FBXO22 Suppresses Oxidative Stress-Induced ASK1 Activation and Cell Death via Ubiquitination-Dependent Degradation of TRIM48
Abstract
1. Introduction
2. Results
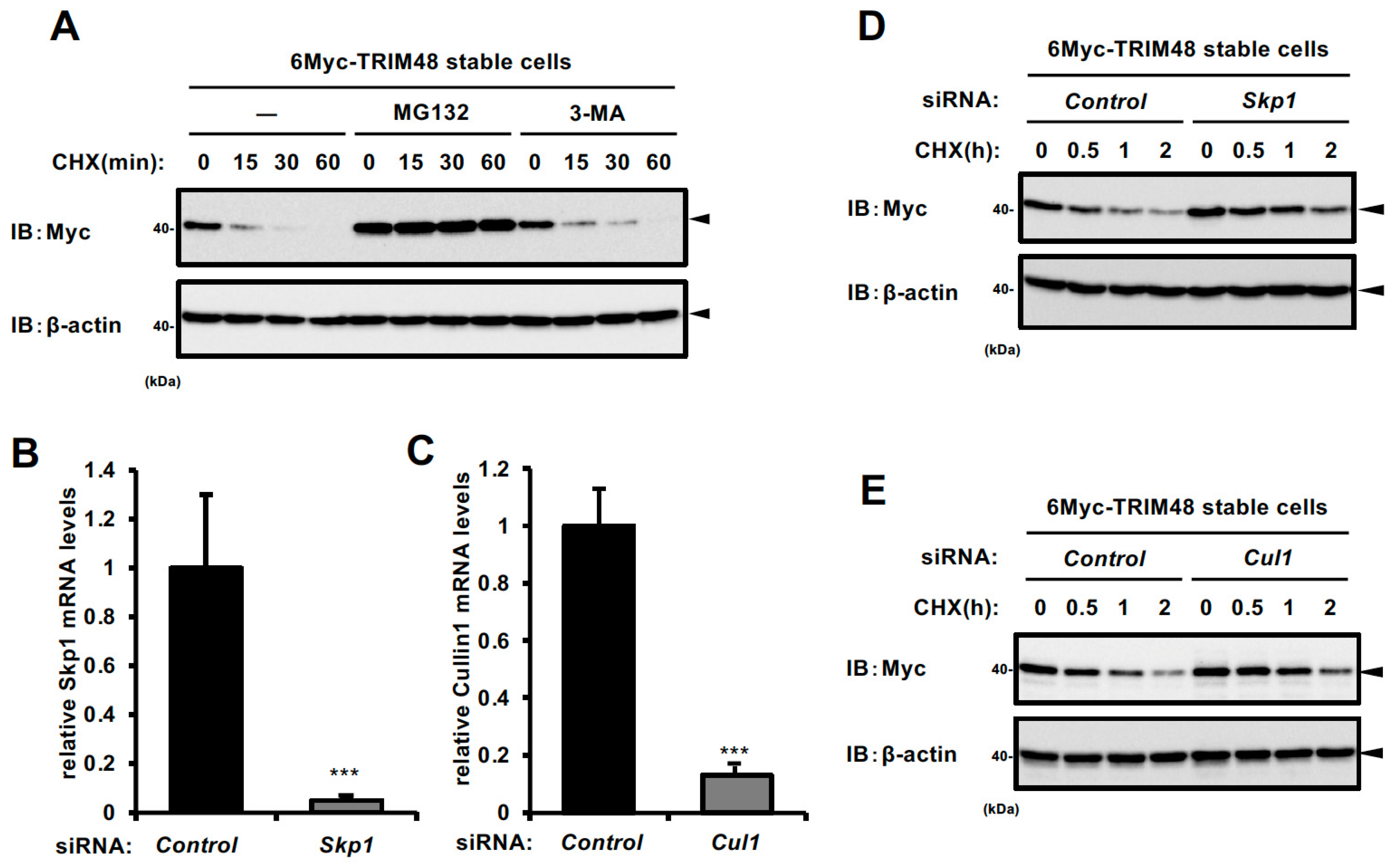
2.1. TRIM48 Protein Undergoes Proteasomal Degradation
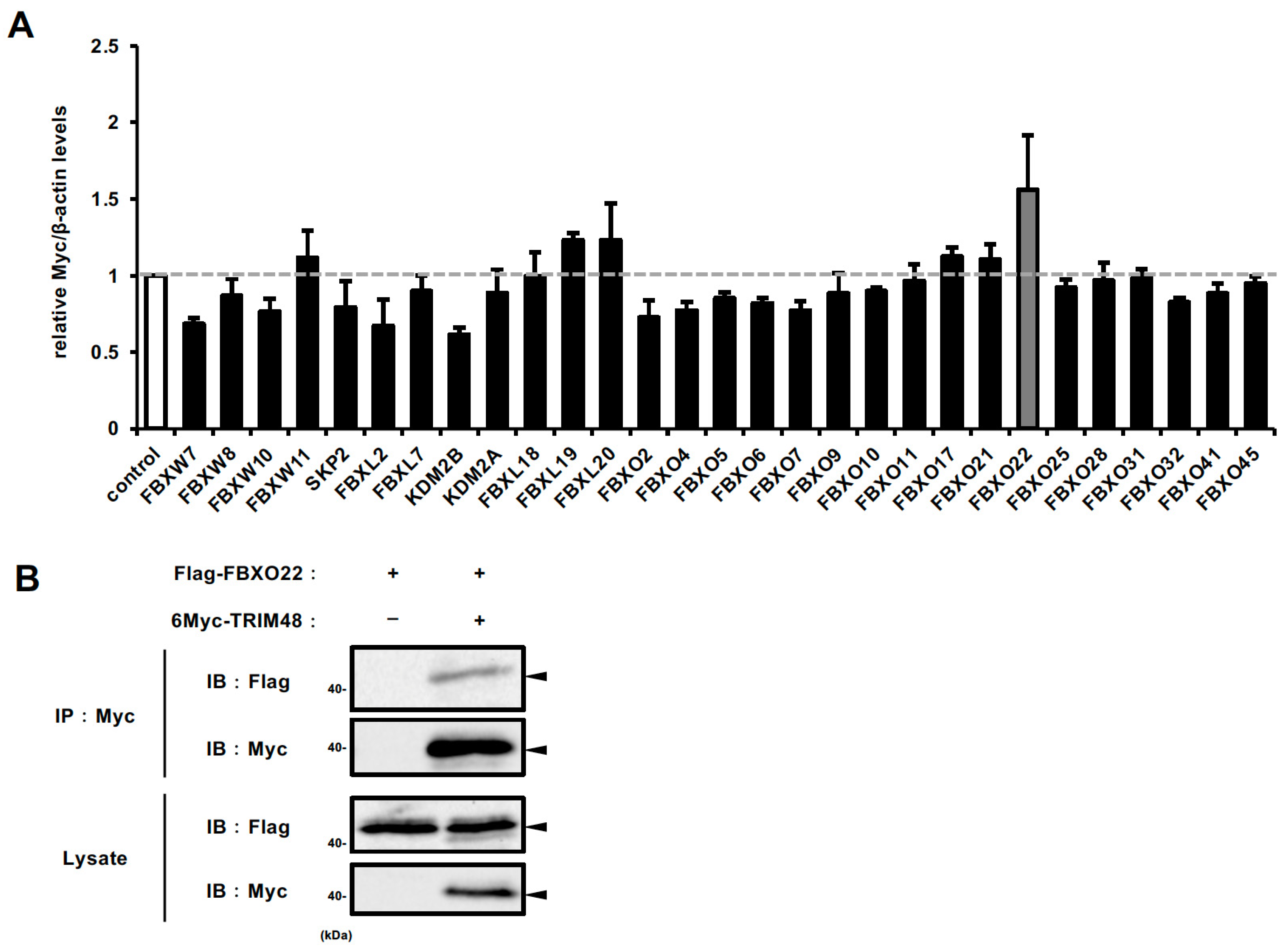
2.2. FBXO22 Serves as a Recognition Subunit for TRIM48 Within the SCF Complex
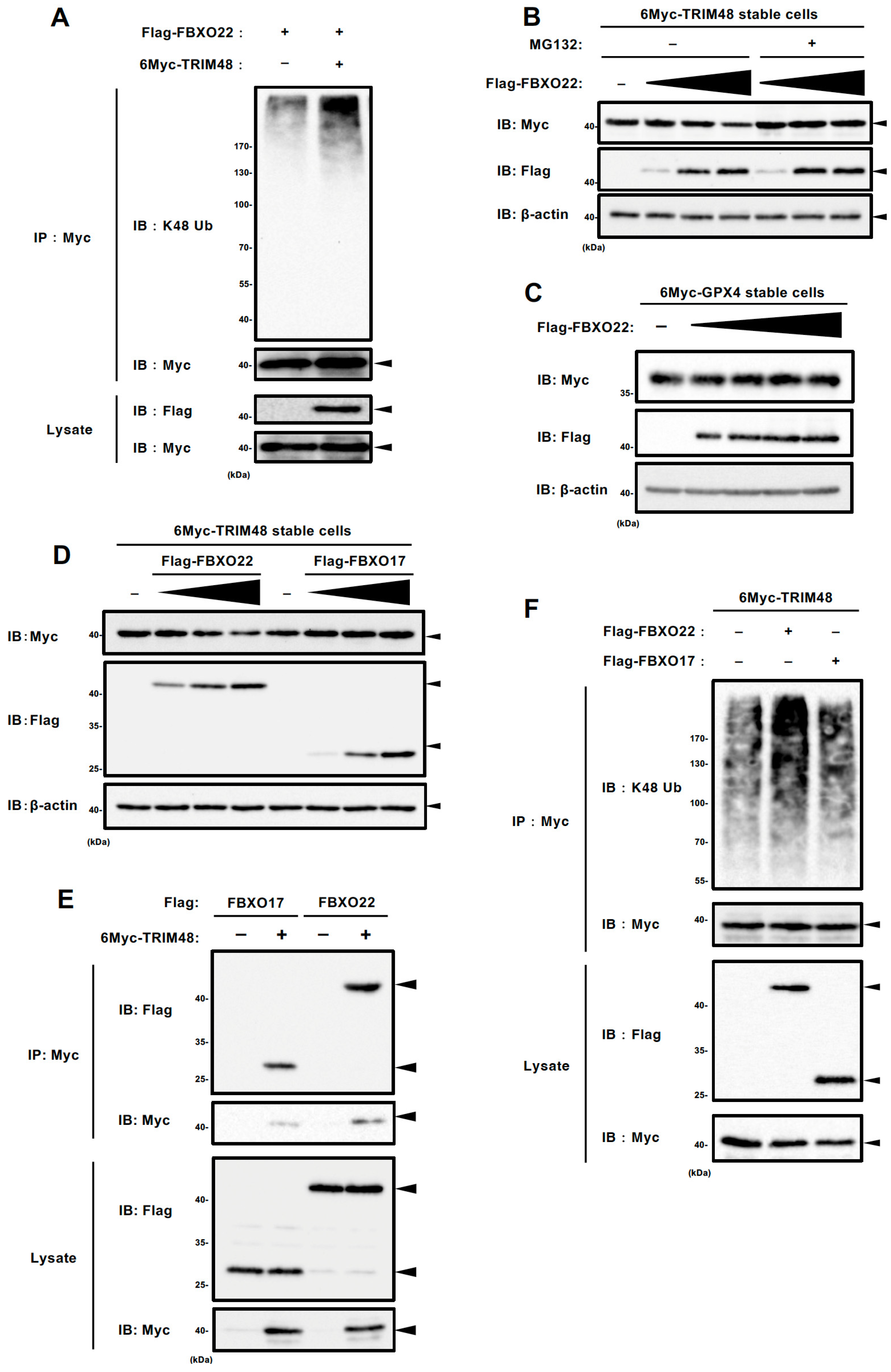
2.3. SCF FBXO22 Promotes TRIM48 Ubiquitination and Degradation
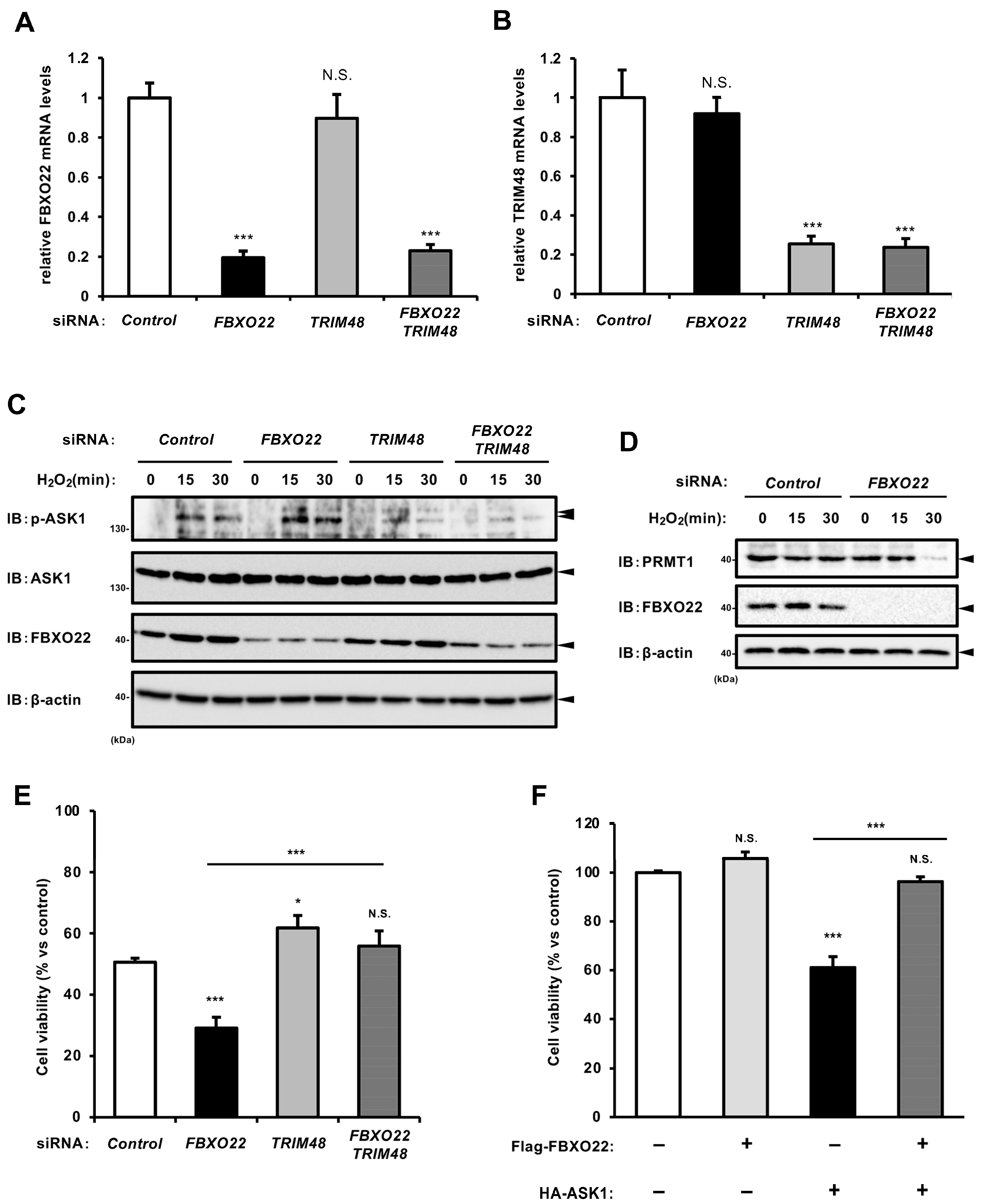
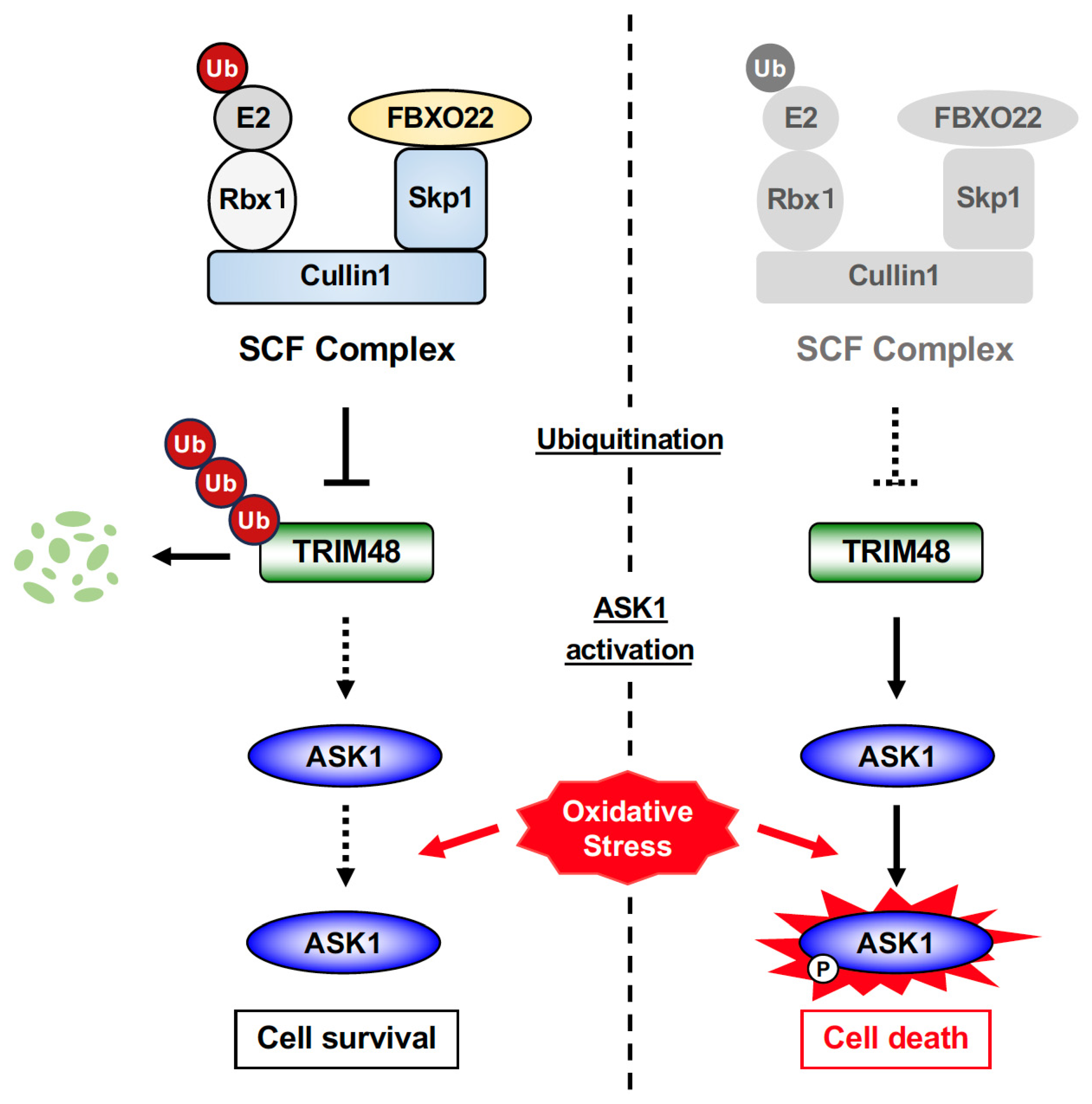
2.4. SCF FBXO22 Protects Against Oxidative Stress-Induced Cell Death by Counteracting TRIM48
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Transfection
4.3. Plasmids
4.4. Immunoblot Assay
4.5. Cell Viability Assay
4.6. RNA Extraction from Cell Lines
4.7. qRT-PCR Analysis
4.8. siRNA Knockdown
4.9. Generation of Stable Cells
4.10. Immunoprecipitation
4.11. In Vivo Ubiquitination Assay
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef]
- Hatakeyama, S. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends Biochem. Sci. 2017, 42, 297–311. [Google Scholar] [CrossRef]
- Han, K.; Lou, D.I.; Sawyer, S.L. Identification of a genomic reservoir for new TRIM genes in primate genomes. PLoS Genet. 2011, 7, e1002388. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, H.; Jian, Z.; Fan, Z.; Liu, S.; Xing, J.; Li, J. Characterization of the primate TRIM gene family reveals the recent evolution in primates. Mol. Genet. Genom. 2020, 295, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Full, F.; van Gent, M.; Sparrer, K.M.J.; Chiang, C.; Zurenski, M.A.; Scherer, M.; Brockmeyer, N.H.; Heinzerling, L.; Sturzl, M.; Korn, K.; et al. Centrosomal protein TRIM43 restricts herpesvirus infection by regulating nuclear lamina integrity. Nat. Microbiol. 2019, 4, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, W.; Cui, H.; Huang, Z.; Ding, R.; Li, N.; Wang, Q.; Wu, F.; Zhao, Y.; Cong, X. TRIM64 promotes ox-LDL-induced foam cell formation, pyroptosis, and inflammation in THP-1-derived macrophages by activating a feedback loop with NF-kappaB via IkappaBalpha ubiquitination. Cell Biol. Toxicol. 2023, 39, 607–620. [Google Scholar] [CrossRef]
- Hirata, Y.; Katagiri, K.; Nagaoka, K.; Morishita, T.; Kudoh, Y.; Hatta, T.; Naguro, I.; Kano, K.; Udagawa, T.; Natsume, T.; et al. TRIM48 Promotes ASK1 Activation and Cell Death through Ubiquitination-Dependent Degradation of the ASK1-Negative Regulator PRMT1. Cell Rep. 2017, 21, 2447–2457. [Google Scholar] [CrossRef]
- Hirata, Y. Reactive Oxygen Species (ROS) Signaling: Regulatory Mechanisms and Pathophysiological Roles. Yakugaku Zasshi 2019, 139, 1235–1241. [Google Scholar] [CrossRef]
- Noguchi, T.; Takeda, K.; Matsuzawa, A.; Saegusa, K.; Nakano, H.; Gohda, J.; Inoue, J.; Ichijo, H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J. Biol. Chem. 2005, 280, 37033–37040. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, L.; Yang, Q.; Li, M.; Pan, X.; Xu, J.; Zhong, C.; Yao, F.; Zhang, R.; Zhou, S.; et al. Thymidine kinase 1 drives hepatocellular carcinoma in enzyme-dependent and -independent manners. Cell Metab. 2023, 35, 912–927.e7. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Peng, S.; Zhou, X.; Tang, H.; Liang, H.; Zhong, X.; Yang, H.; Ke, X.; Lu, M.; et al. PRMT1 promotes the proliferation and metastasis of gastric cancer cells by recruiting MLXIP for the transcriptional activation of the beta-catenin pathway. Genes Dis. 2023, 10, 2622–2638. [Google Scholar] [CrossRef]
- Skaar, J.R.; Pagan, J.K.; Pagano, M. SCF ubiquitin ligase-targeted therapies. Nat. Rev. Drug Discov. 2014, 13, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Hu, L.F.; Zheng, H.F.; Mao, C.J.; Hu, W.D.; Xiong, K.P.; Wang, F.; Liu, C.F. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol. Sin. 2013, 34, 625–635. [Google Scholar] [CrossRef]
- Yumimoto, K.; Yamauchi, Y.; Nakayama, K.I. F-Box Proteins and Cancer. Cancers 2020, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Kincead-Beal, C.; Kulkarni, P.; et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.P.; Lu, B.; Gao, B.B.; Shi, Y.Y.; Xu, J.Q.; Yang, R.; Xu, B.; Ding, P. Overexpression of Tripartite Motif-Containing 48 (TRIM48) Inhibits Growth of Human Glioblastoma Cells by Suppressing Extracellular Signal Regulated Kinase 1/2 (ERK1/2) Pathway. Med. Sci. Monit. 2019, 25, 8422–8429. [Google Scholar] [CrossRef]
- Cheng, J.; Lin, M.; Chu, M.; Gong, L.; Bi, Y.; Zhao, Y. Emerging role of FBXO22 in carcinogenesis. Cell Death Discov. 2020, 6, 66. [Google Scholar] [CrossRef]
- Johmura, Y.; Sun, J.; Kitagawa, K.; Nakanishi, K.; Kuno, T.; Naiki-Ito, A.; Sawada, Y.; Miyamoto, T.; Okabe, A.; Aburatani, H.; et al. SCF(Fbxo22)-KDM4A targets methylated p53 for degradation and regulates senescence. Nat. Commun. 2016, 7, 10574. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Ning, D.; Liu, Q.; Wang, C.; Zhang, Z.; Chu, L.; Yu, C.; Liang, H.F.; Zhang, B.; et al. FBXO22 promotes the development of hepatocellular carcinoma by regulating the ubiquitination and degradation of p21. J. Exp. Clin. Cancer Res. 2019, 38, 101. [Google Scholar] [CrossRef]
- Tian, X.; Dai, S.; Sun, J.; Jin, G.; Jiang, S.; Meng, F.; Li, Y.; Wu, D.; Jiang, Y. F-box protein FBXO22 mediates polyubiquitination and degradation of KLF4 to promote hepatocellular carcinoma progression. Oncotarget 2015, 6, 22767–22775. [Google Scholar] [CrossRef]
- Zhu, X.N.; He, P.; Zhang, L.; Yang, S.; Zhang, H.L.; Zhu, D.; Liu, M.D.; Yu, Y. FBXO22 mediates polyubiquitination and inactivation of LKB1 to promote lung cancer cell growth. Cell Death Dis. 2019, 10, 486. [Google Scholar] [CrossRef]
- Ge, M.K.; Zhang, N.; Xia, L.; Zhang, C.; Dong, S.S.; Li, Z.M.; Ji, Y.; Zheng, M.H.; Sun, J.; Chen, G.Q.; et al. FBXO22 degrades nuclear PTEN to promote tumorigenesis. Nat. Commun. 2020, 11, 1720. [Google Scholar] [CrossRef]
- Sun, R.; Xie, H.Y.; Qian, J.X.; Huang, Y.N.; Yang, F.; Zhang, F.L.; Shao, Z.M.; Li, D.Q. FBXO22 Possesses Both Protumorigenic and Antimetastatic Roles in Breast Cancer Progression. Cancer Res. 2018, 78, 5274–5286. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wu, K.; Cao, M.H.; Yang, Y.; Pan, Y.; Liu, H.; He, Y.; Itahana, Y.; Huang, L.; Zheng, J.N.; et al. SCF(FBXO22) targets HDM2 for degradation and modulates breast cancer cell invasion and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 11754–11763. [Google Scholar] [CrossRef]
- Lignitto, L.; LeBoeuf, S.E.; Homer, H.; Jiang, S.; Askenazi, M.; Karakousi, T.R.; Pass, H.I.; Bhutkar, A.J.; Tsirigos, A.; Ueberheide, B.; et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell 2019, 178, 316–329.e18. [Google Scholar] [CrossRef]
- Ryuno, H.; Naguro, I.; Kamiyama, M. ASK family and cancer. Adv. Biol. Regul. 2017, 66, 72–84. [Google Scholar] [CrossRef]
- Kamiyama, M.; Shirai, T.; Tamura, S.; Suzuki-Inoue, K.; Ehata, S.; Takahashi, K.; Miyazono, K.; Hayakawa, Y.; Sato, T.; Takeda, K.; et al. ASK1 facilitates tumor metastasis through phosphorylation of an ADP receptor P2Y(12) in platelets. Cell Death Differ. 2017, 24, 2066–2076. [Google Scholar] [CrossRef]
- Yin, M.; Zhou, H.J.; Zhang, J.; Lin, C.; Li, H.; Li, X.; Li, Y.; Zhang, H.; Breckenridge, D.G.; Ji, W.; et al. ASK1-dependent endothelial cell activation is critical in ovarian cancer growth and metastasis. JCI Insight 2017, 2, e91828. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chang, S.L.; Fong, Y.C.; Hsu, C.J.; Tang, C.H. Apoptosis signal-regulating kinase 1 is involved in brain-derived neurotrophic factor (BDNF)-enhanced cell motility and matrix metalloproteinase 1 expression in human chondrosarcoma cells. Int. J. Mol. Sci. 2013, 14, 15459–15478. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yu, S.; Xue, Y.; Yan, F. FBXO22, ubiquitination degradation of PHLPP1, ameliorates rotenone induced neurotoxicity by activating AKT pathway. Toxicol. Lett. 2021, 350, 1–9. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Qu, D.; Liu, Y.; Wang, N.; Wang, H.; Fan, Y.; Zhou, Y.; Yu, B.; Xue, Q.; et al. Aberrant expression of FBXO22 is associated with propofol-induced synaptic plasticity and cognitive dysfunction in adult mice. Front. Aging Neurosci. 2022, 14, 1028148. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, W.; Yang, C.; Wei, X.; Fan, L.; Wu, Y.; Gao, X.; Shen, X.; Zhang, Z.; Zhao, J. E3 ubiquitin ligase FBXO22 inhibits SARS-CoV-2 replication via promoting proteasome-dependent degradation of NSP5. J. Med. Virol. 2024, 96, e29891. [Google Scholar] [CrossRef]
- Karpukhina, A.; Tiukacheva, E.; Dib, C.; Vassetzky, Y.S. Control of DUX4 Expression in Facioscapulohumeral Muscular Dystrophy and Cancer. Trends Mol. Med. 2021, 27, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.N.; Yao, Z.; Snider, L.; Fong, A.P.; Cech, J.N.; Young, J.M.; van der Maarel, S.M.; Ruzzo, W.L.; Gentleman, R.C.; Tawil, R.; et al. DUX4 activates germline genes, retroelements, and immune mediators: Implications for facioscapulohumeral dystrophy. Dev. Cell 2012, 22, 38–51. [Google Scholar] [CrossRef]
- Wallace, L.M.; Garwick, S.E.; Mei, W.; Belayew, A.; Coppee, F.; Ladner, K.J.; Guttridge, D.; Yang, J.; Harper, S.Q. DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo. Ann. Neurol. 2011, 69, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Honda, M.; Jonouchi, T.; Arai, M.; Hotta, A.; Mitsuhashi, S.; Nishino, I.; Matsuda, R.; Sakurai, H. A patient-derived iPSC model revealed oxidative stress increases facioscapulohumeral muscular dystrophy-causative DUX4. Hum. Mol. Genet. 2018, 27, 4024–4035. [Google Scholar] [CrossRef]
- Schatzl, T.; Todorow, V.; Kaiser, L.; Weinschrott, H.; Schoser, B.; Deigner, H.P.; Meinke, P.; Kohl, M. Meta-analysis towards FSHD reveals misregulation of neuromuscular junction, nuclear envelope, and spliceosome. Commun. Biol. 2024, 7, 640. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef]
- Tobiume, K.; Saitoh, M.; Ichijo, H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J. Cell. Physiol. 2002, 191, 95–104. [Google Scholar] [CrossRef]
- Kitamura, T.; Koshino, Y.; Shibata, F.; Oki, T.; Nakajima, H.; Nosaka, T.; Kumagai, H. Retrovirus-mediated gene transfer and expression cloning: Powerful tools in functional genomics. Exp. Hematol. 2003, 31, 1007–1014. [Google Scholar] [CrossRef]
- Hirata, Y.; Yamada, Y.; Taguchi, S.; Kojima, R.; Masumoto, H.; Kimura, S.; Niijima, T.; Toyama, T.; Kise, R.; Sato, E.; et al. Conjugated fatty acids drive ferroptosis through chaperone-mediated autophagic degradation of GPX4 by targeting mitochondria. Cell Death Dis. 2024, 15, 884. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashiwabara, N.; Nagaoka, K.; Nakajima, K.; Tsukamoto, H.; Tomioka, Y.; Naguro, I.; Ichijo, H.; Noguchi, T.; Hirata, Y.; Matsuzawa, A. FBXO22 Suppresses Oxidative Stress-Induced ASK1 Activation and Cell Death via Ubiquitination-Dependent Degradation of TRIM48. Int. J. Mol. Sci. 2025, 26, 9472. https://doi.org/10.3390/ijms26199472
Kashiwabara N, Nagaoka K, Nakajima K, Tsukamoto H, Tomioka Y, Naguro I, Ichijo H, Noguchi T, Hirata Y, Matsuzawa A. FBXO22 Suppresses Oxidative Stress-Induced ASK1 Activation and Cell Death via Ubiquitination-Dependent Degradation of TRIM48. International Journal of Molecular Sciences. 2025; 26(19):9472. https://doi.org/10.3390/ijms26199472
Chicago/Turabian StyleKashiwabara, Naoki, Keita Nagaoka, Kenshin Nakajima, Hiroki Tsukamoto, Yoshihisa Tomioka, Isao Naguro, Hidenori Ichijo, Takuya Noguchi, Yusuke Hirata, and Atsushi Matsuzawa. 2025. "FBXO22 Suppresses Oxidative Stress-Induced ASK1 Activation and Cell Death via Ubiquitination-Dependent Degradation of TRIM48" International Journal of Molecular Sciences 26, no. 19: 9472. https://doi.org/10.3390/ijms26199472
APA StyleKashiwabara, N., Nagaoka, K., Nakajima, K., Tsukamoto, H., Tomioka, Y., Naguro, I., Ichijo, H., Noguchi, T., Hirata, Y., & Matsuzawa, A. (2025). FBXO22 Suppresses Oxidative Stress-Induced ASK1 Activation and Cell Death via Ubiquitination-Dependent Degradation of TRIM48. International Journal of Molecular Sciences, 26(19), 9472. https://doi.org/10.3390/ijms26199472






