Molecular Insights into Human Placentation: From Villous Morphogenesis to Pathological Pathways and Translational Biomarkers
Abstract
1. Introduction
2. Molecular and Cellular Basis of Normal Placentation
2.1. Blastocyst Implantation and Trophoblast Differentiation
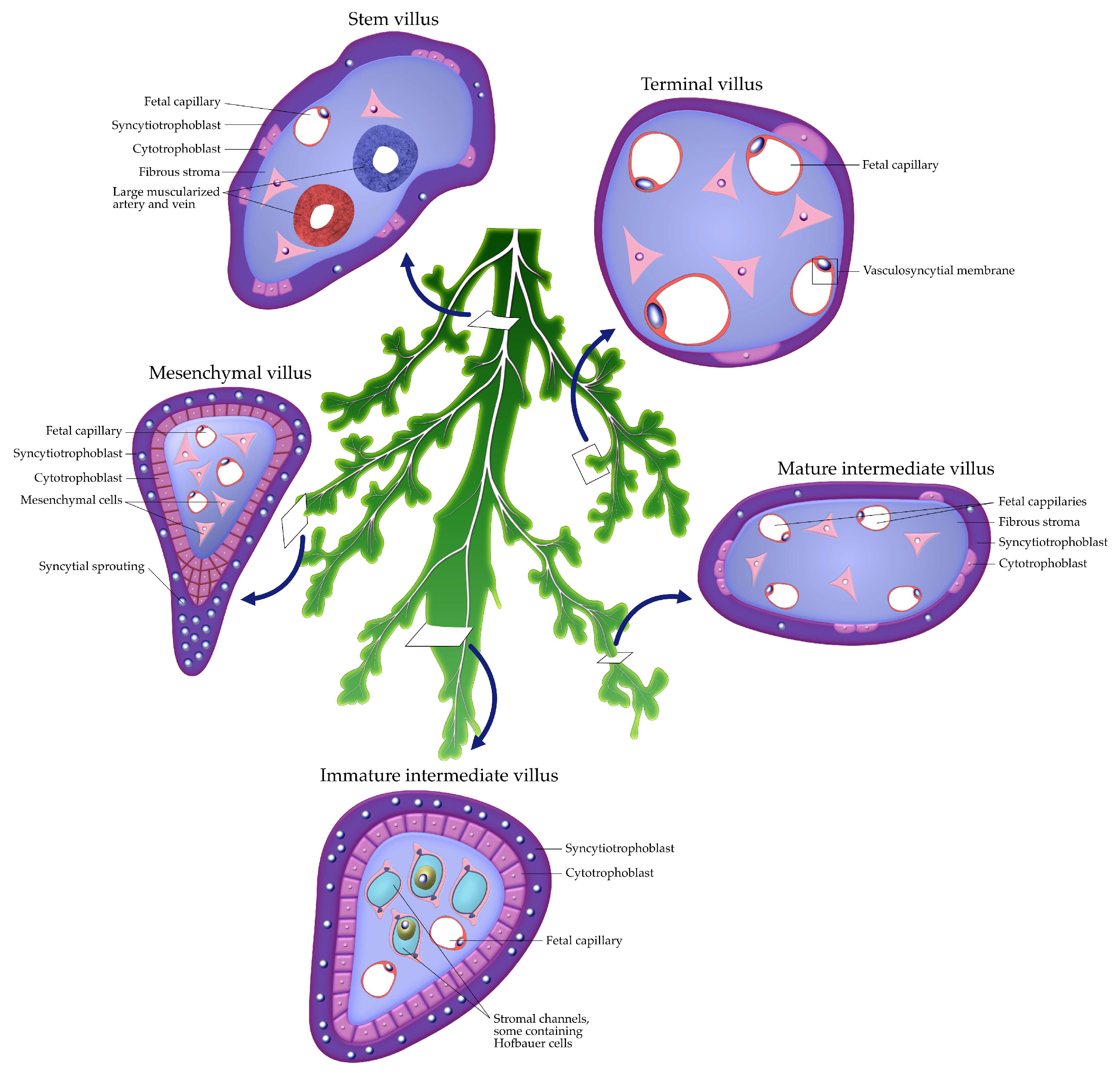
2.2. Chorionic Villous Development
2.3. Trophoblast Invasion and Spiral Artery Remodeling
2.4. Syncytiotrophoblast Formation and Endocrine Functions
2.5. Transition from Histiotrophic to Hemotrophic Nutrition
3. Pathological Pathways in Placental Disease
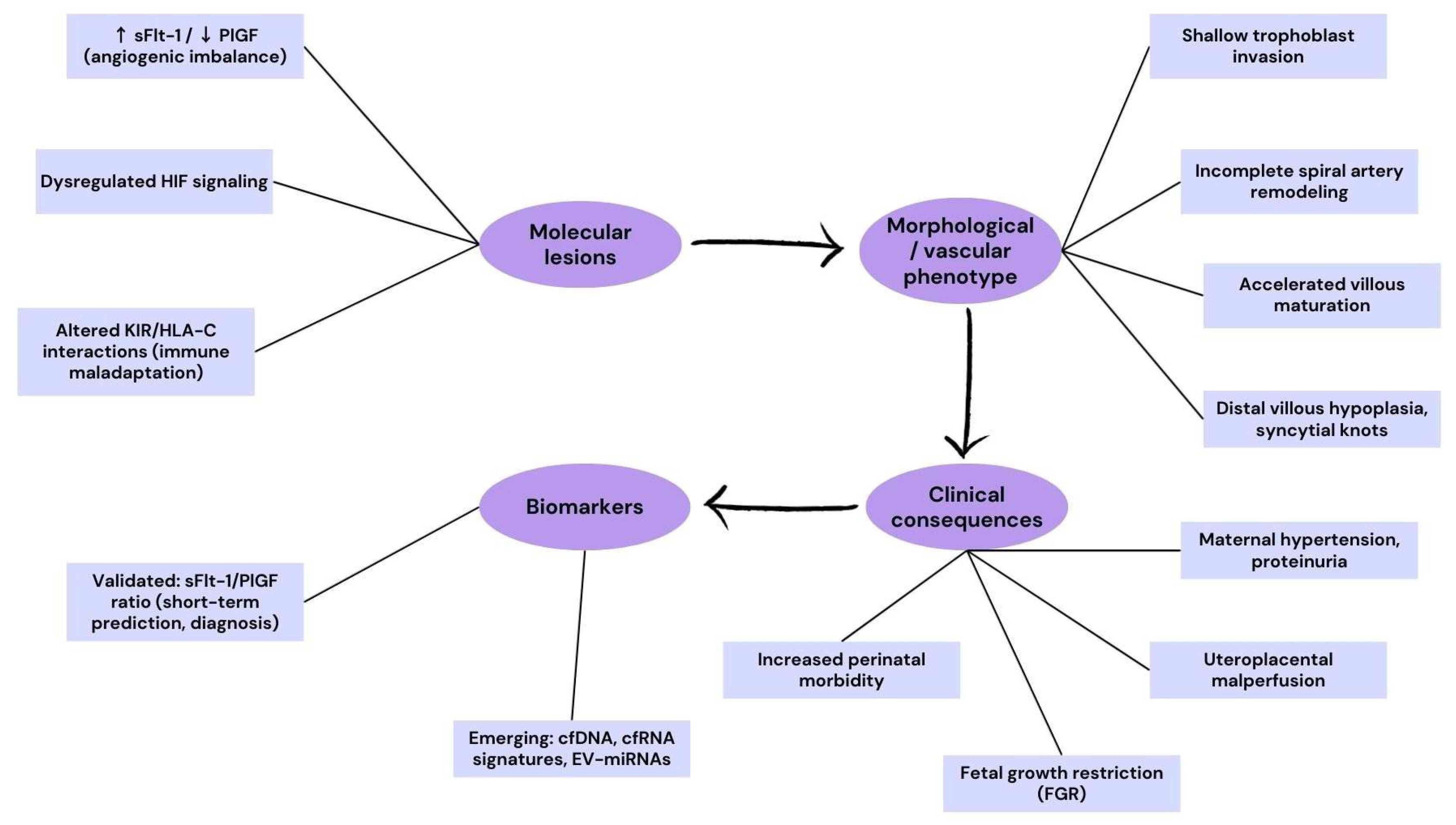
3.1. Preeclampsia and Fetal Growth Restriction
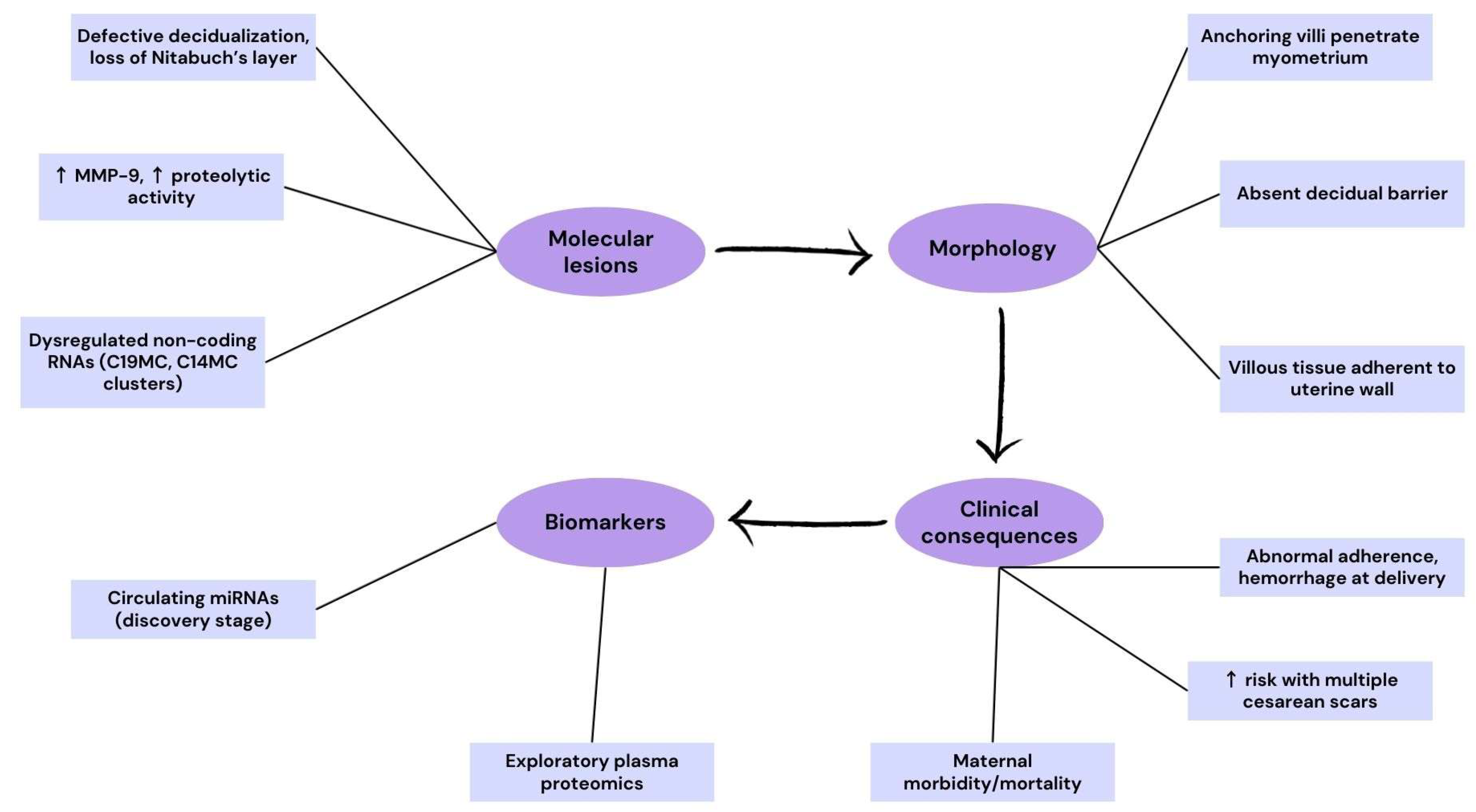
3.2. Placenta Accreta Spectrum (PAS)
3.3. Multiple Gestations and Monochorionic Complications
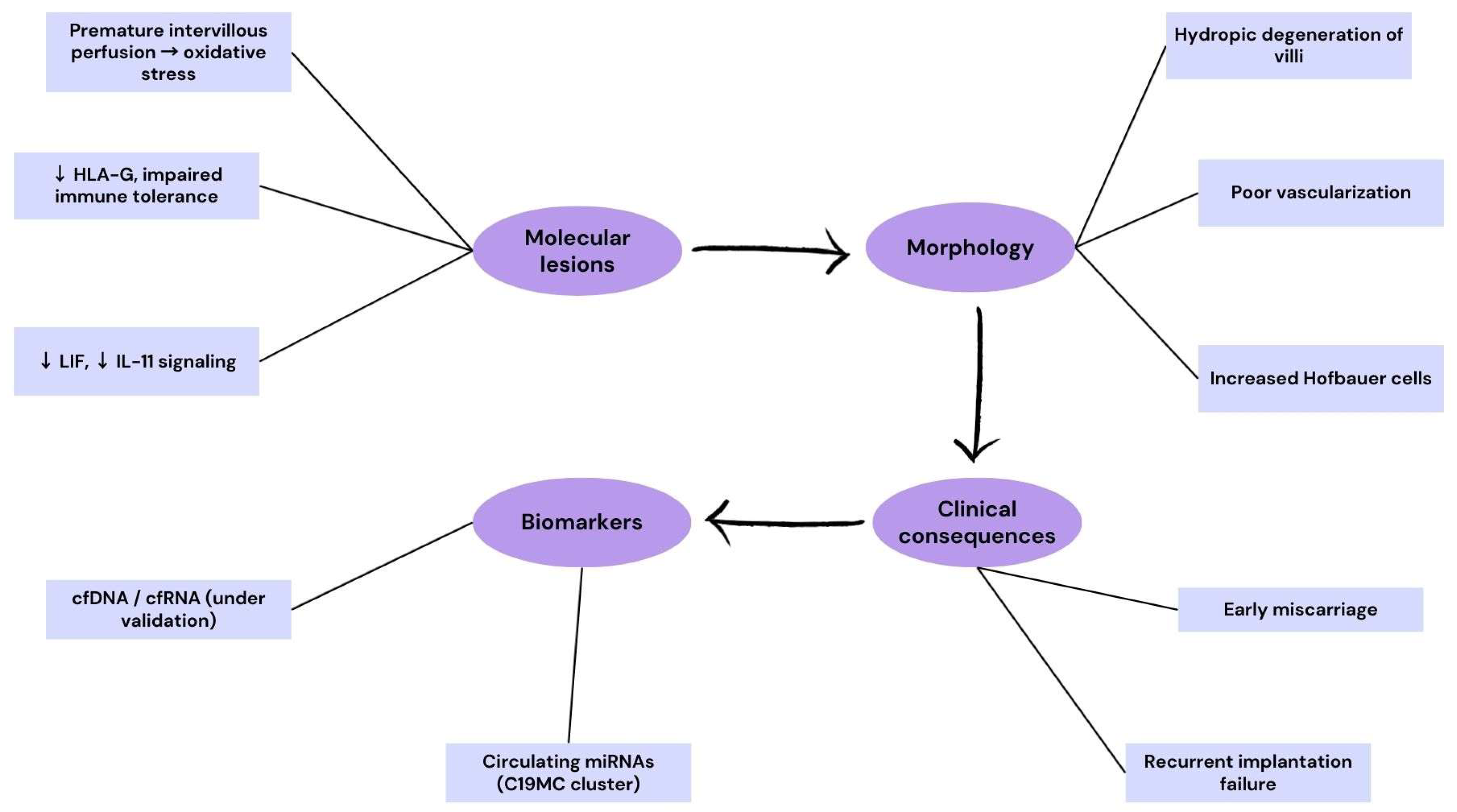
3.4. Pregnancy Loss and Early Implantation Failure
3.5. Structural and Vascular Variants
3.6. Molecular Crossroads of Placental Pathology
4. Diagnostics and Biomarkers
4.1. Angiogenic Markers (sFlt-1/PlGF Ratio)
4.2. Placental Proteins (PAPP-A, PP13, hCG, hPL)
4.3. Nucleic Acid–Based Biomarkers (cfDNA, miRNAs, lncRNAs)
4.4. Extracellular Vesicles and Exosome Signatures
4.5. Multi-Omics Integration
5. Discussion and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Knöfler, M.; Pollheimer, J. Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front. Genet. 2013, 4, 190. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral Artery Remodeling and Trophoblast Invasion in Preeclampsia and Fetal Growth Restriction. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef]
- Moser, G.; Weiss, G.; Sundl, M.; Gauster, M.; Siwetz, M.; Lang-Olip, I.; Huppertz, B. Extravillous trophoblasts invade more than uterine arteries: Evidence for the invasion of uterine veins. Histochem. Cell Biol. 2017, 147, 353–366. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, Y.; Bian, Q.; Zhang, N.; Wang, M.; Wang, J.; Li, X.; Lai, L.; Zhao, Z.; Yu, H. Molecular mechanisms of syncytin-1 in tumors and placental development related diseases. Discov. Oncol. 2023, 14, 104. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef]
- Ietta, F.; Wu, Y.; Winter, J.; Xu, J.; Wang, J.; Post, M.; Caniggia, I. Dynamic HIF1A Regulation During Human Placental Development1. Biol. Reprod. 2006, 75, 112–121. [Google Scholar] [CrossRef]
- Papúchová, H.; Meissner, T.B.; Li, Q.; Strominger, J.L.; Tilburgs, T. The Dual Role of HLA-C in Tolerance and Immunity at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2730. [Google Scholar] [CrossRef]
- de Sá, C.P.N.; Jiménez, M.F.; Rosa, M.W.; Arlindo, E.M.; Ayub, A.C.K.; Cardoso, R.B.; Kreitchmann, R.; El Beitune, P. Evaluation of Angiogenic Factors (PlGF and sFlt-1) in Pre-eclampsia Diagnosis. Rev. Bras. Hematol. Hemoter. 2020, 42, 697–704. [Google Scholar] [CrossRef]
- Zsengellér, Z.K.; Mastyugin, M.; Fusco, A.R.; Vlocskó, B.; Costa, M.; Ferguson, C.; Pintye, D.; Sziva, R.E.; Salahuddin, S.; Young, B.C.; et al. Mitigating Oxidative Stress and Anti-Angiogenic State in an In Vitro Model of Preeclampsia by HY-12, an Organofluorine Hydrazone Antioxidant. Curr. Issues Mol. Biol. 2025, 47, 680. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Ondrackova, M.; Pirkova, P.; Kestlerova, A.; Novotna, V.; Hympanova, L.; Krofta, L. Expression Profile of C19MC microRNAs in Placental Tissue in Pregnancy-Related Complications. DNA Cell Biol. 2015, 34, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Placenta Extracellular Vesicles: Messengers Connecting Maternal and Fetal Systems. Biomolecules 2024, 14, 995. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Turco, M.Y.; Burton, G.J.; Moffett, A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Updat. 2020, 26, 501–513. [Google Scholar] [CrossRef]
- Moore, K.H.; Murphy, H.A.; Chapman, H.; George, E.M. Syncytialization alters the extracellular matrix and barrier function of placental trophoblasts. Am. J. Physiol. Physiol. 2021, 321, C694–C703. [Google Scholar] [CrossRef]
- Gusella, A.; Martignoni, G.; Giacometti, C. Behind the Curtain of Abnormal Placentation in Pre-Eclampsia: From Molecular Mechanisms to Histological Hallmarks. Int. J. Mol. Sci. 2024, 25, 7886. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Espino-Y-Sosa, S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S.; Ruiz-Ramirez, E.; Velasco-Espin, M.; Cerda-Flores, P.; Ramirez-Gonzalez, A.; et al. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef]
- Arutyunyan, A.; Roberts, K.; Troulé, K.; Wong, F.C.K.; Sheridan, M.A.; Kats, I.; Garcia-Alonso, L.; Velten, B.; Hoo, R.; Ruiz-Morales, E.R.; et al. Spatial multiomics map of trophoblast development in early pregnancy. Nature 2023, 616, 143–151. [Google Scholar] [CrossRef]
- Gauster, M.; Moser, G.; Wernitznig, S.; Kupper, N.; Huppertz, B. Early human trophoblast development: From morphology to function. Cell. Mol. Life Sci. 2022, 79, 345. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.-H.; Wang, Y.-X.; Liu, T.-H. A comprehensive review of human trophoblast fusion models: Recent developments and challenges. Cell Death Discov. 2023, 9, 372. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, C.; Wang, P.; Yang, W.; Zhu, H.; Zhang, S. Regulators involved in trophoblast syncytialization in the placenta of intrauterine growth restriction. Front. Endocrinol. 2023, 14, 1107182. [Google Scholar] [CrossRef] [PubMed]
- Priščáková, P.; Svoboda, M.; Feketová, Z.; Hutník, J.; Repiská, V.; Gbelcová, H.; Gergely, L. Syncytin-1, syncytin-2 and suppressyn in human health and disease. J. Mol. Med. 2023, 101, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. The human placenta: New perspectives on its formation and function during early pregnancy. Proc. R. Soc. B Biol. Sci. 2023, 290, 20230191. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Siriwardena, D.; Penfold, C.; Pavlinek, A.; Boroviak, T.E. An integrated atlas of human placental development delineates essential regulators of trophoblast stem cells. Development 2022, 149, dev200171. [Google Scholar] [CrossRef]
- Aye, I.L.M.H.; Tong, S.; Charnock-Jones, D.S.; Smith, G.C.S. The human placenta and its role in reproductive outcomes revisited. Physiol. Rev. 2025, 105, 2305–2376. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, M.; Choi, J. Recent advances in spatially resolved transcriptomics: Challenges and opportunities. BMB Rep. 2022, 55, 113–124. [Google Scholar] [CrossRef]
- Du, J.; Yang, Y.-C.; An, Z.-J.; Zhang, M.-H.; Fu, X.-H.; Huang, Z.-F.; Yuan, Y.; Hou, J. Advances in spatial transcriptomics and related data analysis strategies. J. Transl. Med. 2023, 21, 330. [Google Scholar] [CrossRef]
- Xue, X.; Guo, C.; Fan, C.; Lei, D. The causal role of circulating immunity-inflammation in preeclampsia: A Mendelian randomization. J. Clin. Hypertens. 2024, 26, 474–482. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The Uterine Spiral Arteries In Human Pregnancy: Facts and Controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X. The central role of natural killer cells in preeclampsia. Front. Immunol. 2023, 14, 1009867. [Google Scholar] [CrossRef]
- Yue, S.; Meng, J. Role of Decidual Natural Killer Cells in the Pathogenesis of Preeclampsia. Am. J. Reprod. Immunol. 2024, 93, e70033. [Google Scholar] [CrossRef]
- Dai, W.; Guo, R.; Na, X.; Jiang, S.; Liang, J.; Guo, C.; Fang, Y.; Na, Z.; Li, D. Hypoxia and the endometrium: An indispensable role for HIF-1α as therapeutic strategies. Redox Biol. 2024, 73, 103205. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, L.; Chen, S.; Zheng, L.; Ma, X. Decoding the molecular pathways governing trophoblast migration and placental development; a literature review. Front. Endocrinol. 2024, 15, 1486608. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xiong, L.; Cai, J.; Fan, L.; Huang, C.; Zhang, S.; Jin, Y.; Luo, E.; Xing, S.; Yang, X. Single-cell and spatial transcriptomics: Discovery of human placental development and disease. FASEB BioAdvances 2024, 6, 503–518. [Google Scholar] [CrossRef]
- Levenson, D.; Romero, R.; Miller, D.; Galaz, J.; Garcia-Flores, V.; Neshek, B.; Pique-Regi, R.; Gomez-Lopez, N. The maternal-fetal interface at single-cell resolution: Uncovering the cellular anatomy of the placenta and decidua. Am. J. Obstet. Gynecol. 2025, 232, S55–S79. [Google Scholar] [CrossRef]
- Vasconcelos, S.; Moustakas, I.; Branco, M.R.; Guimarães, S.; Caniçais, C.; van der Helm, T.; Ramalho, C.; Marques, C.J.; Lopes, S.M.C.d.S.; Dória, S. Syncytiotrophoblast Markers Are Downregulated in Placentas from Idiopathic Stillbirths. Int. J. Mol. Sci. 2024, 25, 5180. [Google Scholar] [CrossRef]
- Gehl, A.-L.; Klawitter, D.; Wissenbach, U.; Cole, M.; Wesely, C.; Löhr, H.; Weissgerber, P.; Sota, A.; Meyer, M.R.; Fecher-Trost, C. The proteomic landscape of trophoblasts unravels calcium-dependent syncytialization processes and beta-chorionic gonadotropin (ß-hCG) production. Reprod. Biol. Endocrinol. 2025, 23, 33. [Google Scholar] [CrossRef]
- Keenen, M.M.; Yang, L.; Liang, H.; Farmer, V.J.; E Worota, R.; Singh, R.; Gladfelter, A.S.; Coyne, C.B. Comparative analysis of the syncytiotrophoblast in placenta tissue and trophoblast organoids using snRNA sequencing. eLife 2025, 13, RP101170. [Google Scholar] [CrossRef]
- Jaremek, A.; Jeyarajah, M.J.; Bhattad, G.J.; Renaud, S.J. Omics Approaches to Study Formation and Function of Human Placental Syncytiotrophoblast. Front. Cell Dev. Biol. 2021, 9, 674162. [Google Scholar] [CrossRef]
- Elkin, E.R.; Campbell, K.A.; Lapehn, S.; Harris, S.M.; Padmanabhan, V.; Bakulski, K.M.; Paquette, A.G. Placental single cell transcriptomics: Opportunities for endocrine disrupting chemical toxicology. Mol. Cell. Endocrinol. 2023, 578, 112066. [Google Scholar] [CrossRef]
- O’BRien, K.; Wang, Y. The Placenta: A Maternofetal Interface. Annu. Rev. Nutr. 2023, 43, 301–325. [Google Scholar] [CrossRef]
- Burton, G.J.; Cindrova-Davies, T.; Turco, M.Y. Review: Histotrophic nutrition and the placental-endometrial dialogue during human early pregnancy. Placenta 2020, 102, 21–26. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Sferruzzi-Perri, A.N. Human placental development and function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C. Application of Serum sFlt-1/PlGF Ratio Combined with Uterine Artery Blood Flow Ultrasound in Predicting Early-Onset Preeclampsia. Int. J. Women’s Health 2025, 17, 2561–2567. [Google Scholar] [CrossRef]
- Velegrakis, A.; Kouvidi, E.; Fragkiadaki, P.; Sifakis, S. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia: An update (Review). Int. J. Mol. Med. 2023, 52, 89. [Google Scholar] [CrossRef]
- Sim, M.J.; Long, E.O. The peptide selectivity model: Interpreting NK cell KIR-HLA-I binding interactions and their associations to human diseases. Trends Immunol. 2024, 45, 959–970. [Google Scholar] [CrossRef]
- A Gladstone, R.; Snelgrove, J.W.; McLaughlin, K.; Hobson, S.R.; Windrim, R.C.; Melamed, N.; Hladunewich, M.; Drewlo, S.; Kingdom, J.C. Placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt1): Powerful new tools to guide obstetric and medical care in pregnancy. Obstet. Med. 2025, 18, 143–149. [Google Scholar] [CrossRef]
- Yamazaki, T.; Cerdeira, A.S.; Teraoka, Y.; Oomori, Y.; Mukai, Y.; Sugimoto, J.; Koh, I.; Banno, K.; Vatish, M.; Kudo, Y. Predictive accuracy of sFlt-1/PlGF ratio for preeclampsia and adverse outcomes: Prospective, multicenter including primary, secondary, and tertiary care institutions, observational study in Japan. Hypertens. Res. 2025, 48, 2548–2557. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Chi, X.; Sun, Q.; Li, Y.; Xing, W.; Ding, G. Predictive performance of sFlt-1, PlGF and the sFlt-1/PlGF ratio for preeclampsia: A systematic review and meta-analysis. J. Gynecol. Obstet. Hum. Reprod. 2025, 54, 102925. [Google Scholar] [CrossRef]
- Stepan, H.; Galindo, A.; Hund, M.; Schlembach, D.; Sillman, J.; Surbek, D.; Vatish, M. Clinical utility of sFlt-1 and PlGF in screening, prediction, diagnosis and monitoring of pre-eclampsia and fetal growth restriction. Ultrasound Obstet. Gynecol. 2023, 61, 168–180. [Google Scholar] [CrossRef]
- Dathan-Stumpf, A.; Rieger, A.; Verlohren, S.; Wolf, C.; Stepan, H. sFlt-1/PlGF ratio for prediction of preeclampsia in clinical routine: A pragmatic real-world analysis of healthcare resource utilisation. PLoS ONE 2022, 17, e0263443. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Yuan, Y.; Liu, L.; Meng, T. The Roles of Uterine Natural Killer (NK) Cells and KIR/HLA-C Combination in the Development of Preeclampsia: A Systematic Review. BioMed Res. Int. 2020, 2020, 4808072. [Google Scholar] [CrossRef] [PubMed]
- Erol, F.M.; Häußler, J.A.; Medl, M.; Juhasz-Boess, I.; Kunze, M. Placenta Accreta Spectrum (PAS): Diagnosis, Clinical Presentation, Therapeutic Approaches, and Clinical Outcomes. Medicina 2024, 60, 1180. [Google Scholar] [CrossRef]
- Dorji, N.; Dorji, P.; Pelden, T.; Tshering, S. Management challenges of placenta accreta spectrum and a way forward in a resource limited setting—A case report. Int. J. Surg. Case Rep. 2025, 133, 111644. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Guo, Z.; Sun, R.; Yang, X.; Zheng, W.; Ma, Y.; Rong, Y.; Wang, H.; Yang, H.; et al. The diversity of trophoblast cells and niches of placenta accreta spectrum disorders revealed by single-cell RNA sequencing. Front. Cell Dev. Biol. 2022, 10, 1044198. [Google Scholar] [CrossRef]
- Afshar, Y.; Ligumsky, L.K.; Bartels, H.C.; Krakow, D. Biology and Pathophysiology of Placenta Accreta Spectrum Disorder. Obstet. Gynecol. 2025, 145, 611–620. [Google Scholar] [CrossRef]
- Balasundaram, P.; Farhana, A. Immunology at the Maternal-Fetal Interface; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mirani, P.; Murti, K.; Lestari, P.M.; Liberty, I.A.; Kesty, C.; Andrina, H.; Stevanny, B. The Role of CXCR2, MMP-2, and MMP-9 in the Pathogenesis of Placenta Accreta: A Molecular Expression Study. Medicina 2025, 61, 461. [Google Scholar] [CrossRef]
- Chen, S.; Pang, D.; Li, Y.; Zhou, J.; Liu, Y.; Yang, S.; Liang, K.; Yu, B. Serum miRNA biomarker discovery for placenta accreta spectrum. Placenta 2020, 101, 215–220. [Google Scholar] [CrossRef]
- Melekoglu, R.; Yasar, S.; Colak, C.; Kasap, M.; Dogan, U.K.; Yologlu, S.; Yilmaz, E.; Shazly, S. Determination of biomarker candidates for the placenta accreta spectrum by plasma proteomic analysis. Sci. Rep. 2024, 14, 2803. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S. Potential Serum Biomarkers in Prenatal Diagnosis of Placenta Accreta Spectrum. Front. Med. 2022, 9, 860186. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, K.; Ozawa, K.; Wada, S.; Samura, O. Molecular Mechanisms Underlying Twin-to-Twin Transfusion Syndrome. Cells 2022, 11, 3268. [Google Scholar] [CrossRef]
- Schuchardt, E.L.; Miyamoto, S.D.; Crombleholme, T.; Karimpour-Fard, A.; Korst, A.; Neltner, B.; Howley, L.W.; Cuneo, B.; Sucharov, C.C. Amniotic Fluid microRNA in Severe Twin-Twin Transfusion Syndrome Cardiomyopathy—Identification of Differences and Predicting Demise. J. Cardiovasc. Dev. Dis. 2022, 9, 37. [Google Scholar] [CrossRef]
- Papastefan, S.T.; Langereis, M.M.; Redden, C.R.; Liesman, D.R.; Huerta, C.B.; Turner, L.E.; Kang, H.K.; Stetson, B.T.; Ott, K.C.; Marriott, W.S.; et al. Maternal plasma microRNA profiles in twin-twin transfusion syndrome and normal monochorionic twin pregnancies. Front. Mol. Biosci. 2025, 12, 1597215. [Google Scholar] [CrossRef]
- Brock, C.O.; Baschat, A.A.; Moldenhauer, J.S.; Ryan, G.; Johnson, A. Surveillance of monochorionic twins for detection of twin-twin transfusion syndrome and twin anemia polycythemia sequence: A North American Fetal Therapy Network consensus statement. Am. J. Obstet. Gynecol. 2025. [Google Scholar] [CrossRef]
- Tong, M.; Scott, J.N.; Whirledge, S.D.; Abrahams, V.M. Decidualization dampens toll-like receptor mediated inflammatory responses in human endometrial stromal cells by upregulating IκBα. J. Reprod. Immunol. 2023, 159, 103988. [Google Scholar] [CrossRef]
- Salleh, N.; Giribabu, N. Leukemia Inhibitory Factor: Roles in Embryo Implantation and in Nonhormonal Contraception. Sci. World J. 2014, 2014, 201514. [Google Scholar] [CrossRef]
- Subramanian, A.; Weiss, D.; Nyhan, K.; Dewan, A.; Jukic, A.M.Z. Circulating miRNAs in the first trimester and pregnancy complications: A systematic review. Epigenetics 2022, 18, 2152615. [Google Scholar] [CrossRef]
- Ghafourian, M.; Mahdavi, R.; Jonoush, Z.A.; Sadeghi, M.; Ghadiri, N.; Farzaneh, M.; Salehi, A.M. The implications of exosomes in pregnancy: Emerging as new diagnostic markers and therapeutics targets. Cell Commun. Signal. 2022, 20, 51. [Google Scholar] [CrossRef]
- Kim, O.; Hong, S.; Park, I.Y.; Ko, H.S. Association between placental location and cord insertion site with pre-eclampsia: A retrospective cohort study. J. Matern. Neonatal Med. 2024, 37, 2306189. [Google Scholar] [CrossRef]
- Estrela, D.; Santos, R.F.; Masserdotti, A.; Silini, A.; Parolini, O.; Pinto, I.M.; Cruz, A. Molecular Biomarkers for Timely and Personalized Prediction of Maternal-Fetal Health Risk. Biomolecules 2025, 15, 312. [Google Scholar] [CrossRef]
- Medina, A.C.; Gómez-Acebo, I.; de Largy, C.C.G.; Alonso-Molero, J.; Calvo, S.V.; Feu, J.M.O.; Medina, M.C.; Dierssen-Sotos, T. Study of first-trimester serum levels of β-hCG and PAPP-A as a screening test for fetal development of intrauterine growth restriction. BMC Pregnancy Childbirth 2025, 25, 655. [Google Scholar] [CrossRef]
- Uriel, M.; Infante, X.C.R.; Franco, S.R.; Pinilla, E.A.I.; Rojas, N.A. Higher PAPP-A Values in Pregnant Women Complicated with Preeclampsia Than with Gestational Hypertension. Reprod. Sci. 2023, 30, 2503–2511. [Google Scholar] [CrossRef]
- Côté, M.-L.; Giguère, Y.; Forest, J.-C.; Audibert, F.; Johnson, J.A.; Okun, N.; Guerby, P.; Ghesquiere, L.; Bujold, E. First-Trimester PlGF and PAPP-A and the Risk of Placenta-Mediated Complications: PREDICTION Prospective Study. J. Obstet. Gynaecol. Can. 2024, 47, 102732. [Google Scholar] [CrossRef]
- Mohamed, M.H.; El-Din, L.T.; Nigm, D.A.E.; Farghaly, T. Assessment of placental protein 13 as a new marker for early diagnosis of preeclampsia. J. Curr. Med. Res. Pract. 2021, 6, 399. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Ding, Y. Predictive Performance of Placental Protein 13 for Screening Preeclampsia in the First Trimester: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 756383. [Google Scholar] [CrossRef]
- Atalmis, H.A.; Tekin, S.; Yilmaz, I.; Guler, E.Y.; Guleroglu, F.Y.; Cetin, A. ORAI1, FGF23, PP13, palladin, and supervillin as potential biomarkers in late-onset pre-eclampsia: A comparative study in maternal and cord blood. BMC Pregnancy Childbirth 2025, 25, 786. [Google Scholar] [CrossRef]
- Kiyokoba, R.; Uchiumi, T.; Yagi, M.; Toshima, T.; Tsukahara, S.; Fujita, Y.; Kato, K.; Kang, D. Mitochondrial dysfunction-induced high hCG associated with development of fetal growth restriction and pre-eclampsia with fetal growth restriction. Sci. Rep. 2022, 12, 4056. [Google Scholar] [CrossRef]
- Yuen, N.; Lemaire, M.; Wilson, S.L. Cell-free placental DNA: What do we really know? PLoS Genet. 2024, 20, e1011484. [Google Scholar] [CrossRef]
- Adil, M.; Kolarova, T.R.; Doebley, A.-L.; Chen, L.A.; Tobey, C.L.; Galipeau, P.; Rosen, S.; Yang, M.; Colbert, B.; Patton, R.D.; et al. Preeclampsia risk prediction from prenatal cell-free DNA screening. Nat. Med. 2025, 31, 1312–1318. [Google Scholar] [CrossRef]
- Moufarrej, M.N.; Vorperian, S.K.; Wong, R.J.; Campos, A.A.; Quaintance, C.C.; Sit, R.V.; Tan, M.; Detweiler, A.M.; Mekonen, H.; Neff, N.F.; et al. Early prediction of preeclampsia in pregnancy with cell-free RNA. Nature 2022, 602, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Gekas, J.; Boomer, T.H.; Rodrigue, M.-A.; Jinnett, K.N.; Bhatt, S. Use of cell-free signals as biomarkers for early and easy prediction of preeclampsia. Front. Med. 2023, 10, 1191163. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yan, Y.; Yue, X.; Li, Y.; Mo, Y. Advances in cfDNA research for pregnancy-related diseases. Front. Cell Dev. Biol. 2025, 13, 1600532. [Google Scholar] [CrossRef] [PubMed]
- Giannubilo, S.R.; Cecati, M.; Marzioni, D.; Ciavattini, A. Circulating miRNAs and Preeclampsia: From Implantation to Epigenetics. Int. J. Mol. Sci. 2024, 25, 1418. [Google Scholar] [CrossRef]
- Clément, A.-A.; Légaré, C.; Desgagné, V.; Thibeault, K.; White, F.; Scott, M.S.; Jacques, P.; Fraser, W.D.; Perron, P.; Guérin, R.; et al. First trimester circulating miR-194-5p and miR-1278 improve prediction of preeclampsia. Pregnancy Hypertens. 2023, 34, 95–103. [Google Scholar] [CrossRef]
- Popova, A.K.; Vashukova, E.S.; Illarionov, R.A.; Maltseva, A.R.; Pachuliia, O.V.; Postnikova, T.B.; Glotov, A.S. Extracellular Vesicles as Biomarkers of Pregnancy Complications. Int. J. Mol. Sci. 2024, 25, 11944. [Google Scholar] [CrossRef]
- Ghosh, S.; Thamotharan, S.; Fong, J.; Lei, M.Y.Y.; Janzen, C.; Devaskar, S.U. Circulating extracellular vesicular microRNA signatures in early gestation show an association with subsequent clinical features of pre-eclampsia. Sci. Rep. 2024, 14, 16770. [Google Scholar] [CrossRef]
- Jin, K.B.; Shen, S.B.; Shi, R.B.; Xu, X.B.; Hu, M. Exosomal miRNAs in prenatal diagnosis: Recent advances. Medicine 2024, 103, e38717. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Li, Y.; Chen, Q.; Jiang, S.; Gao, Y.; Wang, J.; Chi, Y.; Liu, J.; Wu, X.; et al. Hierarchical lncRNA regulatory network in early-onset severe preeclampsia. BMC Biol. 2024, 22, 159. [Google Scholar] [CrossRef]
- Ogoyama, M.; Takahashi, H.; Suzuki, H.; Ohkuchi, A.; Fujiwara, H.; Takizawa, T. Non-Coding RNAs and Prediction of Preeclampsia in the First Trimester of Pregnancy. Cells 2022, 11, 2428. [Google Scholar] [CrossRef]
- Grossini, E.; Surico, D.; Venkatesan, S.; Pour, M.M.O.; Aquino, C.I.; Remorgida, V. Extracellular Vesicles and Pregnancy-Related Hypertensive Disorders: A Descriptive Review on the Possible Implications “From Bench to Bedside”. Biology 2025, 14, 240. [Google Scholar] [CrossRef]

| Molecular Pathway | Key Regulators | Placental Pathology | Representative Findings |
|---|---|---|---|
| Oxygen sensing & hypoxia [23,32] | HIF-1α, ROS balance | Preeclampsia, FGR, miscarriage | Premature plug loss, oxidative stress |
| Angiogenic balance [48,50] | VEGF, PlGF, sFlt-1 | Preeclampsia, FGR | sFlt-1/PlGF ratio ↑, villous malperfusion |
| Immune tolerance [31,53] | KIR/HLA-C, HLA-G | Preeclampsia, miscarriage | Shallow invasion, immune rejection |
| Syncytialization [36,40] | GCM1, Syncytin-1/2 | Stillbirth, FGR, PE | ↓ PSG, ↓ CSH family gene expression |
| Proteolytic invasion [59] | MMP-2, MMP-9, NF-κB | PAS | Persistent EVT invasion, myometrial infiltration |
| Vascular patterning [63] | VEGF, Notch, Wnt | TTTS, TAPS, sIUGR | Abnormal vascular anastomoses |
| Biomarker Class | Examples | Molecular Insight | Clinical Application/Status | Gestational Window (Best Performance) | Sample Type | Typical Thresholds | Evidence Grade |
|---|---|---|---|---|---|---|---|
| Angiogenic factors [48,49,50] | sFlt-1, PlGF | Angiogenic imbalance | Validated in PE (prediction, monitoring, delivery timing) | 20–37 weeks (second and third trimester) | Maternal plasma/serum | sFlt-1/PlGF ratio >38 (rule-in), <38 (rule-out within 1 week) | Guideline-endorsed; validated in large cohorts |
| Placental proteins [73,76,79] | PAPP-A, PP13, hCG, hPL | Trophoblast invasion, syncytial & endocrine fn | In screening; complementary with angiogenic testing | First trimester (11–14 weeks) | Maternal serum | Low PAPP-A <0.4 MoM; variable for PP13/hCG | Clinically used for screening; moderate evidence |
| Nucleic acids [80,82] | cfDNA, cfRNA, miRNAs, lncRNAs | Genetic/epigenetic placental signals | Emerging; early prediction of PE and related complications | First–second trimester (10–24 weeks) | Maternal plasma | No standardized thresholds | Exploratory; discovery to early validation |
| Extracellular vesicles [87,88] | Exosomal miRNAs, proteins | Trophoblast stress, immune/vascular signaling | Discovery phase; promising for early non-invasive diagnosis | First–second trimester | Maternal plasma/serum | No validated cut-offs | Discovery phase |
| Multi-omics panels [18,27,83] | scRNA-seq, proteomics, metabolomics | Integrated placental fingerprint | Preclinical; precision obstetrics development | Variable (first–third trimester, depending on platform) | Placental tissue, plasma | Research-dependent | Preclinical/experimental |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vornic, I.; Caprariu, R.; Novacescu, D.; Barb, A.C.; Buciu, V.; Băloi, A.; Szekely, D.; Suciu, C.S.; Dumitru, C.; Patrascu, R.; et al. Molecular Insights into Human Placentation: From Villous Morphogenesis to Pathological Pathways and Translational Biomarkers. Int. J. Mol. Sci. 2025, 26, 9483. https://doi.org/10.3390/ijms26199483
Vornic I, Caprariu R, Novacescu D, Barb AC, Buciu V, Băloi A, Szekely D, Suciu CS, Dumitru C, Patrascu R, et al. Molecular Insights into Human Placentation: From Villous Morphogenesis to Pathological Pathways and Translational Biomarkers. International Journal of Molecular Sciences. 2025; 26(19):9483. https://doi.org/10.3390/ijms26199483
Chicago/Turabian StyleVornic, Ioana, Radu Caprariu, Dorin Novacescu, Alina Cristina Barb, Victor Buciu, Adelina Băloi, Diana Szekely, Cristian Silviu Suciu, Catalin Dumitru, Raul Patrascu, and et al. 2025. "Molecular Insights into Human Placentation: From Villous Morphogenesis to Pathological Pathways and Translational Biomarkers" International Journal of Molecular Sciences 26, no. 19: 9483. https://doi.org/10.3390/ijms26199483
APA StyleVornic, I., Caprariu, R., Novacescu, D., Barb, A. C., Buciu, V., Băloi, A., Szekely, D., Suciu, C. S., Dumitru, C., Patrascu, R., Zara, F., & Dumitru, C. S. (2025). Molecular Insights into Human Placentation: From Villous Morphogenesis to Pathological Pathways and Translational Biomarkers. International Journal of Molecular Sciences, 26(19), 9483. https://doi.org/10.3390/ijms26199483








