Brassica Extracts Prevent Benzo(a)pyrene-Induced Transformation by Modulating Reactive Oxygen Species and Autophagy
Abstract
1. Introduction
2. Results
2.1. Identification and Quantitative Analysis of BSE and RCA Extracts
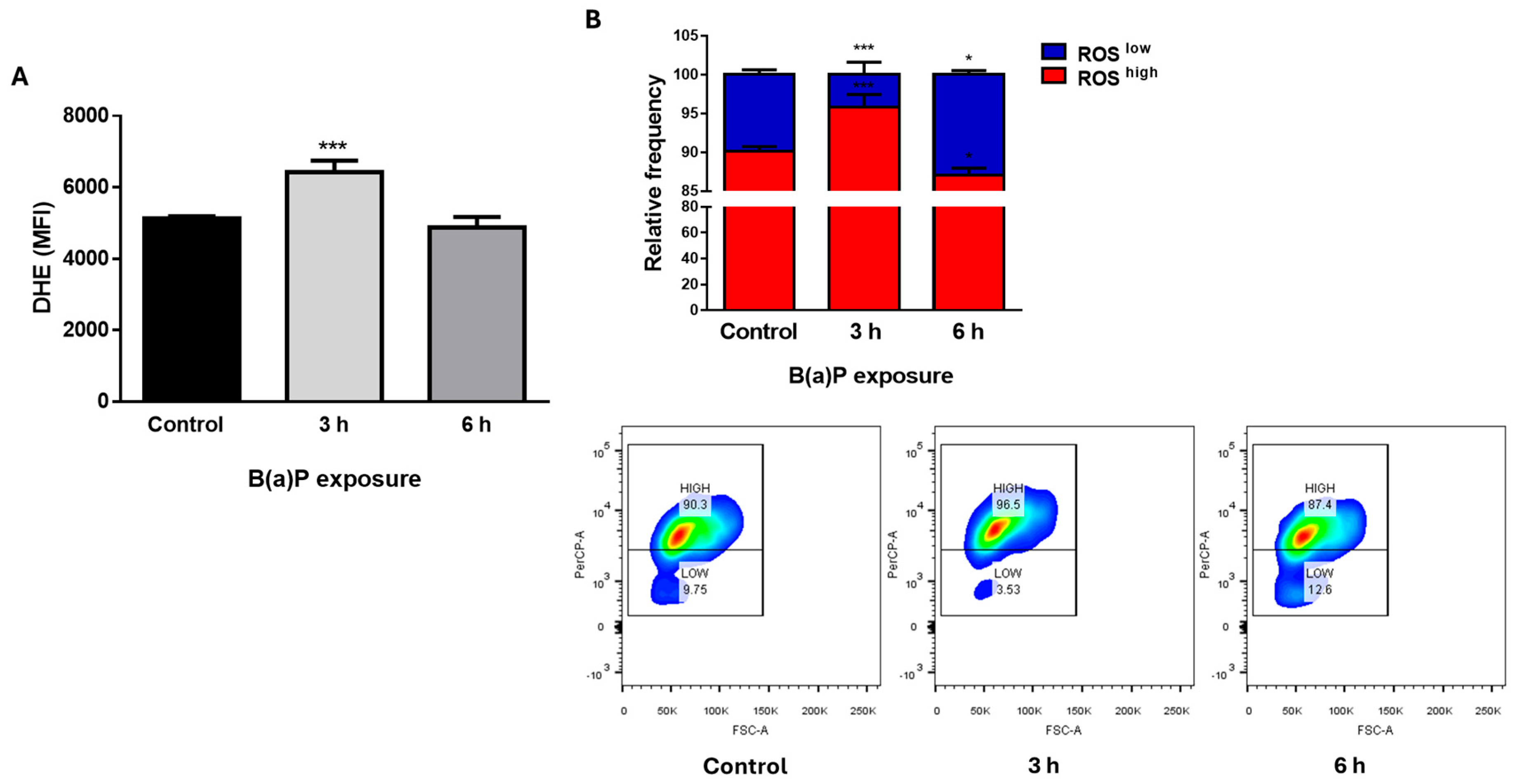
2.2. Benzo(a)pyrene (B(a)P) Induced ROS Production in Mammary Epithelial Cells
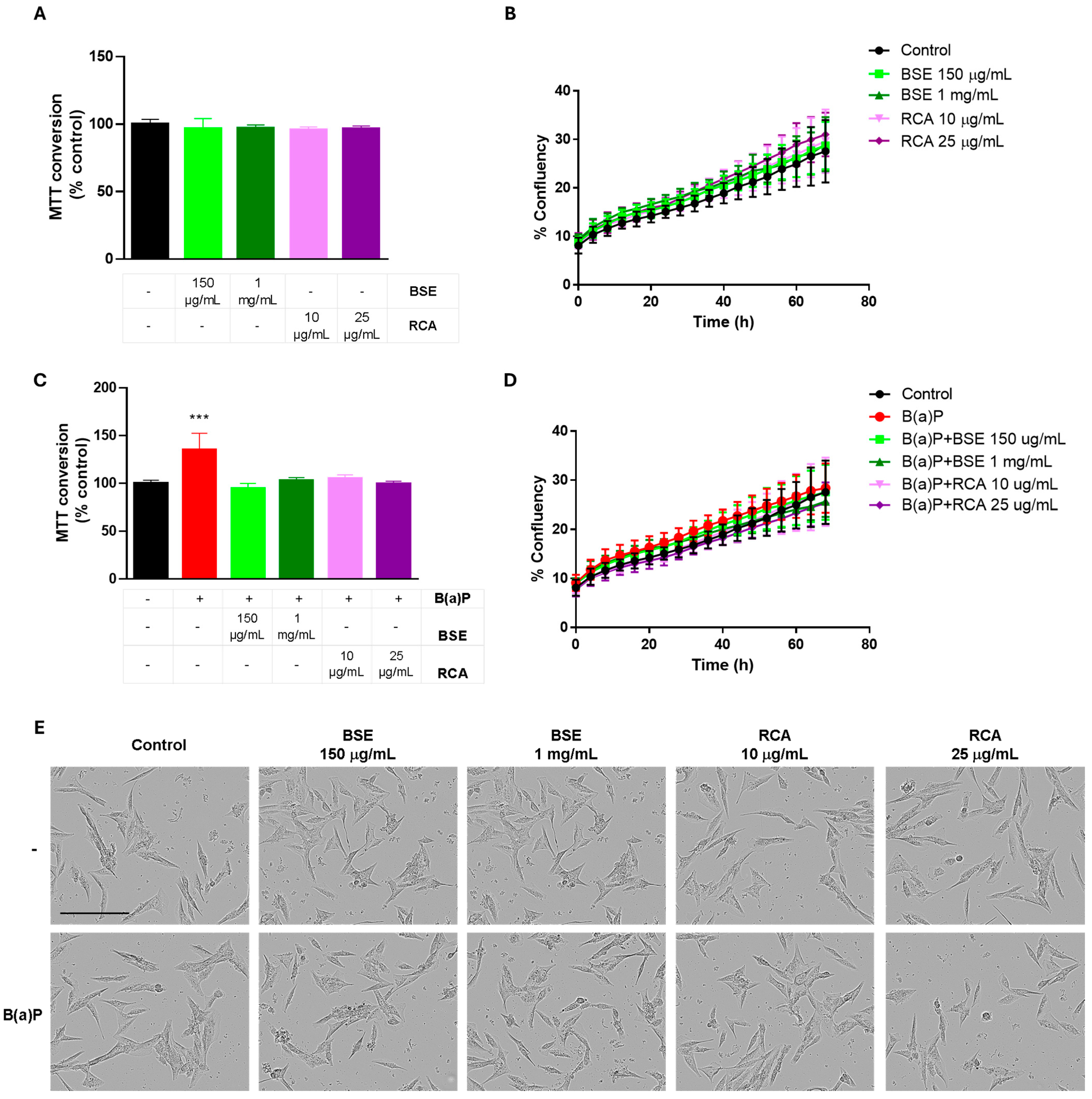
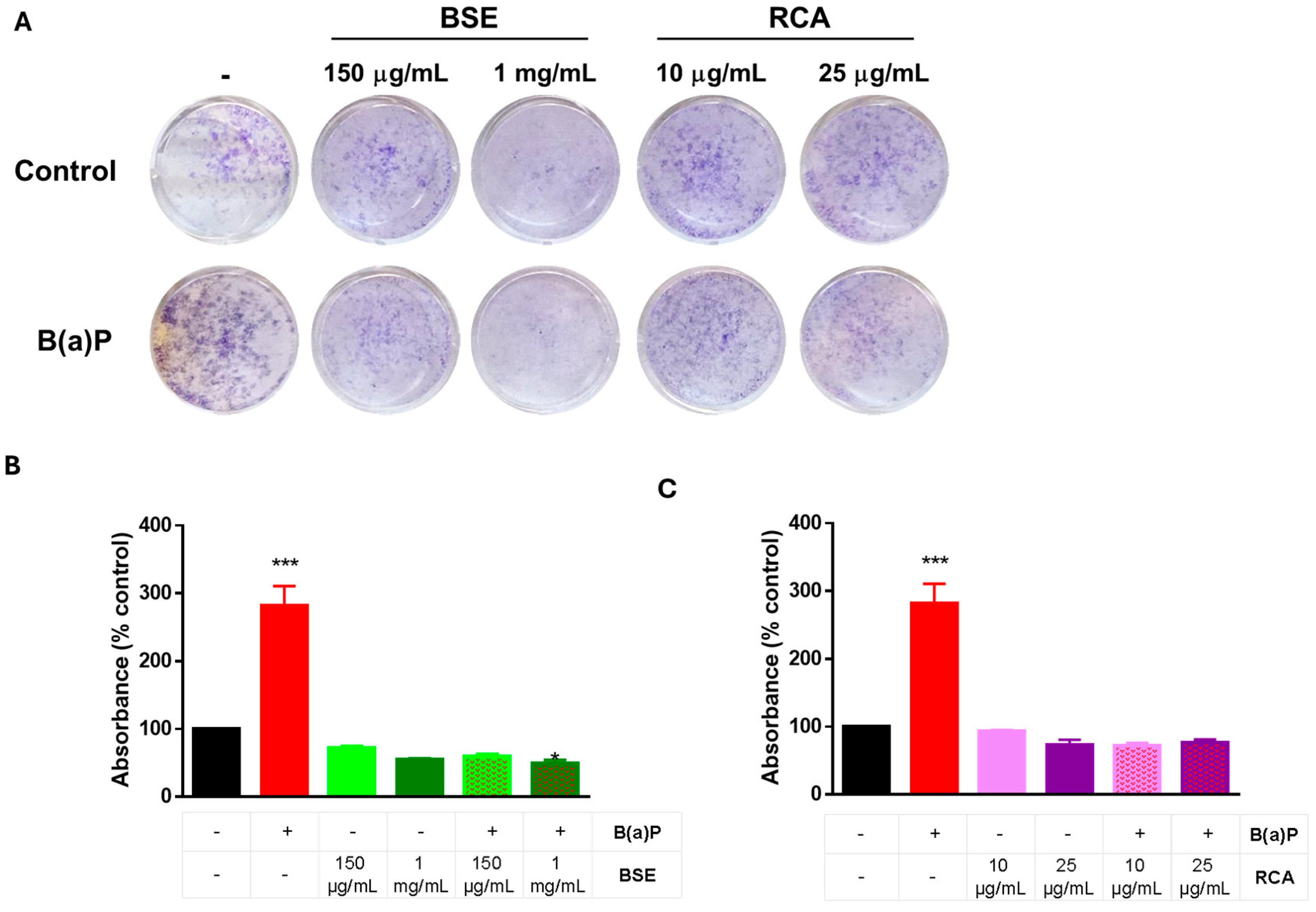
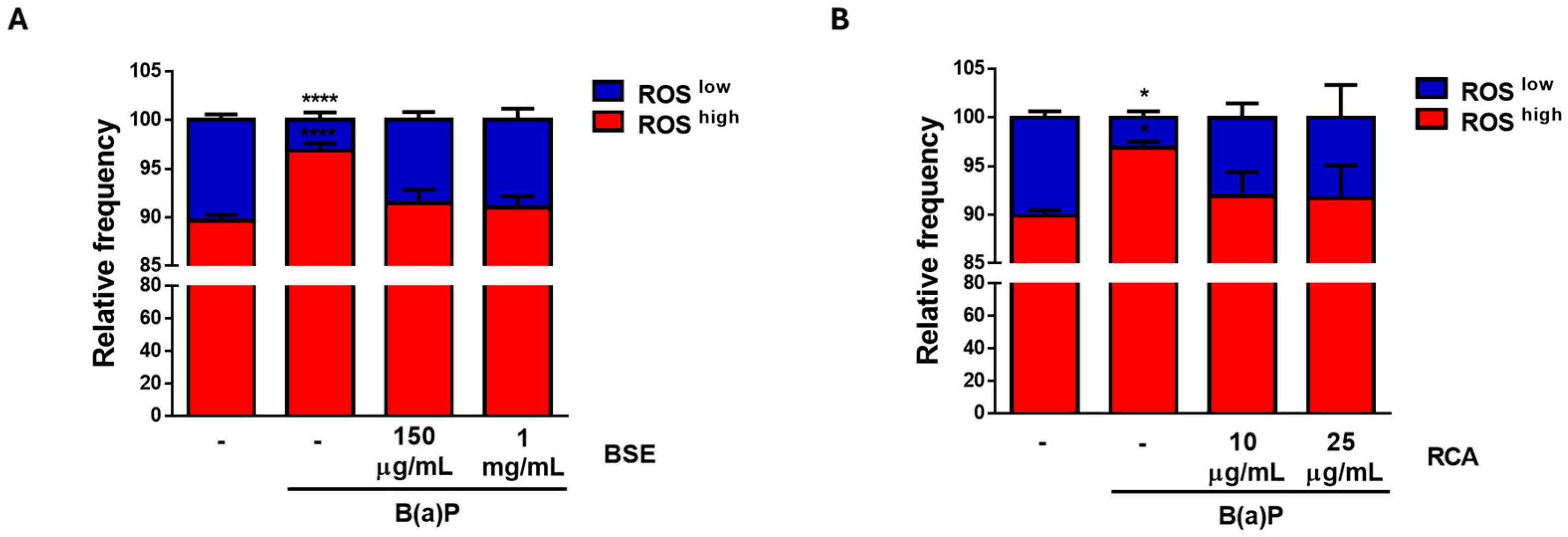
2.3. BSE and RCA Extracts Decreased B(a)P-Induced Proliferation and ROS Production
2.4. RCA Extracts Induced Autophagy in Mammary Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Broccoli Sprout Extract (BSE) and GSL-Hydrolysis Product Determination
4.2. Aqueous Red Cabbage (Brassica oleracea L. var capitata f. rubra) Extract (RCA) and GSL-Hydrolysis Product Determination
4.3. Cell Culture and Cell Viability Evaluation
4.4. Cell Viability
4.5. Clonogenic Assay
4.6. Reactive Oxygen Species (ROS) Determination
4.7. Western Blot
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GSLs | glucosinolates |
| ITCs | isothiocyanates |
| B(a)P | benzo(a)pyrene |
| ROS | Reactive Oxygen Species |
| BSE | broccoli sprout extract |
| RCA | red cabbage aqueous extract |
| TN | triple negative |
| PFAS | per- and polyfluorinated alkyl substances |
| HCAs | heterocyclic amines |
| PAHs | polycyclic aromatic hydrocarbons |
| CQ | chloroquine |
References
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a natural chemical arsenal: More to tell than the myrosinase story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Núñez-Gómez, V.; Baenas, N.; Di Pede, G.; Achour, M.; Manach, C.; Mena, P.; Del Rio, D.; García-Viguera, C.; Moreno, D.A.; et al. Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health. Nutrients 2023, 15, 1424. [Google Scholar] [CrossRef]
- Maycotte, P.; Illanes, M.; Moreno, D.A. Glucosinolates, isothiocyanates, and their role in the regulation of autophagy and cellular function. Phytochem. Rev. 2024, 24, 49–83. [Google Scholar] [CrossRef]
- Arora, R. Glucosinolate Hydrolytic Products-A Multi-Arm Warrior. J. AOAC Int. 2024, 107, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Yang, R.; Li, Y.; Wang, H.; Qin, T.; Yin, X.; Ma, X. Therapeutic progress and challenges for triple negative breast cancer: Targeted therapy and immunotherapy. Mol. Biomed. 2022, 3, 8. [Google Scholar] [CrossRef]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef]
- Maycotte, P.; Medina-Benítez, D.; Ramírez-Torres, N.; López-Muñoz, E.; Mendoza-García, A.V.; Cortés-Hernández, P.; Anaya-Ruiz, M. Molecular diagnosis of breast cancer: Prognostic and therapeutic implications. Rev. Med. Inst. Mex. Seguro Soc. 2020, 58, S62–S74. [Google Scholar] [CrossRef] [PubMed]
- Singletary, S.E. Rating the risk factors for breast cancer. Ann. Surg. 2003, 237, 474–482. [Google Scholar] [CrossRef]
- Stordal, B.; Harvie, M.; Antoniou, M.N.; Bellingham, M.; Chan, D.S.M.; Darbre, P.; Karlsson, O.; Kortenkamp, A.; Magee, P.; Mandriota, S.; et al. Breast cancer risk and prevention in 2024: An overview from the Breast Cancer UK-Breast Cancer Prevention Conference. Cancer Med. 2024, 13, e70255. [Google Scholar] [CrossRef]
- Gabriel, E.M.; Jatoi, I. Breast cancer chemoprevention. Expert Rev. Anticancer Ther. 2012, 12, 223–228. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Dong, Q.; Liu, L.; Wei, Q. Red and processed meat consumption and cancer outcomes: Umbrella review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef]
- Sivasubramanian, B.P.; Dave, M.; Panchal, V.; Saifa-Bonsu, J.; Konka, S.; Noei, F.; Nagaraj, S.; Terpari, U.; Savani, P.; Vekaria, P.H.; et al. Comprehensive Review of Red Meat Consumption and the Risk of Cancer. Cureus 2023, 15, e45324. [Google Scholar] [CrossRef]
- Nkrumah-Elie, Y.M.; Reuben, J.S.; Hudson, A.; Taka, E.; Badisa, R.; Ardley, T.; Israel, B.; Sadrud-Din, S.Y.; Oriaku, E.; Darling-Reed, S.F. Diallyl trisulfide as an inhibitor of benzo(a)pyrene-induced precancerous carcinogenesis in MCF-10A cells. Food Chem. Toxicol. 2012, 50, 2524–2530. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Chen, H.; Xu, P.; Xue, H.; Yuan, J. Dynamic changes of DNA methylation induced by benzo(a)pyrene in cancer. Genes Environ. 2023, 45, 21. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Guzman, X.; Alvarez-Dominguez, L.; Ramirez-Torres, M.F.; Montes-Alvarado, J.B.; Garcia-Ibanez, P.; Moreno, D.A.; Dominguez, F.; Maycotte, P. Cruciferous Plant Extracts, Their Isothyocianate or Indol Derivatives, and Their Effect on Cellular Viability of Breast Cancer Cell Lines. J. Med. Food 2024, 27, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Siangcham, T.; Vivithanaporn, P.; Jantakee, K.; Ruangtong, J.; Thongsepee, N.; Martviset, P.; Chantree, P.; Sornchuer, P.; Sangpairoj, K. Impact of Benzo(a)pyrene and Pyrene Exposure on Activating Autophagy and Correlation with Endoplasmic Reticulum Stress in Human Astrocytes. Int. J. Mol. Sci. 2025, 26, 1748. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Qi, T.; Gao, R.; Xie, H.; Ruan, L.; He, J.; Li, F.; Liu, T.; Xu, H.; et al. Benzo(a)pyrene promotes autophagy to impair endometrial decidualization via inhibiting CXCL12/CXCR4 axis. Chem. Biol. Interact. 2025, 405, 111288. [Google Scholar] [CrossRef]
- Bukowska, B.; Sicinska, P. Influence of Benzo(a)pyrene on Different Epigenetic Processes. Int. J. Mol. Sci. 2021, 22, 13453. [Google Scholar] [CrossRef]
- Sigounas, G.; Hairr, J.W.; Cooke, C.D.; Owen, J.R.; Asch, A.S.; Weidner, D.A.; Wiley, J.E. Role of benzo[alpha]pyrene in generation of clustered DNA damage in human breast tissue. Free Radic. Biol. Med. 2010, 49, 77–87. [Google Scholar] [CrossRef]
- Nkrumah-Elie, Y.M.; Reuben, J.S.; Hudson, A.M.; Taka, E.; Badisa, R.; Ardley, T.; Israel, B.E.; Sadrud-Din, S.Y.; Oriaku, E.T.; Darling-Reed, S.F. The Attenuation of Early Benzo(a)Pyrene-Induced Carcinogenic Insults by Diallyl Disulfide (DADS) in MCF-10A Cells. Nutr. Cancer 2012, 64, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Venturelli, M.; Mastrodomenico, L.; Piombino, C.; Ponzoni, O.; Zaniboni, S.; Barban, S.; Razzaboni, E.; Grandi, G.; Dominici, M.; et al. Chemoprevention strategies in hereditary breast and ovarian cancer syndromes. Tumori 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.M. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol. Biol. 1998, 79, 179–183. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Trevino, S.; Rosas-Murrieta, N.H.; Millan-Perez-Pena, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Quirante-Moya, S.; García-Ibañez, P.; Quirante-Moya, F.; Villaño, D.; Moreno, D.A. The Role of Brassica Bioactives on Human Health: Are We Studying It the Right Way? Molecules 2020, 25, 1591. [Google Scholar] [CrossRef]
- Miller, D.R.; Thorburn, A. Autophagy and organelle homeostasis in cancer. Dev. Cell 2021, 56, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef]
- Statilko, O.; Tsiaka, T.; Sinanoglou, V.J.; Strati, I.F. Overview of Phytochemical Composition of Brassica oleraceae var. capitata Cultivars. Foods 2024, 13, 3395. [Google Scholar]
- Lee, M.G.; Hong, H.J.; Nam, K.S. Anthocyanin Oligomers Induce Apoptosis and Autophagy by Inhibiting the mTOR Signaling Pathway in Human Breast Cancer Cells. Pharmaceuticals 2023, 17, 24. [Google Scholar] [CrossRef]
- Asif Ali, M.; Khan, N.; Kaleem, N.; Ahmad, W.; Alharethi, S.H.; Alharbi, B.; Alhassan, H.H.; Al-Enazi, M.M.; Razis, A.F.A.; Modu, B.; et al. Anticancer properties of sulforaphane: Current insights at the molecular level. Front. Oncol. 2023, 13, 1168321. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Bueno-Solano, C.; Martínez-Ibarra, D.M.; Camacho-Gil, F.; Villa-Lerma, A.G.; Rodríguez-Núñez, J.R.; Lóez-Cervantes, J.; Sánchez-Machado, D.I. Sulforaphane (1-isothiocyanato-4-(methylsulfinyl)-butane) content in cruciferous vegetables. Arch. Latinoam. Nutr. 2009, 59, 95–100. [Google Scholar]
- Baenas-Navarro, N.; Moreno-Fernández, D.A.; García-Viguera, C. Estudio de la bioactividad in vitro e in vivo de brotes de brócoli ricos en glucosinolatos/isotiocianatos. Nereis Rev. Iberoam. Interdiscip. Métodos Model. Y Simulación 2018, 10, 69–78. [Google Scholar]
- Dominguez-Perles, R.; Medina, S.; Moreno, D.; García-Viguera, C.; Ferreres, F.; Gil-Izquierdo, Á. A new ultra-rapid UHPLC/MS/MS method for assessing glucoraphanin and sulforaphane bioavailability in human urine. Food Chem. 2014, 143, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ibañez, P.; Núñez-Sánchez, M.A.; Oliva-Bolarín, A.; Martínez-Sánchez, M.A.; Ramos-Molina, B.; Ruiz-Alcaraz, A.J.; Moreno, D.A. Anti-inflammatory potential of digested Brassica sprout extracts in human macrophage-like HL-60 cells. Food Funct. 2023, 14, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ibañez, P.; Roses, C.; Agudelo, A.; Milagro, F.I.; Barceló, A.M.; Viadel, B.; Nieto, J.A.; Moreno, D.A.; Carvajal, M. The Influence of Red Cabbage Extract Nanoencapsulated with Brassica Plasma Membrane Vesicles on the Gut Microbiome of Obese Volunteers. Foods 2021, 10, 1038. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Delgado-Magallon, A.; Montes-Alvarado, J.B.; Ramirez-Ramirez, D.; Flores-Alonso, J.C.; Cortes-Hernandez, P.; Reyes-Leyva, J.; Herrera-Camacho, I.; Anaya-Ruiz, M.; Pelayo, R.; et al. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef]

| Source | Type of Extract | Metabolite | 150 μg/mL | 1 mg/mL |
| Broccoli sprouts (Brassica oleracea var. italica) | Aqueous (BSE) | SFN | 804 nM | 5360 nM |
| IB | 23.9 nM | 159.2 nM | ||
| I3C | 13.25 nM | 88.3 nM | ||
| Source | Type of Extract | Metabolite | 10 μg/mL | 25 μg/mL |
| Red cabbage (Brassica oleracea L. var. capitata f. rubra) leaf extracts | Aqueous (RCA) | SFN | 210 nM | 525 nM |
| IB | 39.9 nM | 99.8 nM | ||
| I3C | 112.5 nM | 281.3 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Alvarado, J.B.; Garcia-Ibañez, P.; Moreno, D.A.; Sarmiento-Salinas, F.L.; Susano-Hernández, X.E.; Larrauri-Rodríguez, K.A.; García-Hernández, F.J.; Milflores-Flores, L.; Domínguez, F.; Maycotte, P. Brassica Extracts Prevent Benzo(a)pyrene-Induced Transformation by Modulating Reactive Oxygen Species and Autophagy. Int. J. Mol. Sci. 2025, 26, 9519. https://doi.org/10.3390/ijms26199519
Montes-Alvarado JB, Garcia-Ibañez P, Moreno DA, Sarmiento-Salinas FL, Susano-Hernández XE, Larrauri-Rodríguez KA, García-Hernández FJ, Milflores-Flores L, Domínguez F, Maycotte P. Brassica Extracts Prevent Benzo(a)pyrene-Induced Transformation by Modulating Reactive Oxygen Species and Autophagy. International Journal of Molecular Sciences. 2025; 26(19):9519. https://doi.org/10.3390/ijms26199519
Chicago/Turabian StyleMontes-Alvarado, José Benito, Paula Garcia-Ibañez, Diego A. Moreno, Fabiola Lilí Sarmiento-Salinas, Xiadani Edén Susano-Hernández, Karen Andrea Larrauri-Rodríguez, Francisco Jesús García-Hernández, Lorena Milflores-Flores, Fabiola Domínguez, and Paola Maycotte. 2025. "Brassica Extracts Prevent Benzo(a)pyrene-Induced Transformation by Modulating Reactive Oxygen Species and Autophagy" International Journal of Molecular Sciences 26, no. 19: 9519. https://doi.org/10.3390/ijms26199519
APA StyleMontes-Alvarado, J. B., Garcia-Ibañez, P., Moreno, D. A., Sarmiento-Salinas, F. L., Susano-Hernández, X. E., Larrauri-Rodríguez, K. A., García-Hernández, F. J., Milflores-Flores, L., Domínguez, F., & Maycotte, P. (2025). Brassica Extracts Prevent Benzo(a)pyrene-Induced Transformation by Modulating Reactive Oxygen Species and Autophagy. International Journal of Molecular Sciences, 26(19), 9519. https://doi.org/10.3390/ijms26199519







