Alzheimer’s Disease: From Molecular Mechanisms to Promising Therapeutic Strategies
Abstract
1. Introduction
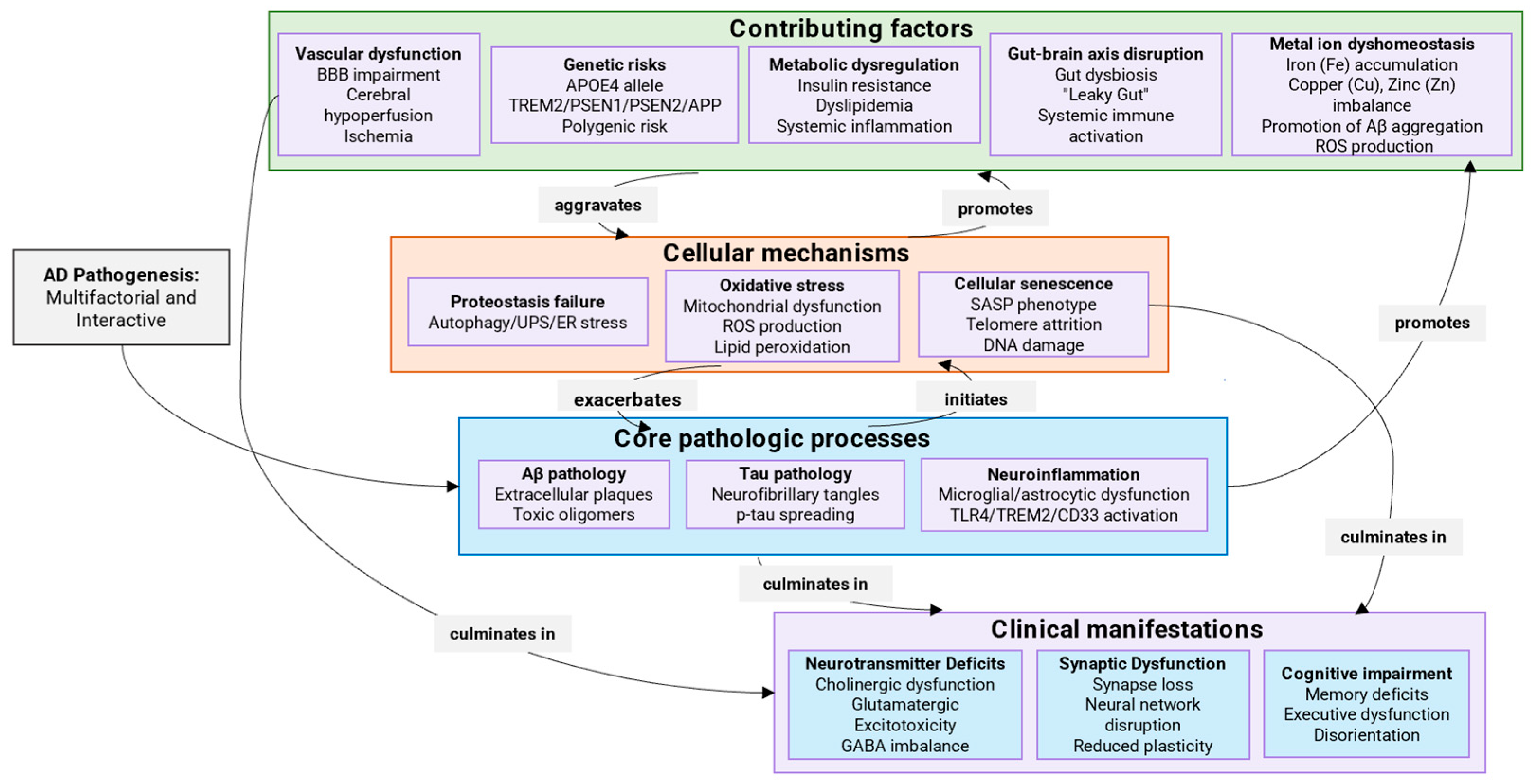
2. Mechanisms of AD Development
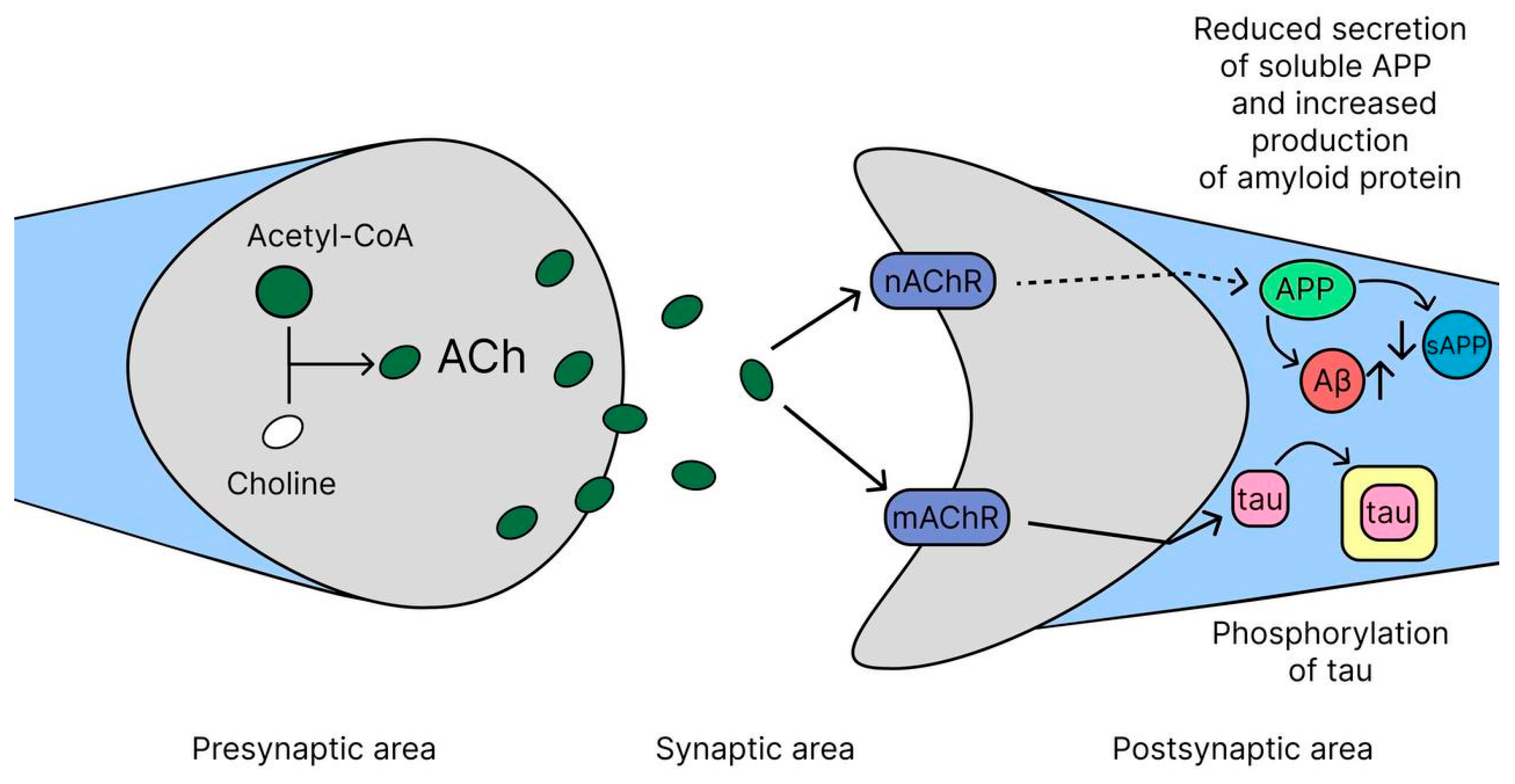
2.1. Cholinergic Hypothesis
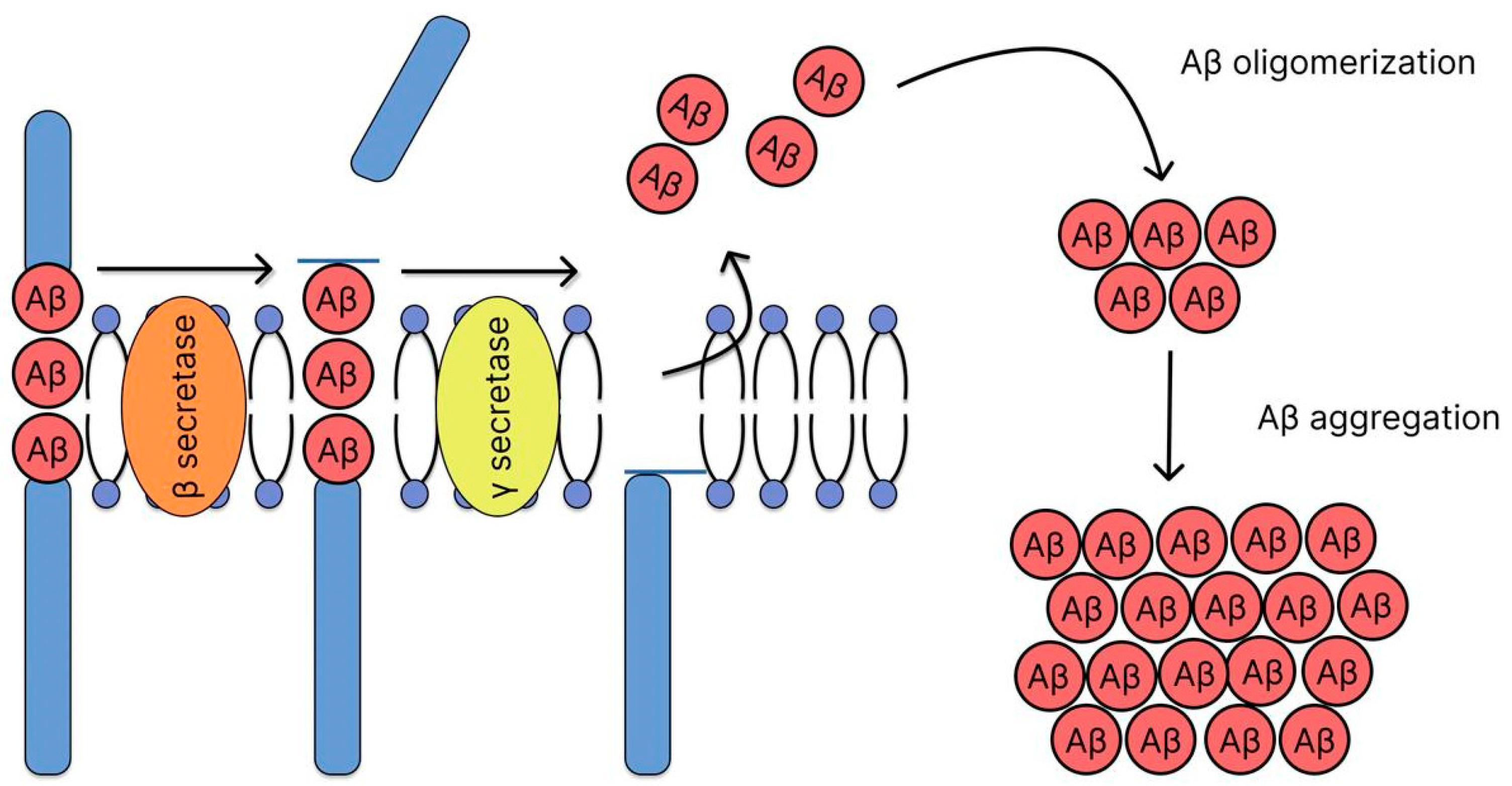
2.2. Amyloid Hypothesis
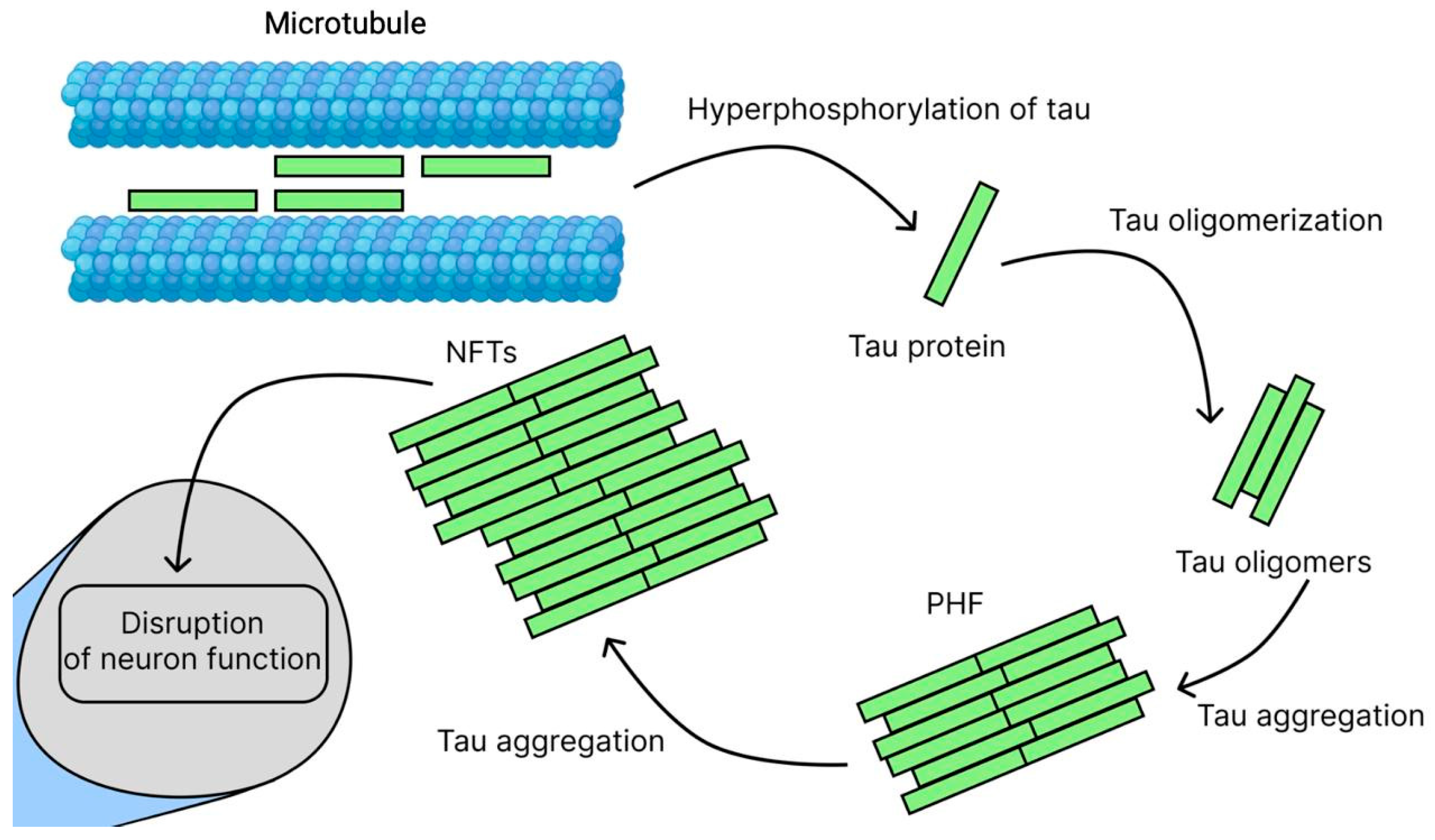
2.3. Tau Hypothesis
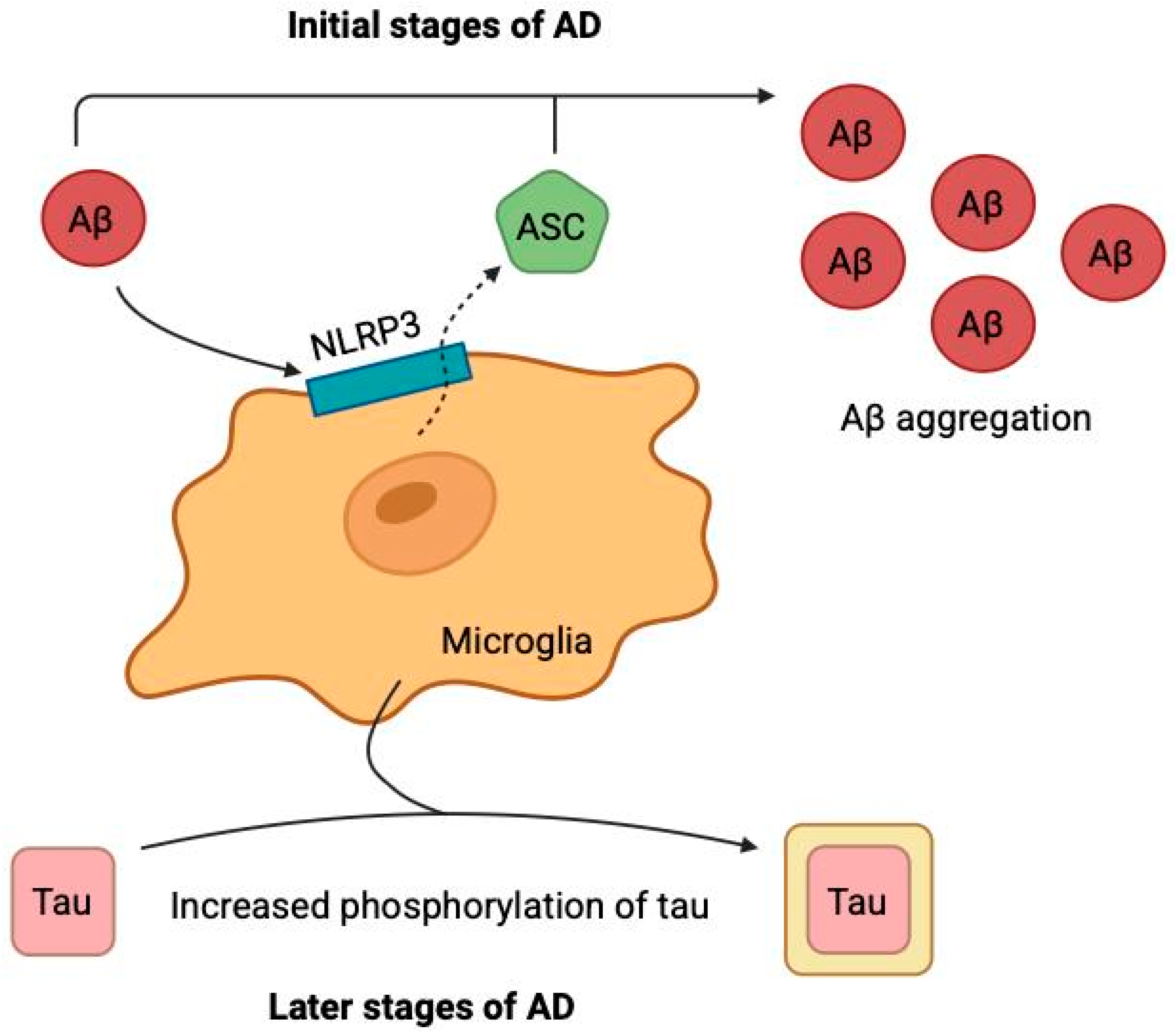
2.4. Neuroinflammation Hypothesis
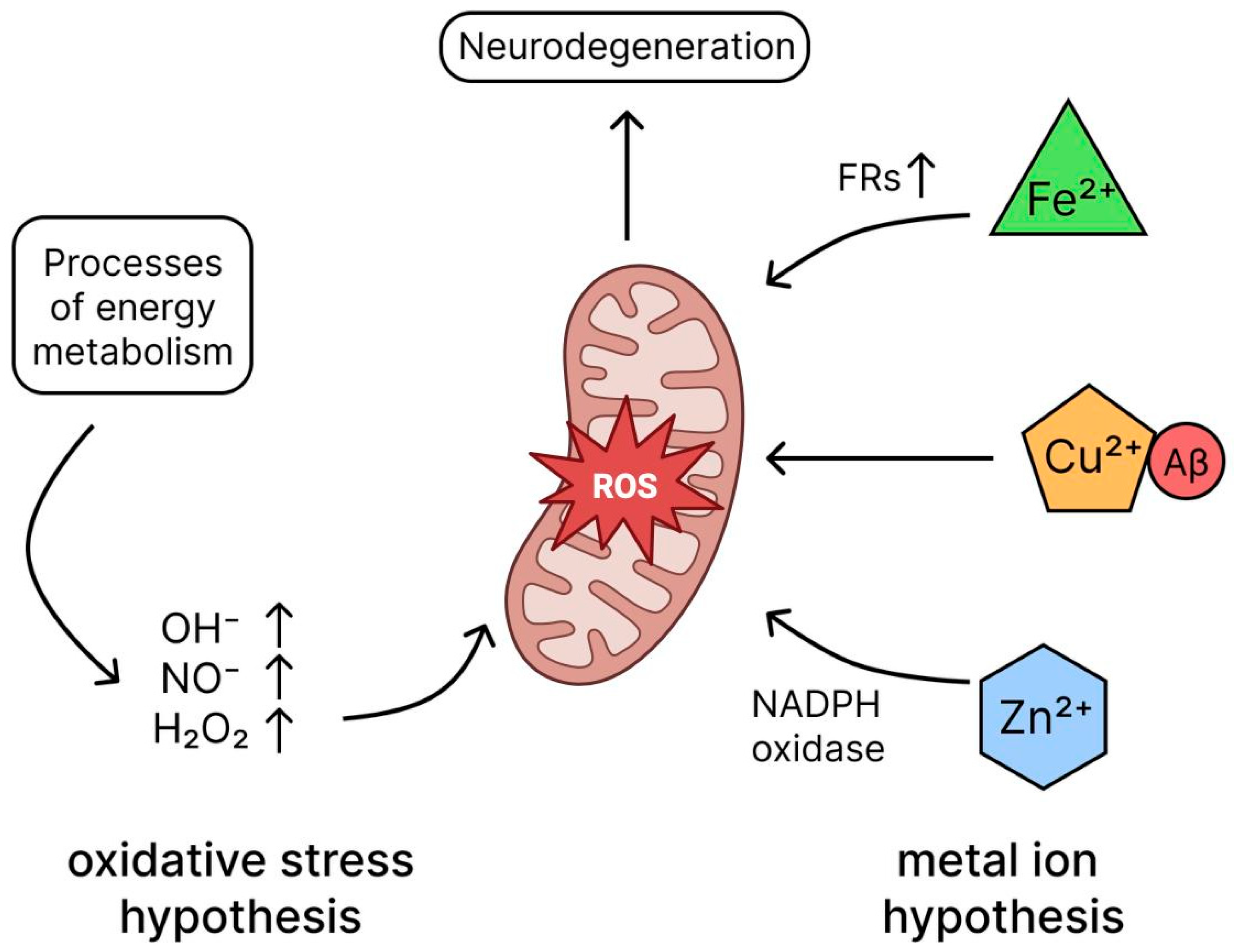
2.5. Oxidative Stress Hypothesis
2.6. Metal Ion Hypothesis
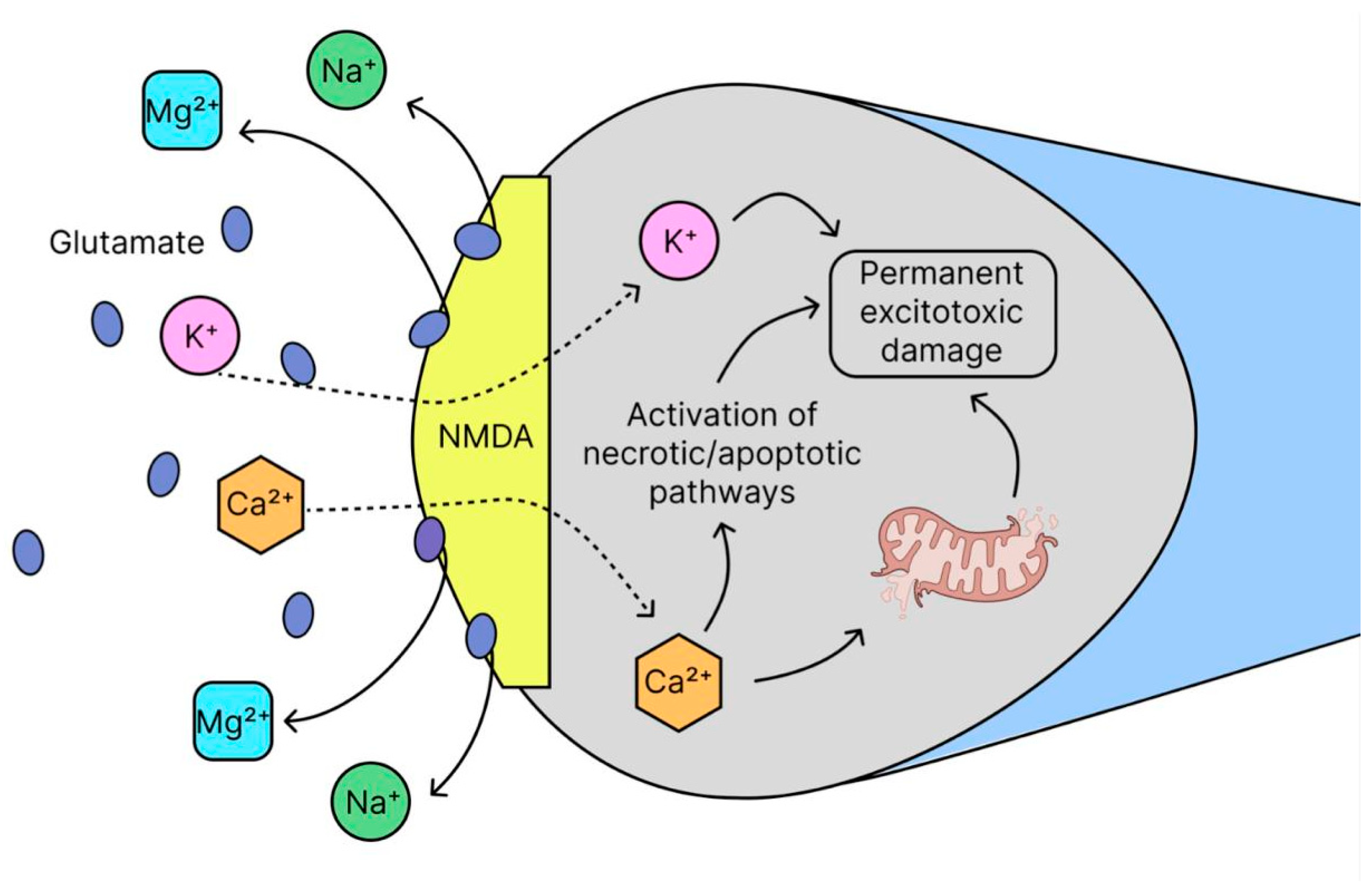
2.7. Glutamate Excitotoxicity
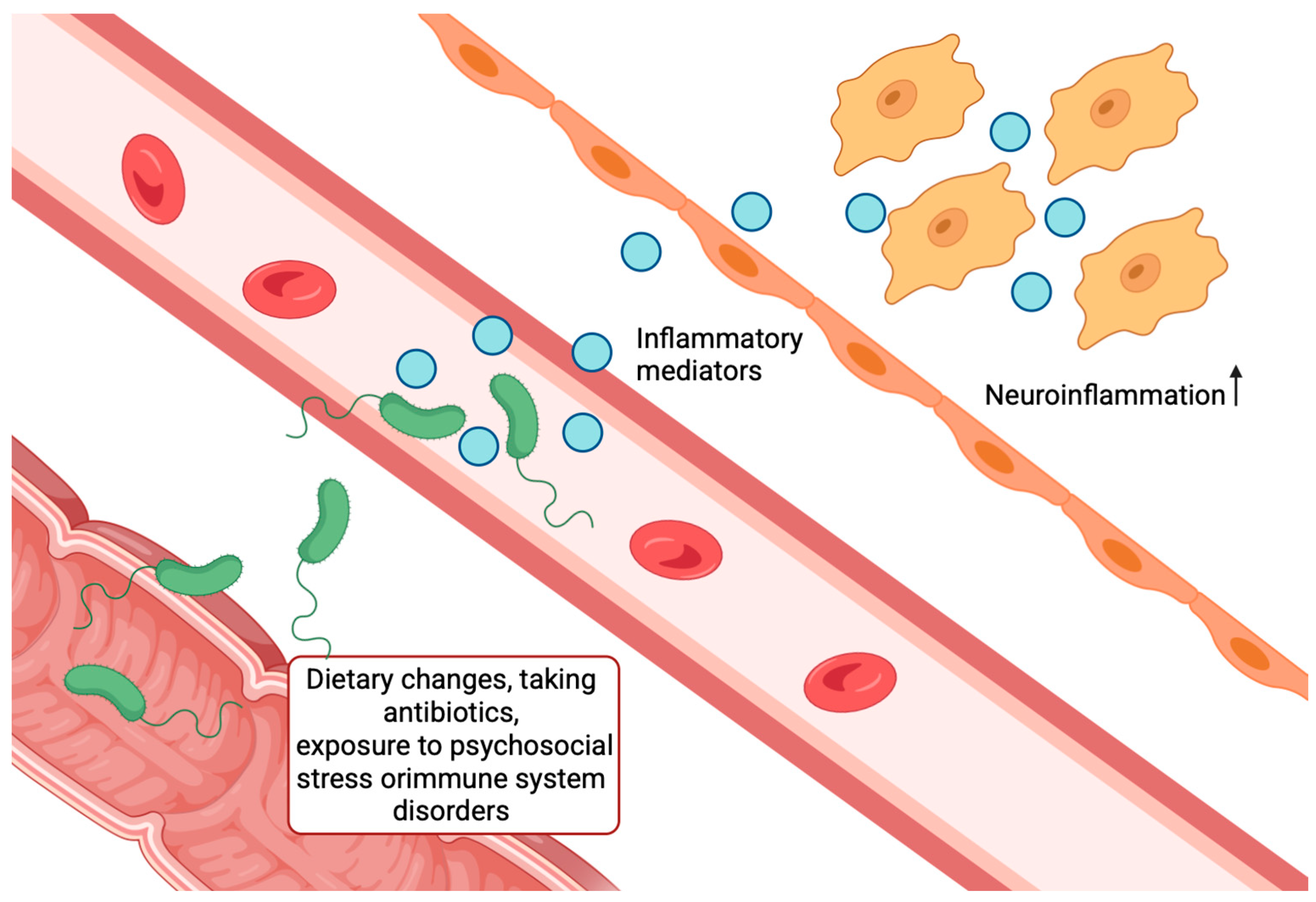
2.8. Microbiota–Gut–Brain Axis Hypothesis
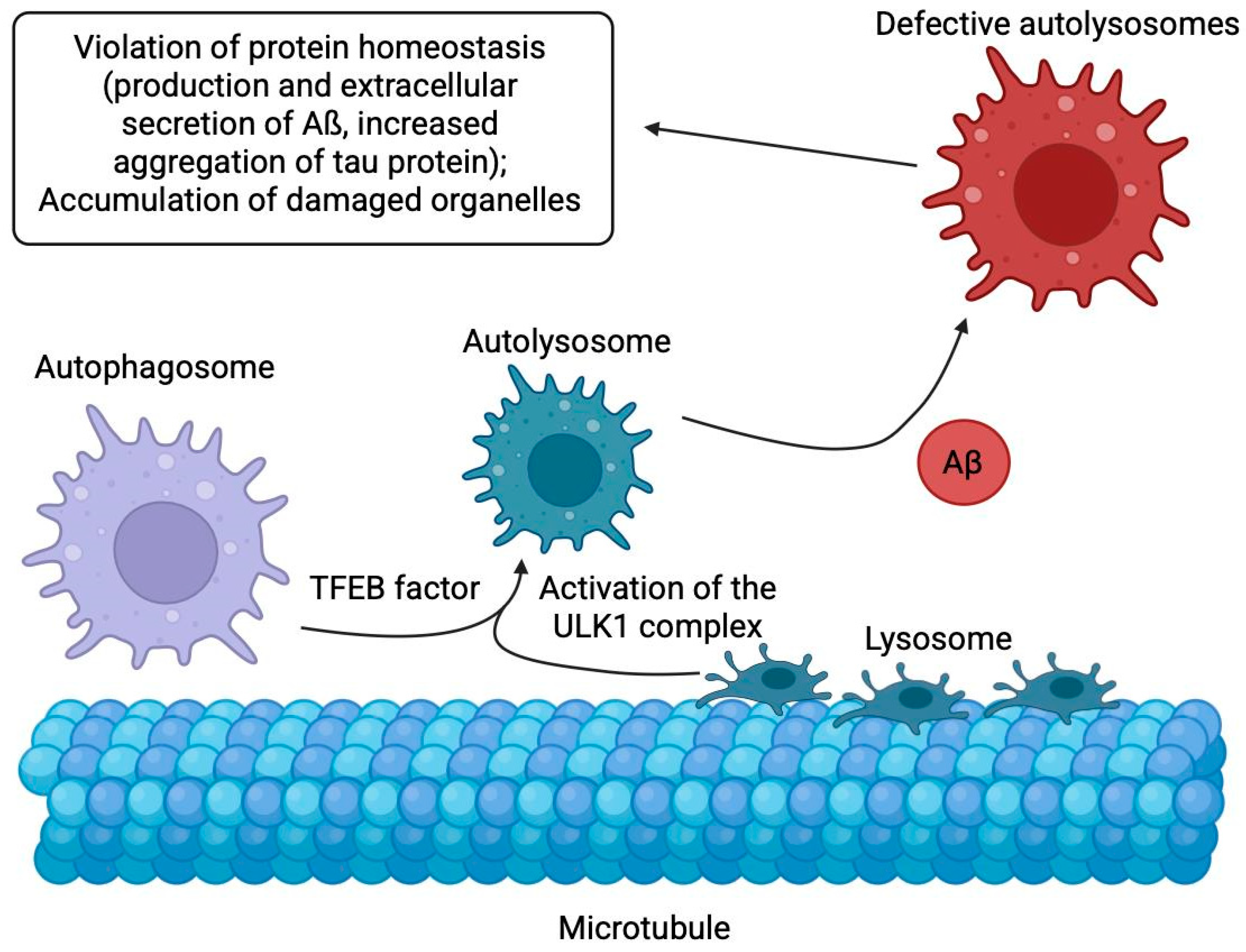
2.9. Abnormal Autophagy
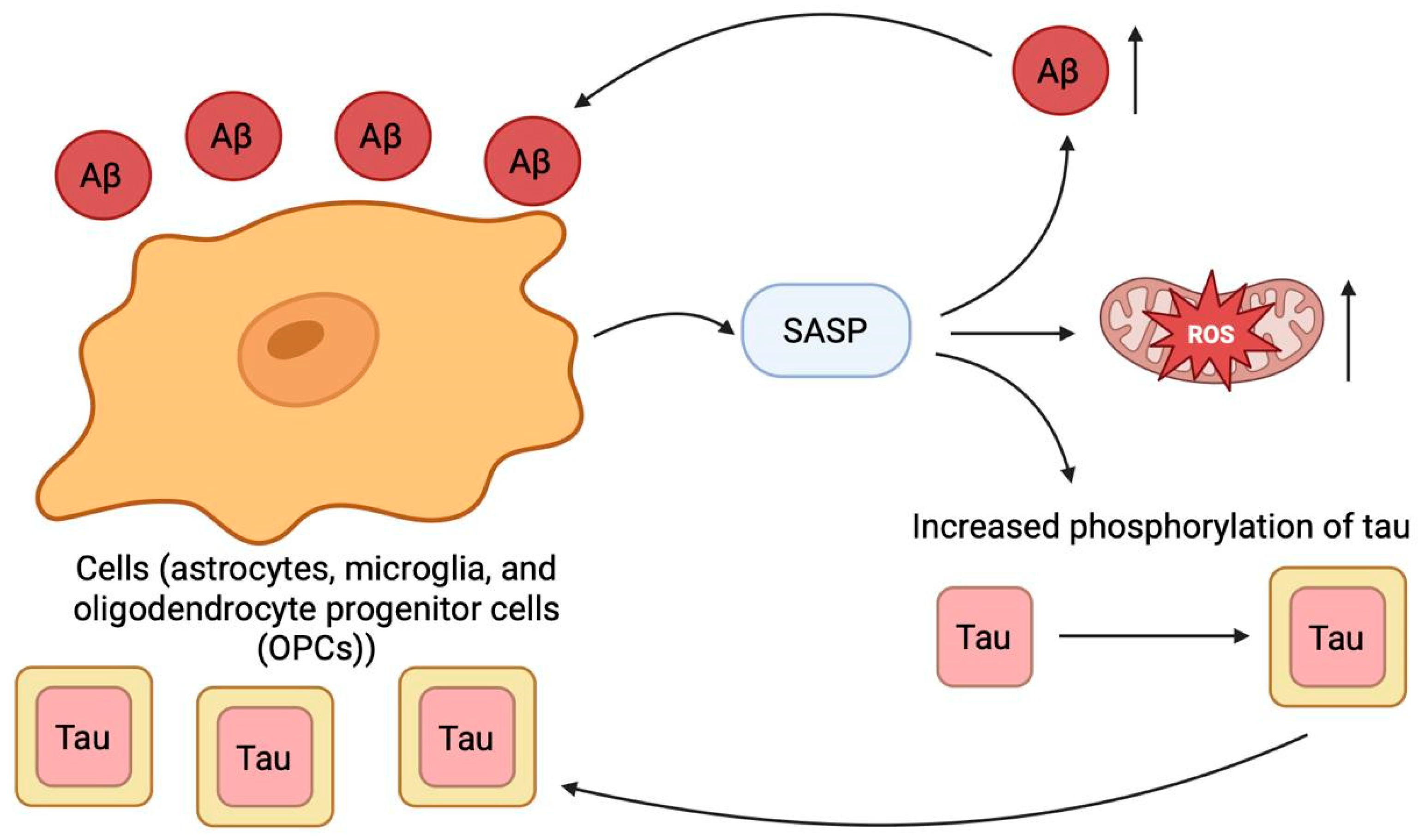
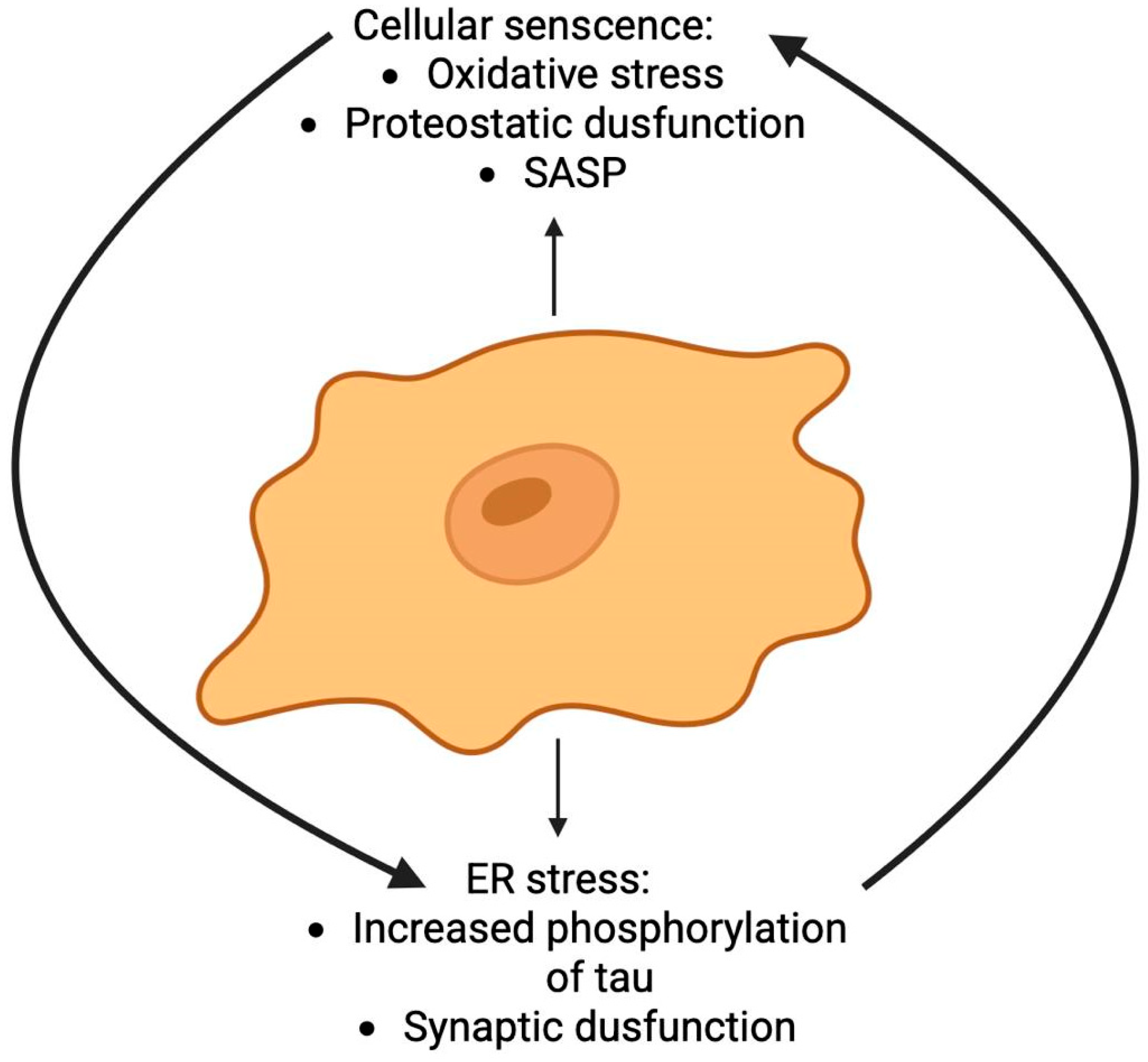
2.10. Cellular Senescence
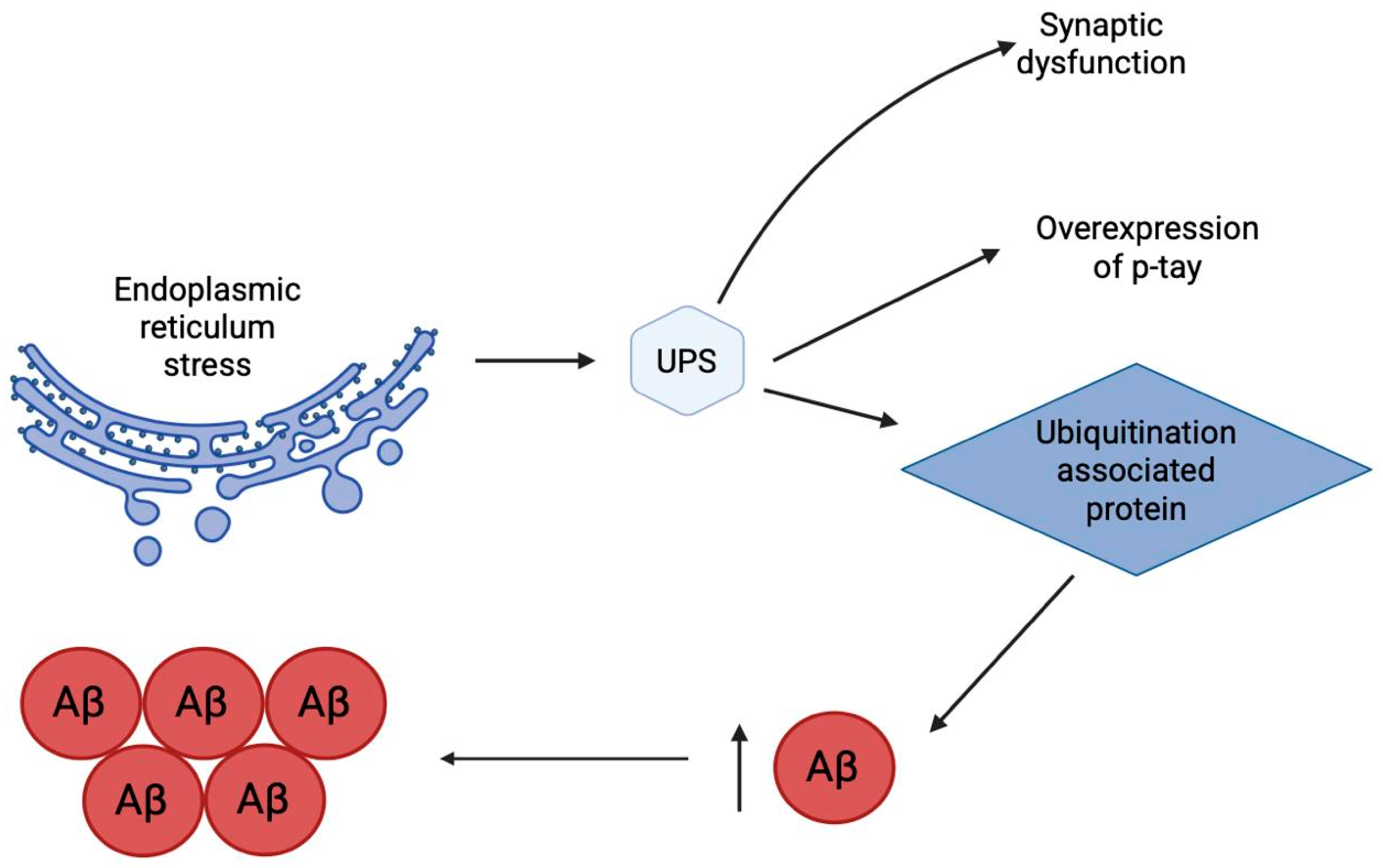
2.11. Endoplasmic Reticulum Stress
2.12. Ubiquitin-Proteasome System
2.13. Comparative Analysis of Therapeutic Targets in AD
3. Targeted Therapeutic Agents for AD
3.1. Small Molecules
3.2. Peptides
3.3. Antibodies and Their Fragments
3.4. Natural Ligands
3.5. Hybrid Multifunctional Molecules
4. Radiopharmaceuticals for the Treatment and Diagnosis of AD
4.1. Radiopharmaceuticals for Aβ Imaging
4.2. Radiopharmaceuticals for Tau Imaging
5. Conclusions
- Theragnostic integration: Future research must focus on developing parallel imaging and therapeutic ligands targeting the same pathway (e.g., a tau-PET ligands and a tau-aggregation inhibitor). This would allow for direct, real-time monitoring of a drug’s distribution and efficacy at its target site.
- Combinatorial biomarker tracking: The true potential of multi-target therapies can only be realized with the ability to simultaneously track multiple pathological processes in a patient. This necessitates the development of novel radiotracers for emerging targets like neuroinflammation (e.g., TSPO), synaptic density, and specific immune responses, enabling a holistic view of treatment effects.
- Personalized treatment algorithms: The ultimate clinical application of this synergy is the creation of personalized treatment algorithms. A patient’s initial PET profile (e.g., high Aβ, low tau; high neuroinflammation) could dictate the choice of combination therapy (e.g., anti-amyloid + anti-inflammatory). Subsequent scans would then objectively guide treatment adjustments, moving away from a one-size-fits-all approach to truly personalized medicine.
- Advanced Quantification and Artificial Intelligence: Overcoming current limitations in PET quantification (e.g., off-target binding, partial volume effect) through advanced modeling and artificial intelligence is critical. This will enhance the sensitivity to detect subtle treatment-induced changes, essential for proving the efficacy of multi-target drugs that may offer modest but clinically meaningful benefits at each targeted pathway.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tackenberg, C.; Kulic, L.; Nitsch, R.M. Familial Alzheimer’s Disease Mutations at Position 22 of the Amyloid β-Peptide Sequence Differentially Affect Synaptic Loss, Tau Phosphorylation and Neuronal Cell Death in an Ex Vivo System. PLoS ONE 2020, 15, e0239584. [Google Scholar] [CrossRef]
- Hayne, D.J.; Lim, S.; Donnelly, P.S. Metal Complexes Designed to Bind to Amyloid-β for the Diagnosis and Treatment of Alzheimer’s Disease. Chem. Soc. Rev. 2014, 43, 6701–6715. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Jayadev, S.; Nochlin, D.; Poorkaj, P.; Steinbart, E.J.; Mastrianni, J.A.; Montine, T.J.; Ghetti, B.; Schellenberg, G.D.; Bird, T.D.; Leverenz, J.B. Familial Prion Disease with Alzheimer Disease-like Tau Pathology and Clinical Phenotype. Ann. Neurol. 2011, 69, 712–720. [Google Scholar] [CrossRef]
- Le, M.N.; Kim, W.; Lee, S.; McKee, A.C.; Hall, G.F. Multiple Mechanisms of Extracellular Tau Spreading in a Non-Transgenic Tauopathy Model. Am. J. Neurodegener. Dis. 2012, 1, 316–333. [Google Scholar] [PubMed]
- Abdelnour, C.; Agosta, F.; Bozzali, M.; Fougère, B.; Iwata, A.; Nilforooshan, R.; Takada, L.T.; Viñuela, F.; Traber, M. Perspectives and Challenges in Patient Stratification in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2022, 14, 112. [Google Scholar] [CrossRef]
- Cummings, J. New Approaches to Symptomatic Treatments for Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 2. Correction in Mol. Neurodegener. 2021, 16, 21. https://doi.org/10.1186/s13024-021-00446-3. [CrossRef] [PubMed]
- Beata, B.K.; Wojciech, J.; Johannes, K.; Piotr, L.; Barbara, M. Alzheimer’s Disease—Biochemical and Psychological Background for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 1059. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Chiriac, S.I.B.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The Significance of the Cholinergic System in the Brain during Aging and in Alzheimer’s Disease. J. Neural Transm. 2006, 113, 1625–1644. [Google Scholar] [CrossRef]
- Crow, T.J.; Cross, A.J.; Cooper, S.J.; Deakin, J.F.W.; Ferrier, I.N.; Johnson, J.A.; Joseph, M.H.; Owen, F.; Poulter, M.; Lofthouse, R.; et al. Neurotransmitter Receptors and Monoamine Metabolites in the Brains of Patients with Alzheimer-Type Dementia and Depression, and Suicides. Neuropharmacology 1984, 23, 1561–1569. [Google Scholar] [CrossRef]
- Hippius, H.; Neundörfer, G. The Discovery of Alzheimer’s Disease. Dialogues Clin. Neurosci. 2003, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hajjo, R.; Sabbah, D.A.; Abusara, O.H.; Al Bawab, A.Q. A Review of the Recent Advances in Alzheimer’s Disease Research and the Utilization of Network Biology Approaches for Prioritizing Diagnostics and Therapeutics. Diagnostics 2022, 12, 2975. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.; Selman, A.; Sehar, U.; Rawat, P.; Reddy, A.P.; Reddy, P.H. Therapeutics of Alzheimer’s Disease: Recent Developments. Antioxidants 2022, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target Ther. 2019, 4, 29, Erratum in Signal Transduct. Target Ther. 2019, 4, 37. [Google Scholar] [CrossRef]
- Fedele, E. Anti-Amyloid Therapies for Alzheimer’s Disease and the Amyloid Cascade Hypothesis. Int. J. Mol. Sci. 2023, 24, 14499. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The Amyloid Cascade Hypothesis: An Updated Critical Review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Rubin, L.; Ingram, L.A.; Resciniti, N.V.; Ashford-Carroll, B.; Leith, K.H.; Rose, A.; Ureña, S.; McCollum, Q.; Friedman, D.B. Genetic Risk Factors for Alzheimer’s Disease in Racial/Ethnic Minority Populations in the U.S.: A Scoping Review. Front. Public Health 2021, 9, 784958. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer Disease: Pathobiology and Targeting Strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New Insights into the Genetic Etiology of Alzheimer’s Disease and Related Dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic Meta-Analysis of Diagnosed Alzheimer’s Disease Identifies New Risk Loci and Implicates Aβ, Tau, Immunity and Lipid Processing. Nat. Genet. 2019, 51, 414–430, Erratum in Nat. Genet. 2019, 51, 1423–1424. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s Disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Ulland, T.K.; Colonna, M. TREM2—A Key Player in Microglial Biology and Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Iturria-Medina, Y.; Sotero, R.C.; Toussaint, P.J.; Mateos-Pérez, J.M.; Evans, A.C.; Weiner, M.W.; Aisen, P.; Petersen, R.; Jack, C.R.; Jagust, W.; et al. Early Role of Vascular Dysregulation on Late-Onset Alzheimer’s Disease Based on Multifactorial Data-Driven Analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Eid, A.; Mhatre, I.; Richardson, J.R. Gene-Environment Interactions in Alzheimer’s Disease: A Potential Path to Precision Medicine. Pharmacol. Ther. 2019, 199, 173–187. [Google Scholar] [CrossRef]
- Boyd, R.J.; Avramopoulos, D.; Jantzie, L.L.; McCallion, A.S. Neuroinflammation Represents a Common Theme amongst Genetic and Environmental Risk Factors for Alzheimer and Parkinson Diseases. J. Neuroinflamm. 2022, 19, 223. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.S.; Uddin, M.J.; Mamum-Or-Rashid, A.N.M.; Pang, M.G.; Rhim, H. Emerging Risk of Environmental Factors: Insight Mechanisms of Alzheimer’s Diseases. Environ. Sci. Pollut. Res. 2020, 27, 44659–44672. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The Cholinergic Hypothesis of Alzheimer’s Disease: A Review of Progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Auld, D.S.; Kornecook, T.J.; Bastianetto, S.; Quirion, R. Alzheimer’s Disease and the Basal Forebrain Cholinergic System: Relations to beta-Amyloid Peptides, Cognition, and Treatment Strategies. Prog. Neurobiol. 2002, 68, 209–245. [Google Scholar] [CrossRef]
- Chen, X.Q.; Mobley, W.C. Exploring the Pathogenesis of Alzheimer Disease in Basal Forebrain Cholinergic Neurons: Converging Insights from Alternative Hypotheses. Front. Neurosci. 2019, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining Cholinergic Therapy for Alzheimer’s Disease. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Bekdash, R.A. The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1273. [Google Scholar] [CrossRef]
- Majdi, A.; Sadigh-Eteghad, S.; Rahigh Aghsan, S.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, Tau, and the Cholinergic System in Alzheimer’s Disease: Seeking Direction in a Tangle of Clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Sale, M.G.F.; Di Lorenzo, M. Towards Timely Alzheimer Diagnosis: A Self-Powered Amperometric Biosensor for the Neurotransmitter Acetylcholine. Biosens. Bioelectron. 2017, 87, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical Trials and Late-Stage Drug Development for Alzheimer’s Disease: An Appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; Berthoumieu, O. Role of Metal Ions in the Self-Assembly of the Alzheimer’s Amyloid-β Peptide. Inorg. Chem. 2013, 52, 12193–12206. [Google Scholar] [CrossRef]
- Lu, P.; Bai, X.C.; Ma, D.; Xie, T.; Yan, C.; Sun, L.; Yang, G.; Zhao, Y.; Zhou, R.; Scheres, S.H.W.; et al. Three-Dimensional Structure of Human γ-Secretase. Nature 2014, 512, 166–170. [Google Scholar] [CrossRef]
- Carter, D.B.; Dunn, E.; Pauley, A.M.; McKinley, D.D.; Fleck, T.J.; Ellerbrook, B.R.; Stratman, N.C.; Zhou, X.; Himes, C.S.; Nye, J.S.; et al. Changes in γ-Secretase Activity and Specificity Caused by the Introduction of Consensus Aspartyl Protease Active Motif in Presenilin 1. Mol. Neurodegener. 2008, 3, 6. [Google Scholar] [CrossRef]
- Carroll, C.M.; Li, Y.M. Physiological and Pathological Roles of the γ-Secretase Complex. Brain Res. Bull. 2016, 126, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble Protein Oligomers in Neurodegeneration: Lessons from the Alzheimer’s Amyloid β-Peptide. Nat. Rev. Mol. Cell. Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Citron, M. Alzheimer’s Disease: Strategies for Disease Modification. Nat. Rev. Drug. Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.L.; Caraci, F.; De Bona, P.; Pappalardo, G.; Nicoletti, F.; Rizzarelli, E.; Copani, A. The Monomer State of Beta-Amyloid: Where the Alzheimer’s Disease Protein Meets Physiology. Rev. Neurosci. 2010, 21, 83–94. [Google Scholar] [CrossRef]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The Physiological Roles of Tau and Aβ: Implications for Alzheimer’s Disease Pathology and Therapeutics. Acta Neuropathol. 2020, 140, 417–447. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Cappai, R.; Barnham, K.J.; Villemagne, V.L. Molecular Mechanisms for Alzheimer’s Disease: Implications for Neuroimaging and Therapeutics. J. Neurochem. 2006, 97, 1700–1725. [Google Scholar] [CrossRef]
- Masters, C.L.; Beyreuther, K. Science, Medicine, and the Future: Alzheimer’s Disease. BMJ 1998, 316, 446–448. [Google Scholar] [CrossRef]
- Roda, A.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-Beta Peptide and Tau Protein Crosstalk in Alzheimer’s Disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Review Interaction Between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef]
- Avila, J.; Lucas, J.J.; Pérez, M.; Hernández, F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, D.; Gong, X.; Zheng, J. A Mechanistic Survey of Alzheimer’s Disease. Biophys. Chem. 2022, 281, 106735. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary Regulation of PI3K/AKT/GSK-3β Pathway in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef]
- Shukla, V.; Skuntz, S.; Pant, H.C. Deregulated Cdk5 Activity Is Involved in Inducing Alzheimer’s Disease. Arch. Med. Res. 2012, 43, 655–662. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M. Tau Pathology and Neurodegeneration. Lancet Neurol. 2013, 12, 609–622. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, C.; Qiu, Y.; Zhou, Z.; Bao, J.; Qian, W. Dendritic/Post-Synaptic Tau and Early Pathology of Alzheimer’s Disease. Front. Mol. Neurosci. 2021, 14, 671779. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Latina, V.; Corsetti, V.; Calissano, P. N-Terminal Tau Truncation in the Pathogenesis of Alzheimer’s Disease (AD): Developing a Novel Diagnostic and Therapeutic Approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165584. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Grundke-Iqbal, I.; Iqbal, K. Glycosylation of Microtubule-Associated Protein Tau: An Abnormal Posttranslational Modification in Alzheimer’s Disease. Nat. Med. 1996, 2, 871–875. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Li, L.; Qin, P.; Iqbal, J.; Deng, Y.; Qing, H. Glycation Alter the Process of Tau Phosphorylation to Change Tau Isoforms Aggregation Property. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 192–201. [Google Scholar] [CrossRef]
- Luo, H.B.; Xia, Y.Y.; Shu, X.J.; Liu, Z.C.; Feng, Y.; Liu, X.H.; Yu, G.; Yin, G.; Xiong, Y.S.; Zeng, K.; et al. SUMOylation at K340 Inhibits Tau Degradation through Deregulating Its Phosphorylation and Ubiquitination. Proc. Natl. Acad. Sci. USA 2014, 111, 16586–16591. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau Biomarkers in Alzheimer’s Disease: Towards Implementation in Clinical Practice and Trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.J.; Buggia-Prevot, V. Novel Targets for Alzheimer’s Disease: A View Beyond Amyloid. Annu. Rev. Med. 2021, 72, 15–28. [Google Scholar] [CrossRef]
- Sala Frigerio, C.; De Strooper, B. Alzheimer’s Disease Mechanisms and Emerging Roads to Novel Therapeutics. Annu. Rev. Neurosci. 2016, 39, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Cras, P.; Kawai, M.; Siedlak, S.; Perry, G. Microglia Are Associated with the Extracellular Neurofibrillary Tangles of Alzheimer Disease. Brain Res. 1991, 558, 312–314. [Google Scholar] [CrossRef]
- El Khoury, J.; Hickman, S.E.; Thomas, C.A.; Cao, L.; Silverstein, S.C.; Loike, J.D. Scavenger Receptor-Mediated Adhesion of Microglia to β-Amyloid Fibrils. Nature 1996, 382, 716–719. [Google Scholar] [CrossRef]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-Derived ASC Specks Crossseed Amyloid-β in Alzheimer’s Disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial Dysfunction and Defective β-Amyloid Clearance Pathways in Aging Alzheimer’s Disease Mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Gualdrini, F.; Natoli, G. Transcriptional Repressors as Guardians of Tissue Macrophage Identity. EMBO J. 2019, 38, e103271. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Reddy, D.H.K. Apoptosis in Alzheimer’s Disease: Insight into the Signaling Pathways and Therapeutic Avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally Persistent Free Radicals: Occurrence, Formation Mechanisms and Implications. Environ. Pollut. 2019, 248, 320–331. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Xiang, Y.; Song, X.; Long, D. Ferroptosis Regulation through Nrf2 and Implications for Neurodegenerative Diseases. Arch. Toxicol. 2024, 98, 579–615. [Google Scholar] [CrossRef]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimer’s Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Yarjanli, Z.; Pakniya, F.; Bidram, E.; Łos, M.J.; Eshraghi, M.; Klionsky, D.J.; Ghavami, S.; Zarrabi, A. Targeting Autophagy, Oxidative Stress, and ER Stress for Neurodegenerative Disease Treatment. J. Control. Release 2022, 345, 147–175. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative Stress: The Core Pathogenesis and Mechanism of Alzheimer’s Disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Ferreira, M.E.S.; de Vasconcelos, A.S.; da Costa Vilhena, T.; da Silva, T.L.; da Silva Barbosa, A.; Gomes, A.R.Q.; Dolabela, M.F.; Percário, S. Oxidative Stress in Alzheimer’s Disease: Should We Keep Trying Antioxidant Therapies? Cell. Mol. Neurobiol. 2015, 35, 595–614. [Google Scholar] [CrossRef]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of Vitamin E and Memantine on Functional Decline in Alzheimer Disease: The TEAM-AD VA Cooperative Randomized Trial. JAMA 2014, 311, 33–44, Erratum in JAMA 2014, 311, 1161. [Google Scholar] [CrossRef]
- Snitz, B.E.; O’Meara, E.S.; Carlson, M.C.; Arnold, A.M.; Ives, D.G.; Rapp, S.R.; Saxton, J.; Lopez, O.L.; Dunn, L.O.; Sink, K.M.; et al. Ginkgo Biloba for Preventing Cognitive Decline in Older Adults a Randomized Trial. JAMA 2009, 302, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral Curcumin for Alzheimer’s Disease: Tolerability and Efficacy in a 24-Week Randomized, Double Blind, Placebo-Controlled Study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.G.; Mandal, P.K.; Maroon, J.C. Oxidative Stress Occurs Prior to Amyloid Aβ Plaque Formation and Tau Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 2023, 14, 2944–2954. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Acc. Chem. Res. 2019, 52, 2026–2035. [Google Scholar] [CrossRef]
- Kepp, K.P. Alzheimer’s Disease: How Metal Ions Define β-Amyloid Function. Coord. Chem. Rev. 2017, 351, 127–159. [Google Scholar] [CrossRef]
- Guilloreau, L.; Combalbert, S.; Sournia-Saquet, M.; Mazarguil, H.; Faller, P. Redox Chemistry of Copper-Amyloid-β: The Generation of Hydroxyl Radical in the Presence of Ascorbate Is Linked to Redox-Potentials and Aggregation State. ChemBioChem 2007, 8, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an Understanding of Amyloid-β Oligomers: Characterization, Toxicity Mechanisms, and Inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- De Stefano, N.; Sormani, M.P. Combining Biomarkers to Profile Multiple Sclerosis Patients. Nat. Rev. Neurol. 2020, 16, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Lei, P.; Bush, A.I. Metallostasis in Alzheimer’s Disease. Free Radic. Biol. Med. 2013, 62, 76–89. [Google Scholar] [CrossRef]
- Sensi, S.L.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef]
- Robert, A.; Liu, Y.; Nguyen, M.; Meunier, B. Regulation of Copper and Iron Homeostasis by Metal Chelators: A Possible Chemotherapy for Alzheimers Disease. Acc. Chem. Res. 2015, 48, 1332–1339. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological Metals and Metal-Targeting Compounds in Major Neurodegenerative Diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef]
- Perez, L.R.; Franz, K.J. Minding Metals: Tailoring Multifunctional Chelating Agents for Neurodegenerative Disease. Dalton Trans. 2010, 7, 2177–2187. [Google Scholar] [CrossRef]
- Chen, L.L.; Fan, Y.G.; Zhao, L.X.; Zhang, Q.; Wang, Z.Y. The Metal Ion Hypothesis of Alzheimer’s Disease and the Anti-Neuroinflammatory Effect of Metal Chelators. Bioorg. Chem. 2023, 131, 106301. [Google Scholar] [CrossRef]
- Choi, D.W. Glutamate Receptors and the Induction of Excitotoxic Neuronal Death. Prog. Brain Res. 1994, 100, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Erdin, D.; Wegmann, W. Lungenmetastase Eines Gutartigen Riesenzelltumors Des Skeletts 27 Jahre Nach Resektion Eines Tumorrezidivs. Pathologe 1996, 17, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Glutamate Neurotoxicity and Diseases of the Nervous System. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Olney, J.W. Glutamate-Induced Neuronal Necrosis in the Infant Mouse Hypothalamus: An Electron Microscopic Study. J. Neuropathol. Exp. Neurol. 1971, 30, 75–90. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Budd, S.L. Mitochondria and Neuronal Survival. Physiol. Rev. 2000, 80, 315–360. [Google Scholar] [CrossRef]
- Caraci, F.; Nicoletti, F.; Copani, A. Metabotropic Glutamate Receptors: The Potential for Therapeutic Applications in Alzheimer’s Disease. Curr. Opin. Pharmacol. 2018, 38, 1–7. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive Review of Mechanisms of Pathogenesis Involved in Alzheimer’s Disease and Potential Therapeutic Strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef]
- Granzotto, A.; Canzoniero, L.M.T.; Sensi, S.L. A Neurotoxic Ménage-à-Trois: Glutamate, Calcium, and Zinc in the Excitotoxic Cascade. Front. Mol. Neurosci. 2020, 13, 600089. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, A.; Berglund, K.; Gu, X.; Jiang, M.Q.; Talati, J.; Zhao, J.; Wei, L.; Yu, S.P. Pathogenesis of Sporadic Alzheimer’s Disease by Deficiency of NMDA Receptor Subunit GluN3A. Alzheimer’s Dement. 2022, 18, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the Physiology and Pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Wen, L.; Wu, Z.; Shen, Y. GABAergic Dysfunction in Excitatory and Inhibitory (E/I) Imbalance Drives the Pathogenesis of Alzheimer’s Disease. Alzheimer’s Dement. 2020, 16, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.L.; Moranth, R.V. Eje Microbiota-Intestino-Cerebro. Cienc. Lat. Rev. Científica Multidiscip. 2023, 14, 4531–4547. [Google Scholar] [CrossRef]
- Bulgart, H.R.; Neczypor, E.W.; Wold, L.E.; Mackos, A.R. Microbial Involvement in Alzheimer Disease Development and Progression. Mol. Neurodegener. 2020, 15, 42. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Asadi, S.; Patel, A.B. Focal Brain Inflammation and Autism. J. Neuroinflamm. 2013, 10, 815. [Google Scholar] [CrossRef]
- Li, C.; Yao, J.; Yang, C.; Yu, S.; Yang, Z.; Wang, L.; Li, S.; He, N. Gut Microbiota-Derived Short Chain Fatty Acids Act as Mediators of the Gut-Liver-Brain Axis. Metab. Brain Dis. 2025, 40, 122. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Liu, B.; Dong, X.; Cai, W.; Zhang, N.; Zhang, H. Gut Microbiota Mediates Neuroinflammation in Alzheimer’s Disease: Unraveling Key Factors and Mechanistic Insights. Mol. Neurobiol. 2025, 62, 3746–3763. [Google Scholar] [CrossRef]
- Gonzalez-Santana, A.; Diaz Heijtz, R. Bacterial Peptidoglycans from Microbiota in Neurodevelopment and Behavior. Trends Mol. Med. 2020, 26, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota–Gut–Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2021, 13, 37. [Google Scholar] [CrossRef]
- Weber, C.; Dilthey, A.; Finzer, P. The Role of Microbiome-Host Interactions in the Development of Alzheimer’s Disease. Front. Cell. Infect. Microbiol. 2023, 13, 1151021. [Google Scholar] [CrossRef]
- Lista, S.; Munafò, A.; Caraci, F.; Imbimbo, C.; Emanuele, E.; Minoretti, P.; Pinto-Fraga, J.; Merino-País, M.; Crespo-Escobar, P.; López-Ortiz, S.; et al. Gut Microbiota in Alzheimer’s Disease: Understanding Molecular Pathways and Potential Therapeutic Perspectives. Ageing Res. Rev. 2025, 104, 102659. [Google Scholar] [CrossRef]
- Cataldo, A.M.; Peterhoff, C.M.; Troncoso, J.C.; Gomez-Isla, T.; Hyman, B.T.; Nixon, R.A. Endocytic Pathway Abnormalities Precede Amyloid β Deposition in Sporadic Alzheimer’s Disease and down Syndrome: Differential Effects of APOE Genotype and Presenilin Mutations. Am. J. Pathol. 2000, 157, 277–286. [Google Scholar] [CrossRef]
- Choi, A.M.K.; Ryter, S.W.; Levine, B. Autophagy in Human Health and Disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Cuervo, A.M. Autophagy: Many Paths to the Same End. Mol. Cell. Biochem. 2004, 263, 55–72. [Google Scholar] [CrossRef]
- Mokas, S.; Mills, J.R.; Garreau, C.; Fournier, M.-J.; Robert, F.; Arya, P.; Kaufman, R.J.; Pelletier, J.; Mazroui, R. Uncoupling Stress Granule Assembly and Translation Initiation Inhibition. Mol. Biol. Cell 2009, 20, 2673–2683. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a Regulated Pathway of Cellular Degradation. Science (1979) 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Bernard, A.; Klionsky, D.J. Autophagosome Formation: Tracing the Source. Dev. Cell. 2013, 25, 116–117. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, D.S.; Goulbourne, C.N.; Im, E.; Stavrides, P.; Pensalfini, A.; Chan, H.; Bouchet-Marquis, C.; Bleiwas, C.; Berg, M.J.; et al. Faulty Autolysosome Acidification in Alzheimer’s Disease Mouse Models Induces Autophagic Build-up of Aβ in Neurons, Yielding Senile Plaques. Nat. Neurosci. 2022, 25, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.S.; Adriaanse, B.A.; Greig, N.H.; Mattson, M.P.; Cader, M.Z.; Bohr, V.A.; Fang, E.F. Mitophagy and Alzheimer’s Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017, 40, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.E.; Oddo, S. Autophagic/Lysosomal Dysfunction in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2013, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.; Bras, J. The Age Factor in Alzheimer’s Disease. Genome Med. 2015, 7, 106. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, C.; Yang, L. Cellular Senescence in Alzheimer’s Disease: From Physiology to Pathology. Transl. Neurodegener. 2024, 13, 55. [Google Scholar] [CrossRef]
- Masaldan, S.; Belaidi, A.A.; Ayton, S.; Bush, A.I. Cellular Senescence and Iron Dyshomeostasis in Alzheimer’s Disease. Pharmaceuticals 2019, 12, 93. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic Therapy Alleviates Aβ-Associated Oligodendrocyte Progenitor Cell Senescence and Cognitive Deficits in an Alzheimer’s Disease Model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Bhat, R.; Crowe, E.P.; Bitto, A.; Moh, M.; Katsetos, C.D.; Garcia, F.U.; Johnson, F.B.; Trojanowski, J.Q.; Sell, C.; Torres, C. Astrocyte Senescence as a Component of Alzheimer’s Disease. PLoS ONE 2012, 7, e45069. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Xing, S.; Chen, C.; Shen, D.; Chen, J. Aβ Promotes CD38 Expression in Senescent Microglia in Alzheimer’s Disease. Biol. Res. 2022, 55, 10. [Google Scholar] [CrossRef] [PubMed]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of Senescent Glial Cells Prevents Tau-Dependent Pathology and Cognitive Decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Ishikawa, F. Proteostasis Failure and Cellular Senescence in Long-Term Cultured Postmitotic Rat Neurons. Aging Cell 2020, 19, e13071. [Google Scholar] [CrossRef] [PubMed]
- Macip, S.; Igarashi, M.; Fang, L.; Chen, A.; Pan, Z.-Q.; Lee, S.W.; Aaronson, S.A. Inhibition of P21 Mediated ROS Accumulation Can Rescue P21-Induced Senescence. EMBO J. 2002, 21, 2180–2188. [Google Scholar] [CrossRef]
- Jaskelioff, M.; Muller, F.L.; Paik, J.H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadiñanos, J.; et al. Telomerase Reactivation Reverses Tissue Degeneration in Aged Telomerase-Deficient Mice. Nature 2011, 469, 102–107. [Google Scholar] [CrossRef]
- Shaerzadeh, F.; Phan, L.; Miller, D.; Dacquel, M.; Hachmeister, W.; Hansen, C.; Bechtle, A.; Tu, D.; Martcheva, M.; Foster, T.C.; et al. Microglia Senescence Occurs in Both Substantia Nigra and Ventral Tegmental Area. Glia 2020, 68, 2228–2245. [Google Scholar] [CrossRef]
- Vanzulli, I.; Papanikolaou, M.; De-La-Rocha, I.C.; Pieropan, F.; Rivera, A.D.; Gomez-Nicola, D.; Verkhratsky, A.; Rodríguez, J.J.; Butt, A.M. Disruption of Oligodendrocyte Progenitor Cells Is an Early Sign of Pathology in the Triple Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2020, 94, 130–139. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, X.C.; Song, Y.; Pan, X.D.; Dai, X.M.; Zhang, J.; Cui, X.L.; Wu, X.L.; Zhu, Y.G. Amyloid β Protein Aggravates Neuronal Senescence and Cognitive Deficits in 5XFAD Mouse Model of Alzheimer’s Disease. Chin. Med. J. (Engl.) 2016, 129, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, T.; Liu, H.; Mi, Y.; Gou, X. Astrocyte Senescence and Alzheimer’s Disease: A Review. Front. Aging Neurosci. 2020, 12, 148. [Google Scholar] [CrossRef]
- Bryant, A.G.; Hu, M.; Carlyle, B.C.; Arnold, S.E.; Frosch, M.P.; Das, S.; Hyman, B.T.; Bennett, R.E. Cerebrovascular Senescence Is Associated with Tau Pathology in Alzheimer’s Disease. Front. Neurol. 2020, 11, 575953. [Google Scholar] [CrossRef]
- Saez-Atienzar, S.; Masliah, E. Cellular Senescence and Alzheimer Disease: The Egg and the Chicken Scenario. Nat. Rev. Neurosci. 2020, 21, 433–444. Correction in Nat. Rev. Neurosci. 2020, 21, 587. https://doi.org/10.1038/s41583-020-0325-z. [CrossRef]
- Zou, P.; Wu, C.; Liu, T.C.Y.; Duan, R.; Yang, L. Oligodendrocyte Progenitor Cells in Alzheimer’s Disease: From Physiology to Pathology. Transl. Neurodegener. 2023, 12, 52. [Google Scholar] [CrossRef]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Faraonio, R. Oxidative Stress and Cell Senescence Process. Antioxidants 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Patil, C.K.; Campisi, J. P38MAPK Is a Novel DNA Damage Response-Independent Regulator of the Senescence-Associated Secretory Phenotype. EMBO J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef]
- Kuk, M.U.; Kim, J.W.; Lee, Y.S.; Cho, K.A.; Park, J.T.; Park, S.C. Alleviation of Senescence via ATM Inhibition in Accelerated Aging Models. Mol. Cells 2019, 42, 210–217. [Google Scholar] [CrossRef]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and Senomorphics: Natural and Synthetic Therapeutics in the Treatment of Aging and Chronic Diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Hou, Y.; Huang, S.; Pei, G. Alzheimer’s Amyloid-β Accelerates Human Neuronal Cell Senescence Which Could Be Rescued by Sirtuin-1 and Aspirin. Front. Cell. Neurosci. 2022, 16, 906270. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Marques Zilli, E.; Petersen, R.C.; Kirkland, J.L.; Tchkonia, T.; Musi, N.; Seshadri, S.; Craft, S.; Orr, M.E. Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD): A Pilot Clinical Trial. J. Prev. Alzheimer’s Dis. 2022, 9, 22–29. [Google Scholar] [CrossRef]
- Sahab Uddin, M.; Tewari, D.; Sharma, G.; Kabir, M.T.; Barreto, G.E.; Bin-Jumah, M.N.; Perveen, A.; Abdel-Daim, M.M.; Ashraf, G.M. Molecular Mechanisms of ER Stress and UPR in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 2902–2919. [Google Scholar] [CrossRef]
- Walter, N.S.; Gorki, V.; Bhardwaj, R.; Punnakkal, P. Endoplasmic Reticulum Stress: Implications in Diseases. Protein J. 2025, 44, 147–161. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K.; Ojala, J. ER Stress in Alzheimer’s Disease: A Novel Neuronal Trigger for Inflammation and Alzheimer’s Pathology. J. Neuroinflamm. 2009, 6, 41. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Lindholm, D.; Ren, J.; Pratico, D. ER Stress and UPR in Alzheimer’s Disease: Mechanisms, Pathogenesis, Treatments. Cell. Death Dis. 2022, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.; He, L. Disrupted Ubiquitin Proteasome System Underlying Tau Accumulation in Alzheimer’s Disease. Neurobiol Aging 2021, 99, 79–85. [Google Scholar] [CrossRef]
- Pohl, C.; Dikic, I. Cellular Quality Control by the Ubiquitin-Proteasome System and Autophagy. Science 2019, 366, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Fu, L. Roles of Ubiquitin Ligases and Deubiquitylases in Alzheimer’s Disease. Mol. Neurobiol. 2025, 62, 7747–7761. [Google Scholar] [CrossRef]
- Ao, C.; Li, C.; Chen, J.; Tan, J.; Zeng, L. The Role of Cdk5 in Neurological Disorders. Front. Cell. Neurosci. 2022, 16, 951202. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Cao, J.; Yao, J.; Fan, X.; Zhao, J.; Zhao, M.; Duan, Q.; Han, B.; Duan, S. KDM1A-Mediated Upregulation of METTL3 Ameliorates Alzheimer’s Disease via Enhancing Autophagic Clearance of p-Tau through M6A-Dependent Regulation of STUB1. Free Radic. Biol. Med. 2023, 195, 343–358. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.R.; Kim, J.; LaFerla, F.M.; Park, J.H.Y.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears Amyloid-β and Tau in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2018, 62, 1800240. [Google Scholar] [CrossRef]
- Cui, H.; Deng, M.; Zhang, Y.; Yin, F.; Liu, J. Geniposide Increases Unfolded Protein Response-Mediating HRD1 Expression to Accelerate APP Degradation in Primary Cortical Neurons. Neurochem. Res. 2018, 43, 669–680. [Google Scholar] [CrossRef]
- Wilcock, G.K.; Gauthier, S.; Frisoni, G.B.; Jia, J.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Schelter, B.O.; Wischik, D.J.; et al. Potential of Low Dose Leuco-Methylthioninium Bis(Hydromethanesulphonate) (LMTM) Monotherapy for Treatment of Mild Alzheimer’s Disease: Cohort Analysis as Modified Primary Outcome in a Phase III Clinical Trial. J. Alzheimer’s Dis. 2018, 61, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Mohamed, T.; Shakeri, A.; Rao, P.P.N.; Soares da Silva, P.; Remião, F.; Borges, F. Repurposing Nitrocatechols: 5-Nitro-α-Cyanocarboxamide Derivatives of Caffeic Acid and Caffeic Acid Phenethyl Ester Effectively Inhibit Aggregation of Tau-Derived Hexapeptide AcPHF6. Eur. J. Med. Chem. 2019, 167, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Viswanathan, G.K.K.; Huber, A.; Arad, E.; Engel, H.; Jelinek, R.; Gazit, E.; Segal, D. Inhibition of Tau Amyloid Formation and Disruption of Its Preformed Fibrils by Naphthoquinone–Dopamine Hybrid. FEBS J. 2021, 288, 4267–4290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, C.; Sawaya, M.R.; Vadla, B.; Khan, S.; Woods, R.J.; Eisenberg, D.; Goux, W.J.; Nowick, J.S. Macrocyclic β-Sheet Peptides That Inhibit the Aggregation of a Tau-Protein-Derived Hexapeptide. J. Am. Chem. Soc. 2011, 133, 3144–3157. Correction in J. Am. Chem. Soc. 2012, 134, 17832. https://doi.org/10.1021/ja308086h. [CrossRef]
- Wang, X.L.; Xiong, Y.; Yang, Y.; Tuo, Q.Z.; Wang, X.C.; Chen, R.; Tian, Q.; Zhang, Z.P.; Yan, X.; Yang, Z.Y.; et al. A Novel Tacrine-Dihydropyridine Hybrid (-)SCR1693 Induces Tau Dephosphorylation and Inhibits Aβ Generation in Cells. Eur. J. Pharmacol. 2015, 754, 134–139. [Google Scholar] [CrossRef]
- Bi, X.; Gall, C.M.; Zhou, J.; Lynch, G. Uptake and Pathogenic Effects of Amyloid Beta Peptide 1-42 Are Enhanced by Integrin Antagonists and Blocked by Nmda Receptor Antagonists Plaques Accompanied by Neuritic Dystrophy, Astrogliosis. Neuroscience 2002, 112, 827–840. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Neuroprotective Effect of Caffeic Acid Phenethyl Ester in a Mouse Model of Alzheimer’s Disease Involves Nrf2/HO-1 Pathway. Aging Dis. 2018, 9, 605–622. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, X.; Lu, B.; Gao, Q.; Chen, Y.; Wu, D.; Zeng, W.; Yang, L.; Li, H.; Yu, B. Roles of Gut Microbiota in Pathogenesis of Alzheimer’s Disease and Therapeutic Effects of Chinese Medicine. Chin. J. Integr. Med. 2022, 28, 1048–1056. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Mehta, K.; Govindaraju, T. Hybrid Multifunctional Modulators Inhibit Multifaceted Aβ Toxicity and Prevent Mitochondrial Damage. ACS Chem. Neurosci. 2018, 9, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Rosenbrock, H.; Giovannini, R.; Schänzle, G.; Koros, E.; Runge, F.; Fuchs, H.; Marti, A.; Reymann, K.G.; Schröder, U.H.; Fedele, E.; et al. The Novel Phosphodiesterase 9A Inhibitor BI 409306 Increases Cyclic Guanosine Monophosphate Levels in the Brain, Promotes Synaptic Plasticity, and Enhances Memory Function in Rodents. J. Pharmacol. Exp. Ther. 2019, 371, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Sanders, O.; Rajagopal, L. Phosphodiesterase Inhibitors for Alzheimer’s Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale. J. Alzheimer’s Dis. Rep. 2020, 4, 185–215. [Google Scholar] [CrossRef]
- Da Silva, P.N.; Da Conceição, R.A.; do Couto Maia, R.; De Castro Barbosa, M.L. Sodium-Glucose Cotransporter 2 (SGLT-2) Inhibitors: A New Antidiabetic Drug Class. Medchemcomm 2018, 9, 1273–1281. [Google Scholar] [CrossRef]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Tang, H.; Donahoo, W.T.; Dekosky, S.T.; Lee, Y.A.; Kotecha, P.; Svensson, M.; Bian, J.; Guo, J. GLP-1RA and SGLT2i Medications for Type 2 Diabetes and Alzheimer Disease and Related Dementias. JAMA Neurol. 2025, 82, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Zhou, D. Inhibition of P38 MAPK Attenuates Ionizing Radiation-Induced Hematopoietic Cell Senescence and Residual Bone Marrow Injury. Radiat. Res. 2011, 176, 743–752. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, L.; Dai, G.; Lu, P.; Zhang, Y.; Li, Y.; Ni, M.; Rui, Y. Inhibition of JAK-STAT Signaling Pathway Alleviates Age-Related Phenotypes in Tendon Stem/Progenitor Cells. Front. Cell. Dev. Biol. 2021, 9, 650250. [Google Scholar] [CrossRef]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK Inhibition Alleviates the Cellular Senescence-Associated Secretory Phenotype and Frailty in Old Age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 Inhibitors as a Novel Class of Senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Gulej, R.; Nyúl-Tóth, Á.; Ahire, C.; DelFavero, J.; Balasubramanian, P.; Kiss, T.; Tarantini, S.; Benyo, Z.; Pacher, P.; Csik, B.; et al. Elimination of Senescent Cells by Treatment with Navitoclax/ABT263 Reverses Whole Brain Irradiation-Induced Blood-Brain Barrier Disruption in the Mouse Brain. Geroscience 2023, 45, 2983–3002. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.; Konigsberg, M.; Silva-Palacios, A. Quercetin and Dasatinib, Two Powerful Senolytics in Age-Related Cardiovascular Disease. Biogerontology 2024, 25, 71–82. Erratum in Biogerontology 2024, 25, 747. https://doi.org/10.1007/s10522-024-10109-7. [CrossRef]
- Hu, Y.; Fryatt, G.L.; Ghorbani, M.; Obst, J.; Menassa, D.A.; Martin-Estebane, M.; Muntslag, T.A.O.; Olmos-Alonso, A.; Guerrero-Carrasco, M.; Thomas, D.; et al. Replicative Senescence Dictates the Emergence of Disease-Associated Microglia and Contributes to Aβ Pathology. Cell Rep. 2021, 35, 109228. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Evans, S.A.; Fielder, E.; Victorelli, S.; Kruger, P.; Salmonowicz, H.; Weigand, B.M.; Patel, A.D.; Pirtskhalava, T.; Inman, C.L.; et al. Whole-Body Senescent Cell Clearance Alleviates Age-Related Brain Inflammation and Cognitive Impairment in Mice. Aging Cell 2021, 20, e13296. [Google Scholar] [CrossRef] [PubMed]
- von Kobbe, C. Targeting Senescent Cells: Approaches, Opportunities, Challenges. Aging 2019, 11, 12844–12861. [Google Scholar] [CrossRef]
- Yoon, M.S. The Role of Mammalian Target of Rapamycin (MTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Chen, J.; Long, Z.; Li, Y.; Luo, M.; Luo, S.; He, G. Alteration of the Wnt/GSK3β/Β-catenin Signalling Pathway by Rapamycin Ameliorates Pathology in an Alzheimer’s Disease Model. Int. J. Mol. Med. 2019, 44, 313–323. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Wang, J.; Sun, S.; Wang, T.; Qiao, X.; Run, X.; Li, H.; Liang, Z. Rapamycin Decreases Tau Phosphorylation at Ser214 Through Regulation of CAMP-Dependent Kinase. Neurochem. Int. 2013, 62, 458–467. [Google Scholar] [CrossRef]
- Hou, S.J.; Zhang, S.X.; Li, Y.; Xu, S.Y. Rapamycin Responds to Alzheimer’s Disease: A Potential Translational Therapy. Clin. Interv. Aging 2023, 18, 1629–1639. [Google Scholar] [CrossRef]
- Spilman, P.; Podlutskaya, N.; Hart, M.J.; Debnath, J.; Gorostiza, O.; Bredesen, D.; Richardson, A.; Strong, R.; Galvan, V. Inhibition of MTOR by Rapamycin Abolishes Cognitive Deficits and Reduces Amyloid-β Levels in a Mouse Model of Alzheimer’s Disease. PLoS ONE 2010, 5, e9979. Erratum in PLoS ONE 2011, 6. https://doi.org/10.1371/annotation/05c1b976-7eab-4154-808d-0526e604b8eb. [CrossRef] [PubMed]
- Shi, Q.; Chang, C.; Saliba, A.; Bhat, M.A. Microglial MTOR Activation Upregulates Trem2 and Enhances B-Amyloid Plaque Clearance in the 5XFAD Alzheimer’s Disease Model. J. Neurosci. 2022, 42, 5294–5313. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin Inhibits the Inflammatory Response Associated with Cellular Transformation and Cancer Stem Cell Growth. Proc. Natl. Acad. Sci. USA 2013, 110, 972–977. [Google Scholar] [CrossRef]
- Ou, Z.; Kong, X.; Sun, X.; He, X.; Zhang, L.; Gong, Z.; Huang, J.; Xu, B.; Long, D.; Li, J.; et al. Metformin Treatment Prevents Amyloid Plaque Deposition and Memory Impairment in APP/PS1 Mice. Brain Behav. Immun. 2018, 69, 351–363. [Google Scholar] [CrossRef]
- Liu, J.; Xu, M.; Ni, B.; Zhang, Z.; Gao, X.; Zhang, D.; Yang, L.; Ye, Z.; Wen, J.; Liu, P. Metformin Therapeutic Targets for Aortic Aneurysms: A Mendelian Randomization and Colocalization Study. Rev. Cardiovasc. Med. 2024, 25, 89. [Google Scholar] [CrossRef]
- Sluggett, J.K.; Koponen, M.; Simon Bell, J.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Uusitupa, M.; Tolppanen, A.M.; Hartikainen, S. Metformin and Risk of Alzheimer’s Disease among Community-Dwelling People with Diabetes: A National Case-Control Study. J. Clin. Endocrinol. Metab. 2020, 105, E963–E972. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Huang, S.; Chen, Q.B.; Zhang, D.; Li, W.; Ao, R.; Leung, F.C.Y.; Zhang, Z.; Huang, J.; Tang, Y.; et al. Metformin Ameliorates A β Pathology by Insulin-Degrading Enzyme in a Transgenic Mouse Model of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2020, 2020, 2315106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Reddy, P.H. MicroRNA-455-3p as a Potential Biomarker for Alzheimer’s Disease: An Update. Front. Aging Neurosci. 2018, 10, 41. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Lu, A.; Han, Y.; Colangelo, D.; Bukata, C.; Scibetta, A.; Yousefzadeh, M.J.; Li, X.; Gurkar, A.U.; et al. ATM Is a Key Driver of NF-ΚB-Dependent DNA-Damage-Induced Senescence, Stem Cell Dysfunction and Aging Correspondence to. Aging 2020, 12, 4688. [Google Scholar] [CrossRef]
- Staderini, M.; Aulić, S.; Bartolini, M.; Tran, H.N.A.; González-Ruiz, V.; Pérez, D.I.; Cabezas, N.; Martínez, A.; Martín, M.A.; Andrisano, V.; et al. A Fluorescent Styrylquinoline with Combined Therapeutic and Diagnostic Activities against Alzheimer’s and Prion Diseases. ACS Med. Chem. Lett. 2013, 4, 225–229. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Gandini, A.; Prati, F.; Uliassi, E. From Companion Diagnostics to Theranostics: A New Avenue for Alzheimer’s Disease? J. Med. Chem. 2016, 59, 7759–7770. [Google Scholar] [CrossRef]
- Gong, C.; Li, X.; Xu, L.; Zhang, Y.H. Target Delivery of a Gene into the Brain Using the RVG29-Oligoarginine Peptide. Biomaterials 2012, 33, 3456–3463. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Rice, K.G. Peptide-Guided Gene Delivery. AAPS J. 2007, 9, 3. [Google Scholar] [CrossRef]
- Seow, W.Y.; Yang, Y.Y.; George, A.J.T. Oligopeptide-Mediated Gene Transfer into Mouse Corneal Endothelial Cells: Expression, Design Optimization, Uptake Mechanism and Nuclear Localization. Nucleic Acids Res. 2009, 37, 6276–6289. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. The Effect of the Human Peptide GHK on Gene Expression Relevant to Nervous System Function and Cognitive Decline. Brain Sci. 2017, 7, 20. [Google Scholar] [CrossRef]
- Bosco, P.; Ferri, R.; Grazia Salluzzo, M.; Castellano, S.; Signorelli, M.; Nicoletti, F.; Di Nuovo, S.; Drago, F.; Caraci, F. Role of the Transforming-Growth-Factor-Β1 Gene in Late-Onset Alzheimer’s Disease: Implications for the Treatment. Curr. Genom. 2013, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Ishimura, M.; Yuba, Y.; Itoh, Y.; Fujimoto, Y. The Peptide Glycyl-ʟ-Histidyl-ʟ-Lysine Is an Endogenous Antioxidant in Living Organisms, Possibly by Diminishing Hydroxyl and Peroxyl Radicals. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 132–138. [Google Scholar]
- Rosenfeld, M.; Nickel, K.; Ladiges, W. GHK Peptide Prevents Sleep-Deprived Learning Impairment in Aging Mice. Aging Pathobiol. Ther. 2023, 5, 33–35. [Google Scholar] [CrossRef]
- Tucker, M.; Liao, G.Y.; Keely, A.; Park, J.Y.; Rosenfeld, M.; Wezeman, J.; Mangalindan, R.; Ratner, D.; Darvas, M.; Ladiges, W. Behavioral and Neuropathological Features of Alzheimer’s Disease Are Attenuated in 5xFAD Mice Treated with Intranasal GHK Peptide. bioRxiv 2023, 4, 567908. [Google Scholar] [CrossRef]
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. N-Acetyl-l-Cysteine Attenuates Oxidative Damage and Neurodegeneration in Rat Brain during Aging. Can. J. Physiol. Pharmacol. 2018, 96, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Sievers, S.A.; Karanicolas, J.; Chang, H.W.; Zhao, A.; Jiang, L.; Zirafi, O.; Stevens, J.T.; Münch, J.; Baker, D.; Eisenberg, D. Structure-Based Design of Non-Natural Amino-Acid Inhibitors of Amyloid Fibril Formation. Nature 2011, 475, 96–100. [Google Scholar] [CrossRef]
- Chu, T.T.; Gao, N.; Li, Q.Q.; Chen, P.G.; Yang, X.F.; Chen, Y.X.; Zhao, Y.F.; Li, Y.M. Specific Knockdown of Endogenous Tau Protein by Peptide-Directed Ubiquitin-Proteasome Degradation. Cell Chem. Biol. 2016, 23, 453–461. [Google Scholar] [CrossRef]
- Soto, C.; Sigurdsson, E.M.; Morelli, L.; Kumar, R.A.; Castaño, E.M.; Frangione, B. Beta-Sheet Breaker Peptides Inhibit Fibrillogenesis in a Rat Brain Model of Amyloidosis: Implications for Alzheimer’s Therapy. Nat. Med. 1998, 4, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, S.; Tonali, N.; Erba, E.; Kaffy, J.; Taverna, M.; Contini, A.; Taylor, M.; Allsop, D.; Gelmi, M.L.; Ongeri, S. β-Hairpin Mimics Containing a Piperidine-Pyrrolidine Scaffold Modulate the β-Amyloid Aggregation Process Preserving the Monomer Species. Chem. Sci. 2017, 8, 1295–1302. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two Phase 3 Trials of Bapineuzumab in Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P.; et al. A Phase III Randomized Trial of Gantenerumab in Prodromal Alzheimer’s Disease. Alzheimer’s Res. Ther. 2017, 9, 95, Erratum in Alzheimer’s Res. Ther. 2017, 10, 99. [Google Scholar] [CrossRef]
- Guthrie, H.; Honig, L.S.; Lin, H.; Sink, K.M.; Blondeau, K.; Quartino, A.; Dolton, M.; Carrasco-Triguero, M.; Lian, Q.; Bittner, T.; et al. Safety, Tolerability, and Pharmacokinetics of Crenezumab in Patients with Mild-to-Moderate Alzheimer’s Disease Treated with Escalating Doses for up to 133 Weeks. J. Alzheimer’s Dis. 2020, 76, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-Amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Kontsekova, E.; Zilka, N.; Kovacech, B.; Novak, P.; Novak, M. First-in-Man Tau Vaccine Targeting Structural Determinants Essential for Pathological Tau-Tau Interaction Reduces Tau Oligomerisation and Neurofibrillary Degeneration in an Alzheimer’s Disease Model. Alzheimer’s Res. Ther. 2014, 6, 44. [Google Scholar] [CrossRef]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N.; et al. ADAMANT: A Placebo-Controlled Randomized Phase 2 Study of AADvac1, an Active Immunotherapy Against Pathological Tau in Alzheimer’s Disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, R.; Cheng, C.; Li, S.; Le, W. New Therapeutics Beyond Amyloid-β and Tau for the Treatment of Alzheimer’s Disease. Acta Pharmacol. Sin. 2021, 42, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Boxer, A.L.; Qureshi, I.; Ahlijanian, M.; Grundman, M.; Golbe, L.I.; Litvan, I.; Honig, L.S.; Tuite, P.; McFarland, N.R.; O’Suilleabhain, P.; et al. Safety of the Tau-Directed Monoclonal Antibody BIIB092 in Progressive Supranuclear Palsy: A Randomised, Placebo-Controlled, Multiple Ascending Dose Phase 1b Trial. Lancet Neurol. 2019, 18, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Eguchi, H.; Kunori, Y.; Matsumoto, Y.; Taniguchi, T.; Mori, H.; Tomiyama, T. Passive Immunotherapy of Tauopathy Targeting PSer413-Tau: A Pilot Study in Mice. Ann. Clin. Transl. Neurol. 2015, 2, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tung, Y.C.; Liu, F.; Gong, C.X.; Iqbal, K. Tau Passive Immunization Inhibits Not Only Tau but Also Aβ Pathology. Alzheimer’s Res. Ther. 2017, 9, 1. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.W.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-Induced Senescence Relayed by an Interleukin-Dependent Inflammatory Network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of Antioxidant Supplement Use and Dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol. 2017, 74, 567–573. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary Flavonols and Risk of Alzheimer Dementia. Neurology 2020, 94, E1749–E1756. [Google Scholar] [CrossRef]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.K.; Garg, G.; Rizvi, S.I. Fisetin as a Caloric Restriction Mimetic Protects Rat Brain against Aging Induced Oxidative Stress, Apoptosis and Neurodegeneration. Life Sci. 2018, 193, 171–179. [Google Scholar] [CrossRef]
- Endres, K.; Fahrenholz, F.; Lotz, J.; Hiemke, C.; Teipel, S.; Lieb, K.; Tüscher, O.; Fellgiebel, A. Increased CSF APPs-a Levels in Patients with Alzheimer Disease Treated with Acitretin. Neurology 2014, 83, 1930–1935. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sodium Oligomannate: First Approval. Drugs 2020, 80, 441–444. Correction in Drugs 2020, 80, 445–446. https://doi.org/10.1007/s40265-020-01274-3. [CrossRef]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Cantus, D.S.; Rochina, M.J.; Aguilar Calpe, M.A.; Yang, I.H. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease After Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimer’s Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Fujita, Y.; Hijikuro, I.; Wada, M.; Uemura, T.; Kobayashi, Y.; Waku, T.; Tanaka, N.; Nishimoto, T.; Izumi, Y.; et al. PE859, A Novel Curcumin Derivative, Inhibits Amyloid-β and Tau Aggregation, and Ameliorates Cognitive Dysfunction in Senescence-Accelerated Mouse Prone 8. J. Alzheimer’s Dis. 2017, 59, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, E.; et al. Treatment of Dementia and Mild Cognitive Impairment with or Without Cerebrovascular Disease: Expert Consensus on the Use of Ginkgo Biloba Extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.C.; Song, Y.Q.; Yao, C.A.; Cheng, I.H.; Chien, C.T.; Lee, G.C.; Yang, W.C.; Lin, Y. Ginkgolide A Prevents the Amyloid-β-Induced Depolarization of Cortical Neurons. J. Agric. Food Chem. 2019, 67, 81–89. [Google Scholar] [CrossRef]
- Plazas, E.; Hagenow, S.; Murillo, M.A.; Stark, H.; Cuca, L.E. Isoquinoline Alkaloids from the Roots of Zanthoxylum Rigidum as Multi-Target Inhibitors of Cholinesterase, Monoamine Oxidase A and Aβ1-42 Aggregation. Bioorg. Chem. 2020, 98, 103722. [Google Scholar] [CrossRef] [PubMed]
- Augustin, N.; Nuthakki, V.K.; Abdullaha, M.; Hassan, Q.P.; Gandhi, S.G.; Bharate, S.B. Discovery of Helminthosporin, an Anthraquinone Isolated from Rumex Abyssinicus Jacq as a Dual Cholinesterase Inhibitor. ACS Omega 2020, 5, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Govindaraju, T. Multipronged Diagnostic and Therapeutic Strategies for Alzheimer’s Disease. Chem. Sci. 2022, 13, 13657–13689. [Google Scholar] [CrossRef]
- Lee, S.; Zheng, X.; Krishnamoorthy, J.; Savelieff, M.G.; Park, H.M.; Brender, J.R.; Kim, J.H.; Derrick, J.S.; Kochi, A.; Lee, H.J.; et al. Rational Design of a Structural Framework with Potential Use to Develop Chemical Reagents That Target and Modulate Multiple Facets of Alzheimer’s Disease. JACS 2014, 136, 299–310. [Google Scholar] [CrossRef]
- Derrick, J.S.; Kerr, R.A.; Nam, Y.; Oh, S.B.; Lee, H.J.; Earnest, K.G.; Suh, N.; Peck, K.L.; Ozbil, M.; Korshavn, K.J.; et al. A Redox-Active, Compact Molecule for Cross-Linking Amyloidogenic Peptides into Nontoxic, off-Pathway Aggregates: In Vitro and In Vivo Efficacy and Molecular Mechanisms. J. Am. Chem. Soc. 2015, 137, 14785–14797. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Samanta, S.; Bagoband, V.; Murugan, N.A.; Govindaraju, T. Antioxidant Berberine-Derivative Inhibits Multifaceted Amyloid Toxicity. iScience 2020, 23, 101005. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cho, H.J.; Sen, S.; Arango, A.S.; Huynh, T.T.; Huang, Y.; Bandara, N.; Rogers, B.E.; Tajkhorshid, E.; Mirica, L.M. Amphiphilic Distyrylbenzene Derivatives as Potential Therapeutic and Imaging Agents for Soluble and Insoluble Amyloid β Aggregates in Alzheimer’s Disease. J. Am. Chem. Soc. 2021, 143, 10462–10476. [Google Scholar] [CrossRef]
- Naiki, H.; Higuchi, K.; Matsushima, K.; Shimada, A.; Chen, W.H.; Hosokawa, M.; Takeda, T. Methods in Laboratory Investigation. Fluorometric Examination of Tissue Amyloid Fibrils in Murine Senile Amyloidosis: Use of the Fluorescent Indicator, Thioflavine, T. Lab. Investig. 1990, 62, 768–773. [Google Scholar]
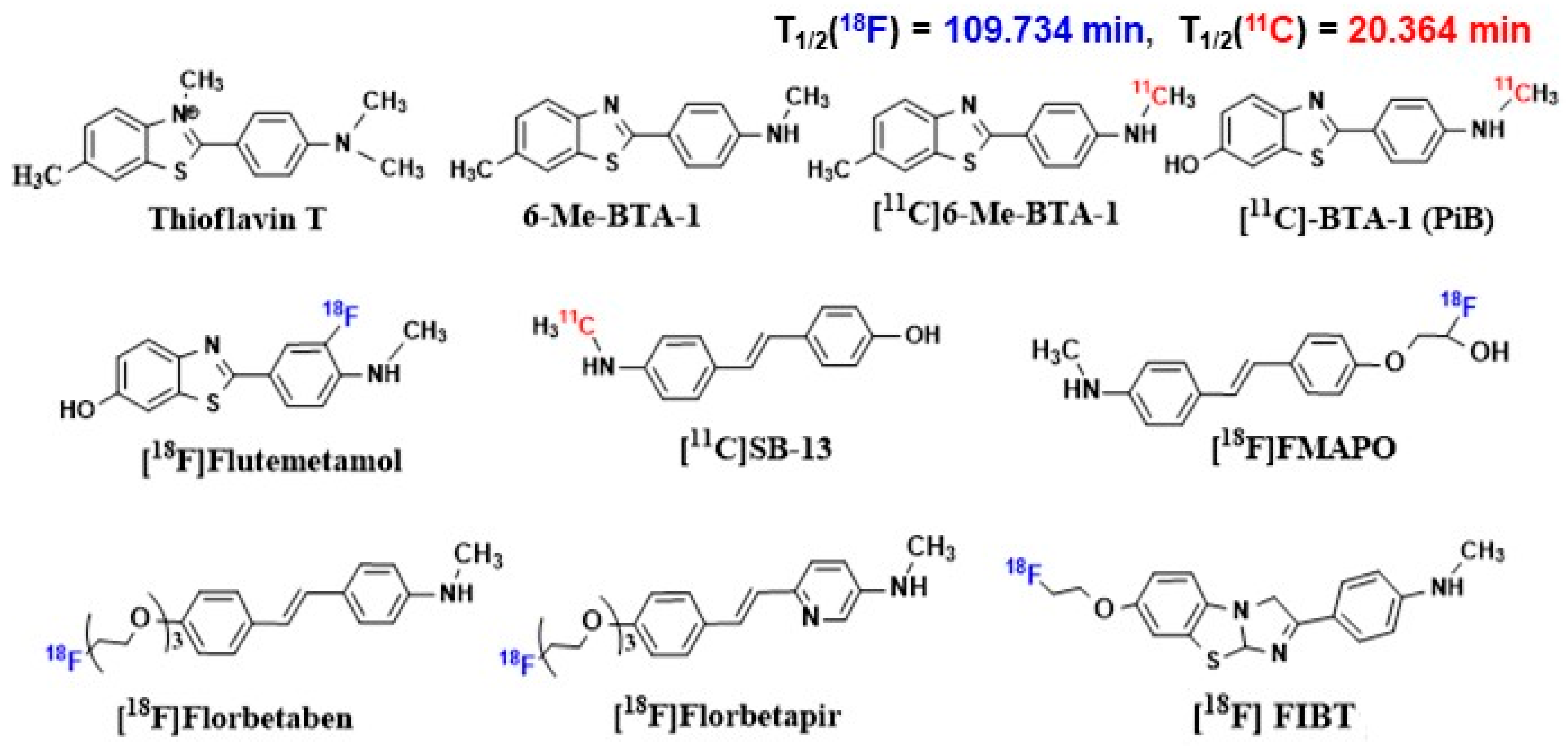
- Klunk, W.E.; Wang, Y.; Huang, G.; Debnath, M.L.; Holt, D.P.; Mathis, C.A. Uncharged Thioflavin-T Derivatives Bind to Amyloid-Beta Protein with High Affinity and Readily Enter the Brain. Life Sci. 2001, 69, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Wang, Y.; Huang, G.; Debnath, M.L.; Holt, D.P.; Shao, L.; Hamilton, R.L.; Ikonomovic, M.D.; DeKosky, S.T.; Mathis, C.A. The Binding of 2-(4′-Methylaminophenyl)Benzothiazole to Postmortem Brain Homogenates Is Dominated by the Amyloid Component. J. Neurosci. 2003, 23, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Mathis, C.A.; Bacskai, B.J.; Kajdasz, S.T.; McLellan, M.E.; Frosch, M.P.; Hyman, B.T.; Holt, D.P.; Wang, Y.; Huang, G.F.; Debnath, M.L.; et al. A Lipophilic Thioflavin-T Derivative for Positron Emission Tomography (PET) Imaging of Amyloid in Brain. Bioorg. Med. Chem. Lett. 2002, 12, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Klunk, W.E.; Abrahamson, E.E.; Mathis, C.A.; Price, J.C.; Tsopelas, N.D.; Lopresti, B.J.; Ziolko, S.; Bi, W.; Paljug, W.R.; et al. Post-Mortem Correlates of in Vivo PiB-PET Amyloid Imaging in a Typical Case of Alzheimer’s Disease. Brain 2008, 131, 1630–1645. [Google Scholar] [CrossRef]
- Juréus, A.; Swahn, B.M.; Sandell, J.; Jeppsson, F.; Johnson, A.E.; Johnström, P.; Neelissen, J.A.M.; Sunnemark, D.; Farde, L.; Svensson, S.P.S. Characterization of AZD4694, a Novel Fluorinated Aβ Plaque Neuroimaging PET Radioligand. J. Neurochem. 2010, 114, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.S.; Mathis, C.A.; Klunk, W.E. Positron Emission Tomography Radioligands for in Vivo Imaging of Aβ Plaques. J. Labelled Comp. Radiopharm. 2013, 56, 89–95. [Google Scholar] [CrossRef]
- Snellman, A.; Rokka, J.; Lopez-Picon, F.R.; Eskola, O.; Wilson, I.; Farrar, G.; Scheinin, M.; Solin, O.; Rinne, J.O.; Haaparanta-Solin, M. Pharmacokinetics of [18F]Flutemetamol in Wild-Type Rodents and Its Binding to Beta Amyloid Deposits in a Mouse Model of Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Jia, H.; Finnema, S.; Cai, Z.; Carson, R.E.; Huang, Y.H. PET Imaging for Early Detection of Alzheimer’s Disease: From Pathologic to Physiologic Biomarkers. PET Clin. 2017, 12, 329–350. [Google Scholar] [CrossRef]
- Zhang, W.; Oya, S.; Kung, M.P.; Hou, C.; Maier, D.L.; Kung, H.F. F-18 Stilbenes as PET Imaging Agents for Detecting β-Amyloid Plaques in the Brain. J. Med. Chem. 2005, 48, 5980–5988. [Google Scholar] [CrossRef]
- Stephenson, K.A.; Chandra, R.; Zhuang, Z.P.; Hou, C.; Oya, S.; Kung, M.P.; Kung, H.F. Fluoro-Pegylated (FPEG) Imaging Agents Targeting Aβ Aggregates. Bioconjug. Chem. 2007, 18, 238–246. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Oya, S.; Kung, M.P.; Hou, C.; Maier, D.L.; Kung, H.F. F-18 Polyethyleneglycol Stilbenes as PET Imaging Agents Targeting Aβ Aggregates in the Brain. Nucl. Med. Biol. 2005, 32, 799–809. [Google Scholar] [CrossRef]
- Kung, H.F.; Choi, S.R.; Qu, W.; Zhang, W.; Skovronsky, D. 18F Stilbenes and Styrylpyridines for PET Imaging of Aβ Plaques in Alzheimer’s Disease: A Miniperspective. J. Med. Chem. 2010, 53, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.C.; Ackerman, U.; Browne, W.; Mulligan, R.; Pike, K.L.; O’Keefe, G.; Tochon-Danguy, H.; Chan, G.; Berlangieri, S.U.; Jones, G.; et al. Imaging of Amyloid β in Alzheimer’s Disease with 18F-BAY94-9172, a Novel PET Tracer: Proof of Mechanism. Lancet Neurol. 2008, 7, 129–135. [Google Scholar] [CrossRef]
- Choi, S.R.; Golding, G.; Zhuang, Z.; Zhang, W.; Lim, N.; Hefti, F.; Benedum, T.E.; Kilbourn, M.R.; Skovronsky, D.; Kung, H.F. Preclinical Properties of 18F-AV-45: A PET Agent for Aβ Plaques in the Brain. J. Nucl. Med. 2009, 50, 1887–1894. [Google Scholar] [CrossRef]
- Yousefi, B.H.; Drzezga, A.; Von Reutern, B.; Manook, A.; Schwaiger, M.; Wester, H.J.; Henriksen, G. A Novel 18F-Labeled Imidazo[2,1-b]Benzothiazole (IBT) for High-Contrast PET Imaging of β-Amyloid Plaques. ACS Med. Chem. Lett. 2011, 2, 673–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yousefi, B.H.; von Reutern, B.; Scherübl, D.; Manook, A.; Schwaiger, M.; Grimmer, T.; Henriksen, G.; Förster, S.; Drzezga, A.; Wester, H.J. FIBT versus Florbetaben and PiB: A Preclinical Comparison Study with Amyloid-PET in Transgenic Mice. EJNMMI Res. 2015, 5, 20. [Google Scholar] [CrossRef]
- van Oostveen, W.M.; de Lange, E.C.M. Imaging Techniques in Alzheimer’s Disease: A Review of Applications in Early Diagnosis and Longitudinal Monitoring. Int. J. Mol. Sci. 2021, 22, 2110. [Google Scholar] [CrossRef]
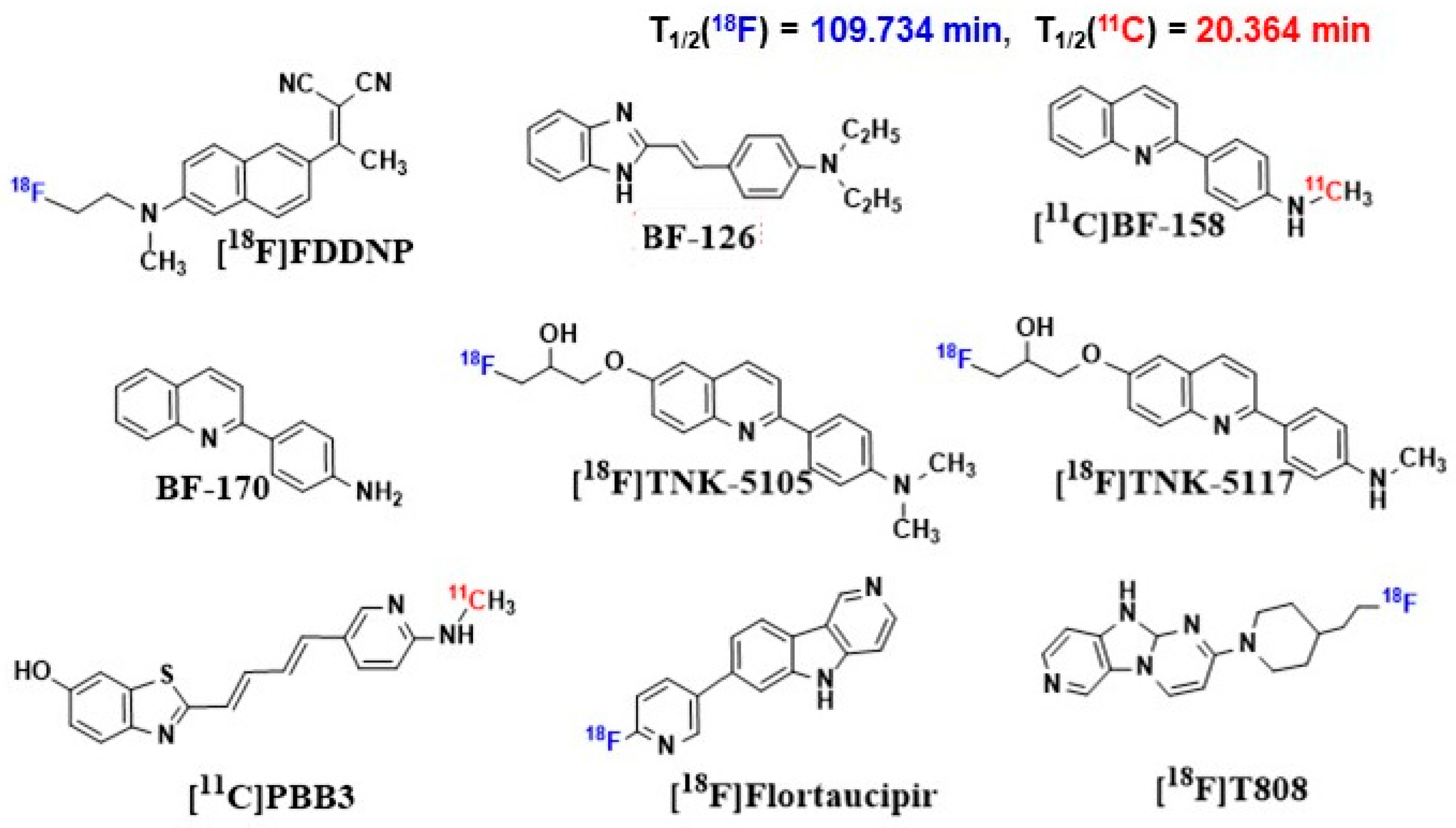
- Okamura, N.; Suemoto, T.; Furumoto, S.; Suzuki, M.; Shimadzu, H.; Akatsu, H.; Yamamoto, T.; Fujiwara, H.; Nemoto, M.; Maruyama, M.; et al. Quinoline and Benzimidazole Derivatives: Candidate Probes for In Vivo Imaging of Tau Pathology in Alzheimer’s Disease. J. Neurosci. 2005, 25, 10857–10862. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Furumoto, S.; Harada, R.; Tago, T.; Yoshikawa, T.; Fodero-Tavoletti, M.; Mulligan, R.S.; Villemagne, V.L.; Akatsu, H.; Yamamoto, T.; et al. Novel 18F-Labeled Arylquinoline Derivatives for Noninvasive Imaging of Tau Pathology in Alzheimer Disease. J. Nucl. Med. 2013, 54, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Furumoto, S.; Fodero-Tavoletti, M.T.; Mulligan, R.S.; Harada, R.; Yates, P.; Pejoska, S.; Kudo, Y.; Masters, C.L.; Yanai, K.; et al. Non-Invasive Assessment of Alzheimer’s Disease Neurofibrillary Pathology Using 18F-THK5105 PET. Brain 2014, 137, 1762–1771. [Google Scholar] [CrossRef]
- Harada, R.; Okamura, N.; Furumoto, S.; Furukawa, K.; Ishiki, A.; Tomita, N.; Hiraoka, K.; Watanuki, S.; Shidahara, M.; Miyake, M.; et al. [18F]THK-5117 PET for Assessing Neurofibrillary Pathology in Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1052–1061. [Google Scholar] [CrossRef]
- Maruyama, M.; Shimada, H.; Suhara, T.; Shinotoh, H.; Ji, B.; Maeda, J.; Zhang, M.R.; Trojanowski, J.Q.; Lee, V.M.Y.; Ono, M.; et al. Imaging of Tau Pathology in a Tauopathy Mouse Model and in Alzheimer Patients Compared to Normal Controls. Neuron 2013, 79, 1094–1108. [Google Scholar] [CrossRef]
- Kimura, Y.; Ichise, M.; Ito, H.; Shimada, H.; Ikoma, Y.; Seki, C.; Takano, H.; Kitamura, S.; Shinotoh, H.; Kawamura, K.; et al. PET Quantification of Tau Pathology in Human Brain with 11C-PBB3. J. Nucl. Med. 2015, 56, 1359–1365. [Google Scholar] [CrossRef]
- Wooten, D.W.; Guehl, N.J.; Verwer, E.E.; Shoup, T.M.; Yokell, D.L.; Zubcevik, N.; Vasdev, N.; Zafonte, R.D.; Johnson, K.A.; El Fakhri, G.; et al. Pharmacokinetic Evaluation of the Tau PET Radiotracer 18F-T807 (18F-AV-1451) in Human Subjects. J. Nucl. Med. 2017, 58, 484–491. [Google Scholar] [CrossRef]
- Chien, D.T.; Szardenings, A.K.; Bahri, S.; Walsh, J.C.; Mu, F.; Xia, C.; Shankle, W.R.; Lerner, A.J.; Su, M.Y.; Elizarov, A.; et al. Early Clinical PET Imaging Results with the Novel PHF-Tau Radioligand [F18]-T808. J. Alzheimer’s Dis. 2014, 38, 171–184. [Google Scholar] [CrossRef] [PubMed]
| Hypothesis | Therapeutic Promise | Challenges and Limitations | Clinical Validation Status | Diagnostic Utility |
|---|---|---|---|---|
| Cholinergic | - rapid symptomatic effect; - well-known and studied target; - several approved drugs; | - does not modify disease course, only symptoms; - effect is temporary and moderate; - side effects; | Very High. AChE inhibitors (donepezil, rivastigmine) are the standard of symptomatic care for decades | Low; Not used for diagnosis. |
| Amyloid (Aβ) | - directly targets a key pathological substrate; - multiple therapeutic approaches (antibodies, BACE inhibitors); | - weak correlation with cognitive decline at late stages; - serious side effects (ARIA); - modest clinical effect; | High. Several drugs approved (aducanumab, lecanemab, donanemab); effect proven but modest; | Very High; Aβ PET imaging and CSF/blood Aβ42/40 ratio is standard diagnostic criteria. |
| Tau pathology | - strongest correlation with cognitive decline and brain atrophy; - more “downstream” process closer to neurodegeneration; | - intracellular pathology makes drug access difficult; - risk of disrupting tau’s normal functions; | Medium/Growing. Many anti-tau antibodies and other approaches in early/mid-stage clinical trials; no approved drugs yet; | Very High. Tau PET imaging and plasma p-tau (p-tau181, p-tau217) are revolutionary biomarkers for diagnosis and staging. |
| Neuroinflammation | - early event in pathogenesis; - validated by human genetics (TREM2, CD33); - potential to boost brain’s “cleanup” system; | - dual role of microglia (protective/destructive); - risk of suppressing necessary immune function; - lack of target specificity; | Low/Medium. Therapies (CD33 antagonists, TREM2 agonists) mostly in early (phase I/II) trials; data is preliminary; | Medium. PET ligands for activated microglia exist but are less specific and not routine. |
| Oxidative stress | - a common node for many pathological processes; - wide range of potential antioxidants; | - process is highly non-specific; - most clinical trials of antioxidants failed; - challenge of brain delivery; | Low. Failures in major clinical trials; new formulations and approaches are in very early stages. | Low; Not used for diagnosis. |
| Metal ion hypothesis | - unique approach targeting both Aβ aggregation and oxidative stress; - potential for drug repurposing (chelators); | - risk of systemic side effects from disrupting metal homeostasis; - poor BBB permeability for chelators; | Low. A few small trials of chelators showed mixed results; no approved therapies. | Low; Not used for diagnosis. |
| Glutamate excitotoxicity | - explains rapid neuronal loss; - approved drug (memantine) with moderate efficacy; | - memantine provides only symptomatic relief and is for moderate-to-severe stages; - difficulty targeting without disrupting physiological signaling; | Medium. Memantine is approved for moderate-to-severe AD; newer receptor-targeting drugs have not succeeded. | Low; Not used for diagnosis. |
| Microbiota–gut–brain axis | - non-invasive intervention via probiotics/prebiotics/diet; - potential for very early prevention; | - extremely difficult to establish causality in humans; - mechanisms of interaction a poorly understood; - high individual variability of microbiome; | Very Low. Mostly preclinical data; clinical trials of probiotics show mixed results. | Very Low; Not used for diagnosis. |
| Abnormal autophagy | - fundamental process: targeting it could clear all types of toxic aggregates (Aβ, p-tau); - very high potential; | - extreme difficulty in targeting brain autophagy specifically without severe systemic side effects; - lack of specific and safe activators; | Low. Preclinical stages. Existing drugs (Rapamycin) are unsuitable due to side effects; search for safe activators is ongoing; | Low; Not used for diagnosis. |
| Cellular senescence | - novel and promising direction; - potential to use senolytics (Dasatinib + Quercetin) to clear senescent cells; | - no direct proof of causal role in human AD; - risk of off-target effects and unpredictable long-term consequences of senolytic therapy; | Very Low. First pilot studies in humans just beginning; for AD, only preclinical data exists; | Low; Not used for diagnosis. |
| Endoplasmic reticulum stress | - a key player in proteostasis failure; - potential for drug repurposing (e.g., TUDCA); | - similar challenge as autophagy: hard to target without disrupting vital UPR functions in other organs; | Low. Preclinical stages. Some studies of TUDCA in other neurodegenerative diseases (e.g., ALS), but not in AD; | Low; Not used for diagnosis. |
| Ubiquitin- proteasome system | - fundamental cellular “quality control” system; - high specificity of potential targets (individual E3 ligases, DUBs); | - high difficulty in developing drugs for specific E3s/DUBs; - risk of globally disrupting UPS with fatal cellular consequences; | Very Low. Purely fundamental and preclinical research; no approaches near clinical trials; | Low; Not used for diagnosis. |
| Class of Agent | Examples | Mechanism of Action/Target | Status | Key Limitations |
|---|---|---|---|---|
| Small Molecules | Methylene Blue (MB), LMTM | reduces tau protein aggregation | clinical trials | mixed efficacy in trials; delivery issues persist. |
| Nitrocatechol derivatives, 5-nitro-α-cyanocarbonamide derivatives | modulate tau protein aggregation | preclinical studies | good anti-aggregation activity in preclinical models (caffeic acid derivatives) | |
| NQ-DA (naphthoquinone-dopamine hybrid) | targets PHF motifs; inhibits tau aggregation | preclinical studies | effective tau aggregation inhibitor | |
| LDN193594 | inhibits kinases CDK5 and GSK3β | preclinical studies (in vivo) | reduced tau pathology, improved cognition (rodent models). | |
| SCR1693 (tacrine-based) | promotes tau dephosphorylation; reduces Aβ production | preclinical studies | dual action: reduces Aβ and promotes tau dephosphorylation. | |
| (R)-[11C]PK11195 | binds TSPO for neuroinflammation imaging | approved (diagnostic) | FDA-approved for neuroinflammation PET imaging | |
| D-APV (D-AP5) | NMDA receptor blocker | preclinical studies (ex vivo) | blocks Aβ uptake and neuroinflammation (ex vivo) | |
| CAPE, TGC86 | antioxidant; modulates Aβ aggregation | preclinical studies | efficacy in mice; suppresses amyloid and mitochondrial damage | |
| PF-04447943 | PDE9 inhibitor; increases cGMP signaling | clinical trials | enhances synaptic plasticity and improves memory in preclinical models; prevents dendritic spine loss in Tg2576 mice | |
| Gliflozins | SGLT2 inhibitor; improves cerebral metabolism, reduces neuroinflammation | clinical trials (repurposing/ phase II) | neuroprotective effects; associated with lower dementia risk and slower cognitive decline in clinical studies | |
| LDN-57444 | inhibitor of ubiquitin-specific protease USP1 | preclinical studies | investigated in oncology; modulates response to DNA damage; neuroprotective potential in AD requires separate study | |
| HBX 41,108 | inhibitor of ubiquitin-specific protease USP7 | preclinical studies | modulates the stability of key proteins; efficacy and safety in AD have not been studied | |
| Navitoclax (ABT-263) | Bcl-2/BCL-XL inhibitor (BH3 mimetic) | clinical trials (phase II) | first-generation senolytic; shows efficacy but causes thrombocytopenia | |
| ABT-737 | Bcl-2/BCL-XL inhibitor (BH3 mimetic) | preclinical studies | prototypical BH3 mimetic; research tool for studying senescence | |
| A-1331852 | selective BCL-XL inhibitor | preclinical studies | more selective for BCL-XL; potentially better safety profile | |
| A-1155463 | selective BCL-XL inhibitor | preclinical studies | highly selective BCL-XL inhibitor; reduces senescent cell burden | |
| Dasatinib + Quercetin | senolytic combination (kinase inhibition + flavonoid) | clinical trials (phase I) | reduces senescent cell burden in patients with diabetic kidney disease | |
| 17-DMAG (Alvespimycin) | HSP90 inhibitor | preclinical studies | reduces senescent cell load in animal models of aging and disease | |
| PIK3R3 inhibitors | p53/p21 signaling pathway inhibitor | preclinical studies | emerging target for s elective senolysis | |
| TRIAP1 inhibitors | p53/p21 signaling pathway inhibitor | preclinical studies | novel approach to target senescent cells | |
| Bocodepsin (OKI-179) | HDAC inhibitor | preclinical studies | epigenetic modulator of senescence; shows potential in cancer models | |
| SB203580 | p38MAPK inhibitor | preclinical studies | reduces SASP production and senescent cell viability | |
| UR13756 | p38MAPK inhibitor | preclinical studies | attenuates senescence- associated inflammation | |
| BIRB796 | p38MAPK inhibitor | preclinical studies | potent p38 inhibitor with senomorphic activity | |
| AG490 | JAK/STAT inhibitor | preclinical studies | reduces inflammatory SASP components | |
| Momelotinib | JAK/STAT inhibitor | preclinical studies | suppresses senescence-associated inflammation | |
| INCB18424 | JAK/STAT inhibitor | preclinical studies | attenuates SASP and chronic inflammation | |
| GW2580 | CSF1R inhibitor (modulates microglia) | preclinical studies | alleviated Aβ accumulation as well as neuritic and synaptic damage by targeting microglia | |
| Genetic clearance (p16-3MR model) | Inducible elimination of p16+ senescent cells | preclinical studies | treatment with AP20187 improved cognitive function, demonstrating proof-of-concept for whole-body senescent cell clearance | |
| Rapamycin | mTOR inhibitor | clinical trials (phase II) | extends health span, reduces SASP, improves function in aged models | |
| Rapalogs (e.g., Everolimus) | mTOR inhibitor | clinical trials | show potential in targeting age-related pathologies | |
| Torin 1 | mTOR inhibitor | preclinical studies | second-generation mTOR inhibitor; potent senomorphic effects | |
| NVP-BEZ235 | PI3K/mTOR inhibitor | preclinical studies | dual inhibitor with potential senomorphic activity | |
| KU-60019 | ATM kinase inhibitor | preclinical studies | modulates DNA damage response to suppress senescence | |
| KU-55933 | ATM kinase inhibitor | preclinical studies | attenuates senescence phenotypes | |
| Loperamide | Ca2+ channel inhibitor | preclinical studies | modulates calcium signaling to disrupt SASP | |
| NDGA | Ca2+ channel inhibitor | preclinical studies | shows senomorphic activity in various models | |
| Isradipine | Ca2+ channel inhibitor | preclinical studies | potential senomorphic effects through calcium modulation | |
| Simvastatin | ERK pathway inhibitor | preclinical studies | pleiotropic effects including potential senomorphic activity | |
| CDD-111 | MAPK inhibitor | preclinical studies | reduces senescence-associated phenotypes | |
| Anakinra | IL-1 receptor antagonist | clinical trials (for other indications) | reduces inflammation; potential senomorphic effects | |
| Ruxolitinib | JAK1/2 inhibitor | approved (for myelofibrosis) | being repurposed for senescence-related inflammation | |
| Metformin | AMPK activator; NF-κB inhibition | approved (for type 2 diabetes) | preclinical and epidemiological data suggest potential benefits for brain health | |
| Aspirin | modulates SIRT1; reduces DNA damage response | repurposed/ investigational | associated with reduced risk of some age-related diseases. | |
| Peptides | RVG29 | targets nAChR for delivery (e.g., BACE1 siRNA) | preclinical studies (in vivo) | 55% Aβ reduction in mice; stability challenges |
| GHK (glycyl-l-histidyl-l-lysine) | antioxidant, improves TGFβ1 signaling | preclinical studies | endogenous antioxidant; improved cognition (aging mice) | |
| GSH (Glutathione) | antioxidant protection | preclinical studies | levels reduced in AD; raising them is a therapeutic goal | |
| NAC (N-acetyl-l-cysteine) | increases GSH levels, antioxidant | preclinical studies | boosts antioxidant enzymes (preclinical) | |
| D-TLKIVW | inhibits tau aggregation (D-amino acid peptide) | preclinical studies | D-amino acid design enhances stability | |
| Ornithine-linked peptidomimetics | inhibits aggregation of PHF motifs | preclinical studies | inhibits PHF aggregation via β-helical conformation | |
| KLVVF, P4, P5 peptides | inhibits tau aggregation | preclinical studies | prevents tau toxicity by retaining random coil state | |
| ALAPYIP (VHL peptide) | induces ubiquitin-dependent tau degradation | preclinical studies | reduces tau levels in primary neurons and transgenic mouse models | |
| iAβ5 | inhibits aggregation of Aβ and tau | preclinical studies | inhibits both Aβ and tau aggregation (in vitro/in vivo) | |
| Hairpin peptide mimetics | inhibits Aβ aggregation | preclinical studies | anti-Aβ aggregation via piperidine-pyrrolidine moieties | |
| Antibodies | Bapineuzumab | targets N-terminals of Aβ42 | clinical trials (phase III completed) | limited efficacy, ARIA side effects |
| Solanezumab | targets Aβ13-28 segment | clinical trials (phase III) | safe, reduces Aβ, but no cognitive benefit | |
| Gantenerumab | targets N-terminal and central regions of Aβ | clinical trials (phase III) | mixed efficacy and safety results | |
| Crenezumab | binds Aβ oligomers, fibrils, and plaques | clinical trials (phase II/III) | inhibits and disrupts Aβ aggregates | |
| Aducanumab | targets conformational epitope of Aβ fibrils | approved (FDA, conditional) | conditionally approved; efficacy remains controversial | |
| DC8E8 | identifies tau epitope (residues 294–305) | preclinical studies | basis for the AADvac1 vaccine | |
| AADvac1 (peptide vaccine) | derived from tau residues 294–305 | clinical trials (phase II completed) | safe but no cognitive improvement | |
| C2N-8E12 (ABBV-8E12) | targets tau protein | clinical trials (phase I completed) | reduced tau, improved cognition; good safety | |
| Gouranemab (BIIB092) | targets N-terminal fragment of tau | clinical trials (phase I completed) | well-tolerated; development ongoing | |
| Ta1505 | targets pSer413 of tau | preclinical studies (in vivo) | reduces tau, improves synapses and cognition (mice) | |
| 43D, 77 × 109 | target tau epitopes 6–18 and 184–195 | preclinical studies | significant tau reduction and cognitive restoration | |
| Tocilizumab | IL-6 inhibitor | approved (for autoimmune diseases) | being explored for neuroinflammation and senescence | |
| Siltuximab | IL-6 inhibitor | approved (for other indications) | potential senomorphic activity through IL-6 neutralization | |
| Sirukumab | IL-6 inhibitor | clinical trials | investigated for inflammatory conditions | |
| Adalimumab | TNF-α inhibitor | approved (for autoimmune diseases) | potential senomorphic effects through TNF-α inhibition | |
| Etanercept | TNF-α inhibitor | approved (for autoimmune diseases) | shows promise in reducing inflammation | |
| Infliximab | TNF-α inhibitor | approved (for autoimmune diseases) | potential application in senescence-associated inflammation | |
| Natural Ligands | Vitamin E, Selenium | antioxidants | clinical studies | minimal improvement in patients; mixed results |
| Flavonols | antioxidants | epidemiological study | epidemiological link to reduced AD risk | |
| Luteolin | antioxidant, anti-inflammatory, inhibits Aβ and tau aggregation | preclinical studies | multifunctional flavonol; shows promise in reducing key pathologies | |
| Resveratrol | antioxidant, anti-inflammatory | clinical trials (phase II) | mixed results in patients | |
| Fisetin | antioxidant, anti-inflammatory | preclinical studies (in vivo) | neuroprotective; reduces oxidative stress (aging rats) | |
| Tauroursodeoxycholic acid (TUDCA) | anti-apoptotic, reduces oxidative stress, improves mitochondrial function, promotes Aβ clearance | clinical trial | neuroprotective bile acid; currently in trials for AD | |
| Acitretin | activates α-secretase | clinical study (pilot) | increases sAPPα, decreases Aβ | |
| Sodium oligomannate | inhibits Aβ aggregation, modulates gut microbiota | approved (China) | modulates microbiota | |
| Coconut oil | source of ketone bodies | clinical trial (pilot) | improved cognition with diet | |
| Curcumin derivative PE859 | targets both Aβ and tau aggregation | preclinical studies (in vivo) | reduces Aβ/tau, improves cognition (mice) | |
| EGb 761 (Ginkgo extract) | AChE inhibition, antioxidant | clinical studies | cognitive improvement debated | |
| Ginkgolide A | attenuates Aβ-induced depolarization, inhibits NMDA receptors | preclinical studies (in vivo) | attenuates Aβ toxicity, inhibits NMDA-R | |
| Nitidine, Avicin | dual AChE and BuChE inhibition | in vitro studies | dual AChE/BuChE inhibition and anti-aggregation | |
| Helminthosporin | Dual AChE and BuChE inhibition | in vitro studies | dual AChE/BuChE inhibition, high BBB permeability | |
| Chrysophanol | AChE and BACE1 inhibition, anti-neuroinflammatory | preclinical studies | anthraquinone with multi-target potential | |
| Hybrid Molecules | APH-1105 | α-secretase activator (nanoparticle) | clinical trials (phase II ongoing) | intranasal delivery |
| ML | controls Aβ aggregation, metal chelation, antioxidant | preclinical studies | reduces Aβ-metal toxicity, decreases ROS, BBB permeable | |
| DMPD | controls Aβ aggregation, metal chelation | preclinical studies (in vivo) | reduced Aβ, reversed memory loss (AD mice) | |
| Ber-D | enhanced metal chelation and antioxidant properties | in vitro studies | antioxidant; protects neuronal cells | |
| Distyrylbenzene-based theranostic | metal chelation, antioxidant, reduces Aβ/tau | preclinical studies | theranostic potential: imaging and therapy |
| Radiopharmaceuticals | Isotope | Advantages | Key Limitations | Status |
|---|---|---|---|---|
| ThT | - | historical marker of Aβ fibrils | low specificity, does not penetrate the BBB | preclinical studies |
| 6-Me-BTA-1 | - | high affinity for Aβ42 | not applicable in vivo use | preclinical studies |
| [11C]6-Me-BTA-1 | 11C | prototype for PiB | short t½ (20 min) | preclinical studies |
| PiB ([11C]PIB) | 11C | gold standard for Aβ-PET | limited scanning time | preclinical studies |
| [18F]Flutemetamol | 18F | analogue of PiB with a long t½ (110 min) | high non-specific binding to white matter | approved (FDA, EMA) |
| [11C]SB-13 | 11C | selectivity for dense plaques | low permeability across the BBB | preclinical studies |
| [18F]FMAPO | 18F | low background in white matter | relatively moderate affinity | preclinical studies |
| [18F]Florbetaben | 18F | high signal to noise ratio | slow accumulation kinetics (60–90 min) | approved (FDA) |
| [18F]Florbetapir | 18F | fast accumulation (20 min) | moderate lipophilicity | approved (FDA, EMA) |
| [18F]FIBT | 18F | ultra-high affinity | complex synthesis, limited clinical data | clinical trials (phase I/II) |
| Radiopharmaceuticals | Isotope | Advantages | Key Limitations | Status |
|---|---|---|---|---|
| [18F]FDDNP | 18F | first ligand for in vivo tau imaging | high binding to Aβ, slow kinetics, low signal-to-noise ratio | preclinical studies |
| BF-126 | 18F/11C | fast accumulation (30–40 min), suitable for dynamic studies | moderate cross-reactivity with Aβ | preclinical studies |
| BF-158/[11C]BF-158 | 18F/11C | good permeability through the BBB, stable pharmacokinetics | limited clinical data | clinical trials (phase I) |
| BF-170 | 18F | better selectivity (almost no binding to Aβ), ultra-high affinity | complex synthesis | clinical trials (phase II) |
| [18F]THK5105 | 18F | sensitivity to early stages of tauopathies | slow clearance, white matter artifacts | preclinical studies |
| [18F]THK5117 | 18F | improved version of THK5105 with less non-specific binding | limited availability | preclinical studies |
| [11C]PBB3 | 11C | 3R/4R-tau imaging, fast clearance (suitable for repeat scans) | short half-life (11C), high cost | clinical trials |
| Flortaucipir ([18F]T807) | 18F | FDA approved (Tauvid), high specificity for parenchymal tau | slow kinetics (60–80 min before scanning) | approved (FDA) |
| [18F]T808 | 18F | fast accumulation (30 min), specificity comparable to T807, fewer artifacts | limited validation data | preclinical studies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, A.V.; Kutuzova, A.D.; Kuzmichev, I.A.; Abakumov, M.A. Alzheimer’s Disease: From Molecular Mechanisms to Promising Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 9444. https://doi.org/10.3390/ijms26199444
Ivanova AV, Kutuzova AD, Kuzmichev IA, Abakumov MA. Alzheimer’s Disease: From Molecular Mechanisms to Promising Therapeutic Strategies. International Journal of Molecular Sciences. 2025; 26(19):9444. https://doi.org/10.3390/ijms26199444
Chicago/Turabian StyleIvanova, Anna V., Alexandra D. Kutuzova, Ilia A. Kuzmichev, and Maxim A. Abakumov. 2025. "Alzheimer’s Disease: From Molecular Mechanisms to Promising Therapeutic Strategies" International Journal of Molecular Sciences 26, no. 19: 9444. https://doi.org/10.3390/ijms26199444
APA StyleIvanova, A. V., Kutuzova, A. D., Kuzmichev, I. A., & Abakumov, M. A. (2025). Alzheimer’s Disease: From Molecular Mechanisms to Promising Therapeutic Strategies. International Journal of Molecular Sciences, 26(19), 9444. https://doi.org/10.3390/ijms26199444







