Curcumin in Inflammatory Complications: Therapeutic Applications and Clinical Evidence
Abstract
1. Introduction
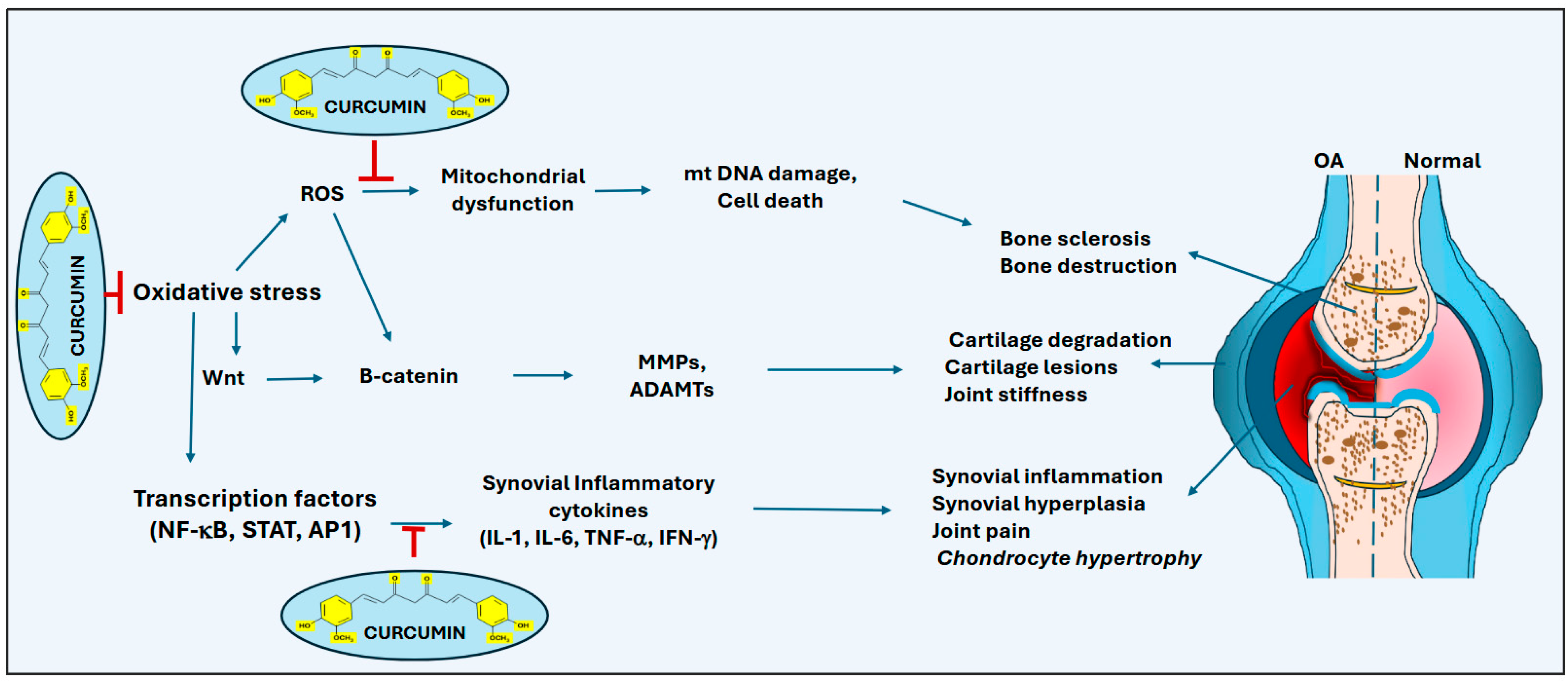
2. Curcumin in Treating Osteoarthritis
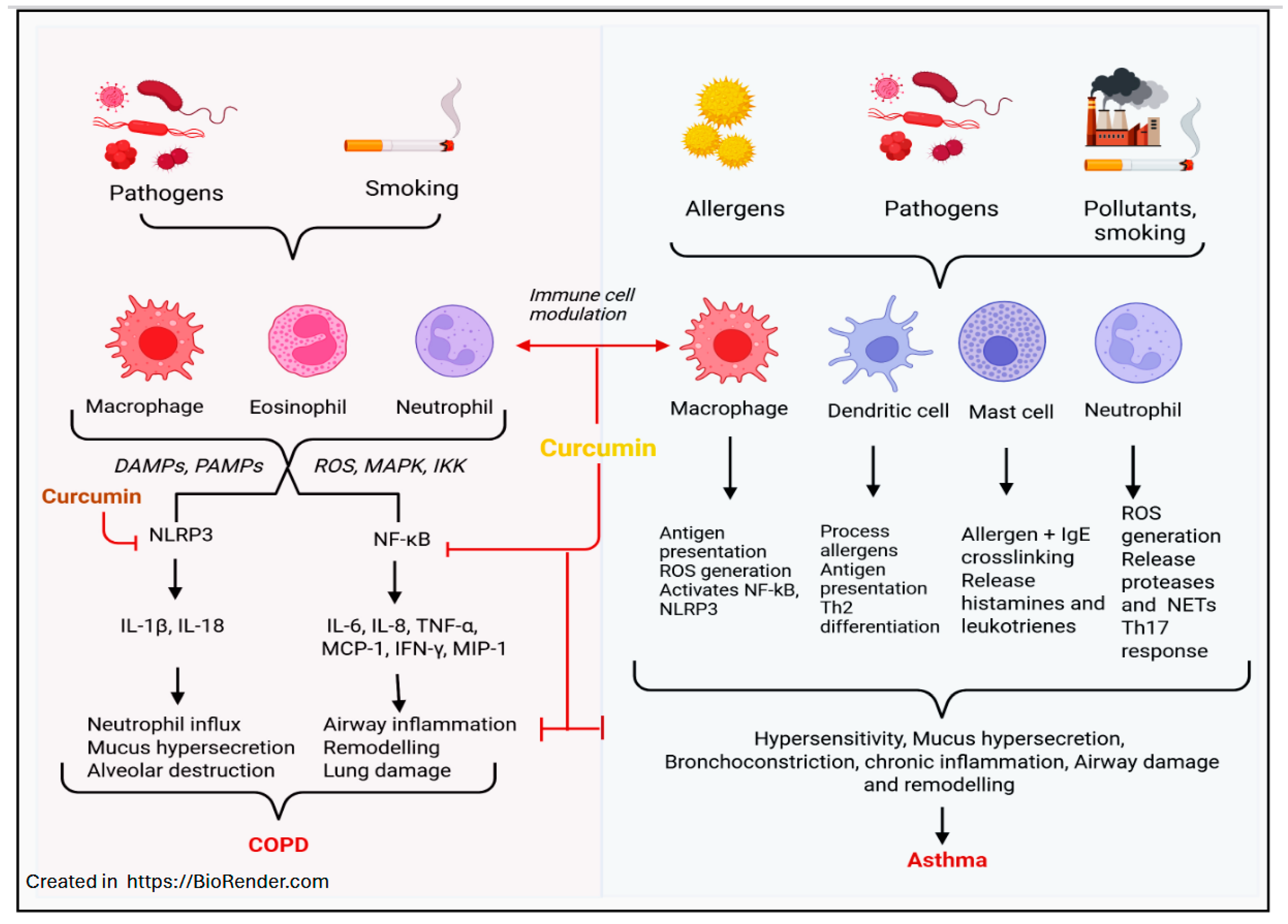
3. Curcumin in Treating Asthma and COPD
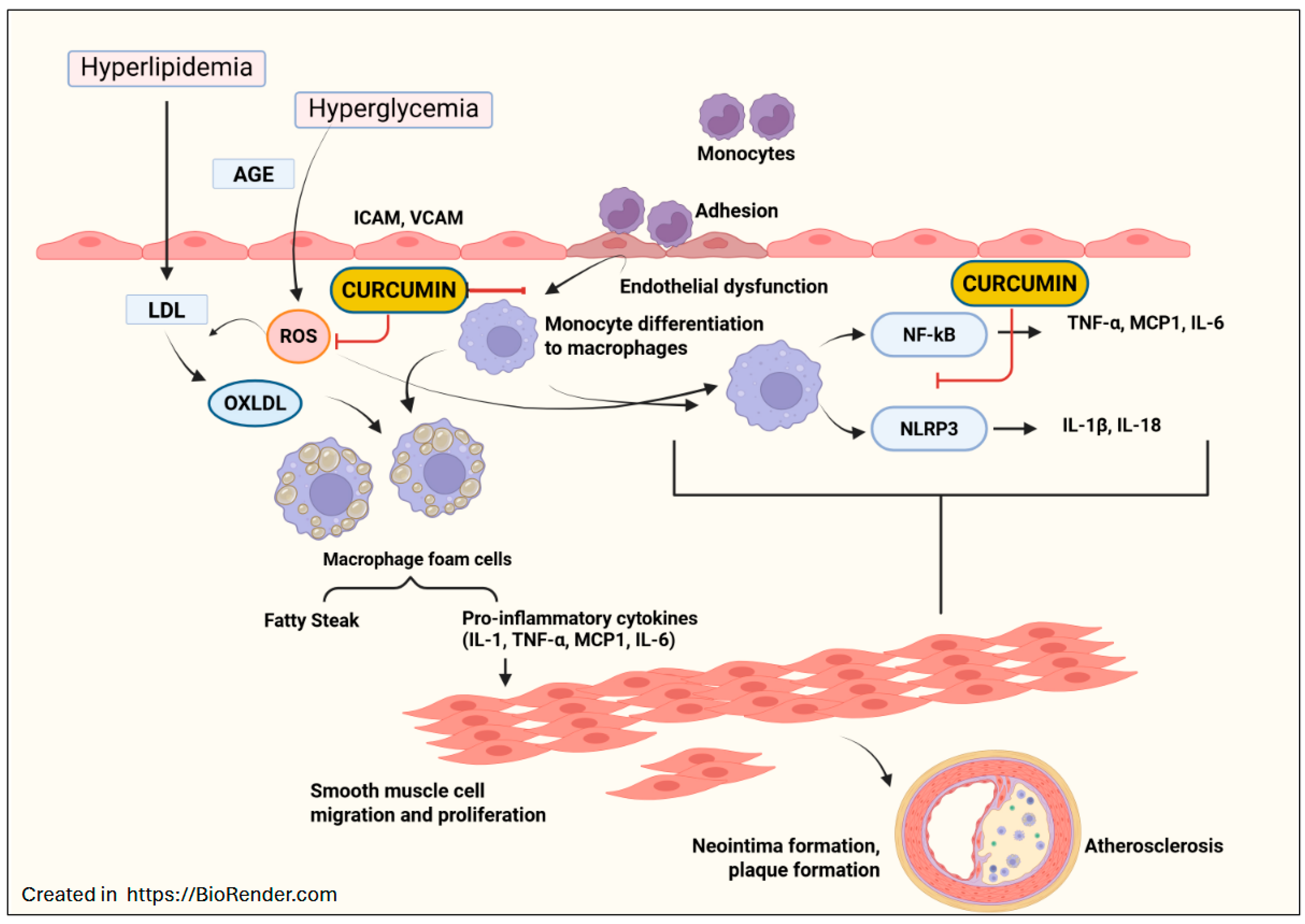
4. Curcumin in Treating Atherosclerosis
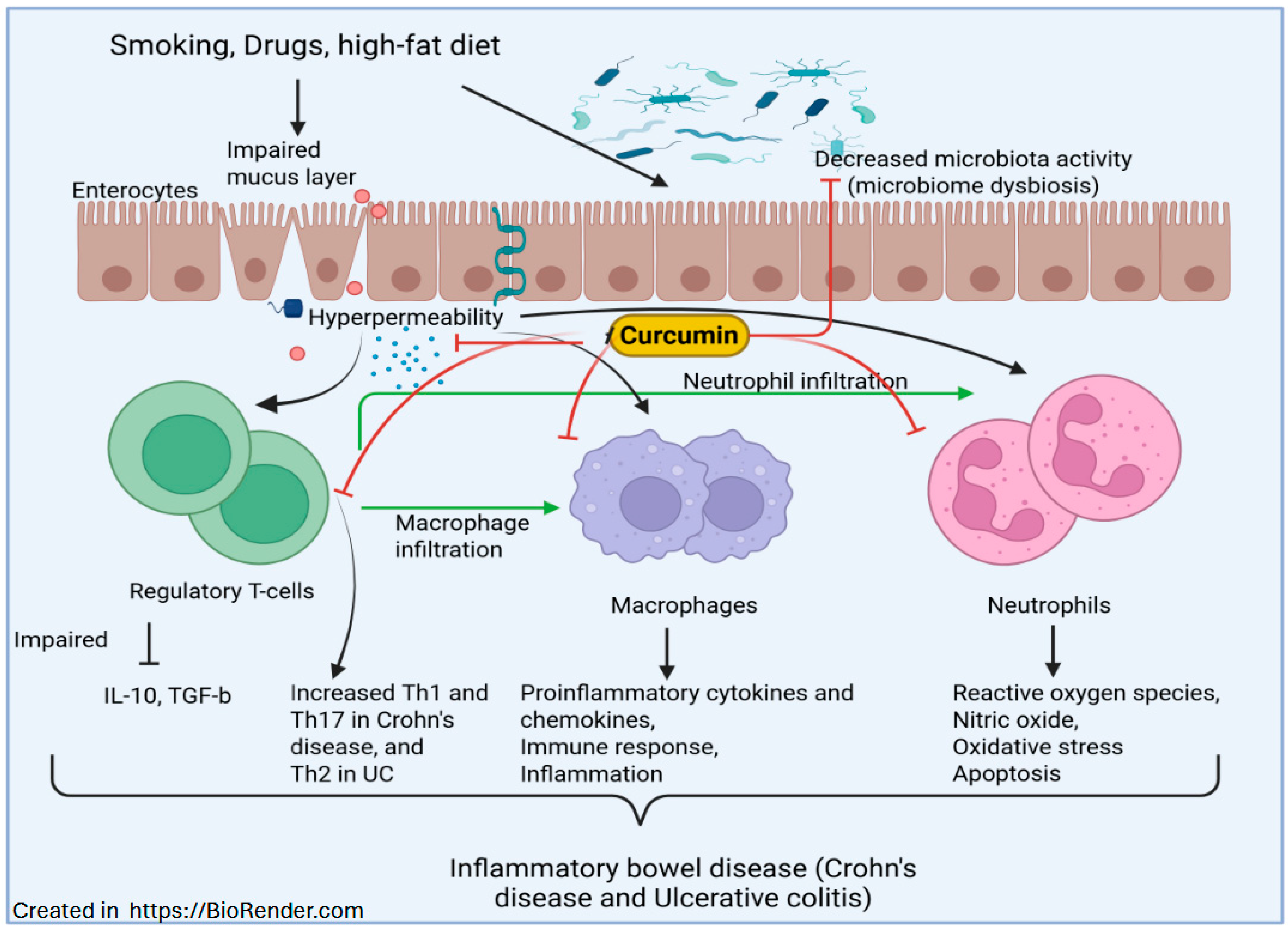
5. Curcumin in Treating IBD
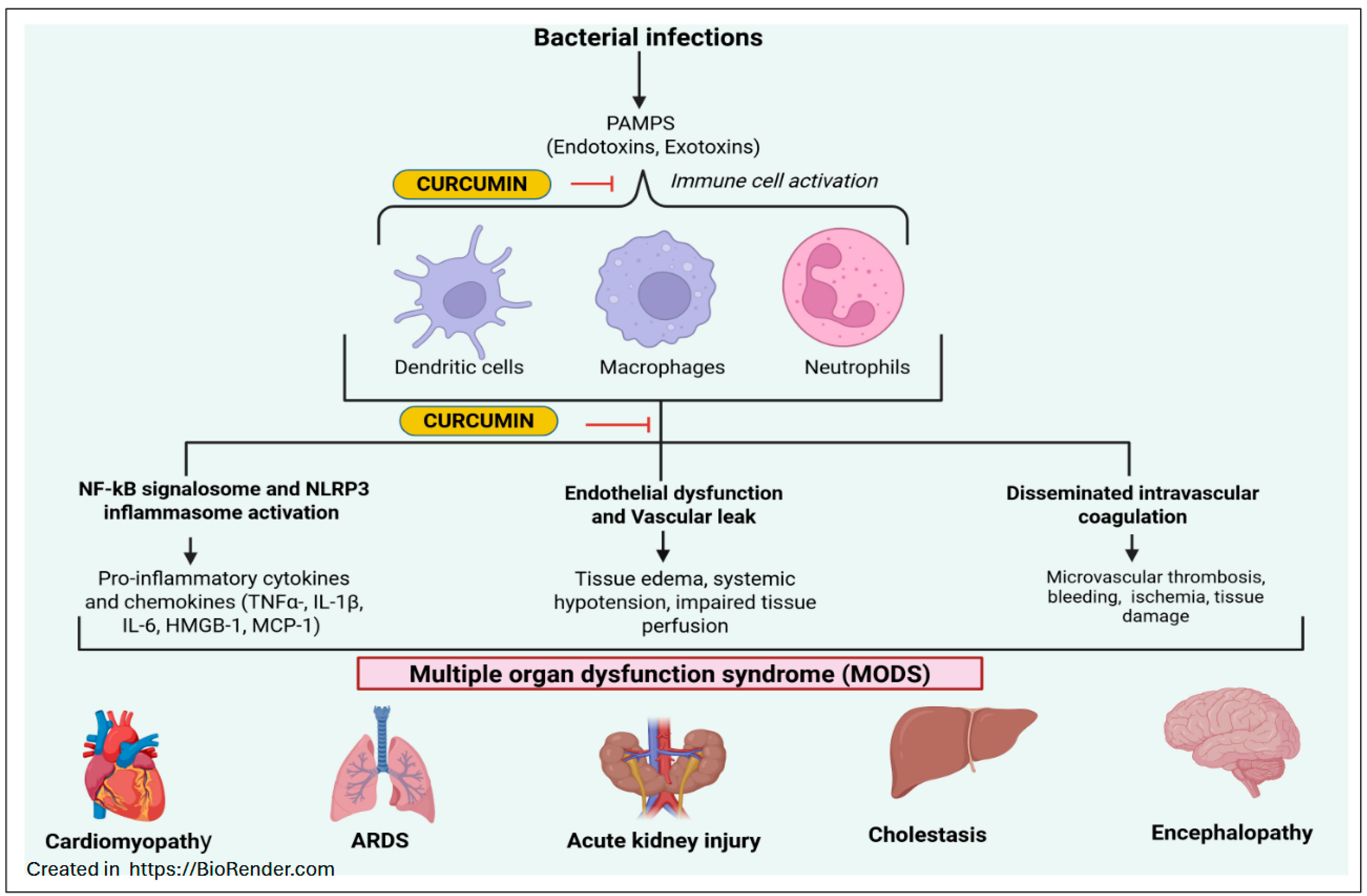
6. Curcumin in Treating Sepsis
7. Curcumin in Treating Psoriasis and Atopic Dermatitis
| References | Design | Formulation | Dose | Duration | Comparator | Primary Outcome |
|---|---|---|---|---|---|---|
| Osteoarthritis | ||||||
| [38] | Active-controlled trial (randomization/blinding; N = 139; ages 38–65) | Curcumin | 1000–2000 mg/day | 28 days | Diclofenac | Pain/function |
| [40] | RCT, double-blind, placebo-controlled, parallel, N = 19, age <80 years | Curcuminoids | 1500 mg/day | 6 weeks | Placebo | Pain/function |
| [44] | RCT, double-blind, placebo controlled, N = 30, ages 40–55 | Curcumin | - | 3 months | Placebo | Immunologic |
| [45] | Clinical study (topical); N = 60 | SNE-PEG organogel | - | 8 weeks | Placebo/standard care | Pain/function |
| [46] | Clinical study | Topical curcumin | 10% | 2 weeks | Placebo/diclofenac | Analgesia |
| Asthma/COPD | ||||||
| [65] | Cross-sectional observational; N = 2478, ages > 55 | Dietary turmeric/curcumin intake | ≥1×/month | — | None | Lung function |
| [69] | Randomized clinical study, N = 77, | Curcumin capsules | 500 mg × 2 | 30 days | Standard asthma therapy | Lung function |
| [83] | RCT, double-blind, controlled, Children and adolescents, ages 7–18 years | Powdered Curcuma longa roots | 30 mg/kg/day | 3- and 6-months follow-ups | Placebo | Control/reliever use |
| [84] | RCT, double-blind, placebo controlled; N = 60 COPD | Nanocurcumin | 80 mg | 3 months | Placebo/standard care | Lung function, cytokines |
| [82] | Randomized clinical study, N = 89, sulfur-mustard lung injury, | Curcuminoids + piperine | 1500 mg/day | 4 weeks | Placebo | Oxidative stress, COPD test |
| Atherosclerosis | ||||||
| [111] | RCT, double-blind, placebo-controlled, N = 227 (T2DM), age > 35 years | Curcumin | 250 mg × 2/day | 12 months | Placebo | Arterial stiffness, lipids |
| [112] | RCT, double-blind, placebo-controlled, N = 64 (T2DM and mild to moderate CAD), | Nano-curcumin | 80 mg/day | 90 days | Placebo | Inflammation, lipoprotein |
| [113] | RCT, double-blind, placebo-controlled, age 20–85 years | Theracurmin® | 90 mg × 2/day | 24 weeks | Placebo | Atherogenic markers |
| IBD | ||||||
| [136] | RCT, double-blind, placebo-controlled (add-on to mesalamine) N = 50 | Curcumin + mesalamine | 3 g/day | One month | Mesalamine + placebo | Remission, biomarkers |
| [138] | RCT, double-blind clinical trial; N = 70 (mild-moderate UC) | Curcumin + drug therapy | 1500 mg/day | 8 weeks | Placebo | Symptoms and inflammation |
| [141] | Multicenter, double-blind RCT pilot study, N = 69 (mild-moderate UC) | Curcumin + mesalamine | 50 mg × 2/day | 6 weeks–3 months | Mesalamine + placebo | Clinical remission |
| [140] | Multicenter, double-blind RCT, N = 30 (Crohn’s) | Theracurmin® | 360 mg/day | 12 weeks | Placebo/standard care | Endoscopic and clinical activity |
| [139] | RCT, double-blind, placebo-controlled, N = 58 (IBD), age > 18 years | Curcumin + piperine | 1000 mg/day | 12 weeks | Placebo | Muscle status |
| Sepsis | ||||||
| [169] | Pilot randomized trial, N = 40 ICU, ages 18–55 years | Nanocurcumin | 160 mg/day | 10 days | Placebo | Inflammation, organ failure |
| [170] | RCT, double-blind, placebo-controlled, N = 40 ICU | Nanocurcumin | 160 mg × 2/day | 10 days | Placebo | Inflammation, oxidative stress |
| [171] | Double-blind RCT, N = 66 ICU, ages 20–75 years | Curcumin + piperine | 500 mg/day | 7 days | Placebo | Inflammation, hematology |
| [172] | Prospective study, N = 52 (MASH + CKD) | Curcumin Meriva® | 2 g/day | 72 weeks | None | Gut barrier, inflammation |
| Psoriasis | ||||||
| [207] | RCT, double-blind, placebo-controlled, moderate to severe psoriasis | Nanocurcumin + acitretin | 3 g/day | 12 weeks | Acitretin | PASI |
| [216] | RCT, double-blind, placebo-controlled, N = 96 (sulfur mustard -chronic pruritus), ages 37–59 years | Curcumin | 1 g/day | 4 weeks | Placebo | Pruritus, biomarkers |
8. Safety Profile and Adverse Events with Curcumin Use
| Formulation | Indication(s) | Typical Dose Range | Duration Range | References |
|---|---|---|---|---|
| Plain oral curcumin | OA, UC/CD, Asthma, Psoriasis | ~1000–2000 mg/day (OA) | 4–12 weeks typical; up to 12 months | [38,40,43,46,65,69,135,138,141,197] |
| Curcumin + piperine | COPD (sulfur-mustard lung injury), IBD (muscle wasting), ICU sepsis | Dose 500 mg/day (5 mg piperine co-administered) | 7 days to multi-week | [82,139,171] |
| Nanocurcumin | ICU sepsis, COPD, Psoriasis | 160 mg/day (ICU) | 10 days (ICU) to multi-week | [84,169,170,207] |
| Theracurmin® | COPD, Crohn’s disease | 360 mg/day | 12–24 weeks | [113,140] |
| Topical (organogel/cream) | Knee OA, Atopic Dermatitis | 10% | 2 weeks | [45,46,212,213,214] |
| Analogs (J147, AI-44) | Sepsis models (preclinical, SAE, endotoxemia) | - | - | [160,161,163] |
9. Conclusions and Future Perspectives
| Pathway/Mechanism | Preclinical Evidence | Human Biomarker Evidence (Clinical Trials) | Limits of Translation from Preclinical to Clinical |
|---|---|---|---|
| NF-κB/AP-1 signaling | Inhibition of NF-κB, AP-1, and downstream cytokines across multiple models (OA chondrocytes, asthma, sepsis, IBD) [18,19,20,21,39,70,122]. | Decreased l inflammatory markers such as IL-6, TNF-α, and CRP in OA, COPD, UC/IBD, and ICU sepsis. [40,43,82,84,136,138,141,169,170,171]. | Clinical data align with NF-κB inhibition and inhibition of inflammatory cytokines. Other upstream signals of NF-kB were not directly measured. |
| NLRP3 inflammasome | Inhibition of NLRP3 activation, inhibition of IL-1β, and caspase-1 inhibition in DSS-colitis, sepsis, endotoxemia models [24,129,163,164]. | Decreased IL-1β observed in ICU sepsis (nanocurcumin) [170]. | Mostly preclinical studies; Il-1b levels in patients. Additional clinical data is required for direct inflammasome readouts (caspase-1 activity, and complex proteins). |
| MAPK/JNK/p38 pathways | Inhibits MAPK phosphorylation and JNK-mediated apoptosis in cells and sepsis models [39,122,157]. | Decreased CRP and TNF-α, improved WOMAC/VAS scores in OA [40]. | Clinical biomarkers do not capture MAPK activity; human confirmation studies are still needed. |
| Oxidative stress/antioxidant defense | Decreased ROS, MDA, MPO; increased Nrf-2, catalase, SOD, TAC in cell and animal models [157,159,160]. | In ICU sepsis trials, decreased MDA, and increased Nrf-2, catalase, SOD, TAC with nanocurcumin [170]; in COPD, improved oxidative stress indices [82]. | Stronger translational connection. Both preclinical and human subject data support antioxidant effects. |
| Endothelial activation | Decreased ICAM-1 and VCAM-1, improved vascular function in atherosclerosis [106,107,108]. | In ICU sepsis, decreased ICAM-1 and VCAM-1 [170]; In atherosclerosis, decreased arterial stiffness, Lp(a), and AT-LDL [111,112,113]. | Both preclinical and human trials confirm endothelial biomarker modulation. |
| Ferroptosis | Curcumin and CeCH analogs decrease ferroptosis markers, restore GPX4, and prevent cardiac/kidney injury in sepsis models [159,160]. | No clinical ferroptosis biomarkers reported. | Translation gap is observed. No human data on GPX4, lipid peroxides, or ferroptosis-specific panels. |
| Immune cell modulation | Inhibition of Th17 cells, increased Tregs, modulation of B/T follicular helper cells in IBD models [123,124,125,126,127]. | In UC trials, CRP/IL-6 decreased and improved remission rates [136,141]. | Clinical trials are limited to measuring the cytokines, no direct immune cell modulation, and Th17/Treg readouts in clinical settings. |
| Disease | Delivery System(s) | Major Effects | References |
|---|---|---|---|
| Osteoarthritis (OA) | Oral capsules/extracts | Comparable efficacy to NSAIDs (diclofenac, naproxen) and reduced pain (VAS, KOOS, WOMAC). | [38,40] |
| Self-nano-emulsifying PEG organogel (SNE-POG) | Improved absorption and efficacy in knee OA. | [45] | |
| Topical curcumin formulations | Analgesic effect and reduced knee pain. | [46] | |
| PGA-curcumin combination (dogs) | Reduced meloxicam dosage (~25%); effective pain control. | [41] | |
| Nanocurcumin/Phytosomes | Improved bioavailability, reduced IL-1β, NO, PGE2, and MMP-3 in chondrocytes. | [39,43,44] | |
| Asthma and COPD | Oral curcumin capsules/extracts | Reduced airway inflammation, NF-κB inhibition and improved FEV1/FVC. | [65,66,70,71] |
| Nanocurcumin | Improved lung function in COPD and decreased IL-6. | [84] | |
| Curcumin + Piperine | Improved bioavailability, oxidative stress and COPD indices. | [79,80,81,82] | |
| Theracurmin® (nano-curcumin) | Reduced AT-LDL in COPD and improved vascular health. | [113] | |
| Atherosclerosis | Nano-curcumin | Reduced hs-CRP, lipoprotein A and improved lipid profiles in CAD. | [112] |
| Theracurmin® | Reduced AT-LDL and improved cardiovascular risk markers. | [113] | |
| Nanoparticles/liposomes | Modulated macrophage polarization (M1→M2) and reduced plaque and cytokines (TNF-α, IL-6). | [101,102,103,104,105,108] | |
| Inflammatory Bowel Disease (IBD) | Oral curcumin + mesalamine | Induced remission in UC and reduced relapse. | [136,141] |
| Nano-curcumin/Theracurmin | Improved mucosal healing and reduced NF-κB activity. | [140] | |
| Curcumin + Piperine | Enhanced absorption, reduced inflammation and muscle depletion. | [139] | |
| Curcumin analogs (C66, derivatives) | Blocked JNK/NF-κB and NLRP3 pathways; reduced colitis severity. | [128,129] | |
| Sepsis | Nanocurcumin (oral/NG tube) | Reduced IL-6, TNF-α, and PCT, and improved SOFA scores. | [169,170] |
| Curcumin + Piperine | Improved inflammatory and hematologic markers in ICU patients. | [171] | |
| Curcumin-loaded exosomes (BMSC-ExoCurcumin) | Reduced oxidative stress and kidney injury in septic models. | [158] | |
| Ceria-curcumin nanozymes (CeCH) | Antioxidant and anti-ferroptotic, reduced heart and kidney inflammation. | [160] | |
| Curcumin analogs (FM0807, J147, AI-44) | Inhibited NF-κB, JNK/MAPK, and NLRP3 pathways, and improved survival. | [157,161,163,164] | |
| Psoriasis | Oral nanocurcumin | Significant PASI reduction | [207] |
| Curcumin-loaded microneedles | Improved lesions and lowered TNF-α, IL-17, IL-22. | [200] | |
| Solid lipid nanoparticles | Reduced keratinocyte proliferation and suppressed psoriatic inflammation. | [202] | |
| Curcumin topical gels | Reduced lesional cytokines (IL-6, TNF-α). | [197,198] | |
| Curcumin-based microbiota modulation | Prevented imiquimod-induced psoriasis in mice. | [201] | |
| Atopic Dermatitis | Tetrahydrocurcumin (THC) SLN gel | Improved hydration and reduced TNF-α, IL-6; promoted healing. | [212] |
| Nanoformulations (lipid nanoparticles, gels) | Reduced inflammatory cell infiltration and prevented thickening. | [209,210,211,213,214,215] | |
| Oral curcumin supplementation | Reduced chronic pruritus and lowered serum substance p. | [216] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malvankar, S.; Jaiswal, P.; Bhat, P.P.; Mehto, S. Regulation of Cancer by Inflammasomes: From Inflammation to Tumorigenesis. Front. Immunol. 2025, 16, 1611719. [Google Scholar] [CrossRef]
- Aleksandrowicz, M.; Konop, M.; Rybka, M.; Mazurek, Ł.; Stradczuk-Mazurek, M.; Kciuk, M.; Bądzyńska, B.; Dobrowolski, L.; Kuczeriszka, M. Dysfunction of Microcirculation in Atherosclerosis: Implications of Nitric Oxide, Oxidative Stress, and Inflammation. Int. J. Mol. Sci. 2025, 26, 6467. [Google Scholar] [CrossRef] [PubMed]
- Vataja, E.; Ratti, G.; Safa, A.; Pagano, M.; Ferrante, L.; Meri, S.; Haapasalo, K. Exploring the Intersection of Atherosclerosis and Alzheimer’s Disease: The Role of Inflammation and Complement Activation. Inflamm. Res. 2025, 74, 102. [Google Scholar] [CrossRef]
- Sankar, S.; Kalidass, B.; Indrakumar, J.; Kodiveri Muthukaliannan, G. NSAID-Encapsulated Nanoparticles as a Targeted Therapeutic Platform for Modulating Chronic Inflammation and Inhibiting Cancer Progression: A Review. Inflammopharmacology 2025, 33, 2493–2522. [Google Scholar] [CrossRef]
- Satapathy, T.; Minj, A.; Verma, M. Impact of NSAIDs, Corticosteroids, DMARDs, Biologics and Their Comparisons with Natural Products in C-Reactive Proteins (CRP) Linked Cardiovascular Disorders. Inflammopharmacology 2025, 33, 2537–2563. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Huscher, D.; Thiele, K.; Gromnica-Ihle, E.; Hein, G.; Demary, W.; Dreher, R.; Zink, A.; Buttgereit, F. Dose-Related Patterns of Glucocorticoid-Induced Side Effects. Ann. Rheum. Dis. 2009, 68, 1119–1124. [Google Scholar] [CrossRef]
- Nakadate, K.; Ito, N.; Kawakami, K.; Yamazaki, N. Anti-Inflammatory Actions of Plant-Derived Compounds and Prevention of Chronic Diseases: From Molecular Mechanisms to Applications. Int. J. Mol. Sci. 2025, 26, 5206. [Google Scholar] [CrossRef]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as Anti-Inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W. The Golden Spice for Life: Turmeric with the Pharmacological Benefits of Curcuminoids Components, Including Curcumin, Bisdemethoxycurcumin, and Demethoxycurcumins. Curr. Org. Synth. 2024, 21, 665–683. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From Ancient Medicine to Current Clinical Trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Slika, L.; Patra, D. Traditional Uses, Therapeutic Effects and Recent Advances of Curcumin: A Mini-Review. Mini Rev. Med. Chem. 2020, 20, 1072–1082. [Google Scholar] [CrossRef]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and Curcumin-Like Molecules: From Spice to Drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef]
- Koroljević, Z.D.; Jordan, K.; Ivković, J.; Bender, D.V.; Perić, P. Curcuma as an Anti-Inflammatory Component in Treating Osteoarthritis. Rheumatol. Int. 2023, 43, 589–616. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Kulsoom; Sun, Y.; Li, Y.; Wang, Z.; Xue, L.; Wang, F. Advancing Cancer Therapy: Nanomaterial-Based Encapsulation Strategies for Enhanced Delivery and Efficacy of Curcumin. Mater. Today Bio 2025, 33, 101963. [Google Scholar] [CrossRef]
- Soleimani Samarkhazan, H.; Noormohamadi, H.; Shafiei, F.S.; Pilehvari, N.; Aghaei, A.H.; Mohammadi, M.H.; Shanaki, M. Curcumin and Acute Myeloid Leukemia: A Golden Hope, Updated Insights. Mol. Biol. Rep. 2025, 52, 583. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhuo, X.; Qi, D.; Ma, H.; Kong, F.; Sun, P.; Zhang, Y.; Xue, P.; Ma, H. Curcumin Alleviates Ovarian Hyperstimulation Syndrome in the Rat Model via Inhibiting the Nuclear Factor Kappa B/Hypoxia-Inducible Factor-1α Signaling Pathway. Histochem. Cell Biol. 2025, 163, 75. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Cicero, A.F.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune Modulation by Curcumin: The Role of Interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Jia, X.; Yang, Q.; Du, Y. AMPK/mTOR/ULK1 Pathway Participates in Autophagy Induction by Curcumin in Colorectal Adenoma Mouse Model. Drug Dev. Res. 2025, 86, e70115. [Google Scholar] [CrossRef]
- Xi, Y.; Zeng, S.; Tan, X.; Deng, X. Curcumin Inhibits the Activity of Ubiquitin Ligase Smurf2 to Promote NLRP3 Dependent Pyroptosis in Non Small Cell Lung Cancer Cells. Int. J. Oncol. 2025, 66, 21. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Wang, Y.; Li, H.; Xu, F.; Zhang, W.; Fu, H.; Yin, L.; Amevor, F.K.; Lin, J.; et al. Investigating Protective Effect of Suspension of Paeoniflorin in Combination with Curcumin Against Acute Liver Injury Based on Inhibition of TLR4/NF-κB/NLRP3 Inflammatory Pathway. Int. J. Mol. Sci. 2025, 26, 6324. [Google Scholar] [CrossRef]
- Marcolin, E.; Chemello, C.; Piovan, A.; Barbierato, M.; Morazzoni, P.; Ragazzi, E.; Zusso, M. A Combination of 5-(3′,4′-Dihydroxyphenyl)-γ-Valerolactone and Curcumin Synergistically Reduces Neuroinflammation in Cortical Microglia by Targeting the NLRP3 Inflammasome and the NOX2/Nrf2 Signaling Pathway. Nutrients 2025, 17, 1316. [Google Scholar] [CrossRef]
- Zhu, K.; Bi, J.; Zhang, Q.; Yang, Y.; Li, J.; Liang, Y. Mechanism of Action of Curcumin Targeting TRPM2/NLRP3 Signaling Axis to Mediate Cell Death in the Treatment of Knee Osteoarthritis. Hum. Exp. Toxicol. 2024, 43, 9603271241308798. [Google Scholar] [CrossRef]
- Greene, M.A.; Loeser, R.F. Aging-Related Inflammation in Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.M.; Al-Hetty, H.R.A.K.; Ahmed, A.T.; Awad, M.M.; Al-Ani, M.Q.; Al-Darraji, M.N.; Salman, D.A.; Ali, L.H. Effect of Curcumin on Lipid Mediators, Glycemic Index, and Oxidative Stress and Inflammation Biomarkers in Polycystic Ovary Syndrome: Future Directions and Current Knowledge—A Systematic Review. Prostaglandins Other Lipid Mediat. 2025, 177, 106947. [Google Scholar] [CrossRef] [PubMed]
- Mokgalaboni, K.; Mashaba, R.G.; Phoswa, W.N.; Lebelo, S.L. Curcumin Attenuates Hyperglycemia and Inflammation in Type 2 Diabetes Mellitus: Quantitative Analysis of Randomized Controlled Trial. Nutrients 2024, 16, 4177. [Google Scholar] [CrossRef]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, H.; Najda, A.; Alanazi, I.S.; Germoush, M.O.; et al. The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant Towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef]
- Lespasio, M.J.; Piuzzi, N.S.; Husni, M.E.; Muschler, G.F.; Guarino, A.; Mont, M.A. Knee Osteoarthritis: A Primer. Perm. J. 2017, 21, e16-183. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.B.; Rockel, J.S.; Mulders, R.; Capellini, T.D.; Appleton, C.T.; Phanstiel, D.H.; Lories, R.; Geurts, J.; Ali, S.A.; Bhutani, N.; et al. From Mechanism to Medicine: The Progress and Potential of Epigenetics in Osteoarthritis. Osteoarthr. Cartil. Open 2025, 7, 100621. [Google Scholar] [CrossRef]
- Lou, Z.; Bu, F. Recent Advances in Osteoarthritis Research: A Review of Treatment Strategies, Mechanistic Insights, and Acupuncture. Medicine 2025, 104, e41335. [Google Scholar] [CrossRef]
- JiaoYi, P.; YongQi, S.; KeChun, G.; XingYu, L.; ZeZhong, L.; Jin Shuai, D.; YouJia, G.; Bing, X.; XiaoFeng, W. Assessing the Efficacy and Safety of Different Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Osteoarthritis: A Systematic Review and Network Meta-Analysis Based on RCT Trials. PLoS ONE 2025, 20, e0320379. [Google Scholar] [CrossRef]
- Wolff, D.G.; Christophersen, C.; Brown, S.M.; Mulcahey, M.K. Topical Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Phys. Sportsmed. 2021, 49, 381–391. [Google Scholar] [CrossRef]
- Sun, J.N.; Shan, Y.Z.; Wu, L.X.; Li, N.; Xu, F.H.; Kong, X.R.; Zhang, B. Preoperative High-Intensity Strength Training Combined with Balance Training Can Improve Early Outcomes After Total Knee Arthroplasty. J. Orthop. Surg. Res. 2023, 18, 692. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and Efficacy of Curcumin Versus Diclofenac in Knee Osteoarthritis: A Randomized Open-Label Parallel-Arm Study. Trials 2019, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Mathy-Hartert, M.; Jacquemond-Collet, I.; Priem, F.; Sanchez, C.; Lambert, C.; Henrotin, Y. Curcumin Inhibits Pro-Inflammatory Mediators and Metalloproteinase-3 Production by Chondrocytes. Inflamm. Res. 2009, 58, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Rahimnia, A.R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid Treatment for Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef]
- della Rocca, G.; Schievano, C.; Di Salvo, A.; Conti, M.B.; della Valle, M.F. Palmitoyl-Glucosamine Co-Micronized with Curcumin for Maintenance of Meloxicam-Induced Pain Relief in Dogs with Osteoarthritis Pain. BMC Vet. Res. 2023, 19, 1594. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Hou, Y.K.; Chen, M.W.; Yu, X.Z.; Chen, S.Y.; Yue, Y.R.; Guo, X.T.; Chen, J.X. A pH-Responsive Metal-Organic Framework for the Co-Delivery of HIF-2α siRNA and Curcumin for Enhanced Therapy of Osteoarthritis. J. Nanobiotechnol. 2023, 21, 6. [Google Scholar] [CrossRef]
- Hsueh, H.C.; Ho, G.R.; Tzeng, S.I.; Liang, K.H.; Horng, Y.S. Effects of Curcumin on Serum Inflammatory Biomarkers in Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Complement. Med. Ther. 2025, 25, 237. [Google Scholar] [CrossRef] [PubMed]
- Noori, E.; Atabaki, M.; Dehnavi, S.; Tavakol Afshari, J.; Mohammadi, M. The Immunomodulatory Effects of Curcumin on Forkhead Box O1 and MicroRNA-873 in Patients with Osteoarthritis. Iran. J. Allergy Asthma Immunol. 2024, 23, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Baharizade, M.; Ghetmiri, S.I.; Mohammady, M.; Mohammadi-Samani, S.; Yousefi, G. Revolutionizing Knee Osteoarthritis Treatment: Innovative Self-Nano-Emulsifying Polyethylene Glycol Organogel of Curcumin for Effective Topical Delivery. AAPS PharmSciTech 2024, 25, 80. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, Z.; Sajedi, F.; Mahboobian, M.M.; Mohammadi, M.; Ataei, S. Analgesic Effect of Curcumin Topical Formulation in Knee Osteoarthritis Patients: A Clinical Trial. J. Basic Clin. Physiol. Pharmacol. 2022, 34, 41–48. [Google Scholar] [CrossRef]
- Wan, Y.; Sun, W.; Yang, J.; Ren, J.; Kou, Q. The Comparison of Curcuminoid Formulations or Its Combination with Conventional Therapies Versus Conventional Therapies Alone for Knee Osteoarthritis. Clin. Rheumatol. 2022, 41, 2153–2169. [Google Scholar] [CrossRef]
- Khanna, A.; Das, S.S.; Smina, T.P.; Thomas, J.V.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M.; Mohanan, R. Curcumagalactomannoside/Glucosamine Combination Improved Joint Health Among Osteoarthritic Subjects as Compared to Chondroitin Sulfate/Glucosamine: Double-Blinded, Randomized Controlled Study. J. Altern. Complement. Med. 2020, 26, 945–955. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Jackson-Michel, S.; Fairchild, T. An Investigation into the Effects of a Curcumin Extract (Curcugen®) on Osteoarthritis Pain of the Knee: A Randomised, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 14, 41. [Google Scholar] [CrossRef]
- Liu, X.; Robbins, S.; Eyles, J.; Fedorova, T.; Virk, S.; Deveza, L.A.; McLachlan, A.J.; Hunter, D.J. Efficacy and Safety of a Supplement Combination on Hand Pain Among People with Symptomatic Hand Osteoarthritis: An Internet-Based, Randomised Clinical Trial—The RADIANT Study. Osteoarthr. Cartil. 2021, 29, 667–677. [Google Scholar] [CrossRef]
- Thomas, J.V.; Smina, T.P.; Khanna, A.; Kunnumakkara, A.B.; Maliakel, B.; Mohanan, R.; Krishnakumar, I.M. Influence of a Low-Dose Supplementation of Curcumagalactomannoside Complex (CurQfen) in Knee Osteoarthritis: A Randomized, Open-Labeled, Active-Controlled Clinical Trial. Phytother. Res. 2021, 35, 1443–1455. [Google Scholar] [CrossRef]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.P. Global Asthma Prevalence in Adults: Findings from the Cross-Sectional World Health Survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.G.; Vogelmeier, C.F.; Agusti, A. Lung Health for All: Chronic Obstructive Lung Disease and World Lung Day 2022. Am. J. Respir. Crit. Care Med. 2022, 206, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Cukic, V.; Lovre, V.; Dragisic, D.; Ustamujic, A. Asthma and Chronic Obstructive Pulmonary Disease (COPD)–Differences and Similarities. Mater. Socio-Med. 2012, 24, 100–105. [Google Scholar] [CrossRef]
- Papi, A.; Blasi, F.; Canonica, G.W.; Morandi, L.; Richeldi, L.; Rossi, A. Treatment Strategies for Asthma: Reshaping the Concept of Asthma Management. Allergy Asthma Clin. Immunol. 2020, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Calhoun, W.J. The Role of Leukotrienes in Airway Inflammation. J. Allergy Clin. Immunol. 2006, 118, 789–798. [Google Scholar] [CrossRef]
- Garzon-Siatoya, W.T.; Carrillo-Martin, I.; Chiarella, S.E.; Gonzalez-Estrada, A. State-of-the-Art Beta-Adrenoreceptor Agonists for the Treatment of Asthma. Expert Opin. Pharmacother. 2022, 23, 243–254. [Google Scholar] [CrossRef]
- Eisner, M.D.; Balmes, J.; Katz, P.P.; Trupin, L.; Yelin, E.H.; Blanc, P.D. Lifetime Environmental Tobacco Smoke Exposure and the Risk of Chronic Obstructive Pulmonary Disease. Environ. Health 2005, 4, 22. [Google Scholar] [CrossRef]
- Agustí, A. Systemic Effects of Chronic Obstructive Pulmonary Disease: What We Know and What We Don’t Know (But Should). Proc. Am. Thorac. Soc. 2007, 4, 522–525. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Stolz, D.; Matera, M.G. Pharmacological Treatment and Current Controversies in COPD. F1000Research 2019, 8, F1000 Faculty Rev-1533. [Google Scholar] [CrossRef]
- Matera, M.G.; Cazzola, M.; Page, C. Prospects for COPD Treatment. Curr. Opin. Pharmacol. 2021, 56, 74–84. [Google Scholar] [CrossRef]
- Page, C.; Cazzola, M. Bifunctional Drugs for the Treatment of Asthma and Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 2014, 44, 475–482. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Comparative Effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: Cohort Study in Real-World Clinical Practice. Chest 2020, 157, 846–855. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Calverley, P.M.; Rabe, K.F. Roflumilast: A Review of Its Use in the Treatment of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Niti, M.; Yap, K.B.; Tan, W.C. Curcumins-Rich Curry Diet and Pulmonary Function in Asian Older Adults. PLoS ONE 2012, 7, e51753. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Tesse, A.; Grossini, E.; Tamma, G.; Brenner, C.; Portincasa, P.; Marinelli, R.A.; Calamita, G. Aquaporins as Targets of Dietary Bioactive Phytocompounds. Front. Mol. Biosci. 2018, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Shahid, H.; Shahzad, M.; Shabbir, A.; Saghir, G. Immunomodulatory and Anti-Inflammatory Potential of Curcumin for the Treatment of Allergic Asthma: Effects on Expression Levels of Pro-Inflammatory Cytokines and Aquaporins. Inflammation 2019, 42, 2037–2047. [Google Scholar] [CrossRef]
- Abidi, A.; Gupta, S.; Agarwal, M.; Bhalla, H.L.; Saluja, M. Evaluation of Efficacy of Curcumin as an Add-On Therapy in Patients of Bronchial Asthma. J. Clin. Diagn. Res. 2014, 8, HC19–HC24. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, Against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lv, J.N.; Li, H.; Jiao, B.; Zhang, Q.H.; Zhang, Y.; Li, Y.L. Curcumin Reduces Lung Inflammation via Wnt/β-Catenin Signaling in Mouse Model of Asthma. J. Asthma 2017, 54, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Safari, S.; Davoodi, P.; Soltani, A.; Fadavipour, M.; Rezaeian, A.; Heydari, F.; Akhlaghdoust, M. Curcumin Effects on Chronic Obstructive Pulmonary Disease: A Systematic Review. Health Sci. Rep. 2023, 6, e1145. [Google Scholar] [CrossRef] [PubMed]
- Belvisi, M.G.; Mitchell, J.A. Targeting PPAR Receptors in the Airway for the Treatment of Inflammatory Lung Disease. Br. J. Pharmacol. 2009, 158, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, R.; Wu, H.; Abudusalamu, A.; Ding, W.; Li, D.; Wei, X.; Niu, L. The Crucial Role of the PPAR Signaling Pathway in the Diagnosis and Treatment of Chronic Obstructive Pulmonary Disease: An Analysis of Gene Expression and Macrophage Polarization. Int. J. Chron. Obstruct. Pulmon. Dis. 2025, 20, 2287–2304. [Google Scholar]
- Beigoli, S.; Kiani, S.; Asgharzadeh, F.; Memarzia, A.; Boskabady, M.H. Promising Role of Peroxisome Proliferator-Activated Receptors in Respiratory Disorders: A Review. Drug Metab. Rev. 2025, 57, 26–50. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, D. Effect of Curcumin on Nasal Symptoms and Airflow in Patients with Perennial Allergic Rhinitis. Ann. Allergy Asthma Immunol. 2016, 117, 697–702.e1. [Google Scholar] [CrossRef]
- Eshaghi Ghalibaf, M.H.; Taghavi Zadeh Yazdi, M.E.; Mansourian, M.; Mohammadian Roshan, N.; Boskabady, M.H. Evaluation of the Protective Effect of Curcuma longa and PPARγ Agonist, Pioglitazone on Paraquat-Induced Lung Injury in Rats. Immun. Inflamm. Dis. 2024, 12, e70001. [Google Scholar] [CrossRef]
- Reis, R.E.N.G.I.N.; Orak, D.; Yilmaz, D.; Cimen, H.; Sipahi, H. Modulation of Cigarette Smoke Extract-Induced Human Bronchial Epithelial Damage by Eucalyptol and Curcumin. Hum. Exp. Toxicol. 2021, 40, 1445–1462. [Google Scholar] [CrossRef]
- Miryan, M.; Soleimani, D.; Askari, G.; Jamialahmadi, T.; Guest, P.C.; Bagherniya, M.; Sahebkar, A. Curcumin and Piperine in COVID-19: A Promising Duo to the Rescue? Adv. Exp. Med. Biol. 2021, 1327, 197–204. [Google Scholar] [CrossRef]
- Askari, G.; Bagherniya, M.; Kiani, Z.; Alikiaii, B.; Mirjalili, M.; Shojaei, M.; Hassanizadeh, S.; Vajdi, M.; Feizi, A.; Majeed, M.; et al. Evaluation of Curcumin-Piperine Supplementation in COVID-19 Patients Admitted to the Intensive Care: A Double-Blind, Randomized Controlled Trial. Adv. Exp. Med. Biol. 2023, 1412, 413–426. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Jaiswal, A.; Subhashini; Singh, R. Combination Therapy with Curcumin Alone Plus Piperine Ameliorates Ovalbumin-Induced Chronic Asthma in Mice. Inflammation 2018, 41, 1922–1933. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghanei, M.; Hajhashemi, A.; Sahebkar, A. Effects of Curcuminoids-Piperine Combination on Systemic Oxidative Stress, Clinical Symptoms and Quality of Life in Subjects with Chronic Pulmonary Complications Due to Sulfur Mustard: A Randomized Controlled Trial. J. Diet. Suppl. 2016, 13, 93–105. [Google Scholar] [CrossRef]
- Manarin, G.; Anderson, D.; Silva, J.M.E.; Coppede, J.D.S.; Roxo-Junior, P.; Pereira, A.M.S.; Carmona, F. Curcuma longa L. Ameliorates Asthma Control in Children and Adolescents: A Randomized, Double-Blind, Controlled Trial. J. Ethnopharmacol. 2019, 238, 111882. [Google Scholar] [CrossRef]
- Zare’i, M.; Rabieepour, M.; Ghareaghaji, R.; Zarrin, R.; Faghfouri, A.H. Nanocurcumin Supplementation Improves Pulmonary Function in Severe COPD Patients: A Randomized, Double Blind, and Placebo-Controlled Clinical Trial. Phytother. Res. 2024, 38, 1224–1234. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Soufi, M.; Sattler, A.M.; Maisch, B.; Schaefer, J.R. Molecular Mechanisms Involved in Atherosclerosis. Herz 2002, 27, 637–648. [Google Scholar] [CrossRef]
- Cho, Y.; Baldán, A. Quest for New Biomarkers in Atherosclerosis. Mo. Med. 2013, 110, 325–330. [Google Scholar] [PubMed]
- Deng, H.; Qiu, W.; Zhang, Y.; Hua, J. Extracellular Vesicles in Atherosclerosis Cardiovascular Disease: Emerging Roles and Mechanisms. Front. Cardiovasc. Med. 2025, 12, 1611557. [Google Scholar] [CrossRef] [PubMed]
- Kowara, M.; Cudnoch-Jedrzejewska, A. Pathophysiology of Atherosclerotic Plaque Development—Contemporary Experience and New Directions in Research. Int. J. Mol. Sci. 2021, 22, 3513. [Google Scholar] [CrossRef]
- Hondros, C.A.B.; Hanseth, S.; Solvik, M.; Pedersen, E.K.R.; Khan, I.; Hovland, S.; Larsen, T.H.; Lønnebakken, M.T. Age-Stratified Differences in Coronary Artery Plaque Phenotypes in Women and Men with Non-Obstructive Coronary Artery Disease. Open Heart 2025, 12, e003371. [Google Scholar] [CrossRef]
- Yao, Z.; Tasdighi, E.; Dardari, Z.A.; Jha, K.K.; Osuji, N.; Rajan, T.; Boakye, E.; Rodriguez, C.J.; Matsushita, K.; Simonsick, E.M.; et al. Association Between Cigarette Smoking and Subclinical Markers of Cardiovascular Harm. J. Am. Coll. Cardiol. 2025, 85, 1018–1034. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, J. Hypertriglyceridemia (>150 mg/dL) as a Marker of Cardiovascular Risk. Clin. Investig. Arterioscler. 2025, 37 (Suppl. S1), 500822. [Google Scholar] [CrossRef] [PubMed]
- Padro, T.; Muñoz-Garcia, N.; Badimon, L. The role of triglycerides in the origin and progression of atherosclerosis. Clin Investig Arterioscler. 2021, 33 (Suppl. S2), 20–28, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Pintó, X.; Fanlo, M.; Esteve, V.; Millán, J.; Grupo de Trabajo Dislipemia Aterogénica, Sociedad Española de Arteriosclerosis (SEA). Remnant Cholesterol, Vascular Risk, and Prevention of Atherosclerosis. Clin. Investig. Arterioscler. 2023, 35, 206–217. [Google Scholar] [CrossRef]
- Rayani, A.; Sharma, G.; Spitz, J.A. Atherosclerotic Cardiovascular Disease Risk Estimates Using the New Predicting Risk of Cardiovascular Disease Events Equations: Implications for Statin Use. Curr. Cardiol. Rep. 2025, 27, 107. [Google Scholar] [CrossRef]
- Hamasaki, M.; Kotani, K. Lipoprotein(a)-Lowering Drugs: A Mini Review. J. Clin. Med. Res. 2025, 17, 181–186. [Google Scholar] [CrossRef]
- Li, X.; Ding, H.; Feng, G.; Huang, Y. Role of Angiotensin Converting Enzyme in Pathogenesis Associated with Immunity in Cardiovascular Diseases. Life Sci. 2024, 352, 122903. [Google Scholar] [CrossRef]
- Shohrati, M.; Abedi, F.; Bagheri, M.; Sahebkar, A. Effects of Curcumin on Vascular Smooth Muscle Cells: Implications for Health and Disease. Pharmacol. Rep. 2025, 77, 1232–1246. [Google Scholar] [CrossRef]
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 2021, 26, 4036. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, T.; Wang, X.; Wei, X.; Chen, Y.; Guo, L.; Zhang, J.; Wang, C. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and Its Signaling Pathways. Cell. Physiol. Biochem. 2015, 36, 631–641. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhong, Y.; Li, Z.; Tang, J.; Pi, C.; Zheng, W.; Shi, P.; Zuo, Y.; Jiang, J.; Yang, Y.; et al. Anti-Atherosclerosis Effect and Mechanism of a Novel Curcumin Analogue CACN136: Regulating Macrophage M1/M2 Polarization and Lipid Metabolism. Front. Pharmacol. 2025, 16, 1632647. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Nikfar, B.; Chaichian, S.; Ekhlasi-Hundrieser, M. Curcumin as a Potential Modulator of M1 and M2 Macrophages: New Insights in Atherosclerosis Therapy. Heart Fail. Rev. 2019, 24, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y.; et al. Curcumin Induces M2 Macrophage Polarization by Secretion of IL-4 and/or IL-13. J. Mol. Cell. Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Um, H.J.; Cho, K.H.; Kwon, T.K. Curcumin Inhibits oxLDL-Induced CD36 Expression and Foam Cell Formation Through the Inhibition of p38 MAPK Phosphorylation. Food Chem. Toxicol. 2013, 58, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Y.; Zhou, J.; Guo, N.; Ma, W.G.; Huang, X.; Wang, H.; Yuan, Z.Y. Curcumin Retunes Cholesterol Transport Homeostasis and Inflammation Response in M1 Macrophage to Prevent Atherosclerosis. Biochem. Biophys. Res. Commun. 2015, 467, 872–878. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.; Li, F.; Ding, H.; Zhang, Q.; Yu, F.; Zhang, H.; Hao, Y.; Liu, B.; Jiang, Y. Biomimetic Nanocomplexes Loading with Evolocumab and Curcumin for Synergistic Anti-Atherosclerosis Therapy in ApoE¯/¯ Mice. J. Nanobiotechnol. 2025, 23, 412. [Google Scholar] [CrossRef]
- Shamsi, M.; Mohammadzadeh, G.; Hatami, M.; Roshanazadeh, M.; Noor-Behbahani, M.; Rashidi, M. Dual Modulation of Canonical and Non-Canonical TGF-β/ROS/Erk1/2 Pathways: Synergistic Activation of Nrf-2 and Antioxidant Enzymes (SOD1, GPx, HO-1) by Quercetin Loaded in Solid Lipid Nanoparticles and Curcumin in Atherosclerosis Therapy. Iran J. Pharm. Res. 2024, 23, e151428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zou, J.; Li, P.; Zheng, X.; Feng, D. Curcumin Protects Against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-Like Receptor 4 Expression. J. Agric. Food Chem. 2018, 66, 449–456. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef]
- Cheng, M.; Ding, F.; Li, L.; Dai, C.; Sun, X.; Xu, J.; Chen, F.; Li, M.; Li, X. Exploring the Role of Curcumin in Mitigating Oxidative Stress to Alleviate Lipid Metabolism Disorders. Front. Pharmacol. 2025, 16, 1517174. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. The Effect of Curcumin on Reducing Atherogenic Risks in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2441. [Google Scholar] [CrossRef] [PubMed]
- Dastani, M.; Rahimi, H.R.; Askari, V.R.; Jaafari, M.R.; Jarahi, L.; Yadollahi, A.; Rahimi, V.B. Three Months of Combination Therapy with Nano-Curcumin Reduces the Inflammation and Lipoprotein (a) in Type 2 Diabetic Patients with Mild to Moderate Coronary Artery Disease: Evidence of a Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Biofactors 2023, 49, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Imaizumi, A.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Komiyama, M.; Wada, H.; et al. Highly Absorptive Curcumin Reduces Serum Atherosclerotic Low-Density Lipoprotein Levels in Patients with Mild COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2029–2034. [Google Scholar] [CrossRef]
- Kaplan, G.G. The Global Burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Karban, A.; Eliakim, R. Effect of Smoking on Inflammatory Bowel Disease: Is It Disease or Organ Specific? World J. Gastroenterol. 2007, 13, 2150–2152. [Google Scholar] [CrossRef]
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastián, V.P.; Álvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef]
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-Based Clinical Practice Guidelines for Inflammatory Bowel Disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic Intestinal Inflammation: Inflammatory Bowel Disease and Colitis-Associated Colon Cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef]
- Meng, Z.W.; Chang, B.; Sang, L.X. Use of Curcumin and Its Nanopreparations in the Treatment of Inflammatory Bowel Disease. World J. Gastroenterol. 2024, 30, 280–282. [Google Scholar] [CrossRef]
- Hanai, H.; Sugimoto, K. Curcumin Has Bright Prospects for the Treatment of Inflammatory Bowel Disease. Curr. Pharm. Des. 2009, 15, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Joseph, N.; Venkataranganna, M.V.; Saxena, A.; Ponemone, V.; Fayad, R. Curcumin, an Active Component of Turmeric in the Prevention and Treatment of Ulcerative Colitis: Preclinical and Clinical Observations. Food Funct. 2012, 3, 1109–1117. [Google Scholar] [CrossRef]
- Wang, J.B.; Qi, L.L.; Zheng, S.D.; Wu, T.X. Curcumin Induces Apoptosis Through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B 2009, 10, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Y.; Wu, T.T.; Huang, J.Q.; Kang, Z.P.; Wang, M.X.; Zhong, Y.B.; Ge, W.; Zhou, B.G.; Zhao, H.M.; Wang, H.Y.; et al. Curcumin Alleviates Experimental Colitis via a Potential Mechanism Involving Memory B Cells and Bcl-6-Syk-BLNK Signaling. World J. Gastroenterol. 2022, 28, 5865–5880. [Google Scholar] [CrossRef]
- Huang, J.; Wu, T.; Zhong, Y.; Huang, J.; Kang, Z.; Zhou, B.; Zhao, H.; Liu, D. Effect of Curcumin on Regulatory B Cells in Chronic Colitis Mice Involving TLR/MyD88 Signaling Pathway. Phytother. Res. 2023, 37, 731–742. [Google Scholar] [CrossRef]
- Song, L.; Deng, Y.; Huang, J.; Zhu, X.; Zhong, Y.; Zhong, Q.; Zhou, W.; Liu, Y.; Zhao, H.; Ge, W.; et al. Effect of Curcumin Regulated Memory Th17 Cells in Mice with DSS-Induced Colitis. Int. Immunopharmacol. 2025, 145, 113770. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Ge, W.; Liu, S.Q.; Long, J.; Jiang, Q.Q.; Zhou, W.; Zuo, Z.Y.; Liu, D.Y.; Zhao, H.M.; Zhong, Y.B. Curcumin Inhibits T Follicular Helper Cell Differentiation in Mice with Dextran Sulfate Sodium (DSS)-Induced Colitis. Am. J. Chin. Med. 2022, 50, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Xu, R.; Huang, X.Y.; Cheng, S.M.; Huang, M.F.; Yue, H.Y.; Wang, X.; Zou, Y.; Lu, A.P.; Liu, D.Y. Curcumin Suppressed Activation of Dendritic Cells via JAK/STAT/SOCS Signal in Mice with Experimental Colitis. Front. Pharmacol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Y.; Zhang, L.; Liu, M.; Yang, J.; Huang, F.; Wang, Y.; Huang, L. Curcumin Analog C66 Alleviates Inflammatory Colitis by Inhibiting the Activation of NF-κB. Inflammopharmacology 2022, 30, 2167–2179. [Google Scholar] [CrossRef]
- Gong, Z.; Zhao, S.; Zhou, J.; Yan, J.; Wang, L.; Du, X.; Li, H.; Chen, Y.; Cai, W.; Wu, J. Curcumin Alleviates DSS-Induced Colitis via Inhibiting NLRP3 Inflammasome Activation and IL-1β Production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, W.; Zhou, X.; Zhu, X.; Suo, F.; Yao, S. The Effectiveness and Safety of Curcumin as a Complementary Therapy in Inflammatory Bowel Disease: A Protocol of Systematic Review and Meta-Analysis. Medicine 2020, 99, e22916. [Google Scholar] [CrossRef]
- Shahinfar, H.; Payandeh, N.; ElhamKia, M.; Abbasi, F.; Alaghi, A.; Djafari, F.; Eslahi, M.; Gohari, N.S.F.; Ghorbaninejad, P.; Hasanzadeh, M.; et al. Administration of Dietary Antioxidants for Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Complement. Ther. Med. 2021, 63, 102787. [Google Scholar] [CrossRef]
- Fallahi, F.; Borran, S.; Ashrafizadeh, M.; Zarrabi, A.; Pourhanifeh, M.H.; Mahabady, M.K.; Sahebkar, A.; Mirzaei, H. Curcumin and Inflammatory Bowel Diseases: From In Vitro Studies to Clinical Trials. Mol. Immunol. 2021, 130, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ghoshal, U.C.; Saigal, V.M.; Chaudhary, M.; Sahu, S.; Pandya, V.; Ghoshal, U. A Randomized Double-Blind, Placebo-Controlled Trial of Artesunate and Curcumin in Patients With Crohn’s Disease: A Pilot Study. JGH Open 2025, 9, e70211. [Google Scholar] [CrossRef]
- Lang, A.; Salomon, N.; Wu, J.C.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.L.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449.e1. [Google Scholar] [CrossRef] [PubMed]
- Alt, F.; Chong, P.W.; Teng, E.; Uebelhack, R. Evaluation of Benefit and Tolerability of IQP-CL-101 (Xanthofen) in the Symptomatic Improvement of Irritable Bowel Syndrome: A Double-Blinded, Randomised, Placebo-Controlled Clinical Trial. Phytother. Res. 2017, 31, 1056–1062. [Google Scholar] [CrossRef]
- Sadeghi, N.; Mansoori, A.; Shayesteh, A.; Hashemi, S.J. The Effect of Curcumin Supplementation on Clinical Outcomes and Inflammatory Markers in Patients with Ulcerative Colitis. Phytother. Res. 2020, 34, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- da Paz Martins, A.S.; de Araújo, O.R.P.; da Silva Gomes, A.; Araujo, F.L.C.; Júnior, J.O.; de Vasconcelos, J.K.G.; Rodrigues Junior, J.I.; Cerqueira, I.T.; Neto, M.Á.F.L.; Bueno, N.B.; et al. Curcumin Plus Piperine Improves Body Composition in Patients with Inflammatory Bowel Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Eur. J. Nutr. 2025, 64, 90. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ikeya, K.; Bamba, S.; Andoh, A.; Yamasaki, H.; Mitsuyama, K.; Nasuno, M.; Tanaka, H.; Matsuura, A.; Kato, M.; et al. Highly Bioavailable Curcumin Derivative Ameliorates Crohn’s Disease Symptoms: A Randomized, Double-Blind, Multicenter Study. J. Crohns Colitis 2020, 14, 1693–1701. [Google Scholar] [CrossRef]
- Banerjee, R.; Pal, P.; Penmetsa, A.; Kathi, P.; Girish, G.; Goren, I.; Reddy, D.N. Novel Bioenhanced Curcumin With Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-to-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-Controlled Pilot Study. J. Clin. Gastroenterol. 2021, 55, 702–708. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Salomon, N.; Karampekos, G.; Viazis, N.; Lahat, A.; Ungar, B.; Eliakim, R.; Kuperstein, R.; Kriger-Sharabi, O.; Reiss-Mintz, H.; et al. Curcumin-QingDai Combination for Patients With Active Ulcerative Colitis: A Randomized, Double-Blinded, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2024, 22, 347–356.e6. [Google Scholar] [CrossRef]
- Erol Doğan, Ö.; Karaca Çelik, K.E.; Baş, M.; Alan, E.H.; Çağın, Y.F. Effects of Mediterranean Diet, Curcumin, and Resveratrol on Mild-to-Moderate Active Ulcerative Colitis: A Multicenter Randomized Clinical Trial. Nutrients 2024, 16, 1504. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The Evolution in Definition, Pathophysiology, and Management. SAGE Open Med. 2019, 7, 2050312119835043. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6429642/ (accessed on 23 September 2025). [CrossRef] [PubMed]
- Li, Y.; Ren, S.; Zhou, S. Advances in Sepsis Research: Insights into Signaling Pathways, Organ Failure, and Emerging Intervention Strategies. Exp. Mol. Pathol. 2025, 142, 104963. [Google Scholar] [CrossRef]
- Richards, G.A.; Zamparini, J.; Kalla, I.; Laher, A.; Murray, L.W.; Shaddock, E.J.; Stacey, S.; Venter, W.F.; Feldman, C. Critical Illness Due to Infection in People Living With HIV. Lancet HIV 2024, 11, e406–e418. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Basodan, N.; Al Mehmadi, A.E.; Aldawood, S.M.; Hawsawi, A.; Fatini, F.; Mulla, Z.M.; Nawwab, W.; Alshareef, A.; Almhmadi, A.H.; Ahmed, A.; et al. Septic Shock: Management and Outcomes. Cureus 2022, 14, e32894. [Google Scholar] [CrossRef]
- Gauer, R.L. Early Recognition and Management of Sepsis in Adults: The First Six Hours. Am. Fam. Physician 2013, 88, 44–53. Available online: https://www.aafp.org/pubs/afp/issues/2013/0701/p44.html (accessed on 23 September 2025).
- Aşuroğlu, T.; Oğul, H. A deep learning approach for sepsis monitoring via severity score estimation. Comput Methods Programs Biomed. 2021, 198, 105816. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.M.; Caetano, M.A.F.; de Carvalho, M.T.; Dos Santos Arruda, F.; Tomé, F.D.; de Oliveira, J.F.; Soave, D.F.; Pereira, J.X.; Celes, M.R.N. Impacts of Curcumin Treatment on Experimental Sepsis: A Systematic Review. Oxid. Med. Cell. Longev. 2023, 2023, 2252213. [Google Scholar] [CrossRef]
- Sundar, J.P.; Calemine, J.; Seth, P.; Sidhu, G.S.; Maheshwari, R.K. Differential Regulation of Cytokines and Transcription Factors in Liver by Curcumin Following Hemorrhage/Resuscitation. Shock 2003, 19, 150–156. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chai, Y.S.; Xie, K.; Yu, F.; Wang, C.J.; Lin, S.H.; Yang, Y.Z.; Xu, F. Curcumin Promotes the Expression of IL-35 by Regulating Regulatory T Cell Differentiation and Restrains Uncontrolled Inflammation and Lung Injury in Mice. Inflammation 2020, 43, 1913–1924. [Google Scholar] [CrossRef]
- Zhao, G.J.; Lu, Z.Q.; Tang, L.M.; Wu, Z.S.; Wang, D.W.; Zheng, J.Y.; Qiu, Q.M. Curcumin Inhibits Suppressive Capacity of Naturally Occurring CD4+CD25+ Regulatory T Cells in Mice In Vitro. Int. Immunopharmacol. 2012, 14, 99–106. [Google Scholar] [CrossRef]
- Wang, J.; Ioan-Facsinay, A.; van der Voort, E.I.H.; Huizinga, T.W.J.; Toes, R.E.M. Transient Expression of FOXP3 in Human Activated Nonregulatory CD4+ T Cells. Eur. J. Immunol. 2007, 37, 129–138. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Wu, W.; Lin, S.; Zhang, N.; Wang, H.; Tan, S.; Lin, P.; Chen, X.; Wu, L.; et al. Effects of FM0807, a Novel Curcumin Derivative, on Lipopolysaccharide-Induced Inflammatory Factor Release via the ROS/JNK/p53 Pathway in RAW264.7 Cells. Biosci. Rep. 2018, 38, BSR20180702. [Google Scholar] [CrossRef]
- Yang, T.; Yu, H.; Xie, Z. Curcumin-Induced Exosomal FTO from Bone Marrow Stem Cells Alleviates Sepsis-Associated Acute Kidney Injury by Modulating the m6A Methylation of OXSR1. Kaohsiung J. Med. Sci. 2025, 41, e12923. [Google Scholar] [CrossRef]
- Chen, K.; Meng, Z.; Min, J.; Wang, J.; Li, Z.; Gao, Q.; Hu, J. Curcumin Alleviates Septic Lung Injury in Mice by Inhibiting TXNIP/TRX-1/GPX4-Mediated Ferroptosis. Nan Fang Yi Ke Da Xue Xue Bao 2024, 44, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Shi, Q.; Yang, J.; Ren, H.; Zhang, L.; Chen, S.; Si, J.; Liu, Y.; Sha, D.; Xu, B.; et al. Ceria Nanozyme Coordination With Curcumin for Treatment of Sepsis-Induced Cardiac Injury by Inhibiting Ferroptosis and Inflammation. J. Adv. Res. 2024, 63, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Zeng, C.; Liu, Y.; Pan, H.; Ke, C. J147 Ameliorates Sepsis-Induced Depressive-Like Behaviors in Mice by Attenuating Neuroinflammation Through Regulating the TLR4/NF-κB Signaling Pathway. J. Mol. Histol. 2023, 54, 725–738. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.; Wang, D.; Sun, Y.; Wang, Q.; Bu, Y.; Niu, F. Curcumin Reduces LPS-Induced Septic Acute Kidney Injury Through Suppression of lncRNA PVT1 in Mice. Life Sci. 2020, 254, 117340. [Google Scholar] [CrossRef]
- Liu, W.; Guo, W.; Zhu, Y.; Peng, S.; Zheng, W.; Zhang, C.; Shao, F.; Zhu, Y.; Hang, N.; Kong, L.; et al. Targeting Peroxiredoxin 1 by a Curcumin Analogue, AI-44, Inhibits NLRP3 Inflammasome Activation and Attenuates Lipopolysaccharide-Induced Sepsis in Mice. J. Immunol. 2018, 201, 2403–2413. [Google Scholar] [CrossRef]
- Gong, Z.; Zhou, J.; Li, H.; Gao, Y.; Xu, C.; Zhao, S.; Chen, Y.; Cai, W.; Wu, J. Curcumin Suppresses NLRP3 Inflammasome Activation and Protects Against LPS-Induced Septic Shock. Mol. Nutr. Food Res. 2015, 59, 2132–2142. [Google Scholar] [CrossRef]
- Ma, F.; Liu, F.; Ding, L.; You, M.; Yue, H.; Zhou, Y.; Hou, Y. Anti-Inflammatory Effects of Curcumin Are Associated With Down Regulating microRNA-155 in LPS-Treated Macrophages and Mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lu, D.; Zou, Y.; Zhou, C.; Liu, H.; Tu, C.; Li, F.; Liu, L.; Zhang, S. Curcumin Protects Against Intestinal Origin Endotoxemia in Rat Liver Cirrhosis by Targeting PCSK9. J. Food Sci. 2017, 82, 772–780. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin Alleviates Lipopolysaccharide-Induced Sepsis and Liver Failure by Suppression of Oxidative Stress-Related Inflammation via PI3K/AKT and NF-κB Related Signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef]
- Rana, M.; Maurya, P.; Reddy, S.S.; Singh, V.; Ahmad, H.; Dwivedi, A.K.; Dikshit, M.; Barthwal, M.K. A Standardized Chemically Modified Curcuma longa Extract Modulates IRAK-MAPK Signaling in Inflammation and Potentiates Cytotoxicity. Front. Pharmacol. 2016, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Mahmoodpoor, A.; Kooshki, F.; Niazkar, H.R.; Shoorei, H.; Tarighat-Esfanjani, A. Effects of Nanocurcumin on Inflammatory Factors and Clinical Outcomes in Critically Ill Patients With Sepsis: A Pilot Randomized Clinical Trial. Eur. J. Integr. Med. 2020, 36, 101122. [Google Scholar] [CrossRef]
- Karimi, A.; Naeini, F.; Niazkar, H.R.; Tutunchi, H.; Musazadeh, V.; Mahmoodpoor, A.; Asghariazar, V.; Mobasserie, M.; Tarighat-Esfanjani, A. Nano-Curcumin Supplementation in Critically Ill Patients With Sepsis: A Randomized Clinical Trial Investigating the Inflammatory Biomarkers, Oxidative Stress Indices, Endothelial Function, Clinical Outcomes and Nutritional Status. Food Funct. 2022, 13, 6596–6612. [Google Scholar] [CrossRef]
- Alikiaii, B.; Khatib, N.; Badpeyma, M.; Hasanzadeh, E.; Abbasi, S.; Amini, S.; Kiani, Z.; Hassanizadeh, S.; Iraj, Z.; Sahebkar, A.; et al. Therapeutic Effects of Curcumin and Piperine Combination in Critically Ill Patients With Sepsis: A Randomized Double-Blind Controlled Trial. Trials 2025, 26, 205. [Google Scholar] [CrossRef]
- Musso, G.; Alberto, M.; Mariano, F.; Cassader, M.; De Michieli, F.; Riva, A.; Petrangolini, G.; Togni, S.; Pinach, S. Impaired Postprandial GLP-2 Response Enhances Endotoxemia, Systemic Inflammation, and Kidney Injury in MASH: Effect of Phospholipid Curcumin Meriva. Gut Microbes 2024, 16, 2424907. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Ghodsi, R.; Kooshki, F.; Karimi, M.; Asghariazar, V.; Tarighat-Esfanjani, A. Therapeutic Effects of Curcumin on Sepsis and Mechanisms of Action: A Systematic Review of Preclinical Studies. Phytother. Res. 2019, 33, 2798–2820. [Google Scholar] [CrossRef]
- Naeini, F.; Tutunchi, H.; Razmi, H.; Mahmoodpoor, A.; Vajdi, M.; Azar, P.S.; Najifipour, F.; Tarighat-Esfanjani, A.; Karimi, A. Does Nano-Curcumin Supplementation Improve Hematological Indices in Critically Ill Patients With Sepsis? A Randomized Controlled Clinical Trial. J. Food Biochem. 2022, 46, e14093. [Google Scholar] [CrossRef]
- Karimi, A.; Pourreza, S.; Vajdi, M.; Mahmoodpoor, A.; Sanaie, S.; Karimi, M.; Tarighat-Esfanjani, A. Evaluating the Effects of Curcumin Nanomicelles on Clinical Outcome and Cellular Immune Responses in Critically Ill Sepsis Patients: A Randomized, Double-Blind, and Placebo-Controlled Trial. Front. Nutr. 2022, 9, 1037861. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, S.C.; Hsu, S.Y.; Lin, Y.A.; Shih, C.M.; Huang, C.Y.; Wang, K.H.; Lee, A.W. Annoying Psoriasis and Atopic Dermatitis: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 4898. [Google Scholar] [CrossRef]
- Wilsmann-Theis, D.; Hagemann, T.; Jordan, J.; Bieber, T.; Novak, N. Facing Psoriasis and Atopic Dermatitis: Are There More Similarities or More Differences? Eur. J. Dermatol. 2008, 18, 172–180. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and Clinical Features of Psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Blauvelt, A.; Callis Duffin, K.; Huang, Y.H.; Savage, L.J.; Guo, L.; Merola, J.F. Psoriasis. Nat. Rev. Dis. Primers 2025, 11, 45. [Google Scholar] [CrossRef]
- Sharma, A.; Dhiman, S.; Singh, T.G.; Bhatia, R.; Awasthi, A. Psoriasis Unveiled: The Cellular Ballet, Molecular Symphony, and Genetic Puzzle. Int. Immunopharmacol. 2025, 161, 115021. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F., IV; Billi, A.C.; Maverakis, E.; Tsoi, L.C.; Gudjonsson, J.E. Novel insights into atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1145–1154. [Google Scholar] [CrossRef]
- Prados-Carmona, A.; Husein-ElAhmed, H.; Navarro-Triviño, F.J.; Ruiz-Villaverde, R. Atopic Dermatitis Beyond Eczema: A Review on Its Systemic Impact Through Pruritus and Associated Comorbidities. Clin. Rev. Allergy Immunol. 2025, 68, 66. [Google Scholar] [CrossRef]
- Lo, Y.; Cheng, T.T.; Huang, C.J.; Cheng, Y.C.; Chyuan, I.T. Advancing Therapeutic Strategies in Atopic Dermatitis: Emerging Targets and Personalized Approaches. Biomolecules 2025, 15, 838. [Google Scholar] [CrossRef]
- Monis, M.; Mathur, P.; Ritu; Mishra, A.K.; Rani, L. Comprehensive Insights into Psoriasis: Pathophysiology, an Advanced Exploration of Current Landscape and Future Prospects in “Therapeutic Strategies”. Recent Adv. Inflamm. Allergy Drug Discov. 2025, 19, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.S.Q.; Sibbald, R.G. Cutaneous Psoriasis: Clinical Aspects and Treatments. Adv. Skin Wound Care 2025, 38, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kupschke, E.; Schenk, M. The Myeloid Switch: Immune Drivers in Atopic Dermatitis—Roles in Pathogenesis and Emerging Therapeutic Targeting. Front. Immunol. 2025, 16, 1608338. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Pithadia, D.J.; Lee, E.B.; Liao, W.; Wu, J.J. Safety and Effectiveness of Anti-Tumor Necrosis Factor-Alpha Biosimilar Agents in the Treatment of Psoriasis. Am. J. Clin. Dermatol. 2020, 21, 483–491. [Google Scholar] [CrossRef]
- Fallahi, N.; Rafiee, M.; Hosseini, S.S.; Sereshki, N.; Anani Sarab, G.; Erfanian, N. Short-Chain Fatty Acids and Their Role in Modulating Autoimmune Responses in Psoriasis: Insights From Recent Microbiota Research. Lett. Appl. Microbiol. 2025, 78, ovaf091. [Google Scholar] [CrossRef]
- Pachauri, A.; Sharma, S. Unravelling the Gut-Skin Axis: The Role of Gut Microbiota in Pathogenesis and Management of Psoriasis. Inflammopharmacology 2025, 33, 3671–3678. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, R.; Li, M.; Lu, M. Current Insights and Trends in Atopic Dermatitis and Microbiota Interactions: A Systematic Review and Bibliometric Analysis. Front. Microbiol. 2025, 16, 1613315. [Google Scholar] [CrossRef]
- Rahinj, S.; Rasal, V.; Modhale, M.; Mate, A.; Kuthval, R. Turmeric in Traditional and Modern Medicine: Comparative Analysis. Int. J. Sci. R. Tech. 2025, 2, 492–494. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Khadem, E. Therapeutic Applications of Turmeric and Its Principal Constituent Curcumin in Wound Healing and Skin Regeneration From the Perspective of Conventional Medicine and Iranian Traditional Medicine (ITM). J. Med. Plants 2017, 16, 12–21. [Google Scholar]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Niziński, P.; Hawrył, A.; Gancarz, M.; Hawrył, D.; Oliwa, W.; Pałka, M.; Markowska, J.; Oniszczuk, A. Potential of Curcumin in the Management of Skin Diseases. Int. J. Mol. Sci. 2024, 25, 3617. [Google Scholar] [CrossRef]
- Liakopoulou, A.; Letsiou, S.; Avgoustakis, K.; Patrinos, G.P.; Lamari, F.N.; Hatziantoniou, S. Curcumin-Loaded Lipid Nanocarriers: A Targeted Approach for Combating Oxidative Stress in Skin Applications. Pharmaceutics 2025, 17, 144. [Google Scholar] [CrossRef]
- Kantasa, T.; Yeerong, K.; Klinjan, P.; Na Takuathung, M.; Settakorn, K.; Chuamanochan, M.; Tovanabutra, N.; Ampasavate, C.; Koonrungsesomboon, N. Therapeutic Potential of Curcumin and Novel Formulations in Psoriasis Treatment: Evidence and Future Prospects. Drug Des. Devel. Ther. 2025, 19, 5387–5414. [Google Scholar] [CrossRef]
- Cai, Z.; Zeng, Y.; Liu, Z.; Zhu, R.; Wang, W. Curcumin Alleviates Epidermal Psoriasis-Like Dermatitis and IL-6/STAT3 Pathway of Mice. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2399–2408. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Liu, L.; Sun, X.; Zhou, Y.; Chen, S.; Lu, Y.; Lu, Y.; Cai, X.; Cai, X.; et al. Efficacy and Safety of Curcumin in Psoriasis: Preclinical and Clinical Evidence and Possible Mechanisms. Front. Pharmacol. 2022, 13, 903160. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lian, H.; Zhou, R.; Gu, Z.; Wu, J.; Wu, Y.; Li, Z. Curcumin and Multiple Health Outcomes: Critical Umbrella Review of Intervention Meta-Analyses. Front. Pharmacol. 2025, 16, 1601204. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, K.; Li, J.; Liu, A.; Xiao, W.; Sun, L. Screening and Preparation of Curcumin Nano-Formulations Combined With Dissolving Microneedles on the Application in the Effective Treatment of Psoriasis. Int. J. Pharm. 2025, 675, 125528. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, W.; Zhang, Y.; Zeng, Y. Curcumin Alleviates Imiquimod-Induced Psoriasis-Like Inflammation and Regulates Gut Microbiota of Mice. Immun. Inflamm. Dis. 2023, 11, e967. [Google Scholar] [CrossRef]
- Serini, S.; Trombino, S.; Cassano, R.; Marino, M.; Calviello, G. Anti-Inflammatory Effects of Curcumin-Based Nanoparticles Containing α-Linolenic Acid in a Model of Psoriasis In Vitro. Nutrients 2025, 17, 692. [Google Scholar] [CrossRef]
- Chen, K.; Yang, H.; Xu, G.; Hu, Y.; Tian, X.; Qin, S.; Jiang, T. Enhanced Skin Penetration of Curcumin by a Nanoemulsion-Embedded Oligopeptide Hydrogel for Psoriasis Topical Therapy. RSC Med. Chem. 2025, 16, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Akhtar, J.; Ahmad, M.; Islam, A.; Badruddeen; Khan, M.I.; Siddiqui, S.; Srivastava, A. Curcumin Nanogel Preparations: A Promising Alternative for Psoriasis Treatment. Curr. Drug Metab. 2024, 25, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, Y.; Yang, C.; Li, F.; Qiu, B.; Ding, W. Enhanced Transdermal Efficiency of Curcumin-Loaded Peptide-Modified Liposomes for Highly Effective Antipsoriatic Therapy. J. Mater. Chem. B 2021, 9, 4846–4856. [Google Scholar] [CrossRef]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Isacchi, B.; Antiga, E.; Caproni, M. Curcumin Nanoparticles Potentiate Therapeutic Effectiveness of Acitrein in Moderate-to-Severe Psoriasis Patients and Control Serum Cholesterol Levels. J. Pharm. Pharmacol. 2018, 70, 919–928. [Google Scholar] [CrossRef]
- Brandt, E.B.; Sivaprasad, U. Th2 Cytokines and Atopic Dermatitis. J. Clin. Cell Immunol. 2011, 2, 110. [Google Scholar] [CrossRef]
- Mo, Z.; Yuan, J.; Guan, X.; Peng, J. Advancements in Dermatological Applications of Curcumin: Clinical Efficacy and Mechanistic Insights in the Management of Skin Disorders. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1083–1092. [Google Scholar] [CrossRef]
- Sharma, S.; Sethi, G.S.; Naura, A.S. Curcumin Ameliorates Ovalbumin-Induced Atopic Dermatitis and Blocks the Progression of Atopic March in Mice. Inflammation 2020, 43, 358–369. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Shao, S.; Shen, Y.; Xiao, F.; Sun, J.; Piao, S.; Zhao, D.; Li, G.; Yan, M. Compound Traditional Chinese Medicine Dermatitis Ointment Ameliorates Inflammatory Responses and Dysregulation of Itch-Related Molecules in Atopic Dermatitis. Chin. Med. 2022, 17, 3. [Google Scholar] [CrossRef]
- Saini, K.; Modgill, N.; Singh, K.K.; Kakkar, V. Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration Into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model. Nanomaterials 2022, 12, 636. [Google Scholar] [CrossRef]
- Cassano, R.; Serini, S.; Curcio, F.; Trombino, S.; Calviello, G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics 2022, 14, 1593. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chang, C.C.; Lin, Y.C.; Chen, M.C. Double-Layered PLGA/HA Microneedle Systems as a Long-Acting Formulation of Polyphenols for Effective and Long-Term Management of Atopic Dermatitis. Biomater. Sci. 2023, 11, 4995–5011. [Google Scholar] [CrossRef]
- Frei, G.; Haimhoffer, Á.; Csapó, E.; Bodnár, K.; Vasvári, G.; Nemes, D.; Lekli, I.; Gyöngyösi, A.; Bácskay, I.; Fehér, P.; et al. In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Self-Nanoemulsifying Drug Delivery System Loaded with Curcumin. Pharmaceutics 2023, 15, 2054. [Google Scholar] [CrossRef]
- Panahi, Y.; Sahebkar, A.; Amiri, M.; Davoudi, S.M.; Beiraghdar, F.; Hoseininejad, S.L.; Kolivand, M. Improvement of Sulphur Mustard-Induced Chronic Pruritus, Quality of Life and Antioxidant Status by Curcumin: Results of a Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Nutr. 2012, 108, 1272–1279. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, A.; Lahori, D.; Namit, A.F.; Paxton, Z.; Ratna, N.; Thornton, D.; Ramana, K.V. Curcumin in Inflammatory Complications: Therapeutic Applications and Clinical Evidence. Int. J. Mol. Sci. 2025, 26, 9366. https://doi.org/10.3390/ijms26199366
Zafar A, Lahori D, Namit AF, Paxton Z, Ratna N, Thornton D, Ramana KV. Curcumin in Inflammatory Complications: Therapeutic Applications and Clinical Evidence. International Journal of Molecular Sciences. 2025; 26(19):9366. https://doi.org/10.3390/ijms26199366
Chicago/Turabian StyleZafar, Amber, Divya Lahori, Aleeza F. Namit, Zackery Paxton, Neha Ratna, Dallin Thornton, and Kota V. Ramana. 2025. "Curcumin in Inflammatory Complications: Therapeutic Applications and Clinical Evidence" International Journal of Molecular Sciences 26, no. 19: 9366. https://doi.org/10.3390/ijms26199366
APA StyleZafar, A., Lahori, D., Namit, A. F., Paxton, Z., Ratna, N., Thornton, D., & Ramana, K. V. (2025). Curcumin in Inflammatory Complications: Therapeutic Applications and Clinical Evidence. International Journal of Molecular Sciences, 26(19), 9366. https://doi.org/10.3390/ijms26199366







