Abstract
Humans have been using lipids for many centuries; these are oils found in plants, particularly in seeds. However, relatively recently, it has become clear that lipids are the primary metabolites of any living organism. Fatty acids (FAs) are a structural component of lipids, and their role in building the framework of the lipid bilayer cannot be overstated. They participate in maintaining homeostasis by controlling membrane permeability. Changes in the FA composition of lipid bilayers can modulate the transition of the membrane from a liquid crystalline to a gel-like state. Thus, knowledge of a plant’s FA profile can aid in understanding the physiological mechanisms underlying their interaction with the environment and the ways in which they adapt to various stress factors. Throughout the colonization of terrestrial habitats, plants evolved, and new phylogenetic groups appeared; at present, some features of the FA composition of their individual representatives are known. However, the overall change in the composition of lipid FAs during the evolution of higher plants is still not understood. Our analysis of the literature showed that the FA diversity tends to decrease from mosses to angiosperms, mainly due to a reduction in polyunsaturated very-long-chain FAs, while the average acyl chain length remains unchanged. It is important to recognize the trends in this process in order to understand the adaptive capabilities of higher plants. This knowledge can be useful not only from a fundamental point of view, but also in practical human activities.
1. Introduction
Only a little over 70 years has passed since Watson and Crick discovered the structure of the DNA molecule in 1953 [1]. However, considerable progress has now been made in understanding the structure of genes and the proteins they encode, and many articles and reviews have been devoted to their evolutionary transformations [2,3,4,5,6,7,8]. Despite the chemical structure of lipids and their constituent fatty acids (FAs) being known for over three centuries, our understanding of how they are synthesized and their evolution in different plant groups is still incomplete [9]. While one can find information in the literature about the evolution of FA desaturases—proteins involved in FA synthesis—there is practically no information about changes in the composition of lipid FAs during the evolution of plant organisms [10,11]. Therefore, in this review, we summarize information on more than 550 higher plants (Embryophyta), including mosses (Marchantiopsida, Jungermanniopsida, Sphagnopsida, Bryopsida, and Polytrichopsida), ferns (Polypodiopsida), gymnosperms (Gnetopsida, Ginkgoopsida, Cycadopsida, and Pinopsida) and angiosperms (Magnoliopsida) (Table A1).
It should be noted that the analysis of literary sources covers a period of more than half a century. All this time, gas chromatography (GC) has been and remains the gold standard for analyzing the FA composition of lipids [12,13]. Only detection methods have undergone a significant change, with gas–liquid chromatography coupled with mass spectrometry leading to the considerable enhancement of analysis and the detection of many rare FAs that were previously only detectable through targeted analysis using specific standards [14,15]. However, the reliability of the GC method enables a comparison of results from different years, as minor FAs rarely account for more than a one percent, and the main composition of FAs is not in doubt. In addition, it is obvious that different plant organs performing different functions may feature in the composition of FAs [16,17]. At the same time, during the evolutionary process, the number of organs and tissues increased, which makes it more difficulty to perform a comparative analysis. Therefore, we used literature sources that mainly studied the composition of FAs in the photosynthetic part of plants. This helped us avoid potential errors in data interpretation (compared to various storage organs (fruits and seeds) or non-photosynthetic organs (for example roots)) and enables us to trace the reasons for phylogenetic changes in the FA composition of Embryophyta.
2. Diversity of Fatty Acids
The monocarboxylic acids comprising the lipids of higher plants are represented by 144 individual FAs (iFAs) (Table S1). The iFAs number in individual plant species ranges from 2 to 69 (Figure 1). The greatest variation is observed in mosses, but this is slightly lower in ferns (from 10 to 65) and lowest in gymnosperms (from 14 to 25) and in angiosperms (from 2 to 30). Generally, (median value), there are 28 iFAs in mosses, 27 in ferns, 20 in gymnosperms, and 12 iFAs in angiosperms. Thus, during the evolution of Embryophyta, there is a clear reduction in the composition diversity of iFA lipids.
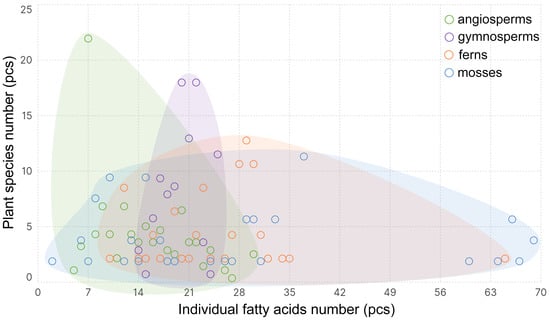
Figure 1.
The frequency with which a certain number of individual fatty acids occur in different Embryophyta clades: mosses, ferns, gymnosperms, and angiosperms.
There are several theories that explain the reasons for this trend. First, mosses, as non-vascular plants, are highly dependent on environmental humidity and are prone to drying out. Their complex lipid composition may play a role in maintaining membrane fluidity and stabilizing it during drying/rehydration cycles. Vascular plants, especially angiosperms, have developed conducting systems, cuticle, stomata and other adaptations that allow them to better control their water balance, reducing the selective pressure required to maintain an extremely complex lipid composition to protect membranes from dehydration [18]. Secondly, as plants evolved and adapted to diverse terrestrial conditions, lipid metabolism became optimized and specialized. Angiosperms developed more efficient and ‘targeted’ lipid synthesis pathways, focusing on the key FA necessary for their complex structures (e.g., developed photosynthetic apparatus, flowers, fruits) and functions [19]. This led to the loss of ‘excessive’ or less critical pathways for the synthesis of exotic FAs, characteristic of more ancient groups. It is known that many angiosperms have lost the ability to synthesize 16:3(7,10,13) in chloroplasts–plastidial or prokaryotic pathway [20,21], and that they have switched to predominantly using the extra-plastidial or eukaryotic pathway (synthesis of 18:1(9) in plastids followed by desaturation in the endoplasmic reticulum) [22,23]; this has undoubtedly led to a reduction in the diversity of the FA profile. Thirdly, it is known that many unique and rare FA mosses can perform specific protective functions (antimicrobial, antifeedant, cryoprotective) in their relatively primitive and vulnerable structures [24,25]. The most striking example of such FAs is acetylenic acids, which are often found in mosses and practically never in other tribes (Table S1); they can therefore be used as marker acids for mosses (Bryophytes) [26]. Acetylenic FAs are credited with powerful antifungal properties [27] and protect plants against being eaten [28]. In angiosperms, these functions could have been partially taken over by other classes of compounds (e.g., flavonoids, alkaloids, terpenoids, complex waxes), thus reducing the need for the synthesis of a wide range of specialized FAs [29,30].
3. Major Fatty Acids
In the vast majority of Embryophyta species, the major FAs are 16:0, 18:2(9,12), and 18:3(9,12,15) (Figure S1, Table S1). In angiosperms, with the exception of Nicotiana [31], the major FAs also include 18:0 and 18:1(9). In addition to the above, the major FAs in gymnosperms include 20:0 and 20:3(5,11,14). In ferns and mosses, the major FAs also include 18:1(9) and 20:4(5,8,11,14) (Figure S1, Table S1). At the same time, the total amount of major FAs in different species ranges from 33% to almost 100%, averaging 85% (median 86%) of all FAs.
The relative uniformity of the major Embryophyta FAs can be explained by the energetic optimization of the pathways of their synthesis. The main pathway of FA biosynthesis in plants is highly conserved, and its enzymes, such as β-ketoacyl-ACP synthases, are optimally adapted to work with precursors of a certain chain length (mainly 16C and 18C). The desaturase enzymes responsible for the double-bond formation (such as FAD2, FAD3, FAD7, FAD8) are also highly conserved and specific to 18C substrates [32]. Thus, the production of longer or more unsaturated FAs requires additional enzymatic steps and the expenditure of ATP and NADPH. In addition it can be assumed that evolution did not “reinvent” the entire metabolic pathway for major FAs, since the existing one perfectly meets the basic needs of plats.
However, it should be noted that there are many variations in the major FAs of different species. For example, in the aforementioned Nicotiana, the major ones are 16:1(9) and 16:3(7,10,13) (Figure S1). There are species in which 12:0 and 14:0 can be classified as the major FAs (Table S1). It is likely that such specific differences between certain species are related to the peculiarities of their biology. For example, in the Solanaceae family, significant amounts of FA have been found in secreted sugar polyesters, which are associated with the broad protective properties of the secretion against pathogens and insect herbivores [33,34,35,36,37].
4. Acyl Chain Length
FAs contain varying numbers of carbon atoms in the acyl chain. Based on this characteristic, all FAs are divided into groups, but it should be noted that there is no single established classification, and these groups of FAs vary greatly between plant and animal objects. During our analysis of the literature, we found that the following are most often considered for plant objects: short-chain: ≤6C [38,39,40], medium-chain: 7C–13C [38,41,42,43,44], long-chain: 14C–19C [41,45,46], and very-long-chain: ≥20C [17,47,48,49].
Short-chain FAs (SCFAs) are represented by two iFAs: 4:0 and 6:0 (Figure 2a). These are extremely rare in Embryophyta, and their median percentage in the FA composition of lipids trends towards zero. Medium-chain FAs (MCFAs) in Embryophyta are represented by 11 iFAs (Figure 2b). In vascular plants, they are mainly represented by saturated FAs (sFAs) such as 10:0, 11:0 and 12:0, while in mosses, the unsaturated FAs (uFAs) 12:1(7) and 13:1(8) are quite common. Ferns usually contain at least one MCFA in their lipid, while in other tribes, they are much less common. However, in gymnosperms, the median value of MCFAs is more than 1%, which is significantly higher than in others (Figure 2b). Long-chain FAs (LCFAs) in Embryophyta are the most diverse group and are represented by 67 iFAs (Figure 2c). They constitute the majority of all lipid FAs (median range from 75% to 95% in different tribes). Angiosperms exhibit the least diversity of LCFAs in a single species, while having the highest percentage content. In gymnosperms and spore plants, the diversity of LCFAs is higher, but the median content is lower. Of particular note is Bryophyta, which stands out for its greatest diversity of LCFAs, sometimes reaching 35 iFAs (Figure 2c). Very-long-chain FAs (VLCFAs) in Embryophyta are represented by 63 iFAs. The greatest diversity is observed in spore plants, slightly lower diversity is observed in gymnosperms, and the lowest diversity is observed in angiosperms. Quantitatively, their content is similar to species diversity by tribe, and in angiosperms, their median content does not exceed 4% (Figure 2d).
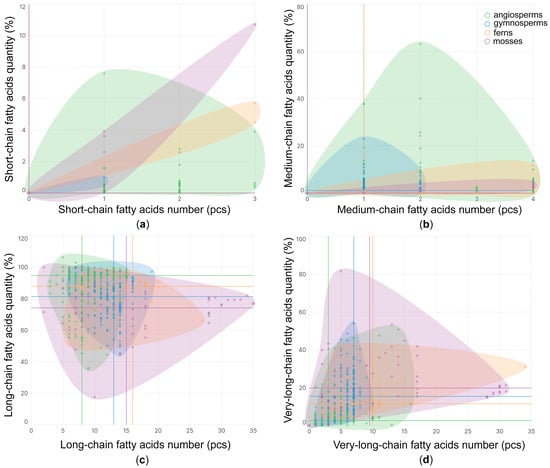
Figure 2.
The number of short-chain (a), medium-chain (b), long-chain (c), and very-long-chain (d) fatty acids in different species of Embryophyta clades: mosses, ferns, gymnosperms, and angiosperms. The lines corresponding to the legend colors indicate the median value of the qualitative and quantitative composition of lipid fatty acids with a specific acyl chain length according to the clades.
The acyl chains of lipid molecules play various roles in regulating the activity of membrane proteins and maintaining membrane function and integrity. The pleiotropic behavior of membrane properties shows a direct correlation with high levels of structural and the compositional complexity of the acyl chains of membrane lipids [50,51]. The length of the acyl chain is the main factor determining the thickness of the membrane [52]. It has been shown that changing the length of the acyl chain from 16 to 24 carbon atoms leads to a slight decrease in the average area per lipid and a clear linear increase in the thickness of the bilayer. Increasing the length of the acyl chain promotes interdigitation (interlinking of biological components that resembles the fingers of two hands being locked together) through the center of the bilayer, which is related to the molecules’ dynamics; this is because lateral diffusion (side-to-side movement of lipids within their respective leaflets of a bilayer membrane) rates decrease slightly as the acyl chain length increases [53].
The composition of membrane FAs determines the temperature of lipid phase transition (Tm) from the lamellar liquid crystalline (Lα) phase to the gel phase (Lβ). The longer the FA chains, the higher the transition temperature. For example, for the diacylphosphatidylethanolamine bilayer model, Tm changes from 30 °C to 90 °C if 12:0 is replaced with 22:0 [54].
Plants regulate the length of acyl chains to adapt to extreme temperatures. At low temperatures, chain shortening (e.g., an increase in the proportion of 16:3(7,10,13) in galactolipids) helps maintain membrane fluidity, preventing phase transition to Lβ [22]. On the contrary, at high temperatures, the elongation of acyl chains stabilizes membranes by strengthening hydrophobic interactions, so Arabidopsis mutants with defects in LCFA synthesis show increased sensitivity to heat stress [55].
In general, the interaction of FAs with lipid membranes is non-specific, weakly selective, and modulated by the physicochemical characteristics of the FA and the lipid bilayer under consideration. The incorporation of FAs into lipid membranes and their inversion across the bilayer occur almost instantaneously [56,57], and this, in turn, increases the curvature stress within the lipid bilayer due to the non-cylindrical geometry of FAs [58,59]. The local accumulation of FAs in the membrane will create small-scale inhomogeneities and defects in the membrane, possibly accompanied by toroidal pores and morphological changes in the membrane, all of which destabilize the membrane and reduce its permeability barrier [60,61]. While FAs with short acyl chains tend to destabilize the lipid bilayer, longer FAs can stabilize gel membranes, making them more rigid [62]. However, abnormal levels of FAs in some organisms, especially sFA, may accompany cellular dysfunction and damage [63,64,65]. FAs can exert their biological and pathological effects through receptor-mediated signaling, as well as through changes in the physical and mechanical properties of lipid bilayers [66]. The length of acyl chains modulates the activity of membrane-integrated proteins [67]. For example, plasma membrane ATPases require a specific lipid environment to function, so shortening the acyl chains of FAs disrupts the conformation of these ion transporters, reducing their activity [68]. Similarly, transport proteins such as aquaporins demonstrate the dependence of the water transport rate on the lipid bilayer thickness, regulated by chain length [69]. FAs play an important signaling role [70], and information about VLCFAs’ involvement in the response of plants to pathogen invasion is particularly noteworthy [71].
Despite the above-described features related to the length of FA chains in Embryophyta clades and the associated features of plant membrane functioning, it is noteworthy that an indicator such as the average chain length (ACL) [72] remains virtually unchanged. ACL consists of 18 carbon atoms and does not change significantly from tribe to tribe, with only a slight tendency towards chain shortening: mosses—18.0, ferns and gymnosperms—17.7, and angiosperms—17.5 (Table S2). This suggests that ACL is crucial in the physiology of Embryophyta, and that rearrangements associated with changes in the chain length of some FAs must be compensated for by others.
5. Even and Odd Numbers of Carbon Atoms in the Acyl Chain
It should be noted that even-chain FAs (even-FAs) are characteristic of all higher plants. Despite the relatively high content of odd-FAs in certain species (for example, the maximum percentage of odd-FAs in angiosperms is 11.45 (sp. № 288), gymnosperms—35.7 (sp. № 344), ferns—18.3 (sp. № 454), mosses—9.3 (sp. № 531)), their median values are extremely low (0.27%, 1.8%, 0.8% and 0.45%, respectively, in the tribes) (Table A1 and Table S2). In plants, FA synthesis primarily occurs in chloroplasts (plastids) and involves a series of enzymatic reactions in which fatty acid synthase (FAS) synthesizes FAs from acetyl-CoA, using malonyl-CoA as a carbon donor [73,74]. The process is cyclical, with two carbon atoms being added by FAS at a time until FA with 16 or 18 carbon atoms is formed [75]. Thus, even-FAs are obtained during de novo synthesis, but it is assumed that odd-FAs can be synthesized in a similar manner. [76], and de novo 15:0 may serve as the ancestor of this branch of the synthesis pathway [77]. It is also known that 18:0 can lose one carbon atom during α-oxidation, forming 17:0 [78,79,80,81]. As a result of the α-oxidation process occurring in peroxisomes, FAs are broken down into smaller molecules, such as acetyl-CoA, which can be used for the synthesis of vital chemicals or for energy production [82,83]. Unlike β-oxidation (which occurs in the mitochondria, during which two carbon atoms are cleaved from FAs molecules, ultimately forming acetyl-CoA and additionally NADH and FADH2 molecules, which are used for energy storage [84,85]), α-oxidation does not directly lead to energy storage [86]. However, there is evidence that α-oxidation is an important pathway for the metabolism of branched-chain FAs and may contribute to the incorporation of intermediate breakdown products into β-oxidation [87]. Nevertheless, it is evident that the appearance of odd-FAs indicates the initiation of energetically unfavorable FA catabolism, which can be observed under stressful conditions [17] and is not normal for Embryophyte.
6. The Double Bonds Number in the Fatty Acid Acyl Chain
Saturated FAs are those that do not contain double bonds in the acyl chain. The median sFA content is 32.5% in angiosperms, 32% in gymnosperms, 27% in ferns, and 22% in mosses (Figure 3; Table S2).
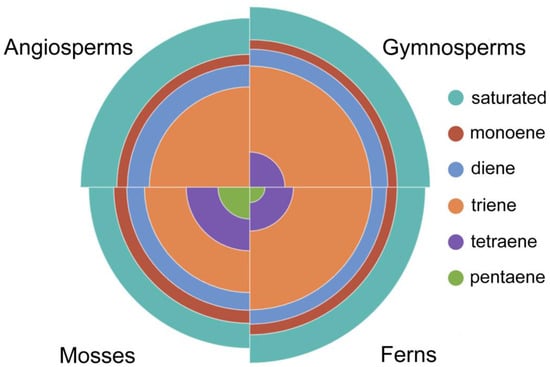
Figure 3.
Median distribution profile of saturated and unsaturated (monoene, diene, triene, tetraene, and pentaene) lipid fatty acids in Embryophyta clades: mosses, ferns, gymnosperms, and angiosperms.
Unsaturated FAs can contain varying numbers of double bonds in their acyl chain, and in Embryophyta they can range from one to six. The number of monoene FAs (mFAs) in the lipids of seed plants was practically equal (≈8%), and slightly higher in ferns and mosses (9–10%) (Figure 3; Table S2). Diene FAs (dFAs) account for a slightly higher percentage of the total lipids in Embryophyta than mFAs, and there are no significant differences between tribes (Figure 3; Table S2). Triene FAs (trFAs) are the most significant group of uFAs, with the highest levels found in gymnosperms and ferns (≈40%), angiosperms having 10% less, and mosses having another 10% less (Figure 3; Table S2). The highest concentration of trFAs is found in the chloroplast membranes of all higher plants, suggesting that these FAs may be primarily necessary for photosynthesis [23,88]. The galactolipids of thylakoid membranes contain predominantly polyunsaturated C18 chains, which provide the flexibility necessary for the organization of photosystems II and I. Replacing 18:3(9,12,15) with 16:0 disrupts the structure of thylakoids and reduces the efficiency of photosynthesis [89,90]. At the same time, it is known that triple mutants of fad3-2 fad7-2 fad8 Arabidopsis, which contain insignificant levels (less than 0.1%) of trFAs, did not differ from wild-type plants, and that their photosynthesis was practically unaffected [91]. However, these mutants were male sterile and did not produce seeds under normal conditions. The authors attribute this to the fact that trFAs are the precursors of oxylipins, which are signaling compounds that regulate the final maturation and release of pollen. At the same time, the exogenous application of jasmonic acid signaling molecules, formed in plants from 18:3(9,12,15) [92], or α-linolenic acid itself restored fertility [91], indicating the crucial role of trFAs in the life cycle of plants.
The most striking difference in uFA content is demonstrated by tetraene FAs (tFAs) (Figure 3; Table S2). They are practically absent in angiosperms (median = 0). The presence of tFAs can be called an archaic feature of Embryophyta: the more evolutionarily the ancient tribe, the more tFAs these plants contain (gymnosperms—3.7%, ferns—5%, and mosses—9%). Pentaene FAs (pFAs) are quite rare in the composition of Embryophyta lipids and are completely uncharacteristic of seed plants. In ferns, their median value is 0.7%, with pFAs most commonly being found in mosses at 3%. (Figure 3; Table S2). Hexaene FAs (hFAs) are not typical regarding the lipid composition of Embryophyta, occurring only in certain species of mosses (sp. № 528), ferns (sps. № 455 and № 457), and angiosperms, where the genus Centaurea should be singled out; in Centaurea, six species (sps. № 104, 105, 107, 111, 118, 121) contain gFAs, but these FAs are also found in species № 12, 15, 78, 163, 272, and 300 from various other families (Table A1, and Tables S1 and S2). Bryophytes often produce large amounts of very-long-chain polyunsaturated FAs (VLPUFAs), mainly including 20:4(5,8,11,14) and 20:5(5,8,11,14,17) [26]. The presence of VLPUFAs is characteristic of aquatic organisms such as algae [93,94,95], but it is rarely found in higher plants. This may indicate that the mosses did not completely lose their marine origins when they entered non-aquatic environments [96].
The presence and position of the double bond is just as important for the state of the membrane as the length of the acyl chain. Thus, on the model of a dioctadecenoyl phosphatidylcholine bilayer, it was shown that changing the position of the double bond from 4 to 9 or 13 in the carbon chains changes Tm from 20 °C to −20 °C or 0 °C, respectively [54]. Under physiological conditions, lipid bilayers are in the Lα state, in which lipids have considerable freedom of movement (they can rotate, swing, and diffuse laterally). When exposed to certain factors (e.g., temperature decrease, dehydration), FA lipid chains begin to pack into a crystal-like structure, forming a more ordered and stable Lβ, where the free movement of lipids is significantly restricted. Thus, the structure and geometry of the lipid acyl chains fundamentally influence the degree of packing within the bilayer. Any conformation of the acyl chain that hinders its tight packing will contribute to an increase in membrane fluidity. For example, compared to a straight sFA chain, the presence of an unsaturated cis bond within the chain will clearly limit the dense packing of the membrane [97]. The inclusion of a cis double bond causes the acyl chain to bend by 30°, which reduces the packing order in the lipid. This disorder is more pronounced when the cis bond is located in the middle of the acyl chains [98]. Thus, the uFA/sFA ratio is directly related to membrane fluidity [99,100,101].
The unsaturation index (UI) is commonly used as an integral indicator that most clearly demonstrates the number of double bonds in the FA composition [102,103]. This indicator illustrates the gradual decrease in unsaturation during the evolutionary process, i.e., from mosses to angiosperms (Figure 4). Moreover, the highest UI values in mosses and ferns are achieved due to tFAS and pFAs, while in gymnosperms, PUFA data are reduced; however, the UI remains quite high due to the high content of trFAs. Thus, the decrease in the amount of VLCFAs (Figure 2d) is accompanied by a significant decrease in the unsaturation of FAs in the lipid composition from mosses to angiosperms. This is quite logical, since it is known that the membrane fluidity will increase both when the FA chain of the membrane is shortened [104] and when the iso bonds in FA are increased [105].
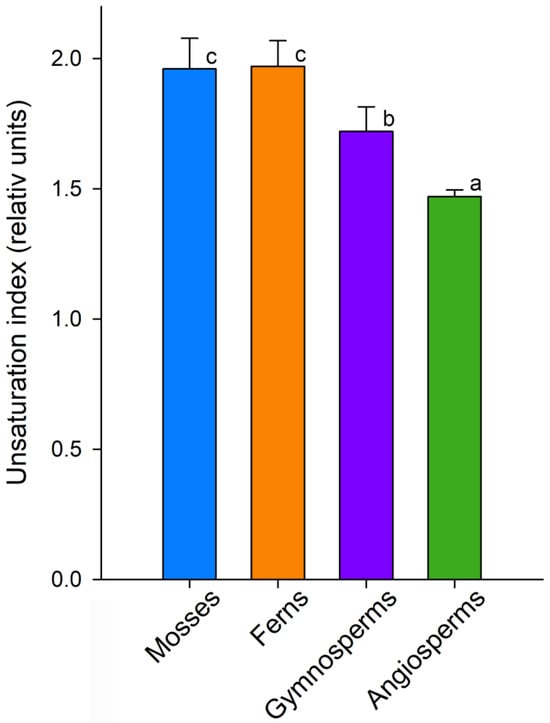
Figure 4.
Unsaturation index of various Embryophyta clades: mosses, ferns, gymnosperms, and angiosperms. Values assigned with different letters indicate a significant difference between the medians (one-way ANOVA followed by post hoc Tukey’s NHSD test, p < 0.05).
With their emergence onto land [106], plants were constantly confronted with changes in climate, with hot periods alternating with prolonged ice ages [107]; it is clear that these stressful conditions promoted the evolution of Embryophyta [108,109]. It can be assumed that repeated periods of decline in the average temperature of the planet [110] contributed to the inclusion of a larger number of shorter-chain FAs in the composition of lipids, since it is known that they help maintain membrane fluidity under cold stress [22]. As one of the compensatory mechanisms for maintaining the physiological parameters of membrane viscosity, plants have been forced to reduce the unsaturation of their membranes during evolution, since these indicators, when changing in one direction, lead to opposite effects on the value of Tm.
It is known that the FA profile is associated with the thermotolerance of plants in different climatic zones [111]. Numerous individual cases of increased FA unsaturation have been identified; this occurs during salinization, drought and UV radiation [112]. The change in membrane fluidity, mediated by changes in unsaturated FA levels, is a function provided in part by the regulated activity of FA desaturases. Various studies have been devoted to the evolution of these enzymes [10,11,113,114,115], so we will not dwell on them separately; however, we cannot fail to mention the important regulatory role of desaturases. Thus, the regulation of membrane fluidity maintains an environment suitable for the functioning of critically important integral proteins during stress. For example, the modulation of 18:1(9) content is central to the normal expression of defense responses to pathogens in Arabidopsis, and the control of 18:1(9) and 18:2(9,12) levels is involved in the regulation of seed development and mycotoxin production against Aspergillus spp. [116].
7. Conclusions
Thus, plants adapt the composition of their membrane FAs to specific conditions, which leads to diversity in FAs and their composition among different species. However, all these changes, consisting of the inclusion of certain FAs in the composition of lipids, are compensated for either by the inclusion/exclusion of other FAs or by a change in their level of unsaturation. This is necessary to maintain certain physiological parameters, such as ACL, and is observed throughout the development of Embryophyta; this ensures optimal Tm values that are directly related to the conductive and protective functions of the lipid bilayer, which constitutes the fundamental physiological functions of the plant organism.
It is also important to note that lipids containing essential FAs are an indispensable component of human nutrition. Therefore, it is necessary to expand research into the FA profile of plants from different taxonomic groups. This will increase breeding options when creating mutants with specified characteristics, considering the physiological capabilities and compensatory functions of the plant organism. At present, in order to comprehensively address this issue, it is necessary to create complete and publicly accessible databases of chromatographic profiles (with mass spectrometric confirmation) for the lipidomes of representatives of all systematic groups of higher plants, since most of the data currently available is scattered and often incomplete.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26199424/s1.
Author Contributions
Conceptualization, A.V. and T.I.; software, A.V. and T.I.; formal analysis, T.I.; data curation, T.I.; writing—original draft preparation, A.V.; writing—review and editing, T.I.; visualization, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out within the state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme № 122042700043-9).
Data Availability Statement
All the data are provided in the review.
Acknowledgments
The authors express their deep gratitude to Maria Breygina for providing plant material for research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACL | Average chain length |
| dFA | Diene fatty acid |
| FA | Fatty acid |
| FAS | Fatty acid synthase |
| GS | Gas chromatography |
| hFA | Hexaene fatty acid |
| iFA | Individual fatty acid |
| LCFA | Long-chain fatty acid |
| Lα | Lamellar liquid crystalline phase |
| Lβ | Gel phase |
| MCFA | Medium-chain fatty acid |
| mFA | Monoene fatty acid |
| pFA | Pentaene fatty acid |
| SCFA | Short-chain fatty acid |
| sFA | Saturated fatty acid |
| tFA | Tetraene fatty acid |
| Tm | Temperature of lipid phase transition |
| trFA | Triene fatty acid |
| uFA | Unsaturated fatty acid |
| UI | Unsaturation index |
| VLCFA | Very-long-chain fatty acid |
| VLPUFA | Very-long-chain polyunsaturated fatty acid |
Appendix A

Table A1.
List of Embryophyta species used. Taxonomy by NCBI https://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi, accessed on 26 May 2025 (*—the first publisher data; see the methodology in [117,118]).
Table A1.
List of Embryophyta species used. Taxonomy by NCBI https://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi, accessed on 26 May 2025 (*—the first publisher data; see the methodology in [117,118]).
| Class | Order | Family | Genus | Species | № |
|---|---|---|---|---|---|
| Magnoliopsida | Gentianales | Apocynaceae | Carissa | C. edulis [119] | 1 |
| Dipsacales | Caprifoliaceae | Lomelosia | L. cretica [120] | 2 | |
| Piperales | Aristolochiaceae | Aristolochia | A. fontanesii [121] | 3 | |
| Zingiberales | Strelitziaceae | Strelitzia | S. reginae [122] | 4 | |
| Malvales | Cistaceae | Cistus | C. ladanifer [123] | 5 | |
| Malvaceae | Abelmoschus | A. esculentus [119] | 6 | ||
| Malvella | M. sherardiana [124] | 7 | |||
| Hibiscus | H. cannabinus [125] | 8 | |||
| H. sabdariffa [125] | 9 | ||||
| Malva | M. neglecta [124] | 10 | |||
| M. sylvestris [126] | 11 | ||||
| Glyphaea | G. brevis [127] | 12 | |||
| Corchorus | C. olitorius [125] | 13 | |||
| Adansonia | A. digitata [119] | 14 | |||
| Sterculia | S. tragacantha [128] | 15 | |||
| Dioscoreales | Dioscoreaceae | Dioscorea | D. communis [129] | 16 | |
| Arecales | Arecaceae | Raphia | R. sudanica [130] | 17 | |
| R. regalis [130] | 18 | ||||
| R. vinifera [130] | 19 | ||||
| R. hookeri [130] | 20 | ||||
| Elaeis | E. guineensis [131] | 21 | |||
| Ranunculales | Papaveraceae | Papaver | P. rhoeas [120] | 22 | |
| Vitales | Vitaceae | Vitis | V. vinifera [132] | 23 | |
| Alismatales | Araceae | Arum | A. elongatum [133] | 24 | |
| Xanthosoma | X. sagittifolium [125] | 25 | |||
| Myrtales | Melastomataceae | Pleroma | P. granulosum [134] | 26 | |
| Combretaceae | Lumnitzera | L. racemose [135] | 27 | ||
| Myrtaceae | Eucalyptus | E. accedens [136] | 28 | ||
| E. patens [136] | 29 | ||||
| E. wandoo [136] | 30 | ||||
| E. marginate [136] | 31 | ||||
| E. diversicolor [136] | 32 | ||||
| Myrtus | M. communis [137] | 33 | |||
| Corymbia | C. calophylla [136] | 34 | |||
| Lythraceae | Punica | P. granatum [132] | 35 | ||
| Fagales | Juglandaceae | Juglans | J. regia [132] | 36 | |
| Fagaceae | Quercus | Q. ilex [138] | 37 | ||
| Castanea | C. sativa [139] | 38 | |||
| Boraginales | Boraginaceae | Cynoglossum | C. creticum [120] | 39 | |
| Anchusa | A. azurea [129] | 40 | |||
| A. officinalis [133] | 41 | ||||
| Borago | B. officinalis [140] | 42 | |||
| Liliales | Liliaceae | Lilium | L. longiflorum * | 43 | |
| Asparagales | Asparagaceae | Asparagus | A. acutifolius [129] | 44 | |
| Asphodelaceae | Xanthorrhoea | X. preissii [122] | 45 | ||
| Eremurus | E. spectabilis [133] | 46 | |||
| Hyacinthaceae | Muscari | M. comosum [120] | 47 | ||
| Amaryllidaceae | Allium | A. orientale [133] | 48 | ||
| A. schoenoprasum [120] | 49 | ||||
| A. sativum [133] | 50 | ||||
| A. ampeloprasum [141] | 51 | ||||
| A. cepa [141] | 52 | ||||
| Poales | Cyperaceae | Carex | C. depressa [142] | 53 | |
| C. cilicica [142] | 54 | ||||
| C. halleriana [142] | 55 | ||||
| C. hartmanii [142] | 56 | ||||
| C. orbicularis [142] | 57 | ||||
| C. punctata [142] | 58 | ||||
| C. pendula [142] | 59 | ||||
| C. sylvatica [142] | 60 | ||||
| C. rostrata [142] | 61 | ||||
| C. liparocarpos [142] | 62 | ||||
| C. paniculate [142] | 63 | ||||
| C. pallescens [142] | 64 | ||||
| C. flacca [142] | 65 | ||||
| C. digitata [142] | 66 | ||||
| C. brevicollis [142] | 67 | ||||
| Poaceae | Zea | Z. mays [143] | 68 | ||
| Ericales | Ericaceae | Leucopogon | L. conostephioides [122] | 69 | |
| Vaccinium | V. myrtillus [144] | 70 | |||
| Arbutus | A. unedo [145] | 71 | |||
| A. andrachne [145] | 72 | ||||
| Primulaceae | Labisia | L. pumila var. pumila [146] | 73 | ||
| L. pumila var. alata [146] | 74 | ||||
| L. pumila var. lanceolata [146] | 75 | ||||
| Aegiceras | A. corniculatum [135] | 76 | |||
| Theaceae | Camellia | C. sinensis [147] | 77 | ||
| Garryales | Eucommiaceae | Eucommia | E. ulmoides [148] | 78 | |
| Asterales | Asteraceae | Artemisia | A. lercheana [16] | 79 | |
| Tragopogon | T. sinuatus [120] | 80 | |||
| Crepis | C. vesicaria [120] | 81 | |||
| Helichrysum | H. stoechas [139] | 82 | |||
| Arctotheca | A. calendula [122] | 83 | |||
| Symphyotrichum | S. novi-belgii [122] | 84 | |||
| Helminthotheca | H. echioides [122] | 85 | |||
| Gundelia | G. tournefortii [149] | 86 | |||
| Stizolophus | S. balsamita [150] | 87 | |||
| Matricaria | M. chamomilla var. recutita [139] | 88 | |||
| Urospermum | U. dalechampii [122] | 89 | |||
| Scolymus | S. hispanicus [120] | 90 | |||
| Dahlia | D. pinnata [120] | 91 | |||
| Silybum | S. marianum [129] | 92 | |||
| Gymnanthemum | G. amygdalinum [151] | 93 | |||
| Rhanterium | R. adpressum [152] | 94 | |||
| Chondrilla | C. juncea [129] | 95 | |||
| Hypochaeris | H. cretensis [120] | 96 | |||
| Telekia | T. speciosa [153] | 97 | |||
| Dittrichia | D. viscosa [154] | 98 | |||
| Stevia | S. rebaudiana [155] | 99 | |||
| Taraxacum | T. obovatum [129] | 100 | |||
| T. officinale [144] | 101 | ||||
| Sonchus | S. oleraceus [129] | 102 | |||
| Reichardia | R. picroides [120] | 103 | |||
| Centaurea | C. pulcherrima [156] | 104 | |||
| C. pseudoscabiosa [156] | 105 | ||||
| C. triumfettii [157] | 106 | ||||
| C. tchihatcheffii [158] | 107 | ||||
| C. saligna [159] | 108 | ||||
| C. iberica [159] | 109 | ||||
| C. calolepis [150] | 110 | ||||
| C. babylonica [156] | 111 | ||||
| C. solstitialis [157] | 112 | ||||
| C. carduiformis [150] | 113 | ||||
| C. virgata [157] | 114 | ||||
| C. paniculate [139] | 115 | ||||
| C. cariensis [150] | 116 | ||||
| C. aggregate [159] | 117 | ||||
| C. patula [158] | 118 | ||||
| C. pterocaula [157] | 119 | ||||
| C. depressa [159] | 120 | ||||
| C. pulchella [158] | 121 | ||||
| C. kotschyi [157] | 122 | ||||
| C. behen [159] | 123 | ||||
| C. cyanus [160] | 124 | ||||
| Calendula | C. officinalis [160] | 125 | |||
| Cichorium | C. spinosum [120] | 126 | |||
| C. intybus [129] | 127 | ||||
| Cynara | C. cornigera [120] | 128 | |||
| C. cardunculus [120] | 129 | ||||
| Lactuca | L. sativa [120] | 130 | |||
| Carthamus | C. tinctorius [161] | 131 | |||
| Lamiales | Acanthaceae | Avicennia | A. officinalis [135] | 132 | |
| Acanthus | A. ilicifolius [135] | 133 | |||
| Bignoniaceae | Spathodea | S. campanulate [134] | 134 | ||
| Verbenaceae | Aloysia | A. citrodora [162] | 135 | ||
| Verbena | V. officinalis [162] | 136 | |||
| Lamiaceae | Nepeta | N. transcaucasica [163] | 137 | ||
| Lavandula | L. intermedia [164] | 138 | |||
| Thymbra | T. capitata [165] | 139 | |||
| Thymus | T. fallax [166] | 140 | |||
| T. kotschyanus var. glabrescens [166] | 141 | ||||
| T. kotschyanus var. kotschyanus [166] | 142 | ||||
| T. haussknechtii [166] | 143 | ||||
| T. pubescens [166] | 144 | ||||
| Vitex | V. altissima [167] | 145 | |||
| V. negundo [167] | 146 | ||||
| V. trifolia [167] | 147 | ||||
| Scutellaria | S. orientalis [163] | 148 | |||
| Satureja | S. boissieri [166] | 149 | |||
| S. macrantha [163] | 150 | ||||
| S. hortensis [164] | 151 | ||||
| Prasium | P. majus [120] | 152 | |||
| Origanum | O. acutidens [166] | 153 | |||
| O. vulgare [166] | 154 | ||||
| Ocimum | O. basilicum [168] | 155 | |||
| Salvia | S. euphratica [163] | 156 | |||
| S. officinalis [169] | 157 | ||||
| Mentha | M. cervine [170] | 158 | |||
| M. piperita [144] | 159 | ||||
| Plantaginaceae | Plantago | P. lanceolata [122] | 160 | ||
| Antirrhinum | A. sp. [122] | 161 | |||
| Oleaceae | Olea | O. europaea [171] | 162 | ||
| Solanales | Solanaceae | Solanum | S. erianthum [127] | 163 | |
| S. macrocarpon [125] | 164 | ||||
| S. nigrum [120] | 165 | ||||
| Nicotiana | N. suaveolens [31] | 166 | |||
| N. arentsii [31] | 167 | ||||
| N. wigandioides [31] | 168 | ||||
| N. pauciflora [31] | 169 | ||||
| N. setchellii [31] | 170 | ||||
| N. raimondii [31] | 171 | ||||
| N. rotundifolia [31] | 172 | ||||
| N. maritima [31] | 173 | ||||
| N. rosulate [31] | 174 | ||||
| N. rosulata subsp. Ingulba [31] | 175 | ||||
| N. occidentalis [31] | 176 | ||||
| N. occidentalis subsp. Hesperis [31] | 177 | ||||
| N. goodspeedii [31] | 178 | ||||
| N. africana [31] | 179 | ||||
| N. corymbose [31] | 180 | ||||
| N. solanifolia [31] | 181 | ||||
| N. undulata [31] | 182 | ||||
| N. umbratical [31] | 183 | ||||
| N. trigonophylla [31] | 184 | ||||
| N. stocktonii [31] | 185 | ||||
| N. simulans [31] | 186 | ||||
| N. noctiflora [31] | 187 | ||||
| N. miersii [31] | 188 | ||||
| N. megalosiphon [31] | 189 | ||||
| N. longiflora [31] | 190 | ||||
| N. langsdorffii [31] | 191 | ||||
| N. knightiana [31] | 192 | ||||
| N. gossei [31] | 193 | ||||
| N. exigua [31] | 194 | ||||
| N. bonariensis [31] | 195 | ||||
| N. amplexicaulis [31] | 196 | ||||
| N. cavicola [31] | 197 | ||||
| N. clevelandii [31] | 198 | ||||
| N. repanda [31] | 199 | ||||
| N. paniculate [31] | 200 | ||||
| N. excelsior [31] | 201 | ||||
| N. tomentosa [31] | 202 | ||||
| N. kawakamii [31] | 203 | ||||
| N. nudicaulis [31] | 204 | ||||
| N. attenuate [31] | 205 | ||||
| N. glutinosa [31] | 206 | ||||
| N. benthamiana [31] | 207 | ||||
| N. tomentosiformis [31] | 208 | ||||
| N. tabacum [172] | 209 | ||||
| N. sylvestris [31] | 210 | ||||
| N. rustica [31] | 211 | ||||
| N. plumbaginifolia [31] | 212 | ||||
| N. otophora [31] | 213 | ||||
| N. glauca [31] | 214 | ||||
| N. debneyi [31] | 215 | ||||
| N. quadrivalvis var. bigelovii [31] | 216 | ||||
| N. alata [31] | 217 | ||||
| N. acuminata [31] | 218 | ||||
| Capsicum | C. annuum [173] | 219 | |||
| Apiales | Araliaceae | Hedera helix | H. helix [122] | 220 | |
| Apiaceae | Anethum | A. foeniculum [120] | 221 | ||
| Helosciadium | H. nodiflorum [129] | 222 | |||
| Centella | C. asiatica [174] | 223 | |||
| Crithmum | C. maritimum [175] | 224 | |||
| Coriandrum | C. sativum [176] | 225 | |||
| Apium | A. graveolens [120] | 226 | |||
| Petroselinum | P. crispum [120] | 227 | |||
| Daucus | D. carota [120] | 228 | |||
| Malpighiales | Salicaceae | Salix | S. sp. [122] | 229 | |
| Rhizophoraceae | Ceriops | C. decandra [135] | 230 | ||
| Bruguiera | B. cylindrica [135] | 231 | |||
| Rhizophora | R. apiculate [135] | 232 | |||
| R. mucronate [135] | 233 | ||||
| Euphorbiaceae | Cnidoscolus | C. aconitifolius [177] | 234 | ||
| Croton | C. zambesicus [178] | 235 | |||
| Excoecaria | E. agallocha [179] | 236 | |||
| Euphorbia | E. lathyrism [180] | 237 | |||
| E. lagascae [180] | 238 | ||||
| Linaceae | Linum | L. usitatissimum [181] | 239 | ||
| Fabales | Fabaceae | Melilotus | M. messanensis [143] | 240 | |
| Senna | S. sophera [182] | 241 | |||
| S. tora [183] | 242 | ||||
| S. siamea [134] | 243 | ||||
| Delonix | D. regia [134] | 244 | |||
| Trifolium | T. angustifolium [139] | 245 | |||
| T. alexandrinum [143] | 246 | ||||
| T. pratense [122] | 247 | ||||
| Cassia | C. fistula [134] | 248 | |||
| Ceratonia | C. siliqua [132] | 249 | |||
| Vigna | V. unguiculata [125] | 250 | |||
| Vicia | V. faba [120] | 251 | |||
| Onobrychis | O. viciifolia [122] | 252 | |||
| Lathyrus | L. ochrus [120] | 253 | |||
| Brassicales | Moringaceae | Moringa | M. oleifera [184] | 254 | |
| Brassicaceae | Arabidopsis | A. helleri [185] | 255 | ||
| A. lyrata [185] | 256 | ||||
| Sinapis | S. arvensis [143] | 257 | |||
| Eruca | E. vesicaria subsp. sativa [120] | 258 | |||
| Brassica | B. napus [186] | 259 | |||
| Cucurbitales | Cucurbitaceae | Cucurbita | C. pepo [187] | 260 | |
| C. pepo subsp. pepo [187] | 261 | ||||
| C. pepo var. melopepo [187] | 262 | ||||
| Bryonia | B. cretica [120] | 263 | |||
| B. dioica [129] | 264 | ||||
| Caryophyllales | Montiaceae | Montia | M. fontana [129] | 265 | |
| Aizoaceae | Sesuvium | S. portulacastrum [188] | 266 | ||
| Amaranthaceae | Amaranthus | A. cruentus [125] | 267 | ||
| Portulacaceae | Portulaca | P. oleracea [189] | 268 | ||
| Caryophyllaceae | Silene | S. vulgaris [120] | 269 | ||
| Polygonaceae | Rumex | R. pulcher [129] | 270 | ||
| R. papillaris [129] | 271 | ||||
| R. patientia [190] | 272 | ||||
| R. obtusifolius [120] | 273 | ||||
| Chenopodiaceae | Salicornia | S. europaea [16] | 274 | ||
| S. brachiate [191] | 275 | ||||
| Beta | B. vulgaris [120] | 276 | |||
| Suaeda | S. monoica [191] | 277 | |||
| S. altissima [192] | 278 | ||||
| S. maritima [191] | 279 | ||||
| Spinacia | S. oleracea [120] | 280 | |||
| Chenopodium | C. album [193] | 281 | |||
| Rosales | Elaeagnaceae | Hippophae | H. rhamnoides [137] | 282 | |
| Rosaceae | Rubus | R. ulmifolius [139] | 283 | ||
| Rosa | R. elliptica subsp. inodora [194] | 284 | |||
| R. dumalis [194] | 285 | ||||
| R. villosa [194] | 286 | ||||
| R. subcanina [194] | 287 | ||||
| R. micrantha [195] | 288 | ||||
| R. rugosa [194] | 289 | ||||
| R. rubiginosa [194] | 290 | ||||
| R. canina [194] | 291 | ||||
| R. majalis [144] | 292 | ||||
| Malus | M. orientalis [196] | 293 | |||
| M. domestica [197] | 294 | ||||
| Mespilus | M. germanica [47] | 295 | |||
| Cydonia | C. oblonga [198] | 296 | |||
| Pyrus | P. communis [17] | 297 | |||
| P. caucasica [17] | 298 | ||||
| Eriobotrya | E. japonica [132] | 299 | |||
| Prunus | P. spinosa [199] | 300 | |||
| P. dulcis [200] | 301 | ||||
| Urticaceae | Urtica | U. dioica [201] | 302 | ||
| Moraceae | Morus | M. nigra [202] | 303 | ||
| M. alba [132] | 304 | ||||
| Ficus | F. carica [132] | 305 | |||
| Cannabaceae | Humulus | H. lupulus [129] | 306 | ||
| Laurales | Lauraceae | Persea | P. americana [132] | 307 | |
| Sapindales | Anacardiaceae | Pistacia | P. lentiscus [203] | 308 | |
| Meliaceae | Azadirachta | A. indica [204] | 309 | ||
| Rutaceae | Citrus | C. limon [132] | 310 | ||
| Gnetopsida | Ephedrales | Ephedraceae | Ephedra | E. chilensis [205] | 311 |
| E. equisetina [205] | 312 | ||||
| E. fragilis [205] | 313 | ||||
| E. gerardiana [205] | 314 | ||||
| E. distachya [205] | 315 | ||||
| Gnetales | Gnetaceae | Gnetum | G. gnemon [205] | 316 | |
| Welwitschiales | Welwitschiaceae | Welwitschia | W. mirabilis [205] | 317 | |
| Ginkgoopsida | Ginkgoales | Ginkgoaceae | Ginkgo | G. biloba [206] | 318 |
| Cycadopsida | Cycadales | Zamiaceae | Ceratozamia | C. robusta [205] | 319 |
| Lepidozamia | L. hopei [205] | 320 | |||
| Macrozamia | M. riedlei [207] | 321 | |||
| M. moorei [205] | 322 | ||||
| Microcycas | M. calocoma [205] | 323 | |||
| Encephalartos | E. lebomboensis [205] | 324 | |||
| Dioon | D. edule [205] | 325 | |||
| Zamia | Z. furfuracea [208] | 326 | |||
| Stangeria | S. eriopus [205] | 327 | |||
| Cycadaceae | Bowenia | B. spectabilis [205] | 328 | ||
| Cycas | C. armstrongii [205] | 329 | |||
| C. siamensis [205] | 330 | ||||
| C. revoluta [209] | 331 | ||||
| Pinopsida | Araucariales | Podocarpaceae | Pectinopitys | P. ferruginea [205] | 332 |
| Podocarpus | P. salignus [205] | 333 | |||
| P. nivalis [205] | 334 | ||||
| P. macrophyllus [205] | 335 | ||||
| P. macrophyllus var. maki [205] | 336 | ||||
| Prumnopity | P. andina [205] | 337 | |||
| P. taxifolia [205] | 338 | ||||
| Pherosphaera | P. fitzgeraldii [205] | 339 | |||
| Dacrydium | D.cupressinum [205] | 340 | |||
| Araucariaceae | Agathis | A. robusta [205] | 341 | ||
| A. australis [205] | 342 | ||||
| A. moorei [205] | 343 | ||||
| Araucaria | A. montana [205] | 344 | |||
| A. luxurians [205] | 345 | ||||
| A. cunninghamii [205] | 346 | ||||
| A. angustifolia [205] | 347 | ||||
| A. araucana [205] | 348 | ||||
| Pinales | Pinaceae | Keteleeria | K. evelyniana [205] | 349 | |
| Abies | A. vejarii [205] | 350 | |||
| A. nordmanniana [208] | 351 | ||||
| A. procera [208] | 352 | ||||
| A. amabilis [208] | 353 | ||||
| A. concolor [205] | 354 | ||||
| A. sachalinensis [205] | 355 | ||||
| A. veitchii [205] | 356 | ||||
| A. cephalonica [205] | 357 | ||||
| A. pinsapo [205] | 358 | ||||
| A. grandis [205] | 359 | ||||
| A. alba [205] | 360 | ||||
| Tsuga | T. canadensis [208] | 361 | |||
| T. heterophylla [208] | 362 | ||||
| Pseudotsuga | P. sinensis var. wilsoniana [208] | 363 | |||
| P. macrocarpa [205] | 364 | ||||
| P. menziesii [205] | 365 | ||||
| Pseudolarix | P. amabilis [205] | 366 | |||
| Pinus | P. strobiformis [205] | 367 | |||
| P. monophyla [205] | 368 | ||||
| P. koraiensis [210] | 369 | ||||
| P. pinaster [205] | 370 | ||||
| P. halepensis [205] | 371 | ||||
| P. bungeana [205] | 372 | ||||
| P. aristata [205] | 373 | ||||
| P. nigra [208] | 374 | ||||
| P. cembra [205] | 375 | ||||
| P. ponderosa [207] | 376 | ||||
| P. jeffreyi [205] | 377 | ||||
| P. resinosa [205] | 378 | ||||
| P. palustris [205] | 379 | ||||
| P. sylvestris [208] | 380 | ||||
| P. pinea [205] | 381 | ||||
| P. wallichiana [205] | 382 | ||||
| P. contorta [208] | 383 | ||||
| Picea | P. obovata [208] | 384 | |||
| P. orientalis [208] | 385 | ||||
| P. asperata [205] | 386 | ||||
| P. chihuahuana [205] | 387 | ||||
| P. torano [205] | 388 | ||||
| P. omorika [205] | 389 | ||||
| P. mariana [208] | 390 | ||||
| P. engelmannii [208] | 391 | ||||
| P. sitchensis [208] | 392 | ||||
| P. pungens [210] | 393 | ||||
| P. glauca [205] | 394 | ||||
| P. abies [208] | 395 | ||||
| Larix | L. gmelinii [211] | 396 | |||
| L. lyallii [205] | 397 | ||||
| L. deciduas [208] | 398 | ||||
| L. sibirica [210] | 399 | ||||
| L. kaempferi [211] | 400 | ||||
| L. laricina [205] | 401 | ||||
| Cedrus | C. atlantica [205] | 402 | |||
| C. libani [205] | 403 | ||||
| C. deodara [208] | 404 | ||||
| Cupressales | Sciadopityaceae | Sciadopitys | S. verticillate [205] | 405 | |
| Taxaceae | Torreya | T. californica [205] | 406 | ||
| Cephalotaxus | C. sinensis [205] | 407 | |||
| C. fortune [205] | 408 | ||||
| C.harringtonia [205] | 409 | ||||
| Taxus | T. cuspidata [205] | 410 | |||
| T. brevifolia [205] | 411 | ||||
| T. baccata [205] | 412 | ||||
| Cupressaceae | Callitris | C. preissii [205] | 413 | ||
| Widdringtonia | W. schwarzii [205] | 414 | |||
| Hesperocyparis | H. bakeri [205] | 415 | |||
| H. lusitanica [205] | 416 | ||||
| H. goveniana [205] | 417 | ||||
| Austrocedrus | A. chilensis [205] | 418 | |||
| Sequoiadendron | S. giganteum [205] | 419 | |||
| Callitropsis | C. nootkatensis [208] | 420 | |||
| C. funebris [205] | 421 | ||||
| Platycladus | P. orientalis [205] | 422 | |||
| Chamaecyparis | C.thyoides [208] | 423 | |||
| C. pisifera [208] | 424 | ||||
| C.hodginsii [205] | 425 | ||||
| C. lawsoniana [208] | 426 | ||||
| Taiwania | T. cryptomerioides [205] | 427 | |||
| Juniperus | J. sabina [205] | 428 | |||
| J. communis [208] | 429 | ||||
| J. chinensis [205] | 430 | ||||
| J. virginiana [208] | 431 | ||||
| Taxodium | T. mucronatum [205] | 432 | |||
| T. distichum var. distichum [205] | 433 | ||||
| T. distichum var. imbricatum [205] | 434 | ||||
| Sequoia | S. sempervirens [205] | 435 | |||
| Cunninghamia | C. lanceolata [205] | 436 | |||
| C. lanceolata var. konishii [205] | 437 | ||||
| Athrotaxis | A. laxifolia [205] | 438 | |||
| Thujopsis | T. dolabrata [205] | 439 | |||
| Tetraclinis | T. articulata [205] | 440 | |||
| Microbiota | M. decussata [205] | 441 | |||
| Diselma | D. archeri [205] | 442 | |||
| Cupressus | C. torulosa [205] | 443 | |||
| C. dupreziana [205] | 444 | ||||
| C. sempervirens [208] | 445 | ||||
| Calocedrus | C. decurrens [205] | 446 | |||
| Metasequoia | M. glyptostroboides [205] | 447 | |||
| Cryptomeria | C. japonica [205] | 448 | |||
| Thuja | T. koraiensis [205] | 449 | |||
| T. standishii [205] | 450 | ||||
| T. occidentalis [205] | 451 | ||||
| T. plicata [208] | 452 | ||||
| Polypodiopsida | Cyatheales | Cyatheaceae | Cyathea | C. dealbata [212] | 453 |
| Hymenophyllales | Hymenophyllaceae | Hymenophyllum | H. plicatum [213] | 454 | |
| H. caudiculatum [213] | 455 | ||||
| Marattiales | Marattiaceae | Ptisana | P. salicina [214] | 456 | |
| Salviniales | Salviniaceae | Salvinia | S. natans [214] | 457 | |
| Azolla | A. caroliniana [215] | 458 | |||
| Schizaeales | Lygodiaceae | Lygodium | L. japonicum [216] | 459 | |
| Osmundales | Osmundaceae | Claytosmunda | C. claytoniana [212] | 460 | |
| Osmunda | O. regalis [217] | 461 | |||
| Osmundastrum | O. cinnamomeum [216] | 462 | |||
| Polypodiales | Woodsiaceae | Woodsia | W. glabella [218] | 463 | |
| Thelypteridaceae | Amauropelta | A. noveboracensis [212] | 464 | ||
| Phegopteris | P. connectilis [212] | 465 | |||
| Polypodiaceae | Pyrrosia | P. eleagnifolia [212] | 466 | ||
| Platycerium | P. bifurcatum [102] | 467 | |||
| Pleopeltis | P. polypodioides [216] | 468 | |||
| Polypodium | P. vulgare [219] | 469 | |||
| Cystopteridaceae | Gymnocarpium | G. dryopteris [218] | 470 | ||
| Cystopteris | C. fragilis [212] | 471 | |||
| Athyriaceae | Deparia | D. pycnosora [212] | 472 | ||
| Athyrium | A. sinense [212] | 473 | |||
| A. distentifolium [218] | 474 | ||||
| A. spinulosum [212] | 475 | ||||
| A. crenulatoserrulatum [212] | 476 | ||||
| A. yokoscense [212] | 477 | ||||
| A. filix-femina [212] | 478 | ||||
| Dennstaedtiaceae | Pteridium | P. esculentum [212] | 479 | ||
| P. aquilinum [220] | 480 | ||||
| Aspleniaceae | Asplenium | A. oblongifolium [212] | 481 | ||
| A. trichomanes [102] | 482 | ||||
| A. scolopendrium [41] | 483 | ||||
| A. nidus [102] | 484 | ||||
| Pteridaceae | Adiantum | A. pedatum [212] | 485 | ||
| Dryopteridaceae | Polystichum | P. tripteron [212] | 486 | ||
| Dryopteris | D. austriaca [221] | 487 | |||
| D. expansa [218] | 488 | ||||
| D. goeringiana [212] | 489 | ||||
| D. crassirhizoma [212] | 490 | ||||
| D. filix-mas [221] | 491 | ||||
| Onocleaceae | Onoclea | O. sensibilis [212] | 492 | ||
| Matteuccia | M. struthiopteris [222] | 493 | |||
| Equisetales | Equisetaceae | Equisetum | E. fluviatile [217] | 494 | |
| E. variegatum [223] | 495 | ||||
| E. hyemale [224] | 496 | ||||
| E. scirpoides [223] | 497 | ||||
| E. arvense [223] | 498 | ||||
| Psilotales | Psilotaceae | Psilotum | P. nudum [217] | 499 | |
| Marchantiopsida | Marchantiales | Ricciaceae | Riccia | R. fluitans [96] | 500 |
| Conocephalaceae | Conocephalum | C. conicum [26] | 501 | ||
| Marchantiaceae | Marchantia | M. polymorpha [26] | 502 | ||
| Jungermanniopsida | Metzgeriales | Metzgeriaceae | Metzgeria | M. pubescens [26] | 503 |
| Ptilidiales | Ptilidiaceae | Ptilidium | P. ciliare [26] | 504 | |
| Jungermanniales | Plagiochilaceae | Plagiochila | P. porelloides [26] | 505 | |
| Pelliales | Pelliaceae | Pellia | P. neesiana [96] | 506 | |
| Sphagnopsida | Sphagnales | Sphagnaceae | Sphagnum | S. majus [225] | 507 |
| S. nemoreum [226] | 508 | ||||
| S. squarrosum [226] | 509 | ||||
| S. magellanicum [225] | 510 | ||||
| S. fimbriatum [225] | 511 | ||||
| Bryopsida | Rhizogoniales | Aulacomniaceae | Aulacomnium | A. palustre [226] | 512 |
| Pottiales | Pottiaceae | Tortella | T. tortuosa [227] | 513 | |
| Bartramiales | Bartramiaceae | Philonotis | P. fontana [226] | 514 | |
| Hypnales | Entodontaceae | Entodon | E. schleicheri [26] | 515 | |
| Thuidiaceae | Thuidium | T. assimile [26] | 516 | ||
| Calliergonaceae | Calliergon | C. cordifolium [96] | 517 | ||
| Scorpidiaceae | Hygrohypnella | H. ochracea [226] | 518 | ||
| Helodiaceae | Helodium | H. blandowii [226] | 519 | ||
| Rhytidiaceae | Rhytidium | R. rugosum [26] | 520 | ||
| Amblystegiaceae | Drepanocladus | D. lycopodioides [96] | 521 | ||
| Amblystegium | A. riparium [228] | 522 | |||
| Cratoneuron | C. filicinum [26] | 523 | |||
| Neckeraceae | Pseudanomodon | P. attenuatus [26] | 524 | ||
| Neckera | N. pennata [26] | 525 | |||
| Myuriaceae | Ctenidium | C. molluscum [227] | 526 | ||
| Climaciaceae | Climacium | C. dendroides [226] | 527 | ||
| Fontinalaceae | Fontinalis | F. antipyretica [229] | 528 | ||
| Hypnaceae | Hypnum | H. andoi [230] | 529 | ||
| H. jutlandicum [231] | 530 | ||||
| H. cupressiforme [232] | 531 | ||||
| Hylocomiaceae | Rhytidiadelphus | R. squarrosus [233] | 532 | ||
| Brachytheciaceae | Kindbergia | K. praelonga [234] | 533 | ||
| Eurhynchium | E. praelongum [228] | 534 | |||
| E. striatum [233] | 535 | ||||
| Rhynchostegium | R. murale [228] | 536 | |||
| Brachythecium | B. erythrorrhizon [228] | 537 | |||
| B. rivulare [26] | 538 | ||||
| B. rutabulum [231] | 539 | ||||
| Pylaisiaceae | Calliergonella | C. cuspidate [235] | 540 | ||
| Bryales | Bryaceae | Bryum | B. sp. [226] | 541 | |
| Ptychostomum | P. moravicum [231] | 542 | |||
| P. pseudotriquetrum [226] | 543 | ||||
| Rhodobryum | R. ontariense [236] | 544 | |||
| Mniaceae | Pseudobryum | P. cinclidioides [226] | 545 | ||
| Plagiomnium | P. confertidens [26] | 546 | |||
| P. medium [226] | 547 | ||||
| P. cuspidatum [237] | 548 | ||||
| Mnium | M. hornum [238] | 549 | |||
| Pseudoditrichales | Ditrichaceae | Ceratodon | C. purpureus [239] | 550 | |
| Dicranales | Rhabdoweisiaceae | Dichodontium | D. pellucidum [227] | 551 | |
| Funariales | Funariaceae | Funaria | F. hygrometrica [240] | 552 | |
| Physcomitrium | P. patens [24] | 553 | |||
| Polytrichopsida | Polytrichales | Polytrichaceae | Pogonatum | P. urnigerum [227] | 554 |
| Atrichum | A. undulatum [230] | 555 | |||
| Polytrichum | P. juniperinum [226] | 556 | |||
| P. commune [26] | 557 |
References
- Watson, J.D.; Crick, F.H.C. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef]
- Ibarra-Laclette, E.; Lyons, E.; Hernández-Guzmán, G.; Pérez-Torres, C.A.; Carretero-Paulet, L.; Chang, T.-H.; Lan, T.; Welch, A.J.; Juárez, M.J.A.; Simpson, J.; et al. Architecture and Evolution of a Minute Plant Genome. Nature 2013, 498, 94–98. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Liu, W.; Li, J.; Xiong, S.; Jia, J.; Lin, Q.; Liu, H.; Cui, P. Evolution of Plant Genome Size and Composition. Genom. Proteom. Bioinform. 2024, 22, qzae078. [Google Scholar] [CrossRef]
- Clark, J.W. Genome Evolution in Plants and the Origins of Innovation. New Phytol. 2023, 240, 2204–2209. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. Plant Genomes: Markers of Evolutionary History and Drivers of Evolutionary Change. Plants People Planet 2021, 3, 74–82. [Google Scholar] [CrossRef]
- Wright, R.C.; Neres, D.F.; Chaisupa, P.; Bryant, J.A. Protein Engineering and Plants: The Evolution of Sustainable Agriculture. Biochemist 2023, 45, 12–17. [Google Scholar] [CrossRef]
- Bartlett, M.E.; Whipple, C.J. Protein Change in Plant Evolution: Tracing One Thread Connecting Molecular and Phenotypic Diversity. Front. Plant Sci. 2013, 4, 382. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Jiang, J.; Hou, X.; Guo, J.; Li, X.; Zhao, D.; Xie, X. Plant Protein-Coding Gene Families: Their Origin and Evolution. Front. Plant Sci. 2022, 13, 995746. [Google Scholar] [CrossRef]
- Cerone, M.; Smith, T.K. A Brief Journey into the History of and Future Sources and Uses of Fatty Acids. Front. Nutr. 2021, 8, 570401. [Google Scholar] [CrossRef] [PubMed]
- Hajiahmadi, Z.; Abedi, A.; Wei, H.; Sun, W.; Ruan, H.; Zhuge, Q.; Movahedi, A. Identification, Evolution, Expression, and Docking Studies of Fatty Acid Desaturase Genes in Wheat (Triticum aestivum L.). BMC Genom. 2020, 21, 778. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, L.; Guan, M.; Liu, J.; Guan, C. Origin and Evolution of Fatty Acid Desaturase Genes in Oil Crop Brassica Napus. Oil Crop Sci. 2022, 7, 200–208. [Google Scholar] [CrossRef]
- Fisk, H.L.; West, A.L.; Childs, C.E.; Burdge, G.C.; Calder, P.C. The Use of Gas Chromatography to Analyze Compositional Changes of Fatty Acids in Rat Liver Tissue during Pregnancy. J. Vis. Exp. 2014, 85, e51445. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Armando, A.M.; Dennis, E.A. High Sensitivity Quantitative Lipidomics Analysis of Fatty Acids in Biological Samples by Gas Chromatography–Mass Spectrometry. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2011, 1811, 648–656. [Google Scholar] [CrossRef]
- Koch, E.; Wiebel, M.; Hopmann, C.; Kampschulte, N.; Schebb, N.H. Rapid Quantification of Fatty Acids in Plant Oils and Biological Samples by LC-MS. Anal. Bioanal. Chem. 2021, 413, 5439–5451. [Google Scholar] [CrossRef]
- Chiu, H.-H.; Kuo, C.-H. Gas Chromatography-Mass Spectrometry-Based Analytical Strategies for Fatty Acid Analysis in Biological Samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Myasoedov, N.A.; Pchelkin, V.P.; Tsydendambaev, V.D.; Vereshchagin, A.G. Increased Content of Very-Long-Chain Fatty Acids in the Lipids of Halophyte Vegetative Organs. Russ. J. Plant Physiol. 2009, 56, 787–794. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K. The Features of the Fatty Acid Composition of Pyrus L. Total Lipids Are Determined by Mountain Ecosystem Conditions. Plant Physiol. Biochem. 2022, 170, 350–363. [Google Scholar] [CrossRef]
- Gasulla, F.; vom Dorp, K.; Dombrink, I.; Zähringer, U.; Gisch, N.; Dörmann, P.; Bartels, D. The Role of Lipid Metabolism in the Acquisition of Desiccation Tolerance in Craterostigma Plantagineum: A Comparative Approach. Plant J. 2013, 75, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. Book 2010, 8, e0133. [Google Scholar] [CrossRef]
- Somerville, C.; Browse, J. Plant Lipids: Metabolism, Mutants, and Membranes. Science 1991, 252, 80–87. [Google Scholar] [CrossRef]
- Roughan, P.G.; Slack, C.R. Cellular Organization of Glycerolipid Metabolism. Annu. Rev. Plant Physiol. 1982, 33, 97–132. [Google Scholar] [CrossRef]
- Browse, J.; Somerville, C. Glycerolipid Synthesis: Biochemistry and Regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 467–506. [Google Scholar] [CrossRef]
- Mongrand, S.; Bessoule, J.-J.; Cabantous, F.; Cassagne, C. The C16:3\C18:3 Fatty Acid Balance in Photosynthetic Tissues from 468 Plant Species. Phytochemistry 1998, 49, 1049–1064. [Google Scholar] [CrossRef]
- Beike, A.K.; Jaeger, C.; Zink, F.; Decker, E.L.; Reski, R. High Contents of Very Long-Chain Polyunsaturated Fatty Acids in Different Moss Species. Plant Cell Rep. 2014, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- de León, I.P.; Hamberg, M.; Castresana, C. Oxylipins in Moss Development and Defense. Front. Plant Sci. 2015, 6, 483. [Google Scholar] [CrossRef] [PubMed]
- Filippova, I.P.; Makhutova, O.N.; Guseynova, V.E.; Gladyshev, M.I. Fatty Acid Profiles of Some Siberian Bryophytes and Prospects of Their Use in Chemotaxonomy. Biomolecules 2023, 13, 840. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Tripathi, S.K.; Feng, Q.; Lorenz, M.C.; Wright, M.A.; Jacob, M.R.; Mask, M.M.; Baerson, S.R.; Li, X.-C.; Clark, A.M.; et al. A Potent Plant-Derived Antifungal Acetylenic Acid Mediates Its Activity by Interfering with Fatty Acid Homeostasis. Antimicrob. Agents Chemother. 2012, 56, 2894–2907. [Google Scholar] [CrossRef]
- Rempt, M.; Pohnert, G. Novel Acetylenic Oxylipins from the Moss Dicranum Scoparium with Antifeeding Activity against Herbivorous Slugs. Angew. Chem. Int. Ed. 2010, 49, 4755–4758. [Google Scholar] [CrossRef]
- Walters, D. Plant Defense: Warding off Attack by Pathogens, Herbivores and Parasitic Plants; Wiley-Blackwell: Hoboken, NJ, USA, 2011; ISBN 978-1-444-34773-9. [Google Scholar]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of Phenols and Polyphenols in Plant Defense Response to Biotic and Abiotic Stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. [Google Scholar]
- Koiwai, A.; Suzuki, F.; Matsuzaki, T.; Kawashima, N. The Fatty Acid Composition of Seeds and Leaves of Nicotiana Species. Phytochemistry 1983, 22, 1409–1412. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef]
- Gentile, A.G.; Stoner, A.K. Resistance in Lycopersicon and Solanum Species to the Potato Aphid1. J. Econ. Entomol. 1968, 61, 1152–1154. [Google Scholar] [CrossRef]
- Gentile, A.G.; Webb, R.E.; Stoner, A.K. Resistance in Lycopersicon and Solanum to Greenhouse Whiteflies1. J. Econ. Entomol. 1968, 61, 1355–1357. [Google Scholar] [CrossRef]
- Gentile, A.G.; Webb, R.E.; Stoner, A.K. Lycopersicon and Solanum Spp. Resistant to the Carmine and the Two-Spotted Spider Mite1. J. Econ. Entomol. 1969, 62, 834–836. [Google Scholar] [CrossRef]
- Juvik, J.A.; Shapiro, J.A.; Young, T.E.; Mutschler, M.A. Acylglucoses from Wild Tomatoes Alter Behavior and Reduce Growth and Survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 1994, 87, 482–492. [Google Scholar] [CrossRef]
- Shapiro, J.A.; Steffens, J.C.; Mutschler, M.A. Acylsugars of the Wild Tomato Lycopersicon Pennellii in Relation to Geographic Distribution of the Species. Biochem. Syst. Ecol. 1994, 22, 545–561. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Evtodienko, V.Y.; Kovbasnjuk, O.N.; Antonenko, Y.N.; Yaguzhinsky, L.S. Effect of the Alkyl Chain Length of Monocarboxylic Acid on the Permeation through Bilayer Lipid Membranes. Biochim. Biophys. Acta-Biomembr. 1996, 1281, 245–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamp, F.; Hamilton, J.A. How Fatty Acids of Different Chain Length Enter and Leave Cells by Free Diffusion. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 149–159. [Google Scholar] [CrossRef]
- Voronkov, A.; Ivanova, T. Significance of Lipid Fatty Acid Composition for Resistance to Winter Conditions in Asplenium Scolopendrium. Biology 2022, 11, 507. [Google Scholar] [CrossRef]
- Reynolds, K.B.; Taylor, M.C.; Zhou, X.; Vanhercke, T.; Wood, C.C.; Blanchard, C.L.; Singh, S.P.; Petrie, J.R. Metabolic Engineering of Medium-Chain Fatty Acid Biosynthesis in Nicotiana Benthamiana Plant Leaf Lipids. Front. Plant Sci. 2015, 6, 164. [Google Scholar] [CrossRef]
- Hänsel, R.; Rimpler, H.; Keller, K.; Schneider, G.; Abel, G.; Bader, G.; Baumann, T.; Bertram, B.; Beyer, G.; Bodesheim, U.; et al. (Eds.) Hagers Handbuch Der Pharmazeutischen Praxis; Springer: Berlin/Heidelberg, Germany, 1994; ISBN 978-3-642-63390-4. [Google Scholar]
- Gu, H.; Jinkerson, R.E.; Davies, F.K.; Sisson, L.A.; Schneider, P.E.; Posewitz, M.C. Modulation of Medium-Chain Fatty Acid Synthesis in Synechococcus sp. PCC 7002 by Replacing FabH with a Chaetoceros Ketoacyl-ACP Synthase. Front. Plant Sci. 2016, 7, 690. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.V.; Mayorov, V.I.; Dikalova, A.E.; Dikalov, S.I. Long-Chain and Medium-Chain Fatty Acids in Energy Metabolism of Murine Kidney Mitochondria. Int. J. Mol. Sci. 2022, 24, 379. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K.; Kozhevnikova, A.D.; Tsydendambaev, V.D. Polyunsaturated and Very-Long-Chain Fatty Acids Are Involved in the Adaptation of Maloideae (Rosaceae) to Combined Stress in the Mountains. Chem. Biodivers. 2020, 17, e1900588. [Google Scholar] [CrossRef]
- De Bigault Du Granrut, A.; Cacas, J.-L. How Very-Long-Chain Fatty Acids Could Signal Stressful Conditions in Plants? Front. Plant Sci. 2016, 7, 1490. [Google Scholar] [CrossRef]
- Clarke, S.D.; Nakamura, M.T. Fatty Acid Structure and Synthesis. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 285–289. [Google Scholar]
- Duché, G.; Sanderson, J.M. The Chemical Reactivity of Membrane Lipids. Chem. Rev. 2024, 124, 3284–3330. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the Diversity of Membrane Lipid Composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Lee, T.-H.; Charchar, P.; Separovic, F.; Reid, G.E.; Yarovsky, I.; Aguilar, M.-I. The Intricate Link between Membrane Lipid Structure and Composition and Membrane Structural Properties in Bacterial Membranes. Chem. Sci. 2024, 15, 3408–3427. [Google Scholar] [CrossRef]
- Niemelä, P.S.; Hyvönen, M.T.; Vattulainen, I. Influence of Chain Length and Unsaturation on Sphingomyelin Bilayers. Biophys. J. 2006, 90, 851–863. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B. Recent Patents on Nonlamellar Liquid Crystalline Lipid Phases in Drug Delivery. Recent Pat. Drug Deliv. Formul. 2013, 7, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat Stress Phenotypes of Arabidopsis Mutants Implicate Multiple Signaling Pathways in the Acquisition of Thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef]
- Høyrup, P.; Davidsen, J.; Jørgensen, K. Lipid Membrane Partitioning of Lysolipids and Fatty Acids: Effects of Membrane Phase Structure and Detergent Chain Length. J. Phys. Chem. B 2001, 105, 2649–2657. [Google Scholar] [CrossRef]
- Kamp, F.; Zakim, D.; Zhang, F.; Noy, N.; Hamilton, J.A. Fatty Acid Flip-Flop in Phospholipid Bilayers Is Extremely Fast. Biochemistry 1995, 34, 11928–11937. [Google Scholar] [CrossRef] [PubMed]
- Cantor, R.S. Lipid Composition and the Lateral Pressure Profile in Bilayers. Biophys. J. 1999, 76, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Fuller, N.; Rand, R.P. The Influence of Lysolipids on the Spontaneous Curvature and Bending Elasticity of Phospholipid Membranes. Biophys. J. 2001, 81, 243–254. [Google Scholar] [CrossRef]
- Arouri, A.; Mouritsen, O.G. Membrane-Perturbing Effect of Fatty Acids and Lysolipids. Prog. Lipid Res. 2013, 52, 130–140. [Google Scholar] [CrossRef]
- Mouritsen, O.G. Lipids, Curvature, and Nano-medicine. Eur. J. Lipid Sci. Technol. 2011, 113, 1174–1187. [Google Scholar] [CrossRef]
- Inoue, T.; Yanagihara, S.; Misono, Y.; Suzuki, M. Effect of Fatty Acids on Phase Behavior of Hydrated Dipalmitoylphosphatidylcholine Bilayer: Saturated versus Unsaturated Fatty Acids. Chem. Phys. Lipids 2001, 109, 117–133. [Google Scholar] [CrossRef]
- Shimano, H. Novel Qualitative Aspects of Tissue Fatty Acids Related to Metabolic Regulation: Lessons from Elovl6 Knockout. Prog. Lipid Res. 2012, 51, 267–271. [Google Scholar] [CrossRef]
- Little, J.P.; Madeira, J.M.; Klegeris, A. The Saturated Fatty Acid Palmitate Induces Human Monocytic Cell Toxicity Toward Neuronal Cells: Exploring a Possible Link Between Obesity-Related Metabolic Impairments and Neuroinflammation. J. Alzheimer’s Dis. 2012, 30, S179–S183. [Google Scholar] [CrossRef]
- Lee, J.Y.; Zhao, L.; Hwang, D.H. Modulation of Pattern Recognition Receptor-Mediated Inflammation and Risk of Chronic Diseases by Dietary Fatty Acids. Nutr. Rev. 2010, 68, 38–61. [Google Scholar] [CrossRef] [PubMed]
- Frasch, S.C.; Bratton, D.L. Emerging Roles for Lysophosphatidylserine in Resolution of Inflammation. Prog. Lipid Res. 2012, 51, 199–207. [Google Scholar] [CrossRef]
- Levental, I.; Lyman, E. Regulation of Membrane Protein Structure and Function by Their Lipid Nano-Environment. Nat. Rev. Mol. Cell Biol. 2023, 24, 107–122, Correction in Nat. Rev.Mol. Cell Biol. 2023, 24, 79. [Google Scholar] [CrossRef]
- Morales-Cedillo, F.; González-Solís, A.; Gutiérrez-Angoa, L.; Cano-Ramírez, D.L.; Gavilanes-Ruiz, M. Plant Lipid Environment and Membrane Enzymes: The Case of the Plasma Membrane H+-ATPase. Plant Cell Rep. 2015, 34, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.P.; Weigle, A.T.; Shukla, D. Functional Regulation of Aquaporin Dynamics by Lipid Bilayer Composition. Nat. Commun. 2024, 15, 1848. [Google Scholar] [CrossRef]
- Bach, L.; Faure, J.-D. Role of Very-Long-Chain Fatty Acids in Plant Development, When Chain Length Does Matter. Comptes Rendus Biol. 2010, 333, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Leger, A.; Roby, D. Very Long Chain Fatty Acid and Lipid Signaling in the Response of Plants to Pathogens. Plant Signal. Behav. 2009, 4, 94–99. [Google Scholar] [CrossRef]
- Liu, J.; An, Z. Comparison of Different Chain N-Fatty Acids in Modern Plants on the Loess Plateau of China. Front. Earth Sci. 2020, 14, 615–624. [Google Scholar] [CrossRef]
- Rajasekharan, R.; Nachiappan, V. Fatty Acid Biosynthesis and Regulation in Plants. In Plant Developmental Biology-Biotechnological Perspectives; Springer: Berlin/Heidelberg, Germany, 2010; pp. 105–115. [Google Scholar]
- Zhang, S.; Wu, S.; Hou, Q.; Zhao, J.; Fang, C.; An, X.; Wan, X. Fatty Acid de Novo Biosynthesis in Plastids: Key Enzymes and Their Critical Roles for Male Reproduction and Other Processes in Plants. Plant Physiol. Biochem. 2024, 210, 108654. [Google Scholar] [CrossRef]
- AID, F. Plant Lipid Metabolism. In Advances in Lipid Metabolism; IntechOpen: London, UK, 2020. [Google Scholar]
- Hajra, A.K.; Radin, N.S. Biosynthesis of the Cerebroside Odd-Numbered Fatty Acids. J. Lipid Res. 1962, 3, 327–332. [Google Scholar] [CrossRef]
- Park, Y.; Ledesma-Amaro, R.; Nicaud, J.-M. De Novo Biosynthesis of Odd-Chain Fatty Acids in Yarrowia lipolytica Enabled by Modular Pathway Engineering. Front. Bioeng. Biotechnol. 2020, 7, 484. [Google Scholar] [CrossRef] [PubMed]
- Croes, K.; Foulon, V.; Casteels, M.; Van Veldhoven, P.P.; Mannaerts, G.P. Phytanoyl-CoA Hydroxylase: Recognition of 3-Methyl-Branched Acyl-CoAs and Requirement for GTP or ATP and Mg2+ in Addition to Its Known Hydroxylation Cofactors. J. Lipid Res. 2000, 41, 629–636. [Google Scholar] [CrossRef]
- Foulon, V.; Sniekers, M.; Huysmans, E.; Asselberghs, S.; Mahieu, V.; Mannaerts, G.P.; Van Veldhoven, P.P.; Casteels, M. Breakdown of 2-Hydroxylated Straight Chain Fatty Acids via Peroxisomal 2-Hydroxyphytanoyl-CoA Lyase. J. Biol. Chem. 2005, 280, 9802–9812. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, D.; Pryse, K.M.; Okunade, A.L.; Su, X. Fatty Acid 2-Hydroxylase Mediates Diffusional Mobility of Raft-Associated Lipids, GLUT4 Level, and Lipogenesis in 3T3-L1 Adipocytes. J. Biol. Chem. 2010, 285, 25438–25447. [Google Scholar] [CrossRef]
- Jenkins, B.J.; Seyssel, K.; Chiu, S.; Pan, P.-H.; Lin, S.-Y.; Stanley, E.; Ament, Z.; West, J.A.; Summerhill, K.; Griffin, J.L.; et al. Odd Chain Fatty Acids; New Insights of the Relationship Between the Gut Microbiota, Dietary Intake, Biosynthesis and Glucose Intolerance. Sci. Rep. 2017, 7, 44845. [Google Scholar] [CrossRef]
- Jansen, G.A.; Wanders, R.J.A. Alpha-Oxidation. Biochim. Biophys. Acta-Mol. Cell Res. 2006, 1763, 1403–1412. [Google Scholar] [CrossRef]
- Wanders, R.J.; Jansen, G.A.; Lloyd, M.D. Phytanic Acid Alpha-Oxidation, New Insights into an Old Problem: A Review. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2003, 1631, 119–135. [Google Scholar] [CrossRef]
- Fillmore, N.; Abo Alrob, O.; Lopaschuk, G.D. Fatty Acid Beta-Oxidation; AOCS Lipid Library: Champaign, IL, USA, 2011. [Google Scholar]
- Kumari, A. Beta Oxidation of Fatty Acids. In Sweet Biochemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 17–19. [Google Scholar] [CrossRef]
- Casteels, M.; Foulon, V.; Mannaerts, G.P.; Van Veldhoven, P.P. Alpha-oxidation of 3-methyl-substituted Fatty Acids and Its Thiamine Dependence. Eur. J. Biochem. 2003, 270, 1619–1627. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Waterham, H.R.; Ferdinandusse, S. Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum. Front. Cell Dev. Biol. 2016, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Los, D.A.; Murata, N. Regulatory Roles in Photosynthesis of Unsaturated Fatty Acids in Membrane Lipids. In Lipids in Photosynthesis: Essential and Regulatory Functions; Springer: Dordrecht, The Netherlands, 2009; pp. 373–388. [Google Scholar] [CrossRef]
- Dörmann, P.; Hoffmann-Benning, S.; Balbo, I.; Benning, C. Isolation and Characterization of an Arabidopsis Mutant Deficient in the Thylakoid Lipid Digalactosyl Diacylglycerol. Plant Cell 1995, 7, 1801–1810. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hartel, H.; Lokstein, H.; Dormann, P.; Grimm, B.; Benning, C. Changes in the Composition of the Photosynthetic Apparatus in the Galactolipid-Deficient Dgd1 Mutant of Arabidopsis Thaliana. Plant Physiol. 1997, 115, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- McConn, M.; Browse, J. The Critical Requirement for Linolenic Acid Is Pollen Development, Not Photosynthesis, in an Arabidopsis Mutant. Plant Cell 1996, 8, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Browse, J. Jasmonate: An Oxylipin Signal with Many Roles in Plants. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2005; pp. 431–456. [Google Scholar]
- Cardoso, C.; Ripol, A.; Afonso, C.; Freire, M.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N. Fatty Acid Profiles of the Main Lipid Classes of Green Seaweeds from Fish Pond Aquaculture. Food Sci. Nutr. 2017, 5, 1186–1194. [Google Scholar] [CrossRef]
- Gnayem, N.; Unis, R.; Gnaim, R.; Israel, Á.; Gnaim, J.; Golberg, A. Fatty Acid Profile in Field-Collected Seaweed, Lipid Extraction Optimization, and Food Functional Properties. Life 2025, 15, 710. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Sidorov, R.A.; Starikov, A.Y.; Voronkov, A.S.; Medvedeva, A.S.; Krivova, Z.V.; Pakholkova, M.S.; Bachin, D.V.; Bedbenov, V.S.; Gabrielyan, D.A.; et al. Assessment of the Biotechnological Potential of Cyanobacterial and Microalgal Strains from IPPAS Culture Collection. Appl. Biochem. Microbiol. 2020, 56, 794–808. [Google Scholar] [CrossRef]
- Lu, Y.; Eiriksson, F.F.; Thorsteinsdóttir, M.; Simonsen, H.T. Valuable Fatty Acids in Bryophytes—Production, Biosynthesis, Analysis and Applications. Plants 2019, 8, 524. [Google Scholar] [CrossRef]
- Loffhagen, N.; Härtig, C.; Babel, W. Suitability of the Trans/Cis Ratio of Unsaturated Fatty Acids in Pseudomonas Putida NCTC 10936 as an Indicator of the Acute Toxicity of Chemicals. Ecotoxicol. Environ. Saf. 2001, 50, 65–71. [Google Scholar] [CrossRef]
- Manni, M.M.; Tiberti, M.L.; Pagnotta, S.; Barelli, H.; Gautier, R.; Antonny, B. Acyl Chain Asymmetry and Polyunsaturation of Brain Phospholipids Facilitate Membrane Vesiculation without Leakage. eLife 2018, 7, e34394. [Google Scholar] [CrossRef]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef] [PubMed]
- Prasertthai, P.; Paethaisong, W.; Theerakulpisut, P.; Dongsansuk, A. High Temperature Alters Leaf Lipid Membrane Composition Associated with Photochemistry of PSII and Membrane Thermostability in Rice Seedlings. Plants 2022, 11, 1454. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B. Changes of Lipid Composition and Saturation Level in Leaves and Roots for Heat-Stressed and Heat-Acclimated Creeping Bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2004, 51, 57–67. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V. Fatty Acids Composition of the Epiphytic Ferns, Platycerium Bifurcatum and Asplenium Nidus, and the Terrestrial Fern, Asplenium Trichomanes. Am. Fern J. 2021, 111, 117–128. [Google Scholar] [CrossRef]
- Lyons, J.M.; Wheaton, T.A.; Pratt, H.K. Relationship between the Physical Nature of Mitochondrial Membranes and Chilling Sensitivity in Plants. Plant Physiol. 1964, 39, 262–268. [Google Scholar] [CrossRef]
- Denich, T.; Beaudette, L.; Lee, H.; Trevors, J. Effect of Selected Environmental and Physico-Chemical Factors on Bacterial Cytoplasmic Membranes. J. Microbiol. Methods 2003, 52, 149–182. [Google Scholar] [CrossRef]
- Kaneda, T. Iso- and Anteiso-Fatty Acids in Bacteria: Biosynthesis, Function, and Taxonomic Significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Verma, K. Geological Records of Climate Change. In Global Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–185. [Google Scholar]
- Waldeck, A.R.; Olson, H.C.; Crockford, P.W.; Couture, A.M.; Cowie, B.R.; Hodgin, E.B.; Bergmann, K.D.; Dewing, K.; Grasby, S.E.; Clark, R.J.; et al. Marine Sulphate Captures a Paleozoic Transition to a Modern Terrestrial Weathering Environment. Nat. Commun. 2025, 16, 2087. [Google Scholar] [CrossRef] [PubMed]
- Hamann, E.; Denney, D.; Day, S.; Lombardi, E.; Jameel, M.I.; MacTavish, R.; Anderson, J.T. Review: Plant Eco-Evolutionary Responses to Climate Change: Emerging Directions. Plant Sci. 2021, 304, 110737. [Google Scholar] [CrossRef]
- Xu, S.Y.; Weng, J. Climate Change Shapes the Future Evolution of Plant Metabolism. Adv. Genet. 2020, 1, e10022. [Google Scholar] [CrossRef] [PubMed]
- Judd, E.J.; Tierney, J.E.; Lunt, D.J.; Montañez, I.P.; Huber, B.T.; Wing, S.L.; Valdes, P.J. A 485-Million-Year History of Earth’s Surface Temperature. Science 2024, 385, eadk3705. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane Fluidity and Its Roles in the Perception of Environmental Signals. Biochim. Biophys. Acta-Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Gigon, A.; Matos, A.-R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.-T. Effect of Drought Stress on Lipid Metabolism in the Leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef]
- López Alonso, D.; García-Maroto, F.; Rodríguez-Ruiz, J.; Garrido, J.A.; Vilches, M.A. Evolution of the Membrane-Bound Fatty Acid Desaturases. Biochem. Syst. Ecol. 2003, 31, 1111–1124. [Google Scholar] [CrossRef]
- Gan, L.; Park, K.; Chai, J.; Updike, E.M.; Kim, H.; Voshall, A.; Behera, S.; Yu, X.-H.; Cai, Y.; Zhang, C.; et al. Divergent Evolution of Extreme Production of Variant Plant Monounsaturated Fatty Acids. Proc. Natl. Acad. Sci. USA 2022, 119, e2201160119. [Google Scholar] [CrossRef] [PubMed]
- Raboanatahiry, N.; Yin, Y.; Chen, K.; He, J.; Yu, L.; Li, M. In Silico Analysis of Fatty Acid Desaturases Structures in Camelina Sativa, and Functional Evaluation of Csafad7 and Csafad8 on Seed Oil Formation and Seed Morphology. Int. J. Mol. Sci. 2021, 22, 10857. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Vereshchagin, M.; Kreslavski, V.; Ivanov, Y.; Kumachova, T.; Ryabchenko, A.; Voronkov, A.; Kosobryukhov, A.; Kuznetsov, V.; Allakhverdiev, S.I. Effect of Phytochrome Deficiency on Photosynthesis, Light-Related Genes Expression and Flavonoid Accumulation in Solanum Lycopersicum under Red and Blue Light. Cells 2022, 11, 3437. [Google Scholar] [CrossRef]
- Voronkov, A.; Ivanova, T.; Kumachova, T. Micromorphological and Biochemical Features of Malus Fruit: Malus Domestica Borkh. and Its Parent Species—Malus Orientalis Uglitzk. Braz. J. Bot. 2020, 43, 21–28. [Google Scholar] [CrossRef]
- Glew, R.H.; Vanderjagt, D.J.; Lockett, C.; Grivetti, L.E.; Smith, G.C.; Pastuszyn, A.; Millson, M. Amino Acid, Fatty Acid, and Mineral Composition of 24 Indigenous Plants of Burkina Faso. J. Food Compos. Anal. 1997, 10, 205–217. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Majchrzak, D.; Wagner, K.H.; Elmadfa, I.; Kafatos, A. Lipid Concentrations of Wild Edible Greens in Crete. Food Chem. 2006, 99, 822–834. [Google Scholar] [CrossRef]
- Dhouioui, M.; Boulila, A.; Jemli, M.; Schiets, F.; Casabianca, H.; Zina, M.S. Fatty Acids Composition and Antibacterial Activity of Aristolochia longa L. and Bryonia dioïca Jacq. Growing Wild in Tunisia. J. Oleo Sci. 2016, 65, 655–661. [Google Scholar] [CrossRef]
- Manning, R. Fatty Acids in Pollen: A Review of Their Importance for Honey Bees. Bee World 2001, 82, 60–75. [Google Scholar] [CrossRef]
- Jerónimo, E.; Cachucho, L.; Soldado, D.; Guerreiro, O.; Bessa, R.J.B.; Alves, S.P. Fatty Acid Content and Composition of the Morphological Fractions of Cistus ladanifer L. and Its Seasonal Variation. Molecules 2020, 25, 1550. [Google Scholar] [CrossRef]
- Hasimi, N.; Ertaş, A.; Varhan Oral, E.; Alkan, H.; Boğa, M.; Yılmaz, M.A.; Yener, İ.; Gazioğlu, I.; Özaslan, C.; Akdeniz, M.; et al. Chemical Profile of Malva Neglecta and Malvella Sherardiana by Lc- MS/MS, GC/MS and Their Anticholinesterase, Antimicrobial and Antioxidant Properties With Aflatoxin-Contents. Marmara Pharm. J. 2017, 21, 471. [Google Scholar] [CrossRef]
- Glew, R.S.; Amoako-Atta, B.; Ankar-Brewoo, G.; Presley, J.; Chuang, L.; Millson, M.; Smith, B.R.; Glew, R.H. Furthering an Understanding of West African Plant Foods. Br. Food J. 2010, 112, 1102–1114. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Leaves, Flowers, Immature Fruits and Leafy Flowered Stems of Malva Sylvestris: A Comparative Study of the Nutraceutical Potential and Composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef]
- Uzoekwe, N.M.; Ukhun, M.E. Fatty Acid and Amino Acid Composition of Two Wild Plants Used as Medicine in Nigeria. J. Chem. Soc. Niger. 2021, 46, 0160–0167. [Google Scholar] [CrossRef]
- Sayuti, M.; Supriatna, I.; Hismayasari, I.B.; Budiadyani, I.G.A.; Yani, A.; Siti Asma, S. Antioxidant Potentials and Fatty Acid Composition of Extracts Sterculia tragacantha Lindl. Leaves From Raja Ampat West Papua Province Indonesia. Int. Res. J. Pharm. 2018, 9, 58–63. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.; Carvalho, A.; Sánchez-Mata, M.; Cámara, M.; Tardío, J. Fatty Acids Profiles of Some Spanish Wild Vegetables. Food Sci. Technol. Int. 2012, 18, 281–290. [Google Scholar] [CrossRef]
- Idiem’ Opute, F. Mesocarp, Seed and Pollen Lipids of Raphia Palms. J. Sci. Food Agric. 1978, 29, 115–120. [Google Scholar] [CrossRef]
- Opute, F.I. Lipid and Sterol Composition of the Pollen of the West African Oilpalm, Elaeis Guineensis. Phytochemistry 1975, 14, 1023–1026. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Aktumsek, A.; Karatas, S. Fatty Acid Composition, Total Sugar Content and Anti-Diabetic Activity of Methanol and Water Extracts of Nine Different Fruit Tree Leaves Collected from Mediterranean Region of Turkey. Int. J. Food Prop. 2015, 18, 2268–2276. [Google Scholar] [CrossRef]
- Ekin, İ. Proximate and Fatty Acid Compositions of Four Therapeutic and Nutritional Plants for Two Consecutive Years. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 92, 929–937. [Google Scholar] [CrossRef]
- Goldson Barnaby, A. Antioxidant Activity, Total Phenolics and Fatty Acid Profile of Delonix Regia, Cassia Fistula, Spathodea Campanulata, Senna Siamea and Tibouchina Granulosa. J. Anal. Pharm. Res. 2016, 3, 00056. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kumar, A.S.; Kannathasan, K.; Venkatesalu, V. Fatty-Acid Composition of Some Mangroves. Chem. Nat. Compd. 2010, 46, 92–94. [Google Scholar] [CrossRef]
- Manning, R.; Harvey, M. Fatty Acids in Honeybee-Collected Pollens from Six Endemic Western Australian Eucalypts and the Possible Significance to the Western Australian Beekeeping Industry. Aust. J. Exp. Agric. 2002, 42, 217. [Google Scholar] [CrossRef]
- Cakir, A. Essential Oil and Fatty Acid Composition of the Fruits of Hippophae rhamnoides L. (Sea Buckthorn) and Myrtus communis L. from Turkey. Biochem. Syst. Ecol. 2004, 32, 809–816. [Google Scholar] [CrossRef]
- Dallali, S.; Zouaouia, R.; Dallalic, D.; Jdidia, S.; Toumia, L. Determination of Some Biochemical Parameters from Leaves of Quercus ilex L. (Fagaceae), Collected in Djabel Zagouan (Tunisia). Arab. J. Med. Aromat. Plants 2021, 7, 1–28. [Google Scholar] [CrossRef]
- Barros, L.; Oliveira, S.; Carvalho, A.M.; Ferreira, I.C.F.R. In Vitro Antioxidant Properties and Characterization in Nutrients and Phytochemicals of Six Medicinal Plants from the Portuguese Folk Medicine. Ind. Crops Prod. 2010, 32, 572–579. [Google Scholar] [CrossRef]
- Montaner, C.; Zufiaurre, R.; Movila, M.; Mallor, C. Evaluation of Borage (Borago officinalis L.) Genotypes for Nutraceutical Value Based on Leaves Fatty Acids Composition. Foods 2021, 11, 16. [Google Scholar] [CrossRef]
- Tsiaganis, M.C.; Laskari, K.; Melissari, E. Fatty Acid Composition of Allium Species Lipids. J. Food Compos. Anal. 2006, 19, 620–627. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Olgun, A. Fatty Acid Composition in Leaf Lipids of Some Carex L. (Cyperaceae) Species from Northeast Anatolia (Turkey). Grasas Aceites 2000, 51, 307–310. [Google Scholar] [CrossRef]
- Shawer, M.B.; Ali, S.M.; Abdellatif, M.A.; El-Refai, A.A. Biochemical Studies of Bee-Collected Pollen in Egypt 2. Fatty Acids and Non-Saponifiables. J. Apic. Res. 1987, 26, 133–136. [Google Scholar] [CrossRef]
- Savych, A.; Basaraba, R.; Muzyka, N.; Ilashchuk, P. Analysis of Fatty Acid Composition Content in the Plant Components of Antidiabetic Herbal Mixture by GC-MS. Pharmacia 2021, 68, 433–439. [Google Scholar] [CrossRef]
- Koukos, D.; Meletiou-Christou, M.-S.; Rhizopoulou, S. Leaf Surface Wettability and Fatty Acid Composition of Arbutus Unedo and Arbutus Andrachne Grown under Ambient Conditions in a Natural Macchia. Acta Bot. Gall. 2015, 162, 225–232. [Google Scholar] [CrossRef][Green Version]
- Karimi, E.; Jaafar, H.Z.; Ghasemzadeh, A.; Ebrahimi, M. Fatty Acid Composition, Antioxidant and Antibacterial Properties of the Microwave Aqueous Extract of Three Varieties of Labisia Pumila Benth. Biol. Res. 2015, 48, 9. [Google Scholar] [CrossRef]
- Lan Anh, N.T. Variations in Fatty Acid Composition of Tea Leaves (Camellia sinensis) Due to Plucking Time and Cultivars. Vietnam J. Sci. Technol. 2018, 54, 284. [Google Scholar] [CrossRef]
- Liu, P.; Qin, L.-J.; Yuan, T.; Yang, Z.-W.; Zhao, D.-G. Fatty Acid Composition of Eucommia Rubber Particles, Content and Molecular Weight Distribution of Eu-Rubber in Eucommia Ulmoides Leaves and Samaras. Authorea 2024. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M. Chemical Evaluation of Flower Bud and Oils of Tumbleweed (Gundelia tourneforti L.) as a New Potential Nutrition Sources. J. Food Biochem. 2011, 35, 1257–1266. [Google Scholar] [CrossRef]
- Tekeli, Y.; Sezgin, M.; Aktumsek, A.; Ozmen Guler, G.; Aydin Sanda, M. Fatty Acid Composition of Six Centaurea Species Growing in Konya, Turkey. Nat. Prod. Res. 2010, 24, 1883–1889. [Google Scholar] [CrossRef]
- Erasto, P.; Grierson, D.S.; Afolayan, A.J. Evaluation of Antioxidant Activity and the Fatty Acid Profile of the Leaves of Vernonia Amygdalina Growing in South Africa. Food Chem. 2007, 104, 636–642. [Google Scholar] [CrossRef]
- Hamia, C.; Gourine, N.; Boussoussa, H.; Saidi, M.; Gaydou, E.M.; Yousfi, M. Chemical Composition and Antioxidant Activity of the Essential Oil and Fatty Acids of the Flowers of Rhanterium Adpressum. Nat. Prod. Commun. 2013, 8, 1934578X1300800837. [Google Scholar] [CrossRef]
- Orhan, I.; Sener, B. Comparative Fatty Acid Analysis of Telekia Speciosa. Chem. Nat. Compd. 2003, 39, 244–245. [Google Scholar] [CrossRef]
- Rhimi, W.; Ben Salem, I.; Iatta, R.; Chaabane, H.; Saidi, M.; Boulila, A.; Cafarchia, C. Dittrichia viscosa L. Leaves Lipid Extract: An Unexploited Source of Essential Fatty Acids and Tocopherols with Antifungal and Anti-Inflammatory Properties. Ind. Crops Prod. 2018, 113, 196–201. [Google Scholar] [CrossRef]
- Manish Tadhani, M.T.; Rema Subhash, R.S. Preliminary Studies on Stevia Rebaudiana Leaves: Proximal Composition, Mineral Analysis and Phytochemical Screening. J. Med. Sci. 2006, 6, 321–326. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Assessment of the Antioxidant Potential and Fatty Acid Composition of Four Centaurea L. Taxa from Turkey. Food Chem. 2013, 141, 91–97. [Google Scholar] [CrossRef]
- Tekeli, Y.; Zengin, G.; Aktumsek, A.; Sezgin, M. Comparison of the Fatty Acid Compositions of Six Centaurea Species. Chem. Nat. Compd. 2013, 49, 496–498. [Google Scholar] [CrossRef]
- Zengin, G.; Cakmak, Y.S.; Guler, G.O.; Aktumsek, A. In Vitro Antioxidant Capacities and Fatty Acid Compositions of Three Centaurea Species Collected from Central Anatolia Region of Turkey. Food Chem. Toxicol. 2010, 48, 2638–2641. [Google Scholar] [CrossRef]
- Erdogan, T.; Gonenc, T.; Cakilcioglu, U.; Kivcak, B. Fatty Acid Composition of the Aerial Parts of Some Centaurea Species in Elazig, Turkey. Trop. J. Pharm. Res. 2014, 13, 613. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R. Nutritional and Chemical Characterization of Edible Petals and Corresponding Infusions: Valorization as New Food Ingredients. Food Chem. 2017, 220, 337–343. [Google Scholar] [CrossRef]
- Harrathi, J.; Hosni, K.; Karray-Bouraoui, N.; Attia, H.; Marzouk, B.; Magné, C.; Lachaâl, M. Effect of Salt Stress on Growth, Fatty Acids and Essential Oils in Safflower (Carthamus tinctorius L.). Acta Physiol. Plant. 2012, 34, 129–137. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Petropoulos, S.A.; Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Plenis, A.; Viapiana, A. Chemical Composition and Antioxidant Properties of Common and Lemon Verbena. Antioxidants 2022, 11, 2247. [Google Scholar] [CrossRef]
- Kilic, Ö. Essential Oil and Fatty Acid Composition of Leaves of Some Lamiaceae Taxa FromTurkey. J. Essent. Oil Bear. Plants 2018, 21, 1706–1711. [Google Scholar] [CrossRef]
- Kokten, K.; Mokhtarzadeh, S.; Cacan, E.; Kutlu, M.A.; Ozdemir, S.; Ucar, R.; Ekmekci, M. Fatty Acid Composition of Stems, Leaves, Flowers, and Seeds of Some Medicinal Plants. Riv. Ital. Sostanze Grasse 2023, 100, 103–108. [Google Scholar]
- Tabti, L.; El Amine Dib, M.; Gaouar Benyelles, N.; Djabou, N.; Bouayad Alam, S.; Paolini, J.; Costa, J.; Muselli, A. Fatty-Acid Composition and Antifungal Activity of Extracts of Thymus Capitatus. J. Herbs. Spices Med. Plants 2015, 21, 203–210. [Google Scholar] [CrossRef]
- Cacan, E.; Kokten, K.; Kilic, O. Leaf Fatty Acid Composition of Some Lamiaceae Taxa from Turkey. Prog. Nutr. 2018, 20, 231–236. [Google Scholar] [CrossRef]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V.; Chandrasekaran, M. Larvicidal Activity of Fatty Acid Methyl Esters of Vitex Species against Culex quinquefasciatus. Parasitol. Res. 2008, 103, 999–1001. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Voronkov, A.S. Lighting Intensity Affects the Fatty Acid Composition of Total Lipids of Basil Leaves and Roots (Ocimum basilicum L.). Russ. J. Plant Physiol. 2023, 70, 94. [Google Scholar] [CrossRef]
- Ben Taarit, M.; Msaada, K.; Hosni, K.; Marzouk, B. Changes in Fatty Acid and Essential Oil Composition of Sage (Salvia officinalis L.) Leaves under NaCl Stress. Food Chem. 2010, 119, 951–956. [Google Scholar] [CrossRef]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.Á.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Characterization and Bioactive Properties of Two Aromatic Plants: Calendula officinalis L. (Flowers) and Mentha cervina L. (Leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Makowska-Wąs, J.; Galanty, A.; Gdula-Argasińska, J.; Tyszka-Czochara, M.; Szewczyk, A.; Nunes, R.; Carvalho, I.S.; Michalik, M.; Paśko, P. Identification of Predominant Phytochemical Compounds and Cytotoxic Activity of Wild Olive Leaves (Olea europaea L. ssp. sylvestris) Harvested in South Portugal. Chem. Biodivers. 2017, 14, e1600331. [Google Scholar] [CrossRef]
- Popov, V.N.; Antipina, O.V.; Pchelkin, V.P.; Tsydendambaev, V.D. Changes in the Content and Composition of Lipid Fatty Acids in Tobacco Leaves and Roots at Low-Temperature Hardening. Russ. J. Plant Physiol. 2012, 59, 177–182. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Garrido-Fernández, J.; Mínguez-Mosquera, M.I.; Lozano-Ruiz, M.; Montero-de-Espinosa, V. Fatty Acid Composition of Two New Pepper Varieties (Capsicum annuum L. cv. Jaranda and Jariza). Effect of Drying Process and Nutritional Aspects. J. Am. Oil Chem. Soc. 1999, 76, 205–208. [Google Scholar] [CrossRef]
- Ogunka-Nnoka, C.; Igwe, F.; Agwu, J.; Peter, O.; Wolugbom, P.H. Nutrient and Phytochemical Composition of Centella Asiatica Leaves. Med. Aromat. Plants 2020, 9, 346. [Google Scholar] [CrossRef]
- Ben Hamed, K.; Ben Youssef, N.; Ranieri, A.; Zarrouk, M.; Abdelly, C. Changes in Content and Fatty Acid Profiles of Total Lipids and Sulfolipids in the Halophyte Crithmum Maritimum under Salt Stress. J. Plant Physiol. 2005, 162, 599–602. [Google Scholar] [CrossRef]
- Neffati, M.; Marzouk, B. Changes in Essential Oil and Fatty Acid Composition in Coriander (Coriandrum sativum L.) Leaves under Saline Conditions. Ind. Crops Prod. 2008, 28, 137–142. [Google Scholar] [CrossRef]
- Kumar, A.; Or-rachid, M.M.; Alzahal, O.; Mcbride, B.W. Fatty Acid Profile of Chaya (Cnidoscolus aconitifolius) Leaves and Fodder. Indian J. Anim. Sci. 2011, 81, 864–869. [Google Scholar]
- Zhang, D.; He, Y. High Precision for Leaf Area Measurement and Instrument Development. Adv. J. Food Sci. Technol. 2014, 6, 167–172. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M.J. Antibacterial and Antifungal Activities of Fatty Acid Methyl Esters of the Blind-Your-Eye Mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.J.; López, M.D. Leaf Lipids from Euphorbia Lagascae Spreng. and Euphorbia lathyris L. Ind. Crops Prod. 2010, 32, 560–565. [Google Scholar] [CrossRef]
- Farag, R.S.; Youssef, A.M.; Ewies, M.A.; Hallabo, S.A.S. Long-Chain Fatty Acids of Six Pollens Collected by Honeybees in Egypt. J. Apic. Res. 1978, 17, 100–104. [Google Scholar] [CrossRef]
- Aziz, S.; Mitu, T.K. Analysis of Fatty Acid and Determination of Total Protein and Phytochemical Content of Cassia Sophera Linn Leaf, Stem, Flower, and Seed. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 3. [Google Scholar] [CrossRef]
- Shukla, S.; Hegde, S.; Kumar, A.; Chaudhary, G.; Tewari, S.K.; Upreti, D.K.; Pal, M. Fatty Acid Composition and Antibacterial Potential of Cassia Tora (Leaves and Stem) Collected from Different Geographic Areas of India. J. Food Drug Anal. 2018, 26, 107–111. [Google Scholar] [CrossRef]
- Saini, R.K.; Shetty, N.P.; Giridhar, P. GC-FID/MS Analysis of Fatty Acids in Indian Cultivars of Moringa Oleifera: Potential Sources of PUFA. J. Am. Oil Chem. Soc. 2014, 91, 1029–1034. [Google Scholar] [CrossRef]
- Seregin, I.V.; Ivanova, T.V.; Voronkov, A.S.; Kozhevnikova, A.D.; Schat, H. Zinc- and Nickel-Induced Changes in Fatty Acid Profiles in the Zinc Hyperaccumulator Arabidopsis halleri and Non-Accumulator Arabidopsis lyrata. Plant Physiol. Biochem. 2023, 197, 107640. [Google Scholar] [CrossRef] [PubMed]
- Rothnie, N.E.; Palmer, M.V.; Burke, D.G.; Sang, J.P.; Hilliard, E.P.; Salisbury, P.A.; Evans, D.E.; Knox, R.B.; Williams, E.G. Comparative Analysis of Fatty Acids in Pollen and Seed of Rapeseed. Phytochemistry 1987, 26, 1895–1897. [Google Scholar] [CrossRef]
- Iosypenko, O.O.; Kyslychenko, V.S.; Omelchenko, Z.I.; Burlaka, I.S. Fatty Acid Composition of Vegetable Marrows and Zucchini Leaves. Pharmacia 2019, 66, 201–207. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and Antifungal Efficacy of Fatty Acid Methyl Esters from the Leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 775–780. [Google Scholar]
- Liu, L.; Howe, P.; Zhou, Y.-F.; Xu, Z.-Q.; Hocart, C.; Zhang, R. Fatty Acids and β-Carotene in Australian Purslane (Portulaca oleracea) Varieties. J. Chromatogr. A 2000, 893, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Akbaş, P.; Ceyhan, G.; Karabekmez Erdem, T.; Alkan, H. Determination the Fatty Acid Composition of the Rumex patientia L. Leaves and in Vitro Antimicrobial Activity of Their Different Extracts. Süleyman Demirel Üniv. Fen Bilim. Enst. Derg. 2020, 24, 362–367. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V. Antimicrobial Activity of Fatty Acid Methyl Esters of Some Members of Chenopodiaceae. Z. Naturforsch. C 2008, 63, 331–336. [Google Scholar] [CrossRef]
- Tsydendambaev, V.D.; Ivanova, T.V.; Khalilova, L.A.; Kurkova, E.B.; Myasoedov, N.A.; Balnokin, Y.V. Fatty Acid Composition of Lipids in Vegetative Organs of the Halophyte Suaeda Altissima under Different Levels of Salinity. Russ. J. Plant Physiol. 2013, 60, 661–671. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Maiorova, O.V.; Orlova, Y.V.; Kuznetsova, E.I.; Khalilova, L.A.; Myasoedov, N.A.; Balnokin, Y.V.; Tsydendambaev, V.D. Cell Ultrastructure and Fatty Acid Composition of Lipids in Vegetative Organs of Chenopodium album L. under Salt Stress Conditions. Russ. J. Plant Physiol. 2016, 63, 763–775. [Google Scholar] [CrossRef]
- Nowak, R. Fatty Acids Composition in Fruits of Wild Rose Species. Acta Soc. Bot. Pol. 2011, 74, 229–235. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Studies on Chemical Constituents and Bioactivity of Rosa Micrantha: An Alternative Antioxidants Source for Food, Pharmaceutical, or Cosmetic Applications. J. Agric. Food Chem. 2010, 58, 6277–6284. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.V.; Voronkov, A.S.; Kumakhova, T.K.; Tsydendambaev, V.D. Distinguishing Features of Fatty Acid Content and Composition in Total Lipids of Malus Orientalis Uglitzk. Pericarp. Russ. J. Plant Physiol. 2020, 67, 463–471. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kuznetsova, E.I.; Kumachova, T.K. Adaptations of Malus domestica Borkh. (Rosaceae) Fruits Grown at Different Altitudes. Russ. J. Plant Physiol. 2019, 66, 922–931. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Voronkov, A.S.; Kuznetsova, E.I.; Kumachova, T.K.; Zhirov, V.K.; Tsydendambaev, V.D. Lipid Fatty Acids from the Pericarp of Cydonia Oblonga Mill. and Mespilus germanica L. Are Involved in Plant Adaptation to Altitudinal Zonality. Dokl. Biochem. Biophys. 2019, 486, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Dmitruk, M.E.; Chrzanowska, E.; Strzałkowska-Abramek, M.; Stawiarz, E. Prunus spinosa L. Pollen-Quantity and Nutritional Quality. Acta Agrobot. 2023, 76, 1–6. [Google Scholar] [CrossRef]
- Loper, G.M.; Standifer, L.N.; Thompson, M.J.; Gilliam, M. Biochemistry and Microbiology of Bee-Collected Almond (Prunus Dulcis) Pollen and Bee Bread. I- Fatty Acids, Sterols, Vitamins and Minerals. Apidologie 1980, 11, 63–73. [Google Scholar] [CrossRef]
- Đurović, S.; Pavlić, B.; Šorgić, S.; Popov, S.; Savić, S.; Petronijević, M.; Radojković, M.; Cvetanović, A.; Zeković, Z. Chemical Composition of Stinging Nettle Leaves Obtained by Different Analytical Approaches. J. Funct. Foods 2017, 32, 18–26. [Google Scholar] [CrossRef]
- Radojković, M.; Zeković, Z.; Mašković, P.; Vidović, S.; Mandić, A.; Mišan, A.; Đurović, S. Biological Activities and Chemical Composition of Morus Leaves Extracts Obtained by Maceration and Supercritical Fluid Extraction. J. Supercrit. Fluids 2016, 117, 50–58. [Google Scholar] [CrossRef]
- Harrat, M.; Benalia, M.; Gourine, N.; Yousfi, M. Variability of the Chemical Compositions of Fatty Acids, Tocopherols and Lipids Antioxidant Activities, Obtained from the Leaves of Pistacia lentiscus L. Growing in Algeria. Med. J. Nutr. Metab. 2018, 11, 199–215. [Google Scholar] [CrossRef]
- Rao, G.N.; Pamidighantam, P.R.; Akula, S. Chemical, Fatty Acid, Volatile Oil Composition and Antioxidant Activity of Shade Dried Neem (Azadirachta indica L.) Flower Powder. Int. Food Res. J. 2014, 21, 807–813. [Google Scholar]
- Mongrand, S.; Badoc, A.; Patouille, B.; Lacomblez, C.; Chavent, M.; Cassagne, C.; Bessoule, J.-J. Taxonomy of Gymnospermae: Multivariate Analyses of Leaf Fatty Acid Composition. Phytochemistry 2001, 58, 101–115. [Google Scholar] [CrossRef]
- Dębski, H.; Mitrus, J.; Góraj-Koniarska, J.; Szablińska-Piernik, J.; Saniewski, M.; Horbowicz, M. The Changes in Fatty Acid Profile during Senescence and Methyl Jasmonate-Induced Senescence of Ginkgo Biloba Leaves. Acta Sci. Pol. Hortorum Cultus 2023, 22, 95–106. [Google Scholar] [CrossRef]
- Ching, T.M.; Ching, K.K. Fatty Acids in Pollen of Some Coniferous Species. Science 1962, 138, 890–891. [Google Scholar] [CrossRef]
- Jamieson, G.R.; Reid, E.H. The Leaf Lipids of Some Conifer Species. Phytochemistry 1972, 11, 269–275. [Google Scholar] [CrossRef]
- Zhukov, A.V.; Kuznetsova, E.I.; Pchelkin, V.P.; Sidorov, R.A.; Tsydendambaev, V.D. Fatty Acid Composition of Lipids from Leaves and Strobila of Cycas Revoluta (Cycas revoluta). Russ. J. Plant Physiol. 2018, 65, 23–29. [Google Scholar] [CrossRef]
- Semenova, N.V.; Makarenko, S.P.; Shmakov, V.N.; Konstantinov, Y.M.; Dudareva, L.V. Fatty Acid Composition of Total Lipids from Needles and Cultured Calluses of Conifers Pinus sylvestris L., Picea pungens Engelm., Pinus koraiensis Siebold & Zucc., and Larix sibirica Ledeb. Biochem. Suppl. Ser. A Membr. Cell Biol. 2017, 11, 287–295. [Google Scholar] [CrossRef]
- Sato, M.; Seki, K.; Kita, K.; Moriguchi, Y.; Yunoki, K.; Kofujita, H.; Ohnishi, M. Prominent Differences in Leaf Fatty Acid Composition in the F 1 Hybrid Compared with Parent Trees Larix gmelinii Var. japonica and L. kaempferi. Biosci. Biotechnol. Biochem. 2008, 72, 2895–2902. [Google Scholar] [CrossRef][Green Version]
- Nekrasov, E.V.; Svetashev, V.I.; Khrapko, O.V.; Vyssotski, M.V. Variability of Fatty Acid Profiles in Ferns: Relation to Fern Taxonomy and Seasonal Development. Phytochemistry 2019, 162, 47–55. [Google Scholar] [CrossRef]
- Rabert, C.; Inostroza, K.; Bravo, S.; Sepúlveda, N.; Bravo, L.A. Exploratory Study of Fatty Acid Profile in Two Filmy Ferns with Contrasting Desiccation Tolerance Reveal the Production of Very Long Chain Polyunsaturated Omega-3 Fatty Acids. Plants 2020, 9, 1431. [Google Scholar] [CrossRef]
- Rozentsvet, O.A.; Rezanka, T.; Bosenko, E.S.; Uzhametskaya, E.A.; Dembitskii, V.M. Fatty Acids, Phospholipids, and the Betaine Lipid DGTS from the Aquatic Fern Salvinia natans. Chem. Nat. Compd. 2005, 41, 487–490. [Google Scholar] [CrossRef]
- Paoletti, C.; Bocci, F.; Lercker, G.; Capella, P.; Materassi, R. Lipid Composition of Azolla Caroliniana Biomass and Its Seasonal Variation. Phytochemistry 1987, 26, 1045–1047. [Google Scholar] [CrossRef]
- Lytle, T.F.; Lytle, J.S.; Caruso, A. Hydrocarbons and Fatty Acids of Ferns. Phytochemistry 1976, 15, 965–970. [Google Scholar] [CrossRef]
- Radunz, A. Über Die Lipide Der Pteridophyten—I. Phytochemistry 1967, 6, 399–406. [Google Scholar] [CrossRef]
- Rozentsvet, O.A.; Bogdanova, E.S.; Golovko, T.K.; Zakhozhiy, I.G. Composition of Lipids of the Leaves of Some Representatives of Polypodiophyta in the Polar Urals. Chem. Plant Raw Mater. 2013, 127–134. [Google Scholar] [CrossRef][Green Version]
- Robinson, P.M.; Smith, D.L.; Safford, R.; Nichols, B.W. Lipid Metabolism in the Fern Polypodium Vulgare. Phytochemistry 1973, 12, 1377–1381. [Google Scholar] [CrossRef]
- Nekrasov, E.V.; Svetashev, V.I. Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids. Foods 2021, 10, 1220. [Google Scholar] [CrossRef]
- Jamieson, G.R.; Reid, E.H. The Fatty Acid Composition of Fern Lipids. Phytochemistry 1975, 14, 2229–2232. [Google Scholar] [CrossRef]
- Nekrasov, E.V.; Shelikhan, L.A.; Svetashev, V.I. Fatty Acid Composition of Gametophytes of Matteuccia struthiopteris (L.) Tod. (Onocleaceae, Polypodiophyta). Bot. Pacifica 2019, 8, 63–66. [Google Scholar] [CrossRef][Green Version]
- Dudareva, L.V.; Nokhsorov, V.V.; Rudikovskaya, E.G.; Petrov, K.A. Fatty-Acid Profiles of Aerial Parts of Three Horsetail Species Growing in Central and Northern Yakutia. Chem. Nat. Compd. 2015, 51, 220–223. [Google Scholar] [CrossRef]
- Schlenk, H.; Gellerman, J.L. Arachidonic, 5, 11, 14, 17-eicosatetraenoic and Related Acids in Plants舒Identification of Unsaturated Fatty Acids. J. Am. Oil Chem. Soc. 1965, 42, 504–511. [Google Scholar] [CrossRef]
- Koskimies, K.; Simola, L.K. The Fatty Acid Composition of Some Sphagnum Species. Can. J. Bot. 1980, 58, 259–263. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T. Distribution of Diacylglycerylhomoserines, Phospholipids and Fatty Acids in Thirteen Moss Species from Southwestern Siberia. Biochem. Syst. Ecol. 1995, 23, 71–78. [Google Scholar] [CrossRef]
- Al-Hasan, R.H.; Ka’wash, H.H.; Radwan, S.S. Enrichment of Mosses with Lipids and Polyunsaturated Fatty Acids by Nitrogen Starvation. Bryologist 1991, 94, 196. [Google Scholar] [CrossRef]
- Ismaeela, M.; Mokraniaand, L.; Ibrahimb, A. Characterization of Fatty Acids Profile in Some Moss Species in Syria. Jordan J. Chem. 2022, 17, 27–33. [Google Scholar] [CrossRef]
- Kalacheva, G.S.; Sushchik, N.N.; Gladyshev, M.I.; Makhutova, O.N. Seasonal Dynamics of Fatty Acids in the Lipids of Water Moss Fontinalis Antipyretica from the Yenisei River. Russ. J. Plant Physiol. 2009, 56, 795–807. [Google Scholar] [CrossRef]
- Pejin, B.; Vujisic, L.; Sabovljevic, M.; Tesevic, V.; Vajs, V. Fatty Acid Chemistry of Atrichum Undulatum and Hypnum Andoi. Hem. Ind. 2012, 66, 207–209. [Google Scholar] [CrossRef]
- Pejin, B.; Vujisic, L.; Sabovljevic, A.; Sabovljevic, M.; Tesevic, V.; Vajs, V. Fatty Acids of Some Moss Species from Germany. Asian J. Chem. 2011, 23, 5187–5188. [Google Scholar]
- Petkova, Z.; Teneva, O.; Antova, G.; Angelova-Romova, M.; Gecheva, G.; Dimitrova-Dyulgerova, I. Chemical Composition, Lipid-Soluble Bioactive Compounds and Potential Health Benefits of the Moss Hypnum Cupressiforme Hedw. Plants 2023, 12, 4190. [Google Scholar] [CrossRef]
- Hansen, C.E.; Rossi, P. Effects of Culture Conditions on Accumulation of Arachidonic and Eicosapentaenoic Acids in Cultured Cells of Rhytidiadelphus Squarrosus and Eurhynchium Striatum. Phytochemistry 1991, 30, 1837–1841. [Google Scholar] [CrossRef]
- Pejin, B.; Vujisic, L.; Sabovljevic, M.; Sabovljevic, A.; Tesevic, V.; Vajs, V. Preliminary Analysis of Fatty Acid Chemistry of Kindbergia praelonga and Kindbergia stokesii (Brachytheciaceae). J. Serbian Chem. Soc. 2010, 75, 1637–1640. [Google Scholar] [CrossRef]
- Pejin, B.; Vujisic, L.; Sabovljevic, M.; Tesevic, V.; Vajs, V. Cuspidata, An Insight into Fatty Acid Composition of Calliergonella. Asian J. Chem. 2011, 23, 5161–5162. [Google Scholar]
- Pejin, B.; Bianco, A.; Newmaster, S.; Sabovljevic, M.; Vujisic, L.; Tesevic, V.; Vajs, V.; De Rosa, S. Fatty Acids of Rhodobryum ontariense (Bryaceae). Nat. Prod. Res. 2012, 26, 696–702. [Google Scholar] [CrossRef]
- Anderson, W.H.; Gellerman, J.L.; Schlenk, H. Arachidonic and Eicosapentaenoic Acids in Developing Gametophores and Sporophytes of the Moss, Mnium cuspidatum. Lipids 1972, 7, 710–714. [Google Scholar] [CrossRef]
- Pejin, B.; Vujisic, L.; Sabovljevic, M.; Tesevic, V.; Vajs, V. The Moss Mnium Hornum, a Promising Source of Arachidonic Acid. Chem. Nat. Compd. 2012, 48, 120–121. [Google Scholar] [CrossRef]
- Gellerman, J.L.; Anderson, W.H.; Schlenk, H. Synthesis and Function of 9,12,15-Octadecatrien-6-Ynoic Acid in the Moss Ceratodon purpureus. Biochemistry 1977, 16, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, R.H.; El-Saadawi, W.E.; Ali, A.M.; Radwan, S.S. Lipids of the Gametophyte and Sporophyte of Funaria Hygrometrica. Comparison with Lipids from Leaves of Vascular Plants. Bryologist 1990, 93, 44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).