1. Introduction
Medicinal plants have long shaped traditional healing systems. In many parts of the world, especially where modern medical services are scarce, they remain the backbone of primary healthcare. However, the increasing interest in phytotherapeutics and integration of such plants into contemporary pharmacological formulations demand a clear understanding of their safety profiles [
1]. This becomes notably important when considering the complex composition of plant matrices, which may contain bioactive compounds with both therapeutic and toxic potential.
Toxicological studies, especially those investigating acute toxicity, cytotoxicity, and genotoxicity, are fundamental for evaluating the safety of traditionally used medicinal plants. Such studies are indispensable when these botanicals are considered for broader pharmacological applications or incorporation into herbal products [
2].
According to taxonomic revisions [
3,
4], the genus
Origanum L. comprises ten sections, nine of which are predominantly distributed across Greece, the southern Balkans, and the eastern Mediterranean. Historically,
Origanum species have been central to both culinary and medicinal traditions. Moreover, their economic and cultural value has persisted for centuries. In modern times, these aromatic plants continue to be extensively utilized in the agricultural, pharmaceutical, and cosmetic sectors, where they are valued for their secondary metabolites with pharmacological activity and significant commercial potential [
5,
6].
Origanum majorana L. (
O. majorana) is found in the flora of Türkiye, especially in Southern Anatolia [
7]. The plant, commonly known as ‘sweet marjoram’, is referred to as ‘Mercanköşk’ in Türkiye, as is the case with other
Origanum species; additionally, it is known by various local names such as ‘Kekik otu’, ‘Göğe kekiği’, ‘Kahve otu’, ‘Guy otu’, ‘Sebso’, and ‘Sebzo’ [
8,
9].
O. majorana is traditionally used as a culinary spice and in folk medicine. The flowering and leafy branches of
O. majorana have been reported to be used in Anatolia as a folk medicine, particularly in the form of decoctions and infusions, for the relief of colds, coughs, flu, sore throat, stomach ache, indigestion, gastric ulcers, nausea, intestinal gas, and diarrhea. They are also traditionally employed as a diuretic and for the treatment of urinary tract diseases, as well as for their sedative effects and in the management of diabetes, hypertension, arteriosclerosis, gallbladder diseases, and to promote sweating [
10]. Its essential oil is characterized by high levels of terpinen-4-ol, often accompanied by cis-sabinene hydrate, γ-terpinene, α-terpineol, linalool, and p-cymene [
11]. Depending on chemotype and geographic origin, minor amounts of thymol and carvacrol may also occur [
12]. These volatile compounds have been associated with antimicrobial, antioxidant, and anti-inflammatory properties, making
O. majorana a valuable source of bioactive metabolites and a subject of growing pharmacological interest [
13].
Despite its growing culinary and medicinal use, detailed in vivo toxicity data on
O. majorana remain scarce, an omission that may lead to overestimating its safety in routine consumption. While low doses of carvacrol can be beneficial, higher concentrations have been linked to adverse outcomes, including DNA damage in certain experimental settings [
14]. Thus, understanding the dose–response relationship and potential cytotoxic or genotoxic risks associated with
O. majorana is crucial, particularly for populations using it frequently or in combination with conventional drugs.
The present study examines the acute oral toxicity of
O. majorana methanol extract in mice, following OECD Test Guideline No. 420 [
15]. It also evaluates in vitro cytotoxicity and genotoxicity via MTT, comet, and micronucleus assays in NIH3T3 cells (a standard non-tumorigenic murine cell line widely used for toxicity screening), aiming to provide a comprehensive safety assessment. These evaluations are intended to establish baseline toxicological data for
O. majorana, supporting its traditional use while ensuring a scientific basis for potential pharmaceutical applications.
3. Discussion
The present study provides a multifaceted evaluation of O. majorana methanolic extract, integrating phytochemical profiling with in vitro cytotoxicity and genotoxicity assays, alongside acute in vivo toxicity assessments in a rodent model. The findings collectively indicate a complex pharmacological profile, where beneficial antioxidant constituents coexist with dose-dependent genotoxic signals at higher concentrations, though without overt systemic toxicity in vivo.
O. majorana is rich in bioactive polyphenolic compounds and flavonoids including rosmarinic acid, apigenin derivatives, luteolin, and chlorogenic acid which have well-documented antioxidant, anti-inflammatory, and organ-protective effects [
16,
17,
18]. Rosmarinic acid, in particular, promotes hepatoprotection through Nrf2 activation and suppression of oxidative stress pathways [
19]. Additionally, hesperidin and related flavonoids display beneficial lipid-modulating and anti-inflammatory properties, offering a mechanistic basis for the metabolic and hematological improvements as observed in this study [
20].
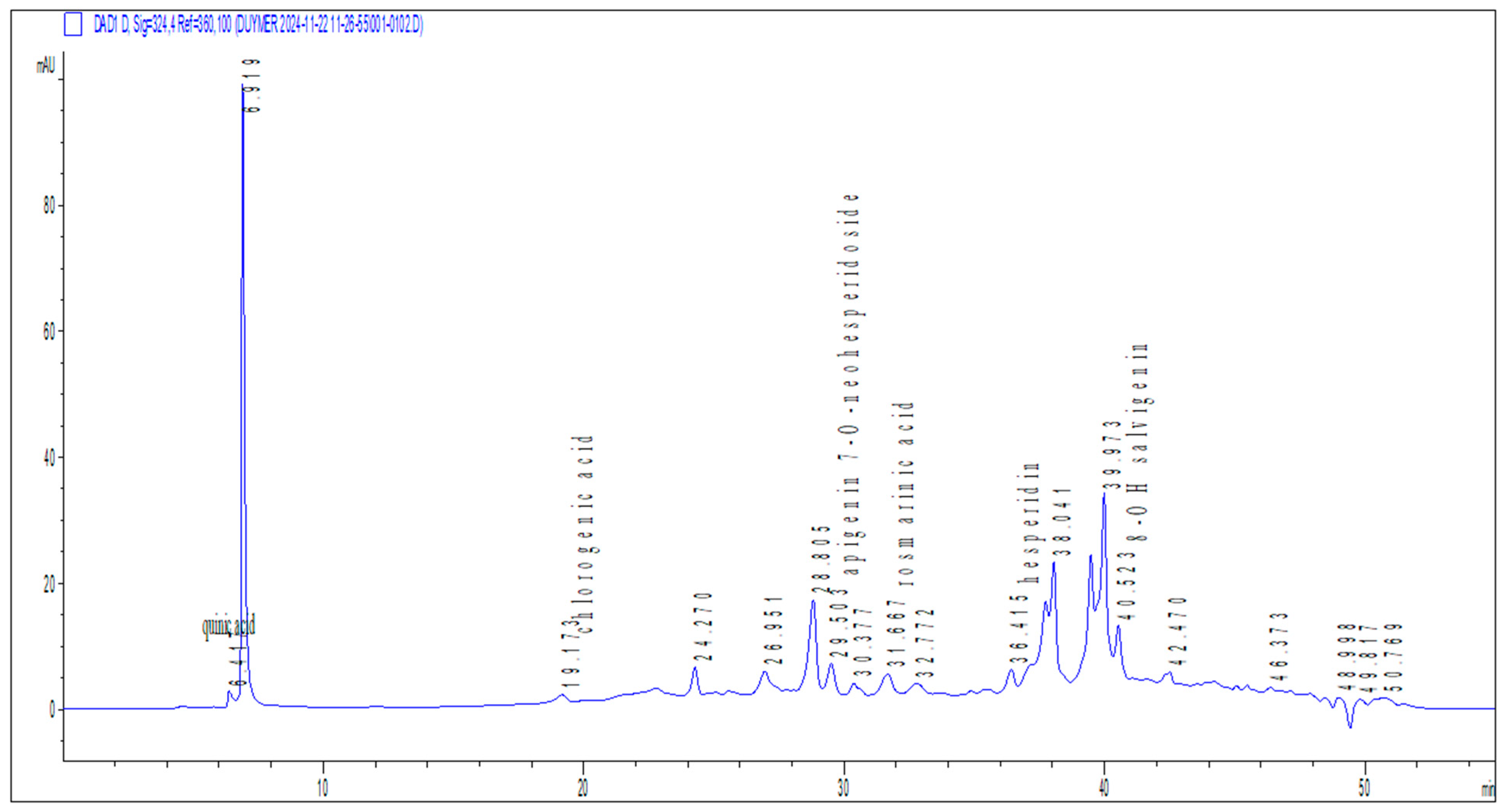
The phytochemical analysis revealed apigenin-7-O-neohesperidoside as the predominant compound in the
O. majorana extract. Previous studies have highlighted its anticancer and cytotoxic properties, suggesting potential therapeutic roles in targeting proliferative disorders [
21]. Interestingly, despite its reported cytotoxic effects against cancerous cells, our in vitro findings showed no significant cytotoxicity toward non-cancerous NIH3T3 fibroblasts, even at the highest concentration tested. This apparent selectivity for malignant cells over normal ones aligns with literature proposing apigenin-7-O-neohesperidoside as a low-toxicity candidate for anticancer drug development, warranting further investigation into its differential cellular mechanisms.
Similarly, rosmarinic acid, another major phenolic constituent identified in our extract, has been associated with hepatoprotective activity in experimental models [
22]. Consistent with this, we observed significant reductions in hepatic enzymes (ALT, AST) and the absence of overt liver toxicity in treated animals, even at the highest administered dose. These findings collectively suggest that
O. majorana’s phytochemical profile may confer protective effects on hepatic tissue, potentially mitigating oxidative or inflammatory insults while exhibiting genotoxic effects only at supratherapeutic concentrations.
In addition, CGA, also detected in methanol extract, may contribute to the metabolic and hepatic improvements observed in this study. Previous studies have shown that CGA significantly reduces ALT and AST levels and suppresses oxidative stress and inflammatory pathways in various hepatic injury models [
23], supporting our findings of lowered hepatic enzyme activities and preserved tissue morphology. Moreover, CGA has been reported to enhance lipid metabolism via inhibition of the FXR/FGF15 signaling pathway and upregulation of CYP7A1 expression, thereby promoting cholesterol catabolism and triglyceride clearance [
24]. These mechanistic insights align with the reduced serum triglycerides and stable glycemic profiles observed in the present study, further highlighting CGA as a key contributor to the extract’s beneficial metabolic effects. Consistently, Lieshchova and Brygadyrenko reported that dietary supplementation with
Origanum vulgare significantly reduced serum triglycerides, lowered the atherogenic index, increased HDL-cholesterol, and improved glycemic control in high-fat diet–induced obese rats [
25]. These findings complement our results by demonstrating that
Origanum species can beneficially modulate lipid and glucose metabolism in vivo. Taken together, the hepatoprotective and lipid-lowering properties observed in our study with
O. majorana are in strong agreement with the independent evidence provided for
O. vulgare, further supporting the therapeutic potential of
O. majorana extract in the management of metabolic disorders.
Importantly,
O. majorana specific studies corroborate our chemical and biological readouts. Targeted HPLC-DAD/ESI-MS profiling consistently identifies rosmarinic acid as predominant, with 5-caffeoylquinic (chlorogenic) acid and apigenin glycosides also present in ethanolic extract [
26]. Functionally,
O. majorana aqueous and organic fractions produce dose-dependent cytotoxicity in cancer cell lines (MDA-MB-231, HT-29) while showing no acute oral toxicity at 5–10 g/kg over 14 days in mice paralleling our observation that no acute oral toxicity over 14 days in rats and normal NIH3T3 fibroblasts retain viability across 10–200 µg/mL [
27]. Consistently, a hydroethanolic extract evaluated under OECD 423 showed no acute toxicity at 2 g/kg with normal clinical observations during 14-day follow-up, supporting a wide acute safety margin in vivo [
28].
With respect to genetic safety, the matrix and dose clearly matter. While our phenolic-rich methanolic extract yielded comet/MN signals at upper in vitro concentrations,
O. majorana essential oil (terpenoid-rich, compositionally distinct) was Ames-negative and V79 MN-negative at non-cytotoxic doses [
29]. Together with our acute rat data, these reports suggest that genotoxic alerts are assay- and concentration-dependent, and they motivate subacute/subchronic in vivo genotoxicity (e.g., bone-marrow MN, organ-specific comet) to determine translational risk at realistic exposure levels.
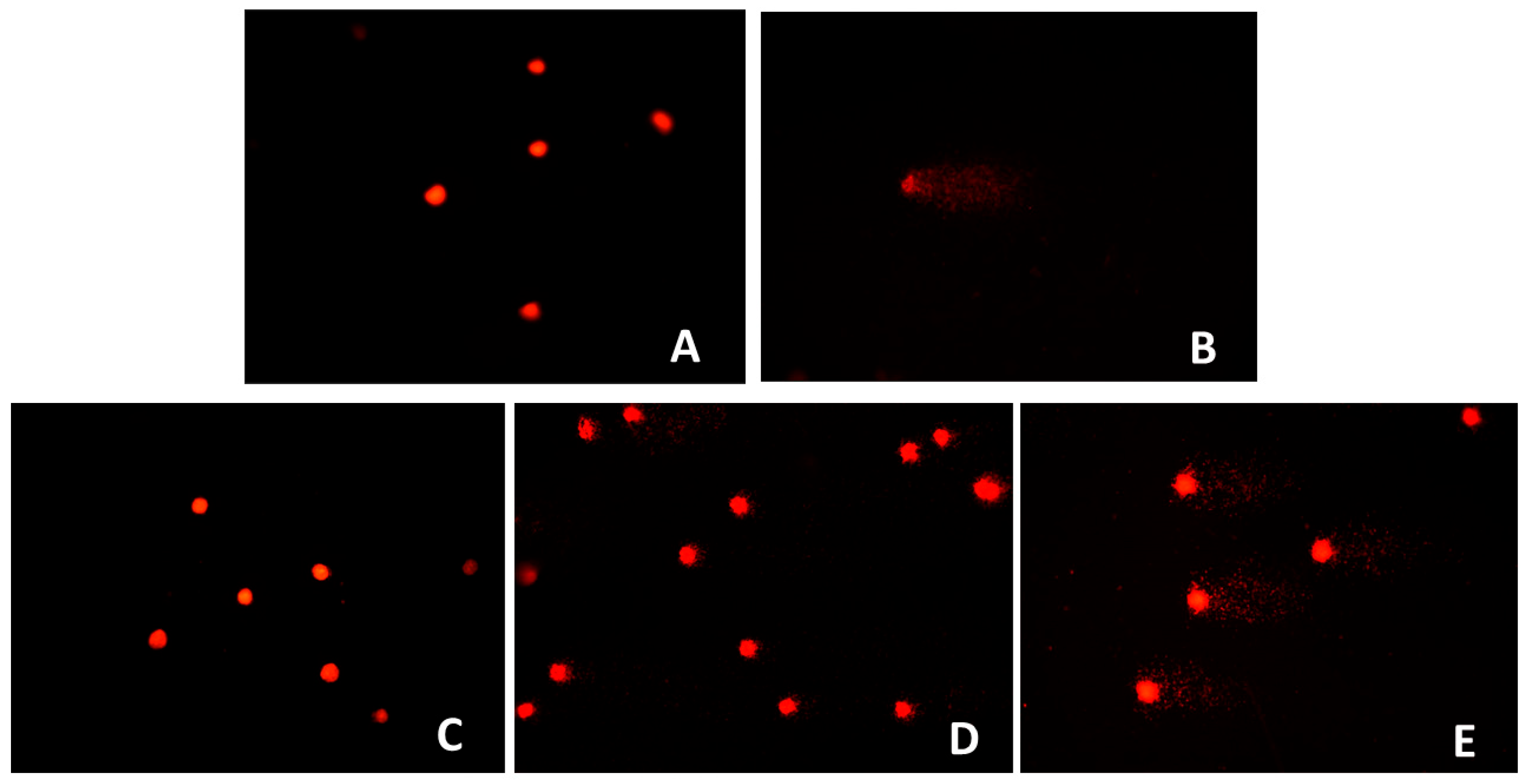
The cytotoxicity assay demonstrated that
O. majorana extracts maintained NIH3T3 fibroblast cell viability above 90% across all tested concentrations (10–200 µg/mL) after 24 h of exposure, confirming the absence of direct cytotoxic effects within this dose range. These findings are consistent with previous evidence that rosmarinic acid, a predominant phenolic compound in
O. majorana, protects mammalian cells against oxidative stress and apoptosis rather than inducing cytotoxicity at moderate concentrations [
19]. Similarly, hesperidin and related flavonoids have been shown to exert cytoprotective and anti-apoptotic effects in vitro, further supporting the biocompatibility observed in our study [
20,
30]. Although there is limited information on in vivo toxicity, several in vitro studies have reported that
O. majorana extracts and their essential oils exhibit dose-dependent cytotoxicity against various cancer cell lines, including breast, colon, and lung carcinoma, generally through induction of apoptosis and oxidative stress [
31,
32,
33]. More importantly, these effects were more pronounced in malignant cells than in normal cells, indicating some degree of selectivity and supporting the safety profile observed in our fibroblast model [
31,
34]. Collectively, these results highlight the safety of
O. majorana extracts under basal conditions and strengthen their potential for safe application in biomedical and nutraceutical formulations.
In contrast, both the comet and MN assays revealed a clear dose-dependent genotoxic effect of
O. majorana extract for 24 h at higher concentrations (100 and 200 µg/mL), with increased DNA strand breaks and elevated MN formation observed. These results suggest that while the extract may not be cytotoxic at these concentrations, it has the potential to compromise genomic integrity under conditions of high exposure. Evidence from a broad review of medicinal plants supports this observation, indicating that DNA damage is a common risk associated with certain herbal preparations especially within the Lamiaceae family when tested in genotoxicity assays [
35]. This consistency across test methodologies underlines the necessity of establishing strict dose limits and safety thresholds for the therapeutic use of
O. majorana extracts in nutraceuticals and clinical applications.
In vivo acute toxicity outcomes demonstrated compelling protective effects of
O. majorana on key hematological and biochemical parameters. Notably, at doses of 300 mg/kg and 2000 mg/kg, we observed elevations in neutrophil and monocyte counts, reductions in eosinophils, and modulated platelet indices. These hematological shifts align with prior evidence, as Ramadan et al. reported that oral administration of
O. majorana leaf extracts significantly attenuated granulocytosis and thrombocytosis in isoproterenol-induced cardiac injury models indicating genuine immunomodulatory potential rather than non-specific toxicity [
36]. Biochemically, our findings revealed substantial reductions in serum ALT, AST, and LDH levels, suggesting hepatoprotective and cardioprotective roles for the extract. Ramadan et al. similarly documented decreases in troponin, LDH, and aminotransferases in treated rats, which they attributed to the antioxidant effects of
O. majorana phytochemicals [
36]. The overlapping patterns between these works reinforce that the observed hematologic normalization and enzyme modulation are likely linked to
O. majorana’s rich antioxidant profile.
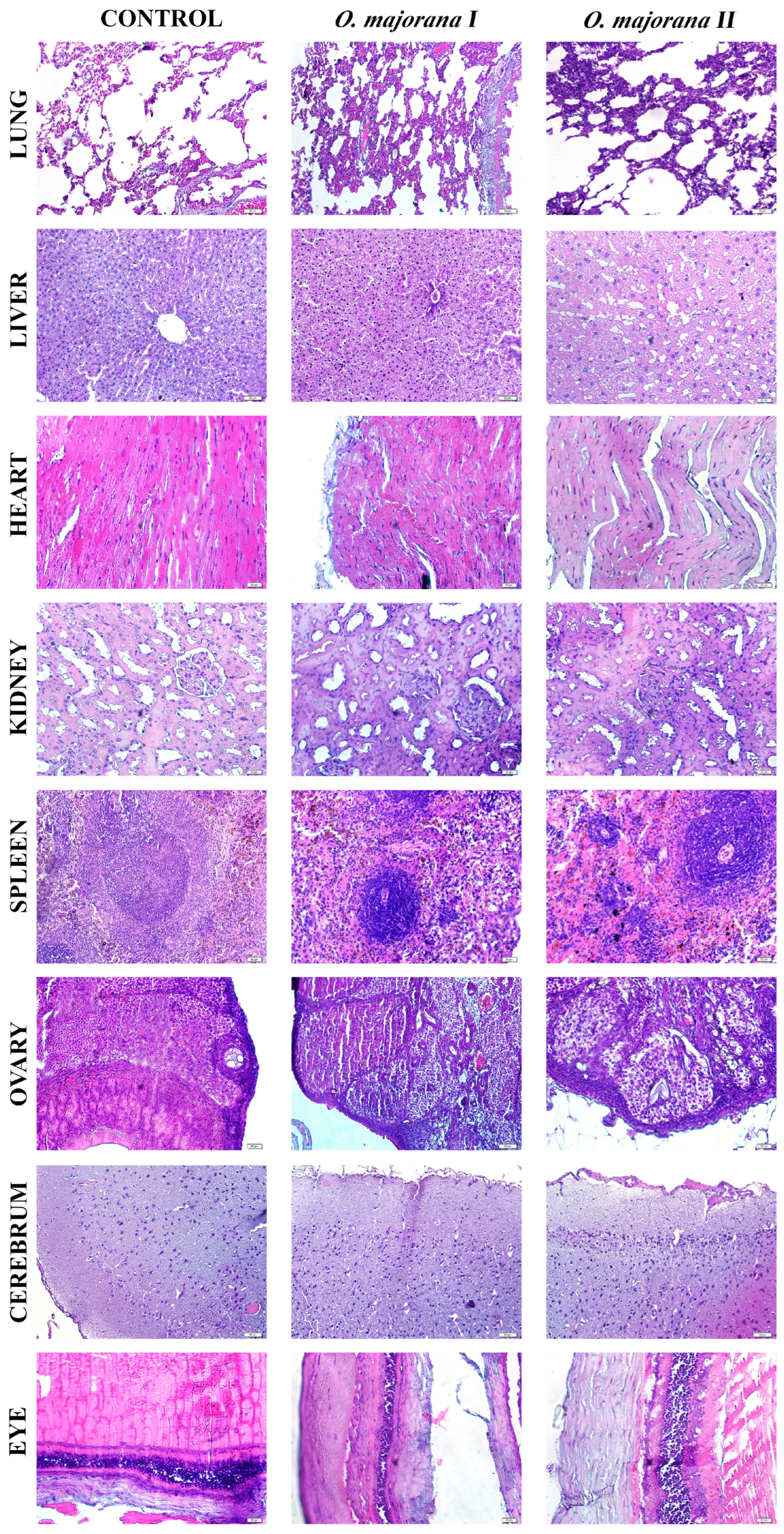
Histopathological examination revealed that acute administration of
O. majorana extract caused mild to moderate morphological alterations in certain organs, despite the absence of gross toxicity as evidenced by unchanged relative organ weights (
p > 0.05). Specifically, hepatic tissues exhibited sinusoidal congestion, hepatocellular vacuolization, and focal inflammatory infiltrates, while renal sections showed tubular dilation and cytoplasmic eosinophilia with preserved glomerular architecture. These findings indicate that although biochemical markers such as ALT and AST suggested hepatoprotection, microscopic changes point to subtle hepatic stress, consistent with reports in related
Origanum species [
37].
Renal histological changes corresponded with normal renal biochemical markers (urea, creatinine, BUN), suggesting adaptive responses rather than overt nephrotoxicity. Moreover, previous studies demonstrated that
Origanum species exert significant nephroprotective and antioxidant effects against chemical-induced renal injury, further corroborating our findings [
38]. Splenic white pulp hyperplasia and follicular disorganization aligned with hematological findings of increased neutrophils and monocytes and reduced eosinophils, supporting an immunomodulatory role of flavonoids such as apigenin and hesperidin. Cardiac muscle showed mild myofiber disorganization and sarcoplasmic rarefaction, which, when considered alongside decreased LDH levels, may reflect cardioprotective effects of rosmarinic acid and related polyphenols. Other tissues, including ovary, cerebrum, and eye, exhibited dose-dependent mild changes such as stromal edema, neuronal shrinkage, and epithelial thinning, but without significant biochemical disturbances, indicating transient adaptive responses rather than irreversible damage. Overall, histopathological observations complement hematological and biochemical results, suggesting that
O. majorana extract exerts functional protection at the systemic level while inducing limited microscopic stress responses in certain organs.
Consistently with stable organ weights and improved biochemistry (ALT/AST, LDH), the mild, focal histological changes are most compatible with subclinical/adaptive responses after acute exposure. To consolidate structure–function alignment, we will provide blinded semi-quantitative scoring with inter-rater documentation and correlate individual scores with biochemical measures. A brief subacute follow-up (both sexes), including pathway markers (hepatic Nrf2/HO-1, FXR–FGF15/CYP7A1; renal KIM-1/NGAL), will clarify persistence or reversibility and further support the protective profile.
Taken together, these data depict a dualistic profile: while O. majorana methanol extract are safe and protective in terms of organ function, oxidative balance, and hematological modulation at low to moderate exposures, they exhibit genotoxic potential in vitro at higher concentrations. This dichotomy underscores the importance of dose in determining the balance between therapeutic and adverse effects. Importantly, the traditional dietary use of O. majorana suggests that low-level exposures are safe, but concentrated extracts for clinical or nutraceutical use require rigorous safety evaluation. Future work should integrate long-term in vivo genotoxicity assays and mechanistic studies on DNA repair pathways to define evidence-based safety thresholds.