Relationship Between the Duration of Intravenous Ketamine Anesthesia and Postoperative Oxidative Stress and Inflammatory Response in Rats
Abstract
1. Introduction
2. Results
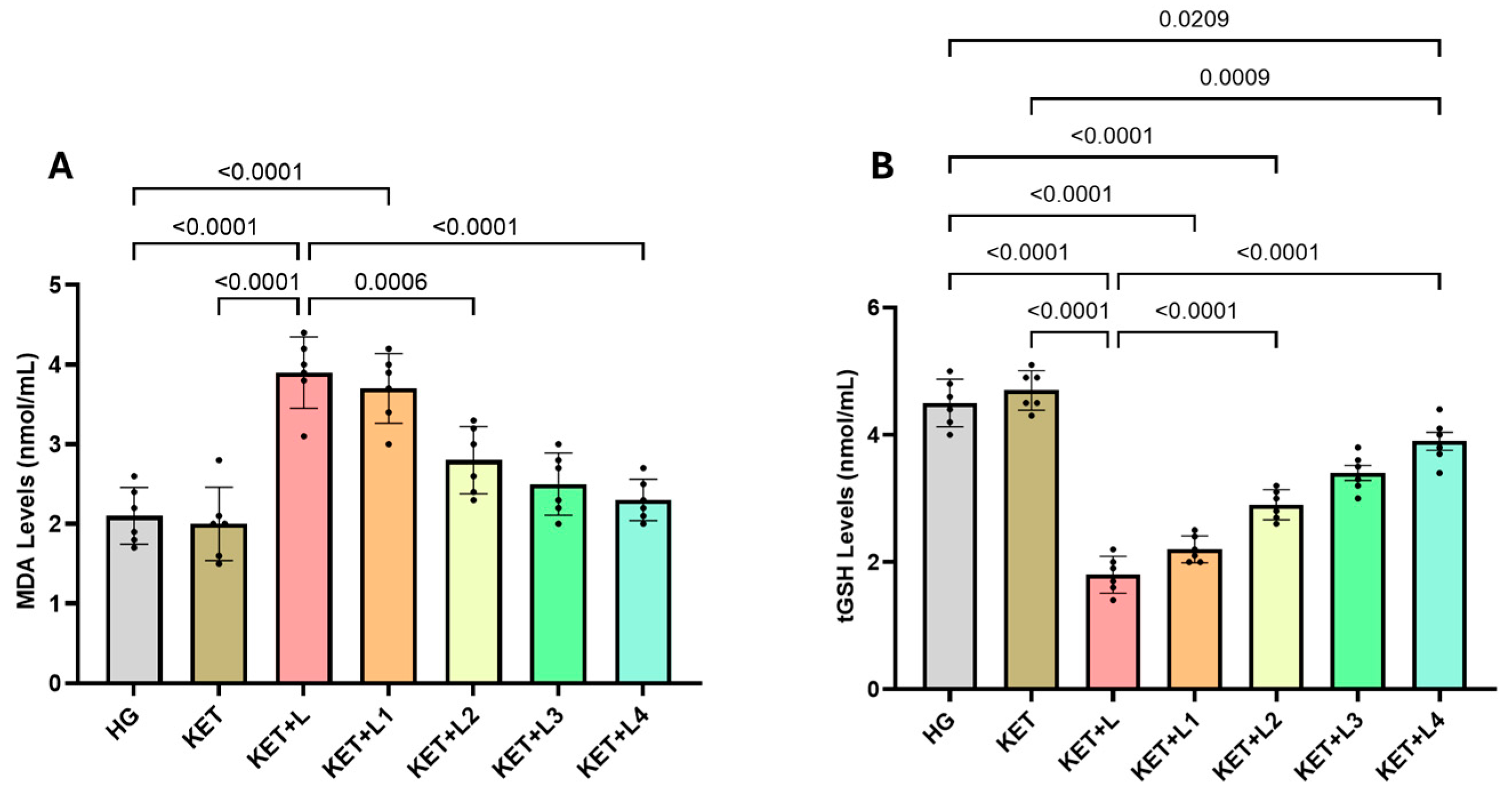
2.1. Blood MDA and tGSH (Total Glutathione) Analysis Results
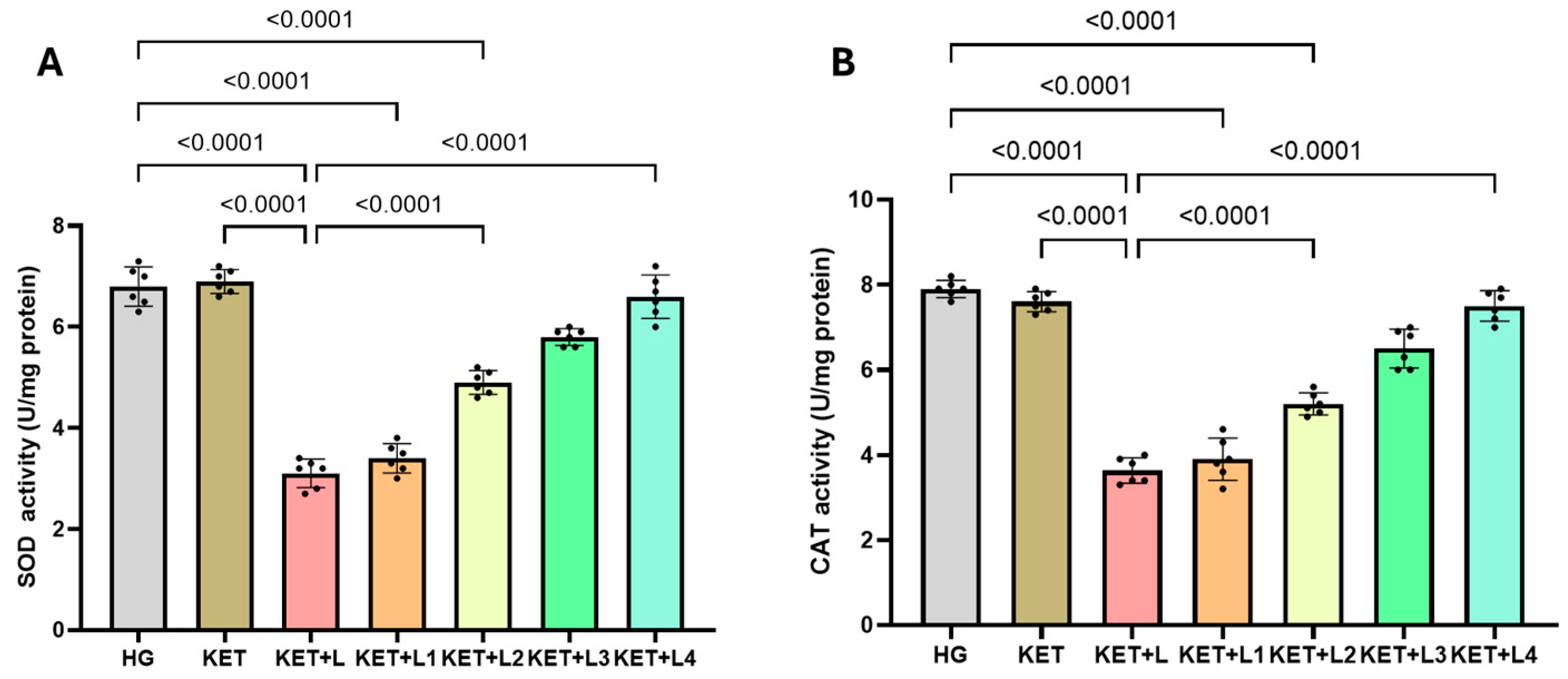
2.2. Blood SOD and CAT Activity Results
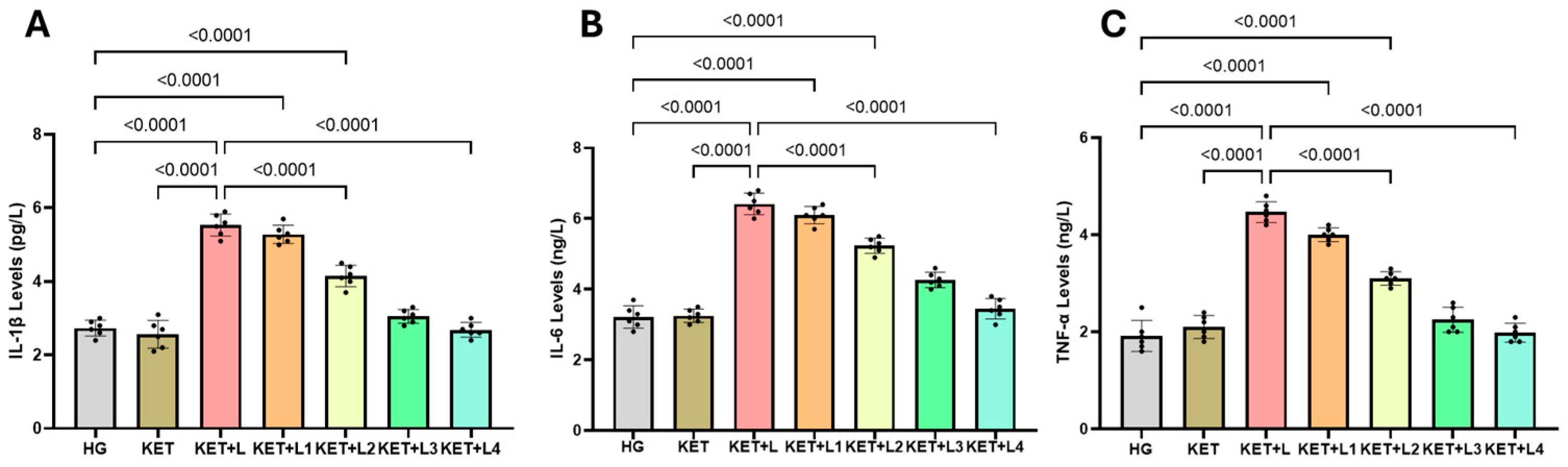
2.3. Blood Inflammatory Cytokine (IL-1β, IL-6, and TNF-α) Results
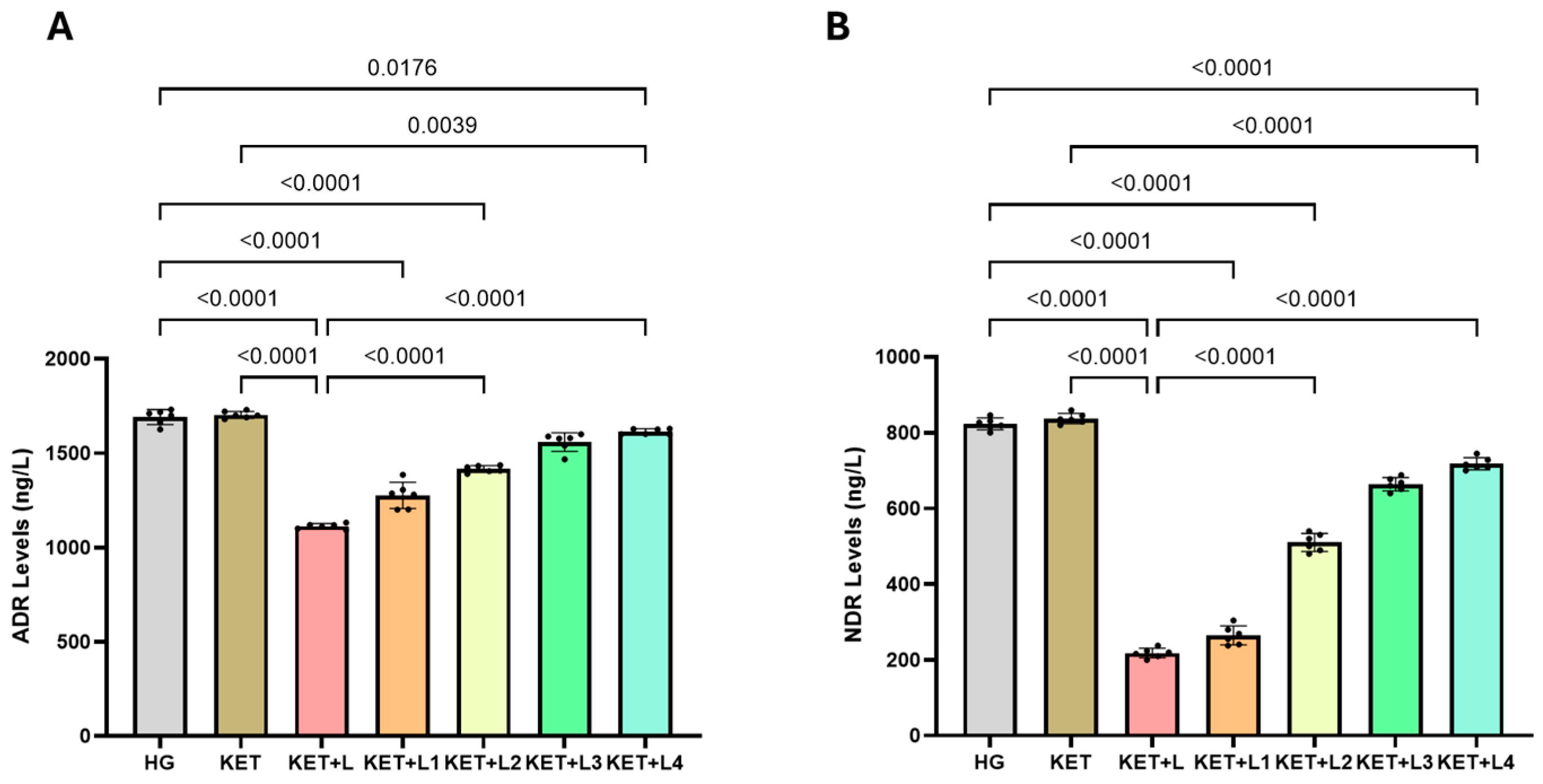
2.4. Blood Catecholamine (ADR and NDR) Results
3. Discussion
4. Methods and Materials
4.1. Animals
4.2. Chemicals
4.3. Experimental Groups
4.4. Experimental Procedure
4.5. Biochemical Analysis Methods
4.5.1. Determination of MDA, GSH, SOD and CAT
4.5.2. Measurement of ADR and NDR Levels in Rats
4.5.3. Blood TNF-α, IL-1β, and IL-6 Determination
4.6. Statistical Analysis—Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | Malondialdehyde |
| ADR | Adrenaline |
| NDR | Noradrenaline |
| tGSH | Total Glutathione |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| ROS | Reactive Oxygen Species |
| LPO | Lipid Peroxidation |
| MPA | Mean Arterial Pressure |
| NMDA | N-methyl-D-aspartate |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-Alpha |
References
- Toro-Pérez, J.; Rodrigo, R. Contribution of oxidative stress in the mechanisms of postoperative complications and multiple organ dysfunction syndrome. Redox Rep. 2021, 26, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sagün, A.; Azizoğlu, M.; Birbiçer, H. Assessment of postoperative complications in a university hospital in Turkey: A retrospective cohort study. Dokuz Eylül Üniversitesi Tıp Fakültesi Derg. 2021, 35, 99–107. [Google Scholar] [CrossRef]
- Li, T.; Ding, J.; Pan, Y.; Shui, W.; Ke, Y.; Pan, Z.; Chen, J. Progress of Perioperative Oxidative Stress in Children. J. Biol. Regul. Homeost. Agents 2023, 37, 6493–6650. [Google Scholar] [CrossRef]
- Yuba, T.; Koyama, Y.; Takahashi, A.; Fujino, Y.; Shimada, S. Association between oxidative stress and postoperative delirium in joint replacement using diacron-reactive oxygen metabolites and biological antioxidant potential tests. Sci. Rep. 2024, 14, 29854. [Google Scholar] [CrossRef]
- Luna-Ortiz, P.; Pilar-Báez, S.; Lazcano-Vázquez, M.F.; Martínez-Rosas, M. Oxidative stress in the perioperative period: Clinical implications. Rev. Mex. Anestesiol. 2024, 47, 23–29. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Shiomoto, K.; Mizutani, K.; Fujioka, K.; Suehiro, K.; Yamada, T.; Sato, E.F.; Nishikawa, K. Reduction of oxidative stress a key for enhanced postoperative recovery with fewer complications in esophageal surgery patients: Randomized control trial to investigate therapeutic impact of anesthesia management and usefulness of simple blood test for prediction of high-risk patients. Medicine 2018, 97, e12845. [Google Scholar] [CrossRef]
- Zivkovic, A.; Jotic, A.; Dozic, I.; Randjelovic, S.; Cirkovic, I.; Medic, B.; Milovanovic, J.; Trivić, A.; Korugic, A.; Vujovic, K.S. Role of Oxidative Stress and Inflammation in Postoperative Complications and Quality of Life After Laryngeal Cancer Surgery. Cells 2024, 13, 1951. [Google Scholar] [CrossRef]
- Plicner, D.; Mazur, P.; Sadowski, J.; Undas, A. Asymmetric dimethylarginine and oxidative stress following coronary artery bypass grafting: Associations with postoperative outcome. Eur. J. Cardio-Thorac. Surg. 2014, 45, e136–e141. [Google Scholar] [CrossRef]
- Wu, J.H.; Marchioli, R.; Silletta, M.G.; Masson, S.; Sellke, F.W.; Libby, P.; Milne, G.L.; Brown, N.J.; Lombardi, F.; Damiano, R.J.; et al. Oxidative stress biomarkers and incidence of postoperative atrial fibrillation in the omega-3 fatty acids for prevention of postoperative atrial fibrillation (OPERA) trial. J. Am. Heart Assoc. 2015, 4, e001886. [Google Scholar] [CrossRef] [PubMed]
- Saugel, B.; Fletcher, N.; Gan, T.J.; Grocott, M.P.; Myles, P.S.; Sessler, D.I.; Auzinger, G.; Chappell, D.; Edwards, M.; Forni, L.G.; et al. Peri Operative Quality Initiative (POQI) international consensus statement on perioperative arterial pressure management. Br. J. Anaesth. 2024, 133, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Liang, S.; Zhou, N.; Zhu, X.; Guo, Q.; Sessler, D.I.; Zou, W. Individualized blood pressure regulation and acute kidney injury in older patients having major abdominal surgery: A pilot randomized trial. Int. J. Surg. 2025, 111, 2894–2902. [Google Scholar] [CrossRef]
- Briesenick, L.; Flick, M.; Saugel, B. Postoperative blood pressure management in patients treated in the ICU after noncardiac surgery. Curr. Opin. Crit. Care 2021, 27, 694–700. [Google Scholar] [CrossRef]
- Alhashemi, S.H.; Mohammadpour, A.H.; Heidari, R.; Nikoo, M.H.; Nemati, M.H.; Vazin, A. The effect of nanocurcumin on the incidence of atrial fibrillation, and markers of inflammation and oxidative stress level after coronary artery bypass graft surgery: A randomized, double-blind, placebo-controlled clinical study. Avicenna J. Phytomed. 2022, 12, 503. [Google Scholar] [CrossRef]
- Ivascu, R.; Torsin, L.I.; Hostiuc, L.; Nitipir, C.; Corneci, D.; Dutu, M. The surgical stress response and anesthesia: A narrative review. J. Clin. Med. 2024, 13, 3017. [Google Scholar] [CrossRef]
- Ritiu, S.A.; Rogobete, A.F.; Sandesc, D.; Bedreag, O.H.; Papurica, M.; Popovici, S.E.; Toma, D.; Ivascu, R.I.; Velovan, R.; Garofil, D.N.; et al. The Impact of General Anesthesia on Redox Stability and Epigenetic Inflammation Pathways: Crosstalk on Perioperative Antioxidant Therapy. Cells 2022, 11, 1880. [Google Scholar] [CrossRef]
- Senoner, T.; Velik-Salchner, C.; Luckner, G.; Tauber, H. Anesthesia Induced Oxidative Stress: Are There Differences between Intravenous and Inhaled Anesthetics? Oxidative Med. Cell. Longev. 2021, 2021, 8782387. [Google Scholar] [CrossRef]
- Orhurhu, V.J.; Vashisht, R.; Claus, L.E.; Cohen, S.P. Ketamine Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Afify, E.; Mohamed, S.; Khedr, M. Ketamine: Recent evidence and current uses. Benha J. Appl. Sci. 2023, 8, 111–117. [Google Scholar] [CrossRef]
- Ghaffari, M.K.; Sefati, N.; Esmaeilpour, T.; Salari, V.; Oblak, D.; Simon, C. The impact of ketamine and thiopental anesthesia on ultraweak photon emission and oxidative-nitrosative stress in rat brains. Front. Syst. Neurosci. 2025, 19, 1502589. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, S.; Xie, W.; He, H. Ketamine ameliorates oxidative stress-induced apoptosis in experimental traumatic brain injury via the Nrf2 pathway. Drug Design. Dev. Ther. 2018, 12, 845–853. [Google Scholar] [CrossRef]
- Ahiskalioglu, E.O.; Aydin, P.; Ahiskalioglu, A.; Suleyman, B.; Kuyrukluyildiz, U.; Kurt, N.; Altuner, D.; Coskun, R.; Suleyman, H. The effects of ketamine and thiopental used alone or in combination on the brain, heart, and bronchial tissues of rats. Arch. Med. Sci. 2018, 14, 645–654. [Google Scholar] [CrossRef]
- Taniguchi, T.; Takemoto, Y.; Kanakura, H.; Kidani, Y.; Yamamoto, K. The dose-related effects of ketamine on mortality and cytokine responses to endotoxin-induced shock in rats. Anesth. Analg. 2003, 97, 1769–1772. [Google Scholar] [CrossRef]
- Réus, G.Z.; Simões, L.R.; Colpo, G.D.; Scaini, G.; Oses, J.P.; Generoso, J.S.; Prossin, A.R.; Kaddurah-Daouk, R.; Quevedo, J.; Barichello, T. Ketamine potentiates oxidative stress and influences behavior and inflammation in response to lipolysaccharide (LPS) exposure in early life. Neuroscience 2017, 353, 17–25. [Google Scholar] [CrossRef]
- Cata, J.P.; Patiño, M.; Lacagnina, M.J.; Li, J.; Gorur, A.; Agudelo-Jimenez, R.; Wei, B.; Hagberg, C.A.; Dougherty, P.M.; Shureiqi, I.; et al. A rat model to investigate quality of recovery after abdominal surgery. Pain Rep. 2021, 6, e943. [Google Scholar] [CrossRef]
- Chang, C.K.; Zdon, M.J. Inflammatory Response of Interleukin-1β and Interleukin-6 in Septic Rats Undergoing Laparotomy and Laparoscopy. Surg. Laparosc. Endosc. Percutaneous Tech. 2005, 15, 124–128. [Google Scholar] [CrossRef]
- Yılmaz, E.M.; Cartı, E.B.; Yenisey, Ç.; Kırnap, M.; Coşkun, M.Ç.; Demirkıran, A.E.; Çetin, N.K. The effect of laparoscopy and laparotomy on hemostasis in experimental colorectal cancer surgery in rats. J. Clin. Med. Kazakhstan 2020, 6, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ryabushko, R.M.; Boiarska, Z.O.; Kostenko, V.O. Combined Effect Of Laparotomy And Experimental Post-Traumatic Stress Disorder On The Sources Of Superoxide Anion Radical In The Rat Heart. Med. Ecol. Probl. 2024, 28, 29–34. [Google Scholar] [CrossRef]
- Senoner, T.; Schindler, S.; Stättner, S.; Öfner, D.; Troppmair, J.; Primavesi, F. Associations of oxidative stress and postoperative outcome in liver surgery with an outlook to future potential therapeutic options. Oxidative Med. Cell. Longev. 2019, 2019, 3950818. [Google Scholar] [CrossRef] [PubMed]
- An, L.N.; Yue, Y.; Guo, W.Z.; Miao, Y.L.; Mi, W.D.; Zhang, H.; Lei, Z.-L.; Han, S.-J.; Dong, L. Surgical trauma induces iron accumulation and oxidative stress in a rodent model of postoperative cognitive dysfunction. Biol. Trace Elem. Res. 2013, 151, 277–283. [Google Scholar] [CrossRef]
- Leimkühler, M.; Bourgonje, A.R.; van Goor, H.; Campmans-Kuijpers, M.J.; de Bock, G.H.; van Leeuwen, B.L. Oxidative stress predicts post-surgery complications in gastrointestinal cancer patients. Ann. Surg. Oncol. 2022, 29, 4540–4547. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Kolios, G.; Manousou, P.; Kazamias, P.; Paramythiotis, D.; Papavramidis, T.S.; Heliadis, S.; Kouroumalis, E.; Eleftheriadis, E. Oxidative stress due to anesthesia and surgical trauma: Importance of early enteral nutrition. Mol. Nutr. Food Res. 2009, 53, 770–779. [Google Scholar] [CrossRef]
- Kärkkäinen, J.; Selander, T.; Purdy, M.; Juvonen, P.; Eskelinen, M. Patients with increased levels of the oxidative stress biomarker SOD1 appear to have diminished postoperative pain after midline laparotomy: A randomised trial with special reference to postoperative pain score (nrs). Anticancer Res. 2018, 38, 1003–1008. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Shibakusa, T.; Mikami, T.; Kurihara, S.; Chiba, Y.; Tsuchiya, T.; Miyachi, T.; Oyama, A.; Tanaka, K.A.; Koyama, N. Enhancement of postoperative recovery by preoperative oral co-administration of the amino acids, cystine and theanine, in a mouse surgical model. Clin. Nutr. 2012, 31, 555–561. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Saimanen, I.; Kärkkäinen, J.; Selander, T.; Purdy, M.; Kokki, M.; Kokki, H.; Eskelinen, M. Plasma catalase in relation to pain following midline laparotomy: A prospective study of patients with benign diseases and patients with cancer. Anticancer Res. 2018, 38, 6479–6484. [Google Scholar] [CrossRef]
- Anadol, E.; Yarım, G.F.; Gültiken, N.; Kazak, F. Effect of ovariohysterectomy on some oxidative stress markers in the rat. Harran Univ. J. Fac. Vet. Med. 2016, 5, 124–128. [Google Scholar]
- Zhao, J.; Zhang, T.; Deng, Z.; Han, X.; Ma, T.; Xie, K. Evaluation of biomarkers from peritoneal fluid as predictors of severity for abdominal sepsis patients following emergency laparotomy. J. Inflamm. Res. 2023, 16, 809–826. [Google Scholar] [CrossRef] [PubMed]
- Müsri, Ö.C.; Oğuz, S.; Gürcan, Ş.; Özgürtaş, T.; Yalta, T.; Tazegul, G.; Müsri, F.Y. Carnosol Alleviates Inflammation And Bacterial Translocation In A Rat Model Of Intestinal Ischemia-Reperfusion Injury. Int. Anatolia Acad. Online J. Health Sci. 2019, 5, 105–118. [Google Scholar]
- Cusack, B.; Buggy, D.J. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Taschner, A.; Kabon, B.; Falkner von Sonnenburg, M.; Graf, A.; Adamowitsch, N.; Fraunschiel, M.; Fleischmann, E.; Reiterer, C. Perioperative supplemental oxygen and plasma catecholamine concentrations after major abdominal surgery—Secondary analysis of a randomized clinical Trial. J. Clin. Med. 2022, 11, 1767. [Google Scholar] [CrossRef]
- Silva, G.N.; Brandão, V.G.; Perez, M.V.; Blum, K.; Lewandrowski, K.U.; Fiorelli, R.K. Neuroinflammatory approach to surgical trauma: Biomarkers and mechanisms of immune and neuroendocrine responses. J. Pers. Med. 2024, 14, 829. [Google Scholar] [CrossRef]
- Carrara, M.; Ferrario, M.; Bollen Pinto, B.; Herpain, A. The autonomic nervous system in septic shock and its role as a future therapeutic target: A narrative review. Ann. Intensive Care 2021, 11, 80. [Google Scholar] [CrossRef]
- Bedir, Z.; Ozkaloglu Erdem, K.; ATEŞ, İ.; Ölmeztürk Karakurt, T.Ü.L.A.Y.; Gürsul, C.; Onk, D.; Kurt, N.; Süleyman, H. Effects of ketamine, thiopental and their combination on the rat liver: A biochemical evaluation. Adv. Clin. Exp. Med. 2022, 31, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Welters, I.D.; Feurer, M.K.; Preiss, V.; Müller, M.; Scholz, S.; Kwapisz, M.; Mogk, M.; Neuhäuser, C. Continuous S-(+)-ketamine administration during elective coronary artery bypass graft surgery attenuates pro-inflammatory cytokine response during and after cardiopulmonary bypass. Br. J. Anaesth. 2011, 106, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H.F.; Berman, R.Y.; Boese, M.; Zhang, M.; Kim, S.Y.; Radford, K.D.; Choi, K.H. Effects of an intravenous ketamine infusion on inflammatory cytokine levels in male and female Sprague–Dawley rats. J. Neuroinflamm. 2022, 19, 75. [Google Scholar] [CrossRef]
- Mostafa, M.; Hasanin, A.; Reda, B.; Elsayad, M.; Zayed, M.; Abdelfatah, M.E. Comparing the hemodynamic effects of ketamine versus fentanyl bolus in patients with septic shock: A randomized controlled trial. J. Anesth. 2024, 38, 756–764. [Google Scholar] [CrossRef]
- Aksoy, M.; Ince, I.; Ahiskalioglu, A.; Dostbil, A.; Celik, M.; Turan, M.I.; Cetin, N.; Suleyman, B.; Alp, H.H.; Suleyman, H. The suppression of endogenous adrenalin in the prolongation of ketamine anesthesia. Med. Hypotheses 2014, 83, 103–107. [Google Scholar] [CrossRef]
- Irwin, M.R.; Curay, C.M.; Choi, S.; Kiyatkin, E.A. Basic physiological effects of ketamine-xylazine mixture as a general anesthetic preparation for rodent surgeries. Brain Res. 2023, 1804, 148251. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Kurt, A.; Isaoglu, U.; Yilmaz, M.; Calik, M.; Polat, B.; Hakan, H.; Ingec, M.; Suleyman, H. Biochemical and histological investigation of famotidine effect on postischemic reperfusion injury in the rat ovary. J. Pediatr. Surg. 2011, 46, 1817–1823. [Google Scholar] [CrossRef]
- Cicek, I.; Esenulku, C.M.; Somuncu, A.M.; Bulut, S.; Yucel, N.; Bal Tastan, T.; Coban, T.A.; Suleyman, H. Sunitinib’s Effect on Bilateral Optic Nerve Damage in Rats Following the Unilateral Clamping and Unclamping of the Common Carotid Artery. Biomedicines 2025, 13, 620. [Google Scholar] [CrossRef] [PubMed]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
| Biochemical Variables | ||||
|---|---|---|---|---|
| Groups | MDA (nmol/mL) | tGSH (nmol/mL) | SOD (U/mg Protein) | CAT (U/mg Protein) |
| HG | 2.10 ± 0.36 | 4.50 ± 0.37 | 6.80 ± 0.39 | 7.90 ± 0.20 |
| KET | 2.00 ± 0.46 | 4.70 ± 0.31 | 6.90 ± 0.24 | 7.60 ± 0.24 |
| KET + L | 3.90 ± 0.45 *,† | 1.80 ± 0.29 *,† | 3.10 ± 0.28 *,† | 3.63 ± 0.30 *,† |
| KET + L1 | 3.70 ± 0.44 *,† | 2.20 ± 0.21 *,† | 3.40 ± 0.29 *,†,‡ | 3.90 ± 0.50 *,†,‡ |
| KET + L2 | 2.80 ± 0.42 *,‡ | 2.90 ± 0.24 *,†,‡ | 4.90 ± 0.24 *,†,‡ | 5.20 ± 0.26 *,†,‡ |
| KET + L3 | 2.50 ± 0.39 ‡ | 3.40 ± 0.29 ‡ | 5.80 ± 0.17 ‡ | 6.50 ± 0.46 ‡ |
| KET + L4 | 2.30 ± 0.26 ‡ | 3.90 ± 0.35 ‡ | 6.60 ± 0.43 ‡ | 7.50 ± 0.36 ‡ |
| Biochemical Variables | |||
|---|---|---|---|
| Groups | IL-1β (pg/L) | IL-6 (ng/L) | TNF-α (ng/L) |
| HG | 2.73 ± 0.22 | 3.22 ± 0.32 | 1.92 ± 0.32 |
| KET | 2.57 ± 0.38 | 3.25 ± 0.19 | 2.10 ± 0.24 |
| KET + L | 5.53 ± 0.30 *† | 6.42 ± 0.31 *† | 4.47 ± 0.22 *† |
| KET + L1 | 5.28 ± 0.25 * | 6.10 ± 0.24 * | 4.00 ± 0.14 * |
| KET + L2 | 4.15 ± 0.29 *‡ | 5.23 ± 0.22 *‡ | 3.10 ± 0.14 *‡ |
| KET + L3 | 3.05 ± 0.19 ‡ | 4.27 ± 0.22 ‡ | 2.25 ± 0.26 ‡ |
| KET + L4 | 2.68 ± 0.20 ‡ | 3.45 ± 0.29 ‡ | 1.98 ± 0.19 ‡ |
| Biochemical Variables | ||
|---|---|---|
| Groups | ADR (ng/L) | NDR (ng/L) |
| HG | 1674.50 ± 38.39 | 823.00 ± 16.97 |
| KET | 1703.33 ± 18.31 | 837.00 ± 14.65 |
| KET + L | 1112.00 ± 13.15 *† | 218.00 ± 13.42 *† |
| KET + L1 | 1276.67 ± 69.24 *‡ | 264.50 ± 25.67 *‡ |
| KET + L2 | 1416.00 ± 16.00 *‡ | 506.67 ± 20.66 *‡ |
| KET + L3 | 1559.00 ± 21.91 ‡ | 664.00 ± 17.78 ‡ |
| KET + L4 | 1614.00 ± 14.52 ‡ | 718.00 ± 16.91 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ince, R.; Ozgodek, H.B.; Kahramanlar, A.A.; Yucel, N.; Sarıgül, C.; Suleyman, H. Relationship Between the Duration of Intravenous Ketamine Anesthesia and Postoperative Oxidative Stress and Inflammatory Response in Rats. Int. J. Mol. Sci. 2025, 26, 9465. https://doi.org/10.3390/ijms26199465
Ince R, Ozgodek HB, Kahramanlar AA, Yucel N, Sarıgül C, Suleyman H. Relationship Between the Duration of Intravenous Ketamine Anesthesia and Postoperative Oxidative Stress and Inflammatory Response in Rats. International Journal of Molecular Sciences. 2025; 26(19):9465. https://doi.org/10.3390/ijms26199465
Chicago/Turabian StyleInce, Ramazan, Habip Burak Ozgodek, Agah Abdullah Kahramanlar, Nurinisa Yucel, Cengiz Sarıgül, and Halis Suleyman. 2025. "Relationship Between the Duration of Intravenous Ketamine Anesthesia and Postoperative Oxidative Stress and Inflammatory Response in Rats" International Journal of Molecular Sciences 26, no. 19: 9465. https://doi.org/10.3390/ijms26199465
APA StyleInce, R., Ozgodek, H. B., Kahramanlar, A. A., Yucel, N., Sarıgül, C., & Suleyman, H. (2025). Relationship Between the Duration of Intravenous Ketamine Anesthesia and Postoperative Oxidative Stress and Inflammatory Response in Rats. International Journal of Molecular Sciences, 26(19), 9465. https://doi.org/10.3390/ijms26199465





