The Significance and Mechanism of Cerebral Enlarged Perivascular Space in Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Results
2.1. Associations Between High-Degree CSO-EPVS and ALS
2.2. Comparison Between Patients with ALS with and Without High-Degree CSO-EPVS
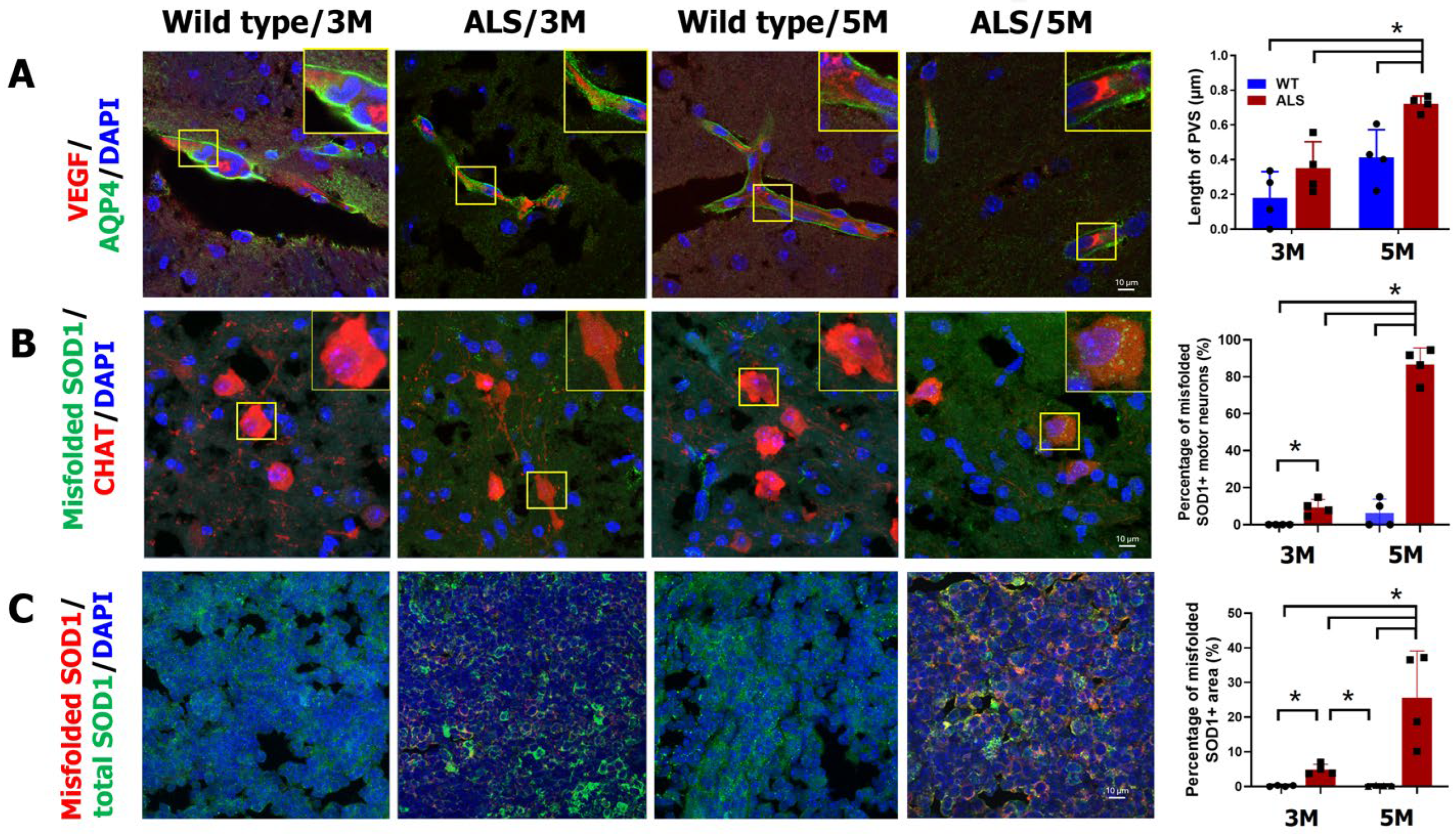
2.3. Increased Cerebral PVS Width and Misfolded Protein in ALS Mice
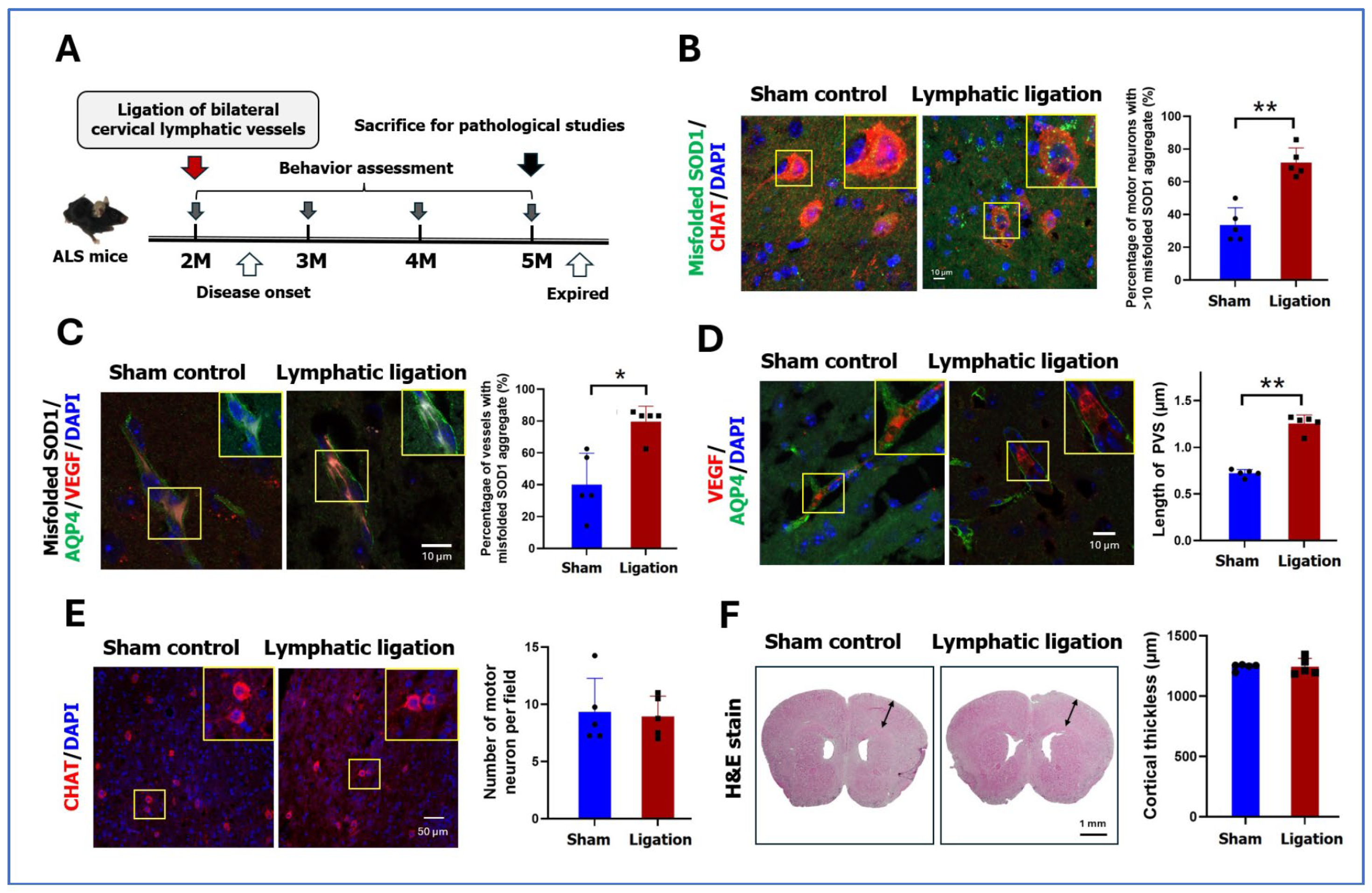
2.4. Pathology and Behaviors After Ligation of Cervical Lymphatic Vessels in ALS Mice
3. Discussion
4. Materials and Methods
4.1. Study Design and Sample Selection
4.2. MRI Acquisition and Analysis
4.3. Animal Model
4.4. Pathological Studies of ALS Mice
4.5. Behavioral Studies of ALS Mice
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ryan, M.; Heverin, M.; McLaughlin, R.L.; Hardiman, O. Lifetime risk and heritability of amyotrophic lateral sclerosis. JAMA Neurol. 2019, 76, 1367–1374. [Google Scholar] [CrossRef]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 480–493. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown Jr, R.H.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Faller, K.M.; Chaytow, H.; Gillingwater, T.H. Targeting common disease pathomechanisms to treat amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2025, 21, 86–102. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Mestre, H.; Kostrikov, S.; Mehta, R.I.; Nedergaard, M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. 2017, 131, 2257–2274. [Google Scholar] [CrossRef]
- Wen, C.; Gan, J.-H.; Liu, S.; Lu, H.; Wang, L.-C.; Wu, H.; Shi, Z.-H.; Ji, Y. Enlarged perivascular spaces correlate with blood-brain barrier leakage and cognitive impairment in Alzheimer’s disease. J. Alzheimer’s Dis. 2025, 104, 382–392. [Google Scholar] [CrossRef]
- Liu, S.; Sun, X.; Ren, Q.; Chen, Y.; Dai, T.; Yang, Y.; Gong, G.; Li, W.; Zhao, Y.; Meng, X. Glymphatic dysfunction in patients with early-stage amyotrophic lateral sclerosis. Brain 2024, 147, 100–108. [Google Scholar] [CrossRef]
- Bown, C.W.; Carare, R.O.; Schrag, M.S.; Jefferson, A.L. Physiology and clinical relevance of enlarged perivascular spaces in the aging brain. Neurology 2022, 98, 107–117. [Google Scholar] [CrossRef]
- Perosa, V.; Oltmer, J.; Munting, L.P.; Freeze, W.M.; Auger, C.A.; Scherlek, A.A.; van der Kouwe, A.J.; Iglesias, J.E.; Atzeni, A.; Bacskai, B.J. Perivascular space dilation is associated with vascular amyloid-β accumulation in the overlying cortex. Acta Neuropathol. 2022, 143, 331–348. [Google Scholar] [CrossRef]
- Watanabe, M.; Dykes-Hoberg, M.; Culotta, V.C.; Price, D.L.; Wong, P.C.; Rothstein, J.D. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol. Dis. 2001, 8, 933–941. [Google Scholar] [CrossRef]
- Nauen, D.W.; Troncoso, J.C. Amyloid-beta is present in human lymph nodes and greatly enriched in those of the cervical region. Alzheimer’s Dement. 2022, 18, 205–210. [Google Scholar] [CrossRef]
- Wang, S.; Melhem, E.R.; Poptani, H.; Woo, J.H. Neuroimaging in amyotrophic lateral sclerosis. Neurotherapeutics 2011, 8, 63–71. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.; Luan, M.; Song, X.; Wang, Y.; Xu, L.; Zhong, M.; Zheng, X. Enlarged perivascular spaces and age-related clinical diseases. Clin. Interv. Aging 2023, 18, 855–867. [Google Scholar] [CrossRef]
- Lynch, K.M.; Sepehrband, F.; Toga, A.W.; Choupan, J. Brain perivascular space imaging across the human lifespan. Neuroimage 2023, 271, 120009. [Google Scholar] [CrossRef]
- Penton, A.A.; Lau, H.; Babikian, V.L.; Shulman, J.; Cervantes-Arslanian, A.; Gangadhara, S.; Greer, D.; Aparicio, H.J.; Romero, J.R. Chronic kidney disease as risk factor for enlarged perivascular spaces in patients with stroke and relation to racial group. Stroke 2020, 51, 3348–3351. [Google Scholar] [CrossRef]
- Gertje, E.C.; van Westen, D.; Panizo, C.; Mattsson-Carlgren, N.; Hansson, O. Association of enlarged perivascular spaces and measures of small vessel and Alzheimer disease. Neurology 2021, 96, e193–e202. [Google Scholar] [CrossRef]
- Kim, S.; Na, H.K.; Sun, Y.; Yoon, Y.J.; Chung, S.J.; Sohn, Y.H.; Lyoo, C.H.; Lee, P.H. Regional burden of enlarged perivascular spaces and cognition and neuropsychiatric symptoms in drug-naive patients with Parkinson disease. Neurology 2024, 102, e209483. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, L.-Y.; Tian, J.; Dai, S.-B.; Chen, Y.; Zheng, R.; Si, X.-L.; Jin, C.-Y.; Song, Z.; Yan, Y.-P. MRI-visible perivascular spaces are associated with cerebrospinal fluid biomarkers in Parkinson’s disease. Aging 2020, 12, 25805. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Pasi, M.; Tsai, L.-K.; Huang, C.-C.; Chen, Y.-F.; Lee, B.-C.; Yen, R.-F.; Gurol, M.E.; Jeng, J.-S. Centrum semiovale perivascular space and amyloid deposition in spontaneous intracerebral hemorrhage. Stroke 2021, 52, 2356–2362. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Boulouis, G.; Pasi, M.; Auriel, E.; van Etten, E.S.; Haley, K.; Ayres, A.; Schwab, K.M.; Martinez-Ramirez, S.; Goldstein, J.N. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017, 88, 1157–1164. [Google Scholar] [CrossRef]
- Mancuso, R.; Navarro, X. Amyotrophic lateral sclerosis: Current perspectives from basic research to the clinic. Prog. Neurobiol. 2015, 133, 1–26. [Google Scholar] [CrossRef]
- Kaur, J.; Fahmy, L.M.; Davoodi-Bojd, E.; Zhang, L.; Ding, G.; Hu, J.; Zhang, Z.; Chopp, M.; Jiang, Q. Waste clearance in the brain. Front. Neuroanat. 2021, 15, 665803. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, C.J.; Cha, J.; Kim, S.-Y.; Lee, S.-K.; Kim, Y.J.; Sohn, Y.H.; Chung, S.J.; Lee, P.H. Choroid plexus volume, amyloid burden, and cognition in the Alzheimer’s disease continuum. Aging Dis. 2024, 16, 552. [Google Scholar] [CrossRef]
- Nakaya, M.; Kamagata, K.; Takabayashi, K.; Uchida, W.; Hagiwara, A.; Christina, A.; Wada, A.; Taoka, T.; Naganawa, S.; Abe, O. Glymphatic System Efficiency, Choroid Plexus Volume, and Lateral Ventricle Volume Variations in Normal Individuals and Throughout the Progression of Alzheimer’s Disease. Alzheimer’s Dement. 2024, 20, e083874. [Google Scholar] [CrossRef]
- Tadayon, E.; Pascual-Leone, A.; Press, D.; Santarnecchi, E. Alzheimer’s Disease Neuroimaging Initiative. Choroid plexus volume is associated with levels of CSF proteins: Relevance for Alzheimer’s and Parkinson’s disease. Neurobiol Aging 2020, 89, 10-1016. [Google Scholar] [CrossRef]
- Municio, C.; Carrero, L.; Antequera, D.; Carro, E. Choroid plexus aquaporins in CSF homeostasis and the glymphatic system: Their relevance for Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 878. [Google Scholar] [CrossRef]
- Oiwa, K.; Watanabe, S.; Onodera, K.; Iguchi, Y.; Kinoshita, Y.; Komine, O.; Sobue, A.; Okada, Y.; Katsuno, M.; Yamanaka, K. Monomerization of TDP-43 is a key determinant for inducing TDP-43 pathology in amyotrophic lateral sclerosis. Sci. Adv. 2023, 9, eadf6895. [Google Scholar] [CrossRef]
- De Carvalho, M.; Dengler, R.; Eisen, A.; England, J.D.; Kaji, R.; Kimura, J.; Mills, K.; Mitsumoto, H.; Nodera, H.; Shefner, J. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008, 119, 497–503. [Google Scholar] [CrossRef]
- Kimura, F.; Fujimura, C.; Ishida, S.; Nakajima, H.; Furutama, D.; Uehara, H.; Shinoda, K.; Sugino, M.; Hanafusa, T. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006, 66, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.-E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S. Neuroimaging standards for research into small vessel disease—Advances since 2013. Lancet Neurol. 2023, 22, 602–618, Erratum in Lancet Neurol. 2023, 22, e10. [Google Scholar] [CrossRef]
- Doubal, F.N.; MacLullich, A.M.; Ferguson, K.J.; Dennis, M.S.; Wardlaw, J.M. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010, 41, 450–454. [Google Scholar] [CrossRef]
- Ding, J.; Sigurðsson, S.; Jónsson, P.V.; Eiriksdottir, G.; Charidimou, A.; Lopez, O.L.; Van Buchem, M.A.; Guðnason, V.; Launer, L.J. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: The age, gene/environment susceptibility–Reykjavik study. JAMA Neurol. 2017, 74, 1105–1112. [Google Scholar] [CrossRef]
- Lai, H.-J.; Kuo, Y.-C.; Ting, C.-H.; Yang, C.-C.; Kao, C.-H.; Tsai, Y.-C.; Chao, C.-C.; Hsueh, H.-W.; Hsieh, P.-F.; Chang, H.-Y. Increase of HCN current in SOD1-associated amyotrophic lateral sclerosis. Brain 2024, 147, 4240–4253. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Hsieh, Y.-C.; Lin, J.S.; Kuo, Z.-T.; Ho, C.-Y.; Chen, C.-H.; Chang, C.-F. Functional investigation of meningeal lymphatic system in experimental intracerebral hemorrhage. Stroke 2022, 53, 987–998. [Google Scholar] [CrossRef]
- Chen, H.H.; Tsai, L.K.; Liao, K.Y.; Wu, T.C.; Huang, Y.H.; Huang, Y.C.; Chang, S.W.; Wang, P.Y.; Tsao, Y.P.; Chen, S.L. Muscle-restricted nuclear receptor interaction protein knockout causes motor neuron degeneration through down-regulation of myogenin at the neuromuscular junction. J. Cachexia Sarcopenia Muscle 2018, 9, 771–785. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Lee, Y.-B.; Lee, B.; Lee, D. A quantitative analysis of spontaneous alternation behaviors on a Y-maze reveals adverse effects of acute social isolation on spatial working memory. Sci. Rep. 2023, 13, 14722. [Google Scholar] [CrossRef]
| ALS (n = 114) | Controls (n = 119) | p Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, year | 60.8 ± 13.3 | 60.1 ± 12.3 | 0.674 |
| Sex, male | 61 (53.5%) | 59 (49.6%) | 0.549 |
| Hypertension | 40 (35.1%) | 34 (28.6%) | 0.285 |
| Diabetes mellitus | 9 (7.9%) | 15 (12.6%) | 0.237 |
| Chronic kidney disease | 1 (0.8%) | 2 (1.7%) | 0.587 |
| Coronary artery disease | 6 (5.3%) | 8 (6.7%) | 0.639 |
| Perivascular space | |||
| BG-EPVS > 20, n (%) | 18 (15.8%) | 12 (10.1%) | 0.194 |
| CSO-EPVS > 20, n (%) | 56 (49.1%) | 18 (15.1%) | <0.001 |
| Hippocampal EPVS, n | 3.8 ± 2.6 | 3.8 ± 1.7 | 0.860 |
| Large EPVS, n (%) | 13 (11.4%) | 8 (6.7%) | 0.212 |
| Whole brain metrics | |||
| White matter hyperintensity (mL) | 5.4 ± 8.7 | 3.5 ± 5.7 | 0.055 |
| Gray matter volume (mL) | 586.3 ± 51.5 | 584.8 ± 54.2 | 0.845 |
| White matter volume (mL) | 470.8 ± 57.2 | 467.7 ± 66.0 | 0.735 |
| Cortical thickness | |||
| Total (mm) | 2.42 ± 0.14 | 2.46 ± 0.14 | 0.298 |
| Frontal lobe (mm) | 2.47 ± 0.16 | 2.53 ± 0.18 | 0.013 |
| Temporal lobe (mm) | 2.78 ± 0.20 | 2.83 ± 0.18 | 0.041 |
| Parietal lobe (mm) | 2.21 ± 0.16 | 2.24 ± 0.19 | 0.239 |
| Occipital lobe (mm) | 2.00 ± 0.26 | 2.20 ± 0.21 | <0.001 |
| Univariable Logistic Regression | Multivariable Logistic Regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age, per 10 years | 1.463 | 1.142–1.874 | 0.003 | 1.589 | 1.176–2.147 | 0.003 |
| Male sex | 2.051 | 1.163–3.615 | 0.013 | 2.155 | 1.128–4.118 | 0.020 |
| ALS | 5.418 | 2.910–10.086 | <0.001 | 6.954 | 3.483–13.884 | <0.001 |
| Hypertension | 1.145 | 0.636–2.061 | 0.651 | 0.48 | 0.221–1.039 | 0.062 |
| Diabetes mellitus | 1.083 | 0.442–2.658 | 0.861 | 1.261 | 0.422–3.764 | 0.678 |
| Chronic kidney disease | 4.389 | 0.392–49.188 | 0.230 | 5.505 | 0.470–64.532 | 0.174 |
| Coronary artery disease | 2.269 | 0.766–6.723 | 0.139 | 2.978 | 0.811–10.931 | 0.100 |
| With High-Degree CSO-EPVS (n = 56) | Without High-Degree CSO-EPVS (n = 58) | p Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age of ALS onset, year | 61.8 ± 10.1 | 51.3 ± 30.0 | 0.014 |
| Age of MRI study, year | 63.1 ± 10.3 | 57.6 ± 15.3 | 0.028 |
| Sex, male | 35 (62.5%) | 26 (44.8%) | 0.059 |
| Bulbar onset | 23 (41.1%) | 16 (27.6%) | 0.129 |
| Mutation of ALS-related genes | 3 (5.4%) | 4 (6.9%) | 1.000 |
| ALSFRS-R | 35.8 ± 10.1 | 32.2 ± 12.4 | 0.088 |
| ALSFRS-R decline (1/year) | 10.9 ± 18.8 | 10.2 ± 9.0 | 0.798 |
| Neuroimaging features | |||
| White matter hyperintensity (mL) | 5.8 ± 9.2 | 5.0 ± 8.3 | 0.606 |
| Gray matter volume (mL) | 580.9 ± 49.6 | 591.5 ± 53.4 | 0.358 |
| White matter volume (mL) | 468.4 ± 62.8 | 473.1 ± 51.8 | 0.714 |
| Cortical thickness (mm) | |||
| Total | 2.40 ± 0.16 | 2.44 ± 0.11 | 0.123 |
| Frontal lobe | 2.44 ± 0.19 | 2.50 ± 0.13 | 0.068 |
| Temporal lobe | 2.75 ± 0.25 | 2.80 ± 0.14 | 0.227 |
| Parietal lobe | 2.18± 0.15 | 2.24 ± 0.15 | 0.078 |
| Occipital lobe | 1.96 ± 0.27 | 2.05 ± 0.24 | 0.146 |
| Choroid plexus volume (mL) | 1.5 ± 0.5 | 1.2 ± 0.5 | 0.009 |
| Lateral ventricle volume (mL) | 30.0 ± 20.0 | 21.8 ± 11.2 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-C.; Kuo, Y.-C.; Cheng, L.-F.; Tsai, Y.-C.; Huang, J.-Z.; Tsai, H.-H.; Lin, J.-S.; Huang, P.-Y.; Ting, C.-H.; Yang, C.-C.; et al. The Significance and Mechanism of Cerebral Enlarged Perivascular Space in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2025, 26, 9474. https://doi.org/10.3390/ijms26199474
Lee B-C, Kuo Y-C, Cheng L-F, Tsai Y-C, Huang J-Z, Tsai H-H, Lin J-S, Huang P-Y, Ting C-H, Yang C-C, et al. The Significance and Mechanism of Cerebral Enlarged Perivascular Space in Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences. 2025; 26(19):9474. https://doi.org/10.3390/ijms26199474
Chicago/Turabian StyleLee, Bo-Ching, Yih-Chih Kuo, Lo-Fan Cheng, Yi-Chieh Tsai, Jia-Zheng Huang, Hsin-Hsi Tsai, Jhih-Syuan Lin, Po-Ya Huang, Chen-Hung Ting, Chih-Chao Yang, and et al. 2025. "The Significance and Mechanism of Cerebral Enlarged Perivascular Space in Amyotrophic Lateral Sclerosis" International Journal of Molecular Sciences 26, no. 19: 9474. https://doi.org/10.3390/ijms26199474
APA StyleLee, B.-C., Kuo, Y.-C., Cheng, L.-F., Tsai, Y.-C., Huang, J.-Z., Tsai, H.-H., Lin, J.-S., Huang, P.-Y., Ting, C.-H., Yang, C.-C., Lai, H.-J., Chao, C.-C., & Tsai, L.-K. (2025). The Significance and Mechanism of Cerebral Enlarged Perivascular Space in Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences, 26(19), 9474. https://doi.org/10.3390/ijms26199474





