Mechanism of Regulation of NaCl Homeostasis in the Distal Colon During Obesity
Abstract
1. Introduction
2. Results
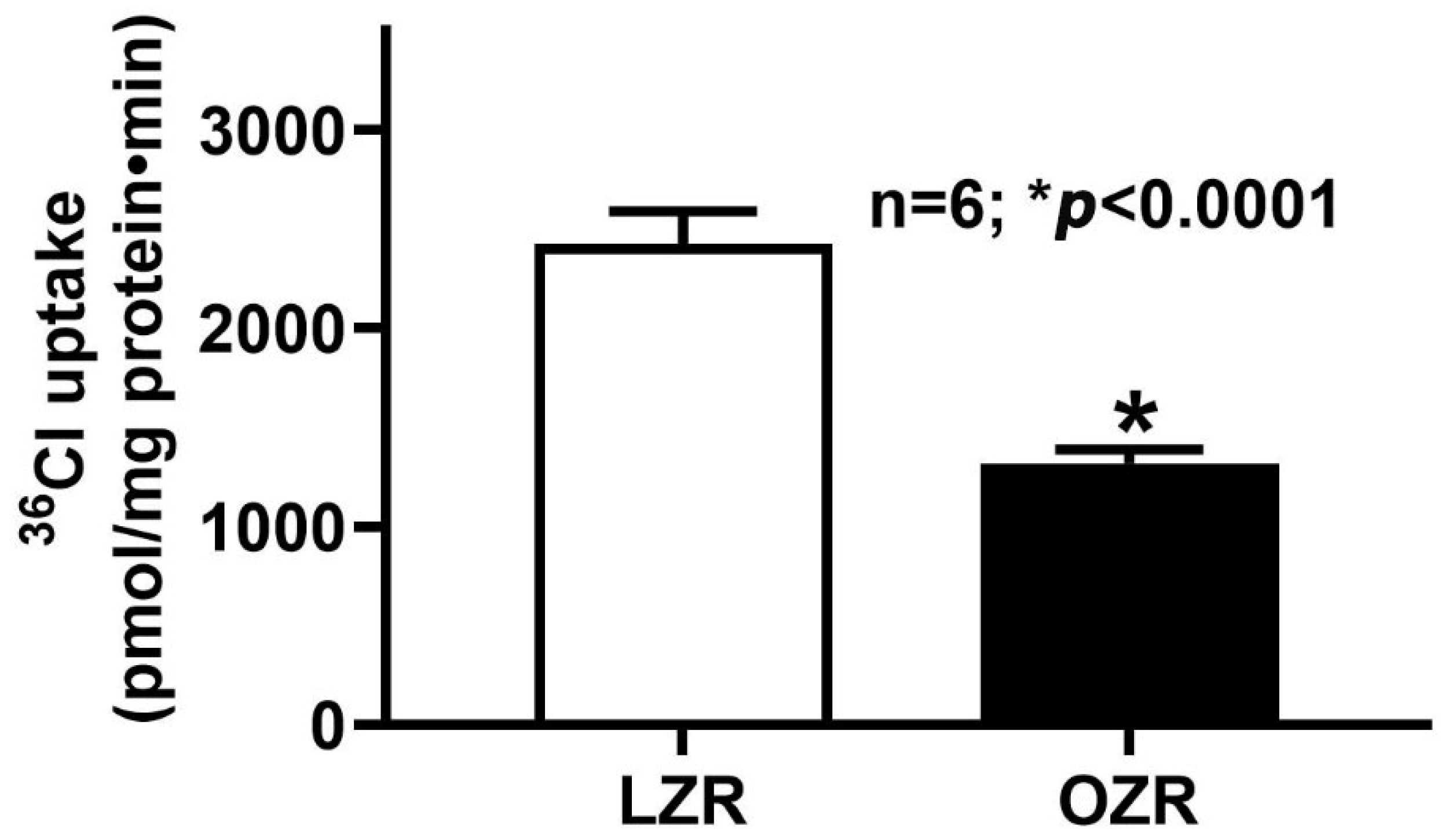
2.1. Cl−/HCO3− Exchange (DRA) Activity in the Colon During Obesity
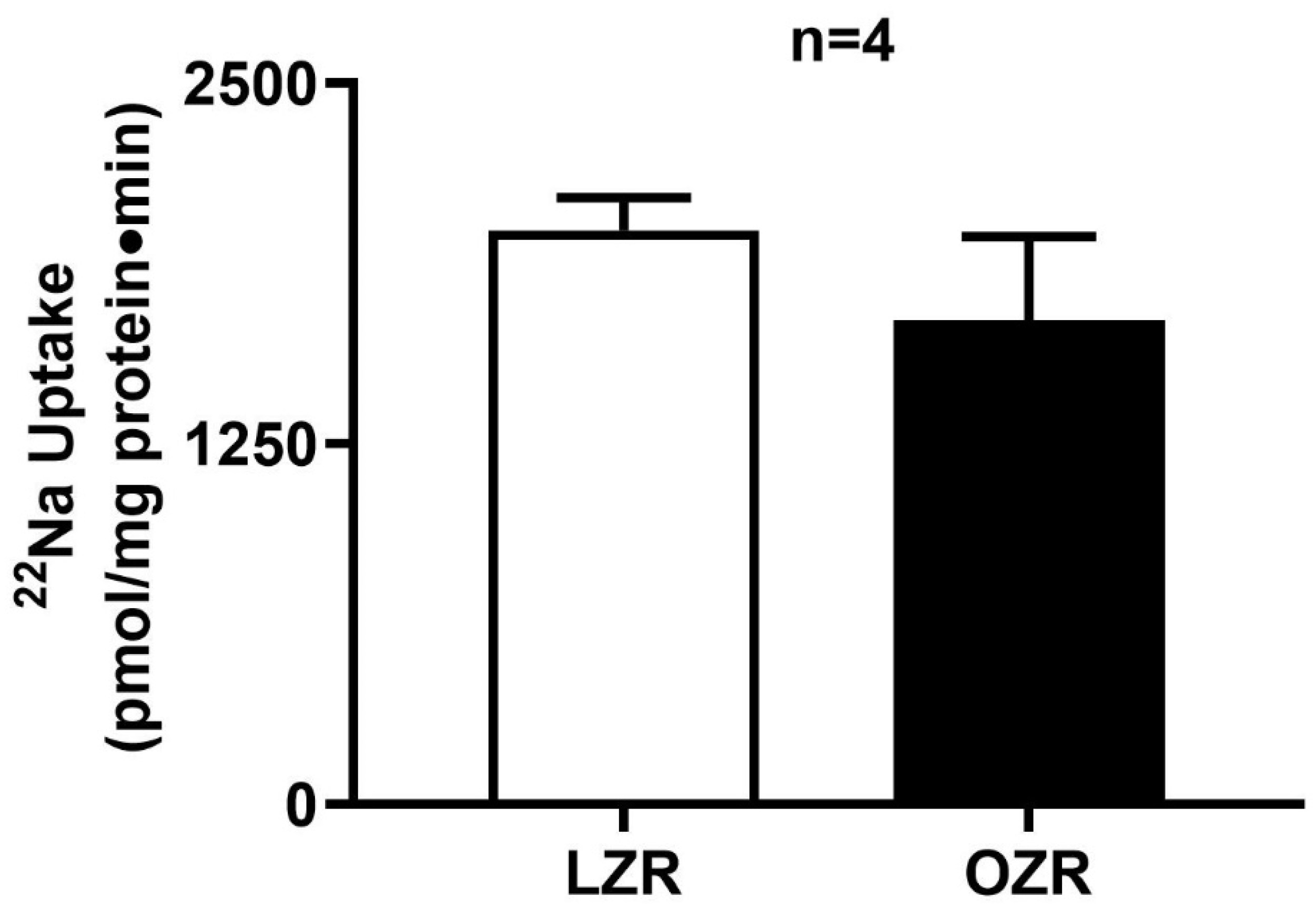
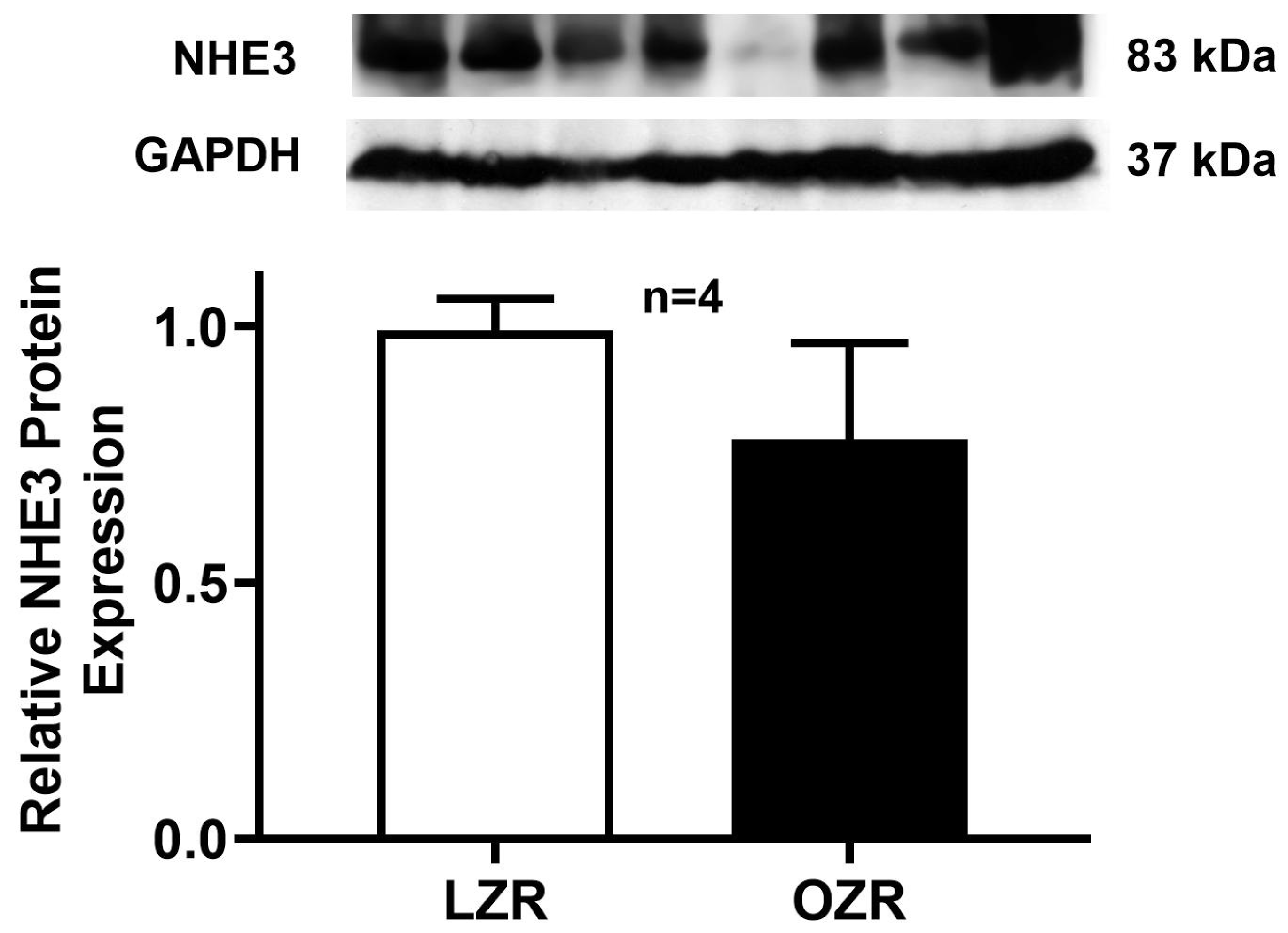
2.2. Na+/H+ Exchange (NHE3) Activity in the Distal Colon in Obesity
2.3. Kinetic Studies of Cl−/HCO3− Exchange in the Distal Colon in Obesity
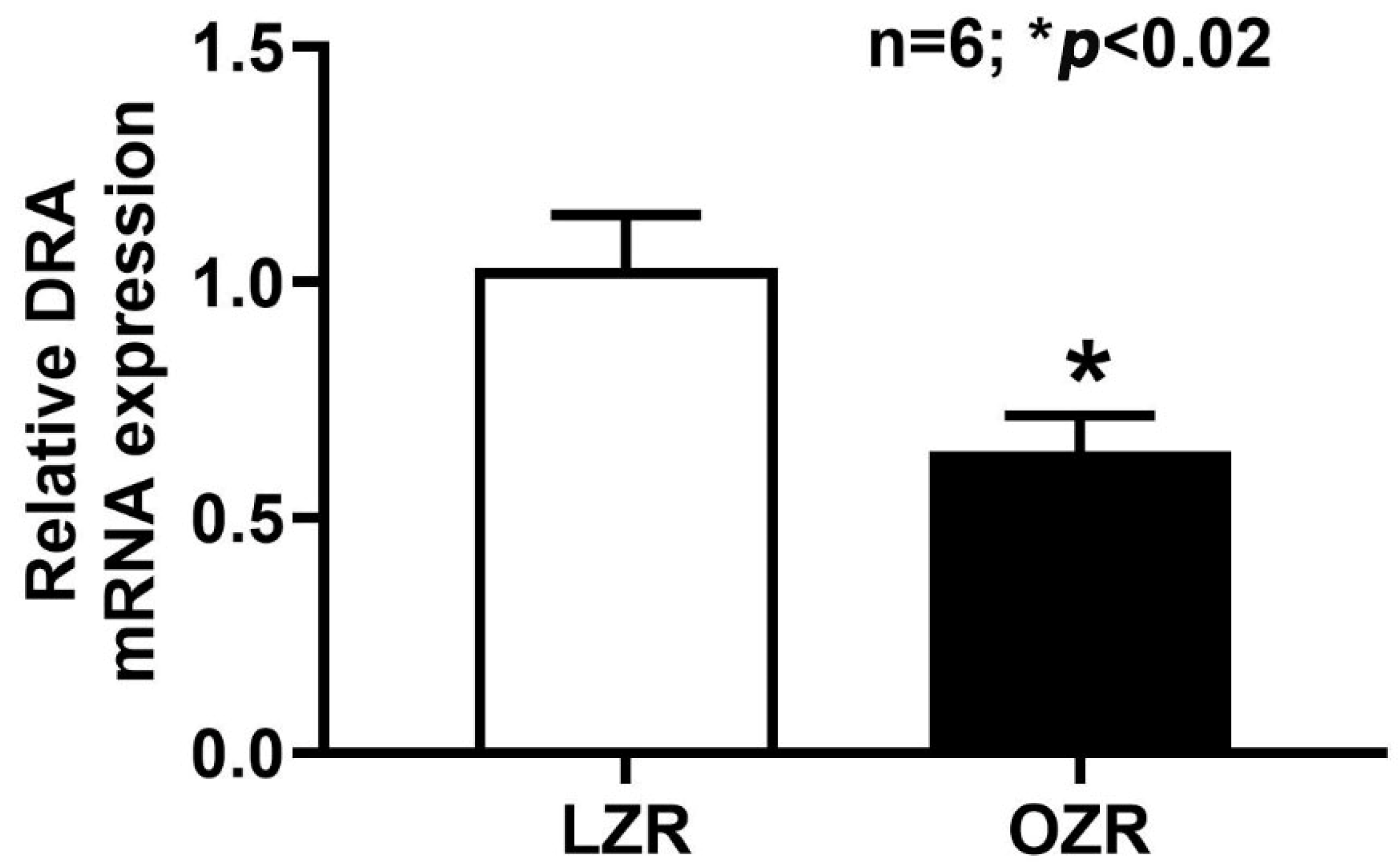
2.4. RT-qPCR Analysis of DRA mRNA Expression in the Obese Zucker Rat
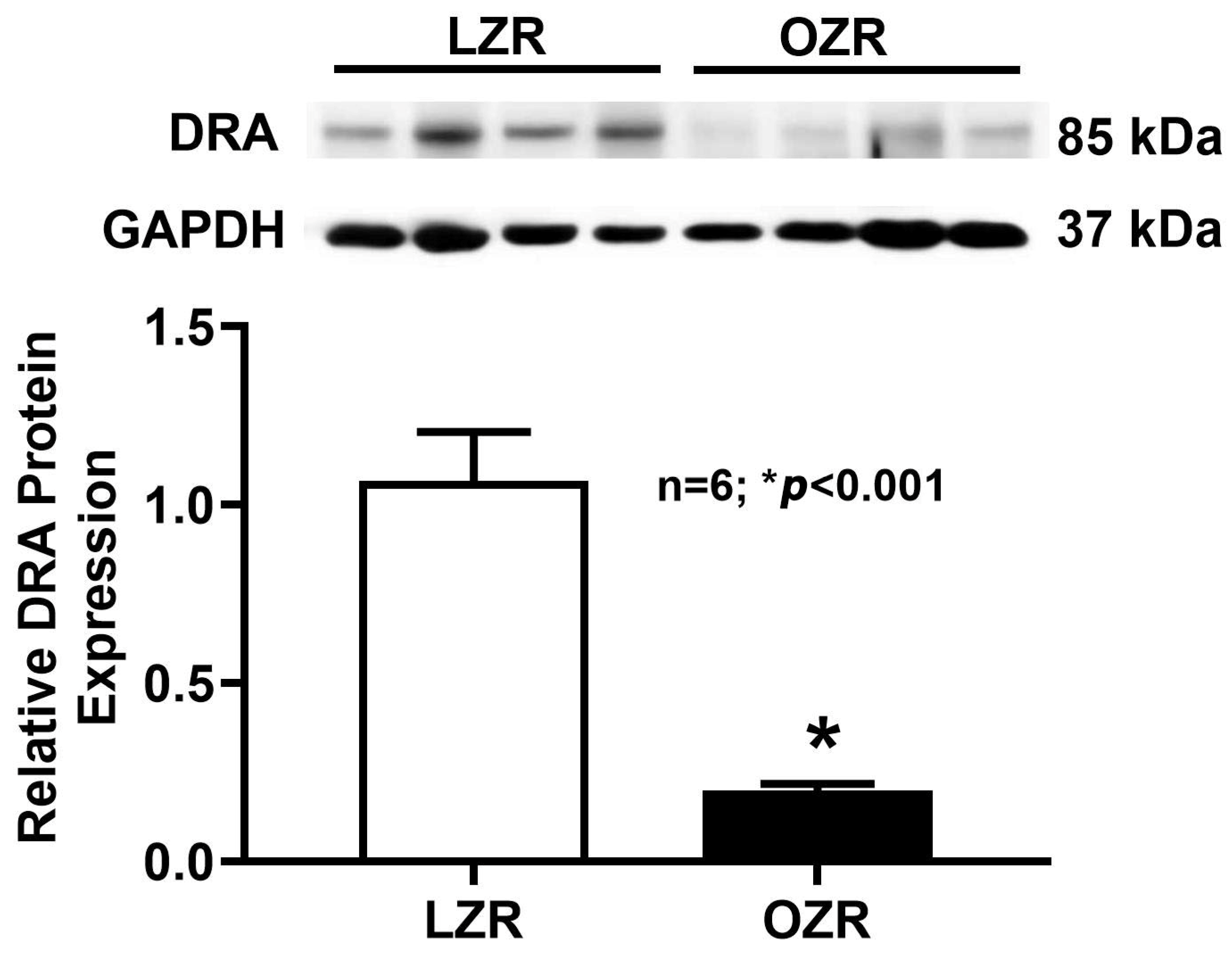
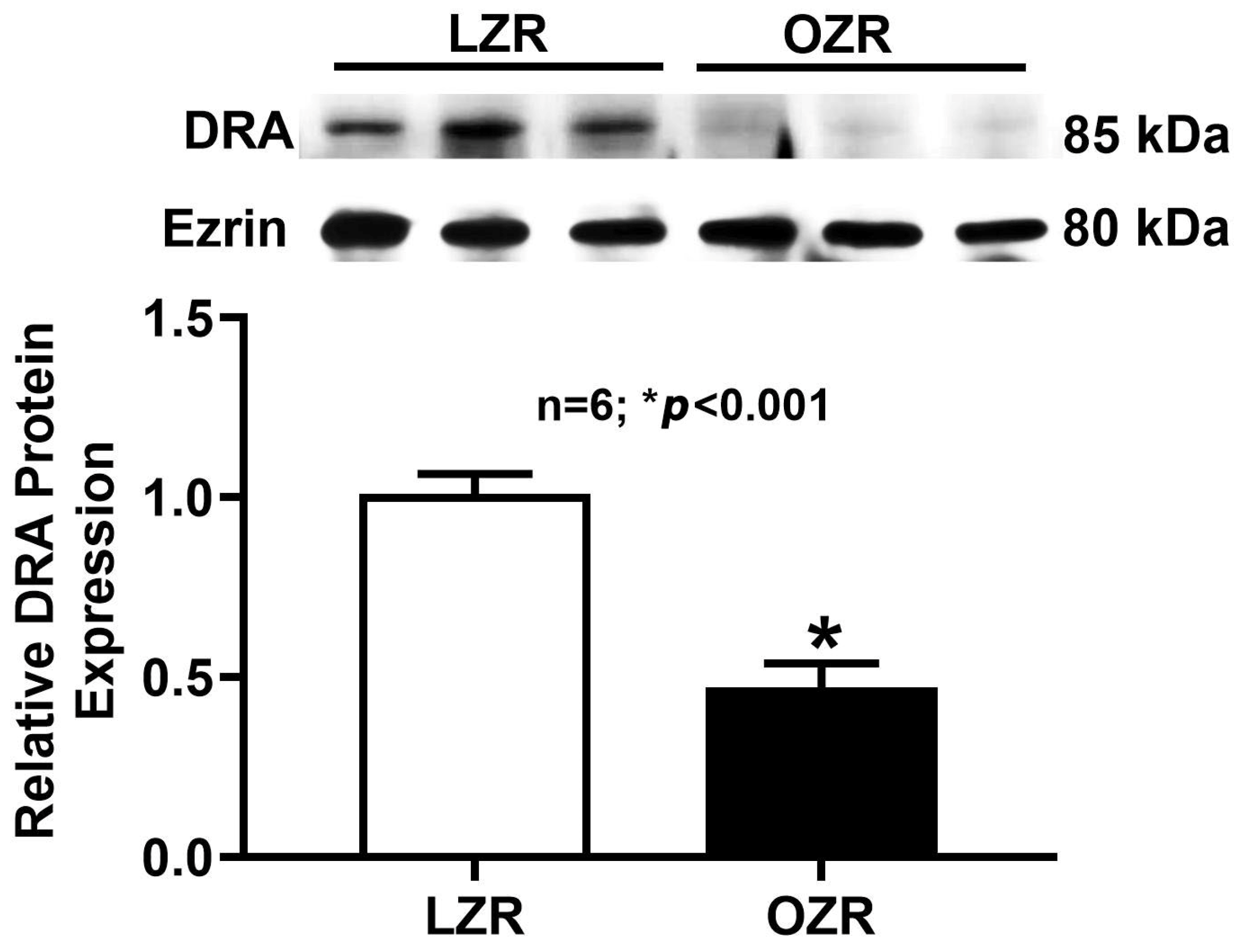
2.5. Western Blot Analysis
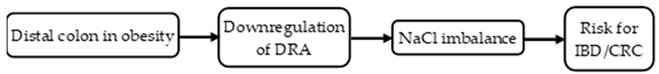
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal Models
4.3. Colonocyte Isolation and Apical Membrane Vesicle Preparation
4.4. Uptake Studies in Intact Ileal Villus Cells and AMV
4.5. Real-Time Quantitative PCR (RT-qPCR) for DRA
4.6. Western Blotting
4.7. Protein Estimation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s Disease |
| UC | Ulcerative colitis |
| NaCl | Sodium chloride |
| DRA | Downregulated in Adenoma |
| NHE3 | Sodium proton exchanger 3 |
| SGLT1 | Sodium-dependent glucose co-transport 1 |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| PAT-1 | Putative Anion Transporter-1 |
| CAC | Colitis-associated colon cancer |
| CRC | Colorectal cancer |
| CLD | Congenital chloride diarrhea |
| DIDS | 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt |
| LZR | Lean Zucker Rat |
| OZR | Obese Zucker Rat |
| AMV | Apical membrane vesicle |
| GI | Gastrointestinal |
| TNFα | Tumor Necrosis Factor alpha |
| INFγ | Interferon gamma |
| IL | Interleukins |
| NO | Nitric oxide |
| NF-kB | Nuclear factor kappa B |
| miRNA | micro ribonucleic acid |
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514–527, Erratum in Curr. Obes. Rep. 2023, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected US state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Wade, K.H.; Zahid, S.; Brancale, J.; Xia, R.; Distefano, M.; Senol-Cosar, O.; Haas, M.E.; Bick, A. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 2019, 177, 587–596.e589. [Google Scholar] [CrossRef]
- Farooqi, I.S. Monogenic obesity syndromes provide insights into the hypothalamic regulation of appetite and associated behaviors. Biol. Psychiatry 2022, 91, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.; Yeo, G.S. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Tarasiuk, A.; Mosińska, P.; Fichna, J. The mechanisms linking obesity to colon cancer: An overview. Obes. Res. Clin. Pract. 2018, 12, 251–259. [Google Scholar] [CrossRef]
- Gibson, T.M.; Park, Y.; Robien, K.; Shiels, M.S.; Black, A.; Sampson, J.N.; Purdue, M.P.; Freeman, L.E.B.; Andreotti, G.; Weinstein, S.J. Body mass index and risk of second obesity-associated cancers after colorectal cancer: A pooled analysis of prospective cohort studies. J. Clin. Oncol. 2014, 32, 4004. [Google Scholar] [CrossRef]
- Palaniappan, B.; Arthur, S.; Sundaram, V.L.; Butts, M.; Sundaram, S.; Mani, K.; Singh, S.; Nepal, N.; Sundaram, U. Inhibition of intestinal villus cell Na/K-ATPase mediates altered glucose and NaCl absorption in obesity-associated diabetes and hypertension. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 9323–9333. [Google Scholar] [CrossRef]
- Crintea, I.N.; Cindrea, A.C.; Mederle, O.A.; Trebuian, C.I.; Timar, R. Electrolyte Imbalances and Metabolic Emergencies in Obesity: Mechanisms and Clinical Implications. Diseases 2025, 13, 69. [Google Scholar] [CrossRef]
- Alper, S.L.; Sharma, A.K. The SLC26 gene family of anion transporters and channels. Mol. Asp. Med. 2013, 34, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, S.; Gomes, R.; Gill, R.K.; Saksena, S.; Alrefai, W.A.; Dudeja, P.K. Mechanisms Underlying Dysregulation of Electrolyte Absorption in Inflammatory Bowel Disease–Associated Diarrhea. Inflamm. Bowel Dis. 2015, 21, 2926–2935. [Google Scholar] [CrossRef]
- Ishiguro, H. HCO3− secretion by SLC 26A3 and mucosal defence in the colon. Acta Physiol. 2014, 211, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Talbot, C.; Lytle, C. Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G358–G367. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Nagai, H.; Ohba, K.-I.; Soleimani, M.; Suzuki, Y. Segmental differences in Slc26a3-dependent Cl− absorption and HCO3− secretion in the mouse large intestine in vitro in Ussing chambers. J. Physiol. Sci. 2021, 71, 5. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef]
- Simpson, J.E.; Schweinfest, C.W.; Shull, G.E.; Gawenis, L.R.; Walker, N.M.; Boyle, K.T.; Soleimani, M.; Clarke, L.L. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1079–G1088. [Google Scholar] [CrossRef]
- Xiao, F.; Yu, Q.; Li, J.; Johansson, M.; Singh, A.; Xia, W.; Riederer, B.; Engelhardt, R.; Montrose, M.; Soleimani, M. Slc26a3 deficiency is associated with loss of colonic HCO3− secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol. 2014, 211, 161–175. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, W.; Furth, E.E.; Wen, X.; Katz, J.P.; Sellon, R.K.; Silberg, D.G.; Antalis, T.M.; Schweinfest, C.W.; Wu, G.D. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G1445–G1453. [Google Scholar] [CrossRef]
- Lohi, H.; Makela, S.; Pulkkinen, K.; Hoglund, P.; Karjalainen-Lindsberg, M.-L.; Puolakkainen, P.; Kere, J. Upregulation of CFTR expression but not SLC26A3 and SLC9A3 in ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G567–G575. [Google Scholar] [CrossRef]
- Asano, K.; Matsushita, T.; Umeno, J.; Hosono, N.; Takahashi, A.; Kawaguchi, T.; Matsumoto, T.; Matsui, T.; Kakuta, Y.; Kinouchi, Y. A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat. Genet. 2009, 41, 1325–1329. [Google Scholar] [CrossRef]
- Schweinfest, C.W.; Henderson, K.W.; Suster, S.; Kondoh, N.; Papas, T.S. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc. Natl. Acad. Sci. USA 1993, 90, 4166–4170. [Google Scholar] [CrossRef] [PubMed]
- Antalis, T.M.; Reeder, J.A.; Gotley, D.C.; Byeon, M.K.; Walsh, M.D.; Henderson, K.W.; Papas, T.S.; Schweinfest, C.W. Down-regulation of the down-regulated in adenoma (DRA) gene correlates with colon tumor progression. Clin. Cancer Res. 1998, 4, 1857–1863. [Google Scholar]
- Zhang, N.; Heruth, D.P.; Wu, W.; Zhang, L.Q.; Nsumu, M.N.; Shortt, K.; Li, K.; Jiang, X.; Wang, B.; Friesen, C. Functional characterization of SLC26A3 c. 392C>G (p. P131R) mutation in intestinal barrier function using CRISPR/CAS9-created cell models. Cell Biosci. 2019, 9, 40. [Google Scholar] [CrossRef]
- Arthur, S.; Palaniappan, B.; Sundaram, U. Mo1798 Unique Regulation of Apical Membrane Cl: HCO3 Exchange (DRA) in Chronic Colitis Associated Cancer in Rats. Gastroenterology 2013, 144, S-666. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.; Abrams, K.; Mayberry, J. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef]
- Canavan, C.; Abrams, K.; Mayberry, J. Meta-analysis: Colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2006, 23, 1097–1104. [Google Scholar] [CrossRef]
- Xu, P.; Tao, Z.; Yang, H.; Zhang, C. Obesity and early-onset colorectal cancer risk: Emerging clinical evidence and biological mechanisms. Front. Oncol. 2024, 14, 1366544. [Google Scholar] [CrossRef]
- Han, K.; Wang, X.; Niu, X.; Li, T.; Linghu, E. Prevalence and associated factors of chronic diarrhea among adults with obesity in the United States: Evidence from the National Health and Nutrition Examination Survey 2005 to 2010. Obes. Res. Clin. Pract. 2024, 18, 328–335. [Google Scholar] [CrossRef]
- Han, K.; Wang, X.; Wang, Y.; Niu, X.; Xiang, J.; Ru, N.; Jia, C.; Sun, H.; He, Z.; Feng, Y.; et al. Prevalence of chronic diarrhea and its association with obesity in a Chinese community-based population. China Med. J. 2024, 138, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Hihnala, S.; Höglund, P.; Lammi, L.; Kokkonen, J.; Örmälä, T.; Holmberg, C. Long-term clinical outcome in patients with congenital chloride diarrhea. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 369–375. [Google Scholar] [CrossRef]
- McCoy, J.; Miller, M.R.; Watson, M.; Crowley, E.; Woolfson, J.P. Paediatric obesity and Crohn’s disease: A descriptive review of disease phenotype and clinical course. Paediatr. Child Health 2024, 29, 158–162. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, L.; Sun, J.; Zhang, Y.; Dai, F. Association of body roundness index with chronic diarrhea and constipation, NHANES 2005–2010. J. Health Popul. Nutr. 2025, 44, 50. [Google Scholar] [CrossRef]
- Ballou, S.; Singh, P.; Rangan, V.; Iturrino, J.; Nee, J.; Lembo, A. Obesity is associated with significantly increased risk for diarrhoea after controlling for demographic, dietary and medical factors: A cross-sectional analysis of the 2009–2010 National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 2019, 50, 1019–1024. [Google Scholar] [CrossRef]
- Kobayashi, M.; Pattarathitwat, P.; Pongprajakand, A.; Kongkaew, S. Association of normal weight obesity with lifestyle and dietary habits in young Thai women: A cross-sectional study. Obes. Pillars 2023, 5, 100055. [Google Scholar] [CrossRef] [PubMed]
- Khakoo, N.S.; Ioannou, S.; Khakoo, N.S.; Vedantam, S.; Pearlman, M. Impact of Obesity on Inflammatory Bowel Disease. Curr. Gastroenterol. Rep. 2022, 24, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Ohno-Machado, L.; Sandborn, W.J.; Singh, S. Obesity is independently associated with higher annual burden and costs of hospitalization in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 709–718.e707. [Google Scholar] [CrossRef]
- Johnson, A.M.; Loftus, E.V. Impact of obesity on the management of inflammatory bowel disease. Gastroenterol. Hepatol. 2020, 16, 350. [Google Scholar]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110. [Google Scholar] [CrossRef]
- Nunziata, A.; Funcke, J.-B.; Borck, G.; Von Schnurbein, J.; Brandt, S.; Lennerz, B.; Moepps, B.; Gierschik, P.; Fischer-Posovszky, P.; Wabitsch, M. Functional and phenotypic characteristics of human leptin receptor mutations. J. Endocr. Soc. 2019, 3, 27–41. [Google Scholar] [CrossRef]
- Camilleri, M.; Malhi, H.; Acosta, A. Gastrointestinal complications of obesity. Gastroenterology 2017, 152, 1656–1670. [Google Scholar] [CrossRef]
- Eslick, G. Gastrointestinal symptoms and obesity: A meta-analysis. Obes. Rev. 2012, 13, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; De Giorgio, R. Letter: Diarrhoea in obese patients-a new nosographic entity? Aliment. Pharmacol. Ther. 2020, 51, 405. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD associated diarrhea. Tissue Barriers 2018, 6, e1463897. [Google Scholar] [CrossRef]
- Kere, J. Overview of the SLC26 family and associated diseases. In Proceedings of the Novartis Foundation Symposium 273, Epithelial Anion Transport in Health and Disease: The Role of the SLC26 Transporters Family, London, UK, 1–3 March 2005; John Wiley: Chichester, UK; Hoboken, NJ, USA, 2006; pp. 2–18. [Google Scholar]
- Kere, J.; Lohi, H.; Höglund, P. Genetic Disorders of Membrane Transport III. Congenital chloride diarrhea. Am. J. Physiol. 1999, 276, G7–G13. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, S.; Kere, J.; Holmberg, C.; Höglund, P. SLC26A3 mutations in congenital chloride diarrhea. Hum. Mutat. 2002, 20, 425–438. [Google Scholar] [CrossRef]
- Schweinfest, C.W.; Spyropoulos, D.D.; Henderson, K.W.; Kim, J.H.; Chapman, J.M.; Barone, S.; Worrell, R.T.; Wang, Z.; Soleimani, M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 2006, 281, 37962–37971. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, D.; Priyamvada, S.; Kageyama, T.; White, Z.; Kumar, A.; Griggs, T.F.; Majumder, A.; Akram, R.; Anbazhagan, A.N.; Sano, T.; et al. Loss of SLC26A3 Results in Colonic Mucosal Immune Dysregulation via Epithelial-Immune Cell Crosstalk. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 903–919. [Google Scholar] [CrossRef]
- Kumar, A.; Jayawardena, D.; Priyamvada, S.; Anbazhagan, A.N.; Chatterjee, I.; Saksena, S.; Dudeja, P.K. SLC26A3 (DRA, the Congenital Chloride Diarrhea Gene): A Novel Therapeutic Target for Diarrheal Diseases. Cell. Mol. Gastroenterol. Hepatol. 2024, 19, 101452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Priyamvada, S.; Ge, Y.; Jayawardena, D.; Singhal, M.; Anbazhagan, A.N.; Chatterjee, I.; Dayal, A.; Patel, M.; Zadeh, K. A novel role of SLC26A3 in the maintenance of intestinal epithelial barrier integrity. Gastroenterology 2021, 160, 1240–1255.e1243. [Google Scholar] [CrossRef]
- Yu, Q. Slc26a3 (DRA) in the gut: Expression, function, regulation, role in infectious diarrhea and inflammatory bowel disease. Inflamm. Bowel Dis. 2021, 27, 575–584. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2020, 70, 313. [Google Scholar]
- Hosseini, S.T.; Nemati, F. Identification of GUCA2A and COL3A1 as prognostic biomarkers in colorectal cancer by integrating analysis of RNA-Seq data and qRT-PCR validation. Sci. Rep. 2023, 13, 17086. [Google Scholar] [CrossRef]
- Barmeyer, C.; Amasheh, S.; Tavalali, S.; Mankertz, J.; Zeitz, M.; Fromm, M.; Schulzke, J.-D. IL-1β and TNFα regulate sodium absorption in rat distal colon. Biochem. Biophys. Res. Commun. 2004, 317, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Kaissi, S.; Homaidan, F.R.; Naim, H.Y.; El-Sabban, M.E. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 2016, 6, 29783. [Google Scholar] [CrossRef] [PubMed]
- Sugi, K.; Musch, M.W.; Chang, E.B.; Field, M. Inhibition of Na+, K+-ATPase by interferon γ down-regulates intestinal epithelial transport and barrier function. Gastroenterology 2001, 120, 1393–1403. [Google Scholar] [CrossRef]
- Rocha, F.; Musch, M.W.; Lishanskiy, L.; Bookstein, C.; Sugi, K.; Xie, Y.; Chang, E.B. IFN-γ downregulates expression of Na+/H+ exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am. J. Physiol.-Cell Physiol. 2001, 280, C1224–C1232. [Google Scholar] [CrossRef]
- Howe, K.; Gauldie, J.; McKay, D.M. TGF-β effects on epithelial ion transport and barrier: Reduced Cl− secretion blocked by a p38 MAPK inhibitor. Am. J. Physiol.-Cell Physiol. 2002, 283, C1667–C1674. [Google Scholar] [CrossRef] [PubMed]
- Greenwood-Van Meerveld, B.; Tyler, K.; Keith, J.C. Recombinant human interleukin-11 modulates ion transport and mucosal inflammation in the small intestine and colon. Lab. Investig. 2000, 80, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.K.; Wilson, K.T. Nitric oxide in inflammatory bowel disease. Inflamm. Bowel Dis. 2003, 9, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chatterjee, I.; Gujral, T.; Alakkam, A.; Coffing, H.; Anbazhagan, A.N.; Borthakur, A.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; et al. Activation of Nuclear Factor-κB by Tumor Necrosis Factor in Intestinal Epithelial Cells and Mouse Intestinal Epithelia Reduces Expression of the Chloride Transporter SLC26A3. Gastroenterology 2017, 153, 1338–1350.e1333. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, P.; Coon, S.; Baseler, W.; Sundaram, S.; Kekuda, R.; Sundaram, U. Prostaglandins, not the leukotrienes, regulate Cl−/HCO3− exchange (DRA, SLC26A3) in villus cells in the chronically inflamed rabbit ileum. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 179–186. [Google Scholar] [CrossRef]
- Arthur, S.; Palaniappan, B.; Afroz, S.; Sundaram, U. Unique Regulation of Coupled NaCl Absorption by Inducible Nitric Oxide in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Intestinal Inflammation. Inflamm. Bowel Dis. 2021, 27, 1804–1812. [Google Scholar] [CrossRef]
- Rahman, M.M.; Borthakur, A.; Afroz, S.; Arthur, S.; Sundaram, U. Unique Regulation of Intestinal Villus Epithelial Cl−/HCO3− Exchange by Cyclooxygenase Pathway Metabolites of Arachidonic Acid in a Mouse Model of Spontaneous Ileitis. Int. J. Mol. Sci. 2021, 22, 4171. [Google Scholar] [CrossRef]
- Rahman, M.M.; Afroz, S.; Arthur, S.; Sundaram, U. Mast cell mediated regulation of small intestinal chloride malabsorption in SAMP1/YitFc mouse model of spontaneous chronic Ileitis. Cells 2021, 10, 697. [Google Scholar] [CrossRef]
- Ding, X.; Li, D.; Li, M.; Wang, H.; He, Q.; Wang, Y.; Yu, H.; Tian, D.; Yu, Q. SLC26A3 (DRA) prevents TNF-alpha-induced barrier dysfunction and dextran sulfate sodium-induced acute colitis. Lab. Investig. J. Tech. Methods Pathol. 2018, 98, 462–476. [Google Scholar] [CrossRef]
- Saksena, S.; Singla, A.; Goyal, S.; Katyal, S.; Bansal, N.; Gill, R.K.; Alrefai, W.A.; Ramaswamy, K.; Dudeja, P.K. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G159–G166. [Google Scholar] [CrossRef]
- Priyamvada, S.; Anbazhagan, A.N.; Gujral, T.; Borthakur, A.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; Dudeja, P.K. All-trans-retinoic acid increases SLC26A3 DRA (down-regulated in adenoma) expression in intestinal epithelial cells via HNF-1β. J. Biol. Chem. 2015, 290, 15066–15077. [Google Scholar] [CrossRef]
- Van der Goten, J.; Vanhove, W.; Lemaire, K.; Van Lommel, L.; Machiels, K.; Wollants, W.-J.; De Preter, V.; De Hertogh, G.; Ferrante, M.; Van Assche, G. Integrated miRNA and mRNA expression profiling in inflamed colon of patients with ulcerative colitis. PLoS ONE 2014, 9, e116117. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Priyamvada, S.; Kumar, A.; Maher, D.B.; Borthakur, A.; Alrefai, W.A.; Malakooti, J.; Kwon, J.H.; Dudeja, P.K. Translational repression of SLC26A3 by miR-494 in intestinal epithelial cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 306, G123–G131. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef]
- Winer, D.A.; Luck, H.; Tsai, S.; Winer, S. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016, 23, 413–426. [Google Scholar] [CrossRef]
- Shemtov, S.J.; Emani, R.; Bielska, O.; Covarrubias, A.J.; Verdin, E.; Andersen, J.K.; Winer, D.A. The intestinal immune system and gut barrier function in obesity and ageing. FEBS J. 2023, 290, 4163–4186. [Google Scholar] [CrossRef]
- Khan, S.; Luck, H.; Winer, S.; Winer, D.A. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat. Commun. 2021, 12, 2598. [Google Scholar] [CrossRef]
- Kaur, K.K.; Allahbadia, G.; Singh, M. Intestinal Immune System in the Regulation of Obesity and Metabolic Syndrome-Therapeutic Implications-A Systematic Review. EC Clin. Exp. Anat. 2020, 3, 7–22. [Google Scholar]
- Spyrou, N.; Vallianou, N.; Kadillari, J.; Dalamaga, M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin. Cancer Biol. 2021, 73, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Acciarino, A.; Diwakarla, S.; Handreck, J.; Bergola, C.; Sahakian, L.; McQuade, R.M. The role of the gastrointestinal barrier in obesity-associated systemic inflammation. Obes. Rev. 2023, 25, e13673. [Google Scholar] [CrossRef] [PubMed]
- Yde, J.; Keely, S.; Wu, Q.; Borg, J.F.; Lajczak, N.; O’Dwyer, A.; Dalsgaard, P.; Fenton, R.A.; Moeller, H.B. Characterization of AQPs in mouse, rat, and human colon and their selective regulation by bile acids. Front. Nutr. 2016, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, U.; Wisel, S.; Rajendren, V.; West, A. Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am. J. Physiol.-Gastrointest. Liver Physiol. 1997, 273, G913–G919. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, U.; West, A.B. Effect of chronic inflammation on electrolyte transport in rabbit ileal villus and crypt cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 272, G732–G741. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, B.; Sundaram, S.; Arthur, S.; Afroz, S.; Sundaram, U. Inducible nitric oxide regulates na-glucose Co-transport in a spontaneous SAMP1/YitFc mouse model of chronic ileitis. Nutrients 2020, 12, 3116. [Google Scholar] [CrossRef]
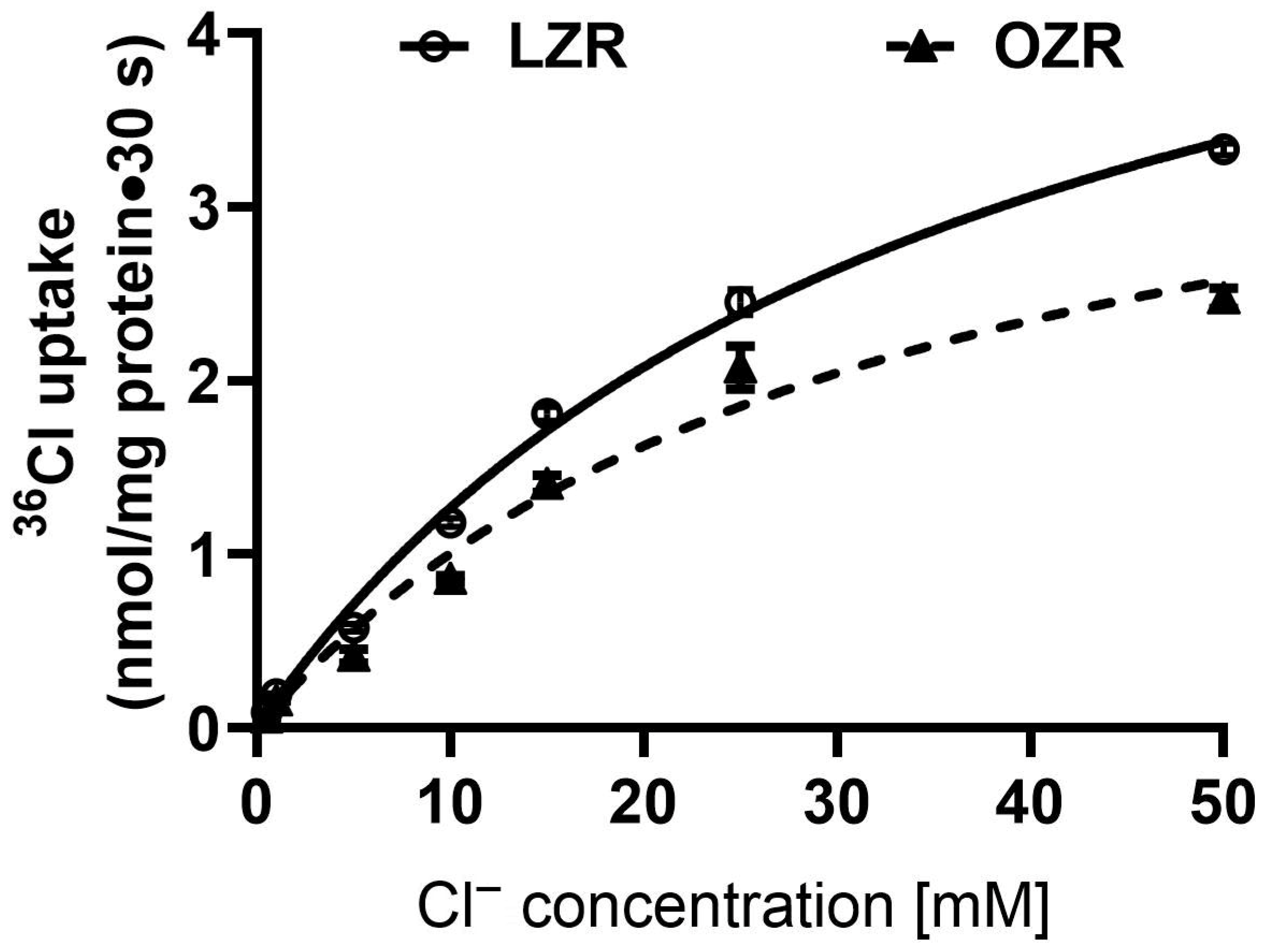
| Km (mM) | Vmax (nmol/mg Protein·30 s) | |
|---|---|---|
| Lean Zucker rat | 35.7 ± 3.2 | 5.8 ± 0.3 |
| Obese Zucker rat | 32.1 ± 5.7 | 4.2 ± 0.4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaniappan, B.; Crutchley, J.; Paulraj, R.S.; Borthakur, A.; Arthur, S. Mechanism of Regulation of NaCl Homeostasis in the Distal Colon During Obesity. Int. J. Mol. Sci. 2025, 26, 9139. https://doi.org/10.3390/ijms26189139
Palaniappan B, Crutchley J, Paulraj RS, Borthakur A, Arthur S. Mechanism of Regulation of NaCl Homeostasis in the Distal Colon During Obesity. International Journal of Molecular Sciences. 2025; 26(18):9139. https://doi.org/10.3390/ijms26189139
Chicago/Turabian StylePalaniappan, Balasubramanian, John Crutchley, Raja Singh Paulraj, Alip Borthakur, and Subha Arthur. 2025. "Mechanism of Regulation of NaCl Homeostasis in the Distal Colon During Obesity" International Journal of Molecular Sciences 26, no. 18: 9139. https://doi.org/10.3390/ijms26189139
APA StylePalaniappan, B., Crutchley, J., Paulraj, R. S., Borthakur, A., & Arthur, S. (2025). Mechanism of Regulation of NaCl Homeostasis in the Distal Colon During Obesity. International Journal of Molecular Sciences, 26(18), 9139. https://doi.org/10.3390/ijms26189139






