Cytokine Networks and Heart Failure Outcomes: CA125 as a Bridge Between Congestion and Inflammation
Abstract
1. Introduction
2. Results
2.1. Study Outcomes
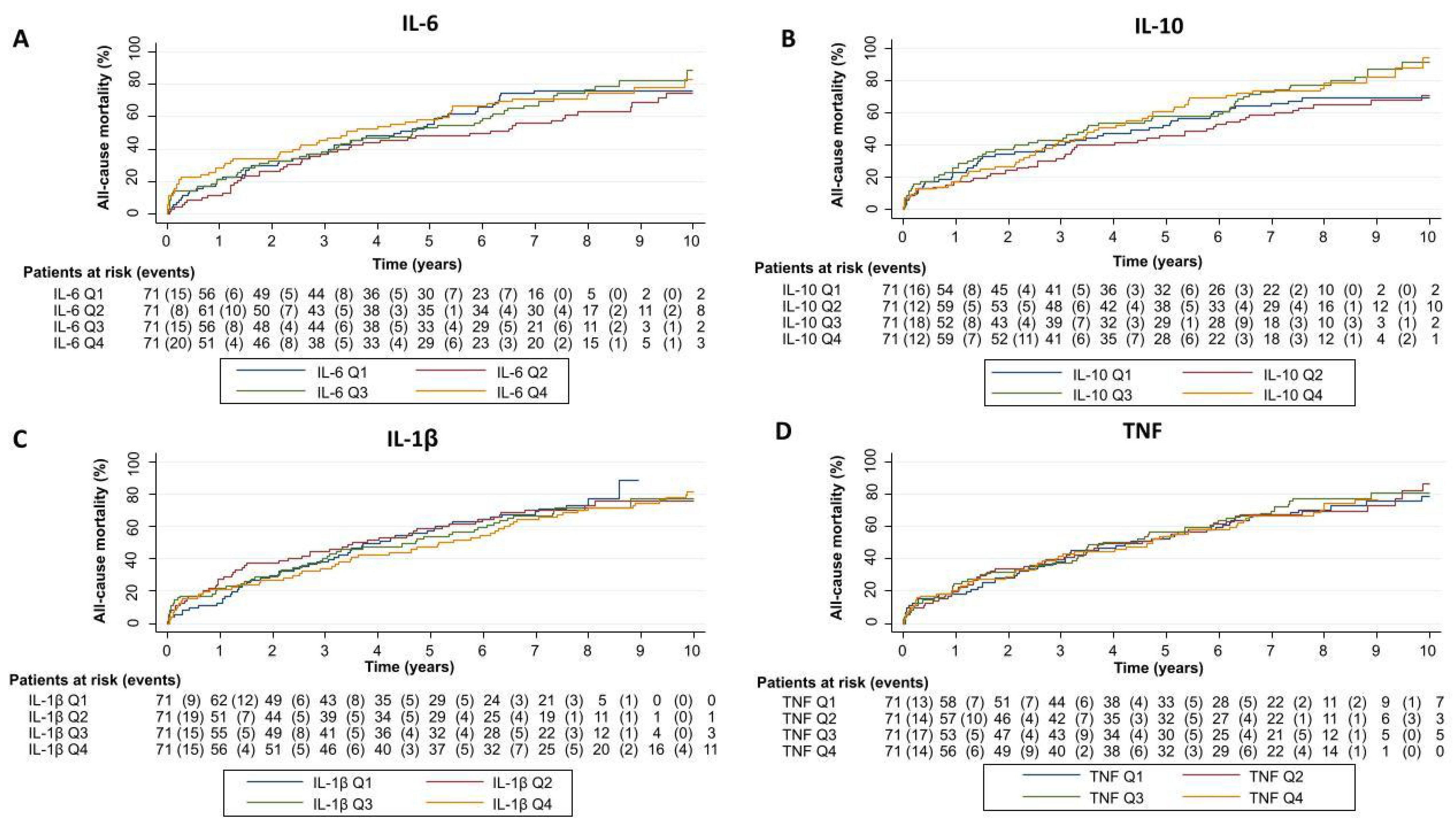
2.2. Cytokine Network and Risk of Adverse Clinical Events in the Whole Sample
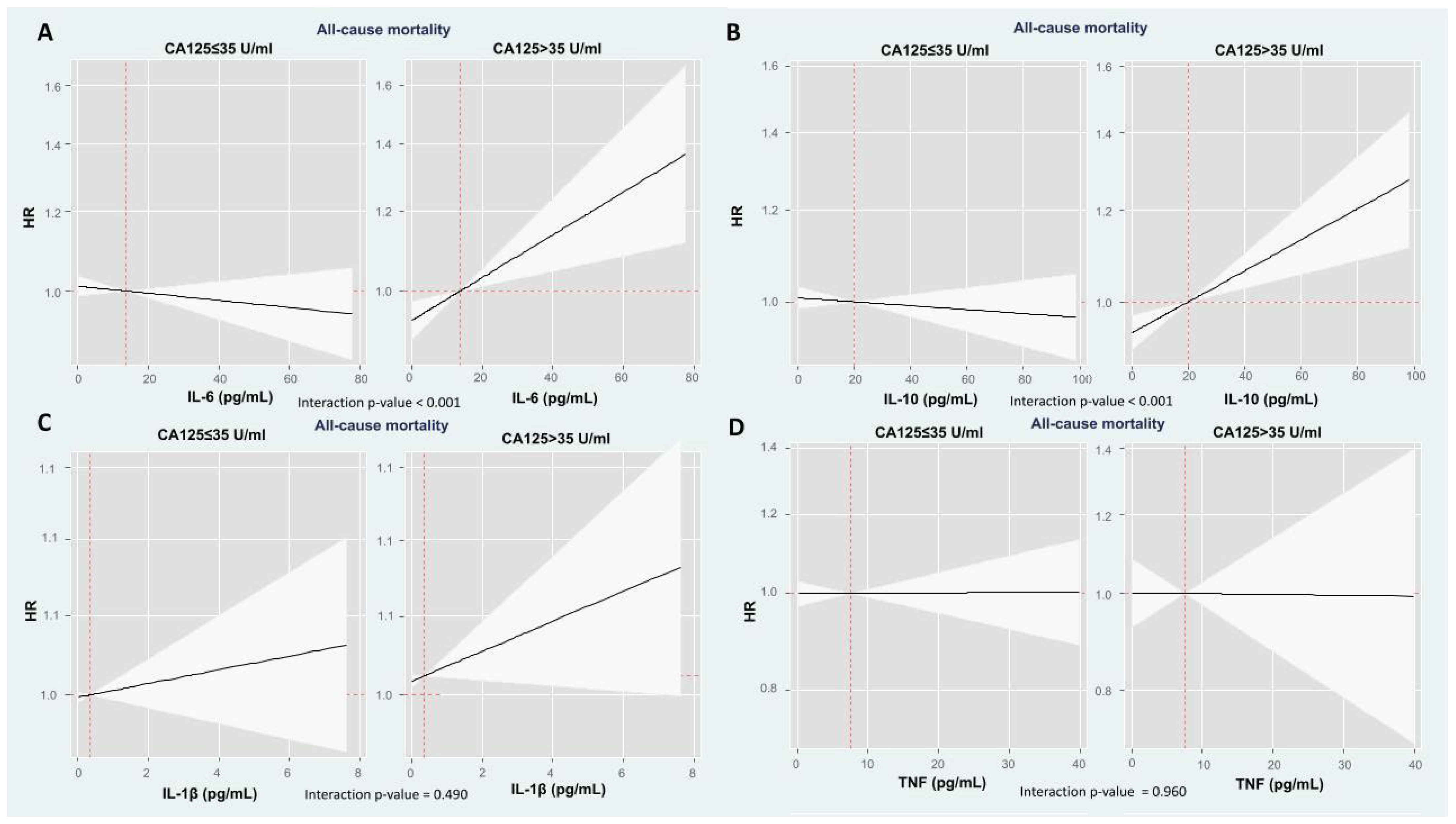
2.3. The Modifying Prognostic Role of Cytokine Network Across CA125: Long-Term Mortality
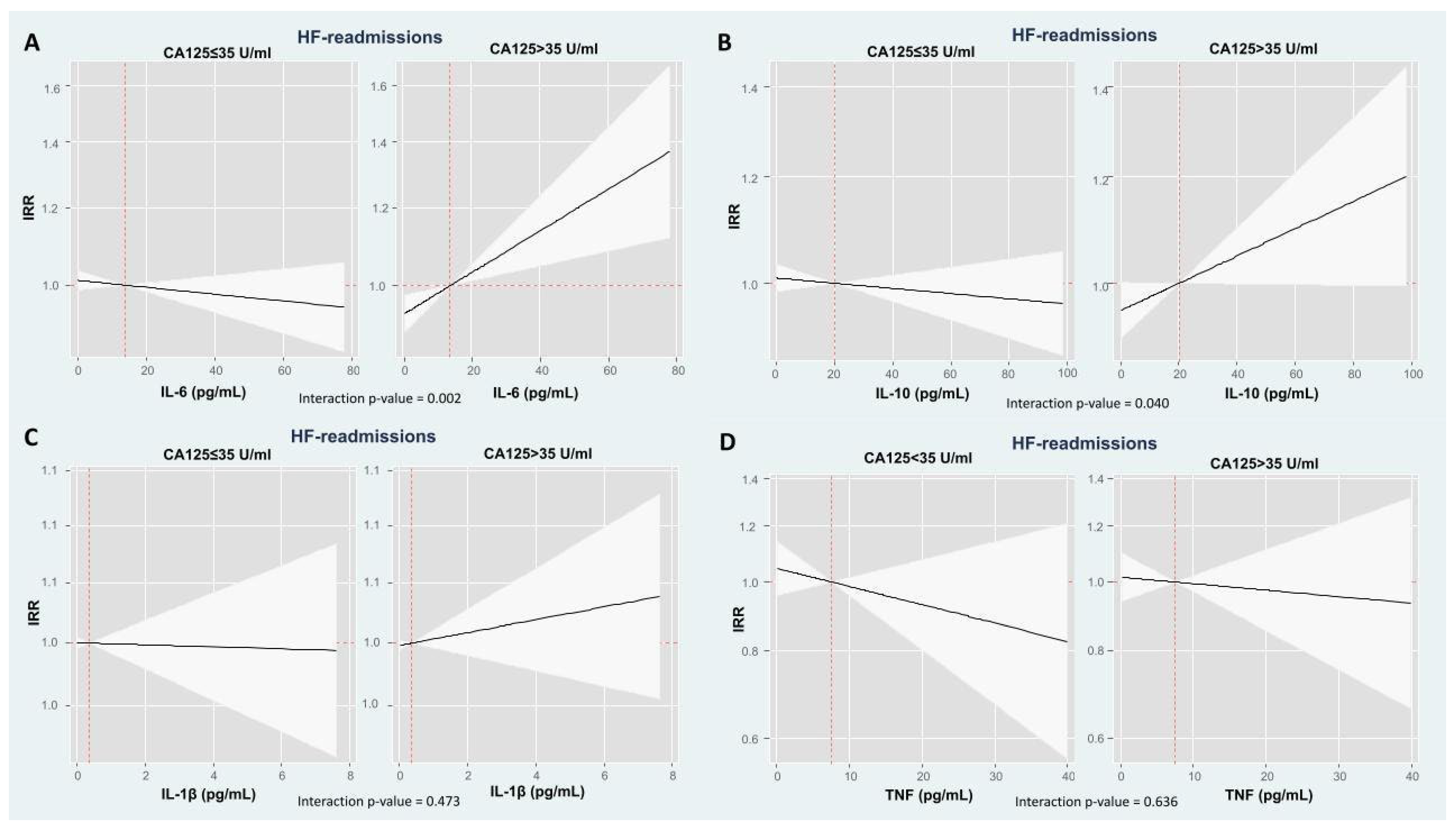
2.4. The Modifying Prognostic Role of Cytokine Network Across CA125: HF Readmissions
3. Discussion
3.1. Prognostic Role of Inflammation in HF
3.2. CA125 as a Potential Inflammatory Modulator in HF
3.3. Clinical Implications
3.4. Limitations
4. Materials and Methods
4.1. Study Group and Protocol
4.2. Biomarker Measurements
4.3. Follow-Up and Endpoints
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HF | Heart failure |
| CA125 | Carbohydrate Antigen 125 |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin 1β |
| TNF | Tumor necrosis factor |
| MUC16 | Mucin 16 |
| Gal-3 | Galectin-3 |
| sST2 | Soluble ST2 |
| SD | Standard deviation |
| HR | Hazard ratio |
| CI | Confidence interval |
| IRR | Incidence rate ratios |
| LVEF | Left ventricular ejection fraction |
| CTD | C-terminal domain |
| EMT | Epithelial-to-mesenchymal transition |
Appendix A
| Total Population (n = 284) | CA125 ≤ 35 U/mL (n = 90) | CA125 > 35 U/mL (n = 194) | p-Value | |
|---|---|---|---|---|
| Epidemiology and clinical findings | ||||
| Current smoker, n (%) | 24 (8.5) | 6 (6.7) | 18 (9.3) | 0.462 |
| PAD, n (%) | 19 (6.7) | 6 (6.7) | 13 (6.7) | 0.991 |
| Charlson index | 2.2 ± 1.9 | 2.0 ± 1.7 | 2.3 ± 1.9 | 0.267 |
| Circulating Biomarkers | ||||
| Hematocrit (%) | 37.5 ± 5.4 | 38.1 ± 5.5 | 37.2 ± 5.4 | 0.205 |
| WBC count, 109/L | 9.4 ± 3.7 | 10.6 ± 4.0 | 8.9 ± 3.4 | <0.001 |
| Neutrophiles, 109/L | 6.9 ± 2.9 | 7.7 ± 3.1 | 6.5 ± 2.7 | 0.002 |
| Iron, µg/dL | 49.6 ± 15.9 | 54.7 ± 14.4 | 47.3 ± 16 | <0.001 |
| Electrocardiography | ||||
| QRS > 120 ms, n (%) | 96 (33.8) | 31 (34.4) | 65 (33.5) | 0.876 |
References
- Murphy, S.P.; Kakkar, R.; McMarthy, C.P.; Januzzi, J.L. Inflammation in heart failure. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–286. [Google Scholar] [CrossRef]
- Colombo, P.C.; Castagna, F.; Onat, D.; Wong, K.Y.; Harxhi, A.; Hayashi, Y.; Friedman, R.A.; Pinsino, A.; Ladanyi, A.; Mebazaa, A.; et al. Experimentally induced peripheral venous congestion exacerbates inflammation, oxidative stress, and neurohormonal and endothelial cell activation in patients with systolic heart failure. J. Card Fail. 2023, 23, 580–591. [Google Scholar] [CrossRef]
- Kittipibul, V.; Fudim, M.; Sobotka, P.A. Congestion and inflammation in heart failure: Beyond the chicken or the egg. J. Card Fail. 2023, 30, 592–595. [Google Scholar] [CrossRef]
- Dick, S.A.; Epelman, S. Chronic heart failure and inflammation: What do we really know? Circ. Res. 2016, 119, 159–171. [Google Scholar] [CrossRef]
- Michou, E.; Wussler, D.; Belkin, M.; Simmen, C.; Strebel, I.; Nowak, A.; Kozhuharov, N.; Shrestha, S.; Lopez-Ayala, P.; Sabti, Z.; et al. Quantifying inflammation using interleukin-6 for improved phenotyping and risk stratification in acute heart failure. Eur. J. Heart Fail. 2023, 25, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; van der Harst, P.; Lang, C.C.; et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019, 21, 965–973. [Google Scholar] [CrossRef]
- Santas, E.; Villar, S.; Palau, P.; Llàcer, P.; de la Espriella, R.; Miñana, G.; Lorenzo, M.; Núñez-Marín, G.; Górriz, J.L.; Carratalá, A.; et al. High-sensitivity C-reactive protein and risk of clinical outcomes in patients with acute heart failure. Sci Rep 2024, 14, 21672. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Tang, W.H.; Hsu, A.; Felker, G.M.; Hernandez, A.F.; Troughton, R.W.; Voors, A.A.; Anker, S.D.; Metra, M.; McMurray, J.J.; et al. High-sensitivity C-reactive protein in acute heart failure: Insights from the ASCEND-HF trial. J. Cardiac Fail. 2014, 20, 319–326. [Google Scholar] [CrossRef]
- Mooney, L.; Jackson, C.E.; Adamson, C.; McConnachie, A.; Welsh, P.; Myles, R.C.; McMurray, J.J.V.; Jhund, P.S.; Petrie, M.C.; Lang, N.N. Adverse outcomes associated with interleukin-6 in patients recently hospitalized for heart failure with preserved ejection fraction. Circ. Heart Fail. 2023, 16, e010051. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.F.; McDowell, K.; Welsh, P.; Petrie, M.C.; Anand, I.; Berg, D.D.; de Boer, R.A.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; et al. Interleukin-6 in Heart Failure With Reduced Ejection Fraction and the Effect of Dapagliflozin An Exploratory Analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial. J. Am. Coll. Cardiol. Heart Fail. 2025, 13, 102393. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Bayes-Genis, A.; Asensio-Lopez, M.C.; Hernández-Vicente, A.; Garrido-Bravo, I.; Pastor-Perez, F.; Díez, J.; Ibáñez, B.; Lax, A. The Interleukin-1 Axis and Risk of Death in Patients With Acutely Decompensated Heart Failure. J. Am. Coll. Cardiol. 2019, 73, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Alogna, A.; Koepp, K.E.; Sabbah, M.; Espindola Netto, J.M.; Jensen, M.D.; Kirkland, J.L.; Lam, C.S.P.; Obokata, M.; Petrie, M.C.; Ridker, P.M.; et al. Interleukin-6 in Patients With Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. Heart Fail. 2023, 11, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; de la Espriella, R.; Rossignol, P.; Voors, A.A.; Mullens, W.; Metra, M.; Chioncel, O.; Januzzi, J.L.; Mueller, C.; Richards, A.M.; et al. Congestion in heart failure: A circulating biomarker-based perspective. A review from the Biomarkers Working Group of the Heart Failure Association, European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1751–1766, Erratum in Eur. J. Heart Fail. 2023, 25, 443; Corrigendum to Eur. J. Heart Fail. 2024, 26, 193; Corrigendum to Eur. J. Heart Fail. 2024, 26, 529.. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Miñana, G.; Santas, E.; Llácer, P.; Núñez, E.; Palau, P.; Bodí, V.; Chorro, F.J.; Sanchis, J.; et al. Antigen carbohydrate 125 as a biomarker in heart failure. A narrative review. Eur. J. Heart Fail. 2021, 23, 1445–1457. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Beard, J.B.; Underwood, L.J.; Dennis, R.A.; Santin, A.D.; York, L. The CA 125 gene: An extracellular superstructure dominated by repeat sequences. Tumour. Biol. 2001, 22, 348–366. [Google Scholar] [CrossRef]
- Zeillemaker, A.M.; Verbrugh, H.A.; Hoynck van Papendrecht, A.A.; Leguit, P. CA 125 secretion by peritoneal mesothelial cells. J. Clin. Pathol. 1994, 47, 263–265. [Google Scholar] [CrossRef]
- Giamougiannis, P.; Martin-Hirsch, P.L.; Martin, F.L. The evolving role of MUC16 (CA125) in the transformation of ovarian cells and the progression of neoplasia. Carcinogenesis 2021, 42, 327–343. [Google Scholar] [CrossRef]
- Eiras, S.; de la Espriella, R.; Fu, X.; Iglesias-Álvarez, D.; Basdas, R.; Núñez-Caamaño, J.R.; Martínez-Cereijo, J.M.; Reija, L.; Fernández, A.L.; Sánchez-López, D.; et al. Carbohydrate antigen 125 on epicardial fat and its association with local inflammation and fibrosis-related markers. J. Transl. Med. 2024, 22, 619. [Google Scholar] [CrossRef]
- Núñez, J.; Rabinovich, G.A.; Sandino, J.; Mainar, L.; Palau, P.; Santas, E.; Villanueva, M.P.; Núñez, E.; Bodí, V.; Chorro, F.J.; et al. Prognostic Value of the Interaction between Galectin-3 and Antigen Carbohydrate 125 in Acute Heart Failure. PLoS ONE 2015, 10, e0122360. [Google Scholar] [CrossRef]
- Revuelta-López, E.; de la Espriella, R.; Miñana, G.; Santas, E.; Villar, S.; Sanchis, J.; Bayés-Genís, A.; Núñez, J. The modulating effect of circulating carbohydrate antigen 125 on ST2 and long-term recurrent morbidity burden. Sci. Rep. 2025, 15, 1905. [Google Scholar] [CrossRef]
- Saggini, R.; Pellegrino, R. MAPK is implicated in sepsis, immunity, and inflammation. Int. J. Infect. 2024, 8, 100–104. [Google Scholar]
- Avivar-Valderas, A. Inhibition of PI3Kβ and mTOR influence the immune response and the defense mechanism against pathogens. Int. J. Infect. 2023, 7, 46–49. [Google Scholar]
- Sims, J.; Smith, D. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Seropian, I.M.; Toldo, S.; Mezzaroma, E.; Abbate, A. Interleukin-1β induces a reversible cardiomyopathy in the mouse. Inflamm. Res. 2013, 62, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Murray, H.M.; Ford, I.; Trompet, S.; de Craen, A.J.; Jukema, J.W.; Stott, D.J.; McInnes, I.B.; Packard, C.J.; Westendorp, R.G.; et al. Circulating Interleukin-10 and Risk of Cardiovascular Events. A Prospective Study in the Elderly at Risk. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2338–2344. [Google Scholar] [CrossRef]
- Vilcek, J.; Lee, T.H. Tumor necrosis factor: New insights into the molecular mechanisms of its multiple actions. J. Biol. Chem. 1991, 266, 7313–7316. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Weston, S.A.; Redfield, M.M.; Killian, J.M.; Roger, V.L. Tumor Necrosis Factor-α and Mortality in Heart Failure. A Community Study. Circulation 2008, 118, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; McMurray, J.J.; Packer, M.; Swedberg, K.; Borer, J.S.; Colucci, W.S.; Djian, J.; Drexler, H.; Feldman, A.; Kober, L.; et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the randomized etanercept worldwide evaluation (RENEWAL). Circulation 2024, 109, 1594–1602. [Google Scholar] [CrossRef]
- Núñez, J.; Bayés-Genís, A.; Revuelta-López, E.; Ter Maaten, J.M.; Miñana, G.; Barallat, J.; Cserkóová, A.; Bodi, V.; Fernández-Cisnal, A.; Núñez, E.; et al. Clinical role of CA125 in worsening heart failure. A BIOSTAT-CHF study subanalysis. J. Am. Coll. Cardiol. Heart Fail. 2020, 8, 386–397. [Google Scholar] [CrossRef]
- Docherty, K.F.; McDowell, K.; Welsh, P.; Osmanska, J.; Anand, I.; de Boer, R.A.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; O’Meara, E.; et al. Association of Carbohydrate Antigen 125 on the Response to Dapagliflozin in Patients With Heart Failure. J. Am. Coll. Cardiol. 2023, 82, 142–157. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Packer, M.; Sattar, N.; Butler, J.; Pocock, S.J.; Anker, S.D.; Maldonado, S.G.; Panova-Noeva, M.; Sumin, M.; Masson, S.; et al. Carbohydrate antigen 125 concentrations across the ejection fraction spectrum in chronic heart failure: The EMPEROR programme. Eur. J. Heart Fail. 2024, 26, 788–802. [Google Scholar] [CrossRef]
- Das, S.; Majhi, P.D.; Al-Mugotir, M.H.; Rachagani, S.; Sorgen, P.; Batra, S.K. Membrane proximal ectodomain cleavage of MUC16 occurs in the acidifyingGolgi/post-Golgi compartments. Sci. Rep. 2015, 5, 9759. [Google Scholar] [CrossRef]
- Gubbels, J.A.; Felder, M.; Horibata, S.; Belisle, J.A.; Kapur, A.; Holden, H.; Petrie, S.; Migneault, M.; Rancourt, C.; Connor, J.P.; et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer 2010, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Rachagani, S.; Torres-Gonzalez, M.P.; Lakshmanan, I.; Majhi, P.D.; Smith, L.M.; Wagner, K.U.; Batra, S.K. Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget 2015, 6, 5772–5787. [Google Scholar] [CrossRef] [PubMed]
- Ballester, B.; Milara, J.; Montero, P.; Cortijo, J. MUC16 Is Overexpressed in Idiopathic Pulmonary Fibrosis and Induces Fibrotic Responses Mediated by Transforming Growth Factor-β1 Canonical Pathway. Int. J. Mol. Sci. 2021, 22, 6502. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Goldman, S.A.; Requena-Ibanez, J.A.; Devesa, A.; Santos-Gallego, C.G.; Badimon, J.J.; Fuster, V. Uncovering the Role of EpicardialAdipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Adv. 2023, 2, 100657. [Google Scholar] [CrossRef]
- Ponnusamy, M.P.; Seshacharyulu, P.; Lakshmanan, I.; Vaz, A.P.; Chugh, S.; Batra, S.K. Emerging role of mucins in epithelial to mesenchymal transition. Curr. Cancer Drug Targets 2013, 9, 945–956. [Google Scholar] [CrossRef]
- Pandhi, P.; Ter Maaten, J.M.; Anker, S.D.; Ng, L.L.; Metra, M.; Samani, N.J.; Lang, C.C.; Dickstein, K.; de Boer, R.A.; van Veldhuisen, D.J.; et al. Pathophysiologic processes and novel biomarkers associated with congestion in heart failure. J. Am. Coll. Cardiol. Heart Fail. 2022, 10, 623–632. [Google Scholar] [CrossRef]
- Colombo, P.C.; Onat, D.; Harxhi, A.; Demmer, R.T.; Hayashi, Y.; Jelic, S.; LeJemtel, T.H.; Bucciarelli, L.; Kebschull, M.; Papapanou, P.; et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur. Heart J. 2014, 35, 448–454. [Google Scholar] [CrossRef] [PubMed]
- De la Espriella, R.; Cobo, M.; Santas, E.; Verbrugge, F.H.; Fudim, M.; Girerd, N.; Miñana, G.; Górriz, J.L.; Bayés-Genís, A.; Núñez, J. Assessment of filling pressures and fluid overload in heart failure: An updated perspective. Rev. Esp. Cardiol. 2023, 76, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Llàcer, P.; Romero, G.; Trullàs, J.C.; de la Espriella, R.; Cobo, M.; Quiroga, B.; Casado, J.; Slon-Roblero, M.F.; Morales-Rull, J.L.; Morgado, J.I.; et al. Consensus on the approach to hydrosaline overload in acute heart failure. SEMI/SEC/S.E.N. recommendations. Rev. Esp. Cardiol. 2024, 77, 556–565. [Google Scholar] [CrossRef] [PubMed]

| Total Population (n = 284) | CA125 ≤ 35 U/mL (n = 90) | CA125 > 35 U/mL (n = 194) | p-Value | |
|---|---|---|---|---|
| Epidemiology and clinical findings | ||||
| Age, years | 72.8 ± 11.4 | 73.8 ± 10.1 | 72.3 ± 11.9 | 0.318 |
| Women, n (%) | 140 (49.3) | 50 (55.6) | 90 (46.4) | 0.151 |
| First HF admission, n (%) | 212 (74.6) | 64 (71.1) | 148 (76.3) | 0.351 |
| Prior NYHA III/IV, n (%) | 52 (18.3) | 20 (22.2) | 32 (16.5) | 0.246 |
| Hypertension, n (%) | 221 (77.8) | 75 (83.3) | 146 (75.3) | 0.128 |
| Diabetes Mellitus, n (%) | 132 (46.5) | 34 (37.8) | 98 (50.5) | 0.045 |
| Dyslipidemia, n (%) | 143 (50.4) | 49 (54.4) | 94 (48.5) | 0.347 |
| Ischemic heart disease, n (%) | 94 (33.1) | 30 (33.3) | 64 (33.0) | 0.954 |
| Valvular heart disease, n (%) | 77 (27.1) | 23 (25.6) | 54 (27.8) | 0.688 |
| Heart rate, beats/min | 97.7 ± 29.0 | 95.2 ± 26.9 | 96.0 ± 27.6 | 0.473 |
| SBP, mmHg | 150.7 ± 34.9 | 157.7 ± 36.4 | 147.5 ± 33.8 | 0.022 |
| DBP, mmHg | 82.1 ± 19.1 | 85.4 ± 19.9 | 80.5 ± 18.6 | 0.044 |
| Peripheral edema, n (%) | 200 (70.4) | 53 (58.9) | 147 (75.8) | 0.044 |
| Pleural effusion, n (%) | 168 (59.2) | 38 (42.4) | 130 (67.0) | <0.001 |
| Circulating Biomarkers | ||||
| Hemoglobin, g/dL | 12.2 ± 1.9 | 12.4 ± 1.9 | 12.1 ± 1.9 | 0.154 |
| NT-proBNP, pg/mL * | 3540 (1894–7490) | 2843 (1253–4775) | 4043 (2064–8702) | 0.006 |
| Serum Creatinine, mg/dL | 1.2 ± 0.6 | 1.2 ± 0.6 | 1.3 ± 0.6 | 0.500 |
| GFR, mL/min/1.73 m2 * | 63.6 ± 25.6 | 63.4 ±22.9 | 63.7 ± 26.8 | 0.906 |
| IL-6, pg/mL * | 13.8 (5.8–42.0) | 17.5 (5.9–54.4) | 13.6 (5.7–32.4) | 0.025 |
| IL-10, pg/mL * | 20.2 (7.1–78.6) | 20.2 (4.9–90.8) | 20.4 (7.8–72.1) | 0.551 |
| IL-1β, pg/mL * | 0.4 (0.1–2.4) | 0.4 (0.1–2.5) | 0.4 (0.1–2.2) | 0.839 |
| TNF, pg/mL * | 7.6 (3.7–19.9) | 7.5 (3.7–22.5) | 7.6 (3.6–18.7) | 0.527 |
| CA125, U/mL * | 69 (30–141) | 19 (14–28) | 111 (67–167) | <0.001 |
| Electrocardiography | ||||
| AF, n (%) | 127 (44.7) | 41 (45.6) | 86 (44.3) | 0.874 |
| Echocardiography | ||||
| LVEF ≥ 50%EF | 157 ± 55.3 | 60 ± 66.7 | 97 ± 50 | 0.009 |
| LAD, mm | 42.6 ± 7.8 | 42.1 ± 6.3 | 42.9 ± 8.4 | 0.428 |
| IVS, mm | 11.3 ± 2.7 | 11.9 ± 2.9 | 11.1 ± 2.6 | 0.028 |
| LV diastolic diameter, mm | 54.8 ± 9.5 | 54.0 ± 9.3 | 55.2 ± 9.7 | 0.358 |
| TAPSE, mm | 18.5 ± 3.5 | 19.6 ± 3.6 | 17.9 ± 3.3 | <0.001 |
| Treatment at discharge | ||||
| Diuretics, n (%) | 275 (96.8) | 87 (96.7) | 188 (96.9) | 0.914 |
| Beta-blockers, n (%) | 205 (72.2) | 59 (65.6) | 146 (75.3) | 0.090 |
| RAAS inhibitors, n (%) | 188 (66.2) | 58 (64.4) | 130 (67.0) | 0.671 |
| MRA, n (%) | 41 (14.4) | 7 (7.8) | 34 (17.5) | 0.030 |
| All Cause Mortality HR (95% CI) | p-Value | HF Readmissions HR (95% CI) | p-Value | |
|---|---|---|---|---|
| IL-6 | 0.245 | 0.989 | ||
| Q1 | 17.9 (13.6–23.4) | 27.5 (12.0–42.9) | ||
| Q2 | 13.1 (0.98–17.4) | 28.2 (13.6–42.6) | ||
| Q3 | 18.0 (13.9–23.4) | 30.9 (15.8–46.1) | ||
| Q4 | 18.4 (14.1–23.9) | 28.0 (19.0–46.2) | ||
| IL-10 | 0.109 | 0.020 | ||
| Q1 | 15.5 (11.7–20.1) | 27.2 (10.9–43.5) | ||
| Q2 | 13.1 (9.9–17.2) | 14.8 (7.7–21.7) | ||
| Q3 | 20.0 (15.4–25.9) | 39.4 (20.8–57.9) | ||
| Q4 | 19.0 (14.7–24.6) | 36.1 (16.7–55.7) | ||
| IL-1β | 0.845 | 0.333 | ||
| Q1 | 17.3 (13.2–22.8) | 24.6 (13.3–35.8) | ||
| Q2 | 17.8 (13.5–23.4) | 42.0 (17.7–66.4) | ||
| Q3 | 16.3 (12.4–21.6) | 21.9 (11.3–31.8) | ||
| Q4 | 15.4 (11.8–19.9) | 29.0 (12.6–45.4) | ||
| TNF | 0.929 | 0.724 | ||
| Q1 | 15.5 (11.9–20.5) | 24.2 (10.4–37.9) | ||
| Q2 | 16.9 (12.9–22.2) | 35.3 (21.1–49.4) | ||
| Q3 | 17.8 (13.7–23.2) | 28.2 (13.7–42.6) | ||
| Q4 | 16.3 (12.4–21.4) | 26.8 (7.5–46.1) |
| IRR (95% CI) | p-Value | |
|---|---|---|
| All-cause mortality | ||
| IL-6, per increase in 10 pg/mL | 1.01 (0.99–1.02) | 0.637 |
| IL-10, per increase in 10 pg/mL | 1.00 (0.99–1.01) | 0.721 |
| IL-1β, per increase in 10 pg/mL | 1.06 (0.99–1.14) | 0.100 |
| TNF, per increase in 10 pg/mL | 1.00 (0.97–1.04) | 0.971 |
| Total HF-readmissions | ||
| IL-6, per increase in 10 pg/mL | 1.01 (0.99–1.02) | 0.752 |
| IL-10, per increase in 10 pg/mL | 1.00 (0.99–1.01) | 0.967 |
| IL-1β, per increase in 10 pg/mL | 1.02 (0.94–1.11) | 0.651 |
| TNF, per increase in 10 pg/mL | 0.962 (0.90–1.03) | 0.250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santas, E.; Martí-Martínez, A.; Villar, S.; de la Espriella, R.; Rodriguez-Borja, E.; Revuelta-López, E.; González-Miqueo, A.; Bayés-Genís, A.; Sanchis, J.; Núñez, J. Cytokine Networks and Heart Failure Outcomes: CA125 as a Bridge Between Congestion and Inflammation. Int. J. Mol. Sci. 2025, 26, 9527. https://doi.org/10.3390/ijms26199527
Santas E, Martí-Martínez A, Villar S, de la Espriella R, Rodriguez-Borja E, Revuelta-López E, González-Miqueo A, Bayés-Genís A, Sanchis J, Núñez J. Cytokine Networks and Heart Failure Outcomes: CA125 as a Bridge Between Congestion and Inflammation. International Journal of Molecular Sciences. 2025; 26(19):9527. https://doi.org/10.3390/ijms26199527
Chicago/Turabian StyleSantas, Enrique, Arancha Martí-Martínez, Sandra Villar, Rafael de la Espriella, Enrique Rodriguez-Borja, Elena Revuelta-López, Arantxa González-Miqueo, Antoni Bayés-Genís, Juan Sanchis, and Julio Núñez. 2025. "Cytokine Networks and Heart Failure Outcomes: CA125 as a Bridge Between Congestion and Inflammation" International Journal of Molecular Sciences 26, no. 19: 9527. https://doi.org/10.3390/ijms26199527
APA StyleSantas, E., Martí-Martínez, A., Villar, S., de la Espriella, R., Rodriguez-Borja, E., Revuelta-López, E., González-Miqueo, A., Bayés-Genís, A., Sanchis, J., & Núñez, J. (2025). Cytokine Networks and Heart Failure Outcomes: CA125 as a Bridge Between Congestion and Inflammation. International Journal of Molecular Sciences, 26(19), 9527. https://doi.org/10.3390/ijms26199527






