Light-Cured Dental Fillings Containing Quinoline and Quinoxaline Derivatives: The Influence of Sorption and Solubility on Color Change—Part III
Abstract
1. Introduction
2. Results and Discussion
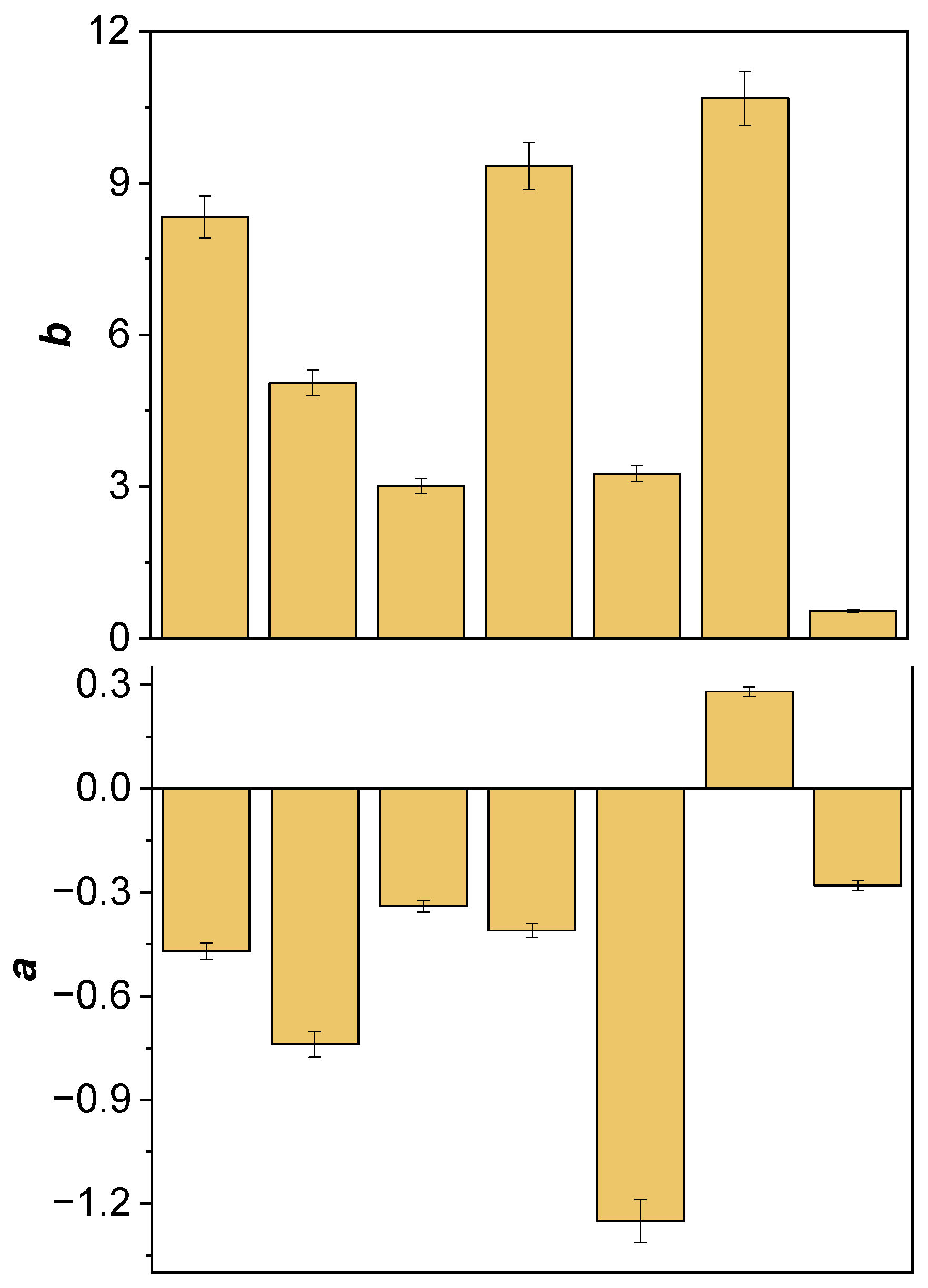
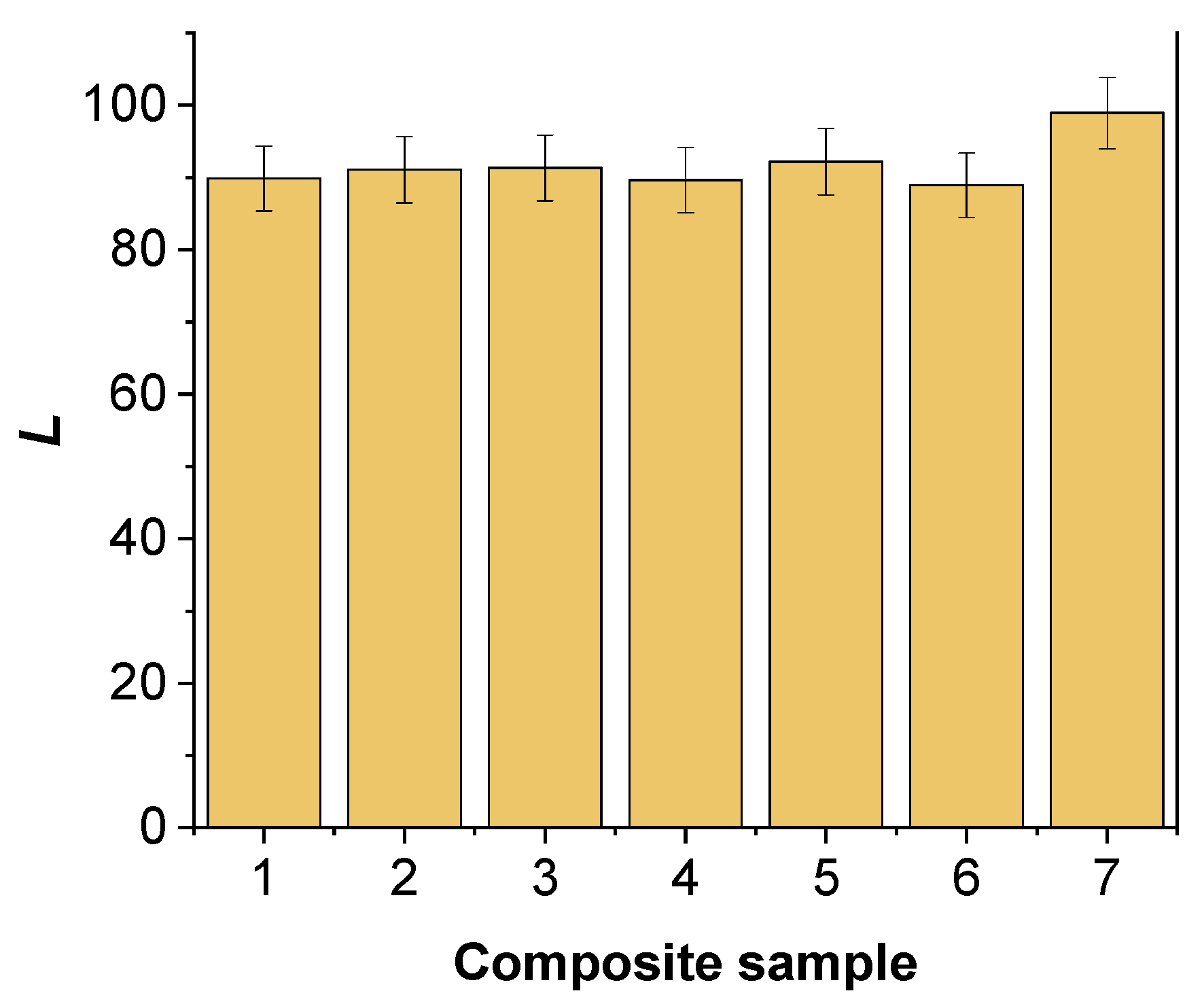
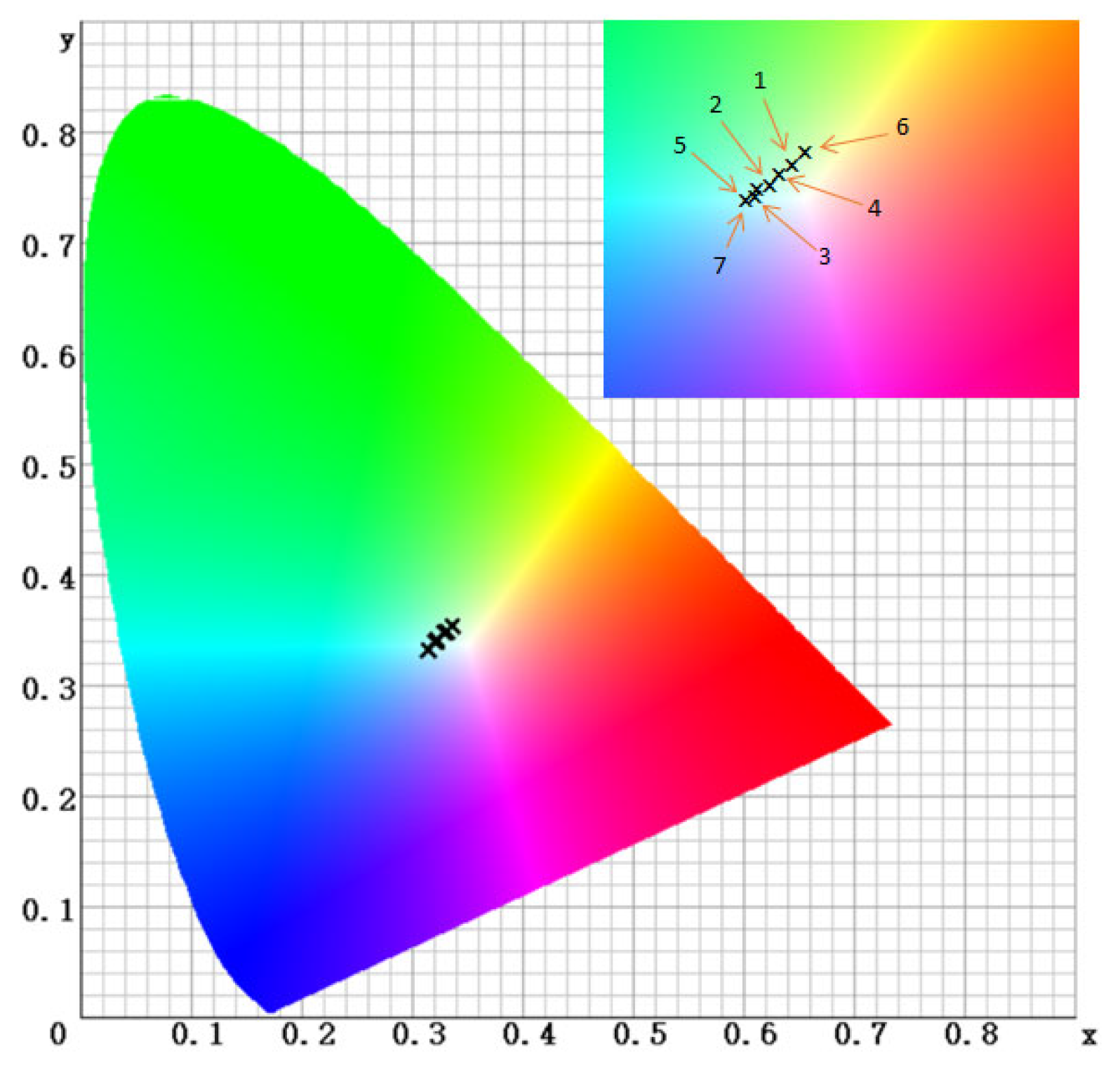
2.1. Spectrophotometric Color Analysis
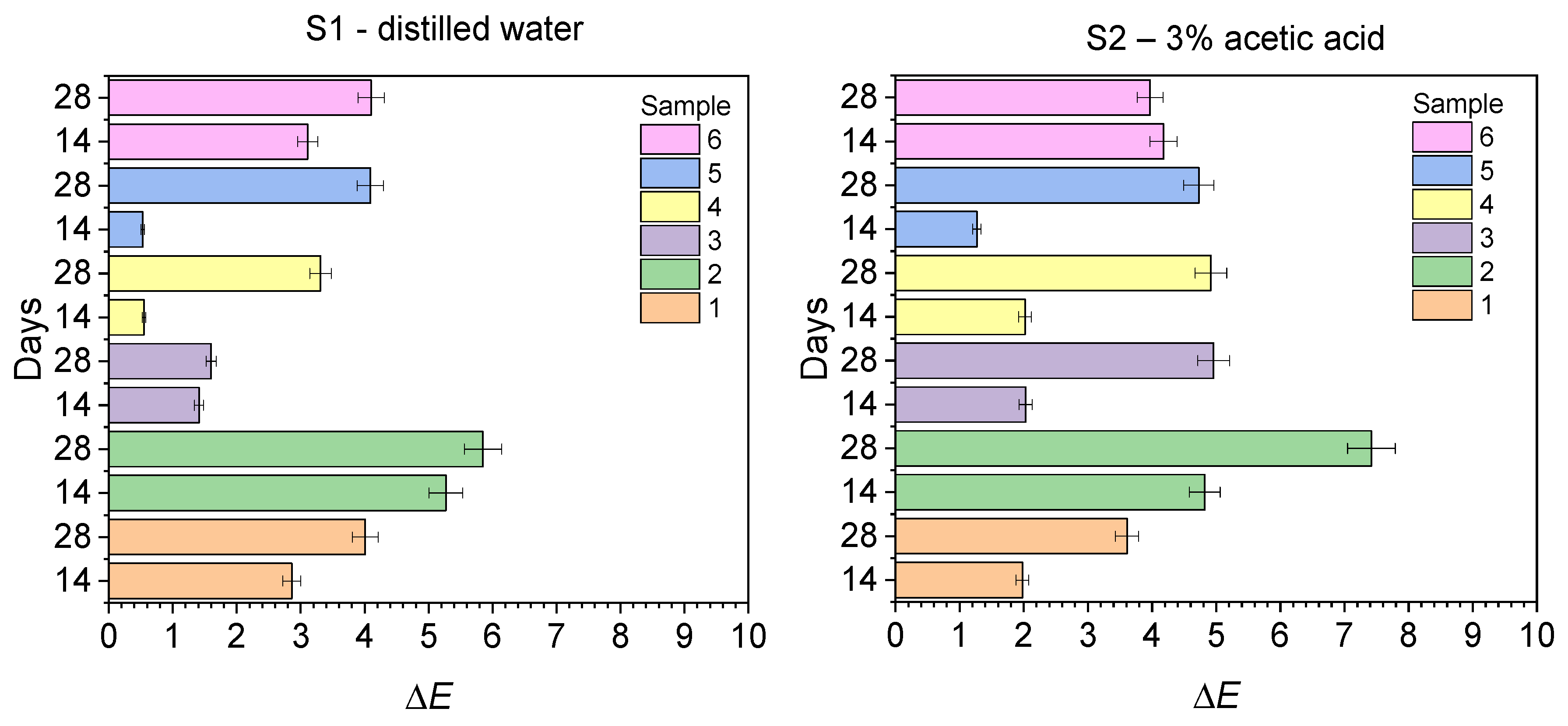
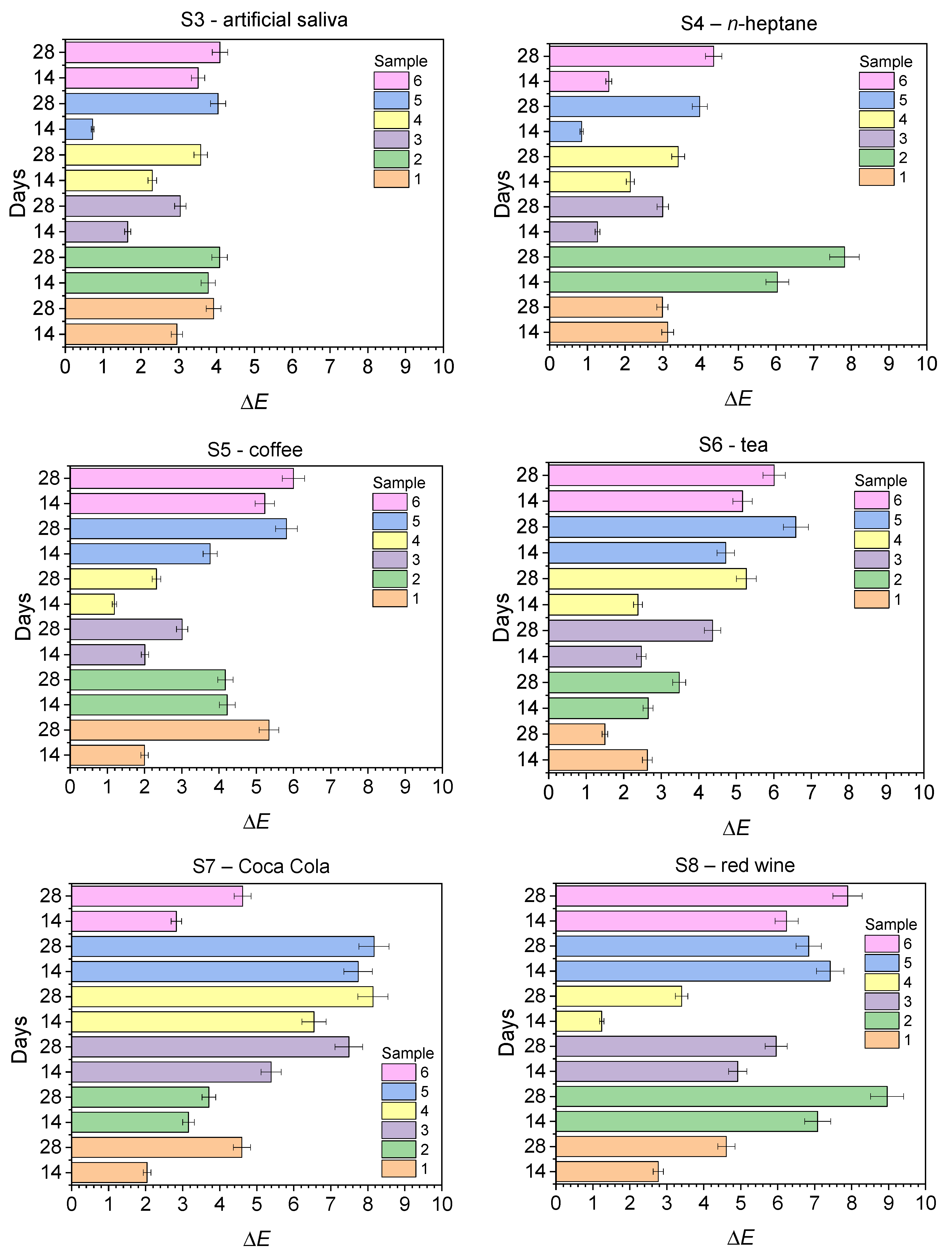
2.2. Discoloration Resistance Tests
2.3. Sorption and Solubility Studies
3. Materials and Methods
3.1. Reagents
3.2. Methods
3.2.1. Sample Preparation
3.2.2. Color Stability Testing Methodology
3.2.3. Sorption and Solubility Test Methodology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferracane, J.L. Current Trends in Dental Composites. Crit. Rev. Oral Biol. Med. 1995, 6, 302–318. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Investigations on a methacrylate-based flowable composite based on the SDR™ technology. Dent. Mater. 2011, 27, 348–355. [Google Scholar] [CrossRef]
- Albuquerque, P.P.A.C.; Moreira, A.D.L.; Moraes, R.R.; Cavalcante, L.M.; Schneider, L.F.J. Color stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J. Dent. 2013, 41, e67–e72. [Google Scholar] [CrossRef]
- Silami, F.D.J.; Mundim, F.M.; Garcia, L.d.F.R.; Sinhoreti, M.A.C.; Pires-de-Souza, F.d.C.P. Color stability of experimental composites containing different photoinitiators. J. Dent. 2013, 41, e62–e66. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.P.A.C.; Bertolo, M.L.; Cavalcante, L.M.A.; Pfeifer, C.; Schneider, L.F.S. Degree of Conversion, Depth of Cure, and Color Stability of Experimental Dental Composite Formulated with Camphorquinone and Phenanthrenequinone Photoinitiators. J. Esthet. Restorat. Dent. 2015, 27, S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Temel, G.; Enginol, B.; Aydin, M.; Balta, D.K.; Arsu, N. Photopolymerization and photophysical properties of amine linked benzophenone photoinitiator for free radical polymerization. J. Photochem. Photobiol. A Chem. 2011, 219, 26–31. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Santini, A.; Miletic, V.; Swift, M.D.; Bradley, M. Degree of conversion and microhardness of TPO-containing resin-based composites cured by polywave and monowave LED units. J. Dent. 2012, 40, 577–584. [Google Scholar] [CrossRef]
- Schroeder, W.F.; Vallo, C.I. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dent. Mater. 2007, 23, 1313–1321. [Google Scholar] [CrossRef]
- Hervás-García, A.; Martínez-Lozano, M.A.; Cabanes-Vila, J.; Barjau-Escribano, A.; Fos-Galve, P. Composite resins: A review of the materials and clinical indications. Med. Oral Patol. Oral Cir. Bucal 2006, 11, E215–E220. [Google Scholar] [PubMed]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The Photoinitiators Used in Resin Based Dental Composite—A Review and Future Perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Brandt, W.C.; Silva, C.G.; Frollini, E.; Souza-Junior, E.J.C.; Sinhoreti, M.A.C. Dynamic mechanical thermal analysis of composite resins with CQ and PPD as photo-initiators photoactivated by QTH and LED units. J. Mech. Behav. Biomed. Mater. 2013, 24, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Hannig, C.; Wiegand, A.; Attin, R. Effect of bleaching on restorative materials and restorations—A systematic review. Dent. Mater. 2004, 20, 852–861. [Google Scholar] [CrossRef]
- Durner, J.; Obermaier, J.; Draenert, M.; Ilie, N. Correlation of the degree of conversion with the amount of elutable substances in nano-hybrid dental composites. Dent. Mater. 2012, 28, 1146–1153. [Google Scholar] [CrossRef]
- Erturk-Avunduk, A.T.; Cengiz-Yanardag, E.; Karakaya, I. The effect of bleaching applications on stained bulk-fill resin composites. BMC Oral Health 2022, 22, 392. [Google Scholar] [CrossRef]
- Pecho, O.E.; Martos, J.; Pinto, K.V.A.; Pinto, K.V.A.; Baldissera, R.A. Effect of hydrogen peroxide on color and whiteness of resin-based composites. J. Esthet. Restor. Dent. 2019, 31, 132–139. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M. Sorption of water, ethanol or ethanol/water solutions by light-cured dental dimethacrylate resins. Dent. Mater. 2011, 27, 1003–1010. [Google Scholar] [CrossRef]
- Rahim, T.N.A.T.; Mohamad, D.; Md Akil, H.; Ab Rahman, I. Water sorption characteristics of restorative dental composites immersed in acidic drinks. Dent. Mater. 2012, 28, e63–e70. [Google Scholar] [CrossRef]
- Örtengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I.E. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J. Oral Rehab. 2001, 28, 1106–1115. [Google Scholar] [CrossRef]
- Miettinen, V.M.; Narva, K.K.; Vallittu, P.K. Water sorption, solubility and effect of post-curing of glass fibre reinforced polymers. Biomaterials 1999, 20, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Yiu, C.K.Y.; King, N.M.; Carrilho, M.R.O.; Sauro, S.; Rueggeberg, F.A.; Prati, C.; Carvalho, R.M.; Pashley, D.H.; Tay, F.R. Effect of resin hydrophilicity and temperature on water sorption of dental adhesive resins. Biomaterials 2006, 27, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Silikas, N.; Satterthwaite, J.D. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent. Mater. 2013, 29, e213–e217. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Salim, N.A.; Satterthwaite, J.D.; Silikas, N. Long-term sorption and solubility of bulk-fill and conventional resin-composites in water and artificial saliva. J. Dent. 2015, 43, 1511–1518. [Google Scholar] [CrossRef]
- Ardu, S.; Duc, O.; Di Bella, E.; Krejci, I. Color stability of recent composite resins. Odontology 2017, 105, 29–35. [Google Scholar] [CrossRef]
- Anfe, T.E.; Agra, C.M.; Vieira, G.F. Evaluation of sorption, solubility and staining of universal and silorane resin-based composites. Eur. J. Prosthodont. Restor. Dent. 2011, 19, 151–154. [Google Scholar]
- Gonulol, N.; Ozer, S.; Sen Tunc, E. Water Sorption, Solubility, and Color Stability of Giomer Restoratives. J. Esthet. Restorat. Dent. 2015, 27, 300–306. [Google Scholar] [CrossRef]
- Powers, J.M.; Dennison, J.B.; Koran, A. Color Stability of Restorative Resins Under Accelerated Aging. J. Dent. Res. 1978, 57, 964–970. [Google Scholar] [CrossRef]
- Mansouri, S.A.; Zidan, A.Z. Effect of Water Sorption and Solubility on Color Stability of Bulk-Fill Resin Composite. J. Contemp. Dent. Pract. 2018, 19, 1129–1134. [Google Scholar] [CrossRef]
- Uchimura, J.Y.T.; Sato, F.; Bianchi, G.; Baesso, M.L.; Santana, R.G.; Pascotto, R.C. Color Stability Over Time of Three Resin-Based Restorative Materials Stored Dry and in Artificial Saliva. J. Esthet. Restorat. Dent. 2014, 26, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Koksal, T.; Dikbas, I. Color Stability of Different Denture Teeth Materials against Various Staining Agents. Dent. Mater. J. 2008, 27, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Dhumwad, S.D. Synthesis, Characterization and Biological Studies of Co(II), Ni(II), Cu(II) and Zn(II) Complexes of Schiff Bases Derived from 3-Formyl-2-Mercaptoquinolines. J. Chem. Pharm. Res. 2011, 3, 504–517. [Google Scholar]
- Jaman, Z.; Karim, M.R.; Siddiquee, T.A.; Mirza, A.H.; Ali, M.A. Synthesis of 5-Substituted 2, 9-Dimethyl-1,10-Phenanthroline Dialdehydes and Their Schiff Bases with Sulfur-Containing Amines. Int. J. Org. Chem. 2013, 3, 214–219. [Google Scholar] [CrossRef]
- Abu Ali, H.; Kamel, S.; Abu Shamma, A. Novel structures of Zn(II) biometal cation with the biologically active substituted acetic acid and nitrogen donor ligands: Synthesis, spectral, phosphate diester catalytic hydrolysis and anti-microbial studies. Appl. Organomet. Chem. 2017, 31, e3829. [Google Scholar] [CrossRef]
- Jabali, B.; Abu Ali, H. New zinc(II) complexes of the Non-steroidal Anti-Inflammatory Drug (indomethacin) and various nitrogen donor ligands. Synthesis, characterization and biological activity. Polyhedron 2016, 117, 249–258. [Google Scholar] [CrossRef]
- Pereira, J.A.; Pessoa, A.M.; Cordeiro, M.N.D.S.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, its derivatives and applications: A State of the Art review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef]
- Pyszka, I.; Jędrzejewska, B. Design of Dyes Based on the Quinoline or Quinoxaline Skeleton towards Visible Light Photoinitiators. Int. J. Mol. Sci. 2024, 25, 4289. [Google Scholar] [CrossRef]
- Pyszka, I.; Jędrzejewska, B. Modification of Light-Cured Composition for Permanent Dental Fillings; Mass Stability of New Composites Containing Quinoline and Quinoxaline Derivatives in Solutions Simulating the Oral Cavity Environment. Materials 2024, 17, 6003. [Google Scholar] [CrossRef]
- Huang, W.; Ren, L.; Cheng, Y.; Xu, M.; Luo, W.; Zhan, D.; Sano, H.; Fu, J. Evaluation of the Color Stability, Water Sorption, and Solubility of Current Resin Composites. Materials 2022, 15, 6710. [Google Scholar] [CrossRef]
- Arregui, M.; Giner, L.; Ferrari, M.; Vallés, M.; Mercadé, M. Six-month color change and water sorption of 9 new-generation flowable composites in 6 staining solutions. Braz. Oral Res. 2016, 30, e123. [Google Scholar] [CrossRef]
- Gawriołek, M.; Sikorska, E.; Ferreira, L.F.V.; Costa, A.I.; Khmelinskii, I.; Krawczyk, A.; Sikorski, M.; Koczorowski, R. Color and Luminescence Stability of Selected Dental Materials In Vitro. J. Prosthodont. 2012, 21, 112–122. [Google Scholar] [CrossRef] [PubMed]
- He, W.-H.; Park, C.J.; Byun, S.; Tan, D.; Lin, C.Y.; Chee, W. Evaluating the relationship between tooth color and enamel thickness, using twin flash photography, cross-polarization photography, and spectrophotometer. J. Esthet. Restor. Dent. 2020, 32, 91–101. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.C.R.S.; Rocha, M.G.; Gatti, A.; Correr, A.B.; Ferracane, J.L.; Sinhoret, M.A.C. Effect of different photoinitiators and reducing agents on cure efficiency and color stability of resin-based composites using different LED wavelengths. J. Dent. 2015, 43, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.J.; Longman, C.M. Water sorption and solubility of resin-based materials following inadequate polymerization by a visible-light curing system. J. Oral Rehab. 1989, 16, 57–61. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J. Mater. Sci. Mater. Med. 2007, 18, 133–137. [Google Scholar] [CrossRef]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R.d.O. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Osorio, E.; Fuentes, V.; Prati, C.; García-Godoy, F. Sorption and solubility of resin-based restorative dental materials. J. Dent. 2003, 31, 43–50. [Google Scholar] [CrossRef]
- ISO 4049; Dentistry—Polymer-Based Restorative Materials, 5th ed. ISO: Geneva, Switzerland, 2019.
- Bagheri, R.; Burrow, M.F.; Tyas, M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J. Dent. 2005, 33, 389–398. [Google Scholar] [CrossRef]
- Johnston, W.M. Color measurement in dentistry. J. Dent. 2009, 37, e2–e6. [Google Scholar] [CrossRef]
- Haselton, D.R.; Diaz-Arnold, A.M.; Dawson, D.V. Color stability of provisional crown and fixed partial denture resins. J. Prosthet. Dent. 2005, 93, 70–75. [Google Scholar] [CrossRef]
- Vichi, A.; Ferrari, M.; Davidson, C.L. Color and opacity variations in three different resin-based composite products after water aging. Dent. Mater. 2004, 20, 530–534. [Google Scholar] [CrossRef]
- Ertas, E.; Gűler, A.U.; Yűcel, A.C.; Köprűlű, H.; Gűler, E. Color Stability of Resin Composites after Immersion in Different Drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef]



| Organic Phase | |
|---|---|
| Photoinitiators: | 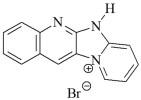 DQ1: quinoline [2,3-b]-1H-imidazo [1,2-a]pyridinium bromide DQ1: quinoline [2,3-b]-1H-imidazo [1,2-a]pyridinium bromide |
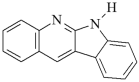 DQ2: 6H-indolo [2,3-b]quinoline DQ2: 6H-indolo [2,3-b]quinoline | |
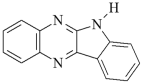 DQ3: 6H-indolo [2,3-b]quinoxaline DQ3: 6H-indolo [2,3-b]quinoxaline | |
 DQ4: 6-methyl-6H-indolo [2,3-b]quinoxaline DQ4: 6-methyl-6H-indolo [2,3-b]quinoxaline | |
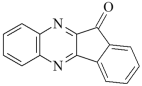 DQ5: 11H-indeno [1,2-b]qunioxalin-11-on DQ5: 11H-indeno [1,2-b]qunioxalin-11-on | |
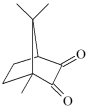 CQ: camphorquinone CQ: camphorquinone | |
| Co-initiators: |  PhTAA: (phenylthio)acetic acid PhTAA: (phenylthio)acetic acid |
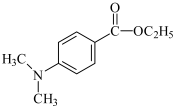 EDMAB: ethyl 4-dimethylaminobenzoate EDMAB: ethyl 4-dimethylaminobenzoate | |
| Monomers: |  TMPTA: trimethylolpropane triacrylate TMPTA: trimethylolpropane triacrylate |
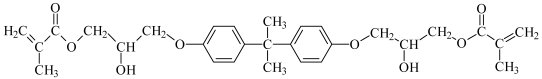 Bis-GMA: Bisphenol A glycerolate dimethacrylate | |
| Solvent: | 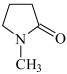 MP: 1-methyl-2-pyrolidinone MP: 1-methyl-2-pyrolidinone |
| Inorganic phase | |
| Dental filler: | SiO2—30, CaO—10, Al2O3—30, F—15, P2O5 < 10, Na2O < 10% mass; ING: Inter Dental Glass |
| Sample | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| ΔE | 8.86 ± 0.56 | 11.72 ± 0.78 | 7.70 ± 0.68 | 12.37 ± 1.05 | 7.02 ± 0.34 | 14.57 ± 1.32 |
| Composite Material, ΔE | ||||||
|---|---|---|---|---|---|---|
| Storage Time, Days | 1 DQ1-PhTAA | 2 DQ2-PhTAA | 3 DQ3-PhTAA | 4 DQ4-PhTAA | 5 DQ5-PhTAA | 6 CQ-EDMAB |
| 0 | ||||||
| S1—distilled water | ||||||
| 14 | 2.86 ± 0.15 | 5.27 ± 0.25 | 1.41 ± 0.37 | 0.55 ± 0.21 | 0.53 ± 0.13 | 3.11 ± 0.24 |
| 28 | 4.01 ± 0.23 | 5.85 ± 0.59 | 1.60 ± 0.09 | 3.31 ± 0.23 | 4.09 ± 0.67 | 4.10 ± 0.71 |
| S2—3% acetic acid | ||||||
| 14 | 1.98 ± 0.09 | 4.82 ± 0.45 | 2.03 ± 0.31 | 2.02 ± 0.32 | 1.27 ± 0.20 | 4.18 ± 0.15 |
| 28 | 3.61 ± 0.35 | 7.42 ± 0.26 | 4.96 ± 0.67 | 4.92 ± 0.45 | 4.73 ± 0.37 | 3.97 ± 0.67 |
| S3—artificial saliva | ||||||
| 14 | 2.95 ± 0.34 | 3.78 ± 0.45 | 1.65 ± 0.08 | 2.30 ± 0.13 | 0.72 ± 0.17 | 3.51 ± 0.34 |
| 28 | 3.92 ± 0.25 | 4.08 ± 0.93 | 3.04 ± 0.87 | 3.58 ± 0.45 | 4.04 ± 0.43 | 4.09 ± 0.32 |
| S4—n-heptane | ||||||
| 14 | 3.13 ± 0.47 | 6.04 ± 0.89 | 1.27 ± 0.05 | 2.14 ± 0.87 | 0.85 ± 0.03 | 1.57 ± 0.06 |
| 28 | 2.99 ± 0.02 | 7.82 ± 0.78 | 3.00 ± 0.76 | 3.41 ± 0.34 | 3.98 ± 0.36 | 4.35 ± 0.25 |
| S5—coffee | ||||||
| 14 | 2.00 ± 0.36 | 4.22 ± 0.41 | 2.01 ± 0.44 | 1.19 ± 0.56 | 3.76 ± 0.55 | 5.23 ± 0.78 |
| 28 | 5.34 ± 0.22 | 4.17 ± 0.34 | 3.01 ± 0.67 | 2.32 ± 0.33 | 5.81 ± 0.26 | 6.00 ± 0.99 |
| S6—tea | ||||||
| 14 | 2.63 ± 0.47 | 2.65 ± 0.89 | 2.47 ± 0.21 | 2.38 ± 0.35 | 4.72 ± 0.22 | 5.17 ± 0.34 |
| 28 | 1.50 ± 0.09 | 3.48 ± 0.45 | 4.37 ± 0.56 | 5.27 ± 0.78 | 6.59 ± 0.66 | 6.01 ± 0.98 |
| S7—Coca Cola | ||||||
| 14 | 2.04 ± 0.54 | 3.16 ± 0.78 | 5.39 ± 0.89 | 6.55 ± 0.45 | 7.74 ± 0.72 | 2.83 ± 0.09 |
| 28 | 4.60 ± 0.66 | 3.71 ± 0.55 | 7.49 ± 0.67 | 8.14 ± 0.89 | 8.17 ± 1.02 | 4.62 ± 0.30 |
| S8—red wine | ||||||
| 14 | 2.77 ± 0.67 | 7.08 ± 0.77 | 4.92 ± 1.01 | 1.24 ± 0.22 | 7.42 ± 0.57 | 6.21 ± 0.98 |
| 28 | 4.61 ± 0.22 | 8.96 ± 0.54 | 5.96 ± 0.32 | 3.40 ± 0.48 | 6.84 ± 0.67 | 7.89 ± 0.84 |
| Sample | Storage Time (Days) | NBS Unit | Color Change | NBS Unit | Color Change |
|---|---|---|---|---|---|
| S1—distilled water | S2—3% acetic acid | ||||
| 1 | 14 | 2.63 | noticeable | 1.82 | noticeable |
| 28 | 3.68 | significant | 3.32 | significant | |
| 2 | 14 | 4.84 | significant | 4.43 | significant |
| 28 | 5.38 | significant | 6.82 | large | |
| 3 | 14 | 1.29 | small | 1.86 | noticeable |
| 28 | 1.47 | small | 4.56 | significant | |
| 4 | 14 | 0.50 | slight | 1.85 | noticeable |
| 28 | 4.51 | significant | 4.52 | significant | |
| 5 | 14 | 0.48 | slight | 1.16 | small |
| 28 | 3.76 | significant | 4.35 | significant | |
| 6 | 14 | 2.86 | noticeable | 3.84 | significant |
| 28 | 3.77 | significant | 3.59 | significant | |
| S3—artificial saliva | S4—n-heptane | ||||
| 1 | 14 | 2.71 | noticeable | 2.87 | noticeable |
| 28 | 3.60 | significant | 2.75 | noticeable | |
| 2 | 14 | 3.47 | significant | 5.55 | significant |
| 28 | 3.75 | significant | 7.19 | large | |
| 3 | 14 | 1.51 | noticeable | 1.16 | small |
| 28 | 2.79 | noticeable | 2.76 | noticeable | |
| 4 | 14 | 2.11 | noticeable | 1.96 | noticeable |
| 28 | 3.29 | significant | 3.13 | significant | |
| 5 | 14 | 0.66 | small | 0.78 | small |
| 28 | 3.71 | significant | 3.66 | significant | |
| 6 | 14 | 3.22 | significant | 1.44 | small |
| 28 | 3.76 | significant | 4.00 | noticeable | |
| S5—coffee | S6—tea | ||||
| 1 | 14 | 1.84 | noticeable | 2.41 | noticeable |
| 28 | 4.91 | significant | 1.38 | small | |
| 2 | 14 | 3.88 | significant | 2.41 | noticeable |
| 28 | 3.83 | significant | 3.20 | significant | |
| 3 | 14 | 1.84 | noticeable | 2.27 | noticeable |
| 28 | 2.76 | noticeable | 4.02 | significant | |
| 4 | 14 | 1.09 | small | 2.18 | noticeable |
| 28 | 2.13 | noticeable | 4.84 | significant | |
| 5 | 14 | 3.45 | significant | 4.34 | significant |
| 28 | 5.34 | significant | 6.06 | large | |
| 6 | 14 | 4.81 | significant | 4.75 | significant |
| 28 | 5.52 | significant | 5.52 | significant | |
| S7—Coca Cola | S8—red wine | ||||
| 1 | 14 | 1.87 | noticeable | 2.54 | noticeable |
| 28 | 4.23 | significant | 4.24 | significant | |
| 2 | 14 | 2.90 | noticeable | 6.51 | large |
| 28 | 3.41 | significant | 8.24 | large | |
| 3 | 14 | 4.86 | significant | 4.52 | significant |
| 28 | 6.89 | large | 5.48 | significant | |
| 4 | 14 | 6.02 | large | 1.14 | small |
| 28 | 7.48 | large | 3.12 | significant | |
| 5 | 14 | 7.12 | large | 6.66 | large |
| 28 | 7.51 | large | 6.29 | large | |
| 6 | 14 | 2.60 | noticeable | 5.71 | significant |
| 28 | 4.25 | significant | 7.25 | large | |
| Sample | Sp (mg/mm3) Mean ± SD | Sl (mg/mm3) Mean ± SD | Sp (mg/mm3) Mean ± SD | Sl (mg/mm3) Mean ± SD |
|---|---|---|---|---|
| Day 14 | Day 28 | |||
| 1 | 11.46 ± 2.24 | 7.22 ± 0.20 | 14.01 ± 1.64 | 14.01 ± 0.74 |
| 2 | 22.92 ± 1.30 | 8.49 ± 0.31 | 25.47 ± 1.03 | 18.68 ± 0.93 |
| 3 | 4.24 ± 1.08 | 2.12 ± 0.08 | 5.52 ± 0.93 | 4.25 ± 0.23 |
| 4 | 16.13 ± 0.92 | 8.91 ± 0.32 | 19.53 ± 0.99 | 24.63 ± 0.95 |
| 5 | 1.27 ± 1.19 | 3.39 ± 0.21 | 3.39 ± 2.23 | 8.06 ± 0.26 |
| 6 | 18.68 ± 1.05 | 4.24 ± 0.35 | 22.92 ± 0.98 | 11.88 ± 0.48 |
| No. | c, M | Organic Phase | Inorganic Phase | |||||
|---|---|---|---|---|---|---|---|---|
| Photoinitiator | Co-Initiator | Monomer | Solvent | |||||
| 1 |  |  | 1.80 × 10−3 | DQ1 | PhTAA | TMPTA | MP | IDG |
| 2 |  |  | 1.35 × 10−3 | DQ2 | PhTAA | TMPTA | MP | IDG |
| 3 |  |  | 1.38 × 10−3 | DQ3 | PhTAA | TMPTA | MP | IDG |
| 4 |  |  | 1.58 × 10−3 | DQ4 | PhTAA | TMPTA | MP | IDG |
| 5 |  |  | 1.54 × 10−3 | DQ5 | PhTAA | TMPTA | MP | IDG |
| 6 |  |  | 0.675 | CQ | EDMAB | Bis-GMA | MP | IDG |
| NBS Units | Color Change |
|---|---|
| 0.0–0.5 | slight |
| 0.5–1.5 | small |
| 1.5–3.0 | noticeable |
| 3.0–6.0 | significant |
| 6.0–12.0 | large |
| ≥12 | very large |
| No | Solution | Simulated Environment | |
|---|---|---|---|
| S1 | distilled water | hydrated foods | |
| S2 | 3% acetic acid | hydrated foods with pH < 4.5 | |
| S3 | artificial saliva | saliva | |
| S4 | n-heptane | fatty foods | |
| S5 | coffee |  | foods containing pigments |
| S6 | tea | ||
| S7 | Coca-Cola | ||
| S8 | red wine | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyszka, I.; Bereźnicki, D.; Jędrzejewska, B. Light-Cured Dental Fillings Containing Quinoline and Quinoxaline Derivatives: The Influence of Sorption and Solubility on Color Change—Part III. Int. J. Mol. Sci. 2025, 26, 9537. https://doi.org/10.3390/ijms26199537
Pyszka I, Bereźnicki D, Jędrzejewska B. Light-Cured Dental Fillings Containing Quinoline and Quinoxaline Derivatives: The Influence of Sorption and Solubility on Color Change—Part III. International Journal of Molecular Sciences. 2025; 26(19):9537. https://doi.org/10.3390/ijms26199537
Chicago/Turabian StylePyszka, Ilona, Dawid Bereźnicki, and Beata Jędrzejewska. 2025. "Light-Cured Dental Fillings Containing Quinoline and Quinoxaline Derivatives: The Influence of Sorption and Solubility on Color Change—Part III" International Journal of Molecular Sciences 26, no. 19: 9537. https://doi.org/10.3390/ijms26199537
APA StylePyszka, I., Bereźnicki, D., & Jędrzejewska, B. (2025). Light-Cured Dental Fillings Containing Quinoline and Quinoxaline Derivatives: The Influence of Sorption and Solubility on Color Change—Part III. International Journal of Molecular Sciences, 26(19), 9537. https://doi.org/10.3390/ijms26199537





