Sympathetic Biomarker Dynamics Post-Myocardial Infarction: TH, PGP9.5, and SYN Expression Discordance in Murine Hearts
Abstract
1. Introduction
2. Results
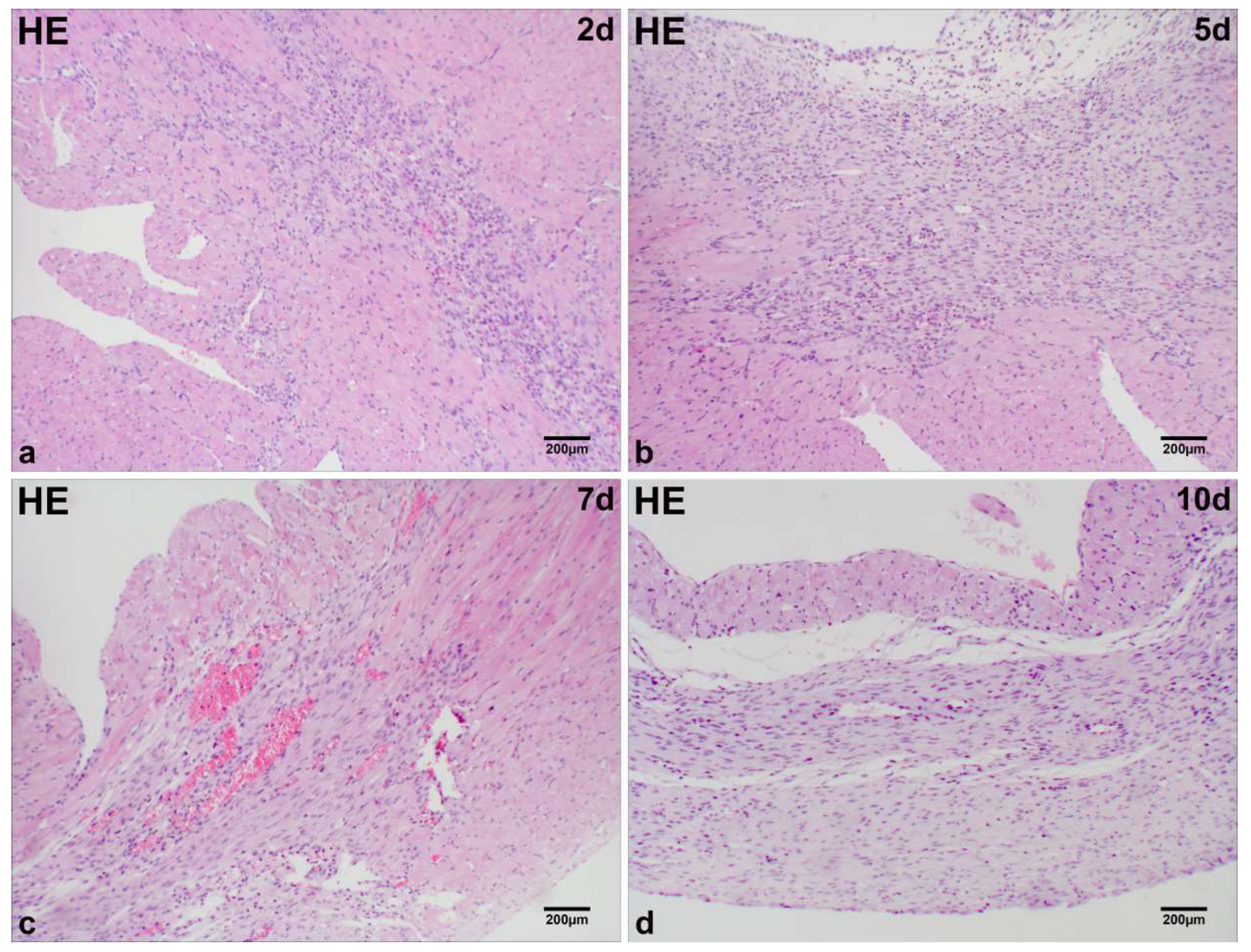
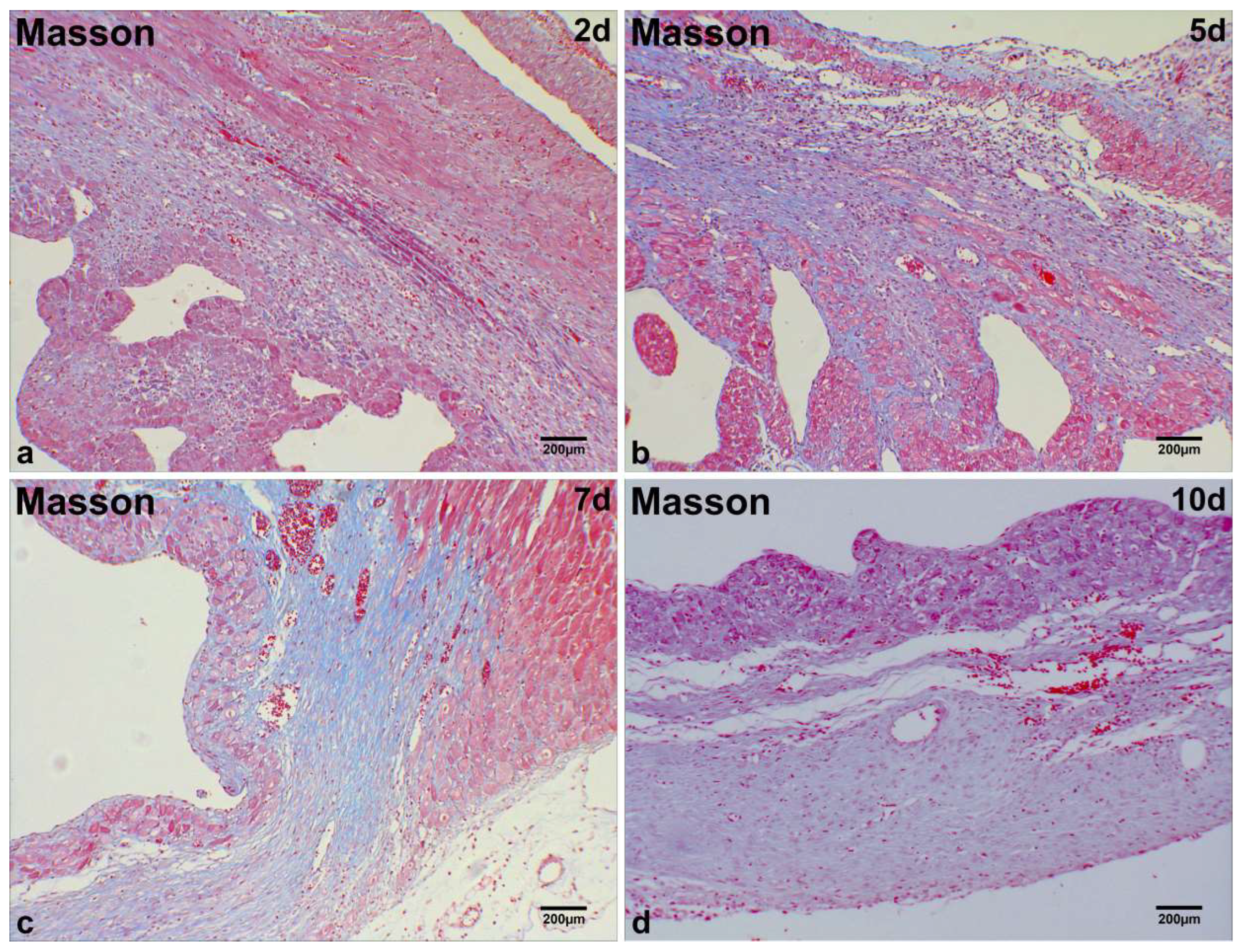
2.1. Microscopic Analysis of MI Tissue in Mice
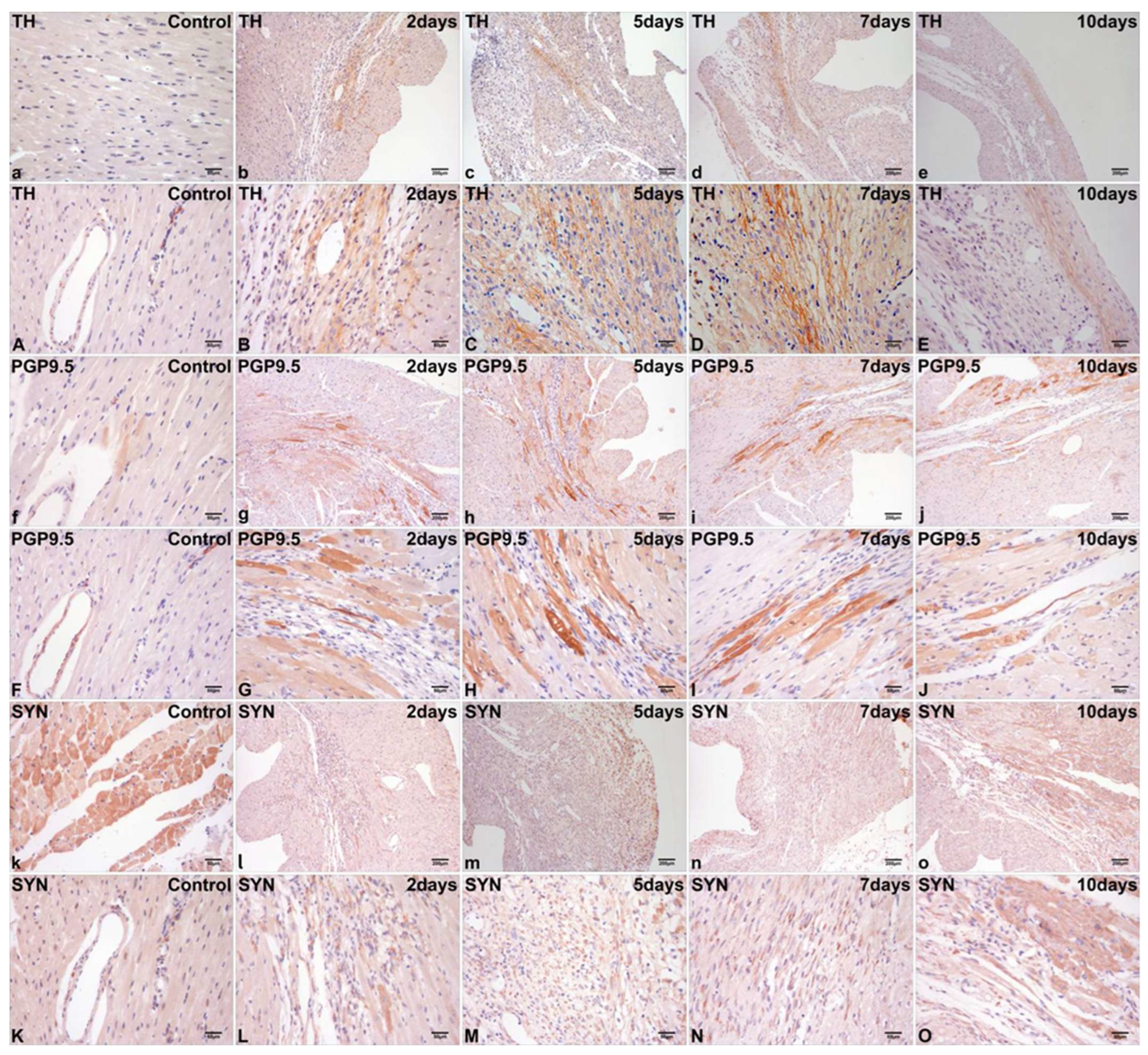
2.2. Immunohistochemical Localization of TH, PGP9.5, and SYN in Normal Cardiac Tissue
2.3. Differential Immunohistochemical Expression of TH, PGP9.5, and SYN in Post-Infarct Hearts
2.4. Dynamic Alterations in Myocardial TH, PGP9.5, and SYN mRNA Levels in Post-MI Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Procedures
4.2. MI Model
4.3. Histological Examination
4.4. Immunohistochemical Staining
4.5. qRT-PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Fukuda, K.; Kanazawa, H.; Aizawa, Y.; Ardell, J.L.; Shivkumar, K. Cardiac Innervation and Sudden Cardiac Death. Circ. Res. 2015, 116, 2005–2019. [Google Scholar] [CrossRef]
- Blake, M.R.; Gardner, R.T.; Jin, H.; Staffenson, M.A.; Rueb, N.J.; Barrios, A.M.; Dudley, G.B.; Cohen, M.S.; Habecker, B.A. Small Molecules Targeting PTPsigma-Trk Interactions Promote Sympathetic Nerve Regeneration. ACS Chem. Neurosci. 2022, 13, 688–699. [Google Scholar] [CrossRef]
- Billman, G.E. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: Effect of endurance exercise training. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1171–H1193. [Google Scholar] [CrossRef]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020, 12, 373–408. [Google Scholar] [CrossRef]
- Huang, Z.H.; Ni, R.J.; Luo, P.H.; Zhou, J.N. Distribution of tyrosine-hydroxylase-immunoreactive neurons in the hypothalamus of tree shrews. J. Comp. Neurol. 2020, 52, 935–952. [Google Scholar] [CrossRef]
- Torres, H.; Huesing, C.; Burk, D.H.; Molinas, A.J.R.; Neuhuber, W.L.; Berthoud, H.R.; Münzberg, H.; Derbenev, A.V.; Zsombok, A. Sympathetic innervation of the mouse kidney and liver arising from prevertebral ganglia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R328–R337. [Google Scholar] [CrossRef]
- Krivova, Y.S.; Proshchina, A.E.; Otlyga, D.A.; Leonova, O.G.; Saveliev, S.V. Prenatal development of sympathetic innervation of the human pancreas. Ann. Anat. 2022, 240, 151880. [Google Scholar] [CrossRef]
- Aschrafi, A.; Berndt, A.; Kowalak, J.A.; Gale, J.R.; Gioio, A.E.; Kaplan, B.B. Angiotensin II mediates the axonal trafficking of tyrosine hydroxylase and dopamine β-hydroxylase mRNAs and enhances norepinephrine synthesis in primary sympathetic neurons. J. Neurochem. 2019, 150, 666–677. [Google Scholar] [CrossRef]
- Konosu-Fukaya, S.; Omata, K.; Tezuka, Y.; Ono, Y.; Aoyama, Y.; Satoh, F.; Fujishima, F.; Sasano, H.; Nakamura, Y. Catecholamine-Synthesizing Enzymes in Pheochromocytoma and Extraadrenal Paraganglioma. Endocr. Pathol. 2018, 29, 302–309. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Ichinose, H.; Kobayashi, K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural. Transm. 2019, 126, 397–409. [Google Scholar] [CrossRef]
- Amalia, S.N.; Baral, H.; Fujiwara, C.; Uchiyama, A.; Inoue, Y.; Yamazaki, S.; Ishikawa, M.; Kosaka, K.; Sekiguchi, A.; Yokoyama, Y.; et al. TRPV4 Regulates the Development of Psoriasis by Controlling Adenosine Triphosphate Expression in Keratinocytes and the Neuroimmune System. J. Investig. Dermatol. 2023, 143, 2356–2365. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Hoang, L.; Yosef, A.; Alotaibi, F.; Allaire, C.; Brotto, L.; Fraser, I.S.; Bedaiwy, M.A.; Ng, T.L.; Lee, A.F.; et al. Nerve Bundles and Deep Dyspareunia in Endometriosis. Reprod. Sci. 2016, 23, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Kolos, Y.A.; Grigoriyev, I.P.; Korzhevskyi, D.E. A synaptic marker synaptophysin. Morfologiia 2015, 147, 78–82. [Google Scholar]
- Kokotos, A.C.; Harper, C.B.; Marland, J.R.K.; Smillie, K.J.; Cousin, M.A.; Gordon, S.L. Synaptophysin sustains presynaptic performance by preserving vesicular synaptobrevin-II levels. J. Neurochem. 2019, 151, 28–37. [Google Scholar] [CrossRef]
- Chang, C.W.; Hsiao, Y.T.; Jackson, M.B. Synaptophysin Regulates Fusion Pores and Exocytosis Mode in Chromaffin Cells. J. Neurosci. 2021, 41, 3563–3578. [Google Scholar] [CrossRef]
- Yu, T.S.; Wang, X.; Zhang, H.D.; Bai, R.F.; Zhao, R.; Guan, D.W. Evaluation of specific neural marker GAP-43 and TH combined with Masson-trichrome staining for forensic autopsy cases with old myocardial infarction. Int. J. Legal. Med. 2018, 132, 187–195. [Google Scholar] [CrossRef]
- Drobysheva, A.; Ahmad, M.; White, R.; Wang, H.W.; Leenen, F.H. Cardiac sympathetic innervation and PGP9.5 expression by cardiomyocytes after myocardial infarction: Effects of central MR blockade. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1817–H1829. [Google Scholar] [CrossRef]
- Han, S.; Kobayashi, K.; Joung, B.; Piccirillo, G.; Maruyama, M.; Vinters, H.V.; March, K.; Lin, S.F.; Shen, C.; Fishbein, M.C.; et al. Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 954–961. [Google Scholar] [CrossRef]
- Cope, A.L.; Schraiber, J.G.; Pennell, M. Macroevolutionary divergence of gene expression driven by selection on protein abundance. Science 2025, 387, 1063–1068. [Google Scholar] [CrossRef]
- Mete, O.; Asa, S.L.; Gill, A.J.; Kimura, N.; de Krijger, R.R.; Tischler, A. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr. Pathol. 2022, 33, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, M.W.; Verkerk, A.O.; van Ginneken, A.C.; Baartscheer, A.; Schumacher, C.; de Jonge, N.; de Bakker, J.M.; Opthof, T. Norepinephrine induces action potential prolongation and early afterdepolarizations in ventricular myocytes isolated from human end-stage failing hearts. Eur. Heart J. 2001, 22, 955–963. [Google Scholar] [CrossRef] [PubMed]
- van Weperen, V.Y.H.; Hoang, J.D.; Jani, N.R.; Khaky, A.; Herring, N.; Smith, C.; Vaseghi, M. Circulating noradrenaline leads to release of neuropeptide Y from cardiac sympathetic nerve terminals via activation of β-adrenergic receptors. J. Physiol. 2025, 603, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Vrabec, T.; Bender, S.; Chan, S.A.; Cha, S.; Haridas, S.; Hanna, P.; Ajijola, O.A.; Shivkumar, K.; Smith, C.; Ardell, J.L. Bioelectronic block of stellate ganglia mitigates pacing-induced heterogeneous release of catecholamine and neuropeptide Y in the infarcted pig heart. J. Physiol. 2025, 603, 2071–2088. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W.; Jama, A.; Donohue, T.; Wernli, G.; Onyszchuk, G.; Al-Hafez, B.; Bilgen, M.; Smith, P.G. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res. 2006, 1124, 142–154. [Google Scholar] [CrossRef]
- Goto, Y.; Zeng, L.; Yeom, C.J.; Zhu, Y.; Morinibu, A.; Shinomiya, K.; Kobayashi, M.; Hirota, K.; Itasaka, S.; Yoshimura, M.; et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1α. Nat. Commun. 2015, 6, 6153. [Google Scholar] [CrossRef]
- Bi, H.L.; Zhang, X.L.; Zhang, Y.L.; Xie, X.; Xia, Y.L.; Du, J.; Li, H.H. The deubiquitinase UCHL1 regulates cardiac hypertrophy by stabilizing epidermal growth factor receptor. Sci. Adv. 2020, 6, eaax4826. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.L.; Fu, T.T.; Li, P.B.; Cong, T.; Li, H.H. Blockage of UCHL1 activity attenuates cardiac remodeling in spontaneously hypertensive rats. Hypertens. Res. 2020, 43, 1089–1098. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Cai, M.; Ye, B.; Geng, B.; Li, F.; Zhu, H.; Liu, J.; Wang, X. Ubiquitin Carboxyl-Terminal Hydrolase L1 of Cardiomyocytes Promotes Macroautophagy and Proteostasis and Protects Against Post-myocardial Infarction Cardiac Remodeling and Heart Failure. Front. Cardiovasc. Med. 2022, 9, 866901. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P.; et al. Molecular anatomy of a trafficking organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef]
- Milovanovic, D.; Wu, Y.; Bian, X.; De Camilli, P. A liquid phase of synapsin and lipid vesicles. Science 2018, 361, 604–607. [Google Scholar] [CrossRef]
- Park, D.; Wu, Y.; Lee, S.E.; Kim, G.; Jeong, S.; Milovanovic, D.; De Camilli, P.; Chang, S. Cooperative function of synaptophysin and synapsin in the generation of synaptic vesicle-like clusters in non-neuronal cells. Nat. Commun. 2021, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Wu, Y.; Wang, X.; Gowrishankar, S.; Baublis, A.; De Camilli, P. Synaptic vesicle proteins and ATG9A self-organize in distinct vesicle phases within synapsin condensates. Nat. Commun. 2023, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hamid, T.; Keith, R.J.; Zhou, G.; Partridge, C.R.; Xiang, X.; Kingery, J.R.; Lewis, R.K.; Li, Q.; Rokosh, D.G.; et al. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation 2010, 121, 1912–1925. [Google Scholar] [CrossRef] [PubMed]

| Groups | Mean (%) ± SD (%) (range %) | ||
|---|---|---|---|
| TH | PGP9.5 | SYN | |
| Control | 1 a | 1 a | 1 a |
| 2 days | 1.284 ± 0.08 (1.222–1.432) a | 1.962 ± 0.19 (1.675–2.126) a | 1.618 ± 0.18 (1.390–1.834) a |
| 5 days | 1.702 ± 0.09 (1.635–1.843) b | 2.864 ± 0.14 (2.675–3.025) e | 3.608 ± 0.28 (3.213–3.982) a |
| 7 days | 1.616 ± 0.08 (1.523–1.735) c | 3.102 ± 0.13 (2.983–3.294) e | 3.050 ± 0.12 (2.898–3.212) f |
| 10 days | 1.515 ± 0.07 (1.430–1.582) d | 2.991 ± 0.13 (2.784–3.132) d | 2.799 ± 0.13 (2.674–2.984) g |
| Gene | Species | Primer | GenBank Accession No. | |
|---|---|---|---|---|
| Gapdh | mouse | Forward: | 5′-GGTGAAGGTCGGTGTGAACG-3′ | NM_017008.3 |
| Reverse: | 5′-CTCGCTCCTGGAAGATGGTG-3′ | |||
| TH | mouse | Forward: | 5′-GTTTCAGTGCACACAGTACATC-3′ | NM_009377.2 |
| Reverse: | 5′-CACCGTGGAGAGTTTTTCAATT-3′ | |||
| PGP9.5 | mouse | Forward: | 5′-ATAGAGCCAAGTGTTTCGAGAA-3′ | NM_011670.2 |
| Reverse: | 5′-ATTCACTTTGTCATCTACCCGA-3′ | |||
| SYN | mouse | Forward: | 5′-CCACTGACCCAGAGAACATTAT-3′ | NM_009305.2 |
| Reverse: | 5′-CTTGAACACGAACCATAGGTTG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Pei, B.; Zhao, D. Sympathetic Biomarker Dynamics Post-Myocardial Infarction: TH, PGP9.5, and SYN Expression Discordance in Murine Hearts. Int. J. Mol. Sci. 2025, 26, 9456. https://doi.org/10.3390/ijms26199456
Yu T, Pei B, Zhao D. Sympathetic Biomarker Dynamics Post-Myocardial Infarction: TH, PGP9.5, and SYN Expression Discordance in Murine Hearts. International Journal of Molecular Sciences. 2025; 26(19):9456. https://doi.org/10.3390/ijms26199456
Chicago/Turabian StyleYu, Tianshui, Baoqing Pei, and Dong Zhao. 2025. "Sympathetic Biomarker Dynamics Post-Myocardial Infarction: TH, PGP9.5, and SYN Expression Discordance in Murine Hearts" International Journal of Molecular Sciences 26, no. 19: 9456. https://doi.org/10.3390/ijms26199456
APA StyleYu, T., Pei, B., & Zhao, D. (2025). Sympathetic Biomarker Dynamics Post-Myocardial Infarction: TH, PGP9.5, and SYN Expression Discordance in Murine Hearts. International Journal of Molecular Sciences, 26(19), 9456. https://doi.org/10.3390/ijms26199456





