How Can Molecules Induce Hemorrhoids? The Role of Genetics and Epigenetics in Hemorrhoidal Disease
Abstract
1. Introduction
2. Why Do Hemorrhoids Develop?
3. Inflammation
4. The Role of Vesicles
5. Nitric Oxide and Varicose Veins
6. Angiogenesis
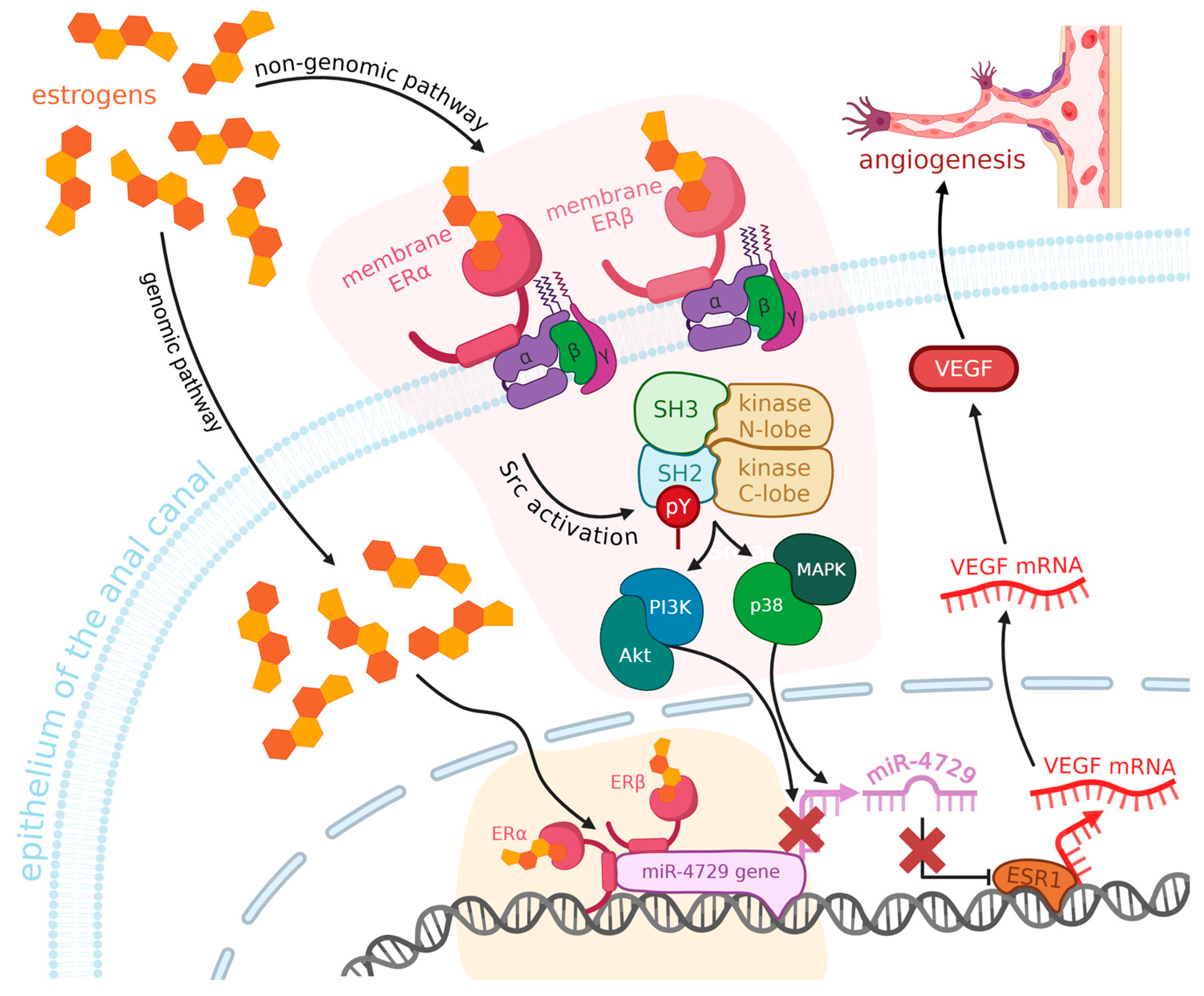
7. A Role of Estrogen in Angiogenesis?
8. Degeneration of Connective Tissue
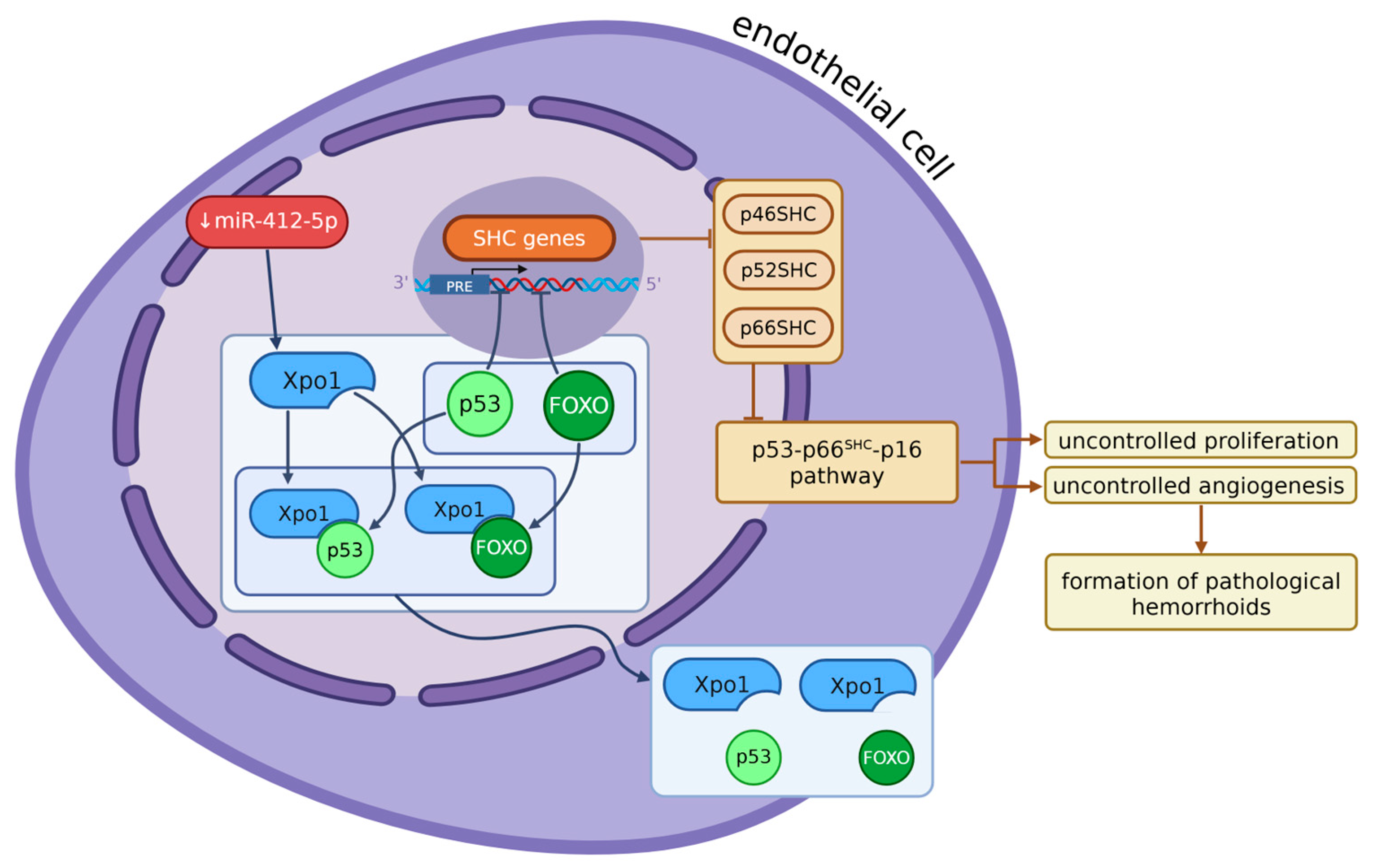
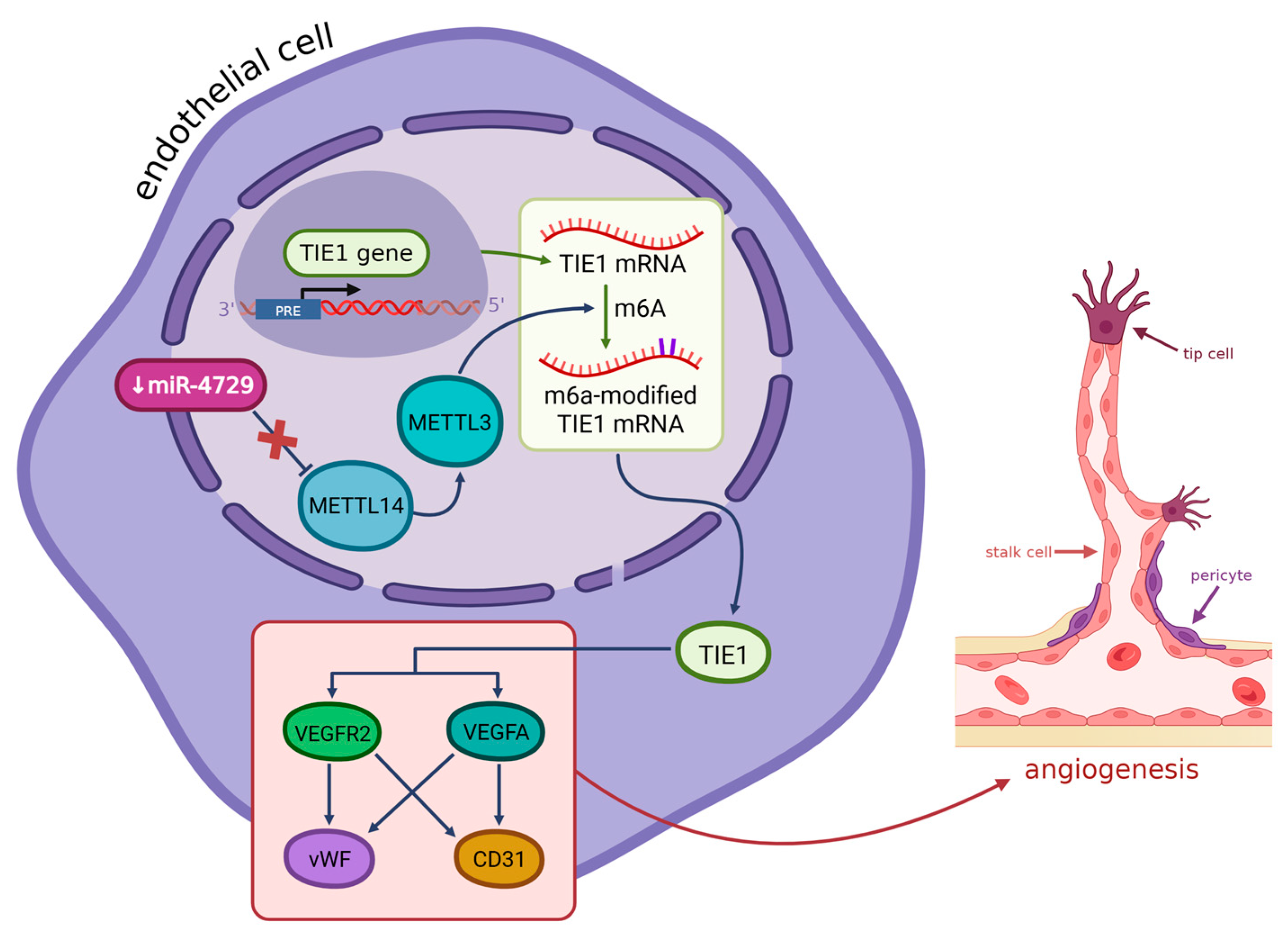
9. Genes Significant in the Development of Hemorrhoids
10. Possible Epigenetic Factors in the Development of Hemorrhoids
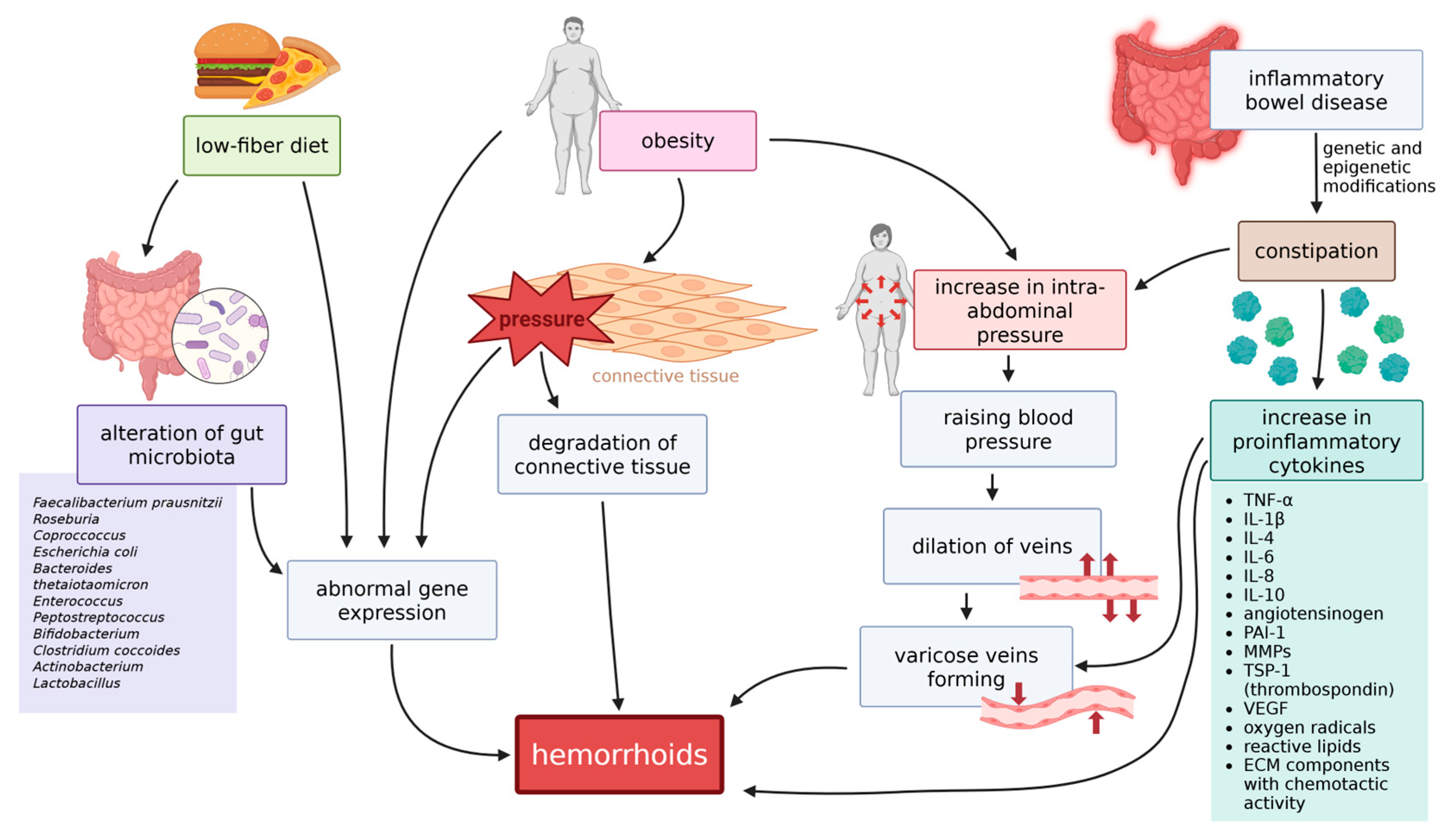
10.1. Diet and Gut Microbiota
10.2. Obesity
10.3. Constipation and Irritable Bowel Syndrome
11. Discussion
12. Conclusions
13. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FOXC2 | Forkhead Box C2 |
| NOX | NADPH Oxidase |
| NOS | Nitric Oxide Synthase |
| CALM3 | Calmodulin 3 |
| miRNA | microRNA |
| mRNA | Messenger RNA |
| DNA | Deoxyribonucleic Acid |
| COX2 | Cyclooxygenase-2 |
| RYBP | Ring1 and YY1-binding protein |
| PRC1 | Polycomb Repressive Complex 1 |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells |
| PGE2 | Prostaglandin E2 |
| ncRNA | Non-coding RNA |
| lncRNA | Long non-coding RNA |
| rRNA | Ribosomal RNA |
| tRNA | Transfer RNA |
| snRNA | Small nuclear RNA |
| UTR | Untranslated Region |
| MMP | Matrix Metalloproteinase |
| RANTES | Regulated on Activation, Normal T-cell Expressed and Secreted |
| CCL-5 | Chemokine (C-C motif) Ligand 5 |
| TNF-α | Tumor Necrosis Factor Alpha |
| VEGF | Vascular Endothelial Growth Factor |
| IL | Interleukin |
| IFN-γ | Interferon Gamma |
| CGRP | Calcitonin Gene-Related Peptide |
| SP | Substance P |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| MAPK | Mitogen-Activated Protein Kinase |
| AMPK | AMP-Activated Protein Kinase |
| PI3K | Phosphoinositide 3-Kinase |
| UBQLN1 | Ubiquilin-1 |
| HERC3 | HECT and RLD Domain Containing E3 Ubiquitin Protein Ligase 3 |
| ECM | Extracellular Matrix |
| vWF | von Willebrand Factor |
| CD | Cluster of Differentiation |
| TGF-β | Transforming Growth Factor Beta |
| Xpo1 | Exportin 1 |
| PI3K/AKT | Phosphoinositide 3-Kinase/Protein Kinase B |
| AP-1 | Activator Protein 1 |
| SHC | Src Homology 2 Domain-Containing |
| m6A | N6-methyladenosine |
| METTL14 | Methyltransferase-Like 14 |
| METTL3 | Methyltransferase-Like 3 |
| WTAP | Wilms Tumor 1-Associated Protein |
| VIRMA | Vir-Like m6A Methyltransferase-Associated |
| RBM15/15B | RNA Binding Motif Protein 15/15B |
| ZC3H13 | Zinc Finger CCCH-Type Containing 13 |
| TIE1 | Tyrosine Kinase with Immunoglobulin-Like and EGF-Like Domains 1 |
| VEGFA | Vascular Endothelial Growth Factor A |
| ESR1 | Estrogen Receptor 1 |
| ERα | Estrogen Receptor Alpha |
| IBS | Irritable Bowel Syndrome |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| TSP-1 | Thrombospondin-1 |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| SANRA | Scale for the Assessment of Narrative Review Articles |
References
- Margetis, N. Pathophysiology of Internal Hemorrhoids. Ann. Gastroenterol. 2019, 32, 264. [Google Scholar] [CrossRef]
- Thomson, W.H.F. The Nature of Haemorrhoids. Br. J. Surg. 1975, 62, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Loder, P.B.; Kamm, M.A.; Nicholls, R.J.; Phillips, R.K.S. Haemorrhoids: Pathology, Pathophysiology and Aetiology. Br. J. Surg 1994, 81, 946–954. [Google Scholar] [CrossRef]
- Pata, F.; Sgró, A.; Ferrara, F.; Vigorita, V.; Gallo, G.; Pellino, G. Anatomy, Physiology and Pathophysiology of Haemorrhoids. Rev. Recent Clin. Trials 2021, 16, 75–80. [Google Scholar] [CrossRef]
- Fox, A.; Tietze, P.H.; Ramakrishnan, K. Anorectal Conditions: Hemorrhoids. FP Essent. 2014, 419, 11–19. [Google Scholar]
- Burkitt, D.P.; Graham-Stewart, C.W. Haemorrhoids–Postulated Pathogenesis and Proposed Prevention. Postgrad. Med. J. 1975, 51, 631–636. [Google Scholar] [CrossRef]
- Lohsiriwat, V. Hemorrhoids: From Basic Pathophysiology to Clinical Management. World J. Gastroenterol. 2012, 18, 2009. [Google Scholar] [CrossRef]
- Sugerman, D.T. Hemorrhoids. J. Am. Med. Assoc. 2014, 312, 2698. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D. Clinical Practice. Hemorrhoids. N. Engl. J. Med. 2014, 371, 944–951. [Google Scholar] [CrossRef]
- Qureshi, W.A. Office Management of Hemorrhoids. Am. J. Gastroenterol. 2018, 113, 795–798. [Google Scholar] [CrossRef]
- Lohsiriwat, V. Treatment of Hemorrhoids: A Coloproctologist’s View. World J. Gastroenterol. 2015, 21, 9245–9252. [Google Scholar] [CrossRef] [PubMed]
- Rakinic, J.; Poola, V.P. Hemorrhoids and Fistulas: New Solutions to Old Problems. Curr. Probl. Surg. 2014, 51, 98–137. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, F. Die Hämorrhoiden Und Andere Krankheiten Des Corpus Cavernosum Recti Und Des Analkanals. DMW-Dtsch. Med. Wochenschr. 1963, 88, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. J. Gastroenterol. 2015, 149, 1731–1741.e3. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, D.A.; Beart, R.W.; Madoff, R.D.; Ault, G.T. Impact of the Aging Population on the Demand for Colo-rectal Procedures. Dis. Colon Rectum 2009, 52, 583–590. [Google Scholar] [CrossRef]
- Műzes, G.; Bohusné Barta, B.; Szabó, O.; Horgas, V.; Sipos, F. Cell-Free DNA in the Pathogenesis and Therapy of Non-Infectious Inflammations and Tumors. Biomedicines 2022, 10, 2853. [Google Scholar] [CrossRef] [PubMed]
- Carrard, J.; Ratajczak, F.; Elsens, J.; Leroy, C.; Kong, R.; Geoffroy, L.; Comte, A.; Fournet, G.; Joseph, B.; Li, X.; et al. Identifying Potent Nonsense-Mediated MRNA Decay Inhibitors with a Novel Screening System. Biomedicines 2023, 11, 2801. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Mailliot, J.; Schaffitzel, C. Nonsense-Mediated MRNA Decay Factor Functions in Human Health and Disease. Biomedicines 2023, 11, 722. [Google Scholar] [CrossRef]
- Vebr, M.; Pomahačová, R.; Sýkora, J.; Schwarz, J. A Narrative Review of Cytokine Networks: Pathophysio-logical and Therapeutic Implications for Inflammatory Bowel Disease Pathogenesis. Biomedicines 2023, 11, 3229. [Google Scholar] [CrossRef]
- Chong, P.S.; Bartolo, D.C.C. Hemorrhoids and Fissure in Ano. Gastroenterol. Clin. N. Am. 2008, 37, 627–644. [Google Scholar] [CrossRef]
- Serra, R.; Gallelli, L.; Grande, R.; Amato, B.; De Caridi, G.; Sammarco, G.; Ferrari, F.; Butrico, L.; Gallo, G.; Rizzuto, A.; et al. Hemorrhoids and Matrix Metalloproteinases: A Multicenter Study on the Predictive Role of Biomarkers. Surgery 2016, 159, 487–494. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Ma, X.; Ge, X.; Deng, Y. Identification of the MicroRNA Alterations in Extracellular Vesicles Derived from Human Haemorrhoids. Exp. Physiol. 2023, 108, 752–761. [Google Scholar] [CrossRef]
- Porwal, A.; Kundu, G.C.; Bhagwat, G.; Butti, R. Herbal Medicine AnoSpray Suppresses Proinflammatory Cytokines COX-2 and RANTES in the Management of Hemorrhoids, Acute Anal Fissures and Perineal Wounds. Available online: https://www.spandidos-publications.com/10.3892/etm.2021.11009 (accessed on 16 February 2024).
- Yoon, S.O.; Park, S.J.; Yun, C.H.; Chung, A.S. Roles of Matrix Metalloproteinases in Tumor Metastasis and Angiogenesis. J. Biochem. Mol. Biol 2003, 36, 128–137. [Google Scholar] [CrossRef]
- Song, C.; Zhou, H.; Lu, H.; Luo, C.; Wang, C.; Wang, Q.; Peng, Y.; Xin, Y.; Liu, T.; Yang, W. Aberrant Expression for MicroRNA Is Potential Crucial Factors of Haemorrhoid. Hereditas 2020, 157, 25. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, H.; Lu, H.; Luo, C.; Wang, Q.; Peng, Y.; Yang, W.; Xin, Y. MiR-4729 Regulates TIE1 MRNA M6A Modification and Angiogenesis in Hemorrhoids by Targeting METTL14. Ann. Transl. Med. 2021, 9, 232. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Luo, C.; Song, C.; Wang, Q.; Peng, Y.; Xin, Y.; Liu, T.; Yang, W. MiR-412-5p Targets Xpo1 to Regulate Angiogenesis in Hemorrhoid Tissue. Gene 2019, 705, 167–176. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, W.; Wang, H.; Dong, J. N6-Methyladenosine-Sculpted Regulatory Landscape of Noncoding RNA. Front. Oncol. 2021, 11, 743990. [Google Scholar] [CrossRef]
- Morgado, P.J.; Suárez, J.A.; Gómez, L.G.; Morgado, P.J. Histoclinical Basis for a New Classification of Hemorrhoidal Disease. Dis. Colon Rectum 1988, 31, 474–480. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E. The Art of MicroRNA Research. Circ. Res. 2011, 108, 219–234. [Google Scholar] [CrossRef]
- Leung, A.K.L.; Sharp, P.A. MicroRNA Functions in Stress Responses. J. Mol. Cell 2010, 40, 205–215. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA Expression Profiles Classify Human Cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Borghini, A.; Andreassi, M.G. Genetic Polymorphisms Offer Insight into the Causal Role of MicroRNA in Coronary Artery Disease. Atherosclerosis 2018, 269, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, D.; Ganju, L.; Kumar, B. MicroRNA in Gastrointestinal Cell Signalling. Inflammopharmacology 2018, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Creugny, A.; Fender, A.; Pfeffer, S. Regulation of Primary MicroRNA Processing. FEBS Lett. 2018, 592, 1980–1996. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.T.; Olson, E.N. MicroRNAs in Stress Signaling and Human Disease. Cell 2012, 148, 1172–1187. [Google Scholar] [CrossRef]
- Yen, Y.P.; Hsieh, W.F.; Tsai, Y.Y.; Lu, Y.L.; Liau, E.S.; Hsu, H.C.; Chen, Y.C.; Liu, T.C.; Chang, M.; Li, J.; et al. Dlk1-Dio3 Locus-Derived LncRNAs Perpetuate Postmitotic Motor Neuron Cell Fate and Subtype Identity. eLife 2018, 7, e38080. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Lin, J.; Chen, J.; Yu, Z.; Chen, C.; Liu, T. MiR-758 Mediates OxLDL-Dependent Vascular Endothelial Cell Damage by Suppressing the Succinate Receptor SUCNR1. Gene 2018, 663, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Shen, H.; Huang, Y.; Liu, T.; Xi, H.; Chen, C. Curcumin Suppresses In Vitro Proliferation and Invasion of Human Prostate Cancer Stem Cells by Modulating DLK1-DIO3 Imprinted Gene Cluster MicroRNAs. Genet. Test. Mol. Biomark. 2018, 22, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, T.; Huang, Y. MicroRNA-134 Suppresses Endometrial Cancer Stem Cells by Targeting POGLUT1 and Notch Pathway Proteins. FEBS Lett. 2015, 589, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Liu, T.; Huang, Y.; Qin, W.; Yang, H.; Chen, J. MicroRNA-134-3p Is a Novel Potential Inhibitor of Human Ovarian Cancer Stem Cells by Targeting RAB27A. Gene 2017, 605, 99–107. [Google Scholar] [CrossRef]
- Xie, P.; Sun, Y.; Ouyang, Q.; Hu, L.; Tan, Y.; Zhou, X.; Xiong, B.; Zhang, Q.; Yuan, D.; Pan, Y.; et al. Physiological Oxygen Prevents Frequent Silencing of the DLK1-DIO3 Cluster during Human Embryonic Stem Cells Culture. Stem Cells 2014, 32, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Porwal, A.; Kundu, G.C.; Bhagwat, G.; Butti, R. Polyherbal Formulation Anoac-H Suppresses the Expression of RANTES and VEGF for the Management of Bleeding Hemorrhoids and Fistula. Available online: https://www.spandidos-publications.com/10.3892/mmr.2021.12376# (accessed on 12 February 2024).
- Shrivastava, L.; da Silva Borges, G.; Shrivastava, R. Clinical Efficacy of a Dual Action, Topical Anti-Edematous and Antiinflammatory Device for the Treatment of External Hemorrhoids. Clin. Exp. Pharmacol. 2018, 8, 1000246. [Google Scholar] [CrossRef]
- Ray, A. Cytokines and Their Role in Health and Disease: A Brief Overview. MOJ Immunol. 2016, 4, 00121. [Google Scholar] [CrossRef]
- Srivastava, A.; Yadav, S.K.; Yachha, S.K.; Thomas, M.A.; Saraswat, V.A.; Gupta, R.K. Pro-Inflammatory Cytokines Are Raised in Extrahepatic Portal Venous Obstruction, with Minimal Hepatic Encephalopathy. J. Gastroenterol. Hepatol. 2011, 26, 979–986. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Jin, W.; Li, P.; Wang, R.; Guo, X. Traditional Chinese Medicine in the Treatment of Hemorrhoids—A Review of Preparations Used and Their Mechanism of Action. Front. Pharmacol. 2023, 14, 1270339. [Google Scholar] [CrossRef]
- Porwal, A.; Kundu, G.; Bhagwat, G.; Nimma, R.; Chowdhury, J. Turmocin Plus Suppresses Vascular Endothelial Growth Factor (VEGF) and Macrophage Infiltration in the Management of Perineal Wounds, Anal Fistula, Acute Anal Fissures and Haemorrhoids. J. Nat. Remedies 2024, 24, 283–291. [Google Scholar] [CrossRef]
- Cuesta, M.C.; Quintero, L.; Pons, H.; Suarez-Roca, H. Substance P and Calcitonin Gene-Related Peptide Increase IL-1β, IL-6 and TNFα Secretion from Human Peripheral Blood Mononuclear Cells. Neurochem. Int. 2002, 40, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Alliger, K.; Weidinger, C.; Yerinde, C.; Wirtz, S.; Becker, C.; Engel, M.A. Functional Role of Transient Receptor Potential Channels in Immune Cells and Epithelia. Front. Immunol. 2018, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Torii, H.; Hosoi, J.; Beissert, S.; Xu, S.; Fox, F.E.; Asahina, A.; Takashima, A.; Rook, A.H.; Granstein, R.D. Regulation of Cytokine Expression in Macrophages and the Langerhans Cell-like Line XS52 by Calcitonin Gene-Related Peptide. J. Leukoc. Biol. 1997, 61, 216–223. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, Y.; Zhang, X.; Li, W.; Zhang, H.; Piao, S.; Li, G.; Yan, M. The Beneficial Effect of Sanhuang Ointment and Its Active Constituents on Experimental Hemorrhoids in Rats. J. Ethnopharmacol. 2024, 319, 117173. [Google Scholar] [CrossRef]
- Klink, C.; Binnebösel, M.; Kämmer, D.; Willis, S.; Prescher, A.; Klinge, U.; Schumpelick, V. Haemorrhoids Are Related to Changes of Cell Function in Mucosa and Submucosa. Int. J. Color. Dis. 2009, 24, 1389–1394. [Google Scholar] [CrossRef]
- Simon, L.S. Role and Regulation of Cyclooxygenase-2 during Inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, T.; Zhong, S.; Yang, W. MicroRNA-770 Promotes Polarization of Macrophages and Hemorrhoids by Suppressing RYBP Expression and Monoubiquitination of Histone H2A on Lys119 Modification. Mol. Immunol. 2025, 182, 41–53. [Google Scholar] [CrossRef]
- Nieuwland, R.; Falcon-Perez, J.M.; Soekmadji, C.; Boilard, E.; Carter, D.; Buzas, E.I. Essentials of Extracellular Vesicles: Posters on Basic and Clinical Aspects of Extracellular Vesicles. J. Extracell Vesicles 2018, 7, 1548234. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Cabral, J.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Extracellular Vesicles as Modulators of Wound Healing. Adv. Drug Deliv. Rev. 2018, 129, 394–406. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018, 175, 695–708.e13. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (US). Gene UBQLN1 Ubiquilin 1 [Homo Sapiens (Human)]. NCBI Gene. Available online: https://www.ncbi.nlm.nih.gov/gene/29979 (accessed on 11 March 2024).
- Hochrainer, K.; Pejanovic, N.; Olaseun, V.A.; Zhang, S.; Iadecola, C.; Anrather, J. The Ubiquitin Ligase HERC3 Attenuates NF-ΚB-Dependent Transcription Independently of Its Enzymatic Activity by Delivering the RelA Subunit for Degradation. Nucleic Acids Res. 2015, 43, 9889–9904. [Google Scholar] [CrossRef]
- Ge, X.; Meng, Q.; Wei, L.; Liu, J.; Li, M.; Liang, X.; Lin, F.; Zhang, Y.; Li, Y.; Liu, Z.; et al. Myocardial Ischemia-reperfusion Induced Cardiac Extracellular Vesicles Harbour Proinflammatory Features and Aggravate Heart Injury. J. Extracell Vesicles 2021, 10, e12072. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Sahún-Español, Á.; Clemente, C.; Jiménez-Loygorri, J.I.; Sierra-Filardi, E.; Herrera-Melle, L.; Gómez-Durán, A.; Sabio, G.; Monsalve, M.; Boya, P.; Arroyo, A.G. P38 MAPK Priming Boosts VSMC Proliferation and Arteriogenesis by Promoting PGC1α-Dependent Mitochondrial Dynamics. Sci. Rep. 2022, 12, 5938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; et al. Signaling Pathways and Targeted Therapy for Myocardial Infarction. Signal. Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef]
- Yong, H.Y.; Koh, M.S.; Moon, A. The P38 MAPK Inhibitors for the Treatment of Inflammatory Diseases and Cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Haviarová, Z.; Janegová, A.; Janega, P.; Durdík, S.; Kováč, P.; Stvrtinová, V.; Mráz, P. Expression of Constitutive Nitric Oxide Synthase Isoforms in Varicose Vein Wall; Preliminary Results. Int. J. Vasc. Med. 2011, 2011, 204723. [Google Scholar] [CrossRef]
- Gokce, A.H.; Gokce, F.S.; Durmus, S.; Hajiyeva, R.; Ersoz, F.; Gelisgen, R.; Uzun, H. The Effect of Nitric Oxide, Endothelial Nitric Oxide Synthetase, and Asymmetric Dimethylarginine in Hemorrhoidal Disease. Rev. Assoc. Med. Bras. 2020, 66, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V.; Wilson, V.G.; Scholefield, J.H.; Dashwood, M.R. Regional Distribution of Nitric Oxide Synthase in Human Anorectal Tissue: A Pilot Study on the Potential Role for Nitric Oxide in Haemorrhoids. Curr. Vasc. Pharmacol. 2018, 18, 43–49. [Google Scholar] [CrossRef]
- Chung, Y.C.; Hou, Y.C.; Pan, A.C.H. Endoglin (CD105) Expression in the Development of Haemorrhoids. Eur. J. Clin. Investig. 2004, 34, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M. Neovascularization in Experimental Retinal Venous Obstruction in Rabbits. JPN. J. Ophthalmol. 2001, 45, 144–150. [Google Scholar] [CrossRef]
- Kang, S.G.; Chung, H.; Hyon, J.Y. Experimental Preretinal Neovascularization by Laser-Induced Thrombosis in Albino Rats. Korean J. Ophthalmol. 1999, 13, 65–70. [Google Scholar] [CrossRef]
- Gravina, G.L.; Senapedis, W.; McCauley, D.; Baloglu, E.; Shacham, S.; Festuccia, C. Nucleo-Cytoplasmic Transport as a Therapeutic Target of Cancer. J. Hematol. Oncol. 2014, 7, 85. [Google Scholar] [CrossRef]
- Chen, Y.; Camacho, S.C.; Silvers, T.R.; Razak, A.R.A.; Gabrail, N.Y.; Gerecitano, J.F.; Kalir, E.; Pereira, E.; Evans, B.R.; Ramus, S.J.; et al. Inhibition of the Nuclear Export Receptor XPO1 as a Therapeutic Target for Platinum-Resistant Ovarian Cancer. Clin. Cancer Res. 2017, 23, 1552–1563. [Google Scholar] [CrossRef]
- Wikström, P.; Lissbrant, I.F.; Stattin, P.; Egevad, L.; Bergh, A. Endoglin (CD105) Is Expressed on Immature Blood Vessels and Is a Marker for Survival in Prostate Cancer. Prostate 2002, 51, 268–275. [Google Scholar] [CrossRef]
- Krupinski, J.; Kaluza, J.; Kumar, P.; Kumar, S.; Wang, J.M. Role of Angiogenesis in Patients with Cerebral Ischemic Stroke. Stroke 1994, 25, 1794–1798. [Google Scholar] [CrossRef]
- Marcovich, A.L.; Morad, Y.; Sandbank, J.; Huszar, M.; Rosner, M.; Pollack, A.; Herbert, M.; Bar-Dayan, Y. Angiogenesis in Pterygium: Morphometric and Immunohistochemical Study. Curr. Eye Res. 2002, 25, 17–22. [Google Scholar] [CrossRef]
- Altomonte, M.; Montagner, R.; Fonsatti, E.; Colizzi, F.; Cattarossi, I.; Brasoveanu, L.I.; Nicotra, M.R.; Cattelan, A.; Natali, P.G.; Maio, M. Expression and Structural Features of Endoglin (CD105), a Transforming Growth Factor Beta1 and Beta3 Binding Protein, in Human Melanoma. Br. J. Cancer 1996, 74, 1586. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Cao, J.; Yan, P.; Li, W.; Sun, Z.; Che, X.; Wang, Q.; Liu, F. Clinical Significance and Distribution Characteristics of Expression of Vascular Endothelial Growth Factor Receptor 2 in Hemorrhoid Mucosa. J. Pract. Med. 2015, 31, 2830–2832. [Google Scholar] [CrossRef]
- Liu, Q.; Han, W.; Wang, L.; Shang, W.; Cao, X. Role of MiR-143-3p in the Development of Hemorrhoids and Postoperative Wound Healing. J. Investig. Surg. 2025, 38, 2480799. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ishizawa, J.; Ruvolo, V.; Dilip, A.; Quintás-Cardama, A.; Mcdonnell, T.J.; Neelapu, S.S.; Kwak, L.W.; Shacham, S.; Kauffman, M.; et al. Induction of P53-Mediated Transcription and Apoptosis by Exportin-1 (XPO1) Inhibition in Mantle Cell Lymphoma. Cancer Sci. 2014, 105, 795–801. [Google Scholar] [CrossRef]
- Camus, V.; Miloudi, H.; Taly, A.; Sola, B.; Jardin, F. XPO1 in B Cell Hematological Malignancies: From Recurrent Somatic Mutations to Targeted Therapy. J. Hematol. Oncol. 2017, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Kisslinger, A.; Iannelli, P.; Vitale, N.; Procaccini, C.; Sparaneo, G.; Chieffi, P.; Ayala, F.; Mancini, F.P.; Tramontano, D. Resveratrol Regulates P66Shc Activation in HaCaT Cells. Exp. Dermatol. 2010, 19, 895–903. [Google Scholar] [CrossRef]
- Migliaccio, E.; Giogio, M.; Mele, S.; Pelicci, G.; Reboldi, P.; Pandolfi, P.P.; Lanfrancone, L.; Pelicci, P.G. The P66shc Adaptor Protein Controls Oxidative Stress Response and Life Span in Mammals. Nature 1999, 402, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hu, Y.; Tang, H.; Hu, H.; Pang, L.; Xing, J.; Liu, Z.; Luo, Y.; Jiang, B.; Liu, T.; et al. RNA Methyltransferase NSUN2 Promotes Stress-Induced HUVEC Senescence. Oncotarget 2016, 7, 19099. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Wu, R.F.; Terada, L.S. P66Shc Restrains Ras Hyperactivation and Suppresses Metastatic Behavior. Oncogene 2010, 29, 5559. [Google Scholar] [CrossRef]
- Bhat, S.S.; Anand, D.; Khanday, F.A. P66Shc as a Switch in Bringing about Contrasting Responses in Cell Growth: Implications on Cell Proliferation and Apoptosis. Mol. Cancer 2015, 14, 76. [Google Scholar] [CrossRef]
- Camici, G.G.; Schiavoni, M.; Francia, P.; Bachschmid, M.; Martin-Padura, I.; Hersberger, M.; Tanner, F.C.; Pelicci, P.G.; Volpe, M.; Anversa, P.; et al. Genetic Deletion of P66Shc Adaptor Protein Prevents Hyperglycemia-Induced Endothelial Dysfunction and Oxidative Stress. Proc. Natl. Acad. Sci. USA 2007, 104, 5217. [Google Scholar] [CrossRef]
- Napoli, C.; Martin-Padura, I.; De Nigris, F.; Giorgio, M.; Mansueto, G.; Somma, P.; Condorelli, M.; Sica, G.; De Rosa, G.; Pelicci, P.G. Deletion of the P66Shc Longevity Gene Reduces Systemic and Tissue Oxidative Stress, Vascular Cell Apoptosis, and Early Atherogenesis in Mice Fed a High-Fat Diet. Proc. Natl. Acad. Sci. USA 2003, 100, 2112–2116. [Google Scholar] [CrossRef]
- Francia, P.; Delli Gatti, C.; Bachschmid, M.; Martin-Padura, I.; Savoia, C.; Migliaccio, E.; Pelicci, P.G.; Schiavoni, M.; Lüscher, T.F.; Volpe, M.; et al. Deletion of P66shc Gene Protects against Age-Related Endothelial Dysfunction. Circulation 2004, 110, 2889–2895, Erratum in Circulation 2005, 111, 3. [Google Scholar] [CrossRef]
- Yamamori, T.; White, A.R.; Mattagajasingh, I.; Khanday, F.A.; Haile, A.; Qi, B.; Byeong, H.J.; Bugayenko, A.; Kasuno, K.; Berkowitz, D.E.; et al. P66shc Regulates Endothelial NO Production and Endothelium-Dependent Vasorelaxation: Implications for Age-Associated Vascular Dysfunction. J. Mol. Cell Cardiol. 2005, 39, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Trinei, M.; Giorgio, M.; Cicalese, A.; Barozzi, S.; Ventura, A.; Migliaccio, E.; Milia, E.; Padura, I.M.; Raker, V.A.; Maccarana, M.; et al. A P53-P66Shc Signalling Pathway Controls Intracellular Redox Status, Levels of Oxidation-Damaged DNA and Oxidative Stress-Induced Apoptosis. Oncogene 2002, 21, 3872–3878. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 Complex Mediates Mammalian Nuclear RNA N6-Adenosine Methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wei, L.; Law, C.T.; Tsang, F.H.C.; Shen, J.; Cheng, C.L.H.; Tsang, L.H.; Ho, D.W.H.; Chiu, D.K.C.; Lee, J.M.F.; et al. RNA N6-Methyladenosine Methyltransferase-like 3 Promotes Liver Cancer Progression through YTHDF2-Dependent Posttranscriptional Silencing of SOCS2. Hepatology 2018, 67, 2254–2270. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-Methyladenosine in Nuclear RNA Is a Major Substrate of the Obesity-Associated FTO. Nat. Chem. Biol. 2011, 7, 885–887, Erratum in Nat. Chem. Biol. 2012, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Klukovich, R.; Peng, H.; Wang, Z.; Yu, T.; Zhang, Y.; Zheng, H.; Klungland, A.; Yan, W. ALKBH5-Dependent M6A Demethylation Controls Splicing and Stability of Long 3’-UTR MRNAs in Male Germ Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E325–E333. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-Methyladenosine-Dependent Regulation of Messenger RNA Stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, I.; Tzelepis, K.; Pandolfini, L.; Shi, J.; Millán-Zambrano, G.; Robson, S.C.; Aspris, D.; Migliori, V.; Bannister, A.J.; Han, N.; et al. Promoter-Bound METTL3 Maintains Myeloid Leukaemia by M6A-Dependent Translation Control. Nature 2017, 552, 126–131. [Google Scholar] [CrossRef]
- Chen, T.; Hao, Y.J.; Zhang, Y.; Li, M.M.; Wang, M.; Han, W.; Wu, Y.; Lv, Y.; Hao, J.; Wang, L.; et al. M(6)A RNA Methylation Is Regulated by MicroRNAs and Promotes Reprogramming to Pluripotency. Cell Stem Cell 2015, 16, 289–301, Erratum in Cell Stem Cell 2015, 16, 338. [Google Scholar] [CrossRef]
- Savant, S.; La Porta, S.; Budnik, A.; Busch, K.; Hu, J.; Tisch, N.; Korn, C.; Valls, A.F.; Benest, A.V.; Terhardt, D.; et al. The Orphan Receptor Tie1 Controls Angiogenesis and Vascular Remodeling by Differentially Regulating Tie2 in Tip and Stalk Cells. Cell Rep. 2015, 12, 1761–1773. [Google Scholar] [CrossRef]
- Garcia, J.; Sandi, M.J.; Cordelier, P.; Binétruy, B.; Pouysségur, J.; Iovanna, J.L.; Tournaire, R. Tie1 Deficiency Induces Endothelial–Mesenchymal Transition. EMBO Rep. 2012, 13, 431. [Google Scholar] [CrossRef]
- Torigata, M.; Yamakawa, D.; Takakura, N. Elevated Expression of Tie1 Is Accompanied by Acquisition of Cancer Stemness Properties in Colorectal Cancer. Cancer Med. 2017, 6, 1378. [Google Scholar] [CrossRef]
- La Porta, S.L.; Roth, L.; Singhal, M.; Mogler, C.; Spegg, C.; Schieb, B.; Qu, X.; Adams, R.H.; Scott Baldwin, H.; Savant, S.; et al. Endothelial Tie1-Mediated Angiogenesis and Vascular Abnormalization Promote Tumor Progression and Metastasis. J. Clin. Investig. 2018, 128, 834–845. [Google Scholar] [CrossRef]
- Tiainen, L.; Korhonen, E.A.; Leppänen, V.M.; Luukkaala, T.; Hämäläinen, M.; Tanner, M.; Lahdenperä, O.; Vihinen, P.; Jukkola, A.; Karihtala, P.; et al. High Baseline Tie1 Level Predicts Poor Survival in Metastatic Breast Cancer. BMC Cancer 2019, 19, 732. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). TIE1 Tyrosine Kinase with Immunoglobulin Like and EGF Like Domains 1 [Homo sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/7075 (accessed on 12 March 2024).
- Meng, F.; Chen, X.; Liu, H.; Zhang, L.; Yu, T.; Xie, M. Fulvestrant, an Estrogen Receptor Inhibitor, Relieves Postoperative Hemorrhoid Edema via up-Regulation of MiR- 424-5p. Trop. J. Pharm. Res. 2022, 21, 1101–1107. [Google Scholar] [CrossRef]
- Parés, D.; Iglesias, M.; Pera, M.; Pascual, M.; Torner, A.; Baró, T.; Alonso, S.; Grande, L. Expression of Estrogen and Progesterone Receptors in the Anal Canal of Women According to Age and Menopause. Dis. Colon Rectum 2010, 53, 1687–1691. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The Protective Role of Estrogen and Estrogen Receptors in Cardiovascular Disease and the Controversial Use of Estrogen Therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). ESR1 Estrogen Receptor 1 [Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=2099 (accessed on 1 September 2025).
- Zhang, J.; Song, H.; Lu, Y.; Chen, H.; Jiang, S.; Li, L. Effects of Estradiol on VEGF and BFGF by Akt in Endometrial Cancer Cells Are Mediated through the NF-ΚB Pathway. Oncol. Rep. 2016, 36, 705–714. [Google Scholar] [CrossRef]
- Losordo, D.W.; Isner, J.M. Estrogen and Angiogenesis: A Review. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 6–12. [Google Scholar] [CrossRef]
- Karas, R.H.; Gauer, E.A.; Bieber, H.E.; Baur, W.E.; Mendelsohn, M.E. Growth Factor Activation of the Estrogen Receptor in Vascular Cells Occurs via a Mitogen-Activated Protein Kinase-Independent Pathway. J. Clin. Investig. 1998, 101, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Shifren, J.L.; Tseng, J.F.; Zaloudek, C.J.; Ryan, I.P.; Meng, Y.G.; Ferrara, N.; Jaffe, R.B.; Taylor, R.N. Ovarian Steroid Regulation of Vascular Endothelial Growth Factor in the Human Endometrium: Implications for Angiogenesis during the Menstrual Cycle and in the Pathogenesis of Endometriosis. J. Clin. Endocrinol. Metab. 1996, 81, 3112–3118. [Google Scholar] [CrossRef]
- Haas, P.A.; Fox, T.A.; Haas, G.P. The Pathogenesis of Hemorrhoids. Dis. Colon Rectum 1984, 27, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Sardiñas, C.; Arreaza, D.D.; Osorio, H. Changes in the Proportions of Types I and III Collagen in Hemorrhoids: The Sliding Anal Lining Theory. J. Coloproctol. 2016, 36, 124–129. [Google Scholar] [CrossRef]
- Willis, S.; Junge, K.; Ebrahimi, R.; Prescher, A.; Schumpelick, V. Haemorrhoids—A Collagen Disease? Color. Dis. 2010, 12, 1249–1253. [Google Scholar] [CrossRef]
- Nasseri, Y.Y.; Krott, E.; Van Groningen, K.M.; Berho, M.; Osborne, M.C.; Wollman, S.; Weiss, E.G.; Wexner, S.D. Abnormalities in Collagen Composition May Contribute to the Pathogenesis of Hemorrhoids: Morphometric Analysis. Tech. Coloproctol. 2015, 19, 83. [Google Scholar] [CrossRef]
- Sun, F. The Effect of Tonifying Qi Method on the Expression of Anti-Aging Fibulin Proteins Family of Internal Prolapsed Hemorrhoids. Ph.D. Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2014. [Google Scholar]
- Sun, F.; Xiao, T.; Chen, H.; Li, Y. Study on Effect of Buzhong Yiqi Tang on Expression of Fibulin-5 in Grade-III Prolapsed Hemorrhoid Tissues. New Chin. Med. 2018, 50, 120–124. [Google Scholar]
- Jin, X.; Wan, X.; Huang, D.; Li, Y.; Sun, F. Therapeutic Efficacy of Buzhong Yiqi Decoction Containing Different Doses of Radix Astragali for Stage i Internal Hemorrhoids Patients with Spleen Deficiency and Sinking of Qi and Its Effect on FIbulin-3 Expression in Hemorrhoid Tissues. J. Guangzhou Univ. Tradit. Chin. Med. 2017, 34, 640–644. [Google Scholar] [CrossRef]
- Albig, A.R.; Schiemann, W.P. Fibulin-5 Antagonizes Vascular Endothelial Growth Factor (VEGF) Signaling and Angiogenic Sprouting by Endothelial Cells. DNA Cell Biol. 2004, 23, 367–379. [Google Scholar] [CrossRef]
- De Vega, S.; Iwamoto, T.; Yamada, Y. Fibulins: Multiple Roles in Matrix Structures and Tissue Functions. Cell Mol. Life Sci. 2009, 66, 1890–1902. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; La Rosa, C.C.D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Kisli, E.; Kemik, A.; Sümer, A.; Kemik, Ö. Matrix Metalloproteinases in Pathogenesis of Hemorrhoidal Disease. Am. Surg. 2013, 79, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Xie, D. Effects of MMP-7 and MMP-9 on the Pathogenesis of Hemorrhoids; Shantou University: Shantou, China, 2008. [Google Scholar]
- Qin, L.; Qin, X. Expression and Significance of Mmp-9 and Vegfr2 in Patients with Internal Hemorrhoids. Chin. J. Cell Biol. 2020, 42, 1800–1805. [Google Scholar]
- Han, W.; Wang, Z.; Zhao, B.; Yang, X.; Wang, D.; Wang, J.; Tang, X.; Zhao, F.; Huang, Y. Pathologic Change of Elastic Fibers with Difference of Microvessel Density and Expression of Angiogenesis-Related Proteins in Internal Hemorrhoid Tissues. Chin. J. Gastrointest. Surg. 2005, 8, 56–59. [Google Scholar]
- Di, Y.; Nie, Q.Z.; Chen, X.L. Matrix Metalloproteinase-9 and Vascular Endothelial Growth Factor Expression Change in Experimental Retinal Neovascularization. Int. J. Ophthalmol. 2016, 9, 804. [Google Scholar] [CrossRef]
- Hollborn, M.; Stathopoulos, C.; Steffen, A.; Wiedemann, P.; Kohen, L.; Bringmann, A. Positive Feedback Regulation between MMP-9 and VEGF in Human RPE Cells. Invest Ophthalmol. Vis. Sci. 2007, 48, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Ellinghaus, D.; Juzenas, S.; Cossais, F.; Burmeister, G.; Mayr, G.; Jørgensen, I.F.; Teder-Laving, M.; Skogholt, A.H.; Chen, S.; et al. Genome-Wide Analysis of 944 133 Individuals Provides Insights into the Etiology of Haemorrhoidal Disease-Record Details-Embase. Gut 2021, 70, 1538–1549. [Google Scholar] [CrossRef]
- Ng, M.Y.M.; Andrew, T.; Spector, T.D.; Jeffery, S. Linkage to the FOXC2 Region of Chromosome 16 for Varicose Veins in Otherwise Healthy, Unselected Siblings Pairs. J. Med. Genet. 2005, 42, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.; Girijamma, A.; Nair, R.; Ramegowda, K.S.; Nair, D.H.; Thulaseedharan, J.V.; Lakkappa, R.B.; Kamalapurkar, G.; Kartha, C.C. Forkhead Box C2promoter Variant C<512C.T Is Associated with Increased Susceptibility to Chronic Venous Diseases. PLoS ONE 2014, 9, e90682. [Google Scholar] [CrossRef]
- Brice, G.; Mansour, S.; Bell, R.; Collin, J.R.O.; Child, A.H.; Brady, A.F.; Sarfarazi, M.; Burnand, K.G.; Jeffery, S.; Mortimer, P.; et al. Analysis of the Phenotypic Abnormalities in Lymphoedema-Distichiasis Syndrome in 74 Patients with FOXC2 Mutations or Linkage to 16q24. J. Med. Genet. 2002, 39, 478–483. [Google Scholar] [CrossRef]
- Kume, T. The Cooperative Roles of Foxcl and Foxc2 in Cardiovascular Development. Adv. Exp. Med. Biol. 2009, 665, 63–77. [Google Scholar] [CrossRef]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Molecular Determinants of Chronic Venous Disease: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 1928. [Google Scholar] [CrossRef]
- Murk, W.; de Wan, A.T. Exhaustive Genome-Wide Search for SNP-SNP Interactions Across 10 Human Diseases. G3 2016, 6, 2043–2050. [Google Scholar] [CrossRef]
- Qar, J.; Al Zoubi, M.S.; Baydoun, I.M.A.; Aljabali, A.A.A.; Al-Trad, B.; Rabi, F.; Al Batayneh, K.M. Truncating Mutation in FOXC2 Gene in Familial Hemorrhoids and Varicose Veins. Int. J. Biol. Biomed. Eng. 2020, 14, 32–38. [Google Scholar] [CrossRef]
- Petrova, T.V.; Karpanen, T.; Norrmén, C.; Mellor, R.; Tamakoshi, T.; Finegold, D.; Ferrell, R.; Kerjaschki, D.; Mortimer, P.; Ylä-Herttuala, S.; et al. Defective Valves and Abnormal Mural Cell Recruitment Underlie Lymphatic Vascular Failure in Lymphedema Distichiasis. Nat. Med. 2004, 10, 974–981. [Google Scholar] [CrossRef]
- Bharath, V.; Kahn, S.R.; Lazo-Langner, A. Genetic Polymorphisms of Vein Wall Remodeling in Chronic Venous Disease: A Narrative and Systematic Review. Blood 2014, 124, 1242–1250. [Google Scholar] [CrossRef]
- Adikusuma, W.; Firdayani, F.; Irham, L.M.; Darmawi, D.; Hamidy, M.Y.; Nopitasari, B.L.; Soraya, S.; Azizah, N. Integrated Genomic Network Analysis Revealed Potential of a Druggable Target for Hemorrhoid Treatment. Saudi Pharm. J. 2023, 31, 101831. [Google Scholar] [CrossRef] [PubMed]
- Schwerd, T.; Bryant, R.V.; Pandey, S.; Capitani, M.; Meran, L.; Cazier, J.B.; Jung, J.; Mondal, K.; Parkes, M.; Mathew, C.G.; et al. NOX1 Loss-of-Function Genetic Variants in Patients with Inflammatory Bowel Disease. Mucosal Immunol. 2017, 11, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Dubois, E.A.; Cohen, A.F. Sapropterin: New Drug Mechanisms. Br. J. Clin. Pharmacol. 2010, 69, 576–577. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Alexander, L.M.; Kenney, W.L. Oral Sapropterin Acutely Augments Reflex Vasodilation in Aged Human Skin through Nitric Oxide-Dependent Mechanisms. J. Appl. Physiol. 2013, 115, 972–978. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xue, M.; Kang, C.; Gao, L.; Zhang, M.; Ma, C.; Jia, W.; Zheng, Y.; Cao, L.; Chen, P.; et al. Increased NOX1 and DUOX2 Expression in the Colonic Mucosa of Patients with Chronic Functional Constipation. Medicine 2022, 101, E30028. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Mason, J.B. Folate Status: Effects on Pathways of Colorectal Carcinogenesis. J. Nutr. 2002, 132, 2413S–2418S. [Google Scholar] [CrossRef] [PubMed]
- Promthet, S.; Pientong, C.; Ekalaksananan, T.; Songserm, N.; Poomphakwaen, K.; Chopjitt, P.; Wiangnon, S.; Tokudome, S. Risk Factors for Rectal Cancer and Methylenetetrahydrofolate Reductase Polymorphisms in a Population in Northeast Thailand. Asian Pac. J. Cancer Prev. 2012, 13, 4017–4023. [Google Scholar] [CrossRef]
- Serra, R.; Ssempijja, L.; Provenzano, M.; Andreucci, M. Genetic Biomarkers in Chronic Venous Disease. Biomark. Med. 2020, 14, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Abuoğlu, H.H.; Gunay, E.; Uzunoğlu, H. Effect of Genetic Factors on the Etiopathogenesis of Thrombosed Hemorrhoidal Disease. Chirurgia 2019, 114, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, H.; Zhou, H.; Song, X.; Yuan, S.; Luo, Y. The Angiogenic Function of Nucleolin Is Mediated by Vascular Endothelial Growth Factor and Nonmuscle Myosin. Blood 2006, 107, 3564–3571. [Google Scholar] [CrossRef]
- Salnikova, L.E.; Khadzhieva, M.B.; Kolobkov, D.S. Biological Findings from the PheWAS Catalog: Focus on Connective Tissue-Related Disorders (Pelvic Floor Dysfunction, Abdominal Hernia, Varicose Veins and Hemorrhoids). Hum. Genet. 2016, 135, 779–795. [Google Scholar] [CrossRef]
- Pecci, A.; Klersy, C.; Gresele, P.; Lee, K.J.D.; De Rocco, D.; Bozzi, V.; Russo, G.; Heller, P.G.; Loffredo, G.; Ballmaier, M.; et al. MYH9-Related Disease: A Novel Prognostic Model to Predict the Clinical Evolution of the Disease Based on Genotype-Phenotype Correlations. Hum. Mutat. 2014, 35, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Hillarp, A.; Dahlbäck, B.; Zöller, B. Actuated Protein C Resistance: From Phenotype to Genotype and Clinical Practice. Blood Rev. 1995, 9, 201–212. [Google Scholar] [CrossRef] [PubMed]
- McGrath, I.M.; Montgomery, G.W.; Mortlock, S. Genomic Characterisation of the Overlap of Endometriosis with 76 Comorbidities Identifies Pleiotropic and Causal Mechanisms Underlying Disease Risk. Hum. Genet. 2023, 142, 1345–1360. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Zafiropoulos, A.; Tzatzarakis, M.N.; Tzanakakis, G.N.; Kafatos, A. Relation of PON1 and CYP1A1 Genetic Polymorphisms to Clinical Findings in a Cross-Sectional Study of a Greek Rural Population Professionally Exposed to Pesticides. Toxicol. Lett. 2009, 186, 66–72. [Google Scholar] [CrossRef]
- Humbert, R.; Adler, D.A.; Disteche, C.M.; Hassett, C.; Omiecinski, C.J.; Furlong, C.E. The Molecular Basis of the Human Serum Paraoxonase Activity Polymorphism. Nat. Genet. 1993, 3, 73–76. [Google Scholar] [CrossRef]
- Hassett, C.; Omiecinski, C.J.; Richter, R.J.; Humbert, R.; Humbert, R.; Furlong, C.E.; Furlong, C.E.; Omiecinski, C.J.; Richter, R.J.; Humbert, R.; et al. Characterization of CDNA Clones Encoding Rabbit and Human Serum Paraoxonase: The Mature Protein Retains Its Signal Sequence. Biochemistry 1991, 30, 10141–10149. [Google Scholar] [CrossRef]
- Blatter Garin, M.C.; James, R.W.; Dussoix, P.; Blanché, H.; Passa, P.; Froguel, P.; Ruiz, J. Paraoxonase Polymorphism Met-Leu54 Is Associated with Modified Serum Concentrations of the Enzyme. A Possible Link between the Paraoxonase Gene and Increased Risk of Cardiovascular Disease in Diabetes. J. Clin. Investig. 1997, 99, 62–66. [Google Scholar] [CrossRef]
- Kato, K.; Isbell, H.M.; Fressart, V.; Denjoy, I.; Debbiche, A.; Itoh, H.; Poinsot, J.; George, A.L.; Coulombe, A.; Shea, M.A.; et al. Novel CALM3 Variant Causing Calmodulinopathy with Variable Expressivity in a 4-Generation Family. Circ. Arrhythmia Electrophysiol. 2022, 15, E010572. [Google Scholar] [CrossRef]
- He, J.; Ni, Z.; Li, Z. CALM3 Affects the Prognosis of Leukemia and Hemorrhoids. Medicine 2023, 102, e36027. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Whittaker, C.A.; Carr, S.A.; Tanabe, K.K.; Hynes, R.O. Extracellular Matrix Signatures of Human Primary Metastatic Colon Cancers and Their Metastases to Liver. BMC Cancer 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Moore, S.W.; Johnson, G. Acetylcholinesterase in Hirschsprung’s Disease. Pediatr. Surg. Int. 2005, 21, 255–263. [Google Scholar] [CrossRef]
- Kwartler, C.S.; Chen, J.; Thakur, D.; Li, S.; Baskin, K.; Wang, S.; Wang, Z.V.; Walker, L.; Hill, J.A.; Epstein, H.F.; et al. Overexpression of Smooth Muscle Myosin Heavy Chain Leads to Activation of the Unfolded Protein Response and Autophagic Turnover of Thick Filament-Associated Proteins in Vascular Smooth Muscle Cells. J. Biol. Chem. 2014, 289, 14075–14088. [Google Scholar] [CrossRef]
- Plackett, T.P.; Kwon, E.; Ronald A Gagliano, J.; Oh, R.C. Ehlers-Danlos Syndrome—Hypermobility Type and Hemorrhoids. Case Rep. Surg. 2014, 2014, 171803. [Google Scholar] [CrossRef] [PubMed]
- Hullar, M.A.J.; Fu, B.C. Diet, the Gut Microbiome, and Epigenetics. Cancer J. 2014, 20, 170–175. [Google Scholar] [CrossRef]
- Palumbo, V.D.; Tutino, R.; Messina, M.; Santarelli, M.; Nigro, C.; Lo Secco, G.; Piceni, C.; Montanari, E.; Barletta, G.; Venturelli, P.; et al. Altered Gut Microbic Flora and Haemorrhoids: Could They Have a Possible Relationship? J. Clin. Med. 2023, 12, 2198. [Google Scholar] [CrossRef] [PubMed]
- Kibret, A.A.; Oumer, M.; Moges, A.M. Prevalence and Associated Factors of Hemorrhoids among Adult Patients Visiting the Surgical Outpatient Department in the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS ONE 2021, 16, e0249736. [Google Scholar] [CrossRef]
- Peery, A.F.; Sandler, R.S.; Galanko, J.A.; Bresalier, R.S.; Figueiredo, J.C.; Ahnen, D.J.; Barry, E.L.; Baron, J.A. Risk Factors for Hemorrhoids on Screening Colonoscopy. PLoS ONE 2015, 10, e0139100. [Google Scholar] [CrossRef] [PubMed]
- Riss, S.; Weiser, F.A.; Schwameis, K.; Riss, T.; Mittlböck, M.; Steiner, G.; Stift, A. The Prevalence of Hemorrhoids in Adults. Int. J. Color. Dis 2012, 27, 215–220. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.E.; Kang, J.H.; Shin, J.Y.; Song, Y.M. Factors Associated with Hemorrhoids in Korean Adults: Korean National Health and Nutrition Examination Survey. Korean J. Fam. Med. 2014, 35, 227. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Role of Extracellular Matrix Remodelling in Adipose Tissue Pathophysiology: Relevance in the Development of Obesity. Histol. Histopathol 2012, 27, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Karaskova, E.; Velganova-Veghova, M.; Geryk, M.; Foltenova, H.; Kucerova, V.; Karasek, D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 4226. [Google Scholar] [CrossRef]
- Kessler, C. Pathophysiology of Obesity. Nurs. Clin. N. Am 2021, 56, 465–478. [Google Scholar] [CrossRef]
- Pigot, F.; Siproudhis, L.; Allaert, F.A. Risk Factors Associated with Hemorrhoidal Symptoms in Specialized Consultation. Gastroenterol. Clin. Biol 2005, 29, 1270–1274. [Google Scholar] [CrossRef]
- Delcò, F.; Sonnenberg, A. Associations between Hemorrhoids and Other Diagnoses. Dis. Colon Rectum 1998, 41, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Davis, K.L.; Baran, R.W. Healthcare Costs and Clinical Sequelae Associated with Constipation in a Managed Care Population. Am. J. Gastroenterol. 2007, 102, S432. [Google Scholar] [CrossRef]
- Johannsson, H.Ö.; Graf, W.; Påhlman, L. Bowel Habits in Hemorrhoid Patients and Normal Subjects. Am. J. Gastroenterol. 2005, 100, 401–406. [Google Scholar] [CrossRef]
- Helvaci, M.R.; Algin, M.C.; Kaya, H. Irritable Bowel Syndrome and Chronic Gastritis, Hemorrhoid, Urolithiasis. Eurasian J. Med. 2009, 41, 158. [Google Scholar] [PubMed]
- Rusu, F.; Dumitraşcu, D.L. Four Years Follow-up of Patients with Irritable Bowel Syndrome. Rom. J. Intern. Med. 2015, 53, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Dothel, G.; Barbaro, M.R.; Di Vito, A.; Ravegnini, G.; Gorini, F.; Monesmith, S.; Coschina, E.; Benuzzi, E.; Fuschi, D.; Palombo, M.; et al. New Insights into Irritable Bowel Syndrome Pathophysiological Mechanisms: Contribution of Epigenetics. J. Gastroenterol. 2023, 58, 605–621. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural Changes in the Gut Microbiome of Constipated Patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef]
- Cao, Y.; Langer, R.; Ferrara, N. Targeting Angiogenesis in Oncology, Ophthalmology and Beyond. Nat. Rev. Drug Discov. 2023, 22, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Fields, G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal Database Combinations for Literature Searches in Systematic Reviews: A Prospective Exploratory Study. Syst. Rev. 2017, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; Cochrane: London, UK, 2023. [Google Scholar]

| Cytokines Expressed in Hemorrhoids | |
|---|---|
| Pro-Inflammatory Cytokines | Anti-Inflammatory Cytokines |
| RANTES | IL-10 |
| TNF-α | ·· |
| VEGF | ·· |
| IL-1β | ·· |
| IL-6 | ·· |
| IL-8 | ·· |
| IL-17 | ·· |
| IFN-γ | ·· |
| MiRNAs in Vesicle Pathways in Hemorrhoids | MiRNAs in Extracellular Vesicle Pathways in Hemorrhoids | |||
|---|---|---|---|---|
| Probable targeted processes * | Endocytosis | Synaptic vesicle pathways | Transcription, protein kinase activity, and ubiquitination | Transcriptional activator activity |
| Status | Upregulated | Downregulated | Upregulated | Downregulated |
| Type of miRNA | miR-375 | miR-376b-3p | miR-6741-3p | miR-548t-5p |
| miR-215-5p | miR-34a-5p | miR-6834-3 | miR-323b-5p | |
| miR-192-5p | miR-152-3p | miR-425 | miR-1322 | |
| miR-143-3p | let-7c-5p | miR-6804-3 | miR-3928-5p | |
| miR-187-3p | miR-107 | miR-744-3 | miR-346 | |
| miR-194-5p | miR-517a-3p | miR-848 | miR-4704-5p | |
| miR-145-5p | miR-517b-3p | miR-299-5 | miR-1913 | |
| miR-490-3p | miR-1307-5p | miR-463 | miR-876-3p | |
| miR-145-3p | miR-190a-5p | miR-317 | miR-4460 | |
| ·· | miR-378a-5p | miR-465 | miR-892a | |
| ·· | miR-708-3p | ·· | ·· | |
| ·· | miR-450a-5p | ·· | ·· | |
| ·· | miR-30e-5p | ·· | ·· | |
| ·· | miR-532-5p | ·· | ·· | |
| Genes Significant to the Development of Hemorrhoids | |||||
|---|---|---|---|---|---|
| Gene | Product | Function | Mechanism of Action | Mutations | Reported Associated Conditions |
| FOXC2 | FOXC2 (forkhead box protein) [137,138] |
| — | ||
| NOX1 and NOS3 | NOX1 (NADPH oxidase) NOS3 (nitric oxide synthase) |
| — | ||
| MTHFR | methylenetetrahydrofolate reductase |
| — |
| |
| MYH9 | heavy chain of non-muscle myosin IIA |
| — |
| |
| F5 | coagulation factor V |
| — |
| |
| CYP1A | aryl hydrocarbon hydroxylase in hepatic and extrahepatic cytochrome P450 | — | — |
|
|
| PON1 | serum paraoxonase 1 | — | — |
|
|
| CALM3 | calmodulin 3 |
| — | — |
|
| ANO1 | anoctamin-1 (voltage-gated calcium-activated anion channel) |
|
|
| — |
| SPRX | — |
|
|
| — |
| ACHE | acetylcholinesterase |
| — |
| |
| SRTT | capped-RNA binding protein | — | — | — |
|
| GSDMC | gasdermin C |
| — |
|
|
| MYH11 | muscle myosin heavy chain 11 |
|
|
|
|
| ELN | elastin |
| — | — |
|
| COL5A2 | type V collagen (regulatory) | — | — | — | |
| PRDM | histone methyltransferase |
| — | — |
|
| Molecular Components Altered in Hemorrhoids | |||
|---|---|---|---|
| Type of Change | Upregulated | Downregulated | Mutated or Altered (*) Genes |
| Molecule | Pro-inflammatory cytokines, especially VEGF | Anti-inflammatory cytokines | FOXC2 |
| CGRP, SP, and TRPV1 | Inhibitory pathways of angiogenesis | MTHFR | |
| COX-2 | Vesicle miRNAs modulating synaptic vesicle pathways and transcriptional activator activity | MYH9 | |
| Vesicle miRNAs modulating endocytosis, transcription, protein kinase activity, and ubiquitination | miR-412-5p, leading to deregulation of the cell cycle | CYP1A | |
| NOS3 | miR-4729, leading to overexpression of TIE1 and angiogenesis via METTL14 regulation | PON1 | |
| NOS and NO | miR-424-5p, caused by estrogens, leading to increased expression of VEGF | ANO1 | |
| vWF, CD31, CD34, endoglin | Fibulin-3 and fibulin-5 | SPRX * | |
| VEGF, VEGFR2 | Collagen levels, collagen I/III ratio | SRTT * | |
| MMPs | ·· | GSDMC * | |
| NOX1 | ·· | COL5A2 | |
| CALM3 | ·· | ·· | |
| ACHE | ·· | ·· | |
| MYH11 | ·· | ·· | |
| ELN | ·· | ·· | |
| PRDM | ·· | ·· | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parol, B.; Sas, O.; Mazurek, M.; Data, K.; Wozniak, S.; Domagala, Z. How Can Molecules Induce Hemorrhoids? The Role of Genetics and Epigenetics in Hemorrhoidal Disease. Int. J. Mol. Sci. 2025, 26, 9394. https://doi.org/10.3390/ijms26199394
Parol B, Sas O, Mazurek M, Data K, Wozniak S, Domagala Z. How Can Molecules Induce Hemorrhoids? The Role of Genetics and Epigenetics in Hemorrhoidal Disease. International Journal of Molecular Sciences. 2025; 26(19):9394. https://doi.org/10.3390/ijms26199394
Chicago/Turabian StyleParol, Barbara, Oliwia Sas, Mateusz Mazurek, Krzysztof Data, Slawomir Wozniak, and Zygmunt Domagala. 2025. "How Can Molecules Induce Hemorrhoids? The Role of Genetics and Epigenetics in Hemorrhoidal Disease" International Journal of Molecular Sciences 26, no. 19: 9394. https://doi.org/10.3390/ijms26199394
APA StyleParol, B., Sas, O., Mazurek, M., Data, K., Wozniak, S., & Domagala, Z. (2025). How Can Molecules Induce Hemorrhoids? The Role of Genetics and Epigenetics in Hemorrhoidal Disease. International Journal of Molecular Sciences, 26(19), 9394. https://doi.org/10.3390/ijms26199394








