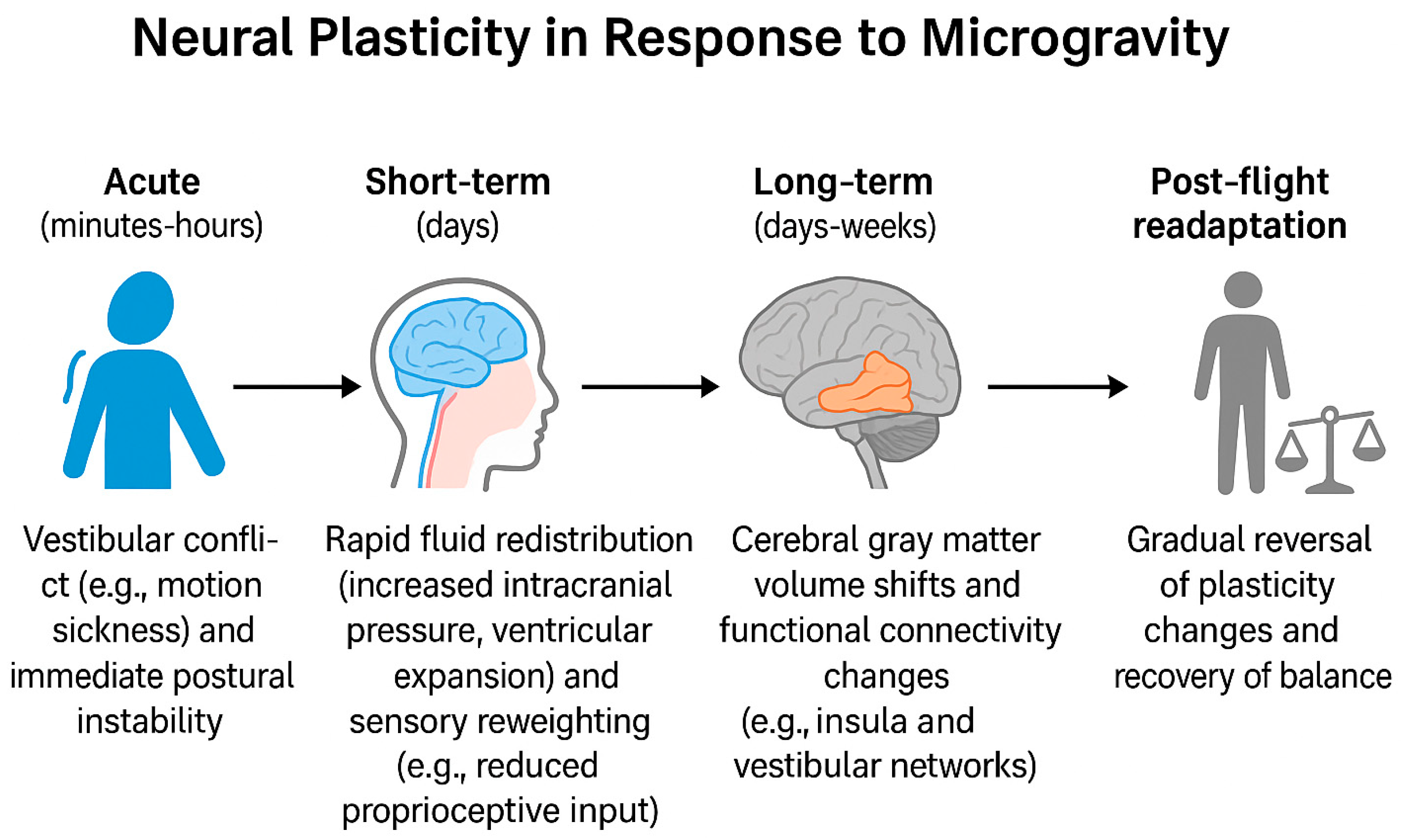
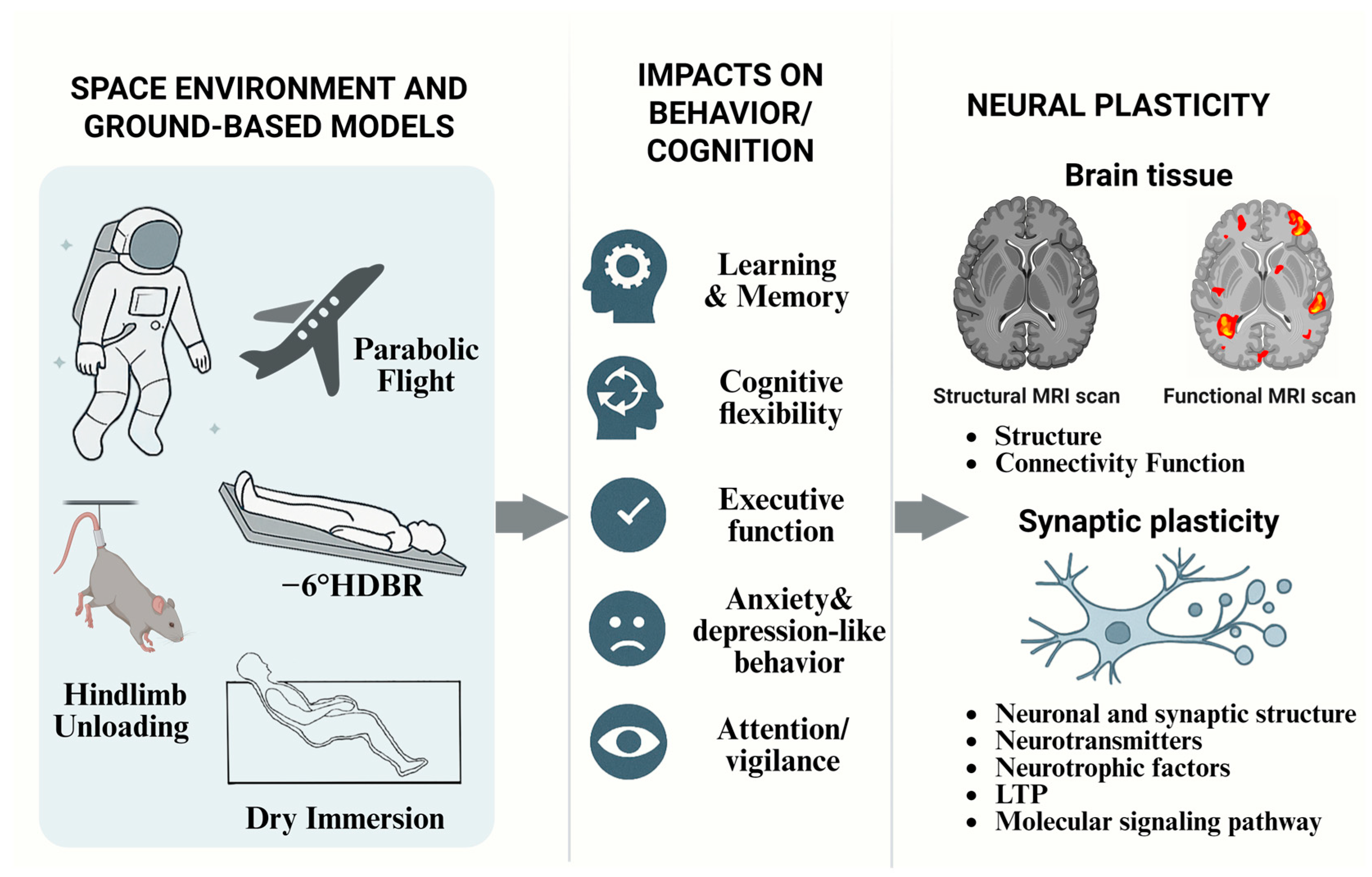
Spaceflight and Ground-Based Microgravity Simulation Impact on Cognition and Brain Plasticity
Abstract
1. Introduction
2. Impact of the Microgravity Environment on Cognitive Function
2.1. Evidence from Spaceflight Missions
2.2. Complementary Evidence from Ground-Based Analog Studies
2.3. Controversies and Future Directions
3. Current Understanding of Microgravity-Induced Effects on Neuroplasticity
3.1. Structural Changes in Brain Tissue
3.2. Synaptic Plasticity Alterations
3.2.1. Structural Synaptic Plasticity
3.2.2. Functional Synaptic Plasticity
3.2.3. Molecular Mechanisms
4. Countermeasures and Future Research Directions
4.1. Countermeasures
4.2. Future Research Directions
4.2.1. Expanding Sample Sizes and Conducting Longitudinal Studies
4.2.2. Standardization and Translational Application of Ground-Based Models
4.2.3. Dynamic Tracking of Synaptic Structural and Functional Plasticity
4.2.4. Investigating Reversibility and Intervention Strategies for Cognitive Impairment
4.2.5. Promoting Interdisciplinary Collaboration and Clinical Translation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinatolo, M.F.; Cohen, L.Y. Monitoring the Impact of Spaceflight on the Human Brain. Life 2022, 12, 1060. [Google Scholar] [CrossRef]
- Mhatre, S.D.; Iyer, J.; Puukila, S.; Paul, A.M.; Tahimic, C.G.T.; Rubinstein, L.; Lowe, M.; Alwood, J.S.; Sowa, M.B.; Bhattacharya, S.; et al. Neuro-consequences of the spaceflight environment. Neurosci. Biobehav. Rev. 2022, 132, 908–935. [Google Scholar] [CrossRef]
- Willey, J.S.; Britten, R.A.; Blaber, E.; Tahimic, C.G.T.; Chancellor, J.; Mortreux, M.; Sanford, L.D.; Kubik, A.J.; Delp, M.D.; Mao, X.W. The individual and combined effects of spaceflight radiation and microgravity on biologic systems and functional outcomes. J. Environ. Sci. Health C Toxicol. Carcinog. 2021, 39, 129–179. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.I.; Limoli, C.L.; Stark, C.E.L.; Stark, S.M. Impact of spaceflight stressors on behavior and cognition: A molecular, neurochemical, and neurobiological perspective. Neurosci. Biobehav. Rev. 2022, 138, 104676. [Google Scholar] [CrossRef]
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red risks for a journey to the red planet: The highest priority human health risks for a mission to Mars. npj Microgravity 2020, 6, 33. [Google Scholar] [CrossRef]
- Wuyts, F.L.; Deblieck, C.; Vandevoorde, C.; Durante, M. Brains in space: Impact of microgravity and cosmic radiation on the CNS during space exploration. Nat. Rev. Neurosci. 2025, 26, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Clément, G.R.; Boyle, R.D.; George, K.A.; Nelson, G.A.; Reschke, M.F.; Williams, T.J.; Paloski4, W.H. Challenges to the central nervous system during human spaceflight missions to Mars. J. Neurophysiol. 2020, 123, 2037–2063. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, J.; Fan, Q.; Zhao, S.; Wu, X.; Wang, J.; Liu, Y.; Li, Y.; Lu, W. Long-term spaceflight composite stress induces depression and cognitive impairment in astronauts—Insights from neuroplasticity. Transl. Psychiatry 2023, 13, 342. [Google Scholar] [CrossRef]
- Faerman, A.; Clark, J.B.; Sutton, J.P. Neuropsychological considerations for long-duration deep spaceflight. Front. Physiol. 2023, 14, 1146096. [Google Scholar] [CrossRef] [PubMed]
- Clement, G.; Ngo-Anh, J.T. Space physiology II: Adaptation of the central nervous system to space flight-past, current, and future studies. Eur. J. Appl. Physiol. 2013, 113, 1655–1672. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; McGregor, H.R.; Reuter-Lorenz, P.A.; Seidler, R.D. Microgravity effects on the human brain and behavior: Dysfunction and adaptive plasticity. Neurosci. Biobehav. Rev. 2021, 122, 176–189. [Google Scholar] [CrossRef]
- Kiffer, F.; Boerma, M.; Allen, A. Behavioral effects of space radiation: A comprehensive review of animal studies. Life Sci. Space Res. 2019, 21, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, R.; Xiu, L.; Chen, S.; Chen, X.; Tan, C. Effects of 45-day-6° head-down bed rest on the time-based prospective memory. Acta Astronaut. 2013, 84, 81–87. [Google Scholar] [CrossRef]
- Roberts, D.R.; Zhu, X.; Tabesh, A.; Duffy, E.W.; Ramsey, D.A.; Brown, T.R. Structural Brain Changes following Long-Term 6° Head-Down Tilt Bed Rest as an Analog for Spaceflight. Am. J. Neuroradiol. 2015, 36, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Seyedmirzaei, H.; Gharepapagh, E.; Mohagheghfard, F.; Hasankhani, Z.; Karbasi, M.; Delavari, S.; Aarabi, M.H. Effect of spaceflight experience on human brain structure, microstructure, and function: Systematic review of neuroimaging studies. Brain Imaging Behav. 2024, 18, 1256–1279. [Google Scholar] [CrossRef]
- Kramer, L.A.; Hasan, K.M.; Stenger, M.B.; Sargsyan, A.; Laurie, S.S.; Otto, C.; Ploutz-Snyder, R.J.; Marshall-Goebel, K.; Riascos, R.F.; Macias, B.R. Intracranial Effects of Microgravity: A Prospective Longitudinal MRI Study. Radiology 2020, 295, 640–648. [Google Scholar] [CrossRef]
- Albadawi, E.A. Structural and functional changes in the hippocampus induced by environmental exposures. Neurosciences 2025, 30, 5–19. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.; Lv, K.; Ji, G.; Zhang, Y.; Wang, Y.; Li, Y.; Qu, L. iTRAQ-based proteomics analysis of hippocampus in spatial memory deficiency rats induced by simulated microgravity. J. Proteom. 2017, 160, 64–73. [Google Scholar] [CrossRef]
- Xiang, S.; Zhou, Y.; Fu, J.; Zhang, T. rTMS pre-treatment effectively protects against cognitive and synaptic plasticity impairments induced by simulated microgravity in mice. Behav. Brain Res. 2019, 359, 639–647. [Google Scholar] [CrossRef]
- Wang, Y.; Javed, I.; Liu, Y.; Lu, S.; Peng, G.; Zhang, Y.; Qing, H.; Deng, Y. Effect of Prolonged Simulated Microgravity on Metabolic Proteins in Rat Hippocampus: Steps toward Safe Space Travel. J. Proteome Res. 2016, 15, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Wang, D.; Avila-Quintero, V.; Bloch, M.H.; Kaffman, A. Deficits in hippocampal-dependent memory across different rodent models of early life stress: Systematic review and meta-analysis. Transl. Psychiatry 2021, 11, 231. [Google Scholar] [CrossRef]
- Hupfeld, K.E.; McGregor, H.R.; Koppelmans, V.; Beltran, N.E.; Kofman, I.S.; De Dios, Y.E.; Riascos, R.F.; Reuter-Lorenz, P.A.; Wood, S.J.; Bloomberg, J.J.; et al. Brain and Behavioral Evidence for Reweighting of Vestibular Inputs with Long-Duration Spaceflight. Cereb. Cortex 2022, 32, 755–769. [Google Scholar] [CrossRef]
- Paloski, W.H.; Reschke, M.F.; Black, F.O.; Doxey, D.D.; Harm, D.L. Recovery of Postural Equilibrium Control Following Spaceflight. Ann. N. Y. Acad. Sci. 1992, 656, 747–754. [Google Scholar] [CrossRef]
- Clement, G.; Skinner, A.; Lathan, C. Distance and Size Perception in Astronauts during Long-Duration Spaceflight. Life 2013, 3, 524–537. [Google Scholar] [CrossRef]
- Albery, W.; Repperger, D. Time and Mass Perception in Nonterrestrial Environments. Acta Astronaut. 1992, 26, 119–126. [Google Scholar] [CrossRef]
- Salazar, A.P.; McGregor, H.R.; Hupfeld, K.E.; Beltran, N.E.; Kofman, I.S.; De Dios, Y.E.; Riascos, R.F.; Reuter-Lorenz, P.A.; Bloomberg, J.J.; Mulavara, A.P.; et al. Changes in working memory brain activity and task-based connectivity after long-duration spaceflight. Cereb. Cortex 2023, 33, 2641–2654. [Google Scholar] [CrossRef]
- Strangman, G.E.; Sipes, W.; Beven, G. Human Cognitive Performance in Spaceflight and Analogue Environments. Aviat. Space Environ. Med. 2014, 85, 1033–1048. [Google Scholar] [CrossRef]
- Tays, G.D.; Hupfeld, K.E.; McGregor, H.R.; Salazar, A.P.; De Dios, Y.E.; Beltran, N.E.; Reuter-Lorenz, P.A.; Kofman, I.S.; Wood, S.J.; Bloomberg, J.J.; et al. The Effects of Long Duration Spaceflight on Sensorimotor Control and Cognition. Front. Neural Circuits 2021, 15, 723504. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.T.; Dilda, V.; Morris, T.R.; Yungher, D.A.; MacDougall, H.G.; Wood, S.J. Long-duration spaceflight adversely affects post-landing operator proficiency. Sci. Rep. 2019, 9, 2677. [Google Scholar] [CrossRef] [PubMed]
- Parihar, V.K.; Allen, B.; Tran, K.K.; Macaraeg, T.G.; Chu, E.M.; Kwok, S.F.; Chmielewski, N.N.; Craver, B.M.; Baulch, J.E.; Acharya, M.M.; et al. What happens to your brain on the way to Mars. Sci. Adv. 2015, 1, e1400256. [Google Scholar] [CrossRef]
- Brandt, T.; Schautzer, F.; Hamilton, D.A.; Brüning, R.; Markowitsch, H.J.; Kalla, R.; Darlington, C.; Smith, P.; Strupp, M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 2005, 128, 2732–2741. [Google Scholar] [CrossRef]
- Smith, P.F. The vestibular system and cognition. Curr. Opin. Neurol. 2017, 30, 84–89. [Google Scholar] [CrossRef]
- Stahn, A.C.; Gunga, H.-C.; Kohlberg, E.; Gallinat, J.; Dinges, D.F.; Kuehn, S. Brain Changes in Response to Long Antarctic Expeditions. N. Engl. J. Med. 2019, 381, 2273–2275. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Grue, K.; Becker, M.; Elizarraras, E.; Frias, E.S.; Halvorsen, A.; Koenig-Zanoff, M.; Frattini, V.; Nimmagadda, H.; Feng, X.; et al. The impact of deep space radiation on cognitive performance: From biological sex to biomarkers to countermeasures. Sci. Adv. 2021, 7, eabg6702. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Ratri, A.; Choi, S.Y.; Tash, J.S.; Ronca, A.E.; Alwood, J.S.; Christenson, L.K. Effects of spaceflight aboard the International Space Station on mouse estrous cycle and ovarian ignore expression. npj Microgravity 2021, 7, 11. [Google Scholar] [CrossRef]
- Hargens, A.R.; Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 2016, 120, 891–903. [Google Scholar] [CrossRef]
- Kermorgant, M.; Nasr, N.; Czosnyka, M.; Arvanitis, D.N.; Helissen, O.; Senard, J.-M.; Pavy-Le Traon, A. Impacts of Microgravity Analogs to Spaceflight on Cerebral Autoregulation. Front. Physiol. 2020, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Pandiarajan, M.; Hargens, A.R. Ground-Based Analogs for Human Spaceflight. Front. Physiol. 2020, 11, 716. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Goswami, N.; Sirek, A.; Green, D.A.; Winnard, A.; Fiebig, L.; Weber, T. Systematic review of the effectiveness of standalone passive countermeasures on microgravity-induced physiologic deconditioning. npj Microgravity 2024, 10, 48. [Google Scholar] [CrossRef]
- Bringoux, L.; Blouin, J.; Coyle, T.; Ruget, H.; Mouchnino, L. Effect of gravity-like torque on goal-directed arm movements in microgravity. J. Neurophysiol. 2012, 107, 2541–2548. [Google Scholar] [CrossRef]
- Jamsek, M.; Kunavar, T.; Blohm, G.; Nozaki, D.; Papaxanthis, C.; White, O.; Babic, J. Effects of Simulated Microgravity and Hypergravity Conditions on Arm Movements in Normogravity. Front. Neural Circuits 2021, 15, 750176. [Google Scholar] [CrossRef]
- Gouvier, W.D.; Pinkston, J.B.; Lovejoy, J.C.; Smith, S.R.; Bray, G.A.; Santa Maria, M.P.; Hammer, J.H.; Hilsabeck, R.C.; Smiroldo, B.; Bentz, B.; et al. Neuropsychological and emotional changes during simulated microgravity: Effects of triiodothyronine, alendronate, and testosterone. Arch. Clin. Neuropsychol. 2004, 19, 595. [Google Scholar] [CrossRef]
- Tomilovskaya, E.; Shigueva, T.; Sayenko, D.; Rukavishnikov, I.; Kozlovskaya, I. Dry Immersion as a Ground-Based Model of Microgravity Physiological Effects. Front. Physiol. 2019, 10, 284. [Google Scholar] [CrossRef]
- Mildren, R.L.; Gomez, L.J.; Cullen, K.E. Convergence of vestibular and proprioceptive signals in the cerebellar nodulus/uvula enhances the encoding of self-motion in primates. Curr. Biol. 2025, 35, 468–482.e3. [Google Scholar] [CrossRef]
- Guillaud, E.; Faure, C.; Doat, E.; Bouyer, L.J.; Guehl, D.; Cazalets, J.-R. Ancestral persistence of vestibulospinal reflexes in axial muscles in humans. J. Neurophysiol. 2020, 123, 2010–2023. [Google Scholar] [CrossRef]
- Lecoq, P.-E.; Dupuis, C.; Mousset, X.; Benoit-Gonnin, X.; Peyrin, J.-M.; Aider, J.-L. Influence of microgravity on spontaneous calcium activity of primary hippocampal neurons grown in microfluidic chips. npj Microgravity 2024, 10, 15. [Google Scholar] [CrossRef]
- Opsomer, L.; Delhaye, B.P.; Theate, V.; Thonnard, J.-L.; Lefevre, P. A haptic illusion created by gravity. Iscience 2023, 26, 107246. [Google Scholar] [CrossRef] [PubMed]
- Carriot, J.; Bringoux, L.; Charles, C.; Mars, F.; Nougier, V.; Cian, C. Perceived body orientation in microgravity: Effects of prior experience and pressure under the feet. Aviat. Space Environ. Med. 2004, 75, 795–799. [Google Scholar] [PubMed]
- Pompeiano, O.; D’Ascanio, P.; Balaban, E.; Centini, C.; Pompeiano, M. Gene expression in autonomic areas of the medulla and the central nucleus of the amygdala in rats during and after space flight. Neuroscience 2004, 124, 53–69. [Google Scholar] [CrossRef]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C.; Potter, M.C.; van Praag, H. All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top. Behav. Neurosci. 2013, 15, 189–210. [Google Scholar] [PubMed]
- Bellone, J.A.; Gifford, P.S.; Nishiyama, N.C.; Hartman, R.E.; Mao, X.W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma radiation on behavior and blood-brain barrier integrity. npj Microgravity 2016, 2, 16019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Chen, H.; Liu, X.; Lv, K.; Wang, T.; Wang, Y.; Ji, G.; Cao, H.; Kan, G.; et al. Involvement of Cholinergic Dysfunction and Oxidative Damage in the Effects of Simulated Weightlessness on Learning and Memory in Rats. Biomed. Res. Int. 2018, 2018, 2547532. [Google Scholar] [CrossRef]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef]
- Gros, A.; Lavenu, L.; Morel, J.-L.; De Deurwaerdere, P. Simulated Microgravity Subtlety Changes Monoamine Function across the Rat Brain. Int. J. Mol. Sci. 2021, 22, 11759. [Google Scholar] [CrossRef]
- Yasuhara, T.; Hara, K.; Maki, M.; Matsukawa, N.; Fujino, H.; Date, I.; Borlongan, C.V. Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience. 2007, 149, 182–191. [Google Scholar] [CrossRef]
- Gros, A.; Furlan, F.M.; Rouglan, V.; Favereaux, A.; Bontempi, B.; Morel, J.-L. Physical exercise restores adult neurogenesis deficits induced by simulated microgravity. npj Microgravity 2024, 10, 69. [Google Scholar] [CrossRef]
- Berezovskaya, A.S.; Tyganov, S.A.; Nikolaeva, S.D.; Naumova, A.A.; Shenkman, B.S.; Glazova, M.V. Plantar Stimulations during 3-Day Hindlimb Unloading Prevent Loss of Neural Progenitors and Maintain ERK1/2 Activity in the Rat Hippocampus. Life 2021, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Temple, M.D.; Kosik, K.S.; Steward, O. Spatial learning and memory is preserved in rats after early development in a microgravity environment. Neurobiol. Learn. Mem. 2002, 78, 199–216. [Google Scholar] [CrossRef]
- Wollseiffen, P.; Klein, T.; Vogt, T.; Abeln, V.; Strueder, H.K.; Stuckenschneider, T.; Sanders, M.; Claassen, J.A.H.R.; Askew, C.D.; Carnahan, H.; et al. Neurocognitive performance is enhanced during short periods of microgravity-Part 2. Physiol. Behav. 2019, 207, 48–54. [Google Scholar] [CrossRef]
- Leach, C.S.; Johnson, P.C. Influence of Spaceflight on Erythrokinetics in Man. Science 1984, 225, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Udden, M.M.; Driscoll, T.B.; Gibson, L.A.; Patton, C.S.; Pickett, M.H.; Jones, J.B.; Nachtman, R.; Allebban, Z.; Ichiki, A.T.; Lange, R.D.; et al. Blood-Volume and Erythropoiesis in the Rat During Spaceflight. Aviat. Space Environ. Med. 1995, 66, 557–561. [Google Scholar] [PubMed]
- Demertzi, A.; Van Ombergen, A.; Tomilovskaya, E.; Jeurissen, B.; Pechenkova, E.; Di Perri, C.; Litvinova, L.; Amico, E.; Rumshiskaya, A.; Rukavishnikov, I.; et al. Cortical reorganization in an astronaut’s brain after long-duration spaceflight. Brain Struct. Funct. 2016, 221, 2873–2876, Erratum in Brain Struct. Funct. 2016, 221, 2877. [Google Scholar] [CrossRef] [PubMed]
- Van Ombergen, A.; Laureys, S.; Sunaert, S.; Tomilovskaya, E.; Parizel, P.M.; Wuyts, F.L. Spaceflight-induced neuroplasticity in humans as measured by MRI: What do we know so far? npj Microgravity 2017, 3, 2. [Google Scholar] [CrossRef]
- Liao, Y.; Lei, M.; Huang, H.; Wang, C.; Duan, J.; Li, H.; Liu, X. The time course of altered brain activity during 7-day simulated microgravity. Front. Behav. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Rao, L.-L.; Liang, Z.-Y.; Chen, X.-P.; Zheng, D.; Tan, C.; Tian, Z.-Q.; Wang, C.-H.; Bai, Y.-Q.; et al. Disrutpted resting-state functional architecture of the brain after 45-day simulated microgravity. Front. Behav. Neurosci. 2014, 8, 200. [Google Scholar] [CrossRef]
- Van Ombergen, A.; Jillings, S.; Jeurissen, B.; Tomilovskaya, E.; Rumshiskaya, A.; Litvinova, L.; Nosikova, I.; Pechenkova, E.; Rukavishnikov, I.; Manko, O.; et al. Brain ventricular volume changes induced by long-duration spaceflight. Proc. Natl. Acad. Sci. USA 2019, 116, 10531–10536. [Google Scholar] [CrossRef]
- Van Ombergen, A.; Jillings, S.; Jeurissen, B.; Tomilovskaya, E.; Ruhl, R.M.; Rumshiskaya, A.; Nosikova, I.; Litvinova, L.; Annen, J.; Pechenkova, E.V.; et al. Brain Tissue-Volume Changes in Cosmonauts. N. Engl. J. Med. 2018, 379, 1678–1680. [Google Scholar] [CrossRef]
- Rappaport, M.B.; Corbally, C.J. Neuroplasticity as a Foundation for Decision-Making in Space. NeuroSci 2022, 3, 457–475. [Google Scholar] [CrossRef]
- Pearson-Fuhrhop, K.M.; Cramer, S.C. Genetic Influences on Neural Plasticity. PM&R 2010, 2, S227–S240. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Rodriguez-Moreno, A. The Impact of Studying Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef]
- Hamaide, J.; De Groof, G.; Van der Linden, A. Neuroplasticity and MRI: A perfect match. Neuroimage 2016, 131, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Stahn, A.C.; Bucher, D.; zu Eulenburg, P.; Denise, P.; Smith, N.; Pagnini, F.; White, O. Paving the way to better understand the effects of prolonged spaceflight on operational performance and its neural bases. npj Microgravity 2023, 9, 59. [Google Scholar] [CrossRef]
- Koppelmans, V.; Bloomberg, J.J.; Mulavara, A.P.; Seidler, R.D. Brain structural plasticity with spaceflight. npj Microgravity 2016, 2, 2, Erratum in npj Microgravity 2017, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Koppelmans, V.; Riascos, R.F.; Hasan, K.M.; Pasternak, O.; Mulavara, A.P.; Bloomberg, J.J.; Seidler, R.D. Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution. JAMA Neurol. 2019, 76, 412–419. [Google Scholar] [CrossRef]
- Jillings, S.; Van Ombergen, A.; Tomilovskaya, E.; Rumshiskaya, A.; Litvinova, L.; Nosikova, I.; Pechenkova, E.; Rukavishnikov, I.; Kozlovskaya, I.B.; Manko, O.; et al. Macro- and microstructural changes in cosmonauts’ brains after long-duration spaceflight. Sci. Adv. 2020, 6, eaaz9488. [Google Scholar] [CrossRef]
- Besnard, S.; Machado, M.L.; Vignaux, G.; Boulouard, M.; Coquerel, A.; Bouet, V.; Freret, T.; Denise, P.; Lelong-Boulouard, V. Influence of vestibular input on spatial and nonspatial memory and on hippocampal NMDA receptors. Hippocampus 2012, 22, 814–826. [Google Scholar] [CrossRef]
- Roberts, D.R.; Albrecht, M.H.; Collins, H.R.; Asemani, D.; Chatterjee, A.R.; Spampinato, M.V.; Zhu, X.; Chimowitz, M.I.; Antonucci, M.U. Effects of Spaceflight on Astronaut Brain Structure as Indicated on MRI. N. Engl. J. Med. 2017, 377, 1746–1753. [Google Scholar] [CrossRef]
- Seidler, R.D.; Mao, X.W.; Tays, G.D.; Wang, T.; zu Eulenburg, P. Effects of spaceflight on the brain. Lancet Neurol. 2024, 23, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; McGregor, H.R.; Lee, J.K.; Beltran, N.E.; Kofman, I.S.; De Dios, Y.E.; Reuter-Lorenz, P.A.; Riascos, R.F.; Pasternak, O.; Wood, S.J.; et al. The Impact of 6 and 12 Months in Space on Human Brain Structure and Intracranial Fluid Shifts. Cereb. Cortex Commun. 2020, 1, tgaa023. [Google Scholar] [CrossRef]
- McGregor, H.R.; Hupfeld, K.E.; Pasternak, O.; Beltran, N.E.; De Dios, Y.E.; Bloomberg, J.J.; Wood, S.J.; Mulavara, A.P.; Riascos, R.F.; Reuter-Lorenz, P.A.; et al. Impacts of spaceflight experience on human brain structure. Sci. Rep. 2023, 13, 7878. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, J.; Huang, Z.; Xi, Y.; Zhang, Q.; Zhu, T.; Liu, X. Altered Baseline Brain Activity with 72 h of Simulated Microgravity—Initial Evidence from Resting-State fMRI. PLoS ONE 2012, 7, e52558. [Google Scholar] [CrossRef]
- Riascos, R.F.; Kamali, A.; Hakimelahi, R.; Mwangi, B.; Rabiei, P.; Seidler, R.D.; Behzad, B.B.; Keser, Z.; Kramer, L.A.; Hasan, K.M. Longitudinal Analysis of Quantitative Brain MRI in Astronauts Following Microgravity Exposure. J. Neuroimaging 2019, 29, 323–330. [Google Scholar] [CrossRef]
- Cassady, K.; Koppelmans, V.; Reuter-Lorenz, P.; De Dios, Y.; Gadd, N.; Wood, S.; Castenada, R.R.; Kofman, I.; Bloomberg, J.; Mulavara, A.; et al. Effects of a spaceflight analog environment on brain connectivity and behavior. Neuroimage 2016, 141, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Guo, X.; Jin, Z.; Ouyang, X.; Zeng, Y.; Feng, J.; Wang, Y.; Yao, L.; Ma, L. Effect of Simulated Microgravity on Human Brain Gray Matter and White Matter—Evidence from MRI. PLoS ONE 2015, 10, e0135835. [Google Scholar] [CrossRef] [PubMed]
- Mann, V.; Sundaresan, A.; Chaganti, M. Cellular changes in the nervous system when exposed to gravitational variation. Neurol. India 2019, 67, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Knierim, J.J.; McNaughton, B.L.; Poe, G.R. Three-dimensional spatial selectivity of hippocampal neurons during space flight. Nat. Neurosci. 2000, 3, 209–210. [Google Scholar] [CrossRef]
- DeFelipe, J.; Arellano, J.I.; Merchán-Pérez, A.; González-Albo, M.C.; Walton, K.; Llinás, R. Spaceflight induces changes in the synaptic circuitry of the postnatal developing neocortex. Cereb. Cortex 2002, 12, 883–891. [Google Scholar] [CrossRef]
- Holstein, G.R.; Kukielka, E.; Martinelli, G.P. Anatomical observations of the rat cerebellar nodulus after 24 hr of spaceflight. J. Gravit. Physiol. A J. Int. Soc. Gravit. Physiol. 1999, 6, P47–P50. [Google Scholar]
- Anken, R.H.; Ibsch, M.; Rahmann, H. Microgravity (STS-90 Neurolab-Mission) influences synapse formation in a vestibular nucleus of fish brain. In Space Life Sciences: Biological Research and Space Radiation; Ijiri, K., Slenzka, K., Kronenberg, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 30, pp. 843–847. [Google Scholar]
- D’Iachkova, L.N. Ultrastructural changes in somatosensory cortex of albino rats during space flight. Izv. Akad. Nauk. Seriia Biol. 2007, 34, 372–375. [Google Scholar] [CrossRef]
- Ranjan, A.; Behari, J.; Mallick, B.N. Cytomorphometric changes in hippocampal CA1 neurons exposed to simulated microgravity using rats as model. Front. Neurol. 2014, 5, 77. [Google Scholar] [CrossRef]
- Brungs, S.; Liemersdorf, C.; Lichterfeld, Y.; Frett, T.; Anken, R.; Jordan, J.; Hemmersbach, R. Hypergravity selectively augments neuronal in vitro differentiation. FASEB J. 2018, 32, 897.1. [Google Scholar] [CrossRef]
- Chomienne, L.; Sainton, P.; Sarlegna, F.R.; Bringoux, L. Hypergravity is more challenging than microgravity for the human sensorimotor system. npj Microgravity 2025, 11, 2. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Q.; Wang, H.; Peng, Y.; Wang, L.; Lu, Y.; Shi, T.; Xiong, L. Electroacupuncture pretreatment ameliorates hypergravity-induced impairment of learning and memory and apoptosis of hippocampal neurons in rats. Neurosci. Lett. 2010, 478, 150–155. [Google Scholar] [CrossRef]
- Mitani, K.; Horii, A.; Kubo, T. Impaired spatial learning after hypergravity exposure in rats. Cogn. Brain Res. 2004, 22, 94–100. [Google Scholar] [CrossRef]
- Kulikova, E.A.; Kulikov, V.A.; Sinyakova, N.A.; Kulikov, A.V.; Popova, N.K. The effect of long-term hindlimb unloading on the expression of risk neurogenes encoding elements of serotonin-, dopaminergic systems and apoptosis; comparison with the effect of actual spaceflight on mouse brain. Neurosci. Lett. 2017, 640, 88–92. [Google Scholar] [CrossRef]
- Popova, N.K.; Kulikov, A.V.; Naumenko, V.S. Spaceflight and brain plasticity: Spaceflight effects on regional expression of neurotransmitter systems and neurotrophic factors encoding genes. Neurosci. Biobehav. Rev. 2020, 119, 396–405. [Google Scholar] [CrossRef]
- Kvetnansky, R.; Culman, J.; Serova, L.V.; Tigranjan, R.A.; Torda, T.; Macho, L. Catecholamines and their enzymes in discrete brain-areas of rats after space-flight on biosatellites cosmos. Acta Astronaut. 1983, 10, 295–300. [Google Scholar] [CrossRef]
- Popova, N.K.; Kulikov, A.V.; Kondaurova, E.M.; Tsybko, A.S.; Kulikova, E.A.; Krasnov, I.B.; Shenkman, B.S.; Bazhenova, E.Y.; Sinyakova, N.A.; Naumenko, V.S. Risk Neurogenes for Long-Term Spaceflight: Dopamine and Serotonin Brain System. Mol. Neurobiol. 2015, 51, 1443–1451. [Google Scholar] [CrossRef]
- Culman, J.; Kvetnansky, T.; Serova, L.V.; Tigranjan, R.A.; Macho, L. Serotonin in individual hypothalamic nuclei of rats after space-flight on biosatellite cosmos-1129. Acta Astronaut. 1985, 12, 373–376. [Google Scholar] [CrossRef]
- Zhu, X.G.; Desiderio, D.M. Effects of space might stress on proopiomelanocortin, proenkephalin-a, and tachykinin neuropeptidergic systems in the rat posterior pituitary. Life Sci. 1994, 55, 347–350. [Google Scholar] [CrossRef]
- Nday, C.M.; Frantzidis, C.; Jackson, G.; Bamidis, P.; Kourtidou-Papadeli, C. Neurophysiological changes in simulated microgravity: An animal model. Neurol. India 2019, 67, 221–226. [Google Scholar] [CrossRef]
- Min, R.; Chen, Z.X.; Wang, Y.; Deng, Z.X.; Zhang, Y.Q.; Deng, Y.L. Quantitative proteomic analysis of cortex in the depressive-like behavior of rats induced by the simulated complex space environment. J. Proteom. 2021, 237, 104144. [Google Scholar] [CrossRef]
- Benarroch, E.E. Brain-derived neurotrophic factor Regulation, effects, and potential clinical relevance. Neurology 2015, 84, 1693–1704. [Google Scholar] [CrossRef]
- Gibon, J.; Barker, P.A. Neurotrophins and Proneurotrophins: Focus on Synaptic Activity and Plasticity in the Brain. Neuroscientist 2017, 23, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.S.; Kulikov, A.V.; Kondaurova, E.M.; Tsybko, A.S.; Kulikova, E.A.; Krasnov, I.B.; Shenkman, B.S.; Sychev, V.N.; Bazhenova, E.Y.; Sinyakova, N.A.; et al. Effect of actual long-term spaceflight on BDNF, TRKB, P75, BAX and BCL-XL genes expression in mouse brain regions. Neuroscience 2015, 284, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Santucci, D.; Kawano, F.; Ohira, T.; Terada, M.; Nakai, N.; Francia, N.; Alleva, E.; Aloe, L.; Ochiai, T.; Cancedda, R.; et al. Evaluation of Gene, Protein and Neurotrophin Expression in the Brain of Mice Exposed to Space Environment for 91 Days. PLoS ONE 2012, 7, e40112. [Google Scholar] [CrossRef]
- Angeloni, D.; Demontis, G.C. Endocrine adaptations across physical and psychological stressors in long-term space flights. Curr. Opin. Endocr. Metab. Res. 2020, 11, 26. [Google Scholar] [CrossRef]
- Maurice, N.; Roussel, B.; Mehier, H.; Gauquelin, G.; Gharib, C. Relationship between hormones and brain water content measured by 1H magnetic resonance spectroscopy during simulated weightlessness in man. Physiol. 1990, 33 (Suppl. 1), S104–S105. [Google Scholar]
- Nicoll, R.A. A Brief History of Long-Term Potentiation. Neuron 2017, 93, 281–290. [Google Scholar] [CrossRef]
- Porte, Y.; Morel, J.-L. Learning on Jupiter, learning on the Moon: The dark side of the G-force. Effects of gravity changes on neurovascular unit and modulation of learning and memory. Front. Behav. Neurosci. 2012, 6, 64. [Google Scholar] [CrossRef]
- Sun, X.-Q.; Xu, Z.-P.; Zhang, S.; Cao, X.-S.; Liu, T.-S. Simulated weightlessness aggravates hypergravity-induced impairment of learning and memory and neuronal apoptosis in rats. Behav. Brain Res. 2009, 199, 197–202. [Google Scholar] [CrossRef]
- Liang, R.; Wang, L.; Yang, Q.; Xu, Q.; Sun, S.F.; Zhou, H.C.; Zhao, M.L.; Gao, J.; Zheng, C.G.; Yang, J.J.; et al. Time-course adaptive changes in hippocampal transcriptome and synaptic function induced by simulated microgravity associated with cognition. Front. Cell. Neurosci. 2023, 17, 1275771. [Google Scholar] [CrossRef]
- Zhai, B.H.; Fu, J.X.; Xiang, S.T.; Shang, Y.C.; Yan, Y.X.; Yin, T.; Zhang, T. Repetitive transcranial magnetic stimulation ameliorates recognition memory impairment induced by hindlimb unloading in mice associated with BDNF/TrkB signaling. Neurosci. Res. 2020, 153, 40–47. [Google Scholar] [CrossRef]
- Wu, X.; Yin, Y.; Liu, J.; Zhu, Y.; Fan, Q.; Zhao, S.; Wang, J.; Gao, J.; Liu, Y.; Jiao, L.; et al. Baoyuan jieyu formula ameliorates depression-like behaviour in rats induced by simulated long-term spaceflight composite stress through regulating MAPK and BDNF pathways. Life Sci. Space Res. 2021, 31, 34–42. [Google Scholar]
- Wu, X.; Li, D.; Liu, J.; Diao, L.; Ling, S.; Li, Y.; Gao, J.; Fan, Q.; Sun, W.; Li, Q.; et al. Dammarane Sapogenins Ameliorates Neurocognitive Functional Impairment Induced by Simulated Long-Duration Spaceflight. Front. Pharmacol. 2017, 8, 315. [Google Scholar] [CrossRef]
- Frigeri, A.; Iacobas, D.A.; Iacobas, S.; Nicchia, G.P.; Desaphy, J.F.; Camerino, D.C.; Svelto, M.; Spray, D.C. Effect of microgravity on gene expression in mouse brain. Exp. Brain Res. 2008, 191, 289–300. [Google Scholar] [CrossRef]
- Chen, H.; Lv, K.; Dai, Z.; Ji, G.; Wang, T.; Wang, Y.; Zhang, Y.; Kan, G.; Li, Y.; Qu, L. Intramuscular injection of mechano growth factor E domain peptide regulated expression of memory-related sod, miR-134 and miR-125b-3p in rat hippocampus under simulated weightlessness. Biotechnol. Lett. 2016, 38, 2071–2080. [Google Scholar] [CrossRef]
- McGregor, H.R.; Lee, J.K.; Mulder, E.R.; Dios, Y.E.D.; Beltran, N.E.; Wood, S.J.; Bloomberg, J.J.; Mulavara, A.P.; Seidler, R.D. Artificial gravity during a spaceflight analog alters brain sensory connectivity. Neuroimage 2023, 278, 120261. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Chang, J.; Deng, Y. Spaceflight and Ground-Based Microgravity Simulation Impact on Cognition and Brain Plasticity. Int. J. Mol. Sci. 2025, 26, 9521. https://doi.org/10.3390/ijms26199521
Hao J, Chang J, Deng Y. Spaceflight and Ground-Based Microgravity Simulation Impact on Cognition and Brain Plasticity. International Journal of Molecular Sciences. 2025; 26(19):9521. https://doi.org/10.3390/ijms26199521
Chicago/Turabian StyleHao, Jiaqi, Jun Chang, and Yulin Deng. 2025. "Spaceflight and Ground-Based Microgravity Simulation Impact on Cognition and Brain Plasticity" International Journal of Molecular Sciences 26, no. 19: 9521. https://doi.org/10.3390/ijms26199521
APA StyleHao, J., Chang, J., & Deng, Y. (2025). Spaceflight and Ground-Based Microgravity Simulation Impact on Cognition and Brain Plasticity. International Journal of Molecular Sciences, 26(19), 9521. https://doi.org/10.3390/ijms26199521