The Role of the MntABC Transporter System in the Oxidative Stress Resistance of Deinococcus radiodurans
Abstract
1. Introduction
2. Results
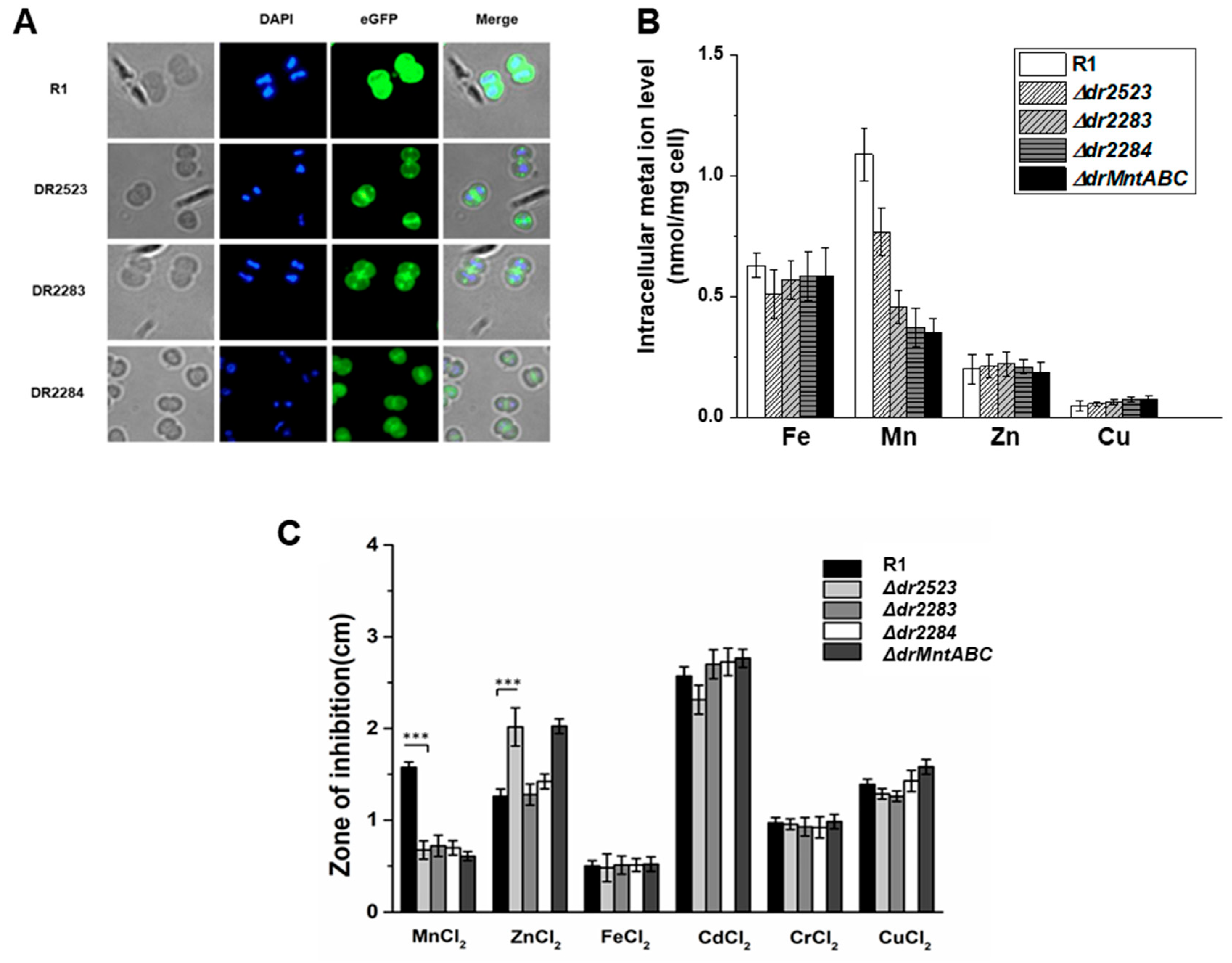
2.1. Identification of the MntABC System in D. radiodurans
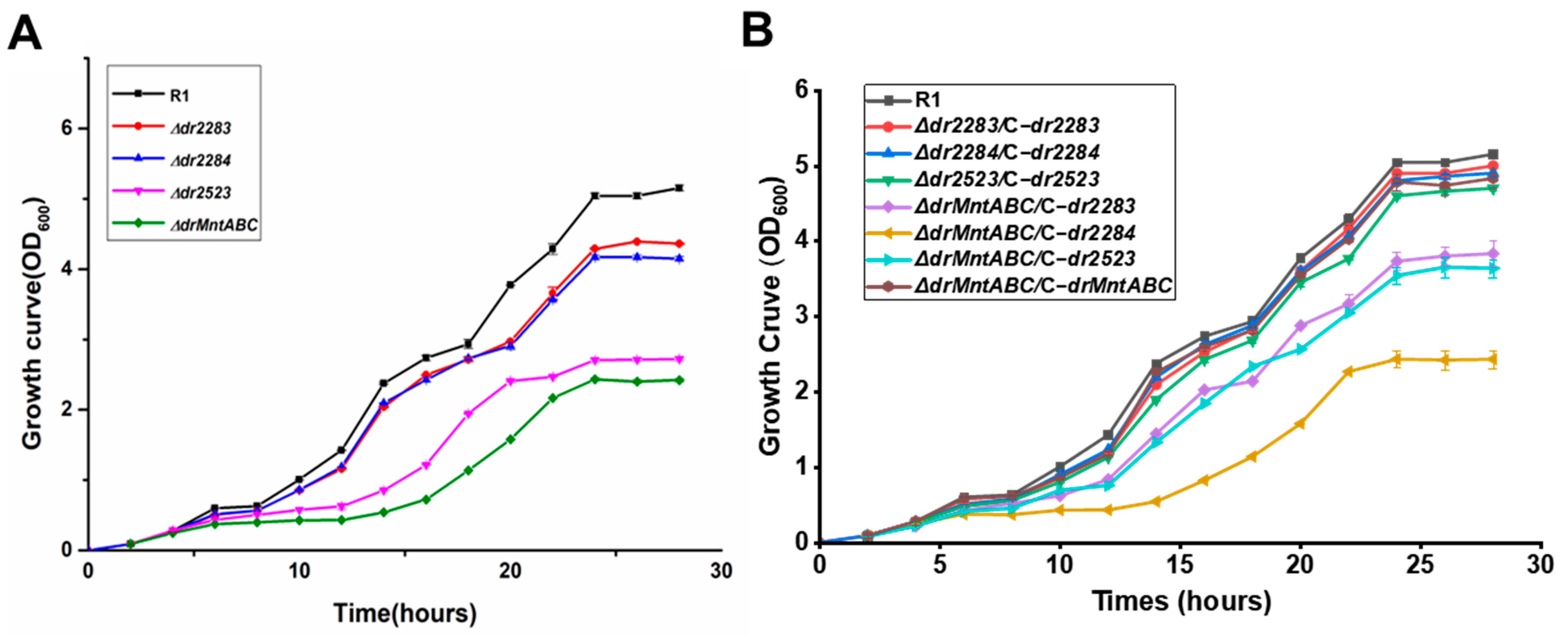
2.2. Mutation Deficiency of the MntABC System Affects Cellular Growth
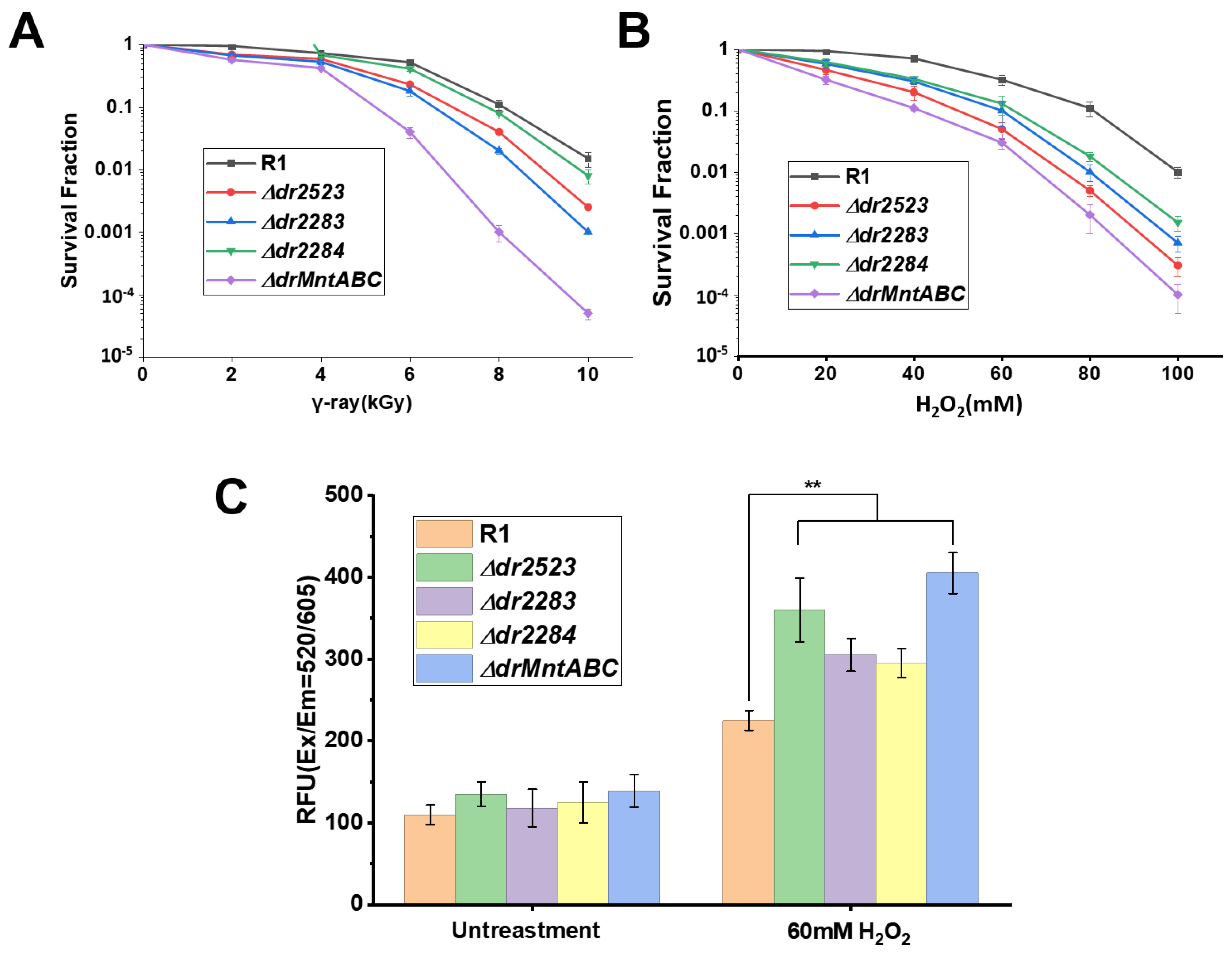
2.3. MntABC Is Involved in the Resistance of D. radiodurans to Oxidative Stress
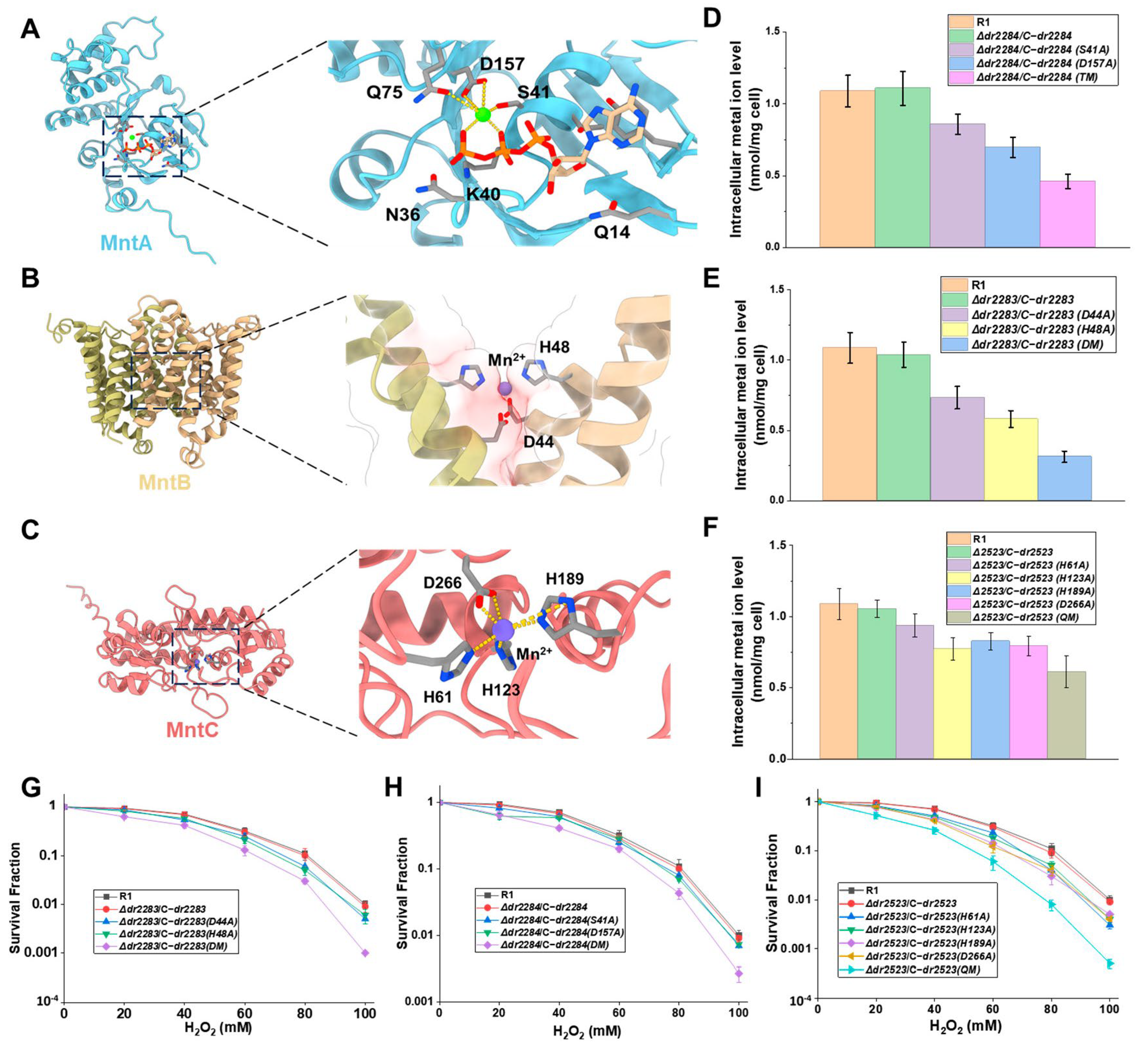
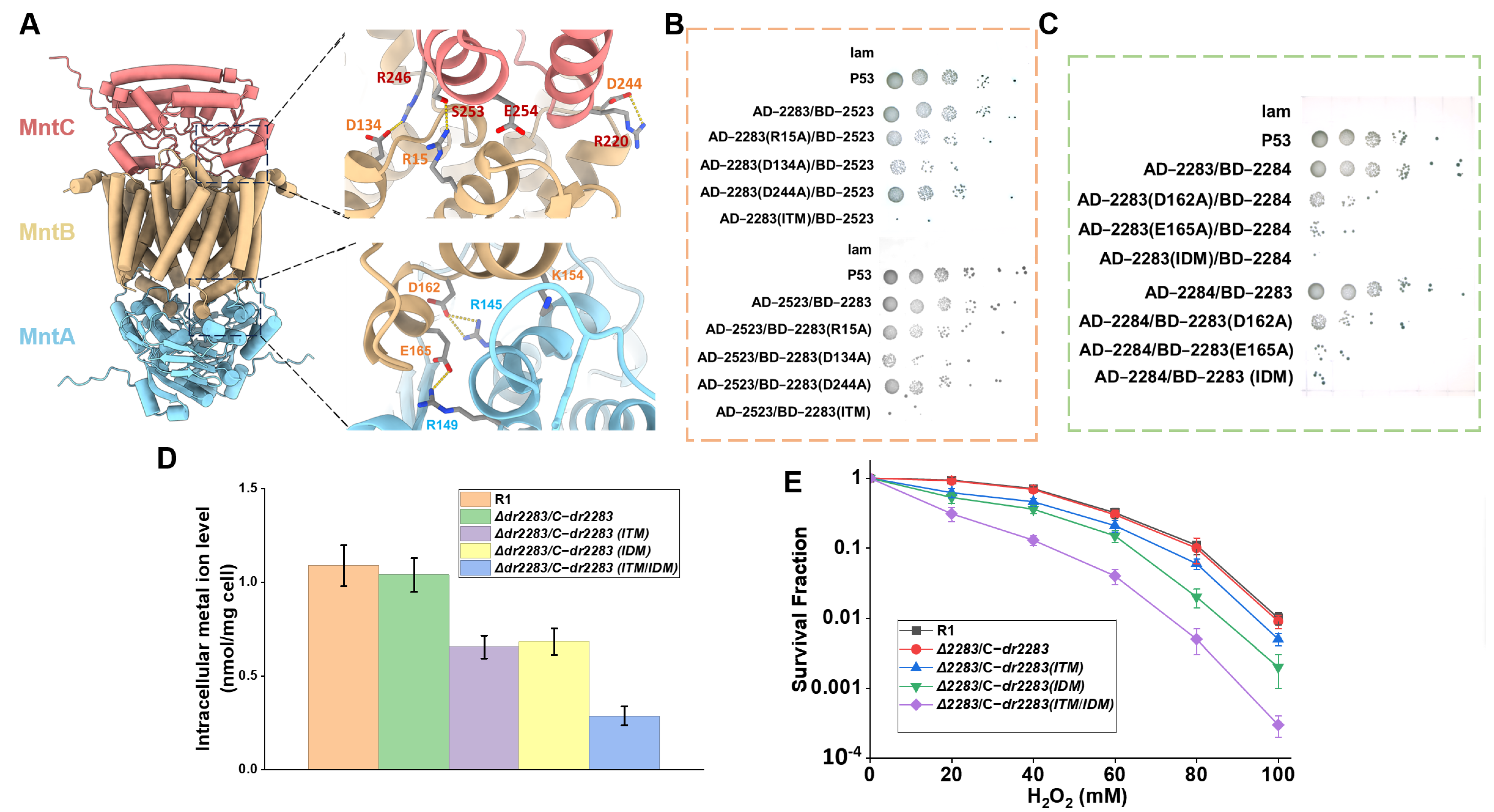
2.4. Key Sites Within MntABC Proteins Involved in Mn Ion Transport and Oxidative Stress Resistance
2.5. Interactions Between the Subunits of the MntABC System Impact on Manganese ion Transport
3. Discussion
4. Material and Methods
4.1. Bacterial Strains and Growth Media
4.2. Bioinformatics and Structural Analysis
4.3. Targeted Gene Disruption via Homologous Recombination
4.4. Gene Complementation
4.5. Site-Directed Mutagenesis
4.6. Subcellular Localization Analysis of DrMntABC
4.7. Metal Cation Sensitivity Assessment
4.8. Intracellular Metal Ion Content Analysis
4.9. Determination of Bacterial Growth Curves
4.10. Real-Time Quantitative PCR Analysis
4.11. Survival Analysis
4.12. Intracellular ROS Accumulation Assay
4.13. Yeast Two-Hybrid Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daly, M.J. Death by Protein Damage in Irradiated Cells. DNA Repair. 2012, 11, 12–21. [Google Scholar] [CrossRef]
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Tatusov, R.L.; Minton, K.W.; Koonin, E.V.; Daly, M.J. Genome of the Extremely Radiation-Resistant Bacterium Deinococcus radiodurans Viewed from the Perspective of Comparative Genomics. Microbiol. Mol. Biol. Rev. 2001, 65, 44–79. [Google Scholar] [CrossRef]
- Rainey, F.A.; Nobre, M.F.; Schumann, P.; Stackebrandt, E.; Da Costa, M.S. Phylogenetic Diversity of the Deinococci as Determined by 16S Ribosomal DNA Sequence Comparison. Int. J. Syst. Evol. Microbiol. 1997, 47, 510–514. [Google Scholar] [CrossRef]
- Makarova, K.S.; Aravind, L.; Daly, M.J.; Koonin, E.V. Specific Expansion of Protein Families in the Radioresistant Bacterium Deinococcus radiodurans. Genetica 2000, 108, 25–34. [Google Scholar] [CrossRef]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Kiang, J.G.; Fukumoto, R.; Lee, D.-Y.; Wehr, N.B.; Viteri, G.A.; Berlett, B.S.; Levine, R.L. Small-Molecule Antioxidant Proteome-Shields in Deinococcus radiodurans. PLoS ONE 2010, 5, e12570. [Google Scholar] [CrossRef]
- Ghosal, D.; Omelchenko, M.V.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Venkateswaran, A.; Zhai, M.; Kostandarithes, H.M.; Brim, H.; Makarova, K.S. How Radiation Kills Cells: Survival of Deinococcus radiodurans and Shewanella Oneidensis under Oxidative Stress. FEMS Microbiol. Rev. 2005, 29, 361–375. [Google Scholar]
- Slade, D.; Radman, M. Oxidative Stress Resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Venkateswaran, A.; Hess, M.; Omelchenko, M.V.; Kostandarithes, H.M.; Makarova, K.S.; et al. Accumulation of Mn(II) in Deinococcus radiodurans Facilitates Gamma-Radiation Resistance. Science 2004, 306, 1025–1028. [Google Scholar] [CrossRef]
- Jeong, S.-W.; Jung, J.-H.; Kim, M.-K.; Seo, H.S.; Lim, H.-M.; Lim, S. The Three Catalases in Deinococcus radiodurans: Only Two Show Catalase Activity. Biochem. Biophys. Res. Commun. 2016, 469, 443–448. [Google Scholar] [CrossRef]
- Qi, H.; Wang, W.; He, J.; Ma, Y.; Xiao, F.; He, S. Antioxidative System of Deinococcus radiodurans. Res. Microbiol. 2020, 171, 45–54. [Google Scholar] [CrossRef]
- Tian, B.; Sun, Z.; Shen, S.; Wang, H.; Jiao, J.; Wang, L.; Hu, Y.; Hua, Y. Effects of Carotenoids from Deinococcus radiodurans on Protein Oxidation. Lett. Appl. Microbiol. 2009, 49, 689–694. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Defense of Deinococcus radiodurans: How Does It Contribute to Extreme Radiation Resistance? Int. J. Radiat. Biol. 2023, 99, 1803–1829. [Google Scholar] [CrossRef]
- Peana, M.; Chasapis, C.T.; Simula, G.; Medici, S.; Zoroddu, M.A. A Model for Manganese Interaction with Deinococcus radiodurans Proteome Network Involved in ROS Response and Defense. J. Trace Elem. Med. Biol. 2018, 50, 465–473. [Google Scholar] [CrossRef]
- Sharma, A.; Gaidamakova, E.K.; Grichenko, O.; Matrosova, V.Y.; Hoeke, V.; Klimenkova, P.; Conze, I.H.; Volpe, R.P.; Tkavc, R.; Gostinčar, C.; et al. Across the Tree of Life, Radiation Resistance Is Governed by Antioxidant Mn2+, Gauged by Paramagnetic Resonance. Proc. Natl. Acad. Sci. USA 2017, 114, E9253–E9260. [Google Scholar] [CrossRef]
- Santos, S.P.; Yang, Y.; Rosa, M.T.G.; Rodrigues, M.A.A.; De La Tour, C.B.; Sommer, S.; Teixeira, M.; Carrondo, M.A.; Cloetens, P.; Abreu, I.A.; et al. The Interplay between Mn and Fe in Deinococcus radiodurans Triggers Cellular Protection during Paraquat-Induced Oxidative Stress. Sci. Rep. 2019, 9, 17217. [Google Scholar] [CrossRef]
- Sun, H.; Li, M.; Xu, G.; Chen, H.; Jiao, J.; Tian, B.; Wang, L.; Hua, Y. Regulation of MntH by a Dual Mn(II)- and Fe(II)-Dependent Transcriptional Repressor (DR2539) in Deinococcus radiodurans. PLoS ONE 2012, 7, e35057. [Google Scholar] [CrossRef]
- Gaidamakova, E.K.; Sharma, A.; Matrosova, V.Y.; Grichenko, O.; Volpe, R.P.; Tkavc, R.; Conze, I.H.; Klimenkova, P.; Balygina, I.; Horne, W.H.; et al. Small-Molecule Mn Antioxidants in Caenorhabditis elegans and Deinococcus radiodurans Supplant MnSOD Enzymes during Aging and Irradiation. mBio 2022, 13, e03394-21. [Google Scholar] [CrossRef]
- Culotta, V.C.; Daly, M.J. Manganese Complexes: Diverse Metabolic Routes to Oxidative Stress Resistance in Prokaryotes and Yeast. Antioxid. Redox Signal. 2013, 19, 933–944. [Google Scholar] [CrossRef]
- Abreu, I.A.; Hearn, A.; An, H.; Nick, H.S.; Silverman, D.N.; Cabelli, D.E. The Kinetic Mechanism of Manganese-Containing Superoxide Dismutase from Deinococcus radiodurans: A Specialized Enzyme for the Elimination of High Superoxide Concentrations. Biochemistry 2008, 47, 2350–2356. [Google Scholar] [CrossRef]
- Waters, L.S. Bacterial Manganese Sensing and Homeostasis. Curr. Opin. Chem. Biol. 2020, 55, 96–102. [Google Scholar] [CrossRef]
- Juttukonda, L.J.; Skaar, E.P. Manganese Homeostasis and Utilization in Pathogenic Bacteria. Mol. Microbiol. 2015, 97, 216–228. [Google Scholar] [CrossRef]
- Chang, S.; Shu, H.; Li, Z.; Wang, Y.; Chen, L.; Hua, Y.; Qin, G. Disruption of Manganese Ions [Mn (II)] Transporter Genes DR1709 or DR2523 in Extremely Radio-Resistant Bacterium Deinococcus radiodurans. Wei Sheng Wu Xue Bao Acta Microbiol. Sin. 2009, 49, 438–444. [Google Scholar]
- Haiyan, S.; Baoming, T. Function Analysis of Two Mn (II) Ion Transporter Genes (DR1709 and DR2523) in Deinococcus radiodurans. Afr. J. Biotechnol. 2010, 9, 2742–2747. [Google Scholar]
- Sun, H.; Xu, G.; Zhan, H.; Chen, H.; Sun, Z.; Tian, B.; Hua, Y. Identification and Evaluation of the Role of the Manganese Efflux Protein in Deinococcus radiodurans. BMC Microbiol. 2010, 10, 319. [Google Scholar] [CrossRef]
- Couñago, R.M.; Ween, M.P.; Begg, S.L.; Bajaj, M.; Zuegg, J.; O’Mara, M.L.; Cooper, M.A.; McEwan, A.G.; Paton, J.C.; Kobe, B.; et al. Imperfect Coordination Chemistry Facilitates Metal Ion Release in the Psa Permease. Nat. Chem. Biol. 2014, 10, 35–41. [Google Scholar] [CrossRef]
- Gribenko, A.; Mosyak, L.; Ghosh, S.; Parris, K.; Svenson, K.; Moran, J.; Chu, L.; Li, S.; Liu, T.; Woods Jr, V.L. Three-Dimensional Structure and Biophysical Characterization of Staphylococcus Aureus Cell Surface Antigen–Manganese Transporter MntC. J. Mol. Biol. 2013, 425, 3429–3445. [Google Scholar]
- Begg, S.L.; Eijkelkamp, B.A.; Luo, Z.; Couñago, R.M.; Morey, J.R.; Maher, M.J.; Ong, C.-L.Y.; McEwan, A.G.; Kobe, B.; O’Mara, M.L.; et al. Dysregulation of Transition Metal Ion Homeostasis Is the Molecular Basis for Cadmium Toxicity in Streptococcus pneumoniae. Nat. Commun. 2015, 6, 6418. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Wong, T.-Y.; Kuo, J.; Liu, J.-K. The Effect of Mn(II) on the Autoinducing Growth Inhibition Factor in Deinococcus radiodurans. Prep. Biochem. Biotechnol. 2014, 44, 645–652. [Google Scholar] [CrossRef]
- Daly, M.J. A New Perspective on Radiation Resistance Based on Deinococcus radiodurans. Nat. Rev. Microbiol. 2009, 7, 237–245. [Google Scholar] [CrossRef]
- Shah, A.M.U.H.; Zhao, Y.; Wang, Y.; Yan, G.; Zhang, Q.; Wang, L.; Tian, B.; Chen, H.; Hua, Y. A Mur Regulator Protein in the Extremophilic Bacterium Deinococcus radiodurans. PLoS ONE 2014, 9, e106341. [Google Scholar] [CrossRef]
- Chen, H.; Xu, G.; Zhao, Y.; Tian, B.; Lu, H.; Yu, X.; Xu, Z.; Ying, N.; Hu, S.; Hua, Y. A Novel OxyR Sensor and Regulator of Hydrogen Peroxide Stress with One Cysteine Residue in Deinococcus radiodurans. PLoS ONE 2008, 3, e1602. [Google Scholar] [CrossRef] [PubMed]
- Gat, O.; Mendelson, I.; Chitlaru, T.; Ariel, N.; Altboum, Z.; Levy, H.; Weiss, S.; Grosfeld, H.; Cohen, S.; Shafferman, A. The Solute-Binding Component of a Putative Mn(II) ABC Transporter (MntA) Is a Novel Bacillus Anthracis Virulence Determinant. Mol. Microbiol. 2005, 58, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Tampé, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Naoe, Y.; Nakamura, N.; Doi, A.; Sawabe, M.; Nakamura, H.; Shiro, Y.; Sugimoto, H. Crystal Structure of Bacterial Haem Importer Complex in the Inward-Facing Conformation. Nat. Commun. 2016, 7, 13411. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Tian, B.; Xu, Z.; Sun, Z.; Lin, J.; Hua, Y. Evaluation of the Antioxidant Effects of Carotenoids from Deinococcus radiodurans through Targeted Mutagenesis, Chemiluminescence, and DNA Damage Analyses. Biochim. Biophys. Acta General. Subj. 2007, 1770, 902–911. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Pang, R.; Cai, C.; Tan, Z.; Dai, S.; Tian, B.; Wang, L. The Role of the MntABC Transporter System in the Oxidative Stress Resistance of Deinococcus radiodurans. Int. J. Mol. Sci. 2025, 26, 9407. https://doi.org/10.3390/ijms26199407
Wang B, Pang R, Cai C, Tan Z, Dai S, Tian B, Wang L. The Role of the MntABC Transporter System in the Oxidative Stress Resistance of Deinococcus radiodurans. International Journal of Molecular Sciences. 2025; 26(19):9407. https://doi.org/10.3390/ijms26199407
Chicago/Turabian StyleWang, Binqiang, Renjiang Pang, Chunhui Cai, Zichun Tan, Shang Dai, Bing Tian, and Liangyan Wang. 2025. "The Role of the MntABC Transporter System in the Oxidative Stress Resistance of Deinococcus radiodurans" International Journal of Molecular Sciences 26, no. 19: 9407. https://doi.org/10.3390/ijms26199407
APA StyleWang, B., Pang, R., Cai, C., Tan, Z., Dai, S., Tian, B., & Wang, L. (2025). The Role of the MntABC Transporter System in the Oxidative Stress Resistance of Deinococcus radiodurans. International Journal of Molecular Sciences, 26(19), 9407. https://doi.org/10.3390/ijms26199407





