Growth Plate Skeletal Stem Cells and Their Actions Within the Stem Cell Niche
Abstract
1. Introduction
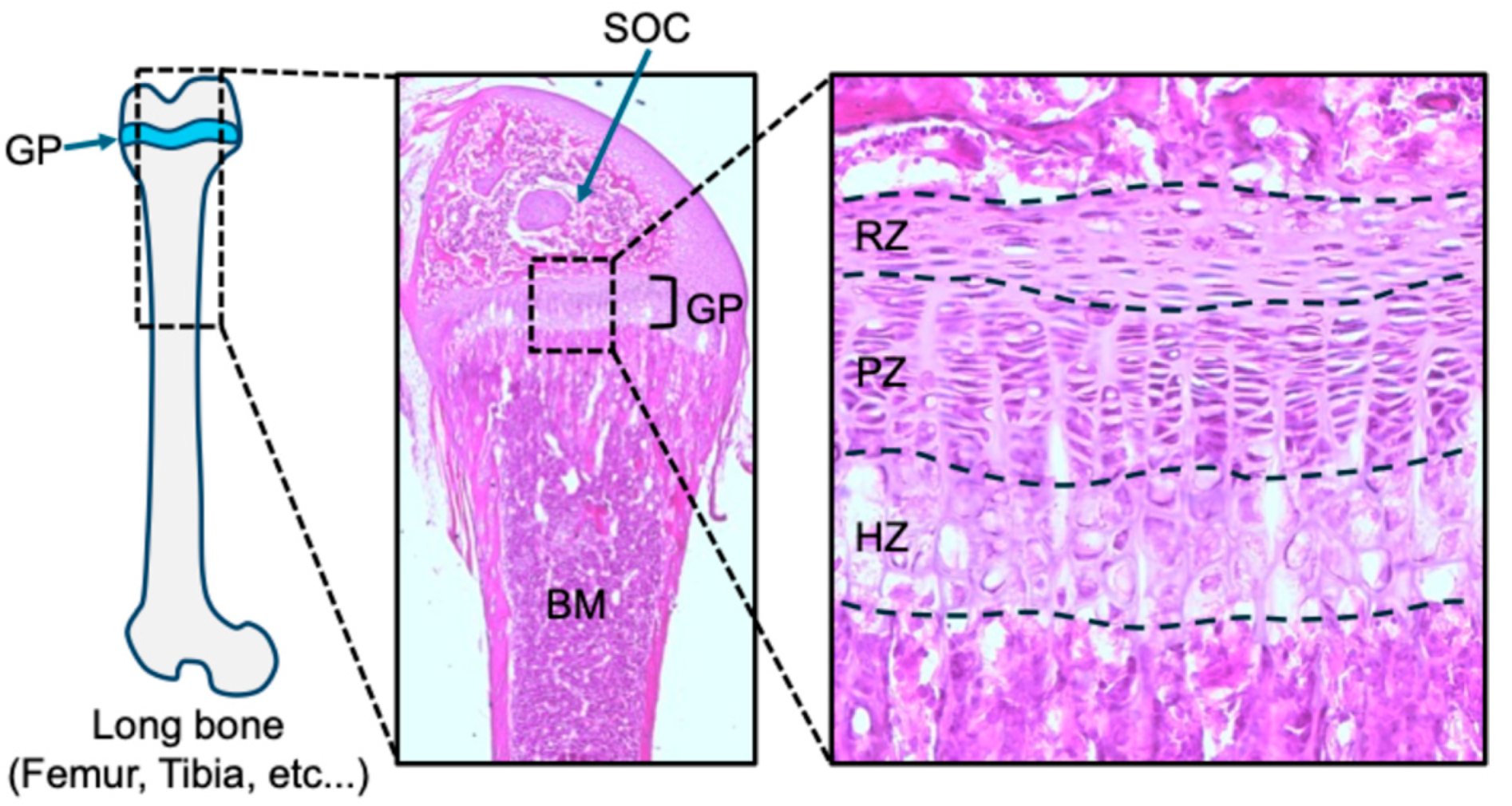
2. Growth Plate Structure and Function
2.1. Characteristics of the Three Zones in Postnatal Growth Plate
2.2. Signaling Pathways Regulating the Growth Plate Activity
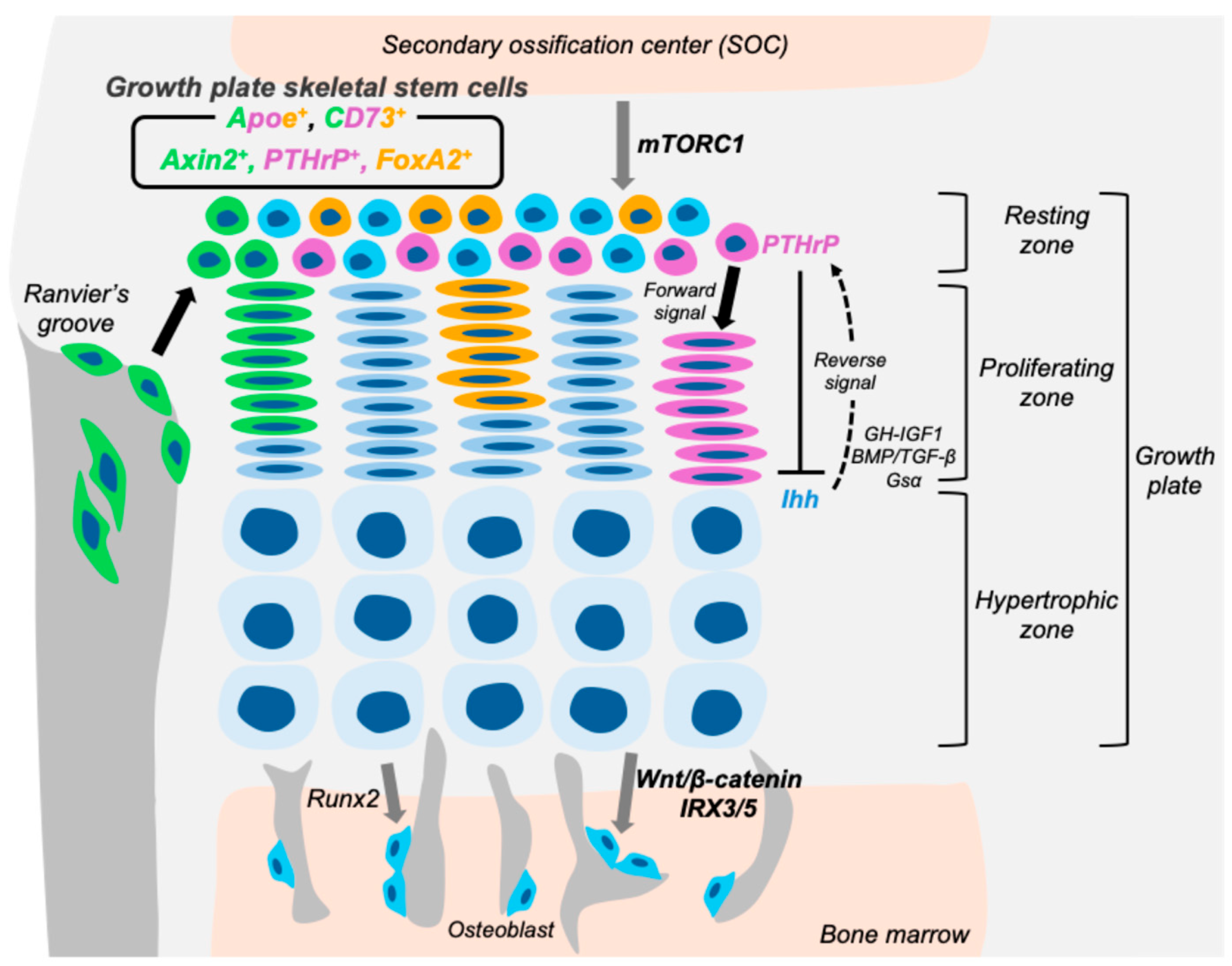
3. Various Growth Plate Skeletal Stem Cells
3.1. PTHrP-Positive Skeletal Stem Cells in the Growth Plate
3.2. CD73-Positive Skeletal Stem Cells in the Growth Plate
3.3. Axin2-Positive Skeletal Stem Cells in the Growth Plate
3.4. FoxA2-Positive Skeletal Stem Cells in the Growth Plate
3.5. ApoE-Positive Skeletal Stem Cells in the Growth Plate
4. Key Signaling Pathway in Growth Plate Skeletal Stem Cells
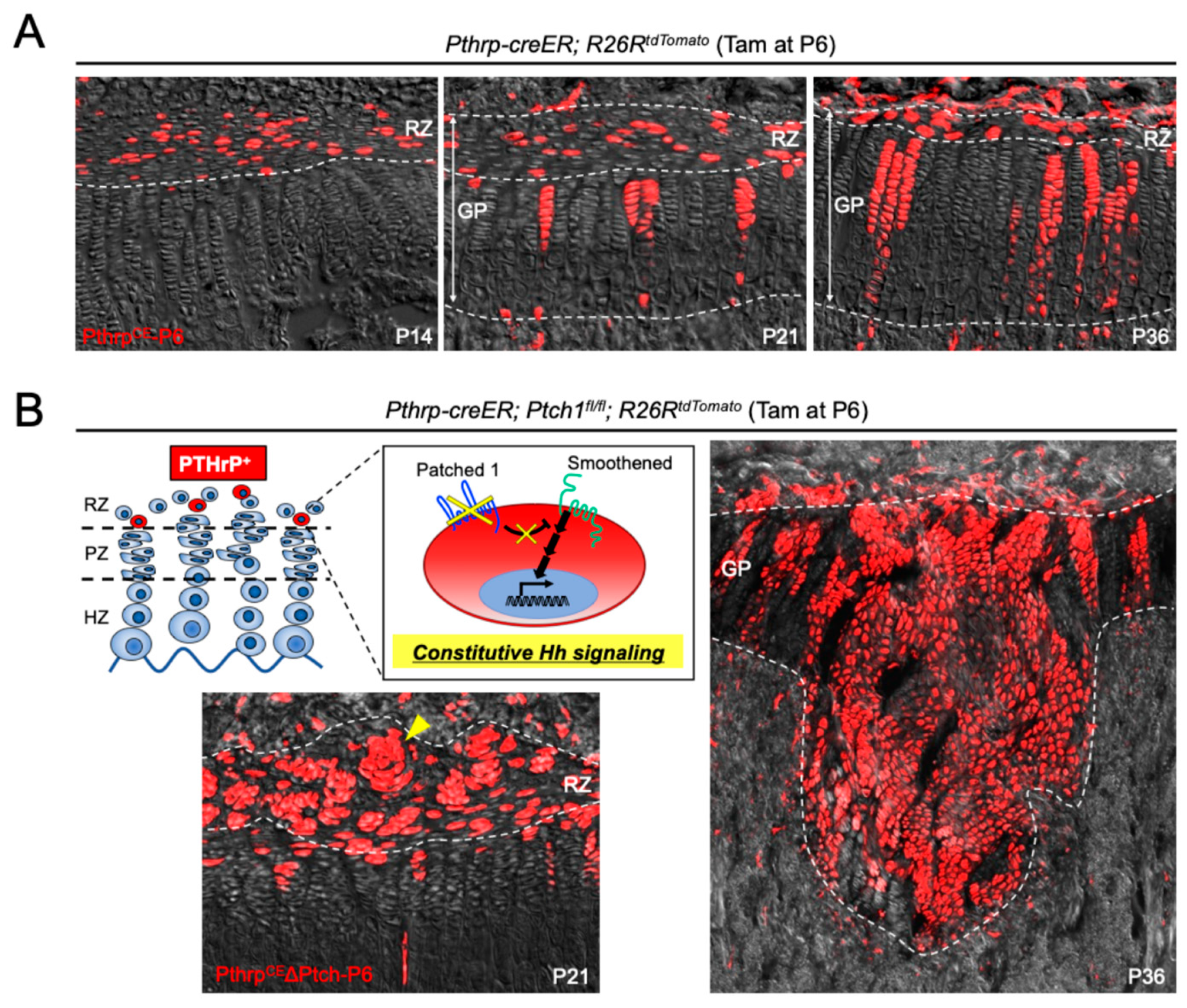
4.1. Stage-Specific Roles of Hedgehog Signaling in the Regulation of Growth Plate Skeletal Stem Cells
4.2. mTORC1 as a Modulator of Skeletal Stem Cell Division and Resting Zone Structure in the Growth Plate
4.3. Wnt-IRX Axis in the Regulation of Growth Plate Skeletal Stem Cell Fate
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Weissman, I.L. Stem Cells: Units of Development, Units of Regeneration, and Units in Evolution. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The Intestinal Crypt, A Prototype Stem Cell Compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef]
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An Evolving Paradigm for Stem Cell Biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef]
- Mizuhashi, K.; Ono, W.; Matsushita, Y.; Sakagami, N.; Takahashi, A.; Saunders, T.L.; Nagasawa, T.; Kronenberg, H.M.; Ono, N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 2018, 563, 254–258. [Google Scholar] [CrossRef]
- Newton, P.T.; Li, L.; Zhou, B.; Schweingruber, C.; Hovorakova, M.; Xie, M.; Sun, X.; Sandhow, L.; Artemov, A.V.; Ivashkin, E.; et al. A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature 2019, 567, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G. Skeletal stem cells. Development 2015, 142, 1023–1027. [Google Scholar] [CrossRef]
- Lui, J.C. Home for a rest: Stem cell niche of the postnatal growth plate. J. Endocrinol. 2020, 246, R1–R11. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Gunawardena, A.T.; Francois, N.B.; Otsuru, S.; Takano, H.; Hirose, K.; Matsuoka, M.; Suzuki, A.; Huang, J.; Qin, L.; et al. Possible Contribution of Wnt-Responsive Chondroprogenitors to the Postnatal Murine Growth Plate. J. Bone Miner. Res. 2019, 34, 964–974. [Google Scholar] [CrossRef]
- Muruganandan, S.; Pierce, R.; Teguh, D.A.; Perez, R.F.; Bell, N.; Nguyen, B.; Hohl, K.; Snyder, B.D.; Grinstaff, M.W.; Alberico, H.; et al. A FoxA2+ long-term stem cell population is necessary for growth plate cartilage regeneration after injury. Nat. Commun. 2022, 13, 2515. [Google Scholar] [CrossRef]
- Kodama, J.; Oichi, T.; Wilkinson, K.J.; Abzug, J.M.; Kaito, T.; Enomoto-Iwamoto, M.; Iwamoto, M.; Otsuru, S. Apolipoprotein E is a marker of all chondrocytes in the growth plate resting zone. Bone Res. 2025, 13, 31. [Google Scholar] [CrossRef]
- Hallett, S.A.; Ono, W.; Matsushita, Y.; Sakagami, N.; Mizuhashi, K.; Tokavanich, N.; Nagata, M.; Zhou, A.; Hirai, T.; Kronenberg, H.; et al. Chondrocytes in the resting zone of the growth plate are maintained in a Wnt-Inhibitory environment. eLife 2020, 10, e64513. [Google Scholar] [CrossRef]
- Orikasa, S.; Matsushita, Y.; Manabe, H.; Fogge, M.; Lee, Z.; Mizuhashi, K.; Sakagami, N.; Ono, W.; Ono, N. Hedgehog activation promotes osteogenic fates of growth plate resting zone chondrocytes through transient clonal competency. JCI Insight 2024, 9, e165619. [Google Scholar] [CrossRef]
- Trompet, D.; Kurenkova, A.D.; Zhou, B.; Li, L.; Dregval, O.; Usanova, A.P.; Chu, T.L.; Are, A.; Nedorubov, A.A.; Kasper, M.; et al. Stimulation of skeletal stem cells in the growth plate promotes linear bone growth. JCI Insight 2024, 9, e165226. [Google Scholar] [CrossRef]
- Trompet, D.; Melis, S.; Chagin, A.S.; Maes, C. Skeletal stem and progenitor cells in bone development and repair. J. Bone Miner. Res. 2024, 39, 633–654. [Google Scholar] [CrossRef]
- Melis, S.; Trompet, D.; Chagin, A.S.; Maes, C. Skeletal stem and progenitor cells in bone physiology, ageing and disease. Nat. Rev. Endocrinol. 2025, 21, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Karaplis, A.C.; Luz, A.; Glowacki, J.; Bronson, R.T.; Tybulewicz, V.L.; Kronenberg, H.M.; Mulligan, R.C. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994, 8, 277–289. [Google Scholar] [CrossRef]
- Lanske, B.; Karaplis, A.C.; Lee, K.; Luz, A.; Vortkamp, A.; Pirro, A.; Karperien, M.; Defize, L.H.; Ho, C.; Mulligan, R.C.; et al. PTH/PTHrP Receptor in Early Development and Indian Hedgehog-Regulated Bone Growth. Science 1996, 273, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Vortkamp, A.; Lee, K.; Lanske, B.; Segre, G.V.; Kronenberg, H.M.; Tabin, C.J. Regulation of Rate of Cartilage Differentiation by Indian Hedgehog and PTH-Related Protein. Science 1996, 273, 613–622. [Google Scholar] [CrossRef]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.C.; Philbrick, W.M.; Amling, M.; Neff, L.A.; Baron, R.; Broadus, A.E. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc. Natl. Acad. Sci. USA 1996, 93, 10240–10245. [Google Scholar] [CrossRef]
- Xie, M.; Gol’din, P.; Herdina, A.N.; Estefa, J.; Medvedeva, E.V.; Li, L.; Newton, P.T.; Kotova, S.; Shavkuta, B.; Saxena, A.; et al. Secondary ossification center induces and protects growth plate structure. eLife 2020, 9, e55212. [Google Scholar] [CrossRef]
- Emons, J.; Chagin, A.S.; Hultenby, K.; Zhivotovsky, B.; Wit, J.M.; Karperien, M.; Sävendahl, L. Epiphyseal Fusion in the Human Growth Plate Does Not Involve Classical Apoptosis. Pediatr. Res. 2009, 66, 654–659. [Google Scholar] [CrossRef]
- Abad, V.; Meyers, J.L.; Weise, M.; Gafni, R.I.; Barnes, K.M.; Nilsson, O.; Bacher, J.D.; Baron, J. The Role of the Resting Zone in Growth Plate Chondrogenesis. Endocrinology 2002, 143, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.L.; Oh, S.; Sung, Y.; Dasari, R.R.; Kirschner, M.W.; Tabin, C.J. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 2013, 495, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S.E. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 12097–12102. [Google Scholar] [CrossRef]
- Zhou, X.; von der Mark, K.; Henry, S.; Norton, W.; Adams, H.; de Crombrugghe, B. Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice. PLoS Genet. 2014, 10, e1004820. [Google Scholar] [CrossRef]
- Mak, K.K.; Kronenberg, H.M.; Chuang, P.T.; Mackem, S.; Yang, Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 2008, 135, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Nakamura, E.; Nguyen, M.T.; Suva, L.J.; Swain, F.L.; Razzaque, M.S.; Mackem, S.; Lanske, B. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc. Natl. Acad. Sci. USA 2007, 104, 6382–6387. [Google Scholar] [CrossRef]
- Su, N.; Jin, M.; Chen, L. Role of FGF/FGFR signaling in skeletal development and homeostasis: Learning from mouse models. Bone Res. 2014, 2, 14003. [Google Scholar] [CrossRef]
- Naski, M.C.; Colvin, J.S.; Coffin, J.D.; Ornitz, D.M. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development 1998, 125, 4977–4988. [Google Scholar] [CrossRef]
- Peters, K.; Ornitz, D.; Werner, S.; Williams, L. Unique Expression Pattern of the FGF Receptor 3 Gene during Mouse Organogenesis. Dev. Biol. 1993, 155, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wynshaw-Boris, A.; Zhou, F.; Kuo, A.; Leder, P. Fibroblast Growth Factor Receptor 3 is a Negative Regulator of Bone Growth. Cell 1996, 84, 911–921. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, Y.; Tang, J.; Huang, J.; Huang, Q.; Xu, W.; Wang, Z.; Luo, F.; Wang, Q.; Chen, H.; et al. FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling. PLoS Genet. 2015, 11, e1005214. [Google Scholar] [CrossRef]
- Ballesteros, M.; Leung, K.C.; Ross, R.J.; Iismaa, T.P.; Ho, K.K. Distribution and Abundance of Messenger Ribonucleic Acid for Growth Hormone Receptor Isoforms in Human Tissues. J. Clin. Endocrinol. Metab. 2000, 85, 2865–2871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, Z.; Elalieh, H.Z.; Nakamura, E.; Nguyen, M.T.; Mackem, S.; Clemens, T.L.; Bikle, D.D.; Chang, W. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J. Bone Miner. Res. 2011, 26, 1437–1446. [Google Scholar] [CrossRef]
- Long, F.; Joeng, K.S.; Xuan, S.; Efstratiadis, A.; McMahon, A.P. Independent regulation of skeletal growth by Ihh and IGF signaling. Dev. Biol. 2006, 298, 327–333. [Google Scholar] [CrossRef]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hui, C.C. Hedgehog Signaling in Development and Cancer. Dev. Cell 2008, 15, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Chen, M.; Kobayashi, T.; Kronenberg, H.M.; Weinstein, L.S. Chondrocyte-Specific Knockout of the G Protein Gsα Leads to Epiphyseal and Growth Plate Abnormalities and Ectopic Chondrocyte Formation. J. Bone Miner. Res. 2005, 20, 663–671. [Google Scholar] [CrossRef]
- Regard, J.B.; Malhotra, D.; Gvozdenovic-Jeremic, J.; Josey, M.; Chen, M.; Weinstein, L.S.; Lu, J.; Shore, E.M.; Kaplan, F.S.; Yang, Y. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat. Med. 2013, 19, 1505–1512. [Google Scholar] [CrossRef]
- Bastepe, M.; Weinstein, L.S.; Ogata, N.; Kawaguchi, H.; Jüppner, H.; Kronenberg, H.M.; Chung, U.I. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 14794–14799. [Google Scholar] [CrossRef]
- Guo, X.; Mak, K.K.; Taketo, M.M.; Yang, Y. The Wnt/β-catenin Pathway Interacts Differentially with PTHrP Signaling to Control Chondrocyte Hypertrophy and Final Maturation. PLoS ONE 2009, 4, e6067. [Google Scholar] [CrossRef]
- Hu, H.; Hilton, M.J.; Tu, X.; Yu, K.; Ornitz, D.M.; Long, F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 2005, 132, 49–60. [Google Scholar] [CrossRef]
- Akiyama, H.; Lyons, J.P.; Mori-Akiyama, Y.; Yang, X.; Zhang, R.; Zhang, Z.; Deng, J.M.; Taketo, M.M.; Nakamura, T.; Behringer, R.R.; et al. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004, 18, 1072–1087. [Google Scholar] [CrossRef]
- Später, D.; Hill, T.P.; O’sullivan, R.J.; Gruber, M.; Conner, D.A.; Hartmann, C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 2006, 133, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a Candidate Gene for Cleidocranial Dysplasia Syndrome, Is Essential for Osteoblast Differentiation and Bone Development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Ueta, C.; Iwamoto, M.; Kanatani, N.; Yoshida, C.; Liu, Y.; Enomoto-Iwamoto, M.; Ohmori, T.; Enomoto, H.; Nakata, K.; Takada, K.; et al. Skeletal Malformations Caused by Overexpression of Cbfa1 or Its Dominant Negative Form in Chondrocytes. J. Cell Biol. 2001, 153, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Yasui, T.; Nomura, S.; Miyake, S.; Deguchi, K.; Himeno, M.; Sato, M.; Yamagiwa, H.; Kimura, T.; Yasui, N.; et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 1999, 214, 279–290. [Google Scholar] [CrossRef]
- Yoshida, C.A.; Yamamoto, H.; Fujita, T.; Furuichi, T.; Ito, K.; Inoue, K.; Yamana, K.; Zanma, A.; Takada, K.; Ito, Y.; et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes. Dev. 2004, 18, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 6, 32–52. [Google Scholar] [CrossRef]
- Kang, Q.; Sun, M.H.; Cheng, H.; Peng, Y.; Montag, A.G.; Deyrup, A.T.; Jiang, W.; Luu, H.H.; Luo, J.; Szatkowski, J.P.; et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004, 11, 1312–1320. [Google Scholar] [CrossRef]
- Storm, E.E.; Huynh, T.V.; Copeland, N.G.; Jenkins, N.A.; Kingsley, D.M.; Lee, S.J. Limb alterations in brachypodism mice due to mutations in a new member of the TGFβ-superfamily. Nature 1994, 368, 639–643. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, M.; Xie, R.; Wang, M.; Jin, H.; Hou, W.; Tang, D.; Harris, S.E.; Mishina, Y.; O’Keefe, R.J.; et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 2011, 124, 3428–3440. [Google Scholar] [CrossRef]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef]
- Estrada, K.D.; Retting, K.N.; Chin, A.M.; Lyons, K.M. Smad6 is essential to limit BMP signaling during cartilage development. J. Bone Miner. Res. 2011, 26, 2498–2510. [Google Scholar] [CrossRef] [PubMed]
- Hojo, H.; Ohba, S.; Taniguchi, K.; Shirai, M.; Yano, F.; Saito, T.; Ikeda, T.; Nakajima, K.; Komiyama, Y.; Nakagata, N.; et al. Hedgehog-Gli Activators Direct Osteo-chondrogenic Function of Bone Morphogenetic Protein toward Osteogenesis in the Perichondrium. J. Biol. Chem. 2013, 288, 9924–9932. [Google Scholar] [CrossRef]
- Minina, E.; Wenzel, H.M.; Kreschel, C.; Karp, S.; Gaffield, W.; McMahon, A.P.; Vortkamp, A. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 2001, 128, 4523–4534. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Koike, C.; Saitoh, K.; Kuroiwa, A.; Iba, H. Developmental patterning in chondrocytic cultures by morphogenic gradients: BMP induces expression of Indian hedgehog and Noggin. Genes Cells 1999, 4, 175–184. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Newton, P.T.; Xie, M.; Medvedeva, E.V.; Sävendahl, L.; Chagin, A.S. Activation of mTORC1 in chondrocytes does not affect proliferation or differentiation, but causes the resting zone of the growth plate to become disordered. Bone Rep. 2018, 8, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Kong, M.; Wen, S.; Tsang, K.Y.; Niu, B.; Hartmann, C.; Chan, D.; Hui, C.-C.; Cheah, K.S.E. IRX3 and IRX5 Inhibit Adipogenic Differentiation of Hypertrophic Chondrocytes and Promote Osteogenesis. J. Bone Miner. Res. 2020, 35, 2444–2457. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, N.K.-a.; Orikasa, S.; Ono, N. Growth Plate Skeletal Stem Cells and Their Actions Within the Stem Cell Niche. Int. J. Mol. Sci. 2025, 26, 9460. https://doi.org/10.3390/ijms26199460
Cheng NK-a, Orikasa S, Ono N. Growth Plate Skeletal Stem Cells and Their Actions Within the Stem Cell Niche. International Journal of Molecular Sciences. 2025; 26(19):9460. https://doi.org/10.3390/ijms26199460
Chicago/Turabian StyleCheng, Natalie Kiat-amnuay, Shion Orikasa, and Noriaki Ono. 2025. "Growth Plate Skeletal Stem Cells and Their Actions Within the Stem Cell Niche" International Journal of Molecular Sciences 26, no. 19: 9460. https://doi.org/10.3390/ijms26199460
APA StyleCheng, N. K.-a., Orikasa, S., & Ono, N. (2025). Growth Plate Skeletal Stem Cells and Their Actions Within the Stem Cell Niche. International Journal of Molecular Sciences, 26(19), 9460. https://doi.org/10.3390/ijms26199460





