Spatiotemporal Transcriptome Profiling Reveals Nutrient Transport Dynamics in Rice Nodes and Roots During Reproductive Development
Abstract
1. Introduction
2. Results
2.1. Transcriptome Sequencing Data Overview
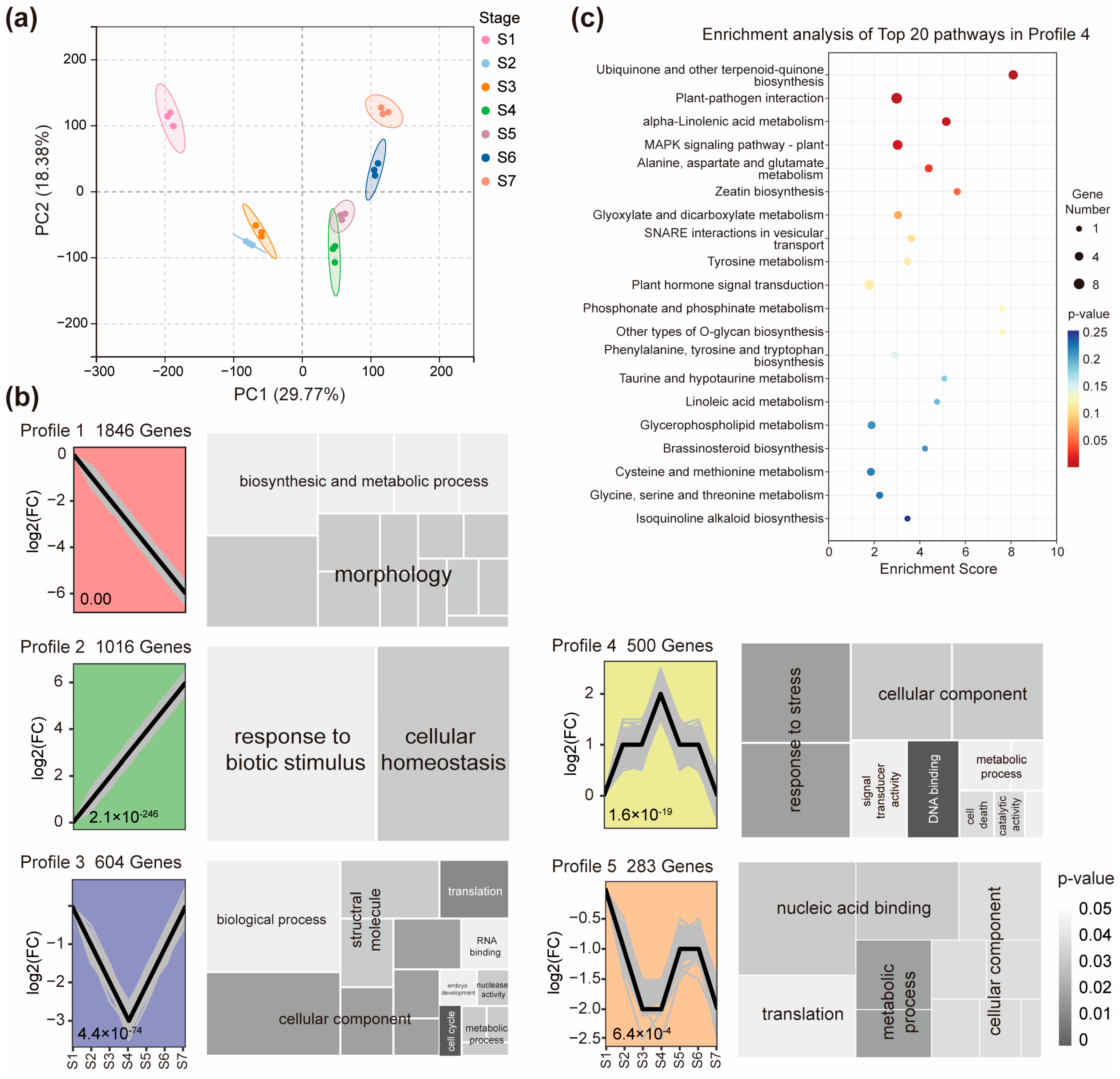
2.2. Gene Expression Pattern of Node I During the Reproductive Growth Stage
2.3. Genes with a Stage-Specific Expression Pattern in Node I Across Reproductive Growth
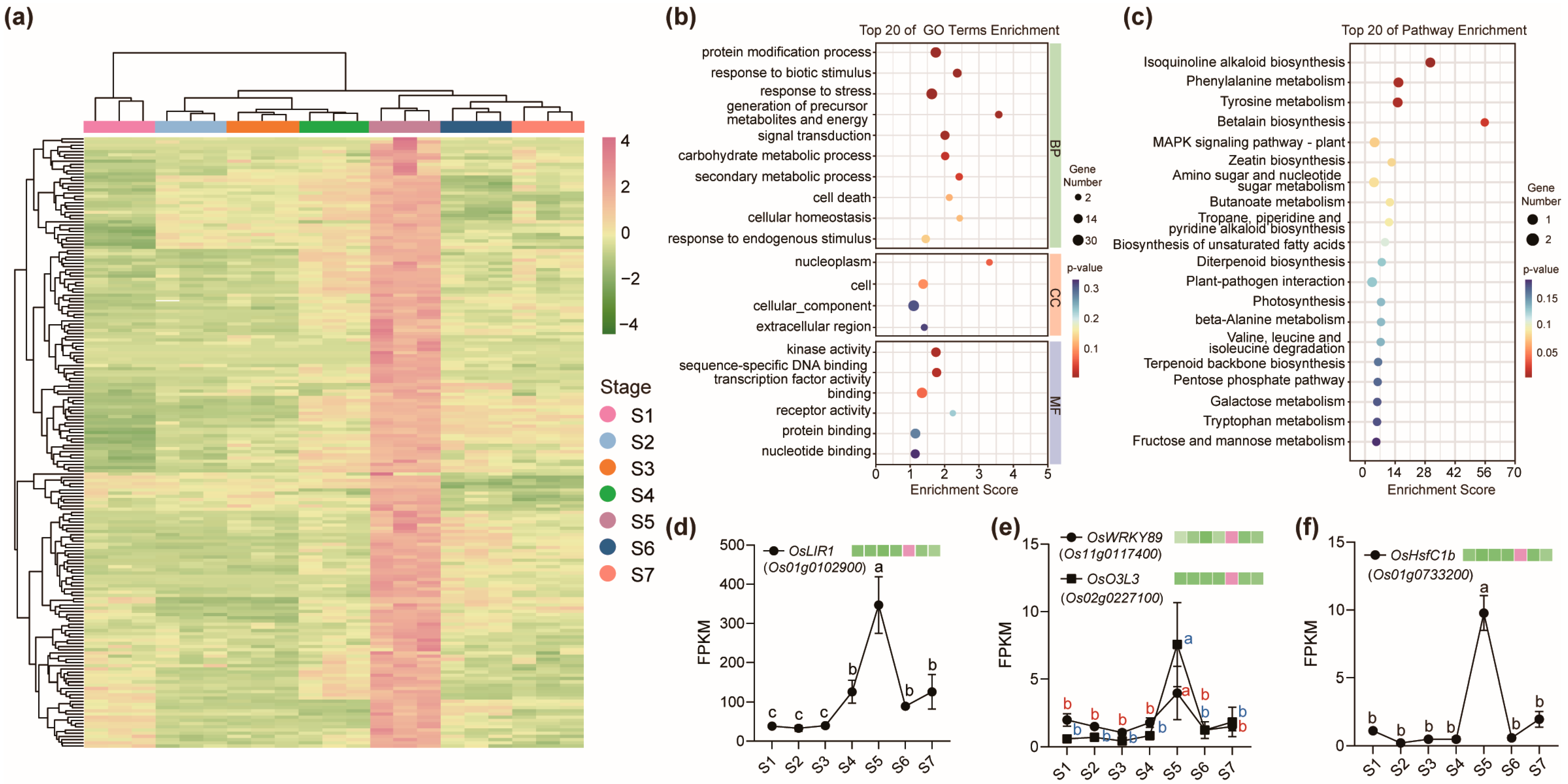
2.4. Genes Specifically Upregulated at the Early Grain Filling Stage (S5) in Node I
2.5. Spatially Distinct Gene Expression in Nodal Tissues During Rice Grain Filling
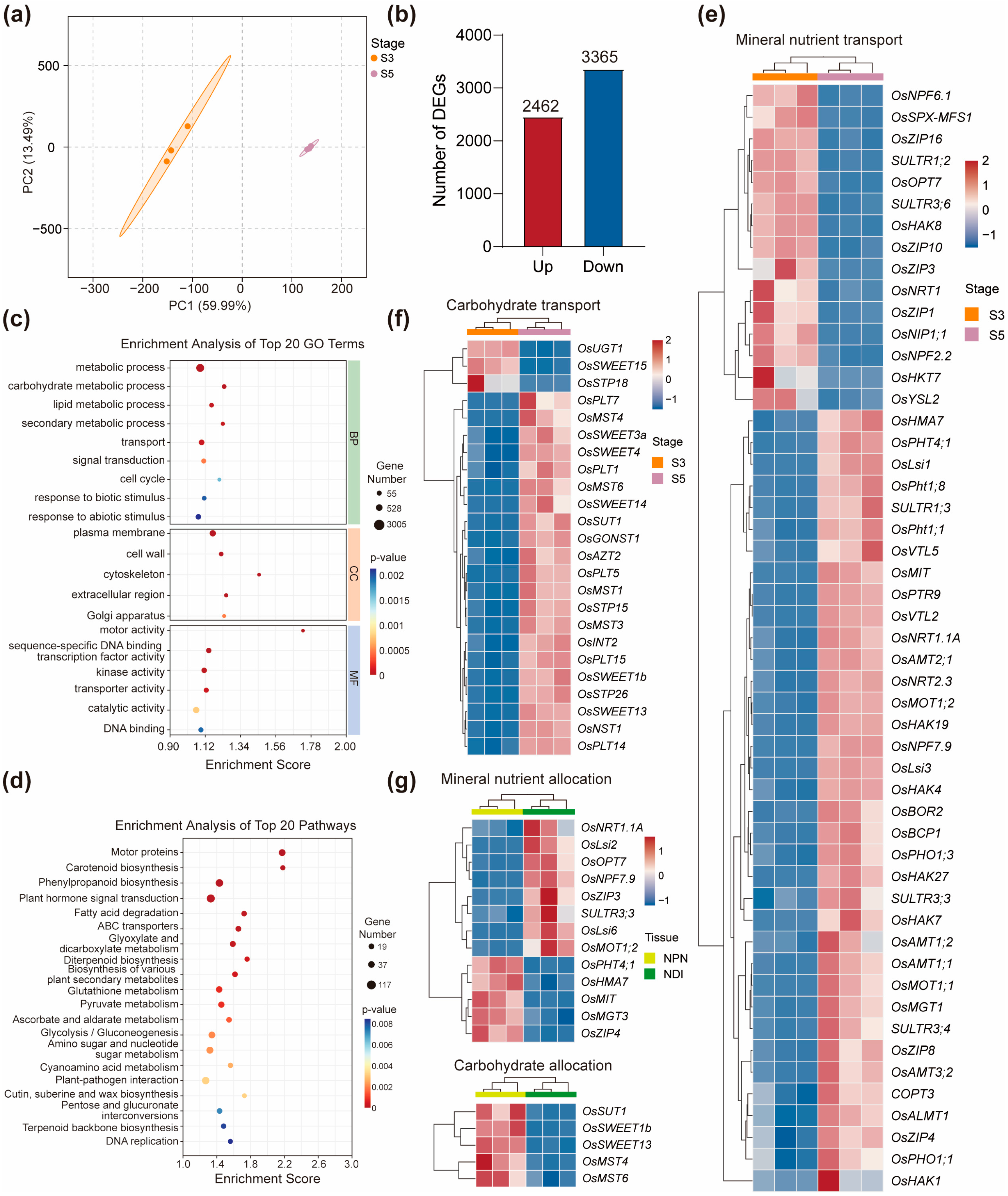
2.6. Differentially Expressed Genes in the Neck-Panicle Node Between the Booting Stage and the Grain Filling Stage
2.7. Differentially Expressed Genes in Roots Between the Early Inflorescence Stage and the Grain Filling Stage
3. Discussion
4. Materials and Methods
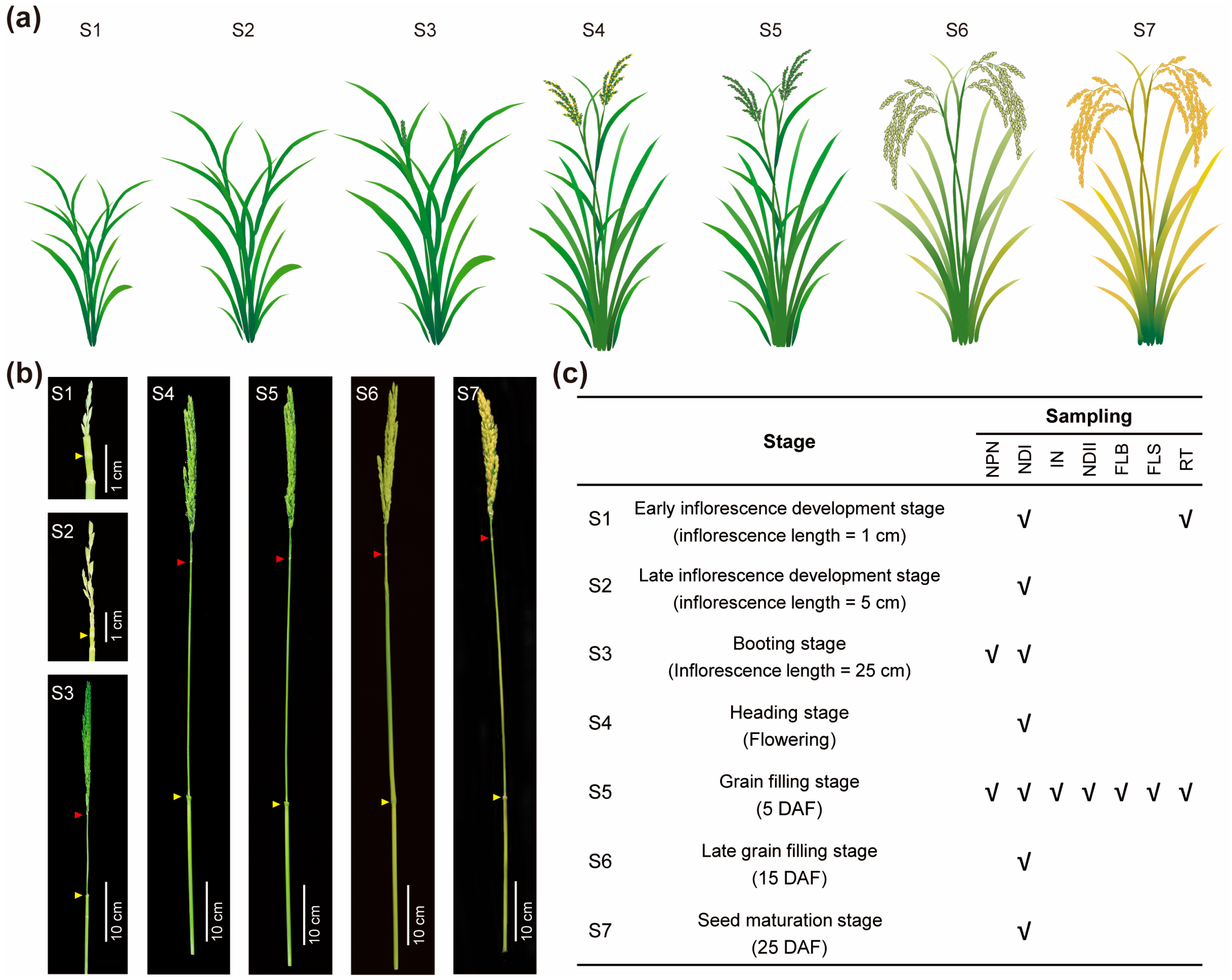
4.1. Plant Materials and Tissue Sampling
4.2. RNA Extraction, cDNA Library Construction, and Transcriptome Sequencing
4.3. RNA-seq Data Processing
4.4. Principal Component Analysis (PCA)
4.5. Short Time-Series Expression Miner (STEM) Analysis
4.6. Venn Diagram Analysis
4.7. Identification of DEGs
4.8. GO and KEGG Analysis
4.9. Validation Genes Specifically Expressed at S5 in Node I by qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NDI | node I |
| NDII | node II |
| NPN | neck-panicle node |
| IN | internode I |
| FLB | flag leaf blade |
| FLS | flag leaf sheath |
| RT | root |
References
- Yamaji, N.; Ma, J.-F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.-F. Node-controlled allocation of mineral elements in Poaceae. Curr. Opin. Plant Biol. 2017, 39, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Ma, J.-F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11401–11406. [Google Scholar] [CrossRef]
- Yamaji, N.; Takemoto, Y.; Miyaji, T.; Mitani-Ueno, N.; Yoshida, K.T.; Ma, J.-F. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 2017, 541, 92–95, Erratum in Nature 2017, 543, 136. [Google Scholar] [CrossRef]
- Fang, Z.; Bai, G.; Huang, W.; Wang, Z.; Wang, X.; Zhang, M. The rice peptide transporter OsNPF7.3 is induced by organic nitrogen, and contributes to nitrogen allocation and grain yield. Front. Plant Sci. 2017, 8, 1338. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Fan, W.; Li, N.; Wu, Q.; Chen, D.; Luan, J.; Zhang, G.; Tian, Q.; Jing, W.; Zhang, Q.; et al. Rice potassium transporter OsHAK18 mediates phloem K+ loading and redistribution. Plant J. 2023, 116, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Ma, J.-F. OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J. Exp. Bot. 2016, 67, 5485–5494. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Masuda, H.; Bashir, K.; Inoue, H.; Tsukamoto, T.; Takahashi, M.; Nakanishi, H.; Aoki, N.; Hirose, T.; Ohsugi, R.; et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010, 62, 379–390. [Google Scholar] [CrossRef]
- Senoura, T.; Sakashita, E.; Kobayashi, T.; Takahashi, M.; Aung, M.S.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol. Biol. 2017, 95, 375–387. [Google Scholar] [CrossRef]
- Che, J.; Yokosho, K.; Yamaji, N.; Ma, J.-F. A vacuolar phytosiderophore transporter alters iron and zinc accumulation in polished rice grains. Plant Physiol. 2019, 181, 276–288. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Sasaki, A.; Xia, J.-X.; Yokosho, K.; Ma, J.-F. A node-based switch for preferential distribution of manganese in rice. Nat. Commun. 2013, 4, 2442. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Mitani-Ueno, N.; Kashino, M.; Ma, J.-F. A node-localized transporter OsZIP3 is responsible for the preferential distribution of Zn to developing tissues in rice. Plant J. 2015, 84, 374–384. [Google Scholar] [CrossRef]
- Huang, W.; Bai, G.; Wang, J.; Zhu, W.; Zeng, Q.; Lu, K.; Sun, S.; Fang, Z. Two splicing variants of OsNPF7.7 regulate shoot branching and nitrogen utilization efficiency in rice. Front. Plant Sci. 2018, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Yamaji, N.; Xia, J.; Ma, J.-F. A member of the heavy metal p-type atpase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 2013, 163, 1353–1362. [Google Scholar] [CrossRef]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Feng, M.-J. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef]
- Li, M.-Z.; Hu, D.-W.; Liu, X.-Q.; Zhang, R.; Liu, H.; Tang, Z.; Zhao, F.-J.; Huang, X.-Y. The OsZIP2 transporter is involved in root-to-shoot translocation and intervascular transfer of cadmium in rice. Plant Cell Environ. 2024, 47, 3865–3881. [Google Scholar] [CrossRef]
- Hao, X.; Zeng, M.; Wang, J.; Zeng, Z.; Dai, J.; Xie, Z.; Yang, Y.; Tian, L.; Chen, L.; Li, D. A node-expressed transporter OsCCX2 is involved in grain cadmium accumulation of rice. Front. Plant Sci. 2018, 9, 476. [Google Scholar] [CrossRef]
- Scofield, G.N.; Hirose, T.; Aoki, N.; Furbank, R.T. Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J. Exp. Bot. 2007, 58, 3155–3169. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.S.; Cho, J.I.; Reinders, A.; Lee, S.W.; Yoo, Y.; Tuan, P.Q.; Choi, S.B.; Bang, G.; Park, Y.I.; Cho, M.H.; et al. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol. 2011, 157, 109–119. [Google Scholar] [CrossRef]
- Wu, Y.; Lee, S.K.; Yoo, Y.; Wei, J.; Kwon, S.Y.; Lee, S.W.; Jeon, J.S.; An, G. Rice transcription factor OsDOF11 modulates sugar transport by promoting expression of sucrose transporter and SWEET genes. Mol. Plant 2018, 11, 833–845. [Google Scholar] [CrossRef]
- Yang, J.; Luo, D.; Yang, B.; Frommer, W.B.; Eom, J.S. SWEET11 and 15 as key players in seed filling in rice. New Phytol. 2018, 218, 604–615. [Google Scholar] [CrossRef]
- Liu, X.-S.; Feng, S.-J.; Zhang, B.-Q.; Wang, M.-Q.; Cao, H.-W.; Rono, J.K.; Chen, X.; Yang, Z.-M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.A.; Lee, J.; Guerinot, M.L.; An, G. Zinc deficiency-inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol. Cells 2010, 29, 551–558. [Google Scholar] [CrossRef]
- Zhao, H.; Frank, T.; Tan, Y.; Zhou, C.; Jabnoune, M.; Arpat, A.B.; Cui, H.; Huang, J.; He, Z.; Poirier, Y.; et al. Disruption of OsSULTR3;3 reduces phytate and phosphorus concentrations and alters the metabolite profile in rice grains. New Phytol. 2016, 211, 926–939. [Google Scholar] [CrossRef]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol. Plant. 2021, 171, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, L.; Liu, H.; Yuan, M. Impaired SWEET-mediated sugar transportation impacts starch metabolism in developing rice seeds. Crop J. 2022, 10, 98–108. [Google Scholar] [CrossRef]
- Luo, S.; Zheng, S.; Li, Z.; Cao, J.; Wang, B.; Xu, Y.; Chong, K. Monosaccharide transporter OsMST6 is activated by transcription factor OsERF120 to enhance chilling tolerance in rice seedlings. J. Exp. Bot. 2024, 75, 4038–4051. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Ludewig, F. Transporters in starch synthesis. Funct. Plant Biol. 2007, 34, 474–479. [Google Scholar] [CrossRef]
- Yin, W.; Yang, H.; Wang, Y.; Feng, P.; Deng, Y.; Zhang, L.; He, G.; Wang, N. Oryza sativa PECTIN DEFECTIVE TAPETUM1 affects anther development through a pectin-mediated signaling pathway in rice. Plant Physiol. 2022, 189, 1570–1586. [Google Scholar] [CrossRef] [PubMed]
- Koumoto, T.; Shimada, H.; Kusano, H.; She, K.-C.; Iwamoto, M.; Takano, M. Rice monoculm mutation moc2, which inhibits outgrowth of the second tillers, is ascribed to lack of a fructose-1,6-bisphosphatase. Plant Biotechnol. 2013, 30, 47–56. [Google Scholar] [CrossRef]
- Qiao, L.; Wu, Q.; Yuan, L.; Huang, X.; Yang, Y.; Li, Q.; Shahzad, N.; Li, H.; Li, W. SMALL PLANT AND ORGAN 1 (SPO1) encoding a Cellulose Synthase-like Protein D4 (OsCSLD4) is an important regulator for plant architecture and organ size in rice. Int. J. Mol. Sci. 2023, 24, 16974. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.S.; Olek, A.T.; Makowski, L.; Badger, J.; Steussy, C.N.; Carpita, N.C.; Stauffacher, C.V. Rice cellulose synthaseA8 plant-conserved region is a coiled-coil at the catalytic core entrance. Plant Physiol. 2017, 173, 482–494. [Google Scholar] [CrossRef]
- Wang, B.; Andargie, M.; Fang, R. The function and biosynthesis of callose in high plants. Heliyon 2022, 8, e09248. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, S.; Yin, L.; Zhao, J.; Guo, B.; Lan, J.; Li, X. A missense mutation in the transmembrane domain of CESA9 affects cell wall biosynthesis and plant growth in rice. Plant Sci. 2012, 196, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Ma, S.; Zong, W.; Yang, N.; Lv, Y.; Yan, C.; Guo, Z.; Li, J.; Li, X.; Xiang, Y.; et al. MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate aba signaling and drought resistance in rice. Plant Cell 2016, 28, 2161–2177. [Google Scholar] [CrossRef]
- Prakash, S.; Rai, R.; Zamzam, M.; Ahmad, O.; Peesapati, R.; Vijayraghavan, U. OsbZIP47 is an integrator for meristem regulators during rice plant growth and development. Front. Plant Sci. 2022, 13, 865928. [Google Scholar] [CrossRef]
- Kim, S.L.; Lee, S.; Kim, H.J.; Nam, H.G.; An, G. OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3A. Plant Physiol. 2007, 145, 1484–1494. [Google Scholar] [CrossRef]
- Wang, K.; Tang, D.; Hong, L.; Xu, W.; Huang, J.; Li, M.; Gu, M.; Xue, Y.; Cheng, Z. DEP and AFO regulate reproductive habit in rice. PLoS Genet. 2010, 6, e1000818. [Google Scholar] [CrossRef]
- Cheng, P.; Zhao, H.; Zhang, S.; Zong, Z.; Li, C.; Ming, L.; Xie, W.; Yu, H. Feedback regulation of m6A modification creates local auxin maxima essential for rice microsporogenesis. Dev. Cell 2025, 60, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, S.; Guo, H.; Wang, S.; Xu, L.; Li, C.; Qian, Q.; Chen, F.; Geisler, M.; Qi, Y.; et al. OsABCB14 functions in auxin transport and iron homeostasis in rice (Oryza sativa L.). Plant J. 2014, 79, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Wu, Y.; Gao, Z.; Chen, H.; Jia, L.; Li, D.; Li, X.; Qian, Q.; Qi, Y. Primary root and root hair development regulation by OsAUX4 and its participation in the phosphate starvation response. J. Integr. Plant Biol. 2021, 63, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Wu, L.; Shao, Y.; Wu, Y.; Mao, C. Functional divergence of PIN1 paralogous genes in rice. Plant Cell Physiol. 2019, 60, 2720–2732. [Google Scholar] [CrossRef]
- Li, G.; Liang, W.; Zhang, X.; Ren, H.; Hu, J.; Bennett, M.J.; Zhang, D. Rice actin-binding protein RMD is a key link in the auxin–actin regulatory loop that controls cell growth. Proc. Natl. Acad. Sci. USA 2014, 111, 10377–10382. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, F.; Zhu, Y.; Lan, D.; Yan, P.; Wang, Y.; Hu, Z.; Zhang, X.; Hu, J.; Niu, F.; et al. Auxin signaling module OsSK41-OsIAA10-OsARF regulates grain yield traits in rice. J. Integr. Plant Biol. 2023, 65, 1753–1766. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, W.; Cui, X.; Chen, M.; Yin, C.; Luo, Z.; Zhu, J.; Lucas, W.J.; Wang, Z.; Zhang, D. Brassinosteroids promote development of rice pollen grains and seeds by triggering expression of Carbon Starved Anther, a MYB domain protein. Plant J. 2015, 82, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Liu, L.; Jin, Y.; Du, L.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-Like kinase to mediate brassinosteroid responses in rice. Plant Cell 2012, 24, 2562–2577. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Cao, X.; Song, X. Epigenetic mutation of RAV6 affects leaf angle and seed size in rice. Plant Physiol. 2015, 169, 2118–2128. [Google Scholar] [CrossRef]
- Je, B.I.; Piao, H.L.; Park, S.J.; Park, S.H.; Kim, C.M.; Xuan, Y.-H.; Park, S.H.; Huang, J.; Do, C.Y.; An, G.; et al. RAV-Like1 maintains brassinosteroid homeostasis via the coordinated activation of BRI1 and biosynthetic genes in rice. Plant Cell 2010, 22, 1777–1791. [Google Scholar] [CrossRef]
- Um, T.Y.; Hong, S.Y.; Han, J.S.; Jung, K.H.; Moon, S.; Choi, B.S.; Basnet, P.; Chung, Y.S.; Lee, S.W.; Yang, W.T.; et al. Gibberellic acid sensitive dwarf encodes an ARPC2 subunit that mediates gibberellic acid biosynthesis, effects to grain yield in rice. Front. Plant Sci. 2022, 13, 1027688. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Wang, X.; Wang, J.; Ye, J.; Xu, S.; Zhang, Y.; Hu, D.; Zhang, M.; Xu, Q.; et al. LG5, a novel allele of EUI1, regulates grain size and flag leaf angle in rice. Plants 2023, 12, 675. [Google Scholar] [CrossRef]
- Rong, C.; Liu, Y.; Chang, Z.; Liu, Z.; Ding, Y.; Ding, C. Cytokinin oxidase/dehydrogenase family genes exhibit functional divergence and overlap in rice growth and development, especially in control of tillering. J. Exp. Bot. 2022, 73, 3552–3568. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, B.; Gao, Z.; Li, H.; Ding, Z. Increased expression of OsSAUR23 and OsRR9 regulates rice plant and organ size. Crop J. 2025, 13, 350–359. [Google Scholar] [CrossRef]
- Kim, H.J.; Kieber, J.J.; Schaller, G.E. The rice F-box protein KISS ME DEADLY2 functions as a negative regulator of cytokinin signalling. Plant Signal. Behav. 2013, 8, e26434. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, R.; Maekawa, M.; Kusano, M.; Tomita, K.; Kondo, H.; Nishimura, H.; Crofts, N.; Fujita, N.; Sakamoto, W. Amyloplast membrane protein SUBSTANDARD STARCH GRAIN6 controls starch grain size in rice endosperm. Plant Physiol. 2016, 170, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, D.; Zhou, S.; Gu, J.; Wang, F.; Dong, J.; Huang, R. The ethylene response factor OsERF109 negatively affects ethylene biosynthesis and drought tolerance in rice. Protoplasma 2017, 254, 401–408. [Google Scholar] [CrossRef]
- Yang, C.; Hu, H.; Ren, H.; Kong, Y.; Lin, H.; Guo, J.; Wang, L.; He, Y.; Ding, X.; Grabsztunowicz, M.; et al. LIGHT-INDUCED RICE1 regulates light-dependent attachment of LEAF-TYPE FERREDOXIN-NADP+ OXIDOREDUCTASE to the thylakoid membrane in rice and Arabidopsis. Plant Cell 2016, 28, 712–728. [Google Scholar] [CrossRef]
- Wang, H.; Hao, J.; Chen, X.; Hao, Z.; Wang, X.; Lou, Y.; Peng, Y.; Guo, Z. Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol. Biol. 2007, 65, 799–815. [Google Scholar] [CrossRef]
- Wang, C.; Guo, W.; Cai, X.; Li, R.; Ow, D.W. Engineering low-cadmium rice through stress-inducible expression of OXS3-family member genes. New Biotechnol. 2019, 48, 29–34. [Google Scholar] [CrossRef]
- Schmidt, R.; Schippers, J.H.; Welker, A.; Mieulet, D.; Guiderdoni, E.; Mueller-Roeber, B. Transcription factor OsHsfC1b regulates salt tolerance and development in Oryza sativa ssp. japonica. AoB Plants 2012, 1, pls011. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.-H.; Yi, H.-Y.; Gong, J.-M. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012, 72, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 5207. [Google Scholar] [CrossRef] [PubMed]
- Agata, A.; Ando, K.; Ota, S.; Kojima, M.; Takebayashi, Y.; Takehara, S.; Doi, K.; Ueguchi-Tanaka, M.; Suzuki, T.; Sakakibara, H.; et al. Diverse panicle architecture results from various combinations of Prl5/GA20ox4 and Pbl6/APO1 alleles. Commun. Biol. 2020, 3, 302. [Google Scholar] [CrossRef]
- Chen, H.; Fang, R.; Deng, R.; Li, J. The OsmiRNA166b-OsHox32 pair regulates mechanical strength of rice plants by modulating cell wall biosynthesis. Plant Biotechnol. J. 2021, 19, 1468–1480. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Xie, Z.; Yang, Y.; Dai, H.; Shi, F.; Ma, L.; Sui, Z.; Xia, C.; Kong, X.; et al. Overexpression of rice OsNRT1.1A/OsNPF6.3 enhanced the nitrogen use efficiency of wheat under low nitrogen conditions. Planta 2024, 259, 127. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, X.; Li, Q.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx. Plant Physiol. 2012, 160, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, Y.; Pei, W.; Jain, A.; Sun, R.; Cao, Y.; Wu, X.; Jiang, T.; Zhang, L.; Fan, X.; et al. Involvement of OsPht1;4 in phosphate acquisition and mobilization facilitates embryo development in rice. Plant J. 2015, 82, 556–569. [Google Scholar] [CrossRef]
- Ding, J.; Ji, C.; Wang, C.; Wang, S.; Ding, G.; Shi, L.; Xu, F.; Cai, H. OsMYB67 knockout promotes rice heading and yield by facilitating copper distribution in panicles. Plant Cell Environ. 2025, 48, 5664–5679. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Y.; Yang, A.; Zhang, W.-H. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant Physiol. 2012, 159, 169–183. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Ying, Y.; Wang, J.; Shao, J.-F.; Yamaji, N.; Whelan, J.; Ma, J.-F.; Shou, H. A transcription factor OsbHLH156 regulates Strategy II iron acquisition through localising IRO2 to the nucleus in rice. New Phytol. 2020, 225, 1247–1260. [Google Scholar] [CrossRef]
- Jiang, M.; Song, Y.; Yang, R.; Zheng, C.; Zheng, Y.; Zhang, H.; Li, S.; Tan, Y.; Huang, J.; Shu, Q.; et al. Melatonin activates the OsbZIP79–OsABI5 module that orchestrates nitrogen and ROS homeostasis to alleviate nitrogen-limitation stress in rice. Plant Commun. 2023, 4, 100674. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Yuan, D.; Liu, Y.; Che, R.; Hu, Y.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H.; et al. Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 2018, 30, 638–651. [Google Scholar] [CrossRef]
- Konishi, N.; Ma, J.-F. Three polarly localized ammonium transporter 1 members are cooperatively responsible for ammonium uptake in rice under low ammonium condition. New Phytol. 2021, 232, 1778–1792. [Google Scholar] [CrossRef]
- Gaur, V.S.; Singh, U.S.; Gupta, A.K.; Kumar, A. Influence of different nitrogen inputs on the members of ammonium transporter and glutamine synthetase genes in two rice genotypes having differential responsiveness to nitrogen. Mol. Biol. Rep. 2012, 39, 8035–8044. [Google Scholar] [CrossRef]
- Suenaga, A.; Moriya, K.; Sonoda, Y.; Ikeda, A.; von Wirén, N.; Hayakawa, T.; Yamaguchi, J.; Yamaya, T. Constitutive expression of a novel-type ammonium transporter OsAMT2 in Rice Plants. Plant Cell Physiol. 2003, 44, 206–211. [Google Scholar] [CrossRef]
- Ruili, L.; Jiaoling, W.; Lei, X.; Meihao, S.; Keke, Y.; Hongyu, Z. Functional analysis of phosphate transporter OsPHT4 family members in rice. Rice Sci. 2020, 27, 493–503. [Google Scholar] [CrossRef]
- Sun, S.; Gu, M.; Cao, Y.; Huang, X.; Zhang, X.; Ai, P.; Zhao, J.; Fan, X.-R.; Xu, G.-H. A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol. 2012, 159, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Ren, H.; Gu, M.; Zhao, J.; Sun, S.; Zhang, X.; Chen, J.; Wu, P.; Xu, G.-H. The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011, 156, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Saenchai, C.; Bouain, N.; Kisko, M.; Prom-u-thai, C.; Doumas, P.; Rouached, H. The involvement of OsPHO1;1 in the regulation of iron transport through integration of phosphate and zinc deficiency signaling. Front. Plant Sci. 2016, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Baumann, A.; Poirier, Y. Characterization of the rice PHO1 gene family reveals a key role for OsPHO1;2 in phosphate homeostasis and the evolution of a distinct clade in dicotyledons. Plant Physiol. 2010, 152, 1693–1704. [Google Scholar] [CrossRef]
- Ishikawa, S.; Hayashi, S.; Tanikawa, H.; Iino, M.; Abe, T.; Kuramata, M.; Feng, Z.; Fujiwara, T.; Kamiya, T. Tonoplast-localized OsMOT1;2 participates in interorgan molybdate distribution in rice. Plant Cell Physiol. 2021, 62, 913–921. [Google Scholar] [CrossRef]
- Li, K.; Zhang, S.; Tang, S.; Zhang, J.; Dong, H.; Yang, S.; Qu, H.; Xuan, W.; Gu, M.; Xu, G. The rice transcription factor Nhd1 regulates root growth and nitrogen uptake by activating nitrogen transporters. Plant Physiol. 2022, 189, 1608–1624. [Google Scholar] [CrossRef]
- Nampei, M.; Ogi, H.; Sreewongchai, T.; Nishida, S.; Ueda, A. Potassium transporter OsHAK17 may contribute to saline-alkaline tolerant mechanisms in rice (Oryza sativa). J. Plant Res. 2024, 137, 505–520. [Google Scholar] [CrossRef]
- Ma, J.-F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Huang, B.-Y.; Zhao, F.-J.; Wang, P. The relative contributions of root uptake and remobilization to the loading of Cd and As into rice grains: Implications in simultaneously controlling grain Cd and As accumulation using a segmented water management strategy. Environ. Pollut. 2022, 293, 118497. [Google Scholar] [CrossRef]
- Li, R.-Y.; Ago, Y.; Liu, W.-J.; Mitani, N.; Feldmann, J.; McGrath, S.P.; Ma, J.-F.; Zhao, F.-J. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009, 150, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.-C.; Zheng, X.-L.; Xiao, Y.-T.; Sun, Z.-F.; Tang, Z.; Zhao, F.-J.; Huang, X.-Y. Spatiotemporal Transcriptome Profiling Reveals Nutrient Transport Dynamics in Rice Nodes and Roots During Reproductive Development. Int. J. Mol. Sci. 2025, 26, 9357. https://doi.org/10.3390/ijms26199357
Lu W-C, Zheng X-L, Xiao Y-T, Sun Z-F, Tang Z, Zhao F-J, Huang X-Y. Spatiotemporal Transcriptome Profiling Reveals Nutrient Transport Dynamics in Rice Nodes and Roots During Reproductive Development. International Journal of Molecular Sciences. 2025; 26(19):9357. https://doi.org/10.3390/ijms26199357
Chicago/Turabian StyleLu, Wan-Chun, Xiu-Lan Zheng, Yue-Tong Xiao, Zhan-Fei Sun, Zhong Tang, Fang-Jie Zhao, and Xin-Yuan Huang. 2025. "Spatiotemporal Transcriptome Profiling Reveals Nutrient Transport Dynamics in Rice Nodes and Roots During Reproductive Development" International Journal of Molecular Sciences 26, no. 19: 9357. https://doi.org/10.3390/ijms26199357
APA StyleLu, W.-C., Zheng, X.-L., Xiao, Y.-T., Sun, Z.-F., Tang, Z., Zhao, F.-J., & Huang, X.-Y. (2025). Spatiotemporal Transcriptome Profiling Reveals Nutrient Transport Dynamics in Rice Nodes and Roots During Reproductive Development. International Journal of Molecular Sciences, 26(19), 9357. https://doi.org/10.3390/ijms26199357







