The Role of Vitamin C in Selected Autoimmune and Immune-Mediated Diseases: Exploring Potential Therapeutic Benefits
Abstract
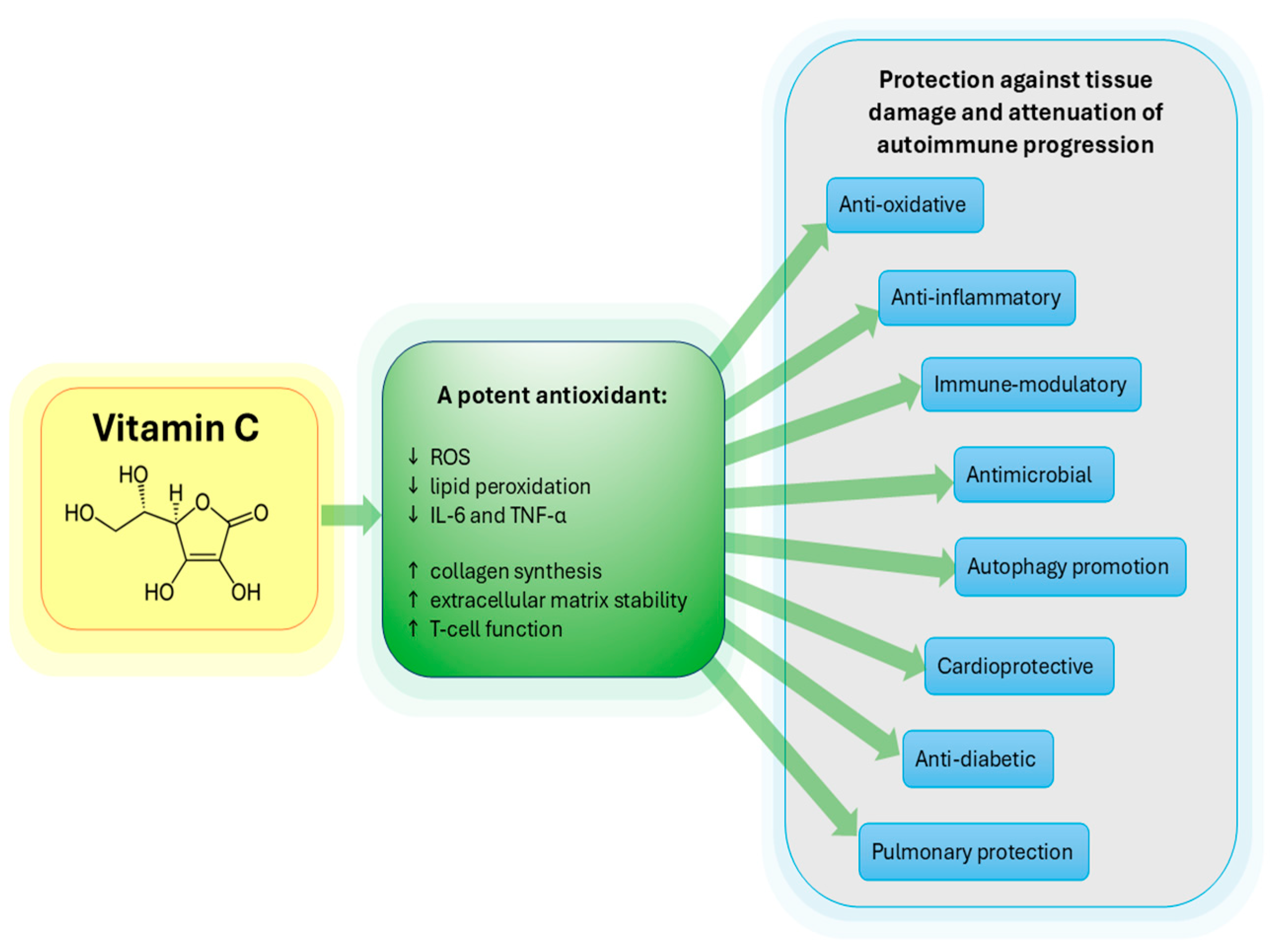
1. Introduction
2. Multiple Sclerosis
3. Rheumatoid Arthritis
4. Sjögren’s Disease
5. Type 1 Diabetes
6. Crohn’s Disease
7. Hashimoto’s Thyroiditis
8. Periodontitis
9. Pernicious Anemia
10. Antiphospholipid Syndrome
11. Alzheimer’s Disease
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APS | antiphospholipid syndrome |
| CD | Crohn’s disease |
| ECM | extracellular matrix |
| HT | Hashimoto’s thyroiditis |
| MS | multiple sclerosis |
| PA | pernicious anemia |
| PD | periodontitis |
| RA | rheumatoid arthritis |
| RCT | randomized controlled trial |
| ROS | reactive oxygen species |
| SD | Sjögren’s disease |
| T1D | type 1 diabetes |
| VitC | vitamin C |
References
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007, 98 (Suppl. 1), S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef]
- Vollbracht, C.; Raithel, M.; Krick, B.; Kraft, K.; Hagel, A.F. Intravenous vitamin C in the treatment of allergies: An interim subgroup analysis of a long-term observational study. J. Int. Med. Res. 2018, 46, 3640–3655. [Google Scholar] [CrossRef]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, M.J. Intravenous Vitamin C Administration Improves Quality of Life in Breast Cancer Patients during Chemo-/radiotherapy and Aftercare: Results of a Retrospective, Multicentre, Epidemiological Cohort Study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar]
- Vollbracht, C.; Kraft, K. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients 2021, 13, 1154. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Plausibility and Feasibility of Intravenous High-Dose Vitamin C in Long COVID Related Fatigue. J. Basic Clin. Pharm. 2021, 12, 1–4. [Google Scholar]
- Schencking, M.; Vollbracht, C.; Weiss, G.; Lebert, J.; Biller, A.; Goyvaerts, B.; Kraft, K. Intravenous Vitamin C in the Treatment of Shingles: Results of a Multicenter Prospective Cohort Study. Med. Sci. Monit. 2012, 18, CR215–CR224. [Google Scholar] [CrossRef] [PubMed]
- Polachini, C.R.N.; Spanevello, R.M.; Zanini, D.; Baldissarelli, J.; Pereira, L.B.; Schetinger, M.R.C.; da Cruz, I.B.M.; Assmann, C.E.; Bagatini, M.D.; Morsch, V.M. Evaluation of Delta-Aminolevulinic Dehydratase Activity, Oxidative Stress Biomarkers, and Vitamin D Levels in Patients with Multiple Sclerosis. Neurotox. Res. 2016, 29, 230–242. [Google Scholar] [CrossRef]
- Tavazzi, B.; Batocchi, A.P.; Amorini, A.M.; Nociti, V.; D’Urso, S.; Longo, S.; Gullotta, S.; Picardi, M.; Lazzarino, G. Serum Metabolic Profile in Multiple Sclerosis Patients. Mult. Scler. Int. 2011, 2011, 167156. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; Salgado-Cámara, P.; García-Martín, E.; Agúndez, J.A.G. Antioxidant Therapies in the Treatment of Multiple Sclerosis. Biomolecules 2024, 14, 1266. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Luchowska-Kocot, D.; Kiełczykowska, M.; Musik, I.; Kurzepa, J. Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders. Nutrients 2017, 9, 659. [Google Scholar] [CrossRef]
- Sotiriou, S.; Gispert, S.; Cheng, J.; Wang, Y.; Chen, A.; Hoogstraten-Miller, S.; Miller, G.F.; Kwon, O.; Levine, M.; Guttentag, S.H.; et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 2002, 8, 514–517. [Google Scholar] [CrossRef]
- Oliani, C.H.P.; Budib, C.L.; Marques, I.P.G.; Silva, A.P.C.; Barboza, M.N.C.; Nogueira, P.F. Supplementation and therapeutic use of vitamin C in multiple sclerosis. Rev. Bras. Oftalmol. 2025, 84, e0054. [Google Scholar] [CrossRef]
- Peng, H.; Wu, X.; Wen, Y.; Lin, J. Plasma circulating vitamin C levels and risk of multiple sclerosis: A two-sample Mendelian randomization analysis. Mult. Scler. Relat. Disord. 2021, 56, 103267. [Google Scholar] [CrossRef] [PubMed]
- Ghadirian, P.; Jain, M.; Ducic, S.; Shatenstein, B.; Morisset, R. Nutritional factors in the aetiology of multiple sclerosis: A case- control study in Montreal, Canada. Int. J. Epidemiol. 1998, 27, 845–852. [Google Scholar] [CrossRef]
- Khosravi-Largani, M.; Pourvali-Talatappeh, P.; Rousta, A.M.; Karimi-Kivi, M.; Noroozi, E.; Mahjoob, A.; Asaadi, Y.; Shahmohammadi, A.; Sadeghi, S.; Shakeri, S.; et al. A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalSci 2018, 10, 37–44. [Google Scholar] [CrossRef]
- Pomary, P.K.; Eichau, S.; Amigó, N.; Barrios, L.; Matesanz, F.; García-Valdecasas, M.; Hrom, I.; Sánchez, M.I.G.; Garcia-Martin, M.L. Multifaceted Analysis of Cerebrospinal Fluid and Serum from Progressive Multiple Sclerosis Patients: Potential Role of Vitamin C and Metal Ion Imbalance in the Divergence of Primary Progressive Multiple Sclerosis and Secondary Progressive Multiple Sclerosis. J. Proteome Res. 2023, 22, 743–757. [Google Scholar] [CrossRef]
- Eldridge, C.F.; Bunge, M.B.; Bunge, R.P.; Wood, P.M. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 1987, 105, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Carlson, N.G.; Rose, J.W. Antioxidants in multiple sclerosis: Do they have a role in therapy? CNS Drugs 2006, 20, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, P.; Huang, S.; Chen, Q.; Wang, X.; Liu, H. Association between rheumatoid arthritis and serum vitamin C levels in Adults: Based on the National health and Nutrition Examination survey database. Prev. Med. Rep. 2024, 44, 102793. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, S.; Luo, Y.; Zhang, C.; Xu, W.; Wang, L. The association between composite dietary antioxidant index and rheumatoid arthritis: Evidence from NHANES 2001–2020. BMC Rheumatol. 2024, 8, 74. [Google Scholar] [CrossRef]
- Baygin, H.; Siriken, F.; Sargın, G.; Çildag, S.; Ozturk, H.; Senturk, T. The relationship between dietary inflammatory index scores and rheumatoid arthritis disease activity. Reum. Clin. 2024, 20, 305–311. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, S.; Xu, H.; Sun, S.; Wang, Z.; Li, M.; Zou, L.; Zhang, Y.; Zhao, Y.; Cui, Y.; et al. Vitamin C alleviates rheumatoid arthritis by modulating gut microbiota balance. Biosci. Trends 2024, 18, 187–194. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, S. Ascorbic acid alleviates rheumatoid arthritis by inhibiting the production of autoantibodies. Cell Commun. Signal. CCS 2024, 22, 373. [Google Scholar] [CrossRef]
- Gomathi, A.; Chenthamarai, G.; Manvizhi, S.; Gowrithilagam, T.G. Effects of Vitamin C and Vitamin E in rheumatoid arthritis—A randomized, open label, and comparative study in a tertiary care hospital. Natl. J. Physiol. Pharm. Pharmacol. 2022, 12, 1463–1465. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Saeedy, S.A.G.; Abdi, A.; Khademi, F.; Lorian, K.; Clark, C.C.; Djafarian, K. Vitamin C reduces interleukin-6 plasma concentration: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. Open Sci. 2021, 40, 1–14. [Google Scholar] [CrossRef]
- Latif, F.A.A.; Ghazali, W.S.W.; Mohamad, S.M.; Lee, L.K. High fiber multigrain supplementation improved disease activity score, circulating inflammatory and oxidative stress biomarkers in rheumatoid arthritis (RA) patients: A randomized human clinical trial. J. Funct. Foods 2023, 100, 105392. [Google Scholar] [CrossRef]
- Hijjawi, N.; Tout, F.S.; Azaizeh, B.; Aljaafreh, B. The role of vitamins D, B12, C, and K in modulating inflammation and disease management in rheumatoid arthritis: A comprehensive review. Clin. Rheumatol. 2025, 44, 591–600. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Vitamin C Supplementation Intervention. Identifier: NCT04036110. Available online: https://www.clinicaltrials.gov/study/NCT04036110 (accessed on 17 September 2025).
- Riitano, G.; Spinelli, F.; Manganelli, V.; Caissutti, D.; Capozzi, A.; Garufi, C.; Garofalo, T.; Misasi, R.; Sorice, M.; Conti, F.; et al. Wnt signaling as a translational target in rheumatoid and psoriatic arthritis. J. Transl. Med. 2025, 23, 158. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, G.-J.; Yoo, H.-S.; Song, D.H.; Chung, K.-H.; Lee, K.-J.; Koo, Y.T.; An, J.H. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.; Cianciulli, A.; Calvello, R.; Porro, C.; De Nuccio, F.; Kashyrina, M.; Miraglia, A.; Lofrumento, D.D.; Panaro, M.A. Ser9p-GSK3β Modulation Contributes to the Protective Effects of Vitamin C in Neuroinflammation. Nutrients 2024, 16, 1121. [Google Scholar] [CrossRef]
- Rharass, T.; Lantow, M.; Gbankoto, A.; Weiss, D.G.; Panáková, D.; Lucas, S. Ascorbic acid alters cell fate commitment of human neural progenitors in a WNT/β-catenin/ROS signaling dependent manner. J. Biomed. Sci. 2017, 24, 78. [Google Scholar] [CrossRef]
- Rodriguez-Trillo, A.; Mosquera, N.; Pena, C.; Rivas-Tobío, F.; Mera-Varela, A.; Gonzalez, A.; Conde, C. Non-Canonical WNT5A Signaling Through RYK Contributes to Aggressive Phenotype of the Rheumatoid Fibroblast-Like Synoviocytes. Front. Immunol. 2020, 11, 555245. [Google Scholar] [CrossRef] [PubMed]
- Laigle, L.; Le Dantec, C.; Soret, P.; Desvaux, E.; Hubert, S.; Foulquier, N.; Moingeon, P.; Guedj, M.; Pers, J.-O. Sjögren’s syndrome: Towards precision medicine. Med. Sci. 2022, 38, 148–151. [Google Scholar] [CrossRef]
- Horai, Y.; Kurushima, S.; Shimizu, T.; Nakamura, H.; Kawakami, A. A Review of the Current Clinical Aspects of Sjögren’s Disease: Geographical Difference, Classification/Diagnostic Criteria, Recent Advancements in Diagnostic Methods, and Molecular Targeted Therapy. J. Clin. Med. 2025, 14, 5577. [Google Scholar] [CrossRef]
- Hyon, J.Y.; Han, S.B. Dry Eye Disease and Vitamins: A Narrative Literature Review. Appl. Sci. 2022, 12, 4567. [Google Scholar] [CrossRef]
- Bu, J.; Liu, Y.; Zhang, R.; Lin, S.; Zhuang, J.; Sun, L.; Zhang, L.; He, H.; Zong, R.; Wu, Y.; et al. Potential New Target for Dry Eye Disease—Oxidative Stress. Antioxidants 2024, 13, 422. [Google Scholar] [CrossRef]
- Machowicz, A.; Hall, I.; de Pablo, P.; Rauz, S.; Richards, A.; Higham, J.; Poveda-Gallego, A.; Imamura, F.; Bowman, S.J.; Barone, F.; et al. Mediterranean diet and risk of Sjögren’s syndrome. Clin. Exp. Rheumatol. 2020, 38, 216–221. [Google Scholar]
- Nesvold, M.B.; Jensen, J.L.; Hove, L.H.; Singh, P.B.; Young, A.; Palm, Ø.; Andersen, L.F.; Carlsen, M.H.; Iversen, P.O. Dietary Intake, Body Composition, and Oral Health Parameters among Female Patients with Primary Sjögren’s Syndrome. Nutrients 2018, 10, 866. [Google Scholar] [CrossRef]
- Benchabane, S.; Sour, S.; Zidi, S.; Hadjimi, Z.; Nabila, L.; Acheli, D.; Bouzenad, A.; Belguendouz, H.; Touil-Boukoffa, C. Exploring the relationship between oxidative stress status and inflammatory markers during primary Sjögren’s syndrome: A new approach for patient monitoring. Int. J. Immunopathol. Pharmacol. 2024, 38, 3946320241263034. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Huang, J.-Y.; Yeh, P.-T. A randomized, double-blind, placebo-controlled study of oral antioxidant supplement therapy in patients with dry eye syndrome. Clin. Ophthalmol. 2016, 10, 813–820. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, Y.; Han, Y.; Wu, Y.; Wang, D.; Zhang, B. Recommendations for nutritional supplements for dry eye disease: Current advances. Front. Pharmacol. 2024, 15, 1388787. [Google Scholar] [CrossRef]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, Z.; Chen, Y.; Lu, H.; Wang, A.; Shi, Y.; Zhang, Q.; Wang, Y.; Li, Y.; Lan, J.; et al. A non-invasive model for diagnosis of primary Sjogren’s disease based on salivary biomarkers, serum autoantibodies, and Schirmer’s test. Arthritis Res. Ther. 2024, 26, 217. [Google Scholar] [CrossRef]
- Navel, V.; Sapin, V.; Henrioux, F.; Blanchon, L.; Labbé, A.; Chiambaretta, F.; Baudouin, C.; Dutheil, F. Oxidative and antioxidative stress markers in dry eye disease: A systematic review and meta-analysis. Acta Ophthalmol. 2022, 100, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Karp, J.; Sun, K.M.; Weaver, C.M. Decreasing Vitamin C Intake, Low Serum Vitamin C Level and Risk for US Adults with Diabetes. Nutrients 2022, 14, 3902. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. Vitamin C Further Improves the Protective Effect of Glucagon-Like Peptide-1 on Acute Hypoglycemia-Induced Oxidative Stress, Inflammation, and Endothelial Dysfunction in Type 1 Diabetes. Diabetes Care 2013, 36, 4104–4108. [Google Scholar] [CrossRef] [PubMed]
- Odermarsky, M.; Lykkesfeldt, J.; Liuba, P. Poor vitamin C status is associated with increased carotid intima-media thickness, decreased microvascular function, and delayed myocardial repolarization in young patients with type 1 diabetes. Am. J. Clin. Nutr. 2009, 90, 447–452. [Google Scholar] [CrossRef]
- Sangani, R.; Naime, M.; Zakhary, I.; Ahmad, S.; Chutkan, N.; Zhu, A.; Ha, Y.; Hamrick, M.; Isales, C.; Elsalanty, M.; et al. Regulation of vitamin C transporter in the type 1 diabetic mouse bone and bone marrow. Exp. Mol. Pathol. 2013, 95, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Piconi, L.; Esposito, K.; Giugliano, D. Telmisartan shows an equivalent effect of vitamin C in further improving endothelial dysfunction after glycemia normalization in type 1 diabetes. Diabetes Care 2007, 30, 1694–1698. [Google Scholar] [CrossRef]
- Mattila, M.; Hakola, L.; Niinistö, S.; Tapanainen, H.; Takkinen, H.-M.; Ahonen, S.; Ilonen, J.; Toppari, J.; Veijola, R.; Knip, M.; et al. Maternal Vitamin C and Iron Intake during Pregnancy and the Risk of Islet Autoimmunity and Type 1 Diabetes in Children: A Birth Cohort Study. Nutrients 2021, 13, 928. [Google Scholar] [CrossRef]
- Juhl, B.; Lauszus, F.F.; Lykkesfeldt, J. Poor Vitamin C Status Late in Pregnancy Is Associated with Increased Risk of Complications in Type 1 Diabetic Women: A Cross-Sectional Study. Nutrients 2017, 9, 186. [Google Scholar] [CrossRef]
- Juhl, B.; Klein, F.; Christiansen, J.S. Vitamin C treatment reduces transcapillary escape rate of albumin in type 1 diabetes. Eur. J. Intern. Med. 2004, 15, 428–435. [Google Scholar] [CrossRef]
- Gordon, B.L.; Galati, J.; Yang, S.; Katz, P.O.; Scherl, E.J. Vitamin C Deficiency: An Under-Recognized Condition in Crohn’s Disease. ACG Case Rep. J. 2020, 7, e00424. [Google Scholar] [CrossRef]
- Verma, K.K.; Deligonul, F.Z.; Tarbox, M.; Chen, H.Z. Vitamin C Deficiency Masquerading as Vasculitis in a Patient With Crohn’s Disease. Cureus 2024, 16, e55295. [Google Scholar] [CrossRef]
- Mortezaei, K.; Gonzales, S.A.B.; Kreitenberg, A.; Arkfeld, D.G. Scurvy in a Patient with Crohn’s Disease: A Case Report. Curr. Rheumatol. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Guarino, L.; Chatelanat, O.; Gressot, P.; Larpin, C.; Serratrice, J.; Coen, M. When a diet is followed too strictly. Scurvy—An old disease in a modern gut: A case report. Medicine 2025, 104, e43688. [Google Scholar] [CrossRef] [PubMed]
- Aghdassi, E.; Wendland, B.E.; Steinhart, A.; Wolman, S.L.; Jeejeebhoy, K.; Allard, J.P. Original contribution Antioxidant vitamin supplementation in Crohn’s disease decreases oxidative stress a randomized controlled trial. Am. J. Gastroenterol. 2003, 98, 348–353. [Google Scholar] [CrossRef]
- Hébuterne, X.; Filippi, J.; Al-Jaouni, R.; Schneider, S. Nutritional consequences and nutrition therapy in Crohn’s disease. Gastroenterol. Clin. Biol. 2009, 33, S235–S244. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.-B.; Hébuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Animashaun, A.; Kelleher, J.; Heatley, R.; Trejdosiewicz, L.; Losowsky, M. The effect of zinc and vitamin C supplementation on the immune status of patients with Crohn’s disease. Clin. Nutr. 1990, 9, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, F.; Iida, M.; Tominaga, M.; Matsumoto, T.; Hirakawa, K.; Sugiyama, S.; Fujishima, M. Multiple vitamin status in Crohn’s disease. Correlation with disease activity. Dig. Dis. Sci. 1993, 38, 1614–1618. [Google Scholar] [CrossRef]
- Harries, A.D.; Heatley, R.V. Nutritional disturbances in Crohn’s disease. Postgrad. Med. J. 1983, 59, 690–697. [Google Scholar] [CrossRef]
- Murphree, J.; Mulherin, D.W.; Morton, C.; Adams, D. High-dose vitamin C therapy for symptomatic deficiency in a patient with myasthenia gravis and Crohn’s disease. Nutr. Clin. Pract. 2021, 37, 1242–1245. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Geerling, B.J.; Badart-Smook, A.; Stockbrügger, R.W.; Brummer, R.J. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am. J. Clin. Nutr. 1998, 67, 919–926. [Google Scholar] [CrossRef]
- Brown, A.C.; Rampertab, S.D.; Mullin, G.E. Existing dietary guidelines for Crohn’s disease and ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 411–425. [Google Scholar] [CrossRef]
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Far, B.F.; Behnoush, A.H.; Ghondaghsaz, E.; Habibi, M.A.; Khalaji, A. The interplay between vitamin C and thyroid. Endocrinol. Diabetes Metab. 2023, 6, e432. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Omrani, G.R. Effects of selenium and vitamin C on the serum level of antithyroid peroxidase antibody in patients with autoimmune thyroiditis. J. Endocrinol. Investig. 2019, 42, 481–487. [Google Scholar] [CrossRef]
- Peepre, K.S.; Deshpandey, U.; Choudhary, P. Role of Antioxidants on Thyroid Hormones in Wister Rats. Int. J. Sci. Res. 2014, 3, 34–38. [Google Scholar]
- Ward, M.H.; Kilfoy, B.A.; Weyer, P.J.; Anderson, K.E.; Folsom, A.R.; Cerhan, J.R. Nitrate Intake and the Risk of Thyroid Cancer and Thyroid Disease. Epidemiology 2010, 21, 389–395. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, H.; Jiang, H.; Lin, H.; He, J.; Fan, S.; Yu, D.; Yang, L.; Tang, H.; Lin, E.; et al. The effect of micronutrient on thyroid cancer risk: A Mendelian randomization study. Front. Nutr. 2024, 11, 1331172. [Google Scholar] [CrossRef]
- Wu, J.; Jia, C.; Wang, Q.; Li, X. Association between vitamin C intake and thyroid function among U.S. adults: A population-based study. Front. Endocrinol. 2024, 15, 1462251. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, E.; Que, H. Association of circulating vitamin levels with thyroid diseases: A Mendelian randomization study. Front. Endocrinol. 2024, 15, 1360851. [Google Scholar] [CrossRef]
- Sarandi, E.; Tsoukalas, D.; Rudofsky, G.; Fragoulakis, V.; Liapi, C.; Paramera, E.; Papakonstantinou, E.; Krasagakis, S.K.; Tsatsakis, A. Identifying the metabolic profile of Hashimoto’s thyroiditis from the METHAP clinical study. Sci. Rep. 2025, 15, 12410. [Google Scholar] [CrossRef]
- Chen, L.; Mao, Y.; Chen, G. Association between total vitamin C intake and hypothyroidism among Hashimoto thyroiditis: National Health and Nutrition Examination Survey, 2007–2012. Br. J. Nutr. 2024, 132, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Anand, S.; Jacobs, R. Salivary Stress Markers, Stress, and Periodontitis: A Pilot Study. J. Periodontol. 2011, 82, 287–292. [Google Scholar] [CrossRef]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ustianowski, Ł.; Ustianowska, K.; Gurazda, K.; Rusiński, M.; Ostrowski, P.; Pawlik, A. The Role of Vitamin C and Vitamin D in the Pathogenesis and Therapy of Periodontitis—Narrative Review. Int. J. Mol. Sci. 2023, 24, 6774. [Google Scholar] [CrossRef]
- Helmersson, J.; Ärnlöv, J.; Larsson, A.; Basu, S. Low dietary intake of β-carotene, α-tocopherol and ascorbic acid is associated with increased inflammatory and oxidative stress status in a Swedish cohort. Br. J. Nutr. 2008, 101, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Miura, H. The Relationship between Vitamin C and Periodontal Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2472. [Google Scholar] [CrossRef]
- Amaliya, V.; Timmerman, M.F.; Abbas, F.; Loos, B.G.; Van der Weijden, G.A.; Van Winkelhoff, A.J.; Winkel, E.G.; Van der Velden, U. Java project on periodontal diseases: The relationship between vitamin C and the severity of periodontitis. J. Clin. Periodontol. 2007, 34, 299–304. [Google Scholar] [CrossRef]
- Assaf, M.; Rabi, H. Assessment of Vitamin C Levels in Periodontal Patients: A Cross-Sectional Study in Palestine. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. 1), S903–S906. [Google Scholar] [CrossRef]
- Munday, M.-R.; Rodricks, R.; Fitzpatrick, M.; Flood, V.M.; Gunton, J.E. A Pilot Study Examining Vitamin C Levels in Periodontal Patients. Nutrients 2020, 12, 2255. [Google Scholar] [CrossRef]
- de Jong, T.M.H.; Stamatelou, E.; Rosema, N.A.M.; Jansen, I.D.C.; Brandt, B.W.; Angelakis, A.; Loos, B.G.; van der Velden, U.; Danser, M.M. Effect of Daily Vitamin C Supplementation with or Without Flavonoids on Periodontal, Microbial, and Systemic Conditions Before and After Periodontal Therapy: A Case Series from an RCT. J. Clin. Med. 2024, 13, 7571. [Google Scholar] [CrossRef]
- Li, W.; Song, J.; Chen, Z. The association between dietary vitamin C intake and periodontitis: Result from the NHANES (2009–2014). BMC Oral Health 2022, 22, 390. [Google Scholar] [CrossRef]
- Van der Velden, U. Vitamin C and Its Role in Periodontal Diseases—The Past and the Present: A Narrative Review. Oral Health Prev. Dent. 2020, 18, 115–123. [Google Scholar] [CrossRef]
- Buzatu, R.; Luca, M.M.; Bumbu, B.A. Does Vitamin C Supplementation Provide a Protective Effect in Periodontal Health? A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 8598. [Google Scholar] [CrossRef]
- Li, X.; Tang, L.; Lin, Y.F.; Xie, G.F. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin. Implant. Dent. Relat. Res. 2018, 20, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Muraglie, S.; Leonardi, R.; Lo Giudice, A. Assessment of Vitamin C and Antioxidant Profiles in Saliva and Serum in Patients with Periodontitis and Ischemic Heart Disease. Nutrients 2019, 11, 2956. [Google Scholar] [CrossRef] [PubMed]
- Hoffbrand, A.V. Megaloblastic Anaemia. In Postgraduate Haematology; Hoffbrand, A.V., Higgs, D.R., Keeling, D.M., Mehta, A.B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Andres, E.; Serraj, K. Optimal management of pernicious anemia. J. Blood Med. 2012, 3, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Dottori, L.; Pivetta, G.; Ligato, I.; Dilaghi, E.; Lahner, E. Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency. Nutrients 2022, 14, 1672. [Google Scholar] [CrossRef]
- Angeli, A.M.; Megna, B.; Mazepa, M.; Ivy, Z.K.; Sultan, S.; Sloan, J.A. Transfusion-dependent anemia secondary to vitamin C deficiency. Am. J. Hematol. 2022, 97, E166–E167. [Google Scholar] [CrossRef]
- Sano, K.; Imoto, N.; Koketsu, H.; Kubo, A.; Ito, R.; Nakashima, M.; Kurahashi, S. Vitamin C Deficiency Megaloblastic Anemia Mimicking Hemolytic Anemia: A Case Report. Intern. Med. 2025. [Google Scholar] [CrossRef]
- Taylor, L.; McCaddon, A.; Wolffenbuttel, B.H.R. Creating a Framework for Treating Autoimmune Gastritis—The Case for Replacing Lost Acid. Nutrients 2024, 16, 662. [Google Scholar] [CrossRef] [PubMed]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J. Gastroenterol. 2017, 23, 563–572. [Google Scholar] [CrossRef]
- Nocella, C.; Bartimoccia, S.; Cammisotto, V.; D’amico, A.; Pastori, D.; Frati, G.; Sciarretta, S.; Rosa, P.; Felici, C.; Riggio, O.; et al. Oxidative Stress in the Pathogenesis of Antiphospholipid Syndrome: Implications for the Atherothrombotic Process. Antioxidants 2021, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Ferro, D.; Iuliano, L.; Rokach, J.; Conti, F.; Valesini, G.; FitzGerald, G.A.; Violi, F. Ongoing prothrombotic state in patients with antiphospholipid antibodies: A role for increased lipid peroxidation. Blood 1999, 93, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- Martinuzzo, M.E.; Forastiero, R.R.; Kordich, L.; Carreras, L.O. Increased lipid peroxidation correlates with platelet activation but not with markers of endothelial cell and blood coagulation activation in patients with antiphospholipid antibodies. Br. J. Haematol. 2001, 114, 845–851. [Google Scholar] [CrossRef]
- Iuliano, L.; Praticò, D.; Ferro, D.; Pittoni, V.; Valesini, G.; Lawson, J.; FitzGerald, G.A.; Violi, F. Enhanced lipid peroxidation in patients positive for antiphospholipid antibodies. Blood 1997, 90, 3931–3935. [Google Scholar] [CrossRef]
- Lefferts, E.C.; Hibner, B.A.; Lefferts, W.K.; Lima, N.S.; Baynard, T.; Haus, J.M.; Lane-Cordova, A.D.; Phillips, S.A.; Fernhall, B. Oral vitamin C restores endothelial function during acute inflammation in young and older adults. Physiol. Rep. 2021, 9, e15104. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Liu, Z. Impact of Neutrophil Extracellular Traps on Thrombosis Formation: New Findings and Future Perspective. Front. Cell. Infect. Microbiol. 2022, 12, 910908. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C. The Role of Physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef]
- Ferro, D.; Saliola, M.; Meroni, P.L.; Valesini, G.; Caroselli, C.; Praticò, D.; Fitzgerald, G.A.; Shoenfeld, Y.; Violi, F. Enhanced monocyte expression of tissue factor by oxidative stress in patients with antiphospholipid antibodies: Effect of antioxidant treatment. J. Thromb. Haemost. JTH 2003, 1, 523–531. [Google Scholar] [CrossRef]
- Isola, S.; Gammeri, L.; Furci, F.; Gangemi, S.; Pioggia, G.; Allegra, A. Vitamin C Supplementation in the Treatment of Autoimmune and Onco-Hematological Diseases: From Prophylaxis to Adjuvant Therapy. Int. J. Mol. Sci. 2024, 25, 7284. [Google Scholar] [CrossRef] [PubMed]
- Kello, N.; Cho, Y.M. Natural supplements in antiphospholipid syndrome: A case for further study. Clin. Immunol. 2024, 258, 109848. [Google Scholar] [CrossRef] [PubMed]
- Štok, U.; Blokar, E.; Lenassi, M.; Holcar, M.; Frank-Bertoncelj, M.; Erman, A.; Resnik, N.; Sodin-Šemrl, S.; Čučnik, S.; Pirkmajer, K.P.; et al. Characterization of Plasma-Derived Small Extracellular Vesicles Indicates Ongoing Endothelial and Platelet Activation in Patients with Thrombotic Antiphospholipid Syndrome. Cells 2020, 9, 1211. [Google Scholar] [CrossRef]
- Tian, W.; Shi, D.; Zhang, Y.; Wang, H.; Tang, H.; Han, Z.; Wong, C.C.L.; Cui, L.; Zheng, J.; Chen, Y. Deep proteomic analysis of obstetric antiphospholipid syndrome by DIA-MS of extracellular vesicle enriched fractions. Commun. Biol. 2024, 7, 99. [Google Scholar] [CrossRef]
- Bonisoli, G.L.; Argentino, G.; Friso, S.; Tinazzi, E. Extracellular Vesicles Analysis as Possible Signatures of Antiphospholipid Syndrome Clinical Features. Int. J. Mol. Sci. 2025, 26, 2834. [Google Scholar] [CrossRef]
- Weaver, D.F. β-Amyloid is an Immunopeptide and Alzheimer’s is an Autoimmune Disease. Curr. Alzheimer Res. 2021, 18, 849–857. [Google Scholar] [CrossRef]
- Weaver, D.F. Alzheimer’s disease as an innate autoimmune disease (AD2): A new molecular paradigm. Alzheimer’s Dement. 2022, 19, 1086–1098. [Google Scholar] [CrossRef]
- Arshavsky, Y.I. Alzheimer’s Disease: From Amyloid to Autoimmune Hypothesis. Neuroscientist 2020, 26, 455–470. [Google Scholar] [CrossRef]
- Severini, C.; Barbato, C.; Di Certo, M.G.; Gabanella, F.; Petrella, C.; Di Stadio, A.; de Vincentiis, M.; Polimeni, A.; Ralli, M.; Greco, A. Alzheimer’s Disease: New Concepts on the Role of Autoimmunity and NLRP3 Inflammasome in the Pathogenesis of the Disease. Curr. Neuropharmacol. 2020, 19, 498–512. [Google Scholar] [CrossRef]
- Hamid, M.; Mansoor, S.; Amber, S.; Zahid, S. A quantitative meta-analysis of vitamin C in the pathophysiology of Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 970263. [Google Scholar] [CrossRef] [PubMed]
- Appiah, D.; Ingabire-Gasana, E.; Appiah, L.; Yang, J. The Relation of Serum Vitamin C Concentrations with Alzheimer’s Disease Mortality in a National Cohort of Community-Dwelling Elderly Adults. Nutrients 2024, 16, 1672. [Google Scholar] [CrossRef]
- He, X.; Lin, Y.; Wu, X.; Li, M.; Zhong, T.; Zhang, Y.; Weng, X. Vitamin C intake and cognitive function in older U.S. adults: Nonlinear dose–response associations and effect modification by smoking status. Front. Nutr. 2025, 12, 1585863. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, J.; Sun, Y.; Wang, Z. Association of antioxidants intake in diet and supplements with risk of Alzheimer’s disease: A systematic review and dose-response meta-analysis of prospective cohort studies. Aging Clin. Exp. Res. 2025, 37, 166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Aran, K.R. Unraveling the molecular mechanisms of vitamin C in Alzheimer’s disease: Oxidative stress, homocysteine metabolism, and amyloid/tau interactions. Aging Health Res. 2025, 5, 100226. [Google Scholar] [CrossRef]
- Murakami, K.; Murata, N.; Ozawa, Y.; Kinoshita, N.; Irie, K.; Shirasawa, T.; Shimizu, T. Vitamin C restores behavioral deficits and amyloid-β oligomerization without affecting plaque formation in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Arlt, S.; Müller-Thomsen, T.; Beisiegel, U.; Kontush, A. Effect of One-Year Vitamin C- and E-Supplementation on Cerebrospinal Fluid Oxidation Parameters and Clinical Course in Alzheimer’s Disease. Neurochem. Res. 2012, 37, 2706–2714. [Google Scholar] [CrossRef]
- Li, F.-J.; Shen, L.; Ji, H.-F. Dietary Intakes of Vitamin E, Vitamin C, and β-Carotene and Risk of Alzheimer’s Disease: A Meta-Analysis. J. Alzheimer’s Dis. 2012, 31, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Harrison, F.E. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 29, 711–726. [Google Scholar] [CrossRef]

| Disease | Evidence Type | Findings | Strength of Evidence * |
|---|---|---|---|
| Multiple sclerosis | Observational, small clinical studies | Reduced serum VitC; some improvement in oxidative stress; limited trial data | Limited |
| Rheumatoid arthritis | Population cohorts, experimental, small RCTs | Inverse associations; antioxidant and immunomodulatory effects; ongoing trials | Moderate |
| Sjögren’s disease | Dietary studies, small interventions | Antioxidant rationale; modest benefits in dry eye; no disease-modifying data | Limited |
| Type 1 diabetes | Animal models, small human interventions | Endothelial and oxidative improvements; no consistent glycemic benefit | Limited |
| Crohn’s disease | Case reports, biochemical analyses | Frequent deficiency; links to oxidative stress; supplementation prevents scurvy | Limited |
| Hashimoto’s thyroiditis | Observational, meta-analyses, animal studies | Possible antibody reduction; mixed population data; no strong RCTs | Limited –Moderate |
| Periodontitis | Cross-sectional, clinical interventions | Consistent link with deficiency; supplementation modestly supports standard therapy | Moderate |
| Pernicious anemia | Case reports, mechanistic studies | Deficiency worsens hematologic profile; supplementation helpful but not curative | Limited |
| Antiphospholipid syndrome | Small biomarker trials, mechanistic rationale | Antioxidant effects demonstrated; no outcome-based clinical data | Limited |
| Alzheimer’s disease | Observational cohorts, meta-analyses | Lower VitC in patients; dietary intake linked to reduced risk; supplementation less consistent | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochol, M.; Jablonowski, L.; Pawlik, A.; Rasławska-Socha, J.; Chamarczuk, A.; Lipski, M.; Mazurek-Mochol, M. The Role of Vitamin C in Selected Autoimmune and Immune-Mediated Diseases: Exploring Potential Therapeutic Benefits. Int. J. Mol. Sci. 2025, 26, 9375. https://doi.org/10.3390/ijms26199375
Mochol M, Jablonowski L, Pawlik A, Rasławska-Socha J, Chamarczuk A, Lipski M, Mazurek-Mochol M. The Role of Vitamin C in Selected Autoimmune and Immune-Mediated Diseases: Exploring Potential Therapeutic Benefits. International Journal of Molecular Sciences. 2025; 26(19):9375. https://doi.org/10.3390/ijms26199375
Chicago/Turabian StyleMochol, Martyna, Lukasz Jablonowski, Andrzej Pawlik, Joanna Rasławska-Socha, Agnieszka Chamarczuk, Mariusz Lipski, and Małgorzata Mazurek-Mochol. 2025. "The Role of Vitamin C in Selected Autoimmune and Immune-Mediated Diseases: Exploring Potential Therapeutic Benefits" International Journal of Molecular Sciences 26, no. 19: 9375. https://doi.org/10.3390/ijms26199375
APA StyleMochol, M., Jablonowski, L., Pawlik, A., Rasławska-Socha, J., Chamarczuk, A., Lipski, M., & Mazurek-Mochol, M. (2025). The Role of Vitamin C in Selected Autoimmune and Immune-Mediated Diseases: Exploring Potential Therapeutic Benefits. International Journal of Molecular Sciences, 26(19), 9375. https://doi.org/10.3390/ijms26199375







