Asiatic Acid Disrupts the Biofilm Virulence of Streptococcus mutans by Transcriptional Reprogramming of Quorum Sensing System
Abstract
1. Introduction
2. Results
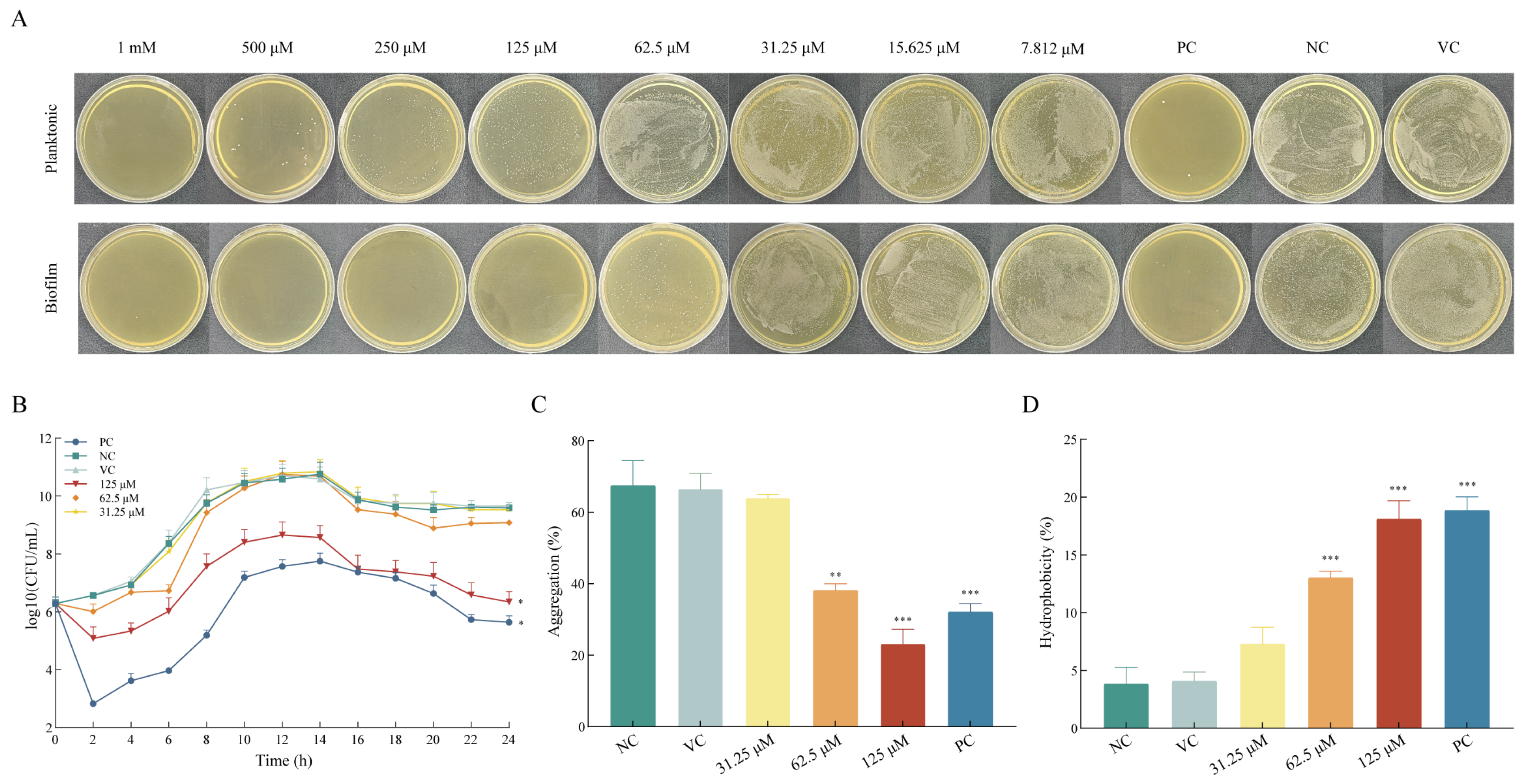
2.1. Asiatic Acid Exhibited Selective Anti-Biofilm Activity Against S. mutans
2.2. Asiatic Acid Decreased Adhesion of S. mutans
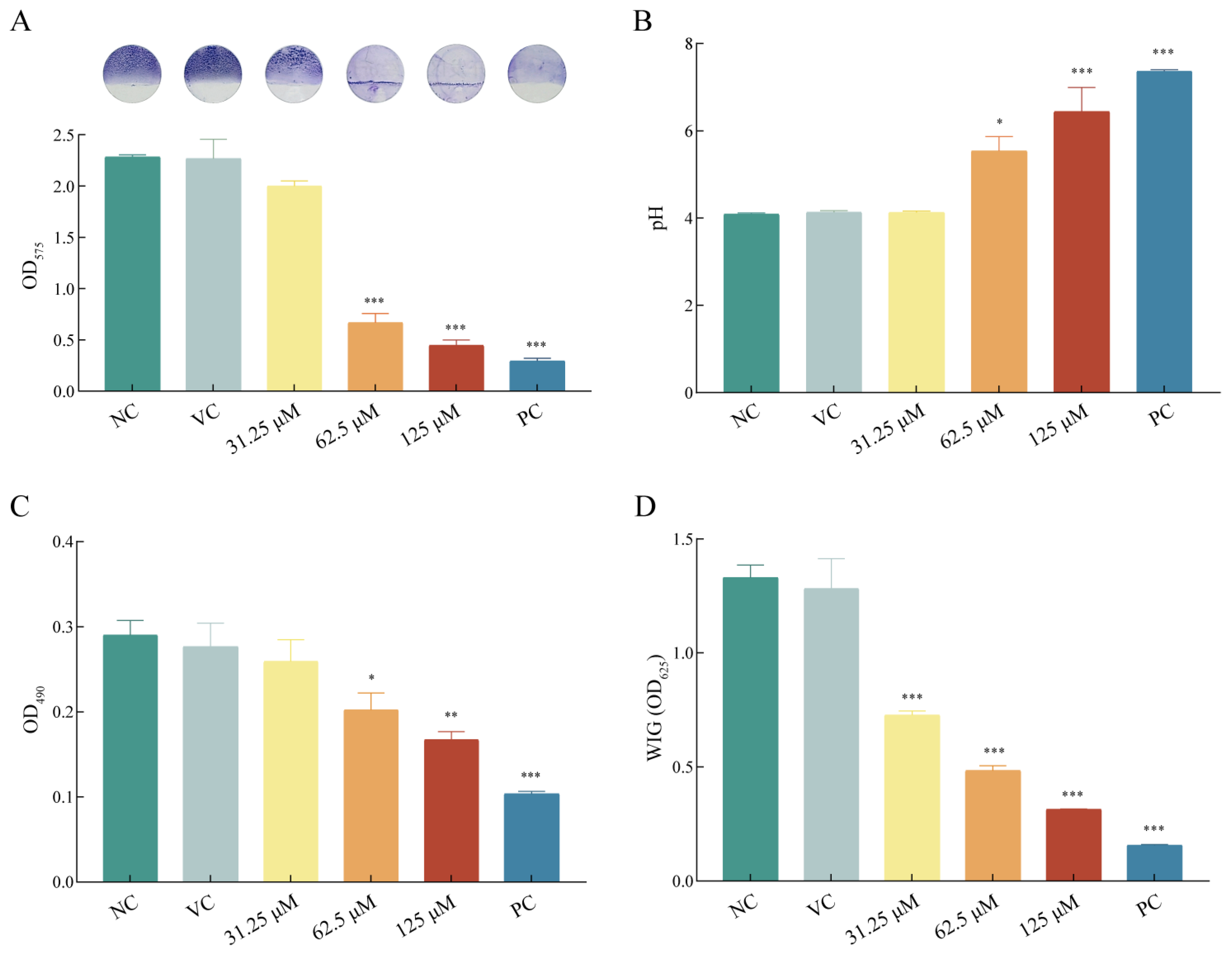
2.3. Asiatic Acid Suppressed Biofilm Formation of S. mutans
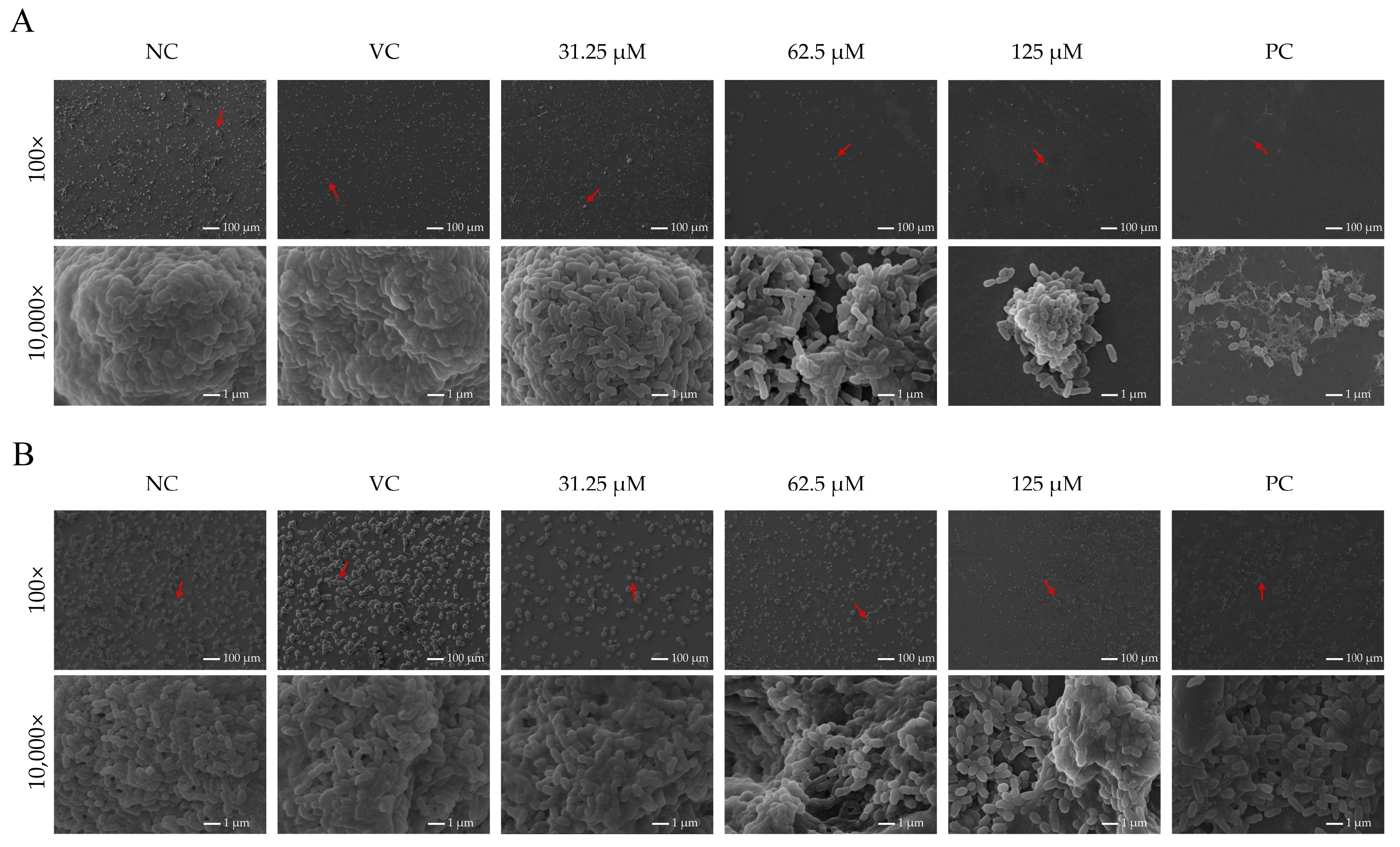
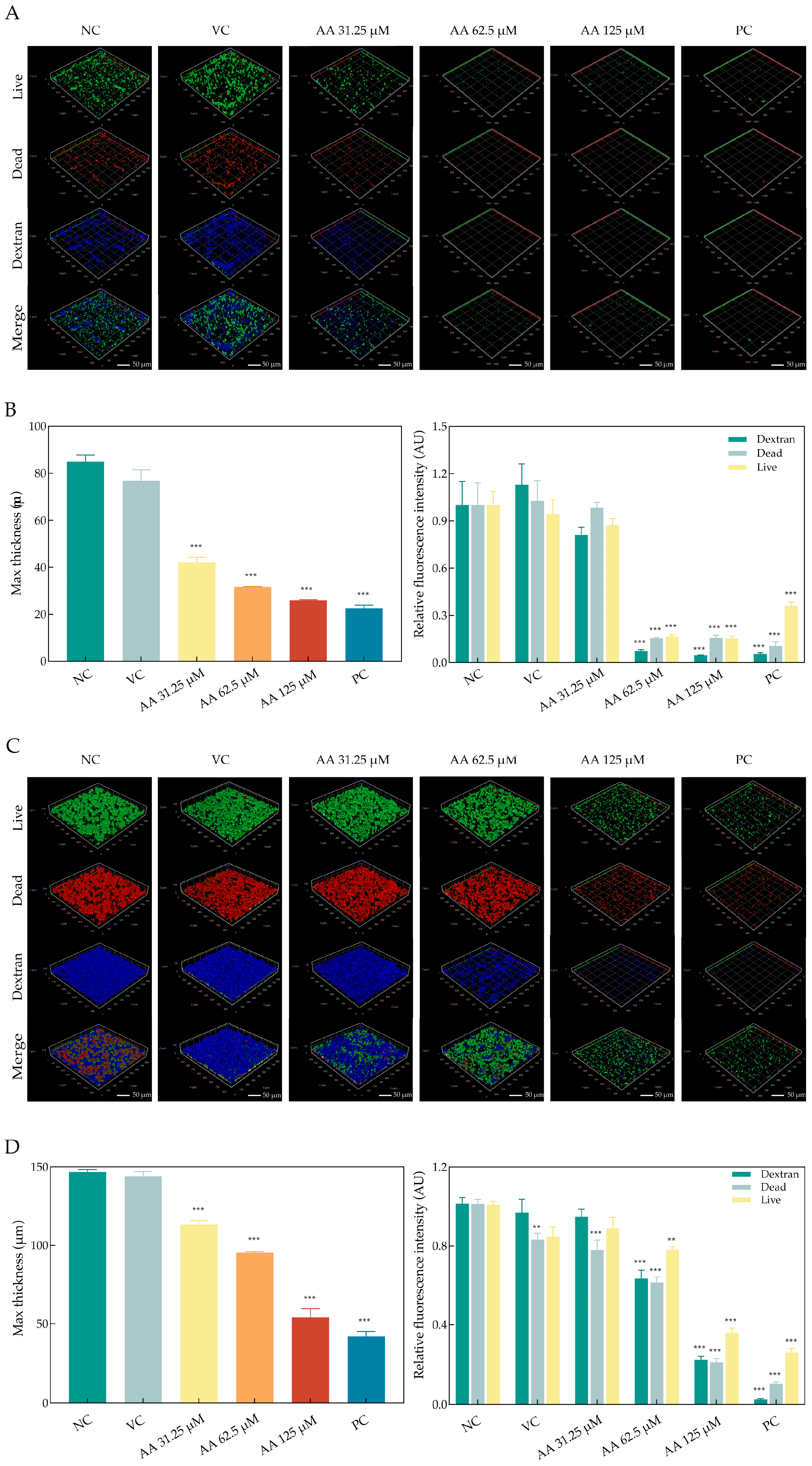
2.4. Asiatic Acid Altered Structural Morphology of S. mutans Biofilms
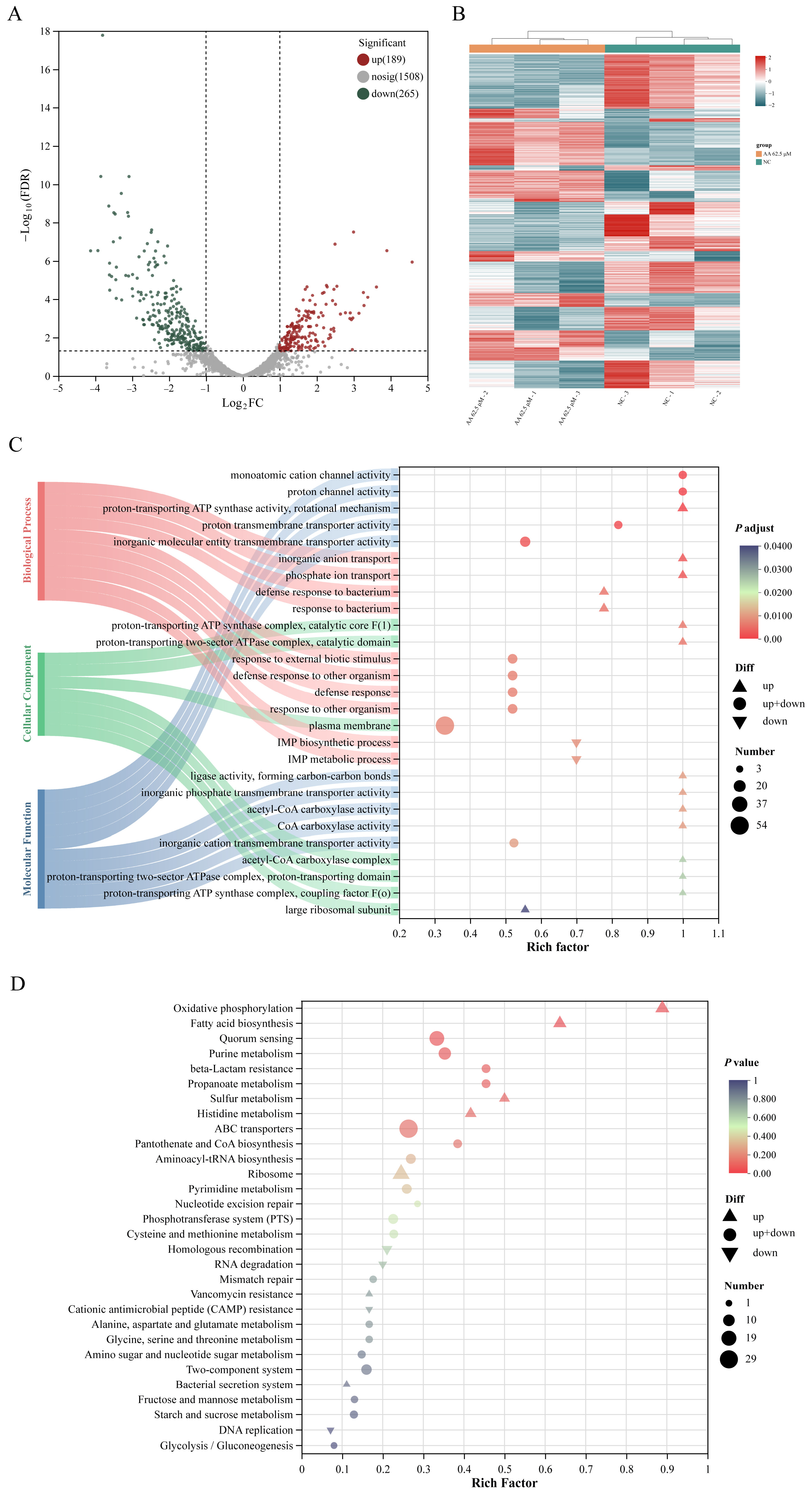
2.5. Transcriptomic Profiling Reveals Key Pathways Modulated by Asiatic Acid
2.6. RT-qPCR Confirms Downregulation of Biofilm-Associated Genes
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Culture Conditions
4.2. Minimal Inhibitory Concentration
4.3. Planktonic Growth Dynamics
4.4. Bacterial Auto-Aggregation
4.5. Bacterial Surface Hydrophobicity
4.6. Biofilm Characterization
4.6.1. Biomass
4.6.2. Acid Production
4.6.3. Metabolic Activity
4.6.4. Exopolysaccharides (EPS)
4.7. Biofilm Structural Imaging
4.7.1. Scanning Electron Microscopy (SEM)
4.7.2. Confocal Laser Scanning Microscopy (CLSM)
4.8. Transcriptomic Profiling and Molecular Validation
4.8.1. RNA Sequencing and Bioinformatics Analysis
4.8.2. Reverse Transcription Quantitative PCR (RT-qPCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chisini, L.A.; Cademartori, M.G.; Conde, M.C.M.; Tovo-Rodrigues, L.; Correa, M.B. Genes in the Pathway of Tooth Mineral Tissues and Dental Caries Risk: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2020, 24, 3723–3738. [Google Scholar] [CrossRef]
- WHO. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030, 1st ed.; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006148-4. [Google Scholar]
- Li, Y.; Xiang, Y.; Ren, H.; Zhang, C.; Hu, Z.; Leng, W.; Xia, L. Association between Periodontitis and Dental Caries: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2024, 28, 306. [Google Scholar] [CrossRef]
- Botelho, J.; Mascarenhas, P.; Viana, J.; Proença, L.; Orlandi, M.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. An Umbrella Review of the Evidence Linking Oral Health and Systemic Noncommunicable Diseases. Nat. Commun. 2022, 13, 7614. [Google Scholar] [CrossRef]
- Tan, L.; Zhong, M.-M.; Zhao, Y.-Q.; Zhao, J.; Dusenge, M.A.; Feng, Y.; Ye, Q.; Hu, J.; Ou-Yang, Z.-Y.; Chen, N.-X.; et al. Type 1 Diabetes, Glycemic Traits, and Risk of Dental Caries: A Mendelian Randomization Study. Front. Genet. 2023, 14, 1230113. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, A.; Stellrecht, E.; Scannapieco, F.A. Associations between Dental Ccaries and Systemic Diseases: A Scoping Review. BMC Oral Health 2021, 21, 472. [Google Scholar] [CrossRef] [PubMed]
- Marruganti, C.; Discepoli, N.; Gaeta, C.; Franciosi, G.; Ferrari, M.; Grandini, S. Dental Caries Occurrence in Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Caries Res. 2021, 55, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.H. Causes and Control of Dental Caries. N. Engl. J. Med. 1987, 317, 996–1004. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Peng, W.; Zhu, Y. Effects of Resveratrol on Cariogenic Virulence Properties of Streptococcus mutans. BMC Microbiol. 2020, 20, 99. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, A.; Xiang, Z.; Lu, M.; Huang, P.; Gong, T.; Pan, Y.; Lin, Y.; Zhou, X.; Li, Y. EpsR Negatively Regulates Streptococcus mutans Exopolysaccharide Synthesis. J. Dent. Res. 2021, 100, 968–976. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef]
- Mansouri, M.; O’Brien, E.P.; Mondal, K.; Chen, C.-C.; Drummond, J.L.; Hanley, L.; Rockne, K.J. Stoichiometric Models of Sucrose and Glucose Fermentation by Oral Streptococci: Implications for Free Acid Formation and Enamel Demineralization. Dent. Mater. 2023, 39, 351–361. [Google Scholar] [CrossRef]
- Van Houte, J.; Sansone, C.; Joshipura, K.; Kent, R. Mutans Streptococci and Non-Mutans Streptococci Acidogenic at Low pH, and in Vitro Acidogenic Potential of Dental Plaque in Two Different Areas of the Human Dentition. J. Dent. Res. 1991, 70, 1503–1507. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Lemos, J.A.C.; Abranches, J.; Gonçalves, R.B.; Burne, R.A. Adaptive Acid Tolerance Response of Streptococcus sobrinus. J. Bacteriol. 2004, 186, 6383–6390. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gong, T.; Ma, Q.; Jing, M.; Zheng, T.; Yan, J.; Chen, J.; Pan, Y.; Sun, Q.; Zhou, X.; et al. Nicotinamide Could Reduce Growth and Cariogenic Virulence of Streptococcus mutans. J. Oral Microbiol. 2022, 14, 2056291. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef]
- Acar, S.; Yetkıner, A.A.; Ersın, N.; Oncag, O.; Aydogdu, S.; Arıkan, C. Oral Findings and Salivary Parameters in Children with Celiac Disease: A Preliminary Study. Med. Princ. Pract. 2012, 21, 129–133. [Google Scholar] [CrossRef]
- Tonelli, A.; Lumngwena, E.N.; Ntusi, N.A.B. The Oral Microbiome in the Pathophysiology of Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Lozano, C.P.; Kreth, J.; Giacaman, R.A. Competition and Caries on Enamel of a Dual-Species Biofilm Model with Streptococcus mutans and Streptococcus sanguinis. Appl. Environ. Microbiol. 2020, 86, e01262-20. [Google Scholar] [CrossRef]
- Mao, X.; Hiergeist, A.; Auer, D.L.; Scholz, K.J.; Muehler, D.; Hiller, K.-A.; Maisch, T.; Buchalla, W.; Hellwig, E.; Gessner, A.; et al. Ecological Effects of Daily Antiseptic Treatment on Microbial Composition of Saliva-Grown Microcosm Biofilms and Selection of Resistant Phenotypes. Front. Microbiol. 2022, 13, 934525. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Cai, J.-N.; Yoo, S.C.; Kim, S.-H.; Jeon, J.-G.; Kim, D. Collagen Peptide in a Combinatorial Treatment with Lactobacillus rhamnosus Inhibits the Cariogenic Properties of Streptococcus mutans: An in Vitro Study. Int. J. Mol. Sci. 2022, 23, 1860. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Zhang, S.; Zhou, W.; Zhang, L.; Huang, X. pH-Activated Antibiofilm Strategies for Controlling Dental Caries. Front. Cell. Infect. Microbiol. 2023, 13, 1130506. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; the International Natural Product Sciences Taskforce; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. Tea Catechin Epigallocatechin Gallate Inhibits Streptococcus mutans Biofilm Formation by Suppressing Gtf Genes. Arch. Oral Biol. 2012, 57, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Dorota, W.; Marta, K.; Dorota, T.-G. Effect of Asiatic and Ursolic Acids on Morphology, Hydrophobicity, and Adhesion of UPECs to Uroepithelial Cells. Folia Microbiol. 2013, 58, 245–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-Planktonic and Anti-Biofilm Properties of Pentacyclic Triterpenes—Asiatic Acid and Ursolic Acid as Promising Antibacterial Future Pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef]
- Garo, E.; Eldridge, G.R.; Goering, M.G.; Pulcini, E.D.; Hamilton, M.A.; Costerton, J.W.; James, G.A. Asiatic Acid and Corosolic Acid Enhance the Susceptibility of Pseudomonas aeruginosa Biofilms to Tobramycin. Antimicrob. Agents Chemother. 2007, 51, 1813–1817. [Google Scholar] [CrossRef]
- Matsumoto-Nakano, M. Role of Streptococcus mutans Surface Proteins for Biofilm Formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef]
- He, X.; Wu, C.; Yarbrough, D.; Sim, L.; Niu, G.; Merritt, J.; Shi, W.; Qi, F. The Cia Operon of Streptococcus mutans Encodes a Unique Component Required for Calcium-mediated Autoregulation. Mol. Microbiol. 2008, 70, 112–126. [Google Scholar] [CrossRef]
- Eguchi, Y.; Kubo, N.; Matsunaga, H.; Igarashi, M.; Utsumi, R. Development of an Antivirulence Drug against Streptococcus mutans: Repression of Biofilm Formation, Acid Tolerance, and Competence by a Histidine Kinase Inhibitor, Walkmycin C. Antimicrob. Agents Chemother. 2011, 55, 1475–1484. [Google Scholar] [CrossRef]
- He, Z.; Huang, Z.; Jiang, W.; Zhou, W. Antimicrobial Activity of Cinnamaldehyde on Streptococcus mutans Biofilms. Front. Microbiol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Lévesque, C.M.; Mair, R.W.; Perry, J.A.; Lau, P.C.Y.; Li, Y.-H.; Cvitkovitch, D.G. Systemic Inactivation and Phenotypic Characterization of Two-Component Systems in Expression of Streptococcus mutans Virulence Properties. Lett. Appl. Microbiol. 2007, 45, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.I.; DeBaz, L.; Agidi, S.; Lee, H.; Xie, G.; Lin, A.H.-M.; Hamaker, B.R.; Lemos, J.A.; Koo, H. Dynamics of Streptococcus mutans Transcriptome in Response to Starch and Sucrose during Biofilm Development. PLoS ONE 2010, 5, e13478. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Wen, Z.T.; Burne, R.A. Multilevel Control of Competence Development and Stress Tolerance in Streptococcus mutans UA159. Infect. Immun. 2006, 74, 1631–1642. [Google Scholar] [CrossRef]
- Hasona, A.; Zuobi-Hasona, K.; Crowley, P.J.; Abranches, J.; Ruelf, M.A.; Bleiweis, A.S.; Brady, L.J. Membrane Composition Changes and Physiological Adaptation by Streptococcus mutans Signal Recognition Particle Pathway Mutants. J. Bacteriol. 2007, 189, 1219–1230. [Google Scholar] [CrossRef]
- Kong, J.; Xia, K.; Su, X.; Zheng, X.; Diao, C.; Yang, X.; Zuo, X.; Xu, J.; Liang, X. Mechanistic Insights into the Inhibitory Effect of Theaflavins on Virulence Factors Production in Streptococcus mutans. AMB Express 2021, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, GPP3-0051-2018. [Google Scholar] [CrossRef] [PubMed]
- Chamlagain, M.; Hu, J.; Sionov, R.V.; Steinberg, D. Anti-Bacterial and Anti-Biofilm Activities of Arachidonic Acid against the Cariogenic Bacterium Streptococcus mutans. Front. Microbiol. 2024, 15, 1333274. [Google Scholar] [CrossRef]
- Harnvoravongchai, P.; Chankhamhaengdecha, S.; Ounjai, P.; Singhakaew, S.; Boonthaworn, K.; Janvilisri, T. Antimicrobial Effect of Asiatic Acid against Clostridium difficile Is Associated with Disruption of Membrane Permeability. Front. Microbiol. 2018, 9, 2125. [Google Scholar] [CrossRef]
- Sui, Y.; Yang, R. Anti-Cariogenic and Anti-Biofilm Effect of Asiatic Acid on Streptococcus mutans. J. Oral Sci. Res. 2021, 37, 401–406. [Google Scholar] [CrossRef]
- Leejae, S.; Pelyuntha, W.; Goodla, L.; Mordmuang, A. Silver Nanoparticles Synthesized from Centella asiatica Extract and Asiatic Acid for Enhanced Biofilm Eradication of Streptococcus Associated with Oral Diseases. Scientifica 2025, 2025, 4867529. [Google Scholar] [CrossRef]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC versus MBIC: The Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef]
- Shen, G.; Yang, L.; Lv, X.; Zhang, Y.; Hou, X.; Li, M.; Zhou, M.; Pan, L.; Chen, A.; Zhang, Z. Antibiofilm Activity and Mechanism of Linalool against Food Spoilage Bacillus Amyloliquefaciens. Int. J. Mol. Sci. 2023, 24, 10980. [Google Scholar] [CrossRef]
- Guan, C.; Che, F.; Zhou, H.; Li, Y.; Li, Y.; Chu, J. Effect of Rubusoside, a Natural Sucrose Substitute, on Streptococcus mutans Biofilm Cariogenic Potential and Virulence Gene Expression in Vitro. Appl. Environ. Microbiol. 2020, 86, e01012–e01020. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, Z.-Z.; Xu, S.-B.; Ma, M.; Wei, X. Farnesol Inhibits Development of Caries by Augmenting Oxygen Sensitivity and Suppressing Virulence-Associated Gene Expression in Streptococcus mutans. J. Biomed. Res. 2017, 31, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, B.; Mao, M.; Chen, H.; Wu, S.; Deng, Y.; Yang, Y.; Zhou, H.; Hu, T. Carbohydrate Metabolism Regulated by Antisense vicR RNA in Cariogenicity. J. Dent. Res. 2020, 99, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Wang, L.; Shui, Y.; Jiang, Q.; Chen, L.; Yang, W.; He, X.; Zeng, J.; Li, Y. Ursolic Acid Inhibits Multi-Species Biofilms Developed by Streptococcus mutans, Streptococcus sanguinis, and Streptococcus gordonii. Arch. Oral Biol. 2021, 125, 105107. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Y.; Qiu, L.; Yu, F.; Hu, X.; Wang, M.; Sun, Y.; Pan, Y.; Zhang, K. The Suppression Effect of SCH-79797 on Streptococcus mutans Biofilm Formation. J. Oral Microbiol. 2022, 14, 2061113. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. Multi-Omics Reveals the Inhibition of Lactiplantibacillus plantarum CCFM8724 in Streptococcus mutans-Candida albicans Mixed-Species Biofilms. Microorganisms 2021, 9, 2368. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Li, F.; Peng, Y.; Sun, Q.; Zhao, H.; Lu, F.; Zhao, H. Asiatic Acid Disrupts the Biofilm Virulence of Streptococcus mutans by Transcriptional Reprogramming of Quorum Sensing System. Int. J. Mol. Sci. 2025, 26, 9510. https://doi.org/10.3390/ijms26199510
Shi Q, Li F, Peng Y, Sun Q, Zhao H, Lu F, Zhao H. Asiatic Acid Disrupts the Biofilm Virulence of Streptococcus mutans by Transcriptional Reprogramming of Quorum Sensing System. International Journal of Molecular Sciences. 2025; 26(19):9510. https://doi.org/10.3390/ijms26199510
Chicago/Turabian StyleShi, Qingying, Fengzhu Li, Yingying Peng, Qiannan Sun, Hong Zhao, Fuping Lu, and Huabing Zhao. 2025. "Asiatic Acid Disrupts the Biofilm Virulence of Streptococcus mutans by Transcriptional Reprogramming of Quorum Sensing System" International Journal of Molecular Sciences 26, no. 19: 9510. https://doi.org/10.3390/ijms26199510
APA StyleShi, Q., Li, F., Peng, Y., Sun, Q., Zhao, H., Lu, F., & Zhao, H. (2025). Asiatic Acid Disrupts the Biofilm Virulence of Streptococcus mutans by Transcriptional Reprogramming of Quorum Sensing System. International Journal of Molecular Sciences, 26(19), 9510. https://doi.org/10.3390/ijms26199510





