Characterization of Rhesus Macaque Embryonic Stem Cells in Primed and Naïve-like Cell States of Pluripotency Using Fourier Transform Infrared (FTIR) Microspectroscopy
Abstract
1. Introduction
2. Results
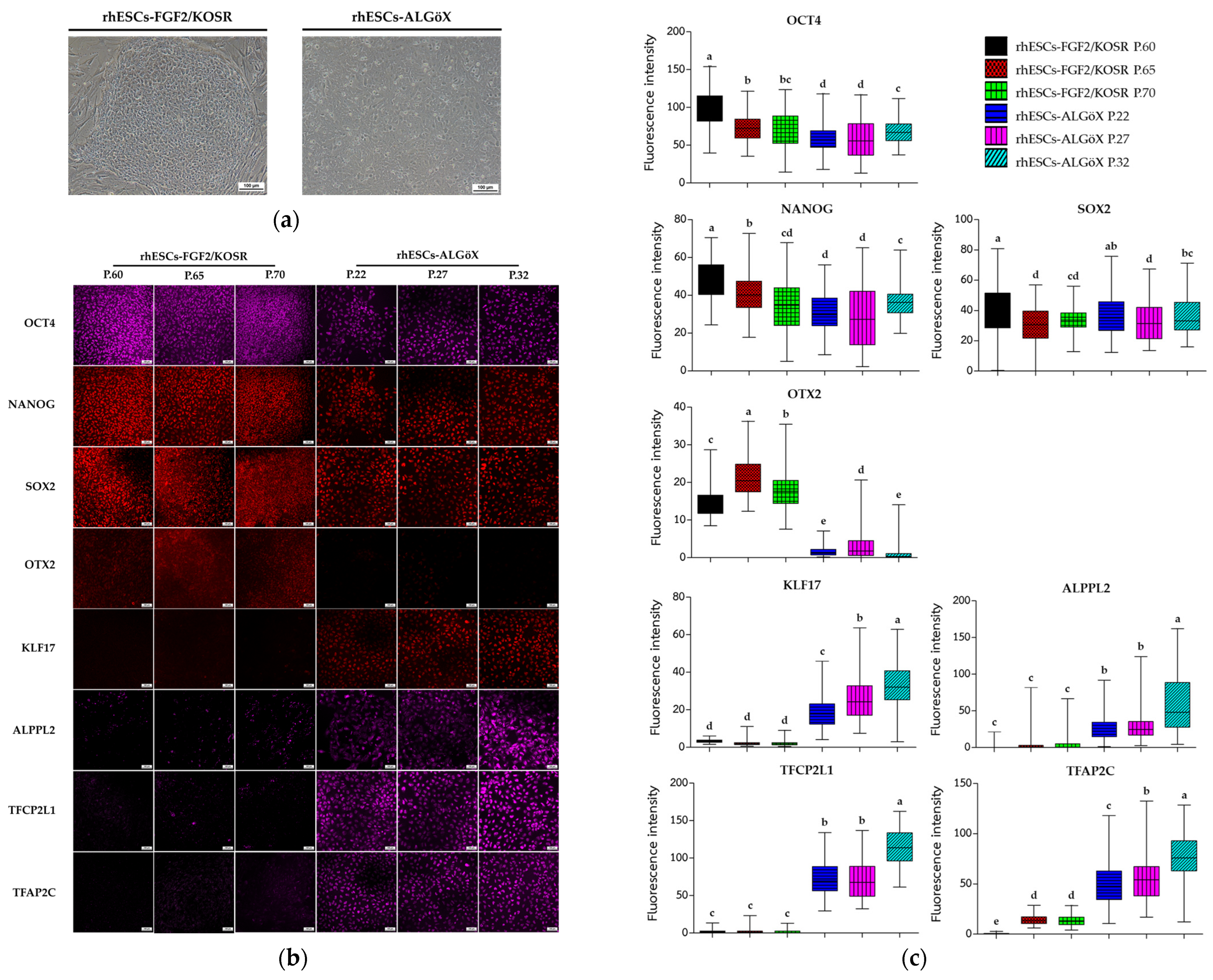
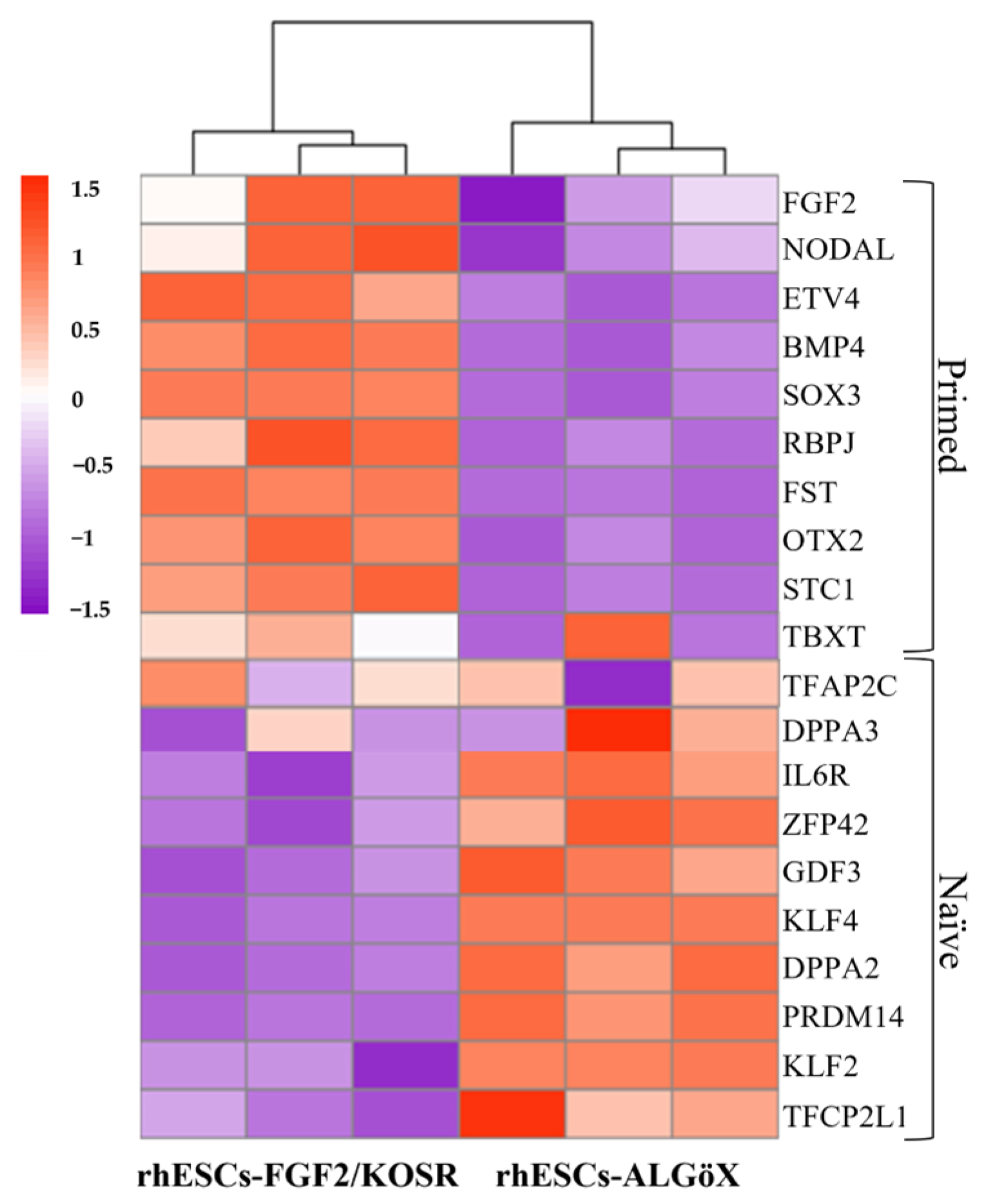
2.1. Characterization of rhESC-FGF2/KOSR and rhESC-ALGöX Cells by Immunocytochemistry and RNA Sequencing
2.2. Characterization of rhESC-FGF2/KOSR and rhESC-ALGöX Cells: FTIR Microspectroscopy Distinguishes Primed and Naïve-like rhESCs
2.2.1. Biochemical Differences Between Cell States
2.2.2. Principal Component Analysis of rhESCs Based on FTIR Spectra
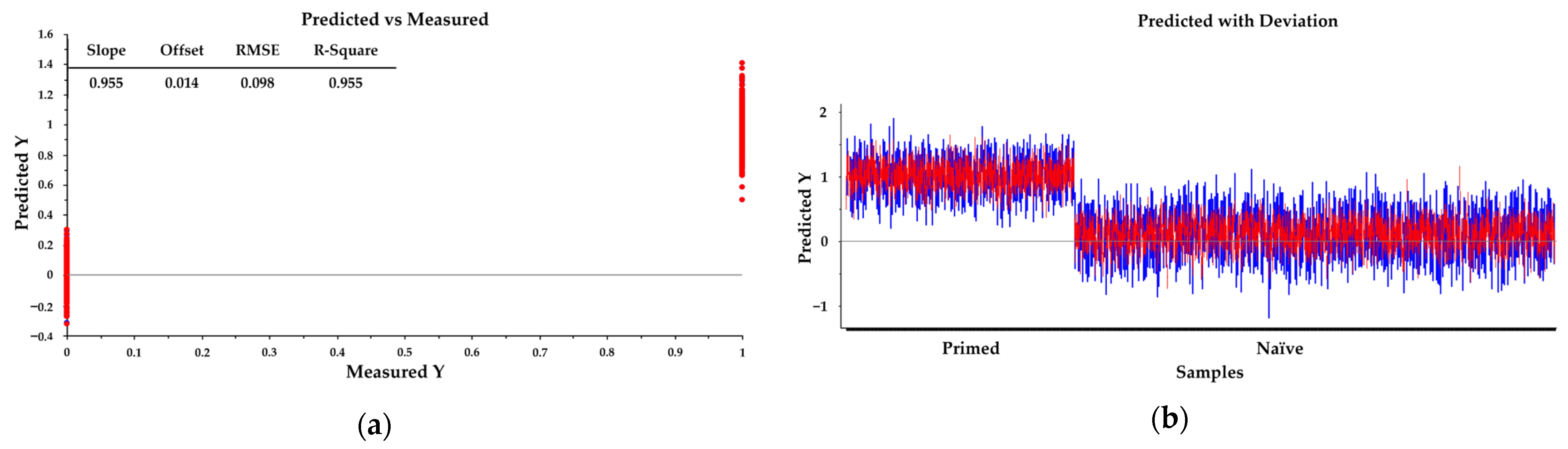
2.2.3. Partial Least Squares Discriminant Analysis (PLS-DA) of rhESCs Based on FTIR Spectra
3. Discussion
4. Materials and Methods
4.1. Preparation of Feeder Cells
4.2. Preparation of Conditioned Medium (CM)
4.3. Culture and Expansion of rhESCs-FGF2/KOSR
4.4. Culture and Expansion of rhESCs-ALGöX
4.5. Immunocytochemistry and Imaging
4.6. RNA Sequencing
4.7. FPA-FTIR Microspectroscopy
4.8. Multivariate Data Analysis of FTIR Spectra
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PSCs | Pluripotent Stem Cells |
| ESCs | Embryonic Stem Cells |
| EpiSCs | Epiblast Stem Cells |
| NHPs | Non-Human Primates |
| FTIR | Fourier-Transform Infrared |
| rhESCs | Rhesus Macaque Embryonic Stem Cells |
| RNA-seq | RNA sequencing |
| FPA | Focal Plane Array |
| PCA | Principal Component Analysis |
| EMSC | Extended Multiplicative Signal Correction |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| MEFs | Mouse embryonic fibroblasts |
| CM | Conditioned Medium |
| P | Passage |
| BaF2 | Barium Fluoride |
| SVM | Support Vector Machine |
References
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Nichols, J.; Smith, A. Naïve and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Anwised, P.; Moorawong, R.; Samruan, W.; Somredngan, S.; Srisutush, J.; Laowtammathron, C.; Aksoy, I.; Parnpai, R.; Savatier, P. An expedition in the jungle of pluripotent stem cells of non-human primates. Stem Cell Rep. 2023, 18, 2016–2037. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, L.; Ayyash, M.; Novershtern, N.; Hanna, J.H. Dynamic stem cell states: Naïve to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016, 17, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.; Perold, F.; Pijoff, Y.; Doerflinger, N.; Rival-Gervier, S.; Givelet, M.; Moulin, A.; Ressaire, M.; Da Silva Fernandes, E.; Bidault, V.; et al. Efficient generation of germline chimeras in a non-rodent species using rabbit induced pluripotent stem cells. Nat. Commun. 2025, 16, 5165. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Q.; Cao, J.; Liu, Y.; Lu, Y.; Sun, Y.; Li, Q.; Huang, Y.; Shang, S.; Bian, X.; et al. Cynomolgus monkey embryo model captures gastrulation and early pregnancy. Cell Stem Cell 2023, 30, 362–377. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Cregger, M.; Berger, A.J.; Rimm, D.L. Immunohistochemistry and Quantitative Analysis of Protein Expression. Arch. Pathol. Lab. Med. 2006, 130, 1026–1030. [Google Scholar] [CrossRef]
- Cao, J.; Ng, E.S.; McNaughton, D.; Stanley, E.G.; Elefanty, A.G.; Tobin, M.J.; Heraud, P. The characterisation of pluripotent and multipotent stem cells using Fourier transform infrared microspectroscopy. Int. J. Mol. Sci. 2013, 14, 17453–17476. [Google Scholar] [CrossRef]
- Matthäus, C.; Bird, B.; Miljković, M.; Chernenko, T.; Romeo, M.; Diem, M. Infrared and Raman microscopy in cell biology. Methods Cell Biol. 2008, 89, 275–308. [Google Scholar] [CrossRef]
- Naumann, D.; Keller, S.; Helm, D.; Schultz, C.; Schrader, B. FT-IR spectroscopy and FT-Raman spectroscopy are powerful analytical tools for the non-invasive characterization of intact microbial cells. J. Mol. Struct. 1995, 347, 399–405. [Google Scholar] [CrossRef]
- Ami, D.; Neri, T.; Natalello, A.; Mereghetti, P.; Doglia, S.M.; Zanoni, M.; Zuccotti, M.; Garagna, S.; Redi, C.A. Embryonic stem cell differentiation studied by FT-IR spectroscopy. Biochim. Biophys. Acta BBA Mol. Cell Res. 2008, 1783, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Bassan, P.; Byrne, H.J.; Bonnier, F.; Lee, J.; Dumas, P.; Gardner, P. Resonant Mie scattering in infrared spectroscopy of biological materials–understanding the ‘dispersion artefact’. Analyst 2009, 134, 1586–1593. [Google Scholar] [CrossRef]
- Thumanu, K.; Tanthanuch, W.; Ye, D.; Sangmalee, A.; Lorthongpanich, C.; Parnpai, R.; Lorthongpanich, C.; Heraud, P. Spectroscopic signature of mouse embryonic stem cell–derived hepatocytes using synchrotron Fourier transform infrared microspectroscopy. J. Biomed. Opt. 2011, 16, 057005. [Google Scholar] [CrossRef]
- Wianny, F.; Bernat, A.; Huissoud, C.; Marcy, G.; Markossian, S.; Cortay, V.; Giroud, P.; Leviel, V.; Kennedy, H.; Savatier, P.; et al. Derivation and cloning of a novel rhesus embryonic stem cell line stably expressing tau-green fluorescent protein. Stem Cells 2008, 26, 1444–1453. [Google Scholar] [CrossRef]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef]
- Dunkhunthod, B.; Thumanu, K.; Eumkeb, G. Application of FTIR microspectroscopy for monitoring and discrimination of the anti-adipogenesis activity of baicalein in 3T3-L1 adipocytes. Vib. Spectrosc. 2017, 89, 92–101. [Google Scholar] [CrossRef]
- Cornacchia, D.; Zhang, C.; Zimmer, B.; Chung, S.Y.; Fan, Y.; Soliman, M.A.; Tchieu, J.; Chambers, S.M.; Shah, H.; Paull, D.; et al. Lipid Deprivation Induces a Stable, Naïve-to-Primed Intermediate State of Pluripotency in Human PSCs. Cell Stem Cell 2019, 25, 120–136. [Google Scholar] [CrossRef]
- Yousefi, M.; Marashi, S.A.; Sharifi, Z.A.; Taleahmad, S. The metabolic network model of primed/naïve human embryonic stem cells underlines the importance of oxidation-reduction potential and tryptophan metabolism in primed pluripotency. Cell Biosci. 2019, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Ng, E.S.; McNaughton, D.; Stanley, E.G.; Elefanty, A.G.; Tobin, M.J.; Heraud, P. Fourier transform infrared microspectroscopy reveals unique phenotypes for human embryonic and induced pluripotent stem cell lines and their progeny. J. Biophotonics 2014, 7, 767–781. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Whelan, D.R.; Bambery, K.R.; Heraud, P.; Tobin, M.J.; Diem, M.; McNaughton, D.; Wood, B.R. Monitoring the reversible B to A-like transition of DNA in eukaryotic cells using Fourier transform infrared spectroscopy. Nucleic Acids Res. 2011, 39, 5439–5448. [Google Scholar] [CrossRef]
- Bassan, P.; Sachdeva, A.; Kohler, A.; Hughes, C.; Henderson, A.; Boyle, J.; Shanks, J.H.; Brown, M.; Clarke, N.W.; Gardner, P. FTIR microscopy of biological cells and tissue: Data analysis using resonant Mie scattering (RMieS) EMSC algorithm. Analyst 2012, 137, 1370–1377. [Google Scholar] [CrossRef]
- Bassan, P.; Kohler, A.; Martens, H.; Lee, J.; Byrne, H.J.; Dumas, P.; Gazi, E.; Brown, M.; Clarke, N.; Gardner, P. Resonant Mie scattering (RMieS) correction of infrared spectra from highly scattering biological samples. Analyst 2010, 135, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, I.; Rognard, C.; Moulin, A.; Marcy, G.; Masfaraud, E.; Wianny, F.; Cortay, V.; Ménard, A.B.; Doerflinger, N.; Dirheimer, M.; et al. Apoptosis, G1 phase stall, and premature differentiation account for low chimeric competence of human and rhesus monkey naïve pluripotent stem cells. Stem Cell Rep. 2021, 16, 56–74. [Google Scholar] [CrossRef]
- Amzal, A.; Pijoff, Y.; Rognard, C.; Marcy, G.; Doerflinger, N.; Bréhier, C.; Anwised, P.; Srisutush, J.; Chimngam, M.; Chatdarong, K.; et al. MEK and AKT signaling Determine the Potential of Pluripotent stem Cells to Colonize Heterologous Preimplantation Embryos. Nature, 2025, in press.
- Heraud, P.; Ng, E.S.; Caine, S.; Yu, Q.C.; Hirst, C.; Mayberry, R.; Bruce, A.; Wood, B.R.; McNaughton, D.; Stanley, E.G.; et al. Fourier transform infrared microspectroscopy identifies early lineage commitment in differentiating human embryonic stem cells. Stem Cell Res. 2010, 4, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Toplak, M.; Read, S.T.; Sandt, C.; Borondics, F. Quasar: Easy machine learning for biospectroscopy. Cells 2021, 10, 2300. [Google Scholar] [CrossRef]
- Afanassieff, M.; Tapponnier, Y.; Savatier, P. Generation of Induced Pluripotent Stem Cells in Rabbits. In Induced Pluripotent Stem (iPS) Cells. Methods and Protocols, 2nd ed.; Turksen, K., Nagy, A., Eds.; Humana Press: New York, NY, USA, 2014; Volume 1357, pp. 149–172. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Heraud, P.; Caine, S.; Sanson, G.; Gleadow, R.; Wood, B.R.; McNaughton, D. Focal plane array infrared imaging: A new way to analyse leaf tissue. New Phytol. 2007, 173, 216–225. [Google Scholar] [CrossRef]


| Band Maxima Second Derivative Spectra (cm−1) | PC−1 Loading (cm−1) | Band Assignments | ||
|---|---|---|---|---|
| rhESCs-FGF2/KOSR | rhESCs-ALGöX | Negative Loading | Positive Loading | |
| P60, P65, P70 | P22, P27, P32 | |||
| 2960 | 2960 | 2940 | CH3 asymmetric stretch due to methyl terminal of membrane phospholipids: mainly lipids [9,17,20,21] | |
| 2921 | 2921 | 2919 | 2900 | CH2 asymmetric stretch of the methylene group of membrane phospholipids: mainly lipids, with some contribution from proteins, carbohydrates, nucleic acids [9,17,18] |
| 2852 | 2852 | 2852 | 2869, 2832 | CH2 symmetric stretching: mainly lipids, with some contribution from proteins, carbohydrates, nucleic acids [9,17,20,21] |
| 1741 | 1741 | 1739, 1704 | C=O stretching vibrations of lipids (triglycerides and cholesterol esters) [9,17,20,21] | |
| 1654 | 1654 | 1614 | 1652 | Amide I: C=O (80%) and C—N (10%) stretching, N—H (10%) bending vibrations: proteins α-helix [9,17,21] |
| 1546 | 1546 | 1544 | Amide II: N—H (60%) bending and C—N (40%) stretching vibrations: proteins α-helix [17,21] | |
| 1463 | 1463 | 1467 | 1450 | CH2 bending vibrations: lipids and proteins [17] Cholesterol methyl band [21] |
| 1396 | 1396 | 1396 | COO− stretching vibrations of amino acid side chains [9,20,21] | |
| 1239 | 1239 | 1232 | PO2-asymmetric stretching vibrations: RNA, DNA, and phospholipids [9,17,20,21] | |
| 1172 | 1157, 1022 | 1180 | 1158 | C–O–C vibrations from glycogen and other carbohydrates [9,17,20,21] |
| 1085 | 1085 | 1083 | PO2-symmetric stretching vibrations: RNA, DNA [9,17,20,21] | |
| 1054 | 1054 | 1068 | C—O vibrations from glycogen and other carbohydrates [9,17] | |
| 966 | 966 | 981 | C—O deoxyribose, C—C DNA [21] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisutush, J.; Samruan, W.; Anwised, P.; Amzal, A.; Rognard, C.; Savatier, P.; Aksoy, I.; Thumanu, K.; Parnpai, R. Characterization of Rhesus Macaque Embryonic Stem Cells in Primed and Naïve-like Cell States of Pluripotency Using Fourier Transform Infrared (FTIR) Microspectroscopy. Int. J. Mol. Sci. 2025, 26, 9514. https://doi.org/10.3390/ijms26199514
Srisutush J, Samruan W, Anwised P, Amzal A, Rognard C, Savatier P, Aksoy I, Thumanu K, Parnpai R. Characterization of Rhesus Macaque Embryonic Stem Cells in Primed and Naïve-like Cell States of Pluripotency Using Fourier Transform Infrared (FTIR) Microspectroscopy. International Journal of Molecular Sciences. 2025; 26(19):9514. https://doi.org/10.3390/ijms26199514
Chicago/Turabian StyleSrisutush, Jittanun, Worawalan Samruan, Preeyanan Anwised, Anaïs Amzal, Cloé Rognard, Pierre Savatier, Irene Aksoy, Kanjana Thumanu, and Rangsun Parnpai. 2025. "Characterization of Rhesus Macaque Embryonic Stem Cells in Primed and Naïve-like Cell States of Pluripotency Using Fourier Transform Infrared (FTIR) Microspectroscopy" International Journal of Molecular Sciences 26, no. 19: 9514. https://doi.org/10.3390/ijms26199514
APA StyleSrisutush, J., Samruan, W., Anwised, P., Amzal, A., Rognard, C., Savatier, P., Aksoy, I., Thumanu, K., & Parnpai, R. (2025). Characterization of Rhesus Macaque Embryonic Stem Cells in Primed and Naïve-like Cell States of Pluripotency Using Fourier Transform Infrared (FTIR) Microspectroscopy. International Journal of Molecular Sciences, 26(19), 9514. https://doi.org/10.3390/ijms26199514








