The HMGB1-RAGE Axis Drives the Proneural-to-Mesenchymal Transition and Aggressiveness in Glioblastoma
Abstract
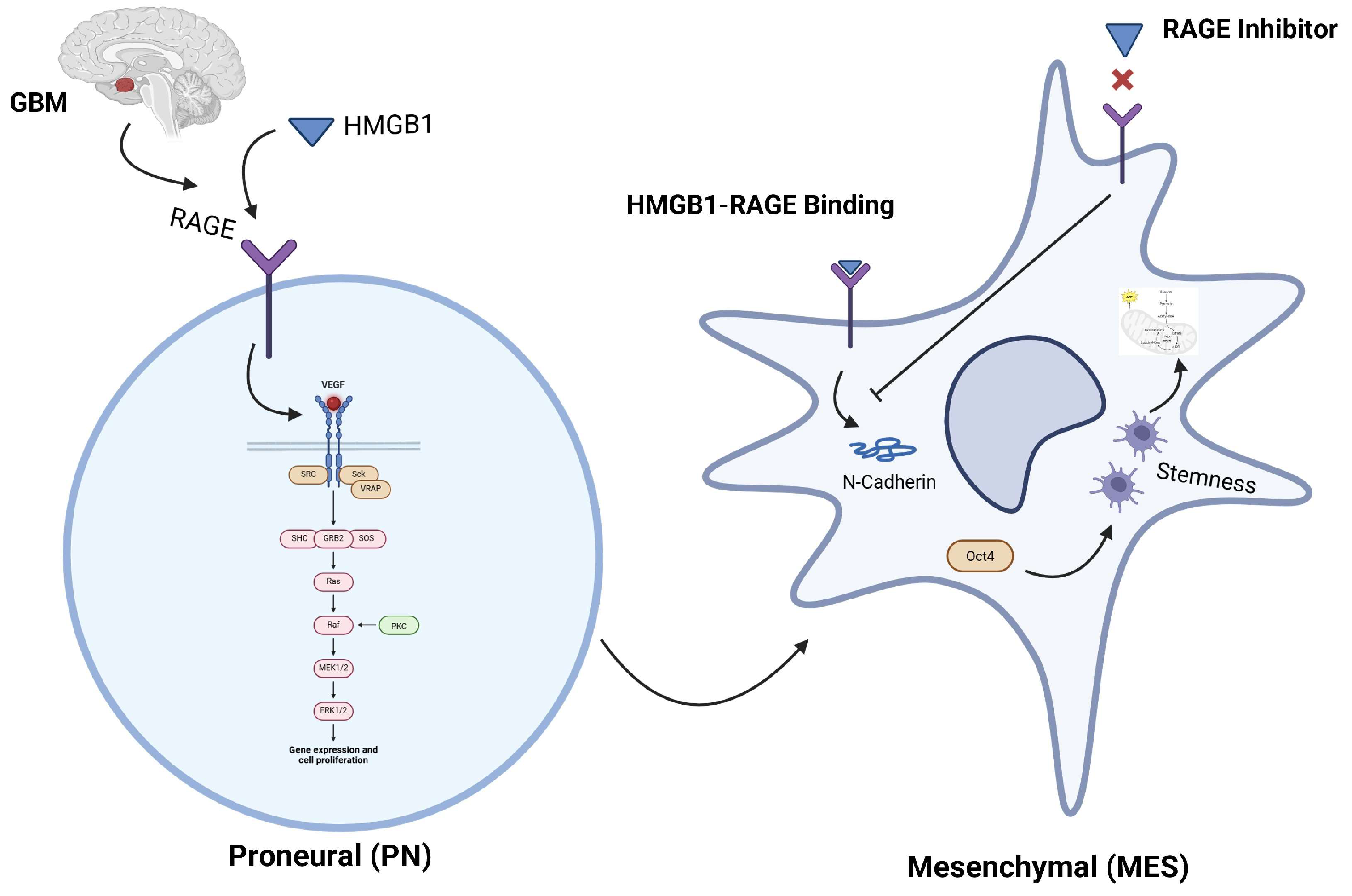
1. Introduction
2. Results
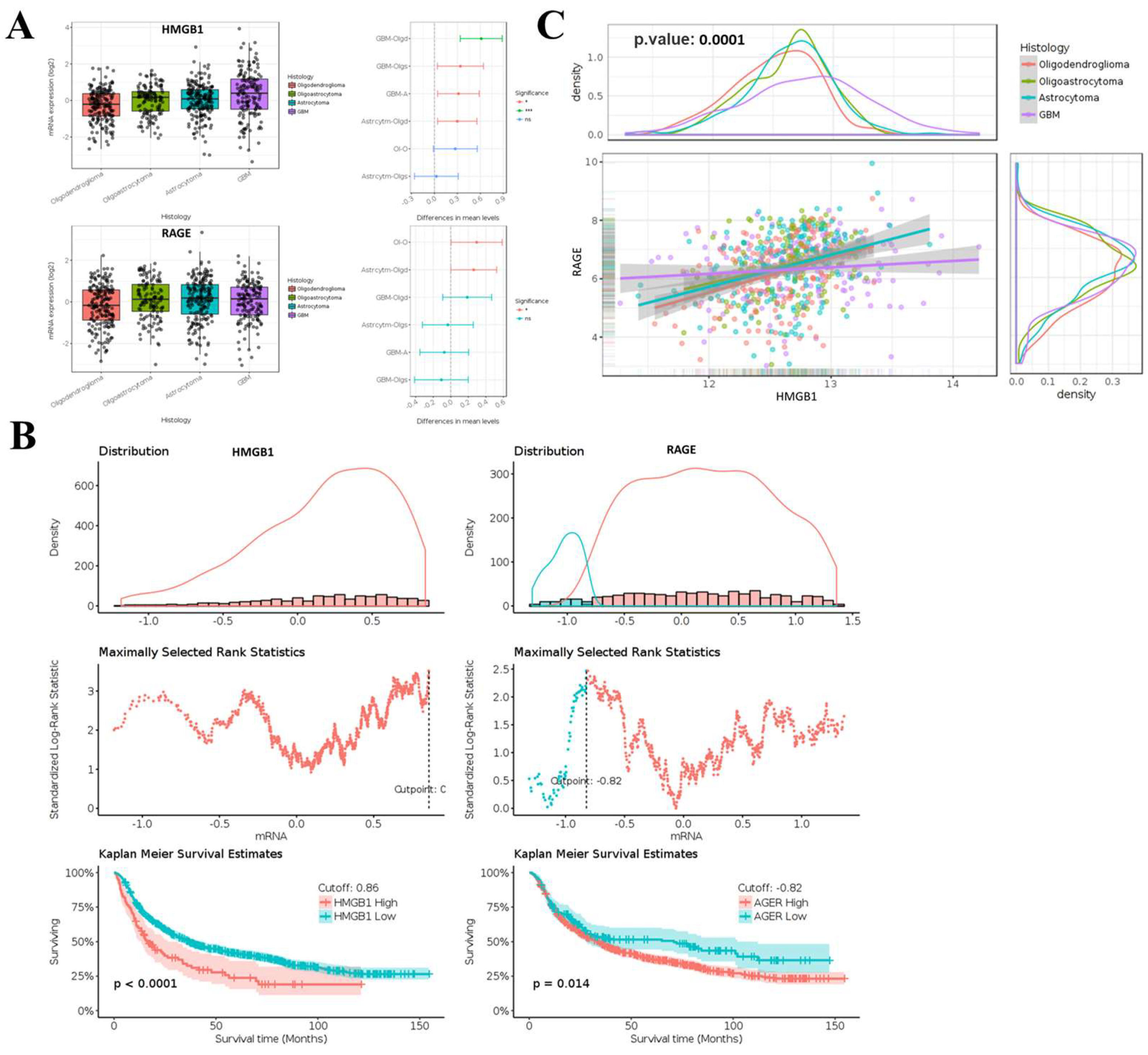
2.1. Aberrant Expression of HMGB1 and RAGE in Glioma and Its Association with Clinical Outcomes
2.2. Immunohistochemical Analysis of HMGB1 and RAGE in GBM SHH In-House Clinical Samples
2.3. HMGB1 Knockdown Attenuates Migration and Invasion of GBM8401 Cells
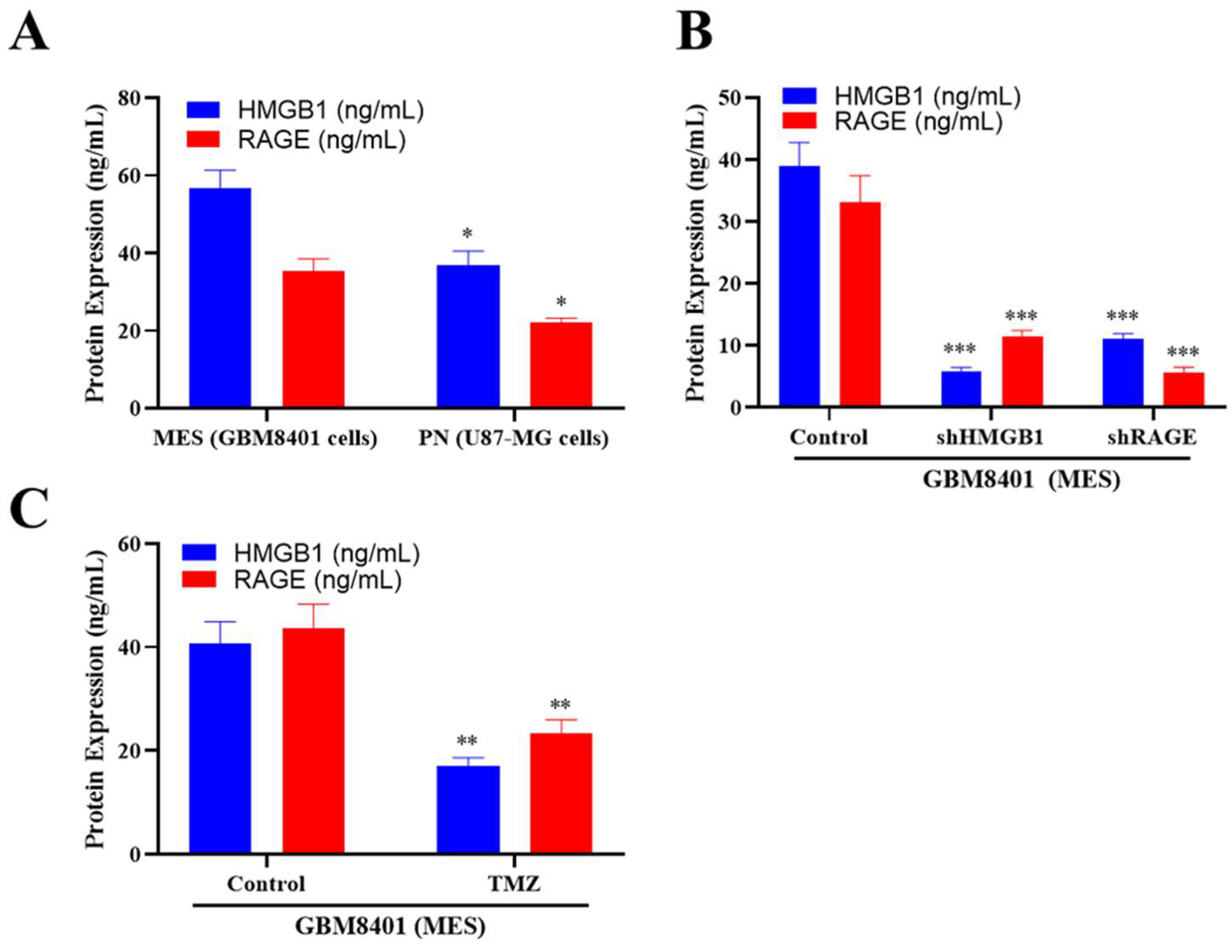
2.4. ELISA Analysis of HMGB1 and RAGE Expression
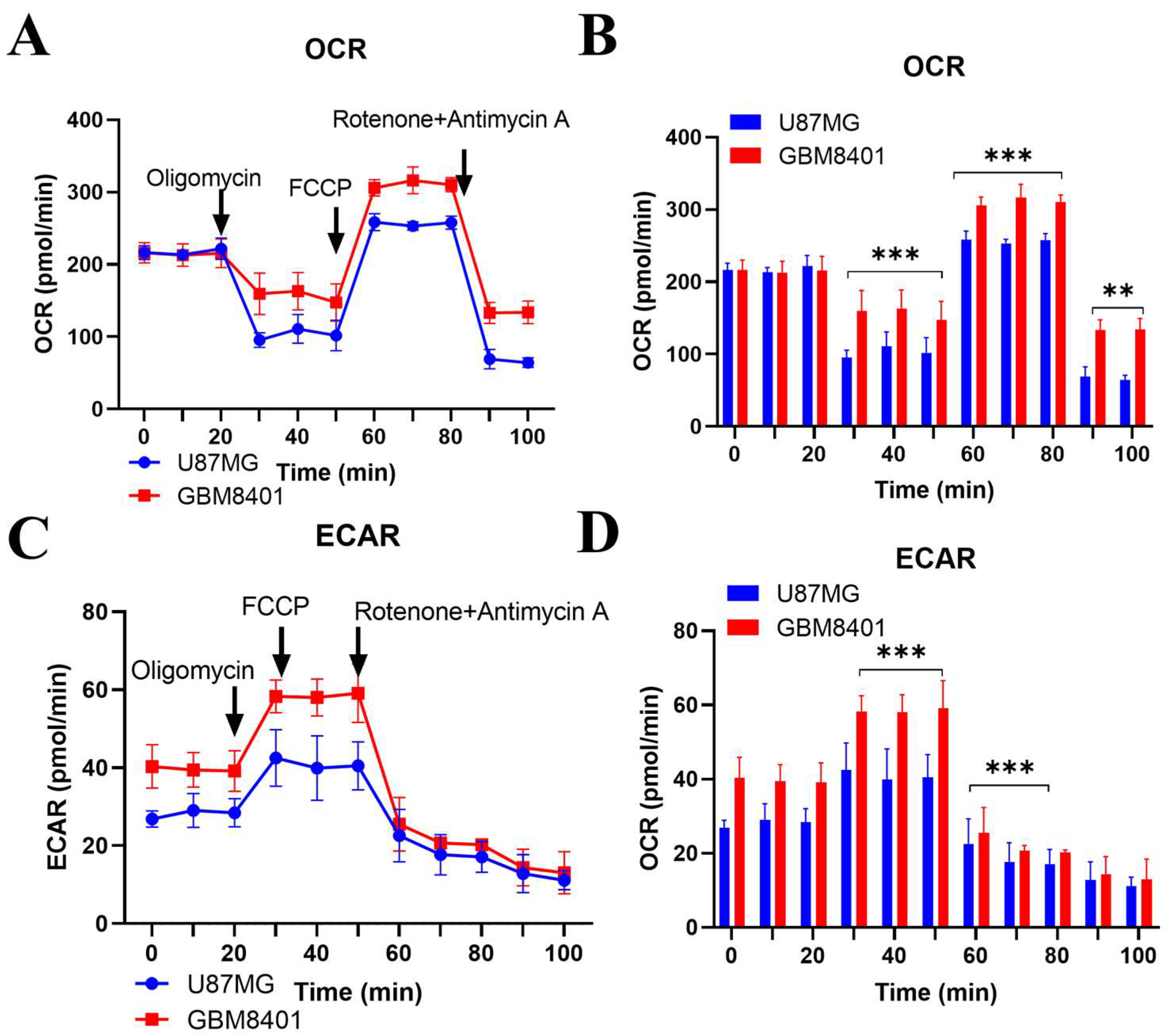
2.5. Comparative Metabolic Analysis of Proneural and Mesenchymal Glioblastoma Cells
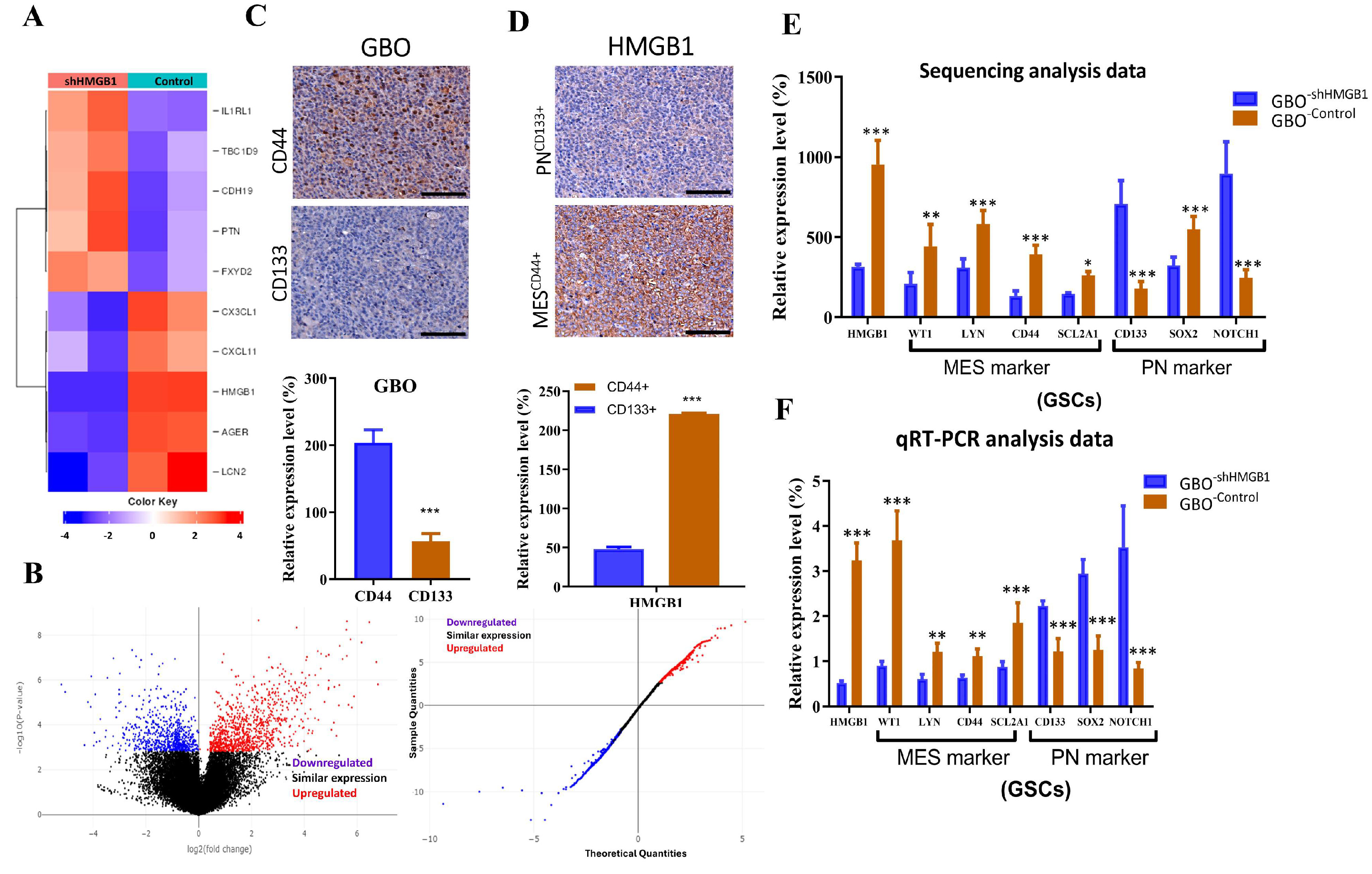
2.6. Silencing RAGE Expression Suppresses the Growth Potential of Glioma Stem Cells In Vitro
2.7. Expression of HMGB1 Associates with Mesenchymal and Proneural Markers in Glioblastoma Behavior and Clinical Outcome
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethical Approval
4.2. Sample Collection and Patient Data Analysis
4.3. Gene Expression Profiling
4.4. Survival Analysis
4.5. Immunohistochemistry (IHC) and Tissue Microarrays
4.6. Cell Culture
4.7. shRNA-Mediated Gene Knockdown
4.8. ELISA
4.9. Invasion and Cell Migration Assays
4.10. Wound-Healing Migration Assay
4.11. Tumor Sphere Formation Assay
4.12. Western Blot Analysis
4.13. Seahorse Extracellular Flux Assay
4.14. qRT-PCR
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 who classification of tumors of the central nervous system: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The who 2021 classification of central nervous system tumours: A practical update on what neurosurgeons need to know—A minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Sandhu, N.; Knox, S.J. A Review of Newly Diagnosed Glioblastoma. Front. Oncol. 2021, 10, 574012. [Google Scholar] [CrossRef] [PubMed]
- Comba, A.; Faisal, S.M.; Varela, M.L.; Hollon, T.; Al-Holou, W.N.; Umemura, Y.; Nunez, F.J.; Motsch, S.; Castro, M.G.; Lowenstein, P.R. Uncovering Spatiotemporal Heterogeneity of High-Grade Gliomas: From Disease Biology to Therapeutic Implications. Front. Oncol. 2021, 11, 703764. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Solanki, C.; Sadana, D.; Arimappamagan, A.; Rao, K.V.L.N.; Rajeswaran, J.; Subbakrishna, D.K.; Santosh, V.; Pandey, P. Impairments in Quality of Life and Cognitive Functions in Long-term Survivors of Glioblastoma. J. Neurosci. Rural Pract. 2017, 8, 228–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, D.; Kang, R.; Zeh, H.J., 3rd; Lotze, M.T. High-mobility group box 1 and cancer. Biochim. Biophys. Acta 2010, 1799, 131–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, B.; Muili, K.; Bolyard, C.; Russell, L.; Lee, T.J.; Banasavadi-Siddegowda, Y.; Yoo, J.Y.; Yan, Y.; Ballester, L.Y.; Bockhorst, K.H.; et al. Suppression of HMGB1 Released in the Glioblastoma Tumor Microenvironment Reduces Tumoral Edema. Mol. Ther. Oncolytics 2018, 12, 93–102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, C.; Li, H.; Li, Y.; Zhang, Y.; Liu, G.; Mi, H.; Li, H.; Xiao, Q.; Niu, L.; Yu, X. Hypoxia-induced HMGB1 promotes glioma stem cells self-renewal and tumorigenicity via RAGE. iScience 2022, 25, 104872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, C.D.; Fuentes, M.K.; Huang, E.H.; Arumugam, T. RAGE and RAGE ligands in cancer. Curr. Mol. Med. 2007, 7, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Blood, D.C.; del Toro, G.; Canet, A.; Lee, D.C.; Qu, W.; Tanji, N.; Lu, Y.; Lalla, E.; Fu, C.; et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qiu, W.; Wang, C.; Qi, Y.; Li, B.; Wang, S.; Zhao, R.; Cheng, B.; Han, X.; Du, H.; et al. Neuronal Activity Promotes Glioma Progression by Inducing Proneural-to-Mesenchymal Transition in Glioma Stem Cells. Cancer Res. 2024, 84, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Zha, C.; Meng, X.; Li, L.; Mi, S.; Qian, D.; Li, Z.; Wu, P.; Hu, S.; Zhao, S.; Cai, J.; et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol. Med. 2020, 17, 154–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, J.; Suarez, J.S.; Minaai, M.; Li, S.; Gaudino, G.; Pass, H.I.; Carbone, M.; Yang, H. HMGB1 as a therapeutic target in disease. J. Cell. Physiol. 2021, 236, 3406–3419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qu, D.; Ling, Z.; Tan, X.; Chen, Y.; Hung, Q.; Li, M.; Liu, T.; Hou, C.; Chen, Y. High mobility group protein B1 (HMGB1) interacts with receptor for advanced glycation end products (RAGE) to promote airway smooth muscle cell proliferation through ERK and NF-κB pathways. Int. J. Clin. Exp. Pathol. 2019, 12, 3268–3278. [Google Scholar] [PubMed] [PubMed Central]
- Otazu, G.K.; Dayyani, M.; Badie, B. Role of RAGE and Its Ligands on Inflammatory Responses to Brain Tumors. Front. Cell. Neurosci. 2021, 15, 770472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varun, B.R.; Ramani, P.; Arya, I.; Palani, J.; Joseph, A.P. Epithelial-mesenchymal transition in cancer stem cells: Therapeutic implications. J. Oral Maxillofac. Pathol. 2023, 27, 359–363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Liu, Y.; Zuo, Z.; Cui, D.; Xu, Y.; Li, L.; Jiang, Y. Dual role of exosomal circCMTM3 derived from GSCs in impeding degradation and promoting phosphorylation of STAT5A to facilitate vasculogenic mimicry formation in glioblastoma. Theranostics 2024, 14, 5698–5724. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jiang, Y.; Xiang, Y.; Wu, B.; Zhao, J.; Huang, R.; Wang, M.; Wang, Y.; Liu, J.; Wu, D.; et al. Super-enhancer-driven LIF promotes the mesenchymal transition in glioblastoma by activating ITGB2 signaling feedback in microglia. Neuro-Oncology 2024, 26, 1438–1452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patra, S.; Roy, P.K.; Dey, A.; Mandal, M. Impact of HMGB1 on cancer development and therapeutic insights focused on CNS malignancy. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189105. [Google Scholar] [CrossRef] [PubMed]
- Bouchart, C.; Trépant, A.L.; Hein, M.; Van Gestel, D.; Demetter, P. Prognostic impact of glioblastoma stem cell markers OLIG2 and CCND2. Cancer Med. 2020, 9, 1069–1078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, W.; Tian, R.; Yu, L.; Bian, S.; Chen, Y.; Yin, B.; Luan, Y.; Chen, S.; Fan, Z.; Yan, R.; et al. Overcoming therapeutic resistance in oncolytic herpes virotherapy by targeting IGF2BP3-induced NETosis in malignant glioma. Nat. Commun. 2024, 15, 131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalkan, R. Glioblastoma Stem Cells as a New Therapeutic Target for Glioblastoma. Clin. Med. Insights Oncol. 2015, 9, 95–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.; Wang, S.; Li, H.L.; Luo, H.; Wu, X.; Lu, J.; Wang, H.W.; Chen, Y.; Chen, D.; Wu, W.T.; et al. FOSL1 promotes proneural-to-mesenchymal transition of glioblastoma stem cells via UBC9/CYLD/NF-κB axis. Mol. Ther. 2022, 30, 2568–2583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seki, H.; Kitabatake, K.; Tanuma, S.I.; Tsukimoto, M. Involvement of RAGE in radiation-induced acquisition of malignant phenotypes in human glioblastoma cells. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130650. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.B.; Beinat, C.; Xie, Y.; Chang, E.; Gambhir, S.S. Tumor treating fields (TTFields) impairs aberrant glycolysis in glioblastoma as evaluated by [18F]DASA-23, a non-invasive probe of pyruvate kinase M2 (PKM2) expression. Neoplasia 2021, 23, 58–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.; Xie, H.M.; Richard, S.A.; Lan, Z. Hemangioblastoma masquerading as a ring enhancing lesion in the cerebellum: A case report. Medicine 2022, 101, e28665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hart, W.S.; Myers, P.J.; Purow, B.W.; Lazzara, M.J. Divergent transcriptomic signatures from putative mesenchymal stimuli in glioblastoma cells. Cancer Gene Ther. 2024, 31, 851–860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Fu, W.J.; Chen, X.Q.; Wang, S.; Deng, R.S.; Tang, X.P.; Yang, K.D.; Niu, Q.; Zhou, H.; Li, Q.R.; et al. Autophagy-based unconventional secretion of HMGB1 in glioblastoma promotes chemosensitivity to temozolomide through macrophage M1-like polarization. J. Exp. Clin. Cancer Res. 2022, 41, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, I.Y.; Liu, S.; Zhang, L.; Liang, R.; Fang, Q.; Zhao, J.; Ren, L.; Medina, E.F.; Filippov, A.; Bonjoc, K.J.; et al. RAGE ablation attenuates glioma progression and enhances tumor immune responses by suppressing galectin-3 expression. Neuro-Oncology 2023, 25, 886–898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Wang, X.; Xu, T.; Xu, F.; Chen, T.; Li, Z.; Wang, Y.; Chen, H.; Ming, J.; Cai, J.; et al. Unveiling the role of TAGLN2 in glioblastoma: From proneural-mesenchymal transition to Temozolomide resistance. Cancer Lett. 2024, 598, 217107. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.B.; Lee, S.; Harmanci, A.S.; Patel, R.; Latha, K.; Yang, Y.; Marisetty, A.; Lee, H.K.; Heimberger, A.B.; Fuller, G.N.; et al. CXCR4 expression is associated with proneural-to-mesenchymal transition in glioblastoma. Int. J. Cancer 2023, 152, 713–724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.; Wang, T.; Ji, N.; Lu, T.; Yuan, R.; Wu, L.; Zhang, J.; Li, M.; Cao, P.; Zhao, J.; et al. Multi-omics and pharmacological characterization of patient-derived glioma cell lines. Nat. Commun. 2024, 15, 6740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alhalabi, O.T.; Göttmann, M.; Gold, M.P.; Schlue, S.; Hielscher, T.; Iskar, M.; Kessler, T.; Hai, L.; Lokumcu, T.; Cousins, C.C.; et al. Integration of transcriptomics, proteomics and loss-of-function screening reveals WEE1 as a target for combination with dasatinib against proneural glioblastoma. Cancer Lett. 2024, 605, 217265. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, G.; Huang, Y.; Zhang, K.; Wu, G.; Xing, W.; Wu, Y.; Zhou, Y.; Sun, C. TAK-242 inhibits glioblastoma invasion, migration, and proneural-mesenchymal transition by inhibiting TLR4 signaling. Exp. Cell Res. 2024, 439, 114091. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Zhang, G.; Da, Q.; Chen, L.; Yu, S.; Zhou, Q.; Weng, Z.; Xin, Z.; Shi, L.; et al. HMGB1-Induced p62 Overexpression Promotes Snail-Mediated Epithelial-Mesenchymal Transition in Glioblastoma Cells via the Degradation of GSK-3β. Theranostics 2019, 9, 1909–1922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, T.; Guan, X.W.; Gribben, J.G.; Liu, F.T.; Jia, L. Blockade of HMGB1 signaling pathway by ethyl pyruvate inhibits tumor growth in diffuse large B-cell lymphoma. Cell Death Dis. 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, Y.; Gao, Z.; Xu, J.; Wang, H.; Guo, Q.; Li, B.; Li, M.; Xu, H.; Qi, Y.; Zhao, S.; et al. SPI1-mediated MIR222HG transcription promotes proneural-to-mesenchymal transition of glioma stem cells and immunosuppressive polarization of macrophages. Theranostics 2023, 13, 3310–3329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.; Hu, L.; Jiang, J.; Li, H.; Wu, Q.; Ooi, K.; Wang, J.; Feng, Y.; Zhu, D.; Xia, C. HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J. Neuroinflamm. 2020, 17, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-C.; Su, Y.-K.; Yadav, V.K.; Fong, I.-H.; Liu, H.-W.; Lin, C.-M. The HMGB1-RAGE Axis Drives the Proneural-to-Mesenchymal Transition and Aggressiveness in Glioblastoma. Int. J. Mol. Sci. 2025, 26, 9352. https://doi.org/10.3390/ijms26199352
Yang H-C, Su Y-K, Yadav VK, Fong I-H, Liu H-W, Lin C-M. The HMGB1-RAGE Axis Drives the Proneural-to-Mesenchymal Transition and Aggressiveness in Glioblastoma. International Journal of Molecular Sciences. 2025; 26(19):9352. https://doi.org/10.3390/ijms26199352
Chicago/Turabian StyleYang, Hao-Chien, Yu-Kai Su, Vijesh Kumar Yadav, Iat-Hang Fong, Heng-Wei Liu, and Chien-Min Lin. 2025. "The HMGB1-RAGE Axis Drives the Proneural-to-Mesenchymal Transition and Aggressiveness in Glioblastoma" International Journal of Molecular Sciences 26, no. 19: 9352. https://doi.org/10.3390/ijms26199352
APA StyleYang, H.-C., Su, Y.-K., Yadav, V. K., Fong, I.-H., Liu, H.-W., & Lin, C.-M. (2025). The HMGB1-RAGE Axis Drives the Proneural-to-Mesenchymal Transition and Aggressiveness in Glioblastoma. International Journal of Molecular Sciences, 26(19), 9352. https://doi.org/10.3390/ijms26199352






