CAR-T in the Treatment of Solid Tumors—A Review of Current Research and Future Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Results
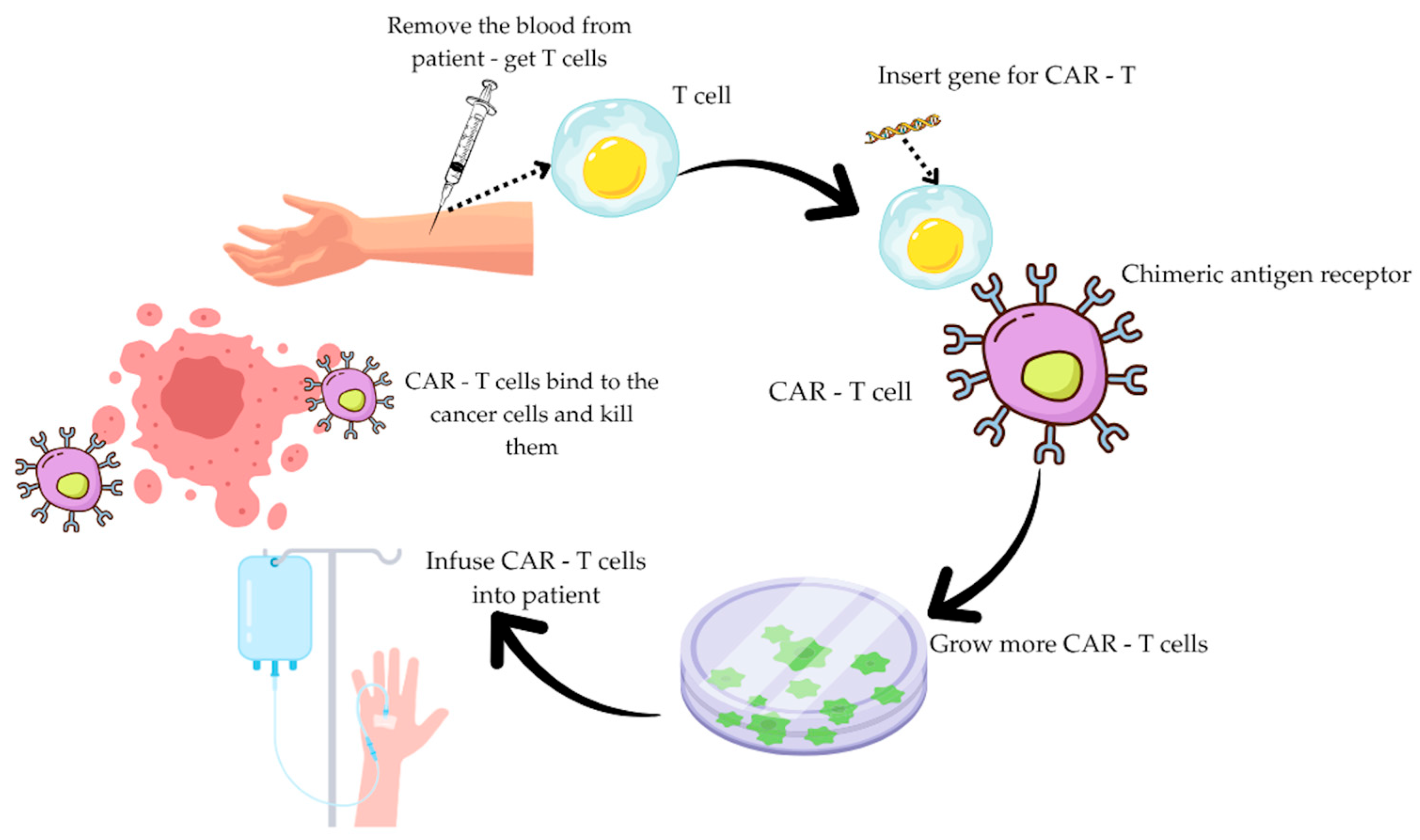
3.1. CAR-T Therapy
3.2. Mechanism of Action of CAR-T Cells
3.3. Currently Approved CAR-T Products and Their Clinical Indications
3.4. Clinical Significance in Practice
3.5. Future Directions and Strategies to Overcome Limitations of CAR-T Therapy in Solid Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | Acute Lymphoblastic Leukemia |
| BCMA | B-Cell Maturation Antigen |
| CAR | Chimeric Antigen Receptor |
| CAR-T | Chimeric Antigen Receptor T-cell |
| Cas9 | CRISPR-associated protein 9 |
| CLDN18.2 | Claudin 18.2 |
| CRS | Cytokine Release Syndrome |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CTLA-4 | Cytotoxic T-lymphocyte Associated Protein 4 |
| CTLs | Cytotoxic T Lymphocytes |
| DCR | Disease Control Rate |
| DD | Death Domain |
| DISC | Death-Inducing Signaling Complex |
| DLBCL | Diffuse Large B-Cell Lymphoma |
| EGFR | Epidermal growth Factor Receptor |
| EMA | European Medicines Agency |
| FADD | Fas-Associated Death Domain Protein |
| FDA | Food and Drug Administration |
| FL | Follicular Lymphoma |
| FNC | Fludarabine, Cyclophosphamide, Nab-paclitaxel |
| GVHD | Graft-versus-host Disease |
| HCC | Hepatocellular Carcinoma |
| HER-2 | Human Epidermal Growth Factor Receptor 2 |
| HGBL | High-Grade B-cell Lymphoma |
| HSCT | Hematopoietic Stem Cell Transplantation |
| ICANS | Immune Effector Cell-associated Neurotoxicity Syndrome |
| IFN-γ | Interferon Gamma |
| IL-6 | Interleukin 6 |
| IS | Immunological Synapse |
| ITAMs | Immunoreceptor Tyrosine-based Activation Motifs |
| LBCL | Large B-cell Lymphoma |
| LFA-1 | Lymphocyte Function-associated Antigen-1 |
| MAV | Metabolically Active Tumor Volume |
| MCL | Mantle Cell Lymphoma |
| MDSCs | Myeloid-Derived Suppressor Cells |
| NKG2D | Natural Killer Group 2 Member D |
| OS | Overall Survival |
| ORR | Objective Response Rate |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PD-L1 | Programmed Death-Ligand 1 |
| PET-CT | Positron Emission Tomography Coupled With Computed Tomography |
| PFS | Progression-Free Survival |
| PMBCL | Primary Mediastinal B-cell Lymphoma |
| PR | Partial Response |
| REMS | Risk Evaluation and Mitigation Strategy |
| SD | Stable Disease |
| SLL | Small Lymphocytic Lymphoma |
| SMACs | Supramolecular Activation Clusters |
| TAM | Tumor-associated Macrophages |
| T-cell | Lymphocyte T |
| TCR/CD3 | T-cell receptor/CD3 complex |
| TNF-α | Tumor Necrosis Factor alpha |
| TRUCK | T cells Redirected for Antigen-Unrestricted Cytokine-initiated Killing |
| TME | Tumor Microenvironment |
| VHH | Variable Domain Of An Antibody Heavy Chain |
References
- Ercilla-Rodríguez, P.; Sánchez-Díez, M.; Alegría-Aravena, N.; Quiroz-Troncoso, J.; Gavira-O’Neill, C.E.; González-Martos, R.; Ramírez-Castillejo, C. CAR-T lymphocyte-based cell therapies; mechanistic substantiation, applications and biosafety enhancement with suicide genes: New opportunities to melt side effects. Front. Immunol. 2024, 15, 1333150. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maus, M.V.; Hinrichs, C.S. CAR T cells and T-cell therapies for cancer: A translational science review. JAMA 2024, 332, 1924–1935. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Nasiri, F.; Safarzadeh Kozani, P.; Salem, F.; Mahboubi Kancha, M.; Dashti Shokoohi, S.; Safarzadeh Kozani, P. Mechanisms of antigen-dependent resistance to chimeric antigen receptor (CAR)-T cell therapies. Cancer Cell Int. 2025, 25, 64. [Google Scholar] [CrossRef]
- Marvin-Peek, J.; Savani, B.N.; Olalekan, O.O.; Dholaria, B. Challenges and advances in chimeric antigen receptor therapy for acute myeloid leukemia. Cancers 2022, 14, 497. [Google Scholar] [CrossRef]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 2020, 38, 473–488. [Google Scholar] [CrossRef]
- Guedan, S.; Calderon, H.; Posey, A.D.; Maus, M.V. Engineering and design of chimeric antigen receptors. Mol. Ther. Methods Clin. Dev. 2018, 12, 145–156. [Google Scholar] [CrossRef]
- Jayaraman, J.; Mellody, M.P.; Hou, A.J.; Desai, R.P.; Fung, A.W.; Pham, A.H.T.; Chen, Y.Y.; Zhao, W. CAR-T design: Elements and their synergistic function. EBioMedicine 2020, 58, 102931. [Google Scholar] [CrossRef] [PubMed]
- Weinkove, R.; George, P.; Dasyam, N.; McLellan, A.D. Selecting costimulatory domains for chimeric antigen receptors: Functional and clinical considerations. Clin. Transl. Immunol. 2019, 8, e1049. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhong, J.F.; Zhang, X. Engineering CAR-T cells. Biomark. Res. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Moghanloo, E.; Mollanoori, H.; Talebi, M.; Pashangzadeh, S.; Faraji, F.; Hadjilooei, F.; Mahmoodzadeh, H. Remote controlling of CAR-T cells and toxicity management: Molecular switches and next generation CARs. Transl. Oncol. 2021, 14, 101070. [Google Scholar] [CrossRef]
- Benmebarek, M.-R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef]
- Khan, A.N.; Chowdhury, A.; Karulkar, A.; Jaiswal, A.K.; Banik, A.; Asija, S.; Purwar, R. Immunogenicity of CAR-T cell therapeutics: Evidence, mechanism and mitigation. Front. Immunol. 2022, 13, 886546. [Google Scholar] [CrossRef]
- Davenport, A.J.; Jenkins, M.R. Programming a serial killer: CAR T cells form non-classical immune synapses. Oncoscience 2018, 5, 69–70. [Google Scholar] [CrossRef]
- Czaplicka, A.; Lachota, M.; Pączek, L.; Zagożdżon, R.; Kaleta, B. Chimeric antigen receptor T cell therapy for pancreatic cancer: A review of current evidence. Cells 2024, 13, 101. [Google Scholar] [CrossRef]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current progress in CAR-T cell therapy for solid tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Volpe, E.; Sambucci, M.; Battistini, L.; Borsellino, G. Fas-Fas ligand: Checkpoint of T cell functions in multiple sclerosis. Front. Immunol. 2016, 7, 382. [Google Scholar] [CrossRef]
- Fischer, J.W.; Bhattarai, N. CAR-T cell therapy: Mechanism, management, and mitigation of inflammatory toxicities. Front. Immunol. 2021, 12, 693016. [Google Scholar] [CrossRef]
- Kagoya, Y.; Tanaka, S.; Guo, T.; Anczurowski, M.; Wang, C.-H.; Saso, K.; O Butler, M.; Minden, M.D.; Hirano, N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018, 24, 352–359. [Google Scholar] [CrossRef]
- Awasthi, R.; Maier, H.J.; Zhang, J.; Lim, S. Kymriah® (tisagenlecleucel)—An overview of the clinical development journey of the first approved CAR-T therapy. Hum. Vaccines Immunother. 2023, 19, 2210046. [Google Scholar] [CrossRef]
- Kymriah. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah (accessed on 31 July 2025).
- Tecartus. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tecartus (accessed on 31 July 2025).
- Center for Biologics Evaluation and Research. TECARTUS. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus (accessed on 31 July 2025).
- Kopmar, N.E.; Cassaday, R.D. Clinical insights on brexucabtagene autoleucel for the treatment of patients with relapsed or refractory B-cell acute lymphoblastic leukemia. Cancer Manag. Res. 2024, 16, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Center for Biologics Evaluation and Research. BREYANZI. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/breyanzi (accessed on 31 July 2025).
- Breyanzi. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/breyanzi (accessed on 31 July 2025).
- Abecma. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/abecma (accessed on 31 July 2025).
- Lin, Y.; Raje, N.; Berddeja, J.G.; Siegel, D.S.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Massaro, M.; et al. Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in patients with relapsed and refractory multiple myeloma: Updated results from phase 1 CRB-401 study. Blood 2020, 136 (Suppl. 1), 26–27. [Google Scholar] [CrossRef]
- Center for Biologics Evaluation and Research. CARVYKTI. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/carvykti (accessed on 31 July 2025).
- Hansen, D.K.; Peres, L.C.; Dima, D.; Richards, A.; Shune, L.; Afrough, A.; Midha, S.; Dhakal, B.; Kocoglu, M.H.; Atrash, S.; et al. Comparison of standard-of-care idecabtagene vicleucel and ciltacabtagene autoleucel in relapsed/refractory multiple myeloma. J. Clin. Oncol. 2025, 43, 1597–1609, Erratum in J. Clin. Oncol. 2025, 43, 1399. [Google Scholar] [CrossRef] [PubMed]
- Yescarta. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta (accessed on 31 July 2025).
- Kite’s Yescarta First CAR T Cell Therapy to Receive European Marketing Authorization for Use in Second Line Diffuse Large B Cell Lymphoma and High Grade B Cell Lymphoma. Available online: https://www.gilead.com/news/news-details/2022/kites-yescarta-first-car-t-cell-therapy-to-receive-european-marketing-authorization-for-use-in-second-line-diffuse-large-b-cell-lymphoma-and-high-grade-b-cell-lymphoma (accessed on 3 September 2025).
- Center for Biologics Evaluation and Research. AUCATZYL. Available online: https://www.fda.gov/vaccines-blood-biologics/aucatzyl (accessed on 3 September 2025).
- Center for Drug Evaluation and Research. FDA Approves Obecabtagene Autoleucel for Adults with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-obecabtagene-autoleucel-adults-relapsed-or-refractory-b-cell-precursor-acute (accessed on 3 September 2025).
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018, 7, e1440169. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Hara, M.H.; Lacey, S.F.; Torigian, D.A.; Nazimuddin, F.; Chen, F.; Kulikovskaya, I.M.; Soulen, M.C.; McGarvey, M.; Nelson, A.M.; et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 2018, 155, 29–32. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Wu, Z.; Feng, K.; Tong, C.; Wang, Y.; Dai, H.; Shi, F.; Yang, Q.; Han, W. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: A phase I clinical trial. Cytotherapy 2020, 22, 573–580. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018, 9, 838–847. [Google Scholar] [CrossRef]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef]
- Sackstein, R. The first step in adoptive cell immunotherapeutics: Assuring cell delivery via glycoengineering. Front. Immunol. 2018, 9, 3084. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Yeku, O.O.; Purdon, T.J.; Koneru, M.; Spriggs, D.; Brentjens, R.J. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 2017, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Springuel, L.; Lonez, C.; Alexandre, B.; van Cutsem, E.; Machiels, J.-P.H.; van Den Eynde, M.; Prenen, H.; Hendlisz, A.; Shaza, L.; Carrasco, J.; et al. Chimeric antigen receptor-T cells for targeting solid tumors: Current challenges and existing strategies. BioDrugs 2019, 33, 515–537. [Google Scholar] [CrossRef]
- Sakemura, R.; Terakura, S.; Watanabe, K.; Julamanee, J.; Takagi, E.; Miyao, K.; Koyama, D.; Goto, T.; Hanajiri, R.; Nishida, T.; et al. A Tet-On inducible system for controlling CD19-chimeric antigen receptor expression upon drug administration. Cancer Immunol. Res. 2016, 4, 658–668. [Google Scholar] [CrossRef]
- Demoulin, B.; Cook, W.J.; Murad, J.; Graber, D.J.; Sentman, M.L.; Lonez, C.; Gilham, D.E.; Sentman, C.L.; Agaugue, S. Exploiting natural killer group 2D receptors for CAR T-cell therapy. Future Oncol. 2017, 13, 1593–1605. [Google Scholar] [CrossRef]
- Lu, L.; Xie, M.; Yang, B.; Zhao, W.-B.; Cao, J. Enhancing the safety of CAR-T cell therapy: Synthetic genetic switch for spatiotemporal control. Sci. Adv. 2024, 10, eadj6251. [Google Scholar] [CrossRef]
- Freyer, C.W.; Porter, D.L. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J. Allergy Clin. Immunol. 2020, 146, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Sek, K.; Chen, A.X.Y.; Cole, T.; Armitage, J.D.; Tong, J.; Yap, K.M.; Munoz, I.; Dunbar, P.A.; Wu, S.; van Elsas, M.J.; et al. Tumor site-directed A1R expression enhances CAR T cell function and improves efficacy against solid tumors. Nat. Commun. 2025, 16, 6123. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-F.; Xie, D. Targeting the gut microbiota to enhance the antitumor efficacy and attenuate the toxicity of CAR-T cell therapy: A new hope? Front. Immunol. 2024, 15, 1362133. [Google Scholar] [CrossRef]
- Second Affiliated Hospital of Guangzhou Medical University. CAR-T Cell Targeting GPC3 for Immunotherapy of Hepatocellular Carcinoma: Phase I Clinical Trial. Available online: https://clinicaltrials.gov/study/NCT03198546 (accessed on 31 July 2025).
- Hou, B.; Tang, Y.; Li, W.; Zeng, Q.; Chang, D. Efficiency of CAR-T Therapy for Treatment of Solid Tumor in Clinical Trials: A Meta-Analysis. Dis. Markers 2019, 2019, 3425291. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Chen, S.; van den Brink, M.R.M. Allogeneic ‘off-the-shelf’ CAR T cells: Challenges and advances. Best Pract. Res. Clin. Haematol. 2024, 37, 101566. [Google Scholar] [CrossRef] [PubMed]
- FDA Eliminates Risk Evaluation and Mitigation Strategies (REMS) for Autologous Chimeric Antigen Receptor (CAR) T Cell Immunotherapies. Available online: https://www.fda.gov/news-events/press-announcements/fda-eliminates-risk-evaluation-and-mitigation-strategies-rems-autologous-chimeric-antigen-receptor (accessed on 31 July 2025).
| Adverse Event | Frequency [%] |
|---|---|
| Lymphocytopenia | 88% |
| Oral mucosal edema | 63% |
| Fever/fatigue | 56% |
| Mucositis oral | 43% |
| The Title of the Research | Intervention | Patients Number | Construct Type | Lymfodepletion | Type of Study/Phase | Dose | Duration of Follow-Up | Cancers | Endpoints | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| CD133-directed CAR-T cells for advanced metastasis malignancies: a phase I trial [38] | CART-CD133 | 23 | Anti-CD133 scFv (derived from HW350341.1) + 4-1BB + CD3ζ Lentivirus vector | Not applied. | I phase | 0.5 to 2 × 106/kg body weight | 24.5 months | HCC, pancreatic cancer, colon cancer | PR PFS SD DCR | PR: 3 patients PFS in HCC: 7months; overall: 5 months SD: 14 patients DCR 3 months 65.2%, DCR 6 months 30.4% Remission: 9 patients |
| Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers [39] | CART-meso | 15 | scFv anty-mesotelin + 4-1BB + CD3ζ; lentiwirus | Some patients received 1.5 g/m2 of cyclophosphamide | I phase | 1–3 × 107 or 108/m2 | Tumor assessments at 1, 3, and 6 months after CART-meso infusion, with additional evaluations every 3 months for up to 2 years | Ovarian adenocarcinoma, pancreatic ductal carcinoma, pleural mesothelioma | SD PFS | SD: 11 patients PFS: 2.1 months In 1 patient reduction of tumor mass—26% (did not meet the RECIST 1.1 criteria) |
| Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial [40] | CART-meso | 6 | anti-mesothelin ss1 scFv + 4-1BB + CD3ζ; lentivirus vector | Not applied. | I phase | 1 to 3 × 108/m2 T cells three times weekly | CT imaging—4 months PET/CT imaging—2 months | Pancreatic adenocarcinoma | SD PFS | SD: 2 patients PFS: 3.8 and 5.4 months MAV: stable in 3 patients and reduced in 1 patient |
| Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: a phase I clinical trial [41] | CART-EGFR | 16 | scFv anty-EGFR + 4-1BB + CD3ζ | 100–200 mg/m2 nab-paklitaksel + 15–35 mg/kg cyklofosfamid | I phase | 1.31–8.9 × 106/kg, total 25 cycles | 49 months | Pancreatic cancer | PR SD DCR PFS OS | PR lasts 2–4 months: 4 patients SD: 8 patients DCR: 85.7%. PFS median: 3 months, OS median: 4.9 months |
| Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers [42] | CART-HER2 | 11 | scFv anti-HER2 + CD8α + CD3ζ | 100–200 mg/m2 nab-paklitaksel + 15–35 mg/kg cyklofosfamid | I phase | 0.76 × 106 to 1.82 × 107 CAR-T cells/kg body weight in one or more infusions | Not reported. | Cancer of the biliary tract and pancreas | PR SD PFS | PR: 1 patient SD: 5 patients PFS: 4.8 months In 1 patient, disappearance of the metastatic lesion and a decrease in the standardized uptake value of the second lesion from 6.5 to 4.7 |
| Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results [43] | CART-CLDN18.2 | 37 | scFv anti-CLDN18.2 + CD3ζ + 4-1BB | Not applied. | I phase | 89 patients were dosed with 2.5 × 108, six with 3.75 × 108 and three with 5.0 × 108 CAR T cells | Median: 32.4 months | Stomach cancer and gastric-esophageal junction cancer, pancreatic cancer | ORR DCR PFS OS | ORR: 48.6% DCR: 73.0% PFS: 3.7 months OS: 6 months: 80.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picheta, N.; Piekarz, J.; Daniłowska, K.; Szklener, K.; Mańdziuk, S. CAR-T in the Treatment of Solid Tumors—A Review of Current Research and Future Perspectives. Int. J. Mol. Sci. 2025, 26, 9486. https://doi.org/10.3390/ijms26199486
Picheta N, Piekarz J, Daniłowska K, Szklener K, Mańdziuk S. CAR-T in the Treatment of Solid Tumors—A Review of Current Research and Future Perspectives. International Journal of Molecular Sciences. 2025; 26(19):9486. https://doi.org/10.3390/ijms26199486
Chicago/Turabian StylePicheta, Natalia, Julia Piekarz, Karolina Daniłowska, Katarzyna Szklener, and Sławomir Mańdziuk. 2025. "CAR-T in the Treatment of Solid Tumors—A Review of Current Research and Future Perspectives" International Journal of Molecular Sciences 26, no. 19: 9486. https://doi.org/10.3390/ijms26199486
APA StylePicheta, N., Piekarz, J., Daniłowska, K., Szklener, K., & Mańdziuk, S. (2025). CAR-T in the Treatment of Solid Tumors—A Review of Current Research and Future Perspectives. International Journal of Molecular Sciences, 26(19), 9486. https://doi.org/10.3390/ijms26199486







