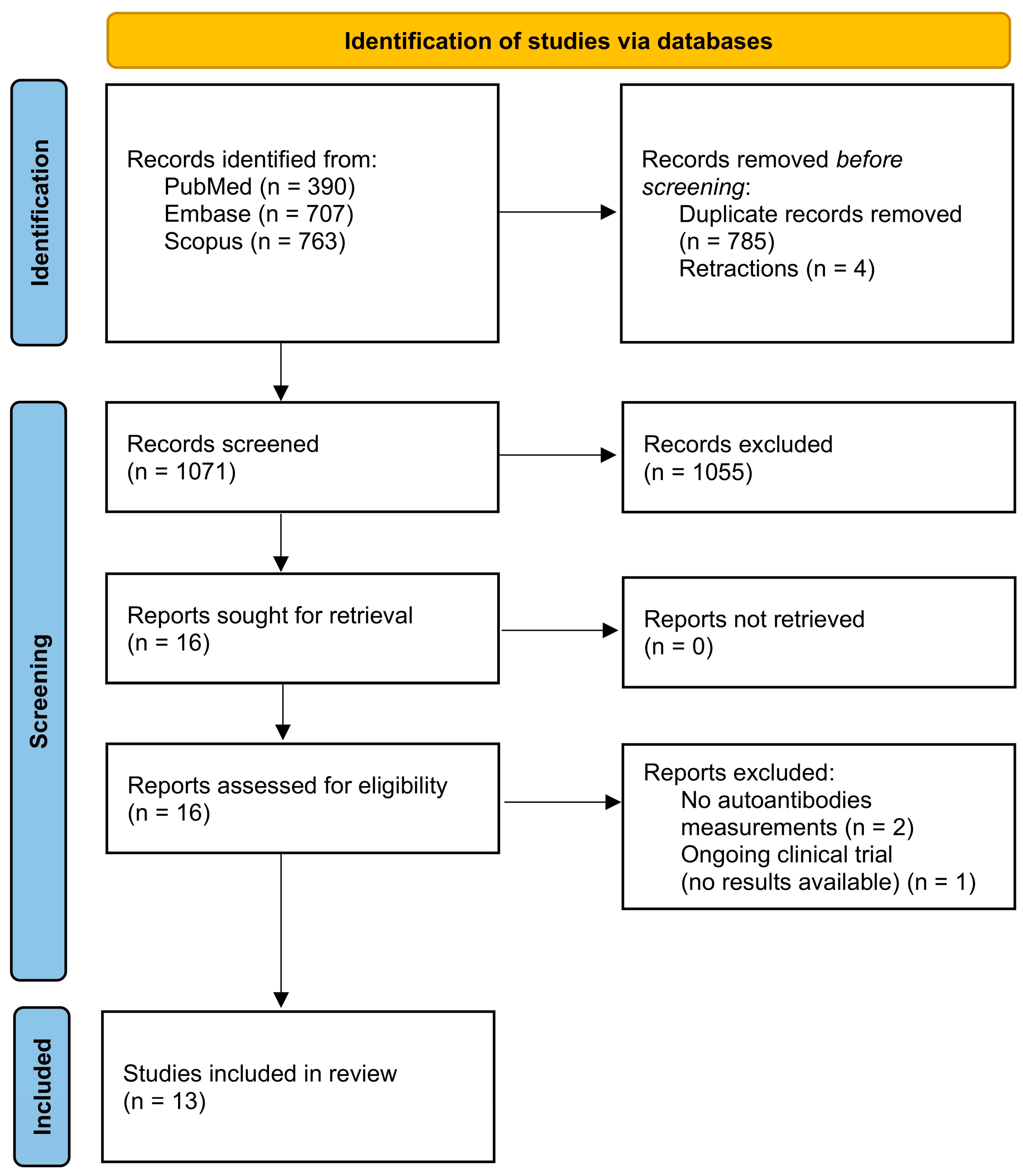
1. Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disorder in women characterized by ovulatory dysfunction and androgen excess [
1]. The diagnosis relies on clinical presentation and biochemical assessment and typically follows the Rotterdam criteria: two of three features (ovulatory dysfunction, clinical/biochemical hyperandrogenism, polycystic ovarian morphology/anti-Müllerian hormone elevation) after exclusion of alternative etiologies of observed symptoms such as thyroid dysfunction, hyperprolactinemia, non-classic congenital adrenal hyperplasia, Cushing’s syndrome, or androgen-secreting tumors [
2]. In the reproductive-age female population, prevalence generally ranges from 8% to 13% depending on diagnostic criteria, with up to 70% of cases remaining undiagnosed [
2,
3]. PCOS has a complex, multifactorial etiology involving genetic susceptibility, developmental and environmental influences, insulin resistance, and dysregulation of hypothalamic–pituitary–ovarian signaling [
1,
4,
5,
6]. Clinically, it is linked to reproductive difficulties (subfertility/infertility, early pregnancy loss, adverse pregnancy outcomes) [
7,
8], hyperandrogenic symptoms (hirsutism, acne, alopecia) [
9], and cardiometabolic risk (insulin resistance, dyslipidemia, obesity, type 2 diabetes) [
10] with substantial psychosocial and quality-of-life impact [
11].
These clinical features coexist with chronic low-grade inflammation and immune perturbations in PCOS, which may increase susceptibility to autoimmunity [
12,
13]. Mechanistically, persistent oxidative stress and dysregulated adaptive immunity could facilitate loss of tolerance and the production of autoantibodies [
12,
14]. Indeed, several reports have demonstrated elevated levels of various autoantibodies in PCOS, including anti-thyroid peroxidase (anti-TPO) [
15], anti-thyroglobulin (anti-TG) [
15], antinuclear antibodies (ANA) [
16], and anti-ovarian antibodies [
17], among others [
18]. The detection of such autoantibodies in PCOS may reflect a state of subclinical immune activation [
19]. Among them, antinuclear antibodies (ANA) are of particular interest. ANA bind to components of the cell nucleus, including proteins, deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and nucleic acid–protein complexes [
20]. In the context of chronic low-grade inflammation, accelerated cellular apoptosis in women with PCOS [
21] and increased exposure to intracellular, including nuclear, antigens may increase the likelihood of ANA formation [
22,
23,
24]. If confirmed, elevated ANA in PCOS, considered a hallmark of generalized autoimmune activation [
25], would support the concept of autoimmune involvement in PCOS and provide a unifying explanation for the heterogeneous autoantibody findings reported by multiple investigators.
Clinically, ANA serve as serological markers of systemic autoimmune rheumatic diseases (e.g., systemic lupus erythematosus, Sjögren’s syndrome, systemic sclerosis) [
26]. Low-titer ANA are also common in otherwise healthy individuals, especially women [
27] and older adults [
28], and may also occur transiently with intercurrent infections [
29], during pregnancy [
30], or with certain medications [
31]. Accordingly, ANA positivity, particularly at low titers, does not in itself establish disease and must be interpreted in a clinical context [
32]. While autoimmune thyroid disease is consistently more prevalent in PCOS [
15,
33,
34], evidence for higher rates of ANA-related systemic autoimmune rheumatic diseases (Sjögren’s syndrome [
35], rheumatoid arthritis [
36,
37], systemic sclerosis [
37], undifferentiated connective tissue disease [
37]) remains limited. Nevertheless, several single-center studies have reported higher ANA positivity in women with PCOS [
16,
18], whereas others found no significant difference [
38]. This heterogeneity leaves an open question: does PCOS represent another clinical state in which, against a background of low-grade inflammation, ANA occur more frequently? To address this gap, we conducted a systematic review quantifying ANA prevalence and levels in PCOS patients versus non-PCOS controls.
4. Discussion
This systematic review is the first study to comprehensively evaluate the occurrence of ANA in the population of women with PCOS. Although original studies suggesting a higher prevalence of these autoantibodies in PCOS compared with the general population have been published over the years, no prior work has systematically synthesized the available evidence.
When focusing on general ANA screening, the results across studies were highly heterogeneous. The prevalence of ANA in women with PCOS ranged from as low as 0% [
38] to as high as 36% [
50], whereas in control groups it ranged from 0% [
38,
52] to 6% [
50]. However, much of this variability appears to be driven by methodological differences and, to a lesser extent, by study quality. When studies with high or moderate risk of bias were excluded [
18,
38,
52], the range slightly narrowed to 0.7–36.0% in PCOS and 2.3–6.0% in controls. Furthermore, when restricting the analysis to studies using ELISA (thereby excluding IIF [
18,
38,
51], studies with unspecified methodology [
55], and those with high/moderate risk of bias [
18,
38,
52]), the prevalence range became more consistent, at 18.4–36.0% in PCOS and 2.3–6.0% in controls. This illustrates that heterogeneity in ANA prevalence is largely attributable to methodological and quality-related factors. Overall, roughly half of the studies reported increased ANA prevalence [
16,
18,
50] or serum levels [
49,
50] in PCOS, while the remainder did not demonstrate significant differences [
38,
47,
51,
52,
55], most likely resulting from methodological variability. Taken together, these findings suggest that ANA may indeed be elevated in at least a subset of women with PCOS.
In contrast, results regarding specific ANA subtypes—particularly anti-dsDNA antibodies—were more consistent. Anti-dsDNA was the most frequently assessed subtype. Although absolute levels varied considerably across studies (with mean values ranging from 4.6 [
47] to 56.3 IU/mL [
50] in PCOS and from 3.8 [
47] to 35.4 IU/mL [
46] in controls), the overall trend strongly suggests an association between PCOS and elevated anti-dsDNA antibodies.
For other subtypes, the evidence is more limited and inconsistent. Anti-nucleosome [
47,
48,
53] and anti-histone antibodies [
47,
53,
54] were each assessed in three studies, with some reporting higher levels in PCOS but others finding no differences. Additional ANA subtypes (SSA, SSB, Sm, RNP, Scl-70, Jo-1, CENP-B) were only rarely investigated, with positive cases occurring sporadically and without reproducible group differences [
46,
51,
52].
From a pathophysiological perspective, the presence of higher ANA levels in PCOS could be explained through several complementary mechanisms: increased availability of nuclear autoantigens due to enhanced apoptosis, oxidative damage, and extracellular DNA release; genetic and epigenetic predisposition favoring aberrant autoantigen presentation; and immune cell imbalances with exaggerated B cell activity, resulting in increased immunoglobulin production and autoantibody generation.
The syndrome is characterized by low-grade chronic inflammation, oxidative stress, hormonal imbalance, and relative hyperestrogenism [
56,
57,
58], all of which may contribute to tissue damage and the subsequent release of intracellular antigens, ultimately triggering ANA production [
12]. Recent evidence further highlights the role of oxidative stress in immune activation. Vale-Fernandes et al. (2024) demonstrated that elevated anti-Müllerian hormone (AMH) levels correlate with increased oxidative stress within the follicular microenvironment in PCOS, and that circulating AMH may serve as a surrogate biomarker of this process [
59]. This oxidative–immune interplay is of particular interest because it links a routine reproductive biomarker with pathways of autoantibody production, and suggests that combined markers such as AMH and ANA could potentially help identify women at higher risk of adverse reproductive outcomes or autoimmune activation. Importantly, several studies have demonstrated increased apoptosis of ovarian granulosa cells in PCOS [
60], accompanied by upregulation of pro-apoptotic markers (Bax, Caspase-3) and downregulation of anti-apoptotic Bcl-2, as well as activation of inflammatory NF-κB pathways, shown in animal and cellular models of PCOS [
21,
60]. This excessive apoptosis within ovarian follicles provides a local source of nuclear antigens, which may become immunogenic if not efficiently cleared [
21,
23]. Furthermore, neutrophil extracellular traps (NETs) and elevated circulating DNA have been detected in PCOS serum and follicular fluid, offering a direct route for nuclear autoantigen exposure and subsequent autoantibody production [
24].
In addition, PCOS has been associated with a higher frequency of certain class II Human Leukocyte Antigen (HLA) allelic variants (e.g.,
HLA-DR and
HLA-DQ) [
61,
62], or expression changes in class I
HLA-B and
HLA-F [
63], which are also linked to autoimmune diseases, suggesting a possible genetic predisposition to autoimmunity in this population [
40]. Since HLA molecules are central to antigen presentation [
64], such alterations may facilitate the display of nuclear self-antigens released in PCOS, thereby lowering the threshold for autoantibody production, including ANA. Interestingly,
HLA-DRB1 and
HLA-DQB1 alleles have been reported both in association with PCOS [
61] and with ANA positivity in independent cohorts [
65]. Although the specific alleles differ across studies and populations, this convergence on the same HLA loci suggests that genetic variation within
DR/
DQ may influence aberrant presentation of nuclear antigens, thereby providing a mechanistic link between PCOS susceptibility and ANA production.
Beyond these factors, the detection of increased ANA and related autoantibodies in PCOS may reflect an exaggerated or dysregulated immune response [
66], supporting the hypothesis that autoimmunity could play a contributory role in the syndrome’s pathogenesis [
4,
67,
68]. Free light chains of immunoglobulins have been found to be elevated in the blood plasma of women with PCOS, providing direct evidence of enhanced overall antibody production and B cell activation in this condition [
69]. Immunophenotyping studies further demonstrate characteristic alterations in immune cell populations in PCOS. These include increased frequencies of activated B cells and higher circulating levels of B cell-activating factor (BAFF), both of which favor antibody and autoantibody production [
70,
71]. In parallel, a shift in T cell subsets has been observed, with reduced regulatory T cells (Treg) and increased proinflammatory type 1 T helper (Th1) and type 17 T helper (Th17) cells, creating an environment permissive for autoreactive B cell help [
72]. Natural killer (NK) cells are often elevated and display enhanced cytotoxicity, potentially contributing to increased cell death and autoantigen release [
73]. Moreover, monocytes and macrophages in PCOS preferentially polarize toward a proinflammatory M1 phenotype, secreting interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α), cytokines that further amplify B cell activation and Th17 differentiation [
74]. Taken together, these cellular abnormalities provide a coherent explanation for the enhanced humoral immune activation in PCOS and the increased likelihood of ANA production.
From a clinical perspective, it is important to underline that higher ANA titers or a higher prevalence of ANA positivity in PCOS does not necessarily imply a high frequency of systemic autoimmune rheumatologic diseases in this population [
27]. Although three of the included studies reported ANA prevalence rates as high as 20–30% in women with PCOS [
16,
18,
50], studies assessing autoimmune comorbidities suggest only a modest numerical increase in certain conditions, such as rheumatoid arthritis or systemic sclerosis, with low prevalence rates of 1–2% in PCOS compared with 1% or less in controls [
35,
36,
37]. Autoimmune connective tissue diseases remain relatively rare in PCOS, and the observed increase in ANA positivity likely reflects immunological activation rather than manifest autoimmune disease. Moreover, ANA positivity can also be detected in several non-pathological conditions, including physiological states such as pregnancy [
30] or aging [
28], and in otherwise healthy individuals without clinical autoimmune disease [
27,
32], as well as in other endocrine and gynecological disorders such as endometriosis [
75] and autoimmune thyroid disease [
76]. Awareness of this association may therefore help clinicians avoid overdiagnosis or unnecessary concern when incidental ANA positivity is identified in PCOS patients [
32]. Thus, ANA positivity in PCOS should be interpreted primarily as a marker of non-specific immune activation, rather than evidence of underlying systemic autoimmune disease. This highlights the need for prospective longitudinal studies to determine whether ANA positivity predicts future autoimmune morbidity or simply reflects the background of chronic low-grade inflammation characteristic of PCOS. At present, to our knowledge, no long-term cohort has assessed the persistence of ANA over time or its progression to clinically manifest autoimmune disease in PCOS. Such studies are essential to distinguish transient, inflammation-related positivity from a stable autoimmune trait. Moreover, integrating emerging biomarkers, such as AMH as a surrogate of oxidative stress [
59], into longitudinal designs could clarify whether oxidative–immune interactions contribute to sustained autoantibody production and adverse reproductive or metabolic outcomes.
To sum up, the current evidence does not support routine ANA screening in all women with PCOS, as ANA positivity most often reflects non-specific immune activation rather than systemic autoimmune disease. However, testing may be clinically relevant in selected subgroups, such as patients presenting with rheumatologic symptoms or women with recurrent pregnancy loss or implantation failure during assisted reproduction, where ANA positivity has been associated with adverse outcomes in some studies [
77,
78,
79]. From a management perspective, the detection of ANA in PCOS should not prompt routine therapeutic interventions in asymptomatic patients, but it may inform closer monitoring, timely referral for rheumatologic evaluation, or individualized reproductive counseling in women at higher clinical risk.
This systematic review has several important limitations that should be considered when interpreting the findings. The most important relates to the high variability in methodologies used to detect ANA: some studies applied indirect immunofluorescence (IIF) on different substrates (e.g., HEp-2 cells vs. animal tissues), while others employed ELISA assays with differing antigen panels. An additional concern is that assay performance characteristics varied markedly across studies. IIF is considered the reference method for ANA detection, but its sensitivity and specificity depend heavily on the substrate used and the positivity threshold. Lower cut-offs increase sensitivity but reduce specificity, yielding more incidental low-titer positives, whereas higher cut-offs may miss clinically relevant cases. Similarly, ELISA assays differ in the antigen panels employed, with some targeting broad nuclear extracts and others focusing on selected antigens, which can yield divergent prevalence estimates. Additionally, positivity thresholds for ELISA were inconsistent, making direct comparisons unreliable. Moreover, the results lacked consistency in reporting: some studies presented mean values, others reported medians without interquartile ranges, and in some cases, results were shown only graphically. Direct comparisons were also hampered by the use of different measurement units (IU/mL vs. optical density). Inconsistencies in terminology were also evident, with certain studies referring to “ANA” when in fact only specific subtypes (e.g., anti-nucleosome antibodies) were measured, further complicating synthesis of the evidence. In addition, there were limitations inherent to PCOS research in general: diagnostic criteria for PCOS were not uniform across studies, ranging from NIH to Rotterdam definitions and local adaptations, thereby contributing to clinical heterogeneity. The studies also varied in inclusion and exclusion criteria, and only a few applied BMI matching between groups. Overall, the risk of bias across studies was mostly low, but several studies with moderate or high risk were identified, and these tended to present the most heterogeneous and extreme results (e.g., ANA prevalence of 0% in one study and 30% in another), further complicating data synthesis.
Nevertheless, this systematic review highlights several important insights. To our knowledge, it is the first systematic review to comprehensively evaluate the occurrence of ANA and their subtypes in women with PCOS, thereby filling an important gap in the literature. In contrast to earlier narrative reports, we considered both general ANA screening and specific ANA subtypes (including anti-dsDNA, anti-nucleosome, and anti-histone antibodies), which provides a more complete overview of potential autoimmune involvement in PCOS. By separately analyzing studies reporting prevalence and those reporting quantitative serum levels, the review offers a nuanced understanding of how autoantibody patterns differ across methodologies. A further strength is the critical appraisal of methodological aspects, including differences in diagnostic criteria for PCOS, assay platforms (IIF vs. ELISA), and units of measurement, which helps to explain the observed heterogeneity and highlights the need for standardization in future research.