp. Rheumatol. 2011, 29, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Kehrl, J.H.; Roberts, A.B.; Wakefield, L.M.; Jakowlew, S.; Sporn, M.B.; Fauci, A.S. Transforming Growth Factor Beta Is an Important Immunomodulatory Protein for Human B Lymphocytes. J. Immunol. 1986, 137, 3855–3860. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Lu, Y.; Zhuang, H.; Gu, W.; Liu, B.; Liu, F.; Sun, J.; Yan, B.; Weng, D.; et al. IL-10-Producing CD1dhiCD5+ Regulatory B Cells May Play a Critical Role in Modulating Immune Homeostasis in Silicosis Patients. Front. Immunol. 2017, 8, 110. [Google Scholar] [CrossRef]
- Bosma, A.; Abdel-Gadir, A.; Isenberg, D.A.; Jury, E.C.; Mauri, C. Lipid-Antigen Presentation by CD1d+ B Cells Is Essential for the Maintenance of Invariant Natural Killer T Cells. Immunity 2012, 36, 477–490. [Google Scholar] [CrossRef]
- Barral, P.; Eckl-Dorna, J.; Harwood, N.E.; De Santo, C.; Salio, M.; Illarionov, P.; Besra, G.S.; Cerundolo, V.; Batista, F.D. B Cell Receptor-Mediated Uptake of CD1d-Restricted Antigen Augments Antibody Responses by Recruiting Invariant NKT Cell Help in Vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 8345–8350. [Google Scholar] [CrossRef]
- Kubo, S.; Yamada, T.; Osawa, Y.; Ito, Y.; Narita, N.; Fujieda, S. Cytosine–Phosphate–Guanosine-DNA Induces CD274 Expression in Human B Cells and Suppresses T Helper Type 2 Cytokine Production in Pollen Antigen-Stimulated CD4-Positive Cells. Clin. Exp. Immunol. 2012, 169, 1–9. [Google Scholar] [CrossRef]
- Zacca, E.R.; Onofrio, L.I.; Acosta, C.D.V.; Ferrero, P.V.; Alonso, S.M.; Ramello, M.C.; Mussano, E.; Onetti, L.; Cadile, I.I.; Stancich, M.I.; et al. PD-L1+ Regulatory B Cells Are Significantly Decreased in Rheumatoid Arthritis Patients and Increase After Successful Treatment. Front. Immunol. 2018, 9, 2241. [Google Scholar] [CrossRef]
- Figueiró, F.; Muller, L.; Funk, S.; Jackson, E.K.; Battastini, A.M.O.; Whiteside, T.L. Phenotypic and Functional Characteristics of CD39high Human Regulatory B Cells (Breg). OncoImmunology 2016, 5, e1082703. [Google Scholar] [CrossRef]
- Kaku, H.; Cheng, K.F.; Al-Abed, Y.; Rothstein, T.L. A Novel Mechanism of B Cell-Mediated Immune Suppression through CD73 Expression and Adenosine Production. J. Immunol. 2014, 193, 5904–5913. [Google Scholar] [CrossRef] [PubMed]
- Van De Veen, W.; Stanic, B.; Yaman, G.; Wawrzyniak, M.; Söllner, S.; Akdis, D.G.; Rückert, B.; Akdis, C.A.; Akdis, M. IgG4 Production Is Confined to Human IL-10–Producing Regulatory B Cells That Suppress Antigen-Specific Immune Responses. J. Allergy Clin. Immunol. 2013, 131, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Okazaki, K. Roles of Regulatory T and B Cells in IgG4-Related Disease. In IgG4-Related Disease; Okazaki, K., Ed.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2016; Volume 401, pp. 93–114. ISBN 978-3-319-52541-9. [Google Scholar]
- Fehres, C.M.; van Uden, N.O.; Yeremenko, N.G.; Fernandez, L.; Franco Salinas, G.; van Duivenvoorde, L.M.; Huard, B.; Morel, J.; Spits, H.; Hahne, M.; et al. APRIL Induces a Novel Subset of IgA+ Regulatory B Cells That Suppress Inflammation via Expression of IL-10 and PD-L1. Front. Immunol. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Veh, J.; Ludwig, C.; Schrezenmeier, H.; Jahrsdörfer, B. Regulatory B Cells—Immunopathological and Prognostic Potential in Humans. Cells 2024, 13, 357. [Google Scholar] [CrossRef]
- Matsumura, Y.; Watanabe, R.; Fujimoto, M. Suppressive Mechanisms of Regulatory B Cells in Mice and Humans. Int. Immunol. 2023, 35, 55–65. [Google Scholar] [CrossRef]
- Zheremyan, E.A.; Ustiugova, A.S.; Radko, A.I.; Stasevich, E.M.; Uvarova, A.N.; Mitkin, N.A.; Kuprash, D.V.; Korneev, K.V. Novel Potential Mechanisms of Regulatory B Cell-Mediated Immunosuppression. Biochem. Mosc. 2023, 88, 13–21. [Google Scholar] [CrossRef]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Zheremyan, E.A.; Ustiugova, A.S.; Uvarova, A.N.; Karamushka, N.M.; Stasevich, E.M.; Gogoleva, V.S.; Bogolyubova, A.V.; Mitkin, N.A.; Kuprash, D.V.; Korneev, K.V. Differentially Activated B Cells Develop Regulatory Phenotype and Show Varying Immunosuppressive Features: A Comparative Study. Front. Immunol. 2023, 14, 1178445. [Google Scholar] [CrossRef]
- Baba, Y.; Saito, Y.; Kotetsu, Y. Heterogeneous Subsets of B-Lineage Regulatory Cells (Breg Cells). Int. Immunol. 2020, 32, 155–162. [Google Scholar] [CrossRef]
- Hong, M.; Liao, Y.; Liang, J.; Chen, X.; Li, S.; Liu, W.; Gao, C.; Zhong, Z.; Kong, D.; Deng, J.; et al. Immunomodulation of Human CD19+CD25high Regulatory B Cells via Th17/Foxp3 Regulatory T Cells and Th1/Th2 Cytokines. Hum. Immunol. 2019, 80, 863–870. [Google Scholar] [CrossRef]
- Menon, M.; Hussell, T.; Ali Shuwa, H. Regulatory B Cells in Respiratory Health and Diseases. Immunol. Rev. 2021, 299, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Rui, K.; Wang, S.; Lu, L. Regulatory B Cells in Autoimmune Diseases. Cell Mol. Immunol. 2013, 10, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.; Ling, G.-S.; Xu, D.; Hussaarts, L.; Romaine, A.; Zhao, H.; Fossati-Jimack, L.; Malik, T.; Cook, H.T.; Botto, M.; et al. IL-10-Producing Regulatory B Cells Induced by IL-33 (BregIL-33) Effectively Attenuate Mucosal Inflammatory Responses in the Gut. J. Autoimmun. 2014, 50, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Yoshizaki, A.; Asano, Y.; Kadono, T.; Tedder, T.F.; Sato, S. IL-10–Producing Regulatory B10 Cells Inhibit Intestinal Injury in a Mouse Model. Am. J. Pathol. 2011, 178, 735–743. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Mizoguchi, E.; Takedatsu, H.; Blumberg, R.S.; Bhan, A.K. Chronic Intestinal Inflammatory Condition Generates IL-10-Producing Regulatory B Cell Subset Characterized by CD1d Upregulation. Immunity 2002, 16, 219–230. [Google Scholar] [CrossRef]
- Yan, H.; He, J.; Lin, X. Heterogeneity of Regulatory B Cells in Autoimmune Diseases: Implications for Immune Equilibrium and Therapeutic Strategies. Immunother. Adv. 2025, 5, ltaf020. [Google Scholar] [CrossRef]
- Wang, L.; Christodoulou, M.-I.; Jin, Z.; Ma, Y.; Hossen, M.; Ji, Y.; Wang, W.; Wang, X.; Wang, E.; Wei, R.; et al. Human Regulatory B Cells Suppress Autoimmune Disease Primarily via Interleukin-37. J. Autoimmun. 2025, 153, 103415. [Google Scholar] [CrossRef]
- Oka, A.; Ishihara, S.; Mishima, Y.; Tada, Y.; Kusunoki, R.; Fukuba, N.; Yuki, T.; Kawashima, K.; Matsumoto, S.; Kinoshita, Y. Role of Regulatory B Cells in Chronic Intestinal Inflammation: Association with Pathogenesis of Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 315–328. [Google Scholar] [CrossRef]
- Lin, Q.R.; Xia, X.P.; Cai, X.N.; Hu, D.Y.; Shao, X.X.; Xia, S.L.; Jiang, Y. Association of Breg cells and Treg cells with the clinical effects of Infliximab in the treatment of Chinese patients with Crohn’s disease. Zhonghua Yi Xue Za Zhi 2020, 100, 3303–3308. [Google Scholar] [CrossRef]
- Lin, W.; Cerny, D.; Chua, E.; Duan, K.; Yi, J.T.J.; Shadan, N.B.; Lum, J.; Maho-Vaillant, M.; Zolezzi, F.; Wong, S.C.; et al. Human Regulatory B Cells Combine Phenotypic and Genetic Hallmarks with a Distinct Differentiation Fate. J. Immunol. 2014, 193, 2258–2266. [Google Scholar] [CrossRef]
- Piper, C.J.M.; Rosser, E.C.; Oleinika, K.; Nistala, K.; Krausgruber, T.; Rendeiro, A.F.; Banos, A.; Drozdov, I.; Villa, M.; Thomson, S.; et al. Aryl Hydrocarbon Receptor Contributes to the Transcriptional Program of IL-10-Producing Regulatory B Cells. Cell Rep. 2019, 29, 1878–1892.e7. [Google Scholar] [CrossRef]
- Lino, A.C.; Dang, V.D.; Lampropoulou, V.; Welle, A.; Joedicke, J.; Pohar, J.; Simon, Q.; Thalmensi, J.; Baures, A.; Flühler, V.; et al. LAG-3 Inhibitory Receptor Expression Identifies Immunosuppressive Natural Regulatory Plasma Cells. Immunity 2018, 49, 120–133.e9. [Google Scholar] [CrossRef]
- Lighaam, L.C.; Unger, P.-P.A.; Vredevoogd, D.W.; Verhoeven, D.; Vermeulen, E.; Turksma, A.W.; Ten Brinke, A.; Rispens, T.; Van Ham, S.M. In Vitro-Induced Human IL-10+ B Cells Do Not Show a Subset-Defining Marker Signature and Plastically Co-Express IL-10 With Pro-Inflammatory Cytokines. Front. Immunol. 2018, 9, 1913. [Google Scholar] [CrossRef]
- Simon, Q.; Pers, J.-O.; Cornec, D.; Le Pottier, L.; Mageed, R.A.; Hillion, S. In-Depth Characterization of CD24 High CD38 High Transitional Human B Cells Reveals Different Regulatory Profiles. J. Allergy Clin. Immunol. 2016, 137, 1577–1584.e10. [Google Scholar] [CrossRef]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19+CD24hiCD38hi B Cells Exhibit Regulatory Capacity in Healthy Individuals but Are Functionally Impaired in Systemic Lupus Erythematosus Patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Iwata, Y.; Matsushita, T.; Horikawa, M.; DiLillo, D.J.; Yanaba, K.; Venturi, G.M.; Szabolcs, P.M.; Bernstein, S.H.; Magro, C.M.; Williams, A.D.; et al. Characterization of a Rare IL-10–Competent B-Cell Subset in Humans That Parallels Mouse Regulatory B10 Cells. Blood 2011, 117, 530–541. [Google Scholar] [CrossRef]
- Margry, B.; Kersemakers, S.C.W.; Hoek, A.; Arkesteijn, G.J.A.; Wieland, W.H.; Van Eden, W.; Broere, F. Activated Peritoneal Cavity B-1a Cells Possess Regulatory B Cell Properties. PLoS ONE 2014, 9, e88869. [Google Scholar] [CrossRef]
- O’Garra, A.; Howard, M. IL-10 Production by CD5 B Cells. Ann. N. Y. Acad. Sci. 1992, 651, 182–199. [Google Scholar] [CrossRef]
- Griffin, D.O.; Rothstein, T.L. Human B1 Cell Frequency: Isolation and Analysis of Human B1 Cells. Front. Immun. 2012, 3, 122. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.-D.; Matsushita, T.; Tsubata, T.; Tedder, T.F. The Development and Function of Regulatory B Cells Expressing IL-10 (B10 Cells) Requires Antigen Receptor Diversity and TLR Signals. J. Immunol. 2009, 182, 7459–7472. [Google Scholar] [CrossRef]
- Zhang, X.; Deriaud, E.; Jiao, X.; Braun, D.; Leclerc, C.; Lo-Man, R. Type I Interferons Protect Neonates from Acute Inflammation through Interleukin 10–Producing B Cells. J. Exp. Med. 2007, 204, 1107–1118. [Google Scholar] [CrossRef]
- Karim, M.; Wang, Y.-F. Phenotypic Identification of CD19+CD5+CD1d+ Regulatory B Cells That Produce Interleukin 10 and Transforming Growth Factor β1 in Human Peripheral Blood. Arch. Med. Sci. 2019, 15, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.A.; Gommerman, J.L.; Rojas, O.L. Plasma Cells: From Cytokine Production to Regulation in Experimental Autoimmune Encephalomyelitis. J. Mol. Biol. 2021, 433, 166655. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-Producing Plasmablasts Exert Regulatory Function in Autoimmune Inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, R.; Zhu, Y.; Deng, S.; Muhuitijiang, B.; Li, C.; Shi, X.; Zhang, L.; Tan, W. Investigating the Impact of Regulatory B Cells and Regulatory B Cell-Related Genes on Bladder Cancer Progression and Immunotherapeutic Sensitivity. J. Exp. Clin. Cancer Res. 2024, 43, 101. [Google Scholar] [CrossRef]
- Yu, N.; Li, X.; Song, W.; Li, D.; Yu, D.; Zeng, X.; Li, M.; Leng, X.; Li, X. CD4+CD25+CD127low/− T Cells: A More Specific Treg Population in Human Peripheral Blood. Inflammation 2012, 35, 1773–1780. [Google Scholar] [CrossRef]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef]
- Pesenacker, A.M.; Bending, D.; Ursu, S.; Wu, Q.; Nistala, K.; Wedderburn, L.R. CD161 Defines the Subset of FoxP3+ T Cells Capable of Producing Proinflammatory Cytokines. Blood 2013, 121, 2647–2658. [Google Scholar] [CrossRef]
- Timmermans, W.M.C.; Van Laar, J.A.M.; Van Der Houwen, T.B.; Kamphuis, L.S.J.; Bartol, S.J.W.; Lam, K.H.; Ouwendijk, R.J.; Sparrow, M.P.; Gibson, P.R.; Van Hagen, P.M.; et al. B-Cell Dysregulation in Crohn’s Disease Is Partially Restored with Infliximab Therapy. PLoS ONE 2016, 11, e0160103. [Google Scholar] [CrossRef]
- Gonzalez, C.G.; Stevens, T.W.; Verstockt, B.; Gonzalez, D.J.; D’Haens, G.; Dulai, P.S. Crohn’s Patient Serum Proteomics Reveals Response Signature for Infliximab but Not Vedolizumab. Inflamm. Bowel Dis. 2024, 30, 1536–1545. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Z.; Yang, H.; Tang, J.; Zuo, T.; Yang, Q.; Huang, Z.; Guo, Q.; Li, M.; Gao, X.; et al. Intestinal mRNA Expression Profiles Associated with Mucosal Healing in Ustekinumab-Treated Crohn’s Disease Patients: Bioinformatics Analysis and Prospective Cohort Validation. J. Transl. Med. 2024, 22, 595. [Google Scholar] [CrossRef]
- Liu, L.; Liang, L.; Liang, H.; Wang, M.; Zhou, W.; Mai, G.; Yang, C.; Chen, Y. Microbiome-Metabolome Generated Bile Acids Gatekeep Infliximab Efficacy in Crohn’s Disease by Licensing M1 Suppression and Treg Dominance. J. Adv. Res. 2025, in press. [CrossRef] [PubMed]
- Saruta, M.; Yu, Q.T.; Fleshner, P.R.; Mantel, P.-Y.; Schmidt-Weber, C.B.; Banham, A.H.; Papadakis, K.A. Characterization of FOXP3+CD4+ Regulatory T Cells in Crohn’s Disease. Clin. Immunol. 2007, 125, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.; Ebrahimpour, S.; Maleki, I.; Abediankenari, S.; Afrouzi, M.M. CD4+ CD25+ CD127low FoxP3+ Regulatory T Cells in Crohn’s Disease. Rom. J. Intern. Med. 2018, 56, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Chamouard, P.; Monneaux, F.; Richert, Z.; Voegeli, A.-C.; Lavaux, T.; Gaub, M.P.; Baumann, R.; Oudet, P.; Muller, S. Diminution of Circulating CD4+CD25high T Cells in Naïve Crohn’s Disease. Dig. Dis. Sci. 2009, 54, 2084–2093. [Google Scholar] [CrossRef]
- Chiba, T.; Endo, M.; Miura, S.; Hayashi, Y.; Asakura, Y.; Oyama, K.; Matsumoto, T. Regulatory T Cells in Crohn’s Disease Following Anti-TNF-α Therapy. JGH Open 2020, 4, 378–381. [Google Scholar] [CrossRef]
- Gibson, D.J.; Elliott, L.; McDermott, E.; Tosetto, M.; Keegan, D.; Byrne, K.; Martin, S.T.; Rispens, T.; Cullen, G.; Mulcahy, H.E.; et al. Heightened Expression of CD39 by Regulatory T Lymphocytes Is Associated with Therapeutic Remission in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2806–2814. [Google Scholar] [CrossRef]
- Rabe, H.; Malmquist, M.; Barkman, C.; Östman, S.; Gjertsson, I.; Saalman, R.; Wold, A.E. Distinct Patterns of Naive, Activated and Memory T and B Cells in Blood of Patients with Ulcerative Colitis or Crohn’s Disease. Clin. Exp. Immunol. 2019, 197, 111–129. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Carsetti, R.; Rosado, M.M.; Ciccocioppo, R.; Cazzola, P.; Morera, R.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Immunoglobulin M Memory B Cell Decrease in Inflammatory Bowel Disease. Eur. Rev. Med. Pharmacol. Sci. 2004, 8, 199–203. [Google Scholar]
- Di Sabatino, A.; Rosado, M.M.; Ciccocioppo, R.; Cazzola, P.; Morera, R.; Corazza, G.R.; Carsetti, R. Depletion of Immunoglobulin M Memory B Cells Is Associated with Splenic Hypofunction in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2005, 100, 1788–1795. [Google Scholar] [CrossRef]
- Pararasa, C.; Zhang, N.; Tull, T.J.; Chong, M.H.A.; Siu, J.H.Y.; Guesdon, W.; Chavele, K.M.; Sanderson, J.D.; Langmead, L.; Kok, K.; et al. Reduced CD27−IgD− B Cells in Blood and Raised CD27−IgD− B Cells in Gut-Associated Lymphoid Tissue in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, S.; Li, S.; Wang, Q.; Li, H.; He, D.; Chen, Y.; Xu, H. The Multi-Organ Landscape of B Cells Highlights Dysregulated Memory B Cell Responses in Crohn’s Disease. Natl. Sci. Rev. 2025, 12, nwaf009. [Google Scholar] [CrossRef] [PubMed]
- Bushara, O.; Escobar, D.J.; Weinberg, S.E.; Sun, L.; Liao, J.; Yang, G.-Y. The Possible Pathogenic Role of IgG4-Producing Plasmablasts in Stricturing Crohn’s Disease. Pathobiology 2022, 89, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Canales-Herrerias, P.; Garcia-Carmona, Y.; Shang, J.; Meringer, H.; Yee, D.S.; Radigan, L.; Buta, S.; Martinez-Delgado, G.; Tankelevich, M.; Helmus, D.; et al. Selective IgA2 Deficiency in a Patient with Small Intestinal Crohn’s Disease. J. Clin. Investig. 2023, 133, e167742. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Zhang, M.; Wang, H.; Jiang, Y.; Gao, P. Ulcerative Colitis Is Characterized by a Decrease in Regulatory B Cells. ECCOJC 2016, 10, 1212–1223. [Google Scholar] [CrossRef]
- Schnell, A.; Schwarz, B.; Wahlbuhl, M.; Allabauer, I.; Hess, M.; Weber, S.; Werner, F.; Schmidt, H.; Rechenauer, T.; Siebenlist, G.; et al. Distribution and Cytokine Profile of Peripheral B Cell Subsets Is Perturbed in Pediatric IBD and Partially Restored During a Successful IFX Therapy. Inflamm. Bowel Dis. 2021, 27, 224–235. [Google Scholar] [CrossRef]
- Mitsialis, V.; Wall, S.; Liu, P.; Ordovas-Montanes, J.; Parmet, T.; Vukovic, M.; Spencer, D.; Field, M.; McCourt, C.; Toothaker, J.; et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology 2020, 159, 591–608.e10. [Google Scholar] [CrossRef]
- Glass, M.C.; Glass, D.R.; Oliveria, J.-P.; Mbiribindi, B.; Esquivel, C.O.; Krams, S.M.; Bendall, S.C.; Martinez, O.M. Human IL-10-Producing B Cells Have Diverse States That Are Induced from Multiple B Cell Subsets. Cell Rep. 2022, 39, 110728. [Google Scholar] [CrossRef]
- Fillatreau, S. Regulatory Functions of B Cells and Regulatory Plasma Cells. Biomed. J. 2019, 42, 233–242. [Google Scholar] [CrossRef]
- Cupi, M.L.; Sarra, M.; Marafini, I.; Monteleone, I.; Franzè, E.; Ortenzi, A.; Colantoni, A.; Sica, G.; Sileri, P.; Rosado, M.M.; et al. Plasma Cells in the Mucosa of Patients with Inflammatory Bowel Disease Produce Granzyme B and Possess Cytotoxic Activities. J. Immunol. 2014, 192, 6083–6091. [Google Scholar] [CrossRef]
- Bouaziz, J.; Yanaba, K.; Tedder, T.F. Regulatory B Cells as Inhibitors of Immune Responses and Inflammation. Immunol. Rev. 2008, 224, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Ishihara, S.; Aziz, M.M.; Oka, A.; Kusunoki, R.; Otani, A.; Tada, Y.; Li, Y.; Moriyama, I.; Oshima, N.; et al. Decreased Production of Interleukin-10 and Transforming Growth Factor-β in Toll-like Receptor-activated Intestinal B Cells in SAMP1/Yit Mice. Immunology 2010, 131, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Povoleri, G.A.M.; Nova-Lamperti, E.; Scottà, C.; Fanelli, G.; Chen, Y.-C.; Becker, P.D.; Boardman, D.; Costantini, B.; Romano, M.; Pavlidis, P.; et al. Human Retinoic Acid-Regulated CD161+ Regulatory T Cells Support Wound Repair in Intestinal Mucosa. Nat. Immunol. 2018, 19, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Thompson-Snipes, L.; Klintmalm, G.; Demetris, A.J.; O’Leary, J.; Oh, S.; Joo, H. CD24hiCD38hi and CD24hiCD27+ Human Regulatory B Cells Display Common and Distinct Functional Characteristics. J. Immunol. 2019, 203, 2110–2120. [Google Scholar] [CrossRef]


| Parametres | CD Patients (n = 43) |
|---|---|
| CDAI, points | 330 [316.0; 381.0] |
| CRP, mg/L | 4.80 [1.60; 17.00] |
| Faecal calprotectin, μg/g | 613.0 [112.0; 2715.0] |
| Platelet, ×109/L | 311 [273.0; 384.0] |
| Before ADA (n = 18) | Before IFX (n = 18) | Before VDZ (n = 7) | |
|---|---|---|---|
| CDAI, points | 345.50 [313; 377] | 330 [317; 384] | 332 [328; 381] |
| CRP, mg/L | 3.75 [0.80; 9.0] | 4.75 [1.20; 29.20] | 9.70 [2.70; 28.60] |
| Faecal calprotectin, μg/g | 493.0 [112; 984] | 307.5 [59; 2764] | 2715 [613; 3720] |
| Platelet, ×109;/L | 334.0 [269; 355] | 297.5 [277; 415] | 332 [285; 384] |
| CD IFX (n = 18) | CD ADA (n = 18) | CD VDZ (n = 7) | Controls (n = 16) | |
|---|---|---|---|---|
| Women | 8 (44.8) | 10 (55.6) | 3 (42.9) | 5 (31.2) |
| Males | 10 (55.6) | 8 (44.4) | 4 (57.1) | 11 (68.8) |
| Age ± SD | 37.8 ± 11.9 | 34.8 ± 9.9 | 42.6 ± 15.9 | 40 ± 10.1 |
| Without therapy | 1 | 2 | 0 | - |
| Therapy with 5-ASA | 2 | 1 | 1 | - |
| Therapy with budesonide | 0 | 1 | 0 | - |
| Therapy with prednisone | 0 | 1 | 0 | - |
| Therapy with immunosuppressants | 2 | 1 | 0 | - |
| Therapy with 5-ASA + prednisone | 3 | 4 | 2 | - |
| Therapy with 5-ASA + immunosuppressants | 2 | 1 | 1 | - |
| Therapy with budesonide + immunosuppressants | 0 | 1 | 0 | - |
| Therapy with prednisone + immunosuppressants | 2 | 4 | 2 | - |
| Therapy with 5-ASA + prednisone + immunosuppressants | 6 | 2 | 1 | - |
| Antibodies | |
|---|---|
| B cell subsets | anti-CD19 FITC (clone 4G7), anti-CD24 PE (clone ML5-RUO), anti-CD27 PE-Cy7 (clone M-T271), anti-CD38 BV421 (clone HIT2), anti-CD5 PerCP-Cyanine 5.5 (clone L17F12), and anti-CD1d APC (clone CD1d42) (all from BD Biosciences) |
| Regulatory T cells and their subsets | anti-CD4 APC-Cy7 (RPA-T4 clone), anti-CD8 V500 (clone RPA-T8), anti-CD25 BV421 (M-A251 clone), anti-CD127 PerCP-Cyanine 5.5 (clone HIL-7R-M21), anti-CD127 FITC (clone R34.34), anti-CD45RA APC (clone HI100), (all from BD Biosciences). CD161 PE (clone 191B8) (Beckman Coulter) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helmin-Basa, A.; Kopoń, M.; Koza, J.; Strzyżewska, E.; Skalska-Bugała, A.; Leśniewski, F.; Wiese-Szadkowska, M.; Balcerowska, S.; Michałkiewicz, J.; Kłopocka, M. Distinct B Cell Subsets Changes as Potential Biomarkers of Response to Biologic Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2025, 26, 9539. https://doi.org/10.3390/ijms26199539
Helmin-Basa A, Kopoń M, Koza J, Strzyżewska E, Skalska-Bugała A, Leśniewski F, Wiese-Szadkowska M, Balcerowska S, Michałkiewicz J, Kłopocka M. Distinct B Cell Subsets Changes as Potential Biomarkers of Response to Biologic Therapy in Crohn’s Disease. International Journal of Molecular Sciences. 2025; 26(19):9539. https://doi.org/10.3390/ijms26199539
Chicago/Turabian StyleHelmin-Basa, Anna, Maria Kopoń, Jarosław Koza, Edyta Strzyżewska, Aleksandra Skalska-Bugała, Fabian Leśniewski, Małgorzata Wiese-Szadkowska, Sara Balcerowska, Jacek Michałkiewicz, and Maria Kłopocka. 2025. "Distinct B Cell Subsets Changes as Potential Biomarkers of Response to Biologic Therapy in Crohn’s Disease" International Journal of Molecular Sciences 26, no. 19: 9539. https://doi.org/10.3390/ijms26199539
APA StyleHelmin-Basa, A., Kopoń, M., Koza, J., Strzyżewska, E., Skalska-Bugała, A., Leśniewski, F., Wiese-Szadkowska, M., Balcerowska, S., Michałkiewicz, J., & Kłopocka, M. (2025). Distinct B Cell Subsets Changes as Potential Biomarkers of Response to Biologic Therapy in Crohn’s Disease. International Journal of Molecular Sciences, 26(19), 9539. https://doi.org/10.3390/ijms26199539






