Molecular Basis, Diagnostic Approaches, and Therapeutic Strategies in Colorectal Cancer—Comprehensive Review
Abstract
1. Introduction
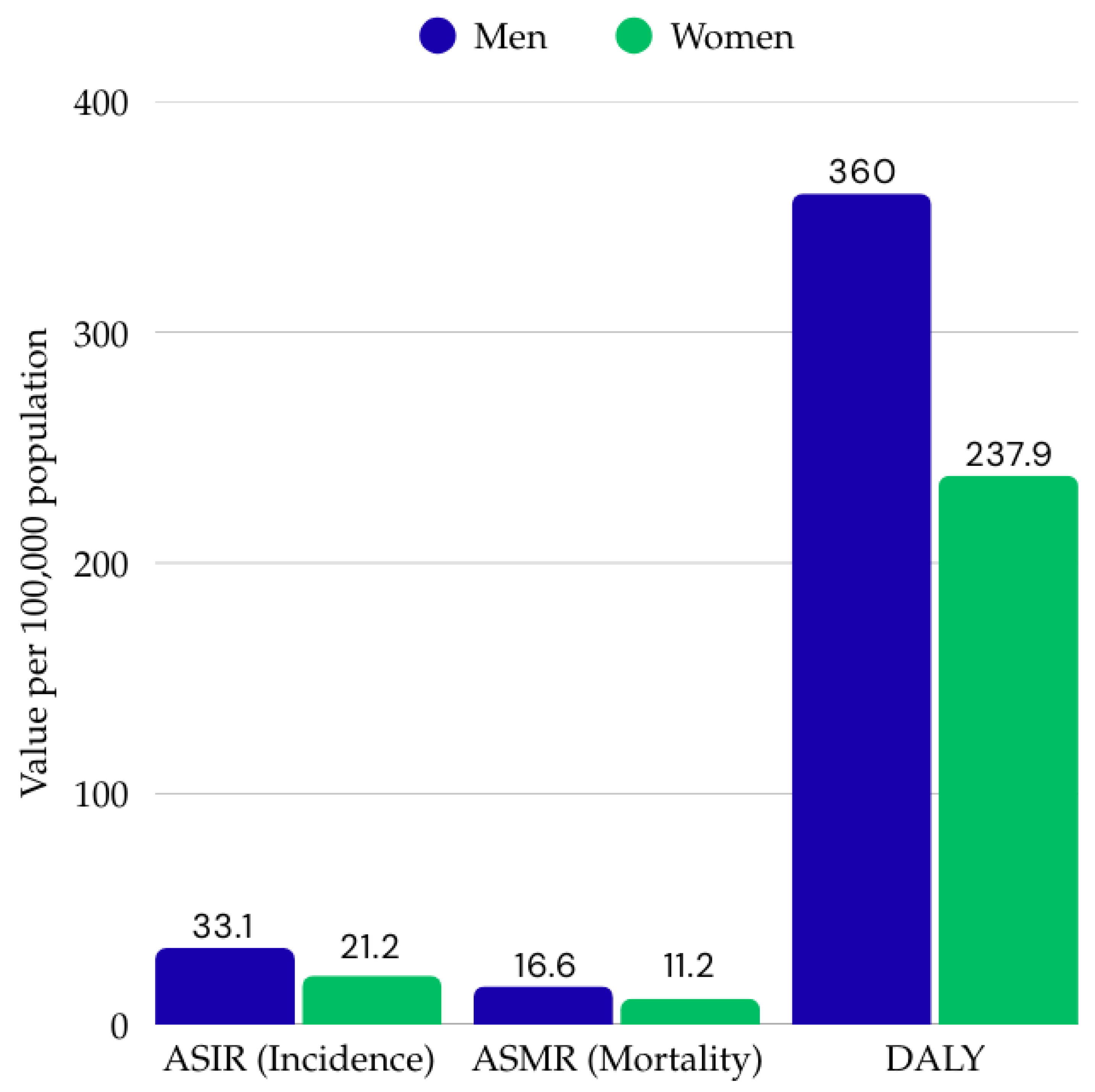
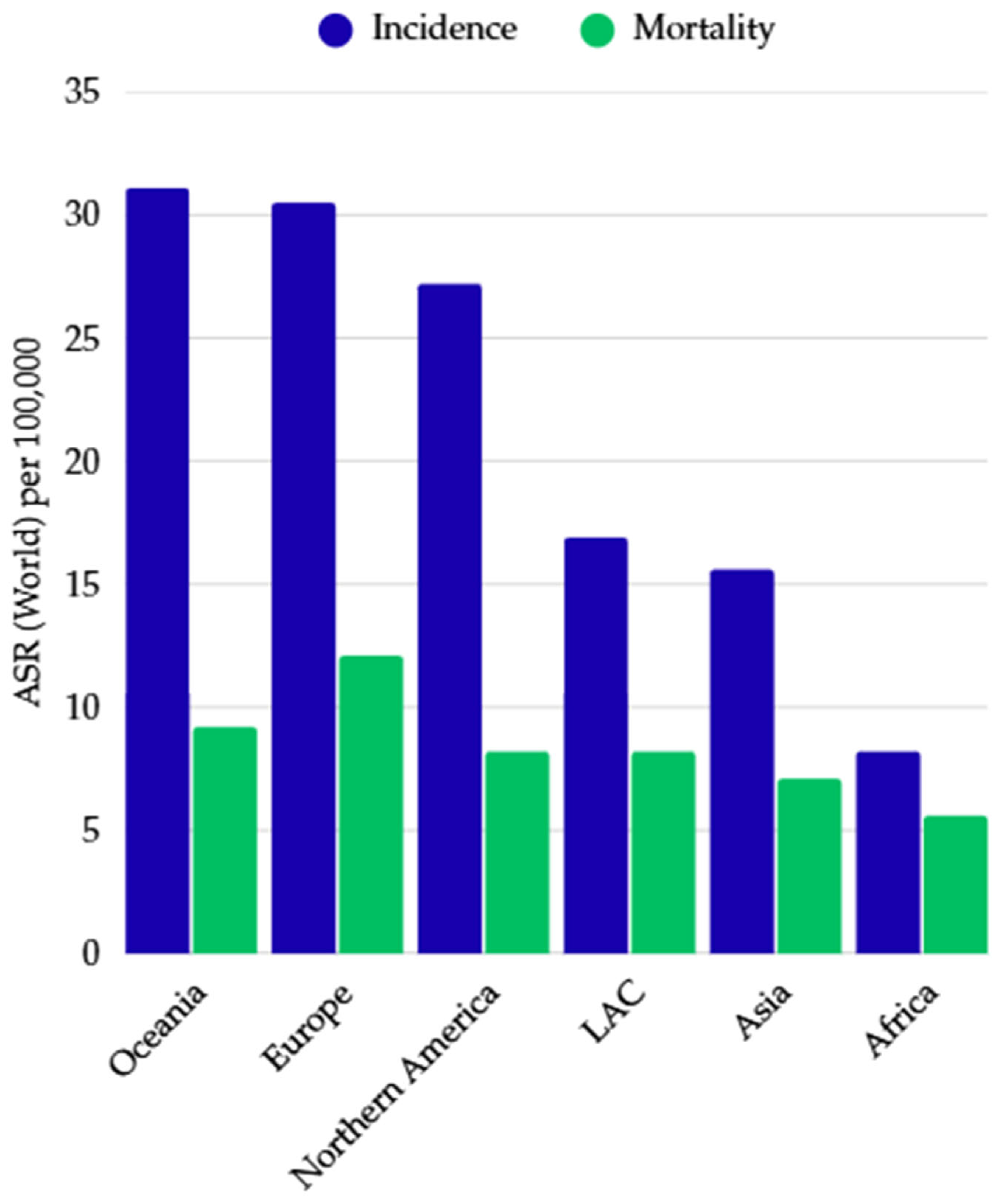
2. Epidemiological Data, Determinants, and Geographic and Demographic Variation in CRC
3. The Pathogenesis of Colorectal Cancer: Theories of Disease Development and Risk Determinants
3.1. Risk Factors for Colorectal Cancer
3.2. Molecular Factors Contributing to the Development of Colorectal Cancer
3.2.1. Chromosomal Instability Pathway (CIN)
3.2.2. Microsatellite Instability Pathway (MSI)
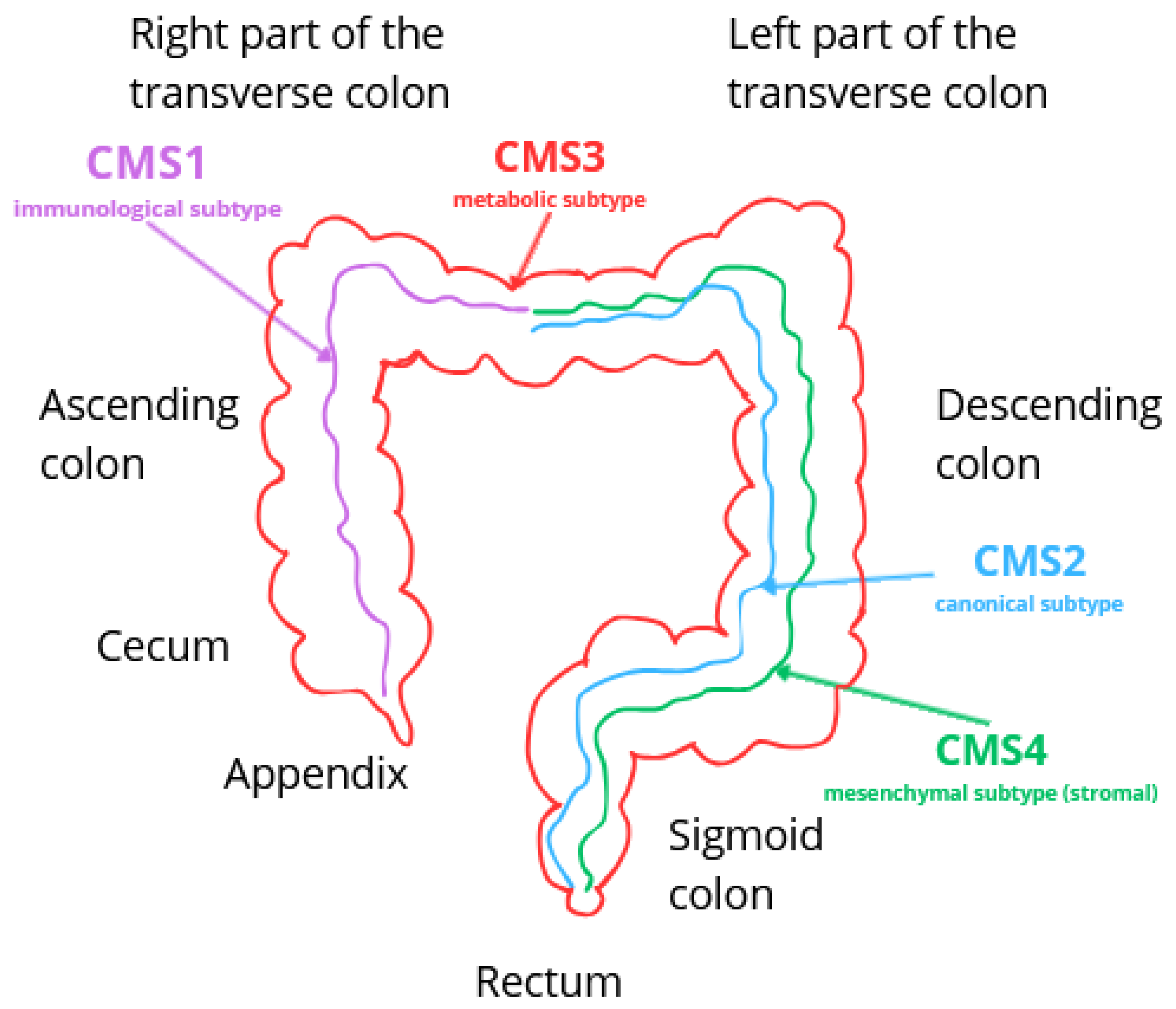
3.3. Classification of Colorectal Cancer
3.3.1. CMS1—Immunological Subtype (MSI)
3.3.2. CMS2—Canonical Subtype
3.3.3. CMS3—Metabolic Subtype
3.3.4. CMS4—Mesenchymal Subtype (Stromal)
4. Secondary Prevention and Diagnosis of Colorectal Cancer
4.1. Sreening Tests
4.2. Diagnostic Tests
4.3. Predictive Biomarkers in Therapy (Companion Diagnostics)
5. Classic Therapeutic Strategies in Colorectal Cancer
6. Modern Methods of Treatment of Colorectal Cancer
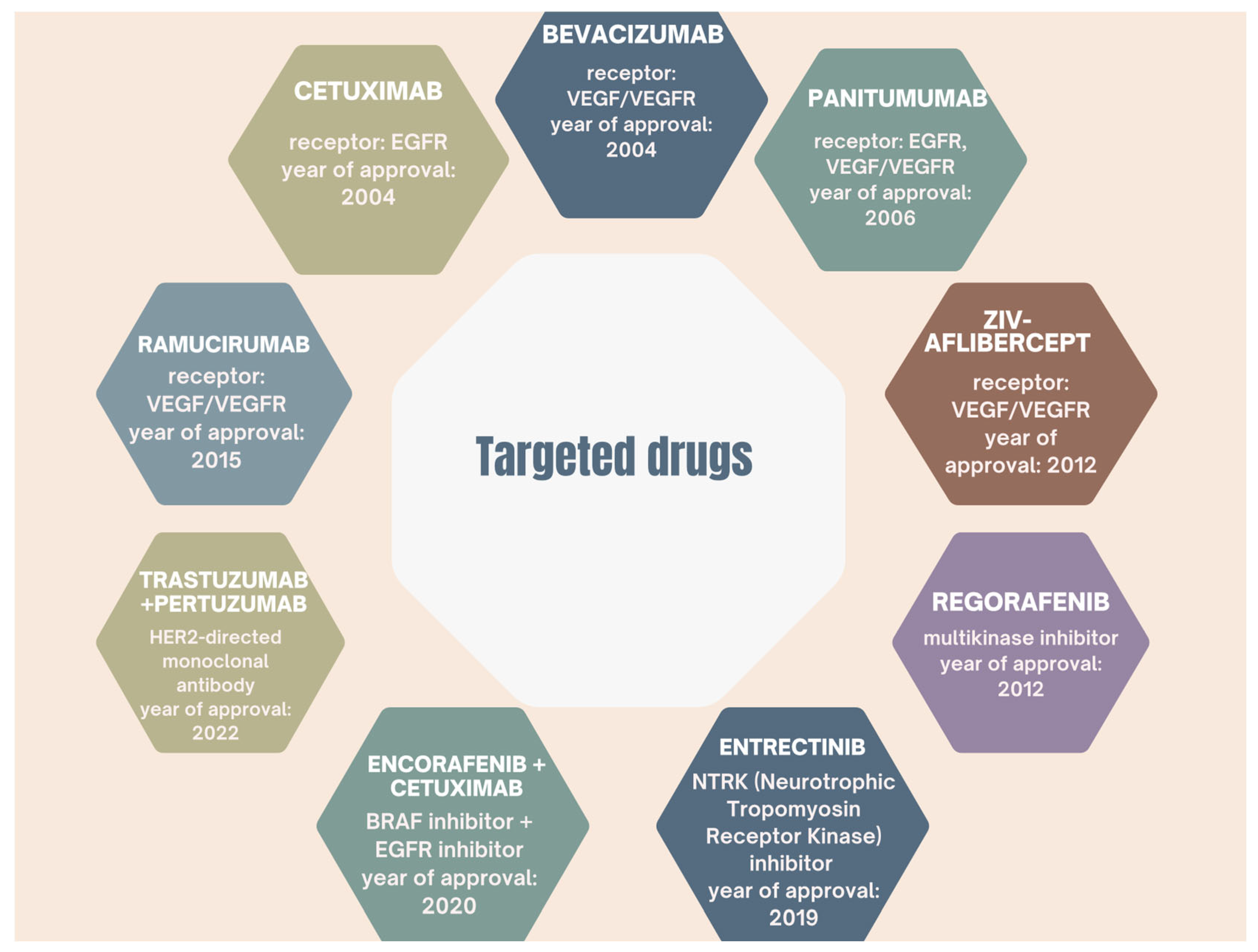
6.1. Molecularly Targeted Therapies and Immunotherapy in Colorectal Cancer
6.1.1. Therapies Involving Anti-VEGF and Anti-EGFR Antibodies and Targeting the VEGF-VEGFR Signaling Pathway
Anti-VEGF Therapies
Anti-EGFR Therapies
6.1.2. BRAF Kinase-Targeted Therapy
6.1.3. PD-1/PD-L1 Checkpoint Inhibitors in the Treatment of Colorectal Cancer
6.1.4. Advances and New Strategies for Targeted Therapy
6.2. Other Systemic Treatments for Colorectal Cancer
7. Contemporary Surgical Techniques—Innovative Approaches in Surgery
7.1. Robotic Surgery
7.2. Innovations in Surgical Planning with Intraoperative Navigation
7.3. Laparoscopic Surgery and Its Advantages over Traditional Surgery
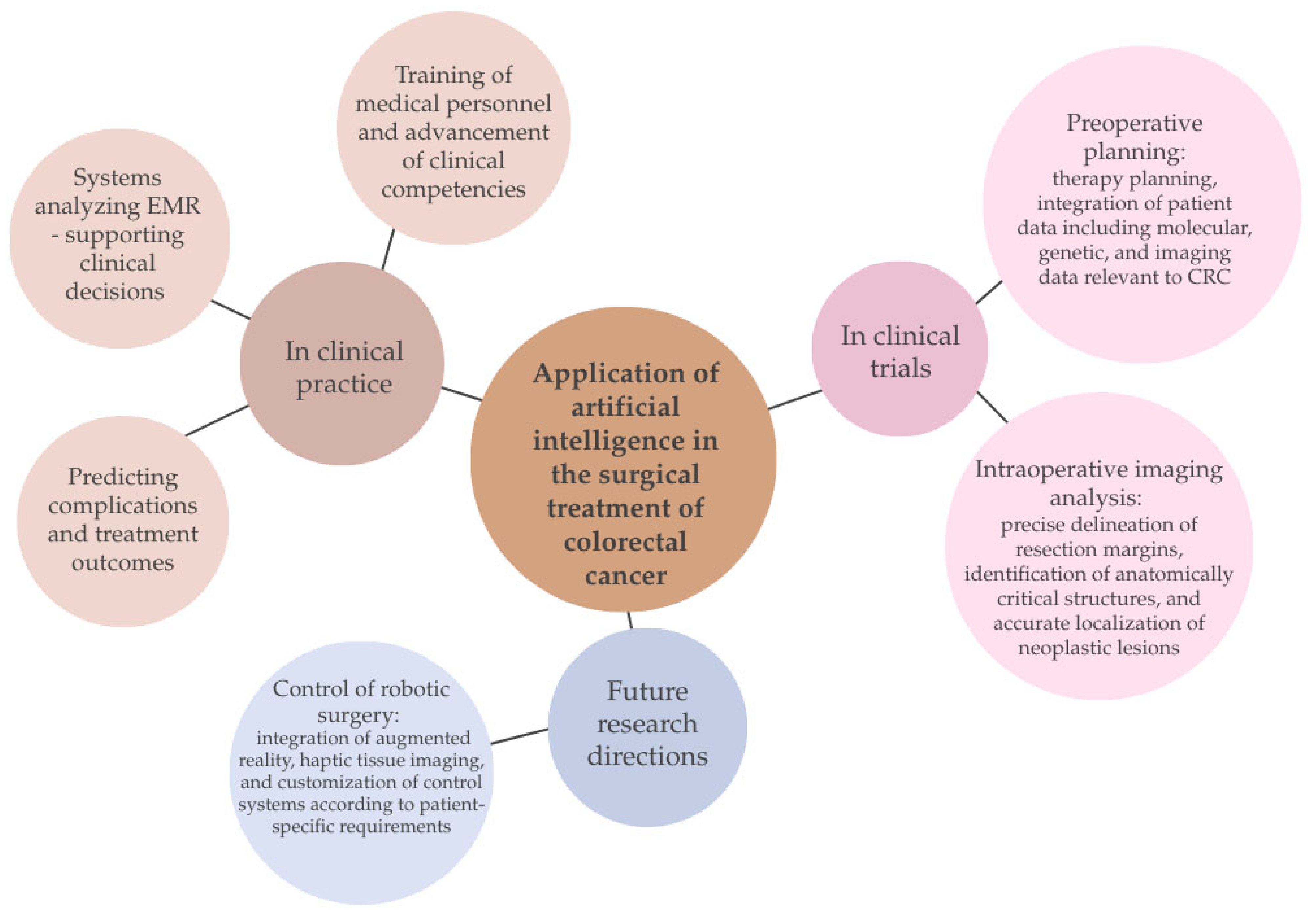
7.4. The Use of AI in the Surgical Treatment of Colorectal Cancer
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tárraga López, P.J.; Albero, J.S.; Rodríguez-Montes, J.A. Primary and secondary prevention of colorectal cancer. Clinical medicine insights. Gastroenterology 2014, 7, 33–46. [Google Scholar] [CrossRef] [PubMed]
- El Hage, M.; Su, Z.; Linnebacher, M. Addressing Challenges in Targeted Therapy for Metastatic Colorectal Cancer. Cancers 2025, 17, 1098. [Google Scholar] [CrossRef]
- Zhai, Z.; Yu, X.; Yang, B.; Zhang, Y.; Zhang, L.; Li, X.; Sun, H. Colorectal cancer heterogeneity and targeted therapy: Clinical implications, challenges and solutions for treatment resistance. Semin. Cell Dev. Biol. 2017, 64, 107–115. [Google Scholar] [CrossRef]
- Srivastava, R. Advancing precision oncology with AI-powered genomic analysis. Front. Pharmacol. 2025, 16, 1591696. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Chen, C. The colorectal cancer epidemic: Challenges and opportunities for primary, secondary and tertiary prevention. Br. J. Cancer 2018, 119, 785–792. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer, World Health Organization. Colorectal Cancer. Available online: https://www.iarc.who.int/cancer-type/colorectal-cancer/#research_websites (accessed on 1 August 2025).
- International Agency for Research on Cancer. Cancer Today; Data Version: Globocan 2022 (Version 1.1)—8 February 2024. Available online: https://gco.iarc.fr/today/en/dataviz/pie?mode=population&group_populations=0&cancers=41 (accessed on 25 August 2025).
- American Institute for Cancer Research; World Cancer Research Fund; Continuous Update Project. Diet, Nutrition, Physical Activity and Colorectal Cancer. 2018. Available online: https://www.wcrf.org/wp-content/uploads/2024/10/Colorectal-cancer-report.pdf (accessed on 8 August 2025).
- GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef]
- World Health Organization-IARC. European Code Against Cancer. 2025. Available online: https://cancer-code-europe.iarc.fr/index.php/en/ecac-12-ways/screening-recommandation/bowel-cancer-screening (accessed on 10 August 2025).
- Darmadi, D.; Mohammadian-Hafshejani, A.; Kheiri, S. Global Disparities in Colorectal Cancer: Unveiling the Present Landscape of Incidence and Mortality Rates, Analyzing Geographical Variances, and Assessing the Human Development Index. J. Prev. Med. Hyg. 2025, 65, E499–E514. [Google Scholar] [CrossRef]
- Kędzia-Berut, R.; Berut, M.; Włodarczyk, M.; Włodarczyk, J.; Dziki, Ł.; Dziki, A.; Mik, M. Colorectal Cancer: Is it Still a Disease of the Elderly? Index Copernic. Int. 2023, 96, 41–45. [Google Scholar] [CrossRef]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153. [Google Scholar] [CrossRef]
- Gonzalez, R.S.; Raza, A.; Propst, R.; Adeyi, O.; Bateman, J.; Sopha, S.C.; Shaw, J.; Auerbach, A. Recent Advances in Digestive Tract Tumors: Updates From the 5th Edition of the World Health Organization “Blue Book”. Arch. Pathol. Lab. Med. 2021, 145, 607–626. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Senore, C.; Segnan, N.; Gunter, M.; Simons, C. 5.5 Colorectal cancer: Decreasing disparities and promoting prevention are policy priorities. In World Cancer Report: Cancer Research for Cancer Prevention; Wild, C.P., Weiderpass, E., Stewart, B.W., Eds.; International Agency for Research on Cancer: Lyon (FR), France, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK606525 (accessed on 20 August 2025).
- Karavin, E.C.; Yılmaz, Z.S.; Yazici, H.; Ersoz, S.; Mungan, S.; Mungan, S. Comparison of Microsatellite Instability with Clinicopathologic Data in Patients with Colon Adenocarcinoma. Cureus 2024, 16, e57814. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.H.; Gerlach, D.; Misale, S.; Petronczki, M.; Kraut, N. Expanding the Reach of Precision Oncology by Drugging All KRAS Mutants. Cancer Discov. 2022, 12, 924–937. [Google Scholar] [CrossRef]
- Goh, A.M.; Coffill, C.R.; Lane, D.P. The role of mutant p53 in human cancer. J. Pathol. 2011, 223, 116–126. [Google Scholar] [CrossRef]
- Lindblom, A. Different mechanisms in the tumorigenesis of proximal and distal colon cancers. Curr. Opin. Oncol. 2001, 13, 63–69. [Google Scholar] [CrossRef]
- Shia, J.; Schultz, N.; Kuk, D.; Vakiani, E.; Middha, S.; Segal, N.H.; Hechtman, J.F.; Berger, M.F.; Stadler, Z.K.; Weiser, M.R.; et al. Morphological Characterization of Colorectal Cancers in The Cancer Genome Atlas Reveals Distinct Morphology-Molecular Associations: Clinical and biological implications. Mod. Pathol. 2016, 30, 599–609. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular Origins of Cancer. Molecular Basis of Colorectal Cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Wodzin, D. Assessment of the Expression of Selected Factors of the TGF-ß Pathway in Colorectal Cancer. Uniwersyet Medyczny w Łodzi, Wydział Farmaceutyczny. 2020. Available online: https://farmacja.umed.pl/wp-content/uploads/2021/03/Damian-Wodziński-Ocena-ekspresji-wybranych-czynników-szlaku-TGFß-w-raku-jelita-grubego.pdf (accessed on 20 August 2025).
- Frąckowiak, M.; Lewandowski, T.; Stelmasiak, P. Molecular subtypes of colorectal cancer as a potential prognostic and predictive factor in the selection of the optimal treatment strategy. Oncol. Clin. Pract. 2019, 15, 320–325. [Google Scholar] [CrossRef]
- Sawayama, H.; Miyamoto, Y.; Ogawa, K.; Yoshida, N.; Baba, H. Investigation of colorectal cancer in accordance with consensus molecular subtype classification. Ann. Gastroenterol. Surg. 2020, 4, 528–539. [Google Scholar] [CrossRef]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal cancer: Epidemiology, risk factors, and prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef] [PubMed]
- Sveen, A.; Kopetz, S.; Lothe, R.A. Biomarker-guided therapy for colorectal cancer: Strength in complexity. Nat. Rev. Clin. Oncol. 2020, 17, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Kolligs, F.T. Diagnostics and epidemiology of colorectal cancer. Visc. Med. 2016, 32, 158–164. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Lișcu, H.-D.; Verga, N.; Atasiei, D.-I.; Badiu, D.-C.; Dumitru, A.V.; Ultimescu, F.; Pavel, C.; Stefan, R.-E.; Manole, D.-C.; Ionescu, A.-I. Biomarkers in colorectal cancer: Actual and future perspectives. Int. J. Mol. Sci. 2024, 25, 11535. [Google Scholar] [CrossRef]
- Bevan, R.; Rutter, M.D. Colorectal cancer screening—Who, how, and when? Clin. Endosc. 2018, 51, 37–49. [Google Scholar] [CrossRef]
- de Assis, J.V.; Coutinho, L.A.; Oyeyemi, I.T.; Oyeyemi, O.T.; Grenfell, R.F.E.Q. Diagnostic and therapeutic biomarkers in colorectal cancer: A review. Am. J. Cancer Res. 2022, 12, 661–680. [Google Scholar]
- Svec, J.; Onhajzer, J.; Korinek, V. Origin, development and therapy of colorectal cancer from the perspective of a biologist and an oncologist. Crit. Rev. Oncol. Hematol. 2024, 204, 104544. [Google Scholar] [CrossRef]
- Ashouri, K.; Wong, A.; Mittal, P.; Torres-Gonzalez, L.; Lo, J.H.; Soni, S.; Algaze, S.; Khoukaz, T.; Zhang, W.; Yang, Y.; et al. Exploring predictive and prognostic biomarkers in colorectal cancer: A comprehensive review. Cancers 2024, 16, 2796. [Google Scholar] [CrossRef]
- Zygulska, A.L.; Pierzchalski, P. Novel diagnostic biomarkers in colorectal cancer. Int. J. Mol. Sci. 2022, 23, 852. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2022, 21, 86. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Wang, Y.; Christie, M.; Simons, K.; Lee, M.; Wong, R.; Kosmider, S.; Ananda, S.; McKendrick, J.; et al. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol. 2019, 5, 1710–1717. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, W.; Zhang, W.; Chen, Y.; Shen, H.; Zhu, H.; Liang, X.; Shen, Z. Tracking of trastuzumab resistance in patients with HER2-positive metastatic gastric cancer by CTC liquid biopsy. Am. J. Cancer Res. 2023, 13, 5684–5697. [Google Scholar] [PubMed]
- Razzaghi, H.; Khabbazpour, M.; Heidary, Z.; Heiat, M.; Shirzad Moghaddam, Z.; Derogar, P.; Khoncheh, A.; Zaki-Dizaji, M. Emerging Role of Tumor-Educated Platelets as a New Liquid Biopsy Tool for Colorectal Cancer. Arch. Iran. Med. 2023, 26, 447–454. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer: Current status, challenges and future prospects. Sig. Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, L.; Blancas, I.; Garrido, J.M.; Mut-Salud, N.; Moya-Jódar, M.; Osuna, A.; Rodríguez-Serrano, F. The role of exosomes on colorectal cancer: A review. J. Gastroenterol. Hepatol. 2018, 33.4, 792–799. [Google Scholar] [CrossRef]
- Raza, A.; Khan, A.Q.; Inchakalody, V.P.; Mestiri, S.; Yoosuf, Z.S.K.; Bedhiafi, T.; El-Ella, D.M.A.; Taib, N.; Hydrose, S.; Akbar, S.; et al. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 99. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Shao, W.; Yan, S.; Ding, S. Efficacy and safety of Omega-3 polyunsaturated fatty acids in adjuvant treatments for colorectal cancer: A meta-analysis of randomized controlled trials. Front. Pharmacol. 2023, 14, 1004465. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Vogel, J.D.; Felder, S.I.; Bhama, A.R.; Hawkins, A.T.; Langenfeld, S.J.; Shaffer, V.O.; Thorsen, A.J.; Weiser, M.R.; Chang, G.J.; Lightner, A.L.; et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of colon cancer. Dis. Colon Rectum 2022, 65, 148–177. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, T.; Tanaka, T.; Nozawa, H.; Emoto, S.; Murono, K.; Kaneko, M.; Sasaki, K.; Otani, K.; Nishikawa, T.; Hata, K.; et al. Comparison of the guidelines for colorectal cancer in Japan, the USA and Europe. Ann. Gastroenterol. Surg. 2017, 2, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2024, 22, e240029. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Gögenur, M.; Rosen, A.W.; Iveson, T.; Kerr, R.S.; Saunders, M.P.; Cassidy, J.; Tabernero, J.; Haydon, A.; Glimelius, B.; Harkin, A.; et al. Time from colorectal cancer surgery to adjuvant chemotherapy: Post hoc analysis of the SCOT randomized clinical trial. JAMA Surg. 2024, 159, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, J.; Han, D.; Ma, X. Artificial Intelligence Applications in the Treatment of Colorectal Cancer: A Narrative Review. Clin. Med. Insights Oncol. 2024, 18, 11795549231220320. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 1139–1167. [Google Scholar] [CrossRef]
- Scott, A.J.; Kennedy, E.B.; Berlin, J.; Brown, G.; Chalabi, M.; Cho, M.T.; Cusnir, M.; Dorth, J.; George, M.; Kachnic, L.A.; et al. Management of Locally Advanced Rectal Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 3355–3375. [Google Scholar] [CrossRef]
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal cancer: Recent advances in management and treatment. World J. Clin. Oncol. 2024, 15, 1136–1156. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General insight into cancer: An overview of colorectal cancer (Review). Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H. Recent advances in therapeutic strategies to improve colorectal cancer treatment. Cancers 2024, 16, 1029. [Google Scholar] [CrossRef]
- Shaham, S.H.; Vij, P.; Tripathi, M.K. Advances in targeted and chemotherapeutic strategies for colorectal cancer: Current insights and future directions. Biomedicines 2025, 13, 642. [Google Scholar] [CrossRef]
- Pathak, P.S.; Chan, G.; Deming, D.A.; Chee, C.E. State-of-the-art management of colorectal cancer: Treatment advances and innovation. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e438466. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, V.; Sandhu, A.; Rawat, K.; Sharma, A.; Saha, L. Current and emerging therapeutic approaches for colorectal cancer: A comprehensive review. World J. Gastrointest. Surg. 2023, 15, 495–519. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Liu, Y.-C.; Chen, C.-C.; Chen, M.-C.; Chiu, T.-Y.; Huang, Y.-L.; Chiang, S.-W.; Lin, C.-L.; Chen, Y.-J.; Lin, C.-Y.; et al. Robotic-assisted colon cancer surgery: Faster recovery and less pain compared to laparoscopy in a retrospective propensity-matched study. Cancers 2025, 17, 243. [Google Scholar] [CrossRef]
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent advances in the treatment of colorectal cancer: A review. J. Nippon Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef]
- Chen, E.; Chen, L.; Zhang, W. Robotic-assisted colorectal surgery in colorectal cancer management: A narrative review of clinical efficacy and multidisciplinary integration. Front. Oncol. 2025, 15, 1502014. [Google Scholar] [CrossRef] [PubMed]
- Kok Wah, J.N. AI-driven robotic surgery in oncology: Advancing precision, personalization, and patient outcomes. J. Robot. Surg. 2025, 19, 382. [Google Scholar] [CrossRef]
- Kok, E.N.D.; van Veen, R.; Groen, H.C.; Heerink, W.J.; Hoetjes, N.J.; van Werkhoven, E.; Beets, G.L.; Aalbers, A.G.J.; Kuhlmann, K.F.D.; Nijkamp, J.; et al. Association of Image-Guided Navigation with Complete Resection Rate in Patients With Locally Advanced Primary and Recurrent Rectal Cancer: A Nonrandomized Controlled Trial. JAMA Netw. Open 2020, 3, e208522. [Google Scholar] [CrossRef]
- Pal, A. The digital horizon in colorectal cancer surgery: A narrative review. Laparosc. Endosc. Robot. Surg. 2025, 8, 67–72. [Google Scholar] [CrossRef]
- Mezger, U.; Jendrewski, C.; Bartels, M. Navigation in surgery. Langenbecks Arch. Surg. 2013, 398, 501–514. [Google Scholar] [CrossRef]
- Kitaguchi, D.; Harai, Y.; Kosugi, N.; Hayashi, K.; Kojima, S.; Ishikawa, Y.; Yamada, A.; Hasegawa, H.; Takeshita, N.; Ito, M. Artificial Intelligence for the Recognition of Key Anatomical Structures in Laparoscopic Colorectal Surgery. Br. J. Surg. 2023, 110, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Eresen, A.; Shangguan, J.; Yang, J.; Benson, A.B., 3rd; Yaghmai, V.; Zhang, Z. Preoperative Prediction of Perineural Invasion and KRAS Mutation in Colon Cancer Using Machine Learning. J. Cancer Res. Clin. Oncol. 2020, 146, 3165–3174. [Google Scholar] [CrossRef]
- Su, Y.; Li, Y.; Chen, W.; Yang, W.; Qin, J.; Liu, L. Automated machine learning-based model for predicting benign anastomotic strictures in patients with rectal cancer who have received anterior resection. Eur. J. Surg. Oncol. 2023, 49, 107113. [Google Scholar] [CrossRef]
- Dadhania, S.; Herrick, B.; Bakshi, B.; Raoof, S.; Haddad, T.C.; Patel, T.; Riaz, I.B.; Payling, M. Shifting the paradigm: Early identification of colorectal cancer with C the Signs clinical decision support. J. Clin. Oncol. 2025, 43, 54. [Google Scholar] [CrossRef]
- Ogbonnaya, C.N.; Li, S.; Tang, C.; Zhang, B.; Sullivan, P.; Erden, M.S.; Tang, B. Exploring the Role of Artificial Intelligence (AI)-Driven Training in Laparoscopic Suturing: A Systematic Review of Skills Mastery, Retention, and Clinical Performance in Surgical Education. Healthcare 2025, 13, 571. [Google Scholar] [CrossRef] [PubMed]
- Negrut, R.L.; Cote, A.; Caus, V.A.; Maghiar, A.M. Systematic Review and Meta-Analysis of Laparoscopic versus Robotic-Assisted Surgery for Colon Cancer: Efficacy, Safety, and Outcomes—A Focus on Studies from 2020–2024. Cancers 2024, 16, 1552. [Google Scholar] [CrossRef]
- Vilsan, J.; Maddineni, S.A.; Ahsan, N.; Mathew, M.; Chilakuri, N.; Yadav, N.; Munoz, E.J.; Nadeem, M.A.; Abbas, K.; Razzaq, W.; et al. Open, Laparoscopic, and Robotic Approaches to Treat Colorectal Cancer: A Comprehensive Review of Literature. Cureus 2023, 15, e38956. [Google Scholar] [CrossRef] [PubMed]
- Schootman, M.; Mutch, M.; Loux, T.; Eberth, J.M.; Davidson, N.O. Differences in effectiveness and use of laparoscopic surgery in locally advanced colon cancer patients. Sci. Rep. 2021, 11, 10022. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Saqib, M.; Zareen, M.; Mumtaz, H. Artificial Intelligence: Revolutionizing Robotic Surgery: Review. Ann. Med. Surg. 2024, 86, 5401–5409. [Google Scholar] [CrossRef] [PubMed]
| Category | MSI (Microsatellite Instability) | CIN (Chromosomal Instability) |
|---|---|---|
| Molecular mechanism | Impairment of the DNA mismatch repair system (MMR—Mismatch Repair) | Numerical and structural changes in chromosomes |
| Genes involved | MLH-1, PMS-2, MSH-6, MSH-2 | APC, TP53, KRAS, SMAD4 |
| Prevalence in colorectal cancer | 12–15% | 70–85% |
| Association with hereditary syndromes | Often associated with Lynch syndrome | Often associated with Lynch syndrome |
| Typical tumor location | Right (proximal) part of the colon | Distal colon and rectum |
| Histological appearance | Mucinous carcinoma with marked lymphocytic infiltration | Well or moderately differentiated adenocarcinoma with characteristic glandular architecture |
| Biomarker | Clinical Significance |
|---|---|
| KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) | An oncogene encoding a GTPase protein (p21) that participates in signal transduction from the cell membrane [36]. Mutations most commonly occur in codons 12 and 13 [37]. The KRAS mutation causes permanent activation of the RAS/RAF/MEK/ERK signaling pathway and resistance to anti-EGFR antibody treatment (e.g., cetuximab). KRAS mutations occur in up to 40% of patients and have been classified as a predictive factor for a poor response to neoadjuvant treatment in patients with locally advanced rectal cancer [34]. Because mutations in the KRAS gene lead to constant activation of the EGFR signaling pathway, epidermal growth factor receptor inhibitors are ineffective [37]. In addition, KRAS mutations are more common in tumors located on the left side of the colon and more common in microsatellite-stable colorectal cancer [37]. |
| BRAF (v-Raf Murine Sarcoma Viral Oncogene Homolog B1) | A gene encoding a serine–threonine kinase that regulates the MAPK pathway associated with cell proliferation [36]. A mutation in codon 600 (i.e., valine to glutamic acid substitution) occurs in 5–9% of patients with colorectal cancer [37]. The frequency of BRAF mutations is higher in women and people over 70 years of age, mainly in the right colon. The BRAF mutation is associated with a poorer prognosis, aggressive growth, unfavorable metastasis, and is an indication for testing in patients with stage IV disease to qualify for targeted therapy [36,37]. |
| TP53 (Tumor Protein p53) | A suppressor gene encoding the cytoplasmic p53 protein, which regulates the cell cycle, apoptosis, aging, and DNA repair. Mutations occur in approximately 60% of CRC patients and are associated with neoplastic transformation (adenoma–carcinoma). However, the detection rate of anti-p53 antibodies in serum has a sensitivity of less than 30%. TP53 mutations are associated with a poorer prognosis and shorter survival time [36]. |
| MSI (Microsatellite Instability) | Occurs in approximately 15% of all colorectal cancers. Caused by a defect in the error correction system during DNA replication—unpaired bases [36,37]. Microsatellite instability is a prognostic marker of a good prognosis and a predictive marker for eligibility for immunotherapy [36]. High microsatellite instability is associated with improved overall survival and a lower rate of disease spread [34]. Knowledge of MSI mutations is crucial in the treatment of colorectal cancer and indicates Lynch syndrome [37]. |
| APC (Adenomatous Polyposis Coli) | APC mutations occur in 70–80% of patients with CRC (both in familial adenomatous polyposis and sporadically). The APC protein controls migration, adhesion, transcription, and apoptosis. Methylation of the APC promoter does not correlate with survival, but its mutation in combination with high miR-21 expression indicates a poorer prognosis. It may be a biomarker for early detection and a target for targeted therapy [36]. |
| HER2 (Human Epidermal Growth Factor Receptor 2) | A protein encoded by the erythroblastic oncogene B gene [36]. Increased HER2 expression in patients with colorectal cancer is associated with resistance to anti-EGFR therapy. In addition to its role as a predictive marker for HER2-targeted therapy, it is an indicator of resistance to monoclonal antibodies targeting EGFR [34]. The authors of [37] report that HER2 expression should be assessed in all patients with metastatic colorectal cancer, as it occurs in approximately 2–6% of cases, and its identification is important in terms of therapy and the use of anti-EGFR and anti-HER2 therapies. |
| PD-L1 (Programmed Death-Ligand 1) | A surface protein that binds to the PD-1 receptor on T lymphocytes, leading to the inhibition of the immune response, which suppresses the body’s reaction against cancer cells [37]. PD-L1 expression is variable and depends, among other things, on inflammation and the method of sample collection. Some data suggest that the presence of PD-1 on immune cells is associated with a favorable prognosis, and research into new drugs that could expand the use of PD-1/PD-L1-targeted therapies is ongoing [37]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, M.K.; El-Mallul, A.; Lubojańska, J.E.; Hudecka, W.; Orłowska, S.M.; Lubojański, P.J.; Bednarczyk, Ł. Molecular Basis, Diagnostic Approaches, and Therapeutic Strategies in Colorectal Cancer—Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 9520. https://doi.org/10.3390/ijms26199520
Kowalska MK, El-Mallul A, Lubojańska JE, Hudecka W, Orłowska SM, Lubojański PJ, Bednarczyk Ł. Molecular Basis, Diagnostic Approaches, and Therapeutic Strategies in Colorectal Cancer—Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(19):9520. https://doi.org/10.3390/ijms26199520
Chicago/Turabian StyleKowalska, Małgorzata Katarzyna, Ahmed El-Mallul, Joanna Elżbieta Lubojańska, Weronika Hudecka, Sara Małgorzata Orłowska, Piotr Jan Lubojański, and Łukasz Bednarczyk. 2025. "Molecular Basis, Diagnostic Approaches, and Therapeutic Strategies in Colorectal Cancer—Comprehensive Review" International Journal of Molecular Sciences 26, no. 19: 9520. https://doi.org/10.3390/ijms26199520
APA StyleKowalska, M. K., El-Mallul, A., Lubojańska, J. E., Hudecka, W., Orłowska, S. M., Lubojański, P. J., & Bednarczyk, Ł. (2025). Molecular Basis, Diagnostic Approaches, and Therapeutic Strategies in Colorectal Cancer—Comprehensive Review. International Journal of Molecular Sciences, 26(19), 9520. https://doi.org/10.3390/ijms26199520







