Investigation of Host-Guest Inclusion Complexes Between Carmustine and α-Cyclodextrin: Synthesis, Characterization, and Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of BCNU-α-CD ICs
2.2. Characterization of BCNU-α-CD ICs
2.2.1. DSC Analysis
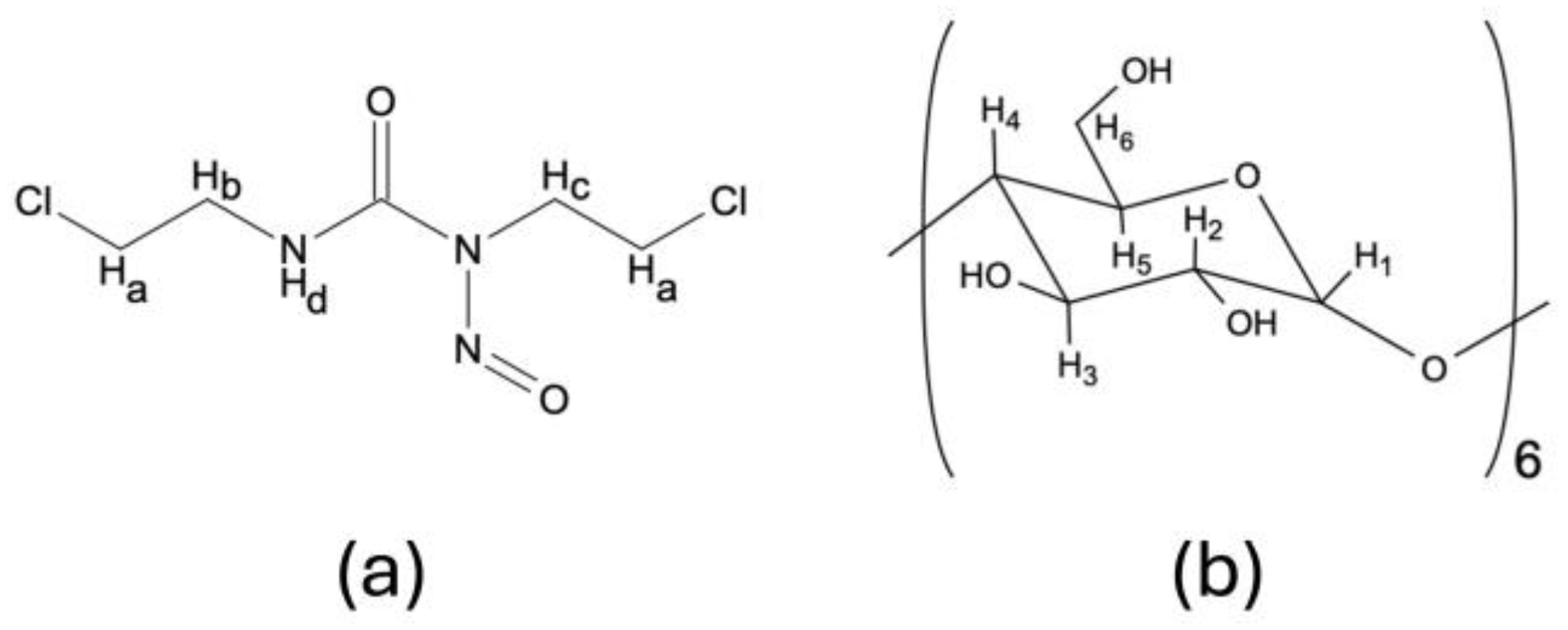
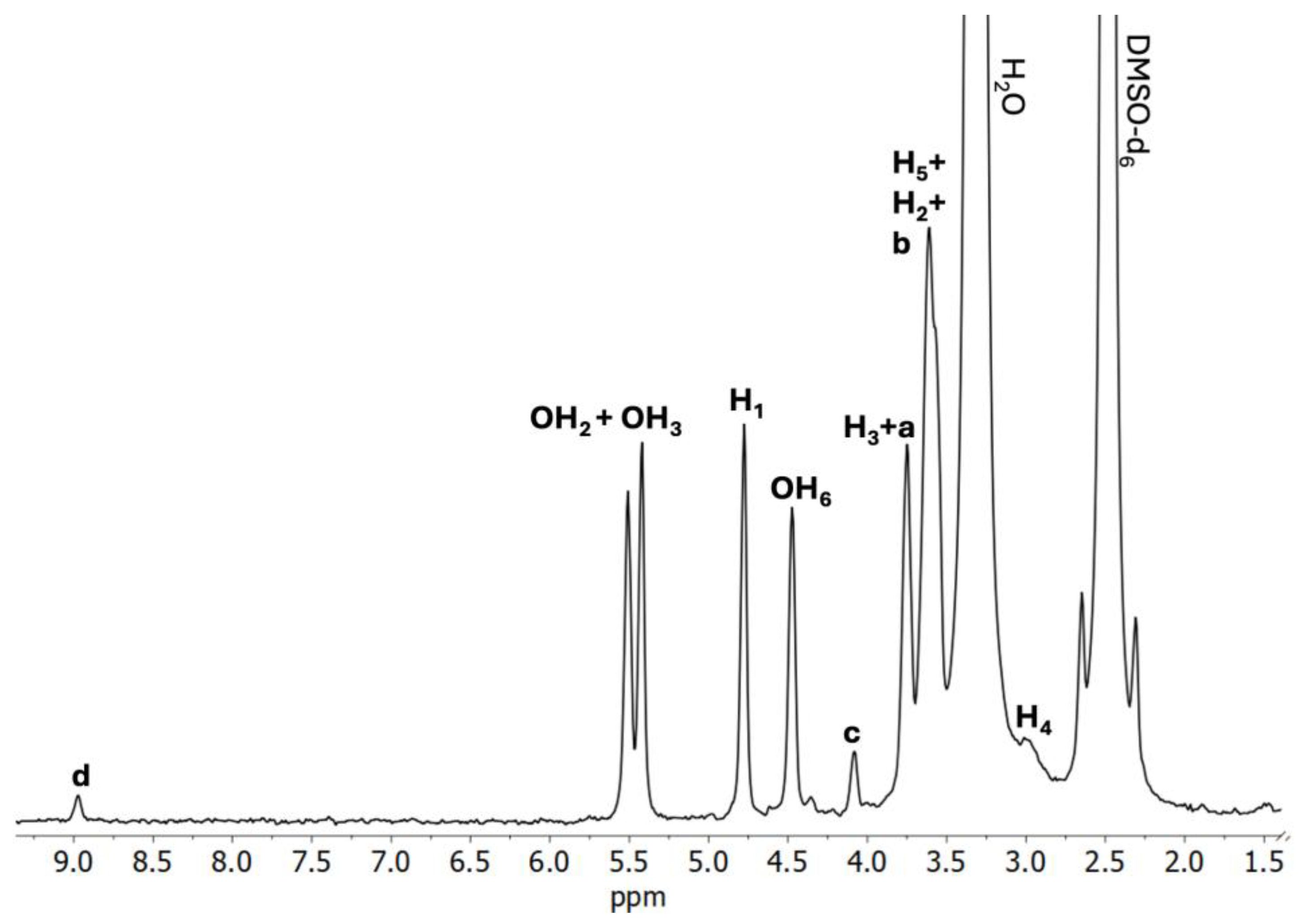
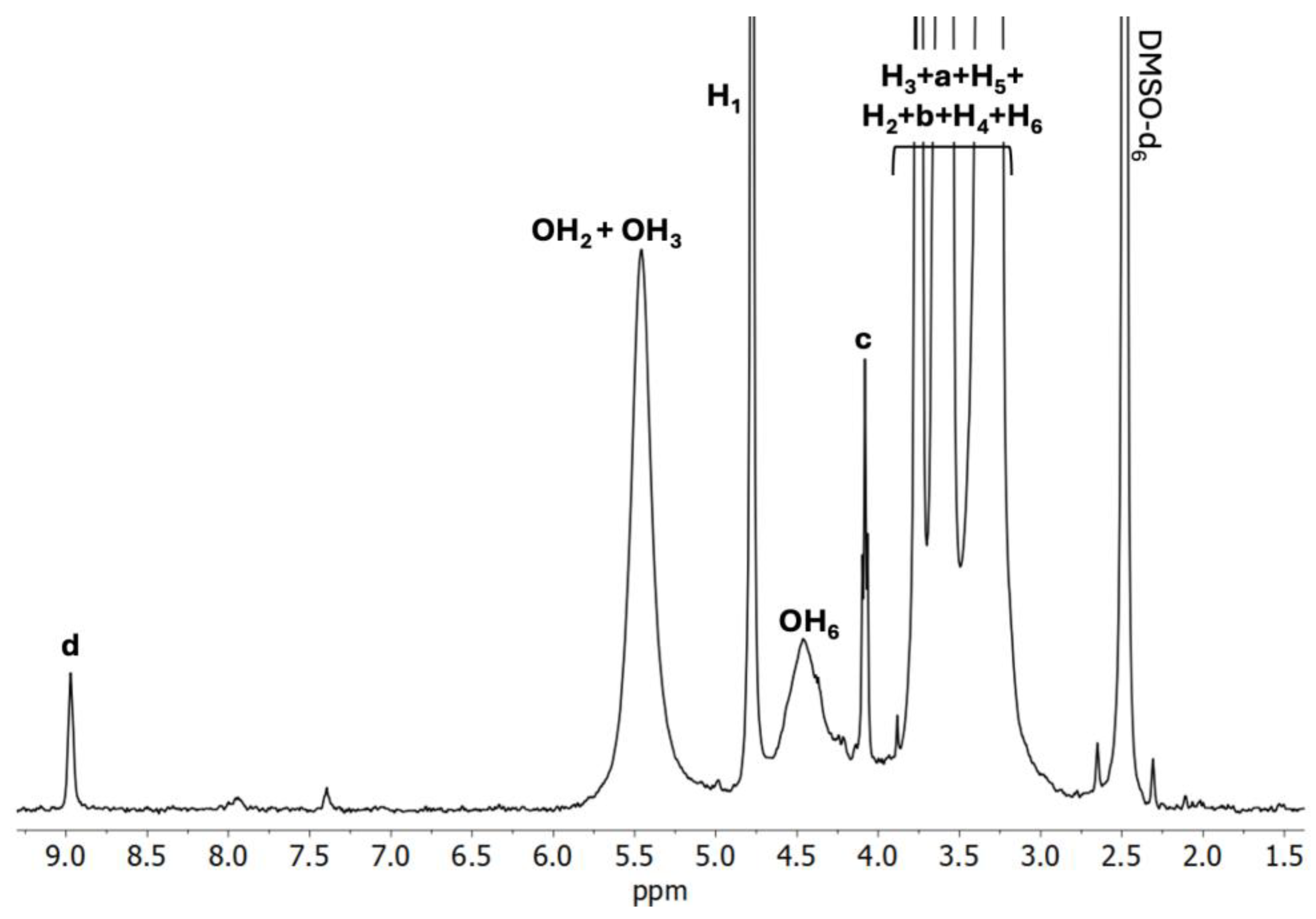
2.2.2. 1H NMR Analysis
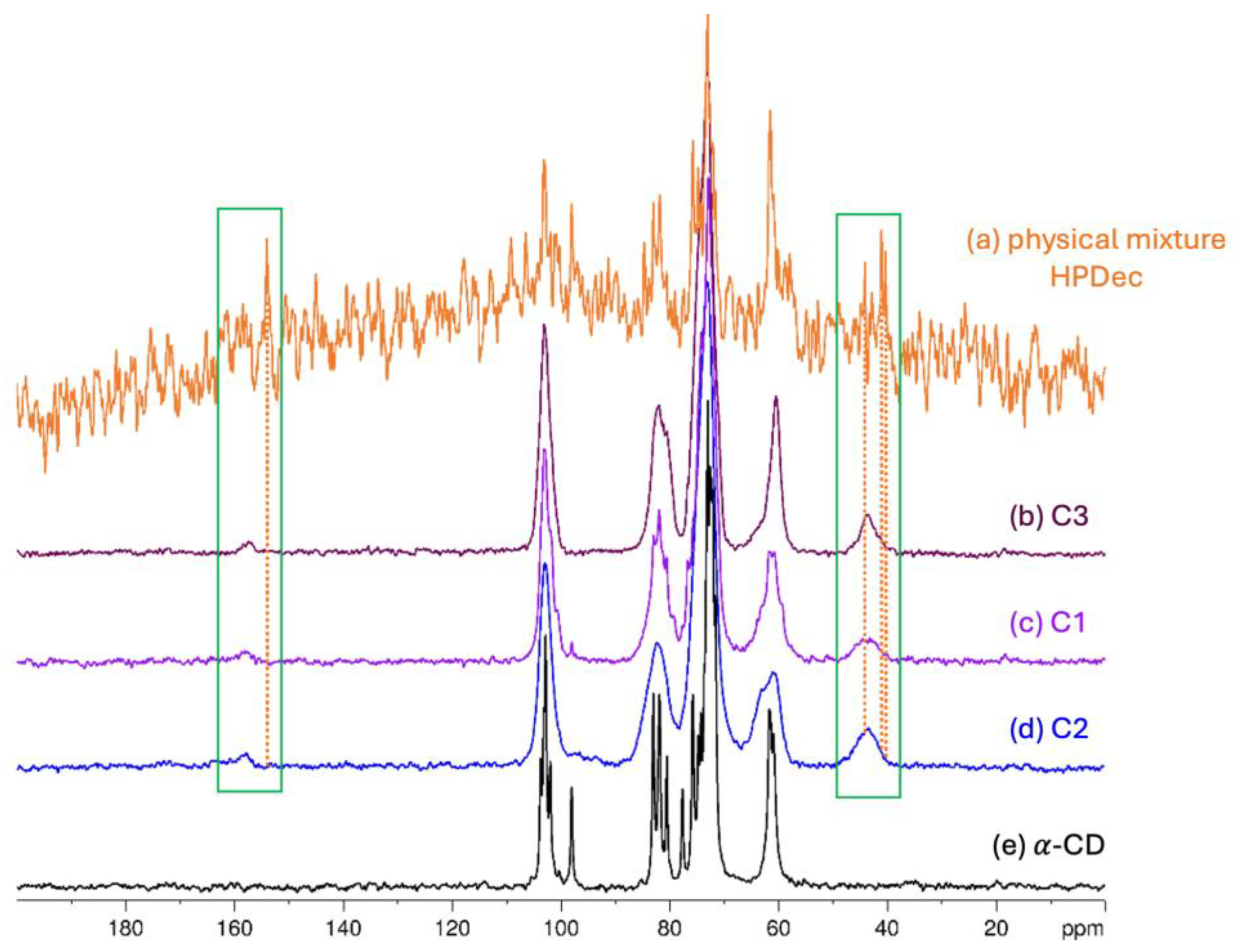
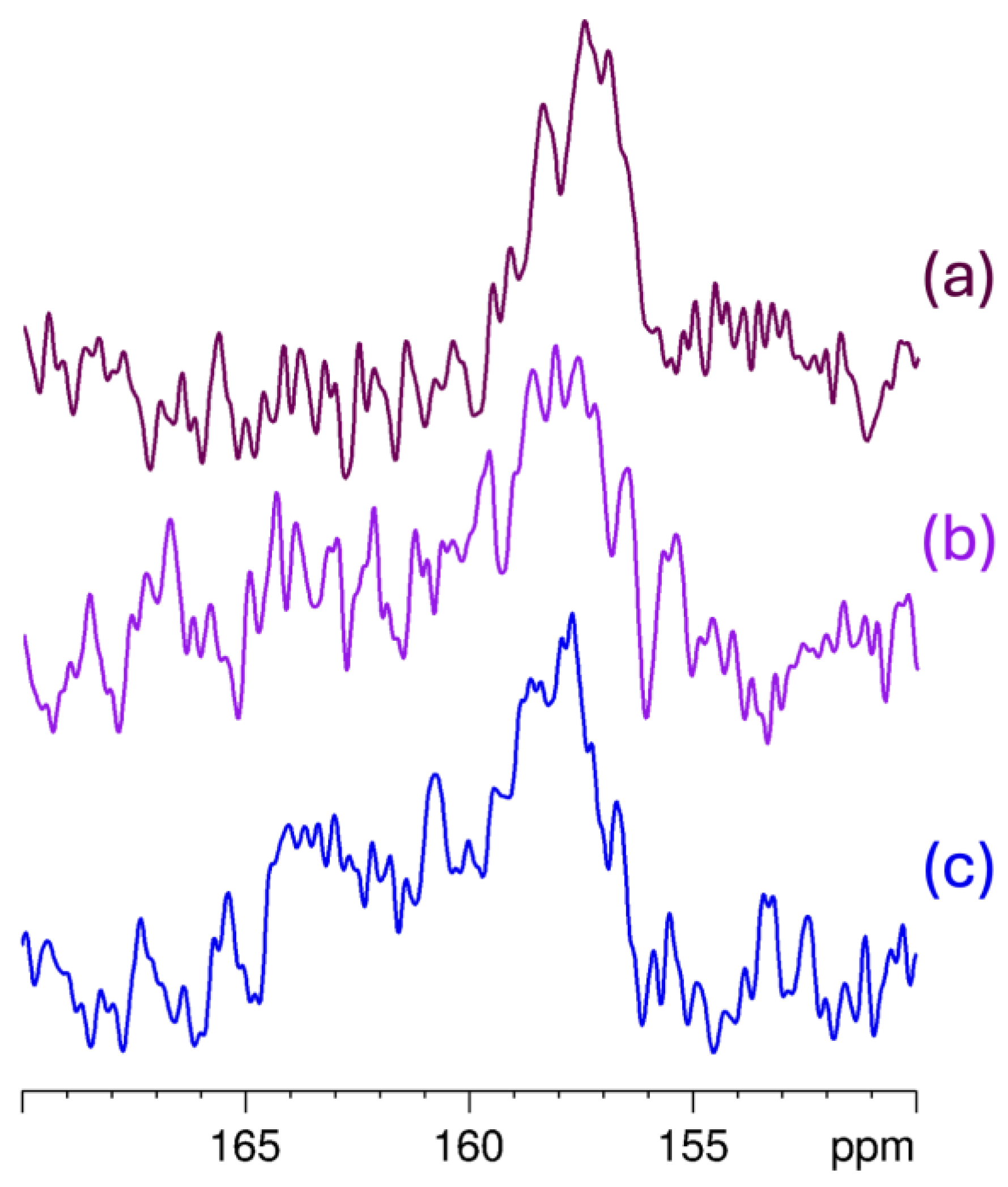
2.2.3. 13C solid-State NMR Analysis
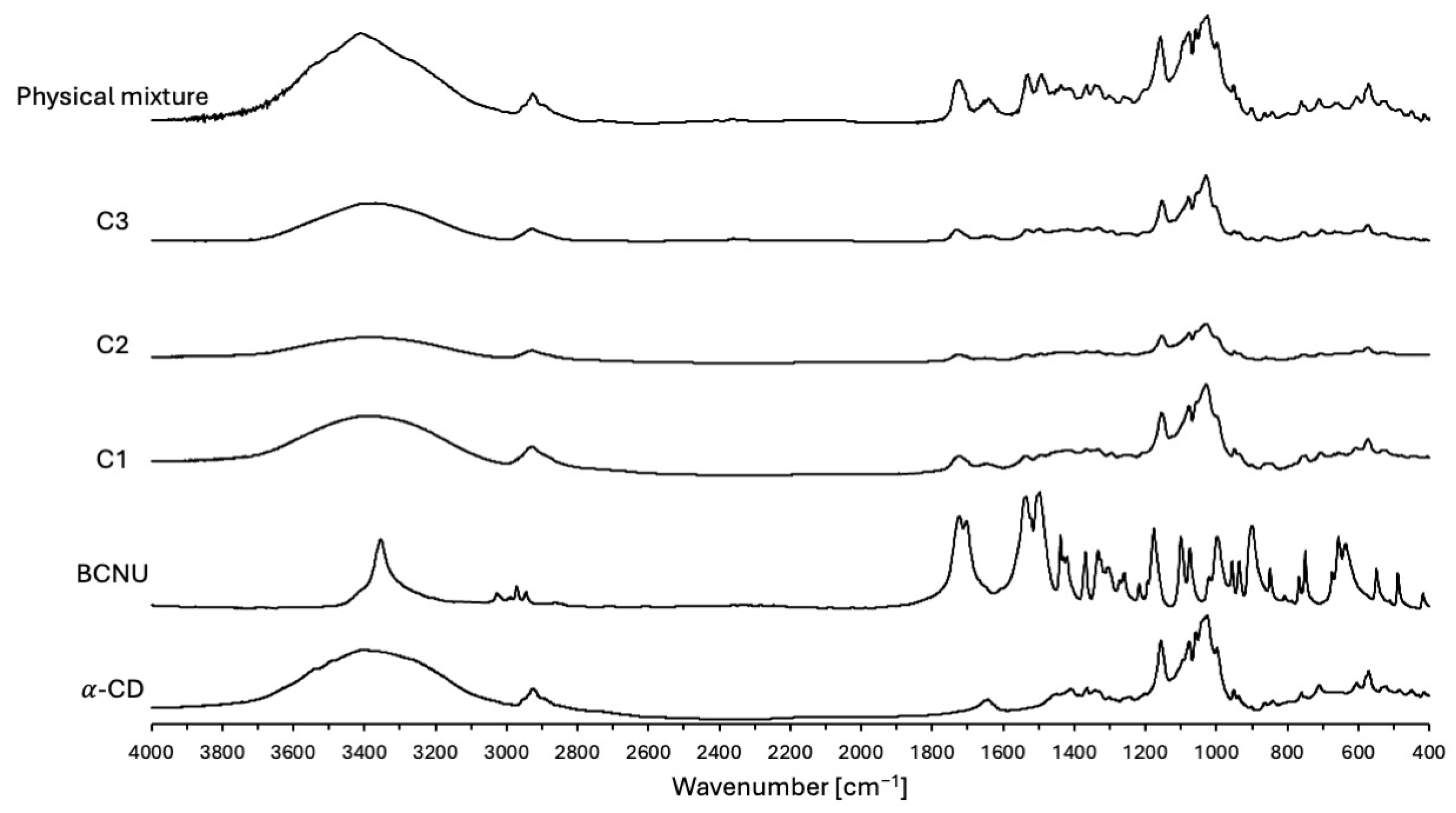
2.2.4. FTIR
2.2.5. UV-Vis Spectroscopy
2.2.6. ESI-MS Analysis
2.3. Determination of Stoichiometry, Stability and Aqueous Solubility
2.3.1. Method of Continuous Variation (Job’s Plot)
2.3.2. Benesi-Hildebrand Method
2.3.3. Aqueous Solubility
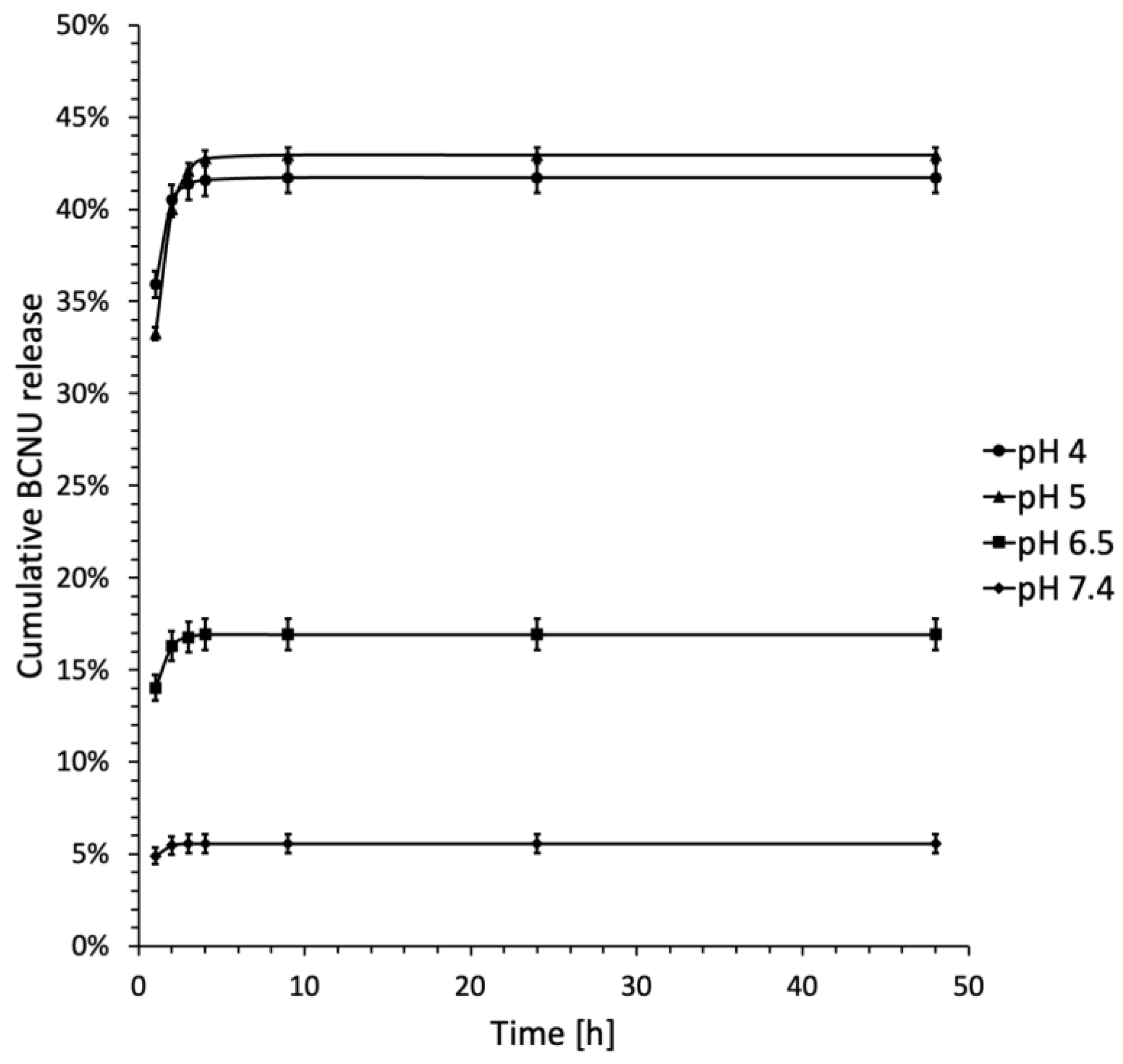
2.4. BCNU Release Studies
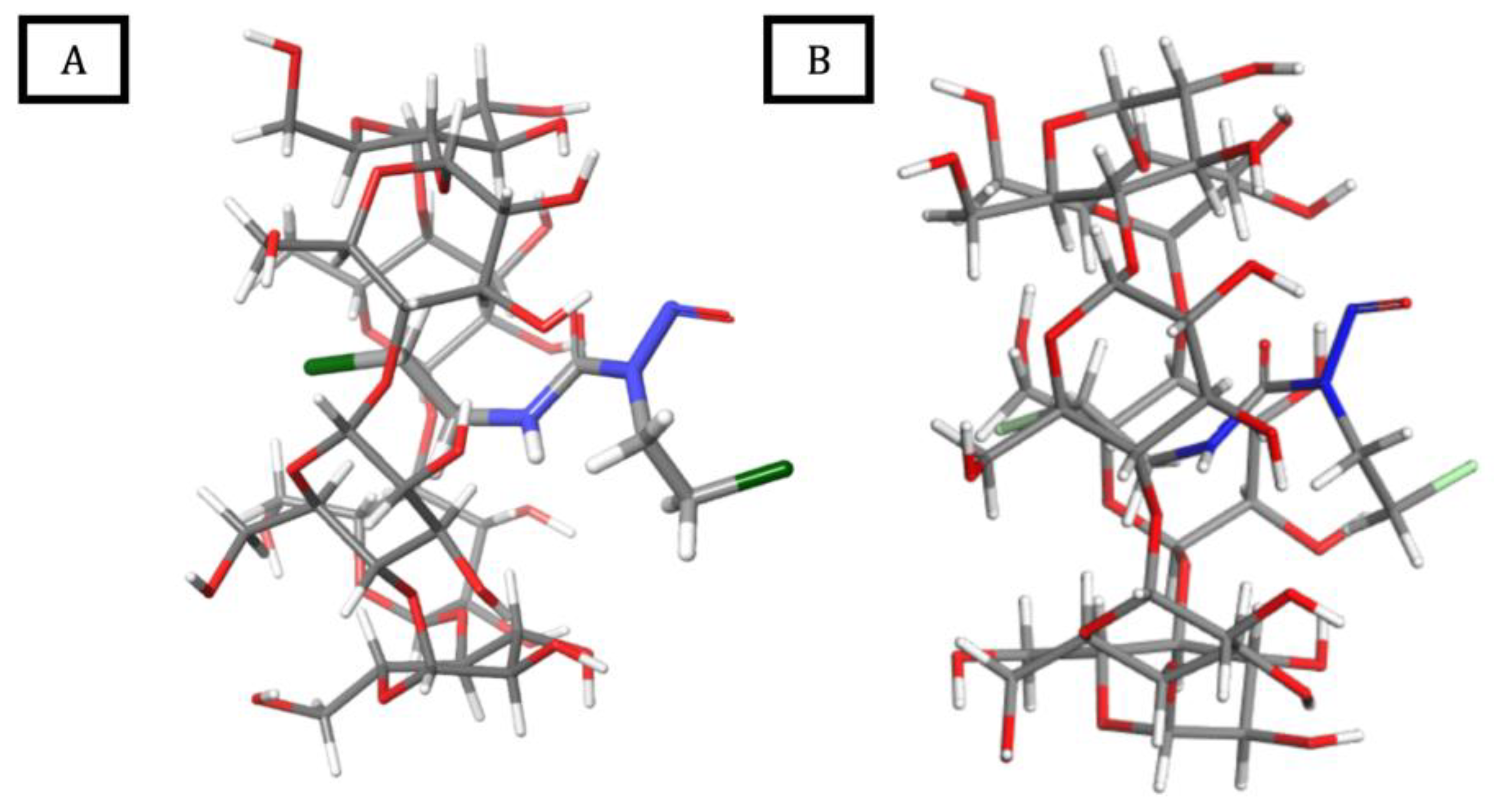
2.5. Molecular Modeling Studies
2.5.1. Molecular Docking and DFT Calculations
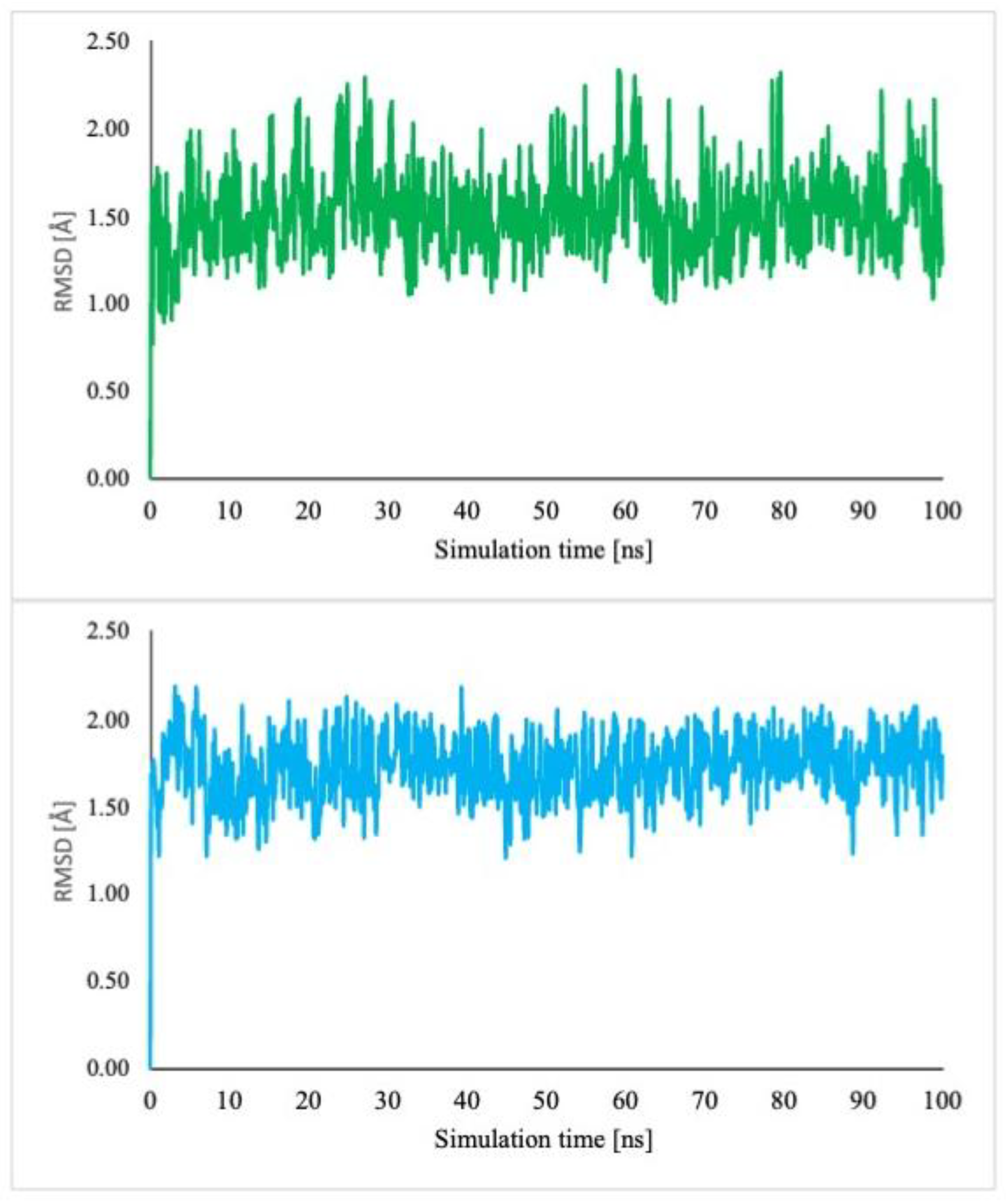
2.5.2. Molecular Dynamics Simulations
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of BCNU-α-CD ICs
Co-Grinding
Cryomilling
Co-Precipitation Method
3.2.2. BCNU Quantification
Determination of Inclusion Ratio and Drug Loading (DL)
Solubility Study
Method of Continuous Variation (Job’s Plot)
Benesi-Hildebrand Method
In Vitro Release Study of BCNU form BCNU-α-CD ICs
- Mathematical models
3.3. Measurements
3.3.1. Structural Examination
3.3.2. UV-Vis
3.3.3. FTIR
3.3.4. DSC
3.3.5. ESI-MS
3.3.6. 13C CP MAS NMR
3.3.7. Molecular Modeling Studies
Initial Structures Preparation
Molecular Docking
- Grid generation
- 2.
- Ligand preparation
- 3.
- Glide XP docking
Molecular Dynamics Simulations
Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Maximum absorption wavelength | |
| 13C CP MAS NMR | Carbon-13 Cross-Polarization Magic Angle Spinning Nuclear Magnetic Resonance |
| 1H NMR | Proton nuclear magnetic resonance |
| A | Absorbance |
| ACN | Acetonitrile |
| BBB | Blood–brain barrier |
| BCNU | Carmustine |
| C1 | Co-grinding method |
| C2 | Cryomilling method |
| C3 | Co-precipitation method |
| DDSs | Drug delivery systems |
| DFT | Density functional theory |
| DL | Drug loading |
| DMSO-d6 | Dimethyl-d6-sulfoxide |
| DSC | Differential scanning calorimetry |
| ESI-MS | Electrospray Ionization Mass Spectrometry |
| F | Fraction of drug released from the matrix after time t |
| F0 | Initial amount of drug |
| FDA | U.S. Food and Drug Administration |
| FT-IR | Fourier-transform infrared spectroscopy |
| G | Gibbs free energy |
| GBM | Glioblastoma multiforme |
| H | Enthalpy |
| HPDec | High-power decoupling |
| HPLC | High-performance liquid chromatography |
| HP--CD | Hydroxypropyl--cyclodextrin |
| HRMS | High-resolution mass spectrometry |
| ICs | Inclusion complexes |
| k | Model constant |
| KD | Dissociation constant |
| KF | Formation constant |
| MD | Molecular dynamics |
| MM | Molecular mechanics |
| MM-GBSA | Molecular Mechanics Generalized Born Surface Area |
| MM-GBSA ΔG | Molecular Mechanics Generalized Born Surface Area binding free energy |
| n | Drug release exponent in Korsmeyer-Peppas model |
| PBS | Phosphate-buffer solution |
| PCM | Polarizable continuum model |
| PES | Potential energy surface |
| QC | Quantum chemical |
| R | Mole fraction |
| RMSD | Root mean square deviation |
| SBE7--CD | Sulfobuthylether--cyclodextrin |
| TFA | Trifluoroacetic acid |
| Tm | Melting temperature |
| TMZ | Temozolomide |
| TS | Temperature-corrected entropy |
| TΔS | Temperature corrected entropy of complexation |
| UV-Vis | Ultraviolet-visible spectroscopy |
| ZPVE | Zero-point vibrational energy |
| α-CD | α-cyclodextrin |
| β-CD | β-cyclodextrin |
| ΔA | Difference in the absorbance |
| ΔE | Energy of complexation |
| ΔG | Gibbs free energy of complexation |
| ΔH | Enthalpy of complexation |
| Chemical shift change |
References
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef]
- American Cancer Society. Global Cancer Facts & Figures 5th Edition; American Cancer Society: Atlanta, GA, USA, 2024. [Google Scholar]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary Brain Tumours in Adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Alifieris, C.; Trafalis, D.T. Glioblastoma Multiforme: Pathogenesis and Treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. An Introduction to Nanotechnology. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 1–27. ISBN 978-0-12-813586-0. [Google Scholar]
- Jain, K.; Mehra, N.K.; Jain, N.K. Potentials and Emerging Trends in Nanopharmacology. Curr. Opin. Pharmacol. 2014, 15, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singhal, S.; Pahwa, S.; Sethi, V.A.; Sharma, S.; Singh, P.; Kale, R.D.; Ali, S.W.; Sagadevan, S. Nanomedicine and Drug Delivery: A Comprehensive Review of Applications and Challenges. Nano-Struct. Nano-Objects 2024, 40, 101403. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Medac. Carmustine Medac. Charakterystyka Produktu Leczniczego Summary of Product Characteristics. 2023. Available online: https://www.ema.europa.eu/pl/documents/product-information/carmustine-medac-epar-product-information_pl.pdf (accessed on 10 March 2025).
- Rani, V.; Venkatesan, J.; Prabhu, A. Carmustine-Loaded Liposomal Delivery Effectively Targets Malignant Glioma Cells and Seizes Endothelial Sprouting In Vitro. J. Clust. Sci. 2024, 35, 1211–1221. [Google Scholar] [CrossRef]
- Mali, A.D.; Bhanwase, A.S. Carmustine: Promising Drug for Treatment of Glioma. Int. J. Drug Deliv. Technol. 2022, 12, 1390–1396. [Google Scholar] [CrossRef]
- Ma, D.Q.; Rajewski, R.A.; Vander Velde, D.; Stella, V.J. Comparative Effects of (SBE)7m-β-CD and HP-β-CD on the Stability of Two Anti-neoplastic Agents, Melphalan and Carmustine. J. Pharm. Sci. 2000, 89, 275–287. [Google Scholar] [CrossRef]
- Montgomery, J.A.; James, R.; McCaleb, G.S.; Johnston, T.P. The Modes of Decomposition of 1,3-Bis(2-Chloroethyl)-1-Nitrosourea and Related Compounds. J. Med. Chem. 1967, 10, 668–674. [Google Scholar] [CrossRef]
- Laskar, P.A.; Ayres, J.W. Degradation of Carmustine in Aqueous Media. J. Pharm. Sci. 1977, 66, 1073–1076. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Gliadel NDA 020637 Approval Letter; U.S. Food and Drug Administration: Silver Spring, MD, USA, 23 September 1996. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020637Orig1s000.pdf (accessed on 18 March 2025).
- Ricciardi, L.; Manini, I.; Cesselli, D.; Trungu, S.; Piazza, A.; Mangraviti, A.; Miscusi, M.; Raco, A.; Ius, T. Carmustine Wafers Implantation in Patients With Newly Diagnosed High Grade Glioma: Is It Still an Option? Front. Neurol. 2022, 13, 884158. [Google Scholar] [CrossRef]
- Shapira-Furman, T.; Serra, R.; Gorelick, N.; Doglioli, M.; Tagliaferri, V.; Cecia, A.; Peters, M.; Kumar, A.; Rottenberg, Y.; Langer, R.; et al. Biodegradable Wafers Releasing Temozolomide and Carmustine for the Treatment of Brain Cancer. J. Control. Release 2019, 295, 93–101. [Google Scholar] [CrossRef]
- Chang, X.; Guo, H.; Li, Y.; Ding, J. Implantable Devices for Resected Glioblastoma Therapy. Asian J. Pharm. Sci. 2025, 20, 101034. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Hanakita, S.; Yoshida, S.; Ikemoto, T.; Oya, S. Unmanageable Cerebrospinal Fluid Leakage With Eosinophilic Meningitis in a Gliadel Wafer Implant Patient. Cureus 2024, 16, e59718. [Google Scholar] [CrossRef]
- Khanizadeh, A.; Ghaemi, A.; Pourmadadi, M.; Javadi, S.; Rahdar, A.; Yazdian, F.; Ghazy, E.; Pandey, S. Advancing Cancer Therapy: Unveiling the Cutting-Edge Potential of Carmustine Nano Carriers for Targeted Treatment. J. Drug Deliv. Sci. Technol. 2024, 99, 105943. [Google Scholar] [CrossRef]
- Honmane, S.M.; Charde, M.S.; Choudhari, P.B.; Jadhav, N.R. Development and in Vitro Evaluation of Folate Conjugated Polydopamine Modified Carmustine-Loaded Liposomes for Improved Anticancer Activity. J. Drug Deliv. Sci. Technol. 2023, 90, 105145. [Google Scholar] [CrossRef]
- Gong, J.; Feng, R.; Fu, X.; Lin, Q.; Wu, B. Fabrication of Co-Delivery Liposomal Formulation Incorporating Carmustine and Cabazitaxel Displays Improved Cytotoxic Potential and Induced Apoptosis in Ovarian Cancer Cells. J. Biomater. Sci. Polym. Ed. 2025, 36, 1–21, Erratum in J. Biomater. Sci. Polym. Ed. 2024, 1. https://doi.org/10.1080/09205063.2024.2436824. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Rajesh, R.; Yen, M.-H.; Paira, P. Inhibition of Glioblastoma and Macrophage Phagocytosis Using Sialic Acid-Grafted Tamoxifen-Carmustine-Polyethyleneimine-Poly(Lactic-Co-Glycolic Acid) Nanoparticles. J. Taiwan Inst. Chem. Eng. 2020, 111, 302–311. [Google Scholar] [CrossRef]
- Yan, C.; Yuan, X.; Kang, C.; Zhao, Y.; Liu, J.; Guo, Y.; Lu, J.; Pu, P.; Sheng, J. Preparation of Carmustine-loaded PLA Ultrasmall-nanoparticles by Adjusting Micellar Behavior of Surfactants. J. Appl. Polym. Sci 2008, 110, 2446–2452. [Google Scholar] [CrossRef]
- Wanjale, M.V.; Sunil Jaikumar, V.; Sivakumar, K.; Ann Paul, R.; James, J.; Kumar, G.V. Supramolecular Hydrogel Based Post-Surgical Implant System for Hydrophobic Drug Delivery Against Glioma Recurrence. Int. J. Nanomed. 2022, 17, 2203–2224. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef] [PubMed]
- Farcas, A.; Resmerita, A.-M.; Balan-Porcarasu, M.; Cojocaru, C.; Peptu, C.; Sava, I. Inclusion Complexes of 3,4-Ethylenedioxythiophene with Per-Modified β- and γ-Cyclodextrins. Molecules 2023, 28, 3404. [Google Scholar] [CrossRef]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in the Solid State: A Review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł. A Review of Applications of Solid-State Nuclear Magnetic Resonance (ssNMR) for the Analysis of Cyclodextrin-Including Systems. Int. J. Mol. Sci. 2023, 24, 3648. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; Caja, M.D.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Jug, M.; Mura, P. Grinding as Solvent-Free Green Chemistry Approach for Cyclodextrin Inclusion Complex Preparation in the Solid State. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins Inclusion Complex: Preparation Methods, Analytical Techniques and Food Industry Applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Pereva, S.; Sarafska, T.; Bogdanova, S.; Spassov, Т. Efficiency of “Cyclodextrin-Ibuprofen” Inclusion Complex Formation. J. Drug Deliv. Sci. Technol. 2016, 35, 34–39. [Google Scholar] [CrossRef]
- Ho, B.T.; Joyce, D.C.; Bhandari, B.R. Encapsulation of Ethylene Gas into α-Cyclodextrin and Characterisation of the Inclusion Complexes. Food Chem. 2011, 127, 572–580. [Google Scholar] [CrossRef]
- Ho, T.M.; Howes, T.; Bhandari, B.R. Encapsulation of CO2 into Amorphous and Crystalline α-Cyclodextrin Powders and the Characterization of the Complexes Formed. Food Chem. 2015, 187, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Czapnik, P.; Stępniak, A.; Buczkowski, A.; Zawisza, A.; Małecka, M. Inclusion Complexes of α-Cyclodextrin with p-Aminobenzoic Acid and Nicotinic Acid: Crystal Structure, DSC and IR Spectroscopy Analysis in Solid State and 1H NMR and ITC Calorimetric Studies of Complexation in Solutions. J. Mol. Struct. 2025, 1320, 139671. [Google Scholar] [CrossRef]
- Lizy Roselet, S.; Prema Kumari, J. Inclusion studies on α-cyclodextrin complexes of Glipizide and Gliclazide with effect of pH. Asian J. Pharm. Clin. Res. 2016, 10, 273–280. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical Properties of Catechin/β-Cyclodextrin Inclusion Complex Obtained via Co-Precipitation. CyTA—J. Food 2019, 17, 544–551. [Google Scholar] [CrossRef]
- Vertzoni, M.; Kartezini, T.; Reppas, C.; Archontaki, H.; Valsami, G. Solubilization and Quantification of Lycopene in Aqueous Media in the Form of Cyclodextrin Binary Systems. Int. J. Pharm. 2006, 309, 115–122. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yu, T.; Yuan, L.; Rao, G.; Li, D.; Mu, C. Preparation, Physicochemical Characterization and Release Behavior of the Inclusion Complex of Trans -Anethole and β-Cyclodextrin. Food Res. Int. 2015, 74, 55–62. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, X.; Xiao, Z.; Chen, H.; Ji, H. Preparation and Release Behaviour of the Inclusion Complexes of Phenylethanol with β -cyclodextrin. Flavour Fragr. J. 2016, 31, 206–216. [Google Scholar] [CrossRef]
- Lizy Roselet, S.; Prema Kumari, J. 1H NMR—A Validation Tool for Supramolecular Complexes of α-Cyclodextrin with Antidiabetic Drugs. Mater. Today Proc. 2021, 37, 88–93. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of Cyclodextrins and Cyclodextrin Complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef] [PubMed]
- Greatbanks, D.; Pickford, R. Cyclodextrins as Chiral Complexing Agents in Water, and Their Application to Optical Purity Measurements. Magn. Reson. Chem. 1987, 25, 208–215. [Google Scholar] [CrossRef]
- Arya, P.; Raghav, N. In-Vitro Studies of Curcumin-β-Cyclodextrin Inclusion Complex as Sustained Release System. J. Mol. Struct. 2021, 1228, 129774. [Google Scholar] [CrossRef]
- Singh, P.; Prabaharan, A.; Ahmad, H.; Islam, S.S. Spectroscopic (Ft-Ir, Ft-Raman and Uv-Vis) Study, Homo-Lumo, Nbo and Molecular Docking Analysis of Alkylating Agent: Bis-Chloroethylnitrosourea. Int. J. Curr. Res. 2018, 10, 66290–66307. [Google Scholar]
- Li, N.; Liu, J.; Zhao, X.; Gao, Y.; Zheng, L.; Zhang, J.; Yu, L. Complex Formation of Ionic Liquid Surfactant and β-Cyclodextrin. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 196–201, Erratum in Colloids Surf. A Physicochem. Eng. Asp. 2008, 324, 237. https://doi.org/10.1016/j.colsurfa.2008.04.034. [Google Scholar] [CrossRef]
- Sambasevam, K.; Mohamad, S.; Sarih, N.; Ismail, N. Synthesis and Characterization of the Inclusion Complex of β-Cyclodextrin and Azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef]
- Rusa, C.C.; Luca, C.; Tonelli, A.E. Polymer−Cyclodextrin Inclusion Compounds: Toward New Aspects of Their Inclusion Mechanism. Macromolecules 2001, 34, 1318–1322. [Google Scholar] [CrossRef]
- Tang, B.; Chen, Z.-Z.; Zhang, N.; Zhang, J.; Wang, Y. Synthesis and Characterization of a Novel Cross-Linking Complex of β-Cyclodextrin-o-Vanillin Furfuralhydrazone and Highly Selective Spectrofluorimetric Determination of Trace Gallium. Talanta 2006, 68, 575–580. [Google Scholar] [CrossRef]
- Knoll, L.; Kraemer, I.; Thiesen, J. Physicochemical Stability of Carmustine-Containing Medicinal Products after Reconstitution and after Dilution to Ready-to-Administer Infusion Solutions Stored Refrigerated or at Room Temperature. Eur. J. Hosp Pharm. 2023, 30, 11–16. [Google Scholar] [CrossRef]
- Dotsikas, Y.; Loukas, Y.L. Efficient Determination and Evaluation of Model Cyclodextrin Complex Binding Constants by Electrospray Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1123–1129. [Google Scholar] [CrossRef]
- Silion, M.; Fifere, A.; Lungoci, A.L.; Marangoci, N.L.; Ibanescu, S.A.; Zonda, R.; Rotaru, A.; Pinteală, M. Mass Spectrometry as a Complementary Approach for Noncovalently Bound Complexes Based on Cyclodextrins. In Advancements of Mass Spectrometry in Biomedical Research; Woods, A.G., Darie, C.C., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1140, pp. 685–701. ISBN 978-3-030-15949-8. [Google Scholar]
- Yilmaz, A.S.; Ozturk, S.; Salih, B.; Ayyala, R.S.; Sahiner, N. ESI-IM-MS Characterization of Cyclodextrin Complexes and Their Chemically Cross-Linked Alpha (α-), Beta (β-) and Gamma (γ-) Cyclodextrin Particles as Promising Drug Delivery Materials with Improved Bioavailability. Colloids Surf. B Biointerfaces 2023, 230, 113522. [Google Scholar] [CrossRef]
- Faria, S.H.D.M.; Teleschi, J.G.; Teodoro, L.; Almeida, M.O. Computational Investigation of the Carmustine (BCNU) Alkylation Mechanism Using the QTAIM, IQA, and NBO Models. Struct. Chem. 2021, 32, 79–96. [Google Scholar] [CrossRef]
- Kate, A.S.; Kohl, A.C.; Kerr, R.G. A simple and sensitive APCI-LC-MS method for the detection of the antitumor agent, carmustine (BCNU) in rat plasma. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 818–824. [Google Scholar] [CrossRef]
- Sforza, S.; Galaverna, G.; Corradini, R.; Dossena, A.; Marchelli, R. ESI-Mass Spectrometry Analysis of Unsubstituted and Disubstituted β-Cyclodextrins: Fragmentation Mode and Identification of the AB, AC, AD Regioisomers. J. Am. Soc. Mass Spectrom. 2003, 14, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, J.B.; Vouros, P. False Positives and the Detection of Cyclodextrin Inclusion Complexes by Electrospray Mass Spectrometry. J. Am. Soc. Mass Spectrom. 1995, 6, 437–447. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Sadaquat, H.; Akhtar, M. Comparative Effects of β-Cyclodextrin, HP-β-Cyclodextrin and SBE7-β-Cyclodextrin on the Solubility and Dissolution of Docetaxel via Inclusion Complexation. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 333–351. [Google Scholar] [CrossRef]
- Lewicki, J. Karmustyna—Właściwości Farmakologiczne i Zastosowania w Chemioterapii Onkologicznej u Psów. Życie Weter. 2007, 82, 679–687. [Google Scholar]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, A.; Delyagina, E.; Agafonov, M.; Khodov, I.; Terekhova, I. Effect of pH, Temperature and Native Cyclodextrins on Aqueous Solubility of Baricitinib. J. Mol. Liq. 2022, 360, 119548. [Google Scholar] [CrossRef]
- Kang, J.; Kumar, V.; Yang, D.; Chowdhury, P.R.; Hohl, R.J. Cyclodextrin Complexation: Influence on the Solubility, Stability, and Cytotoxicity of Camptothecin, an Antineoplastic Agent. Eur. J. Pharm. Sci. 2002, 15, 163–170. [Google Scholar] [CrossRef]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Wu, Q.; Heddleston, J.M.; Choudhary, G.S.; MacSwords, J.; Lathia, J.D.; McLendon, R.; Lindner, D.; Sloan, A.; Rich, J.N. Acidic Stress Promotes a Glioma Stem Cell Phenotype. Cell Death Differ. 2011, 18, 829–840. [Google Scholar] [CrossRef]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The Impact of Tumour pH on Cancer Progression: Strategies for Clinical Intervention. Explor. Target. Anti-Tumor Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating Extracellular Tumour pH: An Effective Target for Cancer Therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef] [PubMed]
- Athmakur, H.; Kondapi, A.K. Carmustine loaded lactoferrin nanoparticles demonstrates an enhanced antiproliferative activity against glioblastoma in vitro. Int. J. App. Pharm. 2018, 10, 234. [Google Scholar] [CrossRef]
- Loo, T.L.; Dion, R.L.; Dixon, R.L.; Rall, D.P. The Antitumor Agent, 1,3-Bis(2-Chloroethyl)-1-Nitrosourea. J. Pharm. Sci. 1966, 55, 492–497. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Talevi, A.; Ruiz, M.E. Korsmeyer-Peppas, Peppas-Sahlin, and Brazel-Peppas: Models of Drug Release. In The ADME Encyclopedia; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–9. ISBN 978-3-030-51519-5. [Google Scholar]
- Rezaei, A.; Nasirpour, A. Evaluation of Release Kinetics and Mechanisms of Curcumin and Curcumin-β-Cyclodextrin Inclusion Complex Incorporated in Electrospun Almond Gum/PVA Nanofibers in Simulated Saliva and Simulated Gastrointestinal Conditions. BioNanoScience 2019, 9, 438–445. [Google Scholar] [CrossRef]
- Szabó, Z.-I.; Orbán, G.; Borbás, E.; Csicsák, D.; Kádár, S.; Fiser, B.; Dobó, M.; Horváth, P.; Kiss, E.; Budai, L.; et al. Inclusion Complexation of the Anticancer Drug Pomalidomide with Cyclodextrins: Fast Dissolution and Improved Solubility. Heliyon 2021, 7, e07581. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Benito, J.; González-Mancebo, S.; Calle, E.; García-Santos, M.P.; Casado, J. A Practical Integrated Approach to Supramolecular Chemistry. I. Equilibria in Inclusion Phenomena. J. Chem. Educ. 1999, 76, 419. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Kowalczyk, S.; Plichta, A.; Zgadzaj, A.; Oledzka, E.; Sobczak, M. Synthesis and Characterization of New Biodegradable Injectable Thermosensitive Smart Hydrogels for 5-Fluorouracil Delivery. Int. J. Mol. Sci. 2021, 22, 8330. [Google Scholar] [CrossRef]
- Bialik, M.; Proc, J.; Zgadzaj, A.; Mulas, K.; Kuras, M.; Sobczak, M.; Oledzka, E. Development and Comprehensive Characteristics of Thermosensitive Liquid Suppositories of Metoprolol Based on Poly(Lactide-Co-Glycolide) Nanoparticles. Int. J. Mol. Sci. 2022, 23, 13743. [Google Scholar] [CrossRef]
- Bialik, M.; Kurkowski, P.; Strzelecka, K.; Kuras, M.; Sobczak, M.; Mulas, K.; Zgadzaj, A.; Czerwińska, M.E.; Gniadek, M.; Oledzka, E. Dual-Controlled Delivery of Furosemide and Atenolol Using a Biodegradable Nanosystem for Antihypertensive Therapy. J. Drug Deliv. Sci. Technol. 2023, 89, 105006. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Bethanis, K.; Christoforides, E.; Dudek, M.K.; Wielgus, E.; Pisklak, D.M. 17-β-Estradiol—β-Cyclodextrin Complex as an Aqueous Solution: Structural and Physicochemical Characterization Supported by MM and QM Calculations. J. Mol. Struct. 2024, 1313, 138710. [Google Scholar] [CrossRef]




| Sample | Synthesis Method | Yield [%] | Inclusion Ratio 1 [%] | DL 2 [%] |
|---|---|---|---|---|
| C1 | Co-grinding | 96 | 50.31 | 2.71 |
| C2 | Cryomilling | 72 | 49.27 | 2.95 |
| C3 | Co-precipitation | 92 | 85.65 | 15.2 |
| H | -CD | BCNU-α-CD IC | |||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | |||||
| H1 | 4.78 | 4.77 | −0.01 | 4.77 | −0.01 | 4.78 | 0.00 |
| H2 | 3.37 | 3.40 | +0.03 | 3.39 | +0.02 | 3.38 | +0.01 |
| H3 | 3.75 | 3.75 | 0.00 | 3.74 | −0.01 | 3.74 | −0.01 |
| H4 | 3.26 | 3.01 | −0.25 | 3.27 | +0.01 | 3.24 | −0.02 |
| H5 | 3.55 | 3.57 | +0.02 | 3.58 | +0.03 | 3.58 | +0.03 |
| H6 | 3.62 | 3.61 | −0.01 | 3.60 | −0.01 | 3.66 | +0.04 |
| OH2 | 5.50 | 5.51 | +0.01 | 5.46 | −0.04 | 5.46 | −0.04 |
| OH3 | 5.42 | 5.42 | 0.00 | 5.46 | +0.04 | 5.46 | +0.04 |
| OH6 | 4.46 | 4.48 | +0.02 | 4.56 | +0.10 | 4.46 | 0.00 |
| H | BCNU | BCNU-α-CD IC | |||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | |||||
| a | 3.75 | 3.77 | +0.02 | 3.74 | −0.01 | 3.74 | −0.01 |
| b | 3.61 | 3.60 | −0.01 | 3.60 | −0.01 | 3.61 | 0.00 |
| c | 4.08 | 4.08 | 0.00 | 4.07 | −0.01 | 4.07 | −0.01 |
| d | 8.97 | 8.99 | +0.02 | 8.98 | +0.01 | 8.97 | 0.00 |
| No. | pH | Zero-Order Model | First-Order Model | Second-Order Model | Higuchi Model | Korsmeyer-Peppas Model | Release Kinetics | Drug Transport Mechanism | |
|---|---|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | ||||
| C3 | 4 | 0.306 | 0.325 | 0.347 | 0.640 | 0.685 | 0.06 | * | rather Fickian diffusion |
| C3 | 5 | 0.357 | 0.387 | 0.420 | 0.696 | 0.731 | 0.11 | * | Fickian diffusion |
| C3 | 6.5 | 0.637 | 0.648 | 0.659 | 0.881 | 0.899 | 0.14 | * | Fickian diffusion |
| C3 | 7.4 | 0.700 | 0.703 | 0.705 | 0.912 | 0.939 | 0.13 | * | Fickian diffusion |
| MM Level | QC Level—DFT | |||||
|---|---|---|---|---|---|---|
| BCNU-α-CD ICs | Glide Score | MM-GBSA ΔG | ΔE | ΔG | ΔH | TΔS |
| −1.833 | −38.54 | −22.95 | −5.74 | −20.75 | −15.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzelecka, K.; Janiec, D.; Sobieraj, J.; Kasiński, A.; Kuras, M.; Zalewska, A.; Szeleszczuk, Ł.; Sobczak, M.; Dudek, M.K.; Oledzka, E. Investigation of Host-Guest Inclusion Complexes Between Carmustine and α-Cyclodextrin: Synthesis, Characterization, and Evaluation. Int. J. Mol. Sci. 2025, 26, 9386. https://doi.org/10.3390/ijms26199386
Strzelecka K, Janiec D, Sobieraj J, Kasiński A, Kuras M, Zalewska A, Szeleszczuk Ł, Sobczak M, Dudek MK, Oledzka E. Investigation of Host-Guest Inclusion Complexes Between Carmustine and α-Cyclodextrin: Synthesis, Characterization, and Evaluation. International Journal of Molecular Sciences. 2025; 26(19):9386. https://doi.org/10.3390/ijms26199386
Chicago/Turabian StyleStrzelecka, Katarzyna, Dominika Janiec, Jan Sobieraj, Adam Kasiński, Marzena Kuras, Aldona Zalewska, Łukasz Szeleszczuk, Marcin Sobczak, Marta K. Dudek, and Ewa Oledzka. 2025. "Investigation of Host-Guest Inclusion Complexes Between Carmustine and α-Cyclodextrin: Synthesis, Characterization, and Evaluation" International Journal of Molecular Sciences 26, no. 19: 9386. https://doi.org/10.3390/ijms26199386
APA StyleStrzelecka, K., Janiec, D., Sobieraj, J., Kasiński, A., Kuras, M., Zalewska, A., Szeleszczuk, Ł., Sobczak, M., Dudek, M. K., & Oledzka, E. (2025). Investigation of Host-Guest Inclusion Complexes Between Carmustine and α-Cyclodextrin: Synthesis, Characterization, and Evaluation. International Journal of Molecular Sciences, 26(19), 9386. https://doi.org/10.3390/ijms26199386










