Balanced Translocations Involving the DMD Gene as a Cause of Muscular Dystrophy in Female Children: A Description of Three Cases
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Molecular Genetic Diagnostics
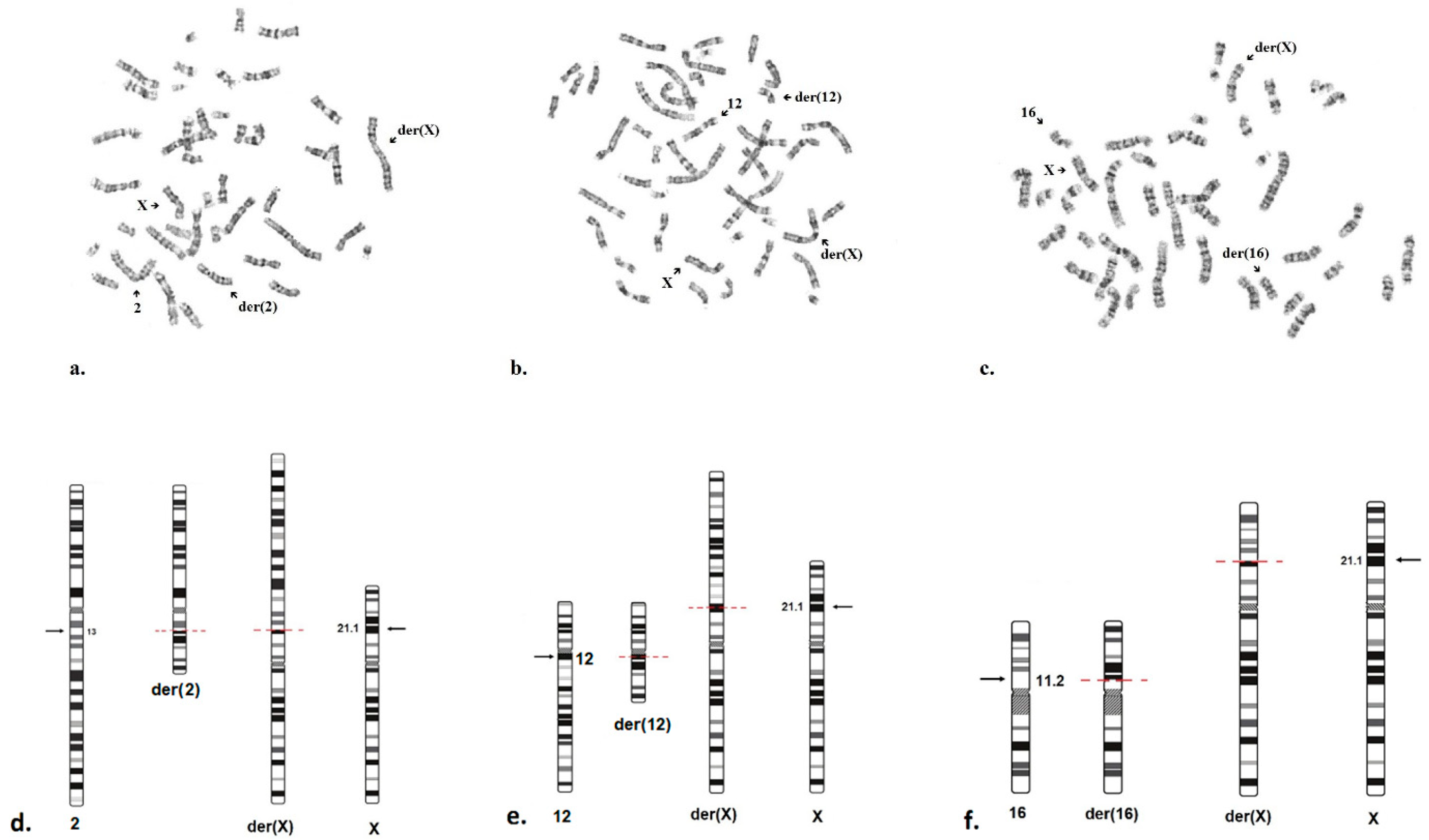
2.3. Cytogenetic Analysis
3. Discussion
4. Patients and Methods
4.1. Clinical Evaluation
4.2. Genetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMD | Duchenne muscular dystrophy |
| LGMD | Limb-Girdle Muscular Dystrophy |
| MLPA | Multiplex Ligation-dependent Probe Amplification |
| CK | Creatine kinase |
| WGS | Whole genome sequencing |
| US | Ultrasound |
| EMG | Electromyography |
| MRI | Magnetic resonance imaging |
| ECG | Electrocardiography |
| EDTA | Ethylenediaminetetraacetic acid |
| DNA | Deoxyribonucleic acid |
| PCR | Polymerase chain reaction |
References
- Salari, N.; Fatahi, B.; Valipour, E.; Kazeminia, M.; Fatahian, R.; Kiaei, A.; Shohaimi, S.; Mohammadi, M. Global Prevalence of Duchenne and Becker Muscular Dystrophy: A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2022, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, J.; Lu, Y.; Lu, Y.; Mai, J.; Bai, L.; Wang, Y.; Zheng, Y.; Yu, M.; Zheng, Y.; et al. Clinical, Pathological, and Genetic Characterization in a Large Chinese Cohort with Female Dystrophinopathy. Neuromuscul. Disord. 2023, 33, 728–736. [Google Scholar] [CrossRef]
- Sarkozy, A.; Quinlivan, R.; Bourke, J.P.; Ferlini, A.; ENMC 263rd Workshop Study Group. 263rd ENMC International Workshop: Focus on Female Carriers of Dystrophinopathy: Refining Recommendations for Prevention, Diagnosis, Surveillance, and Treatment. Hoofddorp, The Netherlands, 13–15 May 2022. Neuromuscul. Disord. 2023, 33, 274–284. [Google Scholar] [CrossRef]
- Houwen-van Opstal, S.L.S.; Tak, R.O.; Pelsma, M.; van den Heuvel, F.M.A.; van Duyvenvoorde, H.A.; Cup, E.H.C.; Sie, L.T.L.; Vles, J.S.H.; de Groot, I.J.M.; Voermans, N.C.; et al. Long-Term Outcomes for Females with Early-Onset Dystrophinopathy. Dev. Med. Child. Neurol. 2023, 65, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Salgado, D.; Monges, S.; Foncuberta, M.E.; Kekou, K.; Kosma, K.; Dawkins, H.; Lamont, L.; Roy, A.J.; Chamova, T.; et al. The TREAT-NMD DMD Global Database: Analysis of More than 7,000 Duchenne Muscular Dystrophy Mutations. Hum. Mutat. 2015, 36, 395–402. [Google Scholar] [CrossRef]
- Trippe, H.; Wieczorek, S.; Kötting, J.; Kress, W.; Schara, U. Xp21/A Translocation: A Rarely Considered Genetic Cause for Manifesting Carriers of Duchenne Muscular Dystrophy. Neuropediatrics 2014, 45, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Segarra-Casas, A.; Yépez, V.A.; Demidov, G.; Laurie, S.; Esteve-Codina, A.; Gagneur, J.; Parkhurst, Y.; Muni-Lofra, R.; Harris, E.; Marini-Bettolo, C.; et al. An Integrated Transcriptomics and Genomics Approach Detects an X/Autosome Translocation in a Female with Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2024, 25, 7793. [Google Scholar] [CrossRef]
- Justel, M.; Jou, C.; Sariego-Jamardo, A.; Juliá-Palacios, N.A.; Ortez, C.; Poch, M.L.; Hedrera-Fernandez, A.; Gomez-Martin, H.; Codina, A.; Dominguez-Carral, J.; et al. Expanding the Phenotypic Spectrum of TRAPPC11-Related Muscular Dystrophy: 25 Roma Individuals Carrying a Founder Variant. J. Med. Genet. 2023, 60, 965–973. [Google Scholar] [CrossRef]
- Jokela, M.; Lehtinen, S.; Palmio, J.; Saukkonen, A.-M.; Huovinen, S.; Vihola, A.; Udd, B. A Novel COL6A2 Mutation Causing Late-Onset Limb-Girdle Muscular Dystrophy. J. Neurol. 2019, 266, 1649–1654. [Google Scholar] [CrossRef]
- Bönnemann, C.G. The Collagen VI-Related Myopathies: Muscle Meets Its Matrix. Nat. Rev. Neurol. 2011, 7, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Corona-Rivera, J.R.; Martínez-Duncker, I.; Morava, E.; Ranatunga, W.; Salinas-Marin, R.; González-Jaimes, A.M.; Castillo-Reyes, K.A.; Peña-Padilla, C.; Bobadilla-Morales, L.; Corona-Rivera, A.; et al. TRAPPC11-CDG Muscular Dystrophy: Review of 54 Cases Including a Novel Patient. Mol. Genet. Metab. 2024, 142, 108469. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Sokoda, T.; Misaki, M.; Shimomura, H.; Takeshima, Y. EP.59A Manifesting Carrier of Duchenne Muscular Dystrophy with a Balanced X- Autosome Translocation with a Breakpoint in the Dystrophin Gene. Neuromuscul. Disord. 2019, 29, S169–S170. [Google Scholar] [CrossRef]
- Vacca, M.; Della Ragione, F.; Scalabrì, F.; D’Esposito, M. X Inactivation and Reactivation in X-Linked Diseases. Semin. Cell Dev. Biol. 2016, 56, 78–87. [Google Scholar] [CrossRef]
- Sharp, A.J.; Spotswood, H.T.; Robinson, D.O.; Turner, B.M.; Jacobs, P.A. Molecular and Cytogenetic Analysis of the Spreading of X Inactivation in X;Autosome Translocations. Hum. Mol. Genet. 2002, 11, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Zatz, M.; Vianna-Morgante, A.M.; Campos, P.; Diament, A.J. Translocation (X;6) in a Female with Duchenne Muscular Dystrophy: Implications for the Localisation of the DMD Locus. J. Med. Genet. 1981, 18, 442–447. [Google Scholar] [CrossRef]
- Pluta, N.; von Moers, A.; Pechmann, A.; Stenzel, W.; Goebel, H.-H.; Atlan, D.; Wolf, B.; Nanda, I.; Zaum, A.-K.; Rost, S. Whole-Genome Sequencing Identified New Structural Variations in the DMD Gene That Cause Duchenne Muscular Dystrophy in Two Girls. Int. J. Mol. Sci. 2023, 24, 13567. [Google Scholar] [CrossRef]
- Szűcs, Z.; Pinti, É.; Haltrich, I.; Szén, O.P.; Nagy, T.; Barta, E.; Méhes, G.; Bidiga, L.; Török, O.; Ujfalusi, A.; et al. An Ultra-Rare Manifestation of an X-Linked Recessive Disorder: Duchenne Muscular Dystrophy in a Female Patient. Int. J. Mol. Sci. 2022, 23, 13076. [Google Scholar] [CrossRef]
- Nozoe, K.T.; Akamine, R.T.; Mazzotti, D.R.; Polesel, D.N.; Grossklauss, L.F.; Tufik, S.; Andersen, M.L.; Moreira, G.A. Phenotypic Contrasts of Duchenne Muscular Dystrophy in Women: Two Case Reports. Sleep Sci. 2016, 9, 129–133. [Google Scholar] [CrossRef]
- Demirci, H.; Durmus, H.; Toksoy, G.; Uslu, A.; Parman, Y.; Hanagasi, H. Cognition of the Mothers of Patients with Duchenne Muscular Dystrophy. Muscle Nerve 2020, 62, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, F.; Torelli, S.; Ferlini, A. Dystrophin and Mutations: One Gene, Several Proteins, Multiple Phenotypes. Lancet Neurol. 2003, 2, 731–740. [Google Scholar] [CrossRef]
- Preethish-Kumar, V.; Shah, A.; Polavarapu, K.; Kumar, M.; Safai, A.; Vengalil, S.; Nashi, S.; Deepha, S.; Govindaraj, P.; Afsar, M.; et al. Disrupted Structural Connectome and Neurocognitive Functions in Duchenne Muscular Dystrophy: Classifying and Subtyping Based on Dp140 Dystrophin Isoform. J. Neurol. 2022, 269, 2113–2125. [Google Scholar] [CrossRef]
- Politano, L.; Nigro, V.; Nigro, G.; Petretta, V.R.; Passamano, L.; Papparella, S.; Di Somma, S.; Comi, L.I. Development of Cardiomyopathy in Female Carriers of Duchenne and Becker Muscular Dystrophies. JAMA 1996, 275, 1335–1338. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, W.; Du, J.; Jin, S.; Li, S.; Zhao, Y.; Xu, C.; Wang, Z.; Lv, H.; Zhang, W.; et al. The Trefoil with Single Fruit Sign in Muscle Magnetic Resonance Imaging Is Highly Specific for Dystrophinopathies. Eur. J. Radiol. 2015, 84, 1992–1998. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Dixon, L.D.; Kokesh, K.J.; Zingariello, C.D.; Vandenborne, K.; Walter, G.A.; Barnard, A.M. Skeletal Muscle Symptoms and Quantitative MRI in Females with Dystrophinopathy. Muscle Nerve 2024, 70, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ye, L.; Yang, Z.; Chen, H.; Yuan, J.; Wang, H.; Guo, X.; Li, Y.; Wang, J.; Chen, F.; et al. Balanced Chromosomal Rearrangement Detection by Low-Pass Whole-Genome Sequencing. Curr. Protoc. Hum. Genet. 2018, 96, 8.18.1–8.18.16. [Google Scholar] [CrossRef]
- Elangkovan, N.; Dickson, G. Gene Therapy for Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, S303–S316. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Muntoni, F.; McDonald, C.M.; Mercuri, E.M.; Ciafaloni, E.; Komaki, H.; Leon-Astudillo, C.; Nascimento, A.; Proud, C.; Schara-Schmidt, U.; et al. AAV Gene Therapy for Duchenne Muscular Dystrophy: The EMBARK Phase 3 Randomized Trial. Nat. Med. 2025, 31, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Kourakis, S.; Timpani, C.A.; Campelj, D.G.; Hafner, P.; Gueven, N.; Fischer, D.; Rybalka, E. Standard of Care versus New-Wave Corticosteroids in the Treatment of Duchenne Muscular Dystrophy: Can We Do Better? Orphanet J. Rare Dis. 2021, 16, 117. [Google Scholar] [CrossRef]
- Bourke, J.; Turner, C.; Bradlow, W.; Chikermane, A.; Coats, C.; Fenton, M.; Ilina, M.; Johnson, A.; Kapetanakis, S.; Kuhwald, L.; et al. Cardiac Care of Children with Dystrophinopathy and Females Carrying DMD-Gene Variations. Open Heart 2022, 9, e001977. [Google Scholar] [CrossRef]
- Boyd, Y.; Buckle, V.; Holt, S.; Munro, E.; Hunter, D.; Craig, I. Muscular Dystrophy in Girls with X;Autosome Translocations. J. Med. Genet. 1986, 23, 484–490. [Google Scholar] [CrossRef]
- Wizard® Genomic DNA Purification Kit Protocol. Available online: https://www.promega.in/resources/protocols/technical-manuals/0/wizard-genomic-dna-purification-kit-protocol/ (accessed on 4 September 2025).
- MRC Holland—MRC Holland. Available online: https://www.mrcholland.com/ (accessed on 1 September 2025).
- 100 000, Я. Available online: https://biotechcampus.ru/ (accessed on 4 September 2025).
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Picard Tools—By Broad Institute. Available online: https://broadinstitute.github.io/picard/ (accessed on 4 September 2025).
- Poplin, R.; Chang, P.-C.; Alexander, D.; Schwartz, S.; Colthurst, T.; Ku, A.; Newburger, D.; Dijamco, J.; Nguyen, N.; Afshar, P.T.; et al. A Universal SNP and Small-Indel Variant Caller Using Deep Neural Networks. Nat. Biotechnol. 2018, 36, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.-F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E.M.; et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Бескoрoвайный, Н.С. Прoграмма “NGS-Data-Genome”. Available online: https://www.elibrary.ru/item.asp?id=46483810 (accessed on 4 September 2025).
- Home—OMIM—(OMIM.ORG). Available online: https://www.omim.org/ (accessed on 4 September 2025).
- The DMD Gene Homepage—Global Variome Shared LOVD. Available online: https://databases.lovd.nl/shared/genes/DMD (accessed on 4 September 2025).
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Yorifuji, T.; Mituyoshi, I. Skewed X Inactivation in Manifesting Carriers of Duchenne Muscular Dystrophy. Clin. Genet. 1998, 53, 102–107. [Google Scholar] [CrossRef]
- Orstavik, K.H. X Chromosome Inactivation in Clinical Practice. Hum. Genet. 2009, 126, 363–373. [Google Scholar] [CrossRef]

| Patient | Gender | Family History | Genotype (WGS+ Karyotype) | Segregation | Age of Examination | Age of Clinical Manifestation | First Symptoms | Delayed Walking | Proximal Weakness | Waddling Gait | Gowers’ Sign | Calf Muscle Hypertrophy | Intellectual Disability |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Female | no | 46,X,t(X;2) (p21;q13) | de novo | 4 years | 1 years | Speech developmental delay, strabismus | No | Yes | Yes | Yes | Yes | Yes |
| N2 | Female | no | 46,Х,t(X;12) (p21.1;q11-q12) | de novo | 6 years | 6 months | Increased levels of CK, ALT and AST | No | Yes | Yes | Yes | Yes | Yes |
| N3 | Female | no | 46,X,t(X;16) (p21.1;p11.2) | de novo | 9 years | 5 years | Hyperlordosis, gait disturbance | No | Yes | Yes | Yes | Yes | Yes |
| Patient | CK (U/L) | ALT and AST (U/L) | ECG | Cardiac US | Lower Limb Muscle MRI | EMG |
|---|---|---|---|---|---|---|
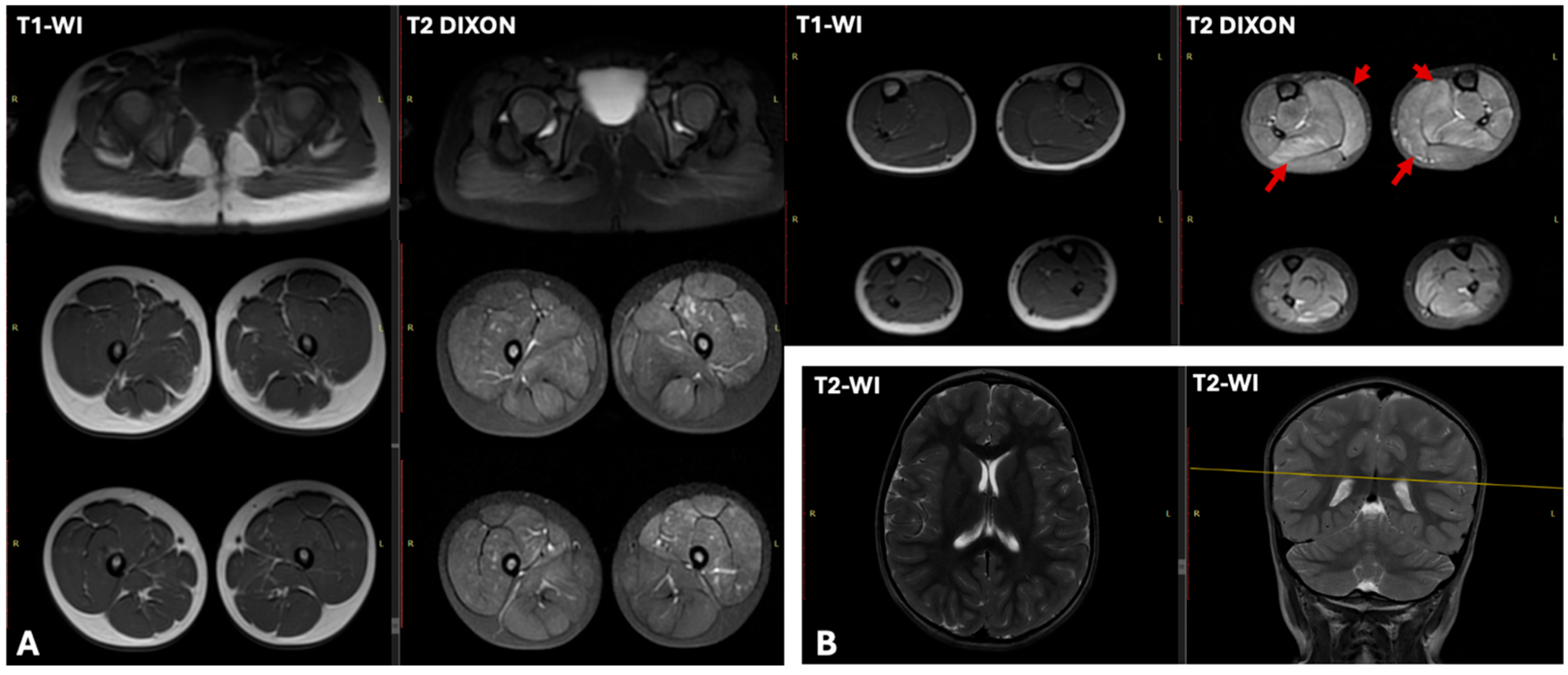
| N1 | 26,694 | x11 | Moderate sinus arrhythmia, incomplete RBBB. Deep Q wave in leads III and aVF. Slight impairment of repolarization in the myocardium, manifested as T wave flattening in lead III | LVEF—61.4% (normal >60%), LVEDD—37.4 mm (normal <37 mm). | T1-WI showed no fatty infiltration. T2 DIXON images revealed clear hyperintensity within the soleus and medial gastrocnemius muscles | myogenic pattern |
| N2 | 23,000 | x6 | Incomplete RBBB, moderate hypoplasia of the pulmonary artery trunk | Ebstein’s anomaly | No data | myogenic pattern |
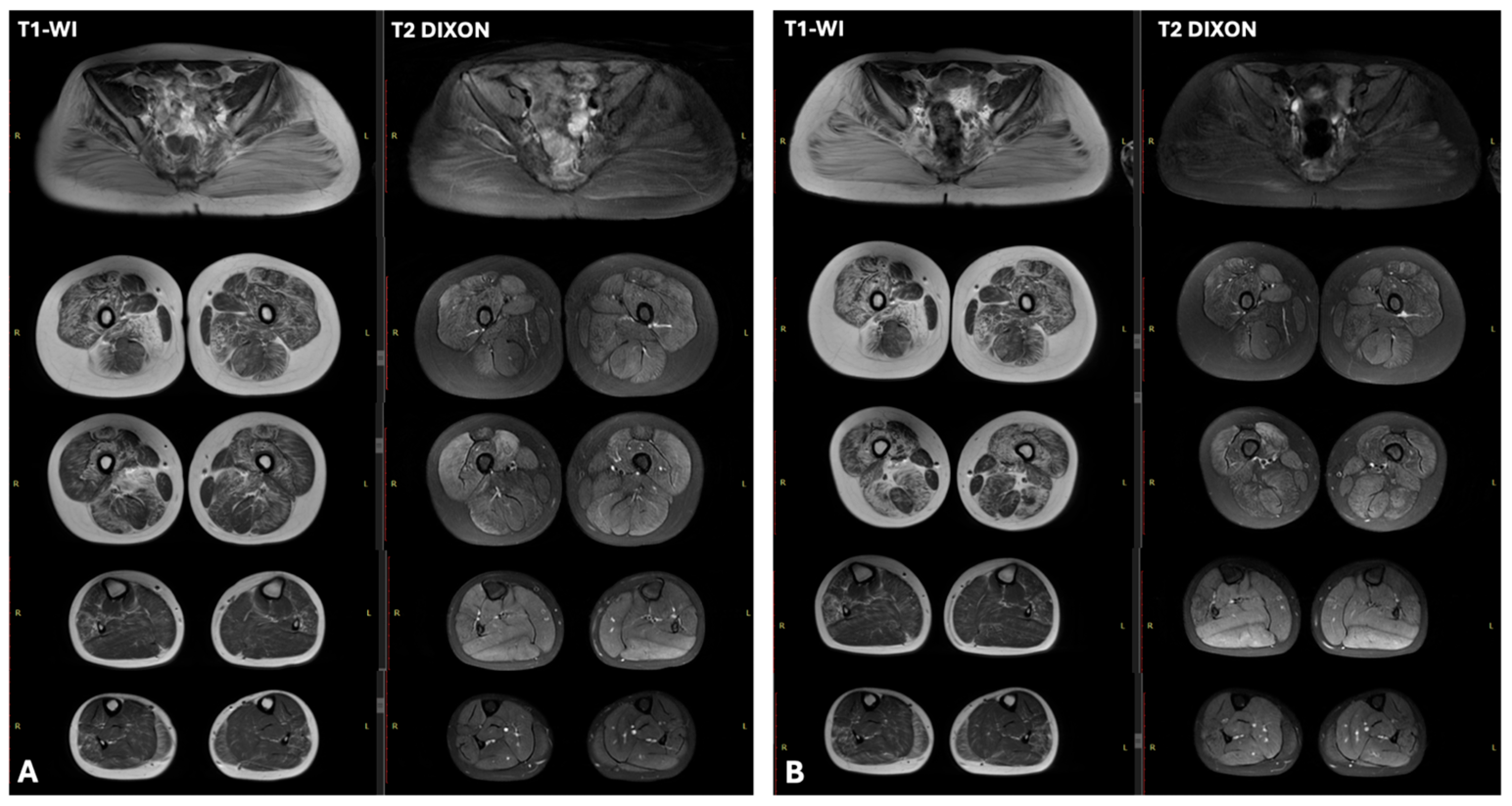
| N3 | 16,000 | x3–5 | Abnormal ventricular repolarization | No changes | Characteristic “trefoil with a single fruit” sign, with severe involvement of the adductor magnus and biceps femoris and relative sparing of the gracilis, sartorius, adductor longus, and semitendinosus. | myogenic pattern |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorontsova, E.O.; Murtazina, A.; Zinina, E.; Polyakov, A.V.; Sumina, M.; Rybakova, O.A.; Vlodavets, D.; Kazakov, D.; Suvorova, Y.; Sharkova, I.V.; et al. Balanced Translocations Involving the DMD Gene as a Cause of Muscular Dystrophy in Female Children: A Description of Three Cases. Int. J. Mol. Sci. 2025, 26, 9389. https://doi.org/10.3390/ijms26199389
Vorontsova EO, Murtazina A, Zinina E, Polyakov AV, Sumina M, Rybakova OA, Vlodavets D, Kazakov D, Suvorova Y, Sharkova IV, et al. Balanced Translocations Involving the DMD Gene as a Cause of Muscular Dystrophy in Female Children: A Description of Three Cases. International Journal of Molecular Sciences. 2025; 26(19):9389. https://doi.org/10.3390/ijms26199389
Chicago/Turabian StyleVorontsova, Ekaterina O., Aysylu Murtazina, Elena Zinina, Alexander V. Polyakov, Maria Sumina, Olga A. Rybakova, Dmitry Vlodavets, Dmitry Kazakov, Yulia Suvorova, Inna V. Sharkova, and et al. 2025. "Balanced Translocations Involving the DMD Gene as a Cause of Muscular Dystrophy in Female Children: A Description of Three Cases" International Journal of Molecular Sciences 26, no. 19: 9389. https://doi.org/10.3390/ijms26199389
APA StyleVorontsova, E. O., Murtazina, A., Zinina, E., Polyakov, A. V., Sumina, M., Rybakova, O. A., Vlodavets, D., Kazakov, D., Suvorova, Y., Sharkova, I. V., Demina, N. A., Repina, S. A., Bulanova, V. A., Antonova, M., Dadali, E., Marakhonov, A. V., Shilova, N. V., Kutsev, S. I., & Shchagina, O. A. (2025). Balanced Translocations Involving the DMD Gene as a Cause of Muscular Dystrophy in Female Children: A Description of Three Cases. International Journal of Molecular Sciences, 26(19), 9389. https://doi.org/10.3390/ijms26199389







