Cardiac Myosin Inhibitors in Hypertrophic Cardiomyopathy: From Sarcomere to Clinic
Abstract
1. Introduction: What Is Hypertrophic Cardiomyopathy (HCM)?
2. Epidemiology, Diagnosis, and Treatment of HCM
2.1. Epidemiology
2.2. Diagnosis
2.3. Treatment
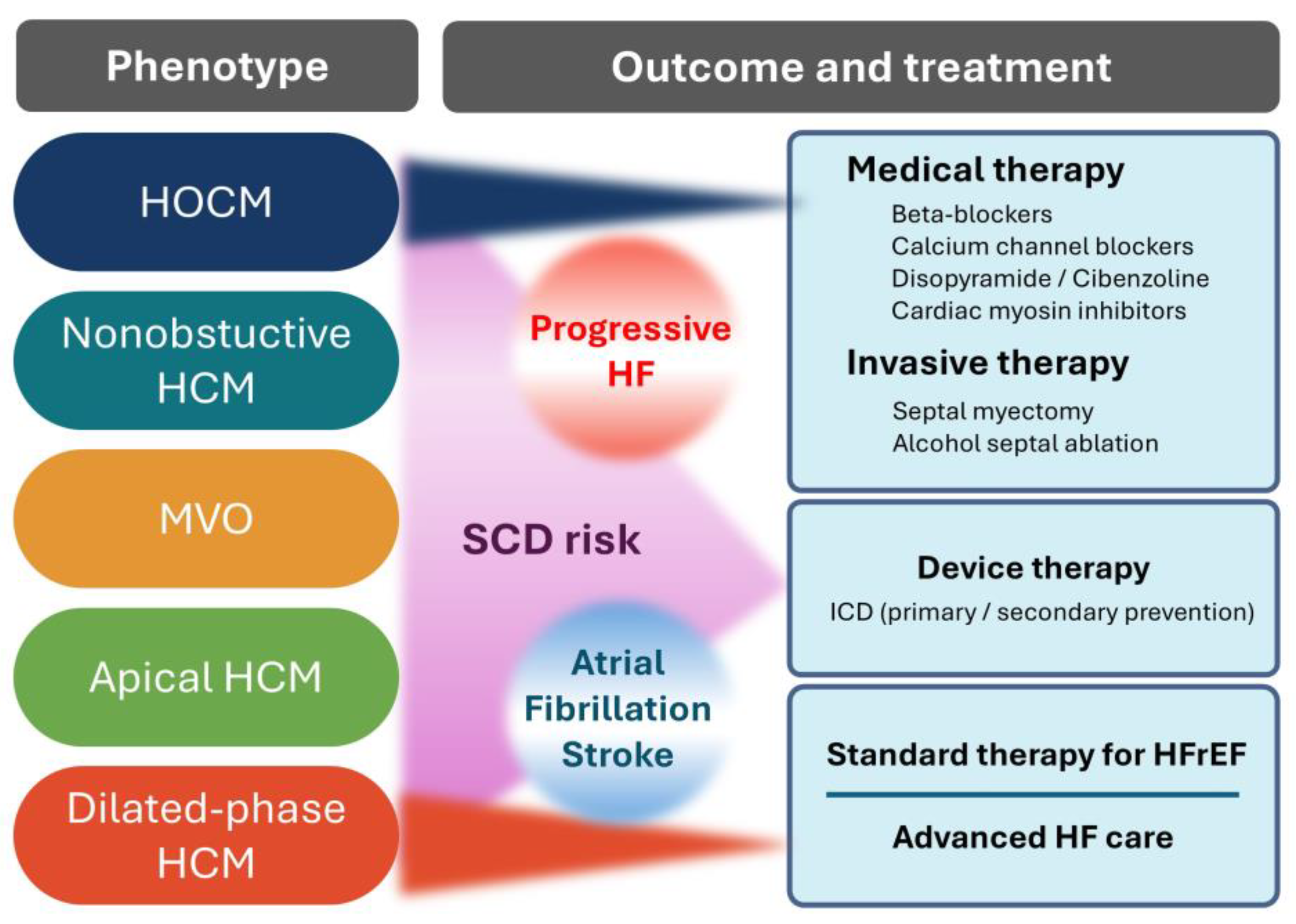
3. Pathology of HCM
4. History of Myosin Inhibitor Development
4.1. Elucidation of the Molecular Mechanisms of HCM (1950s–2000s)
4.2. Conceptual Shift Toward Myosin Modulation (2010s–Present)
4.3. Clinical Advancement of Myosin Inhibitors
4.4. Future Developments
5. Myosin Regulation and Inhibition in HCM
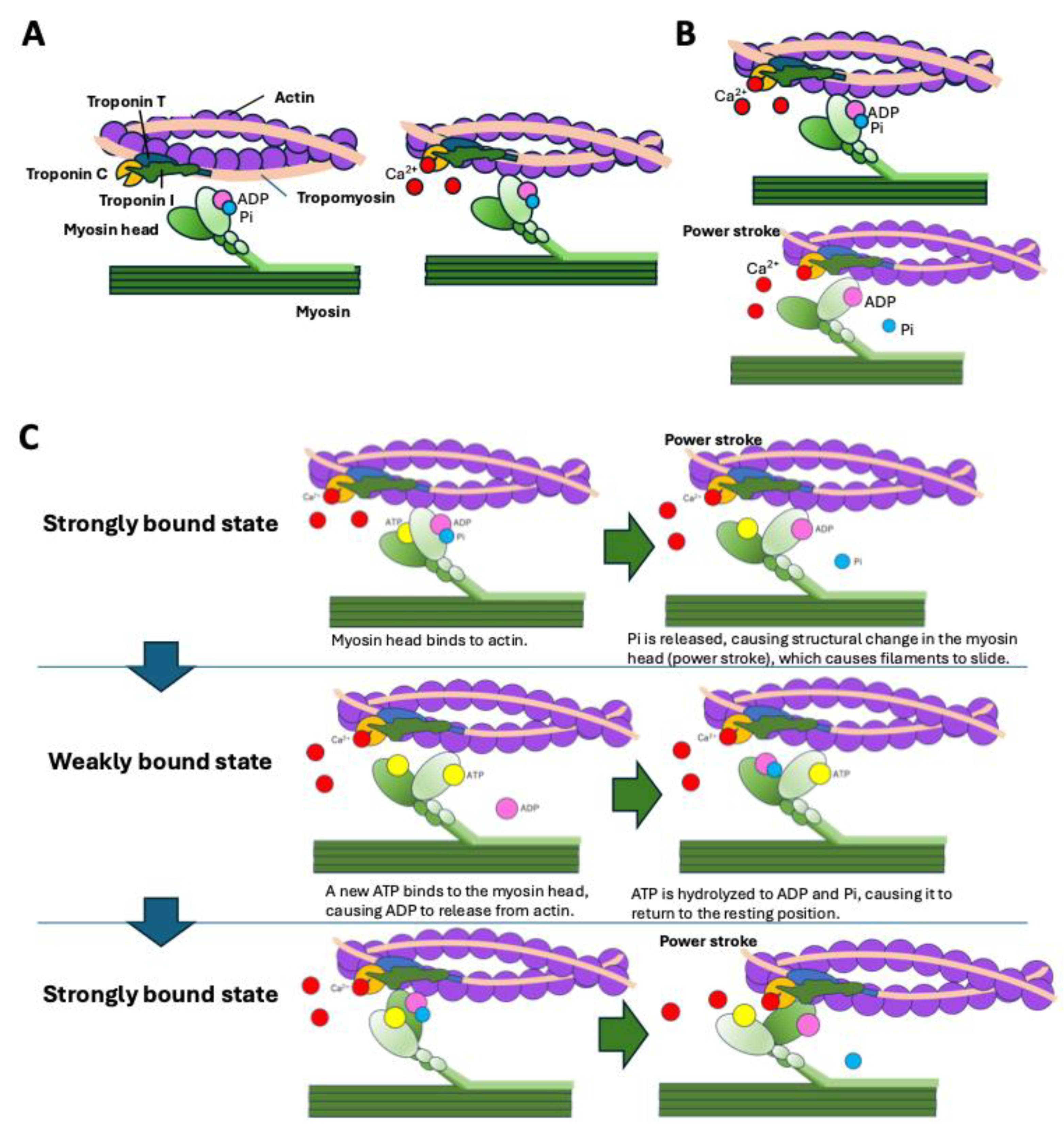
5.1. Molecular Mechanism of Sarcomere Contraction
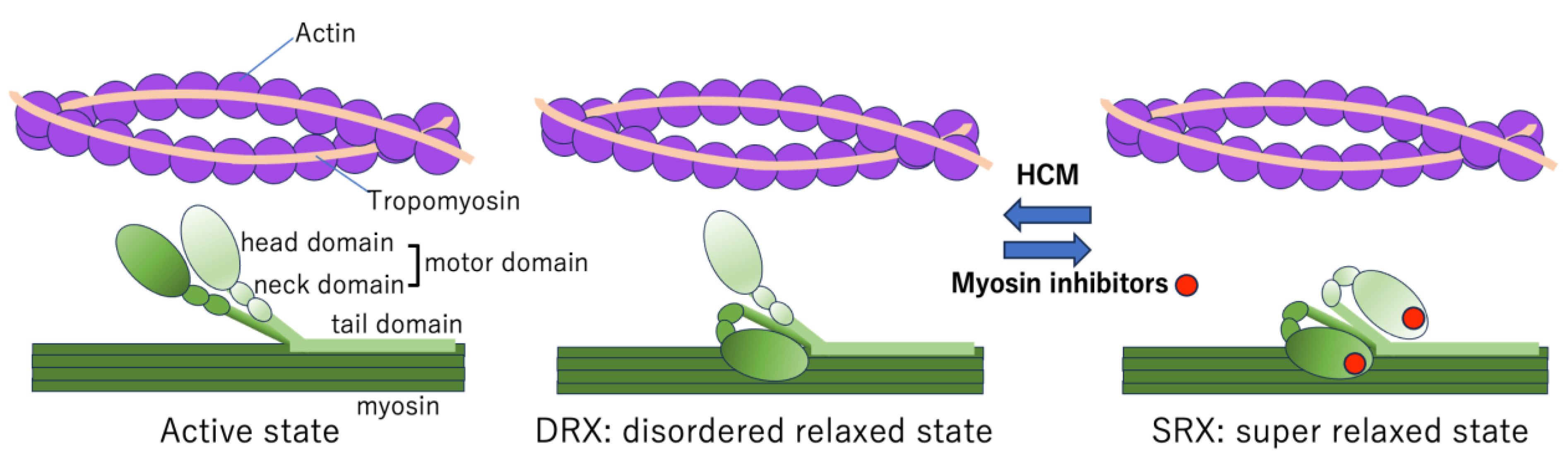
5.2. Myosin Head Conformations
5.3. Conformations of Myosin Heads in HCM
5.4. Mechanism of Action of Myosin Inhibitors
6. Cardiac Myosin Inhibitors and Therapeutic Evidence for HOCM
6.1. Mavacamten
6.2. Aficamten
6.3. Mechanisms Underlying the Therapeutic Effect of Cardiac Myosin Inhibitors on HOCM
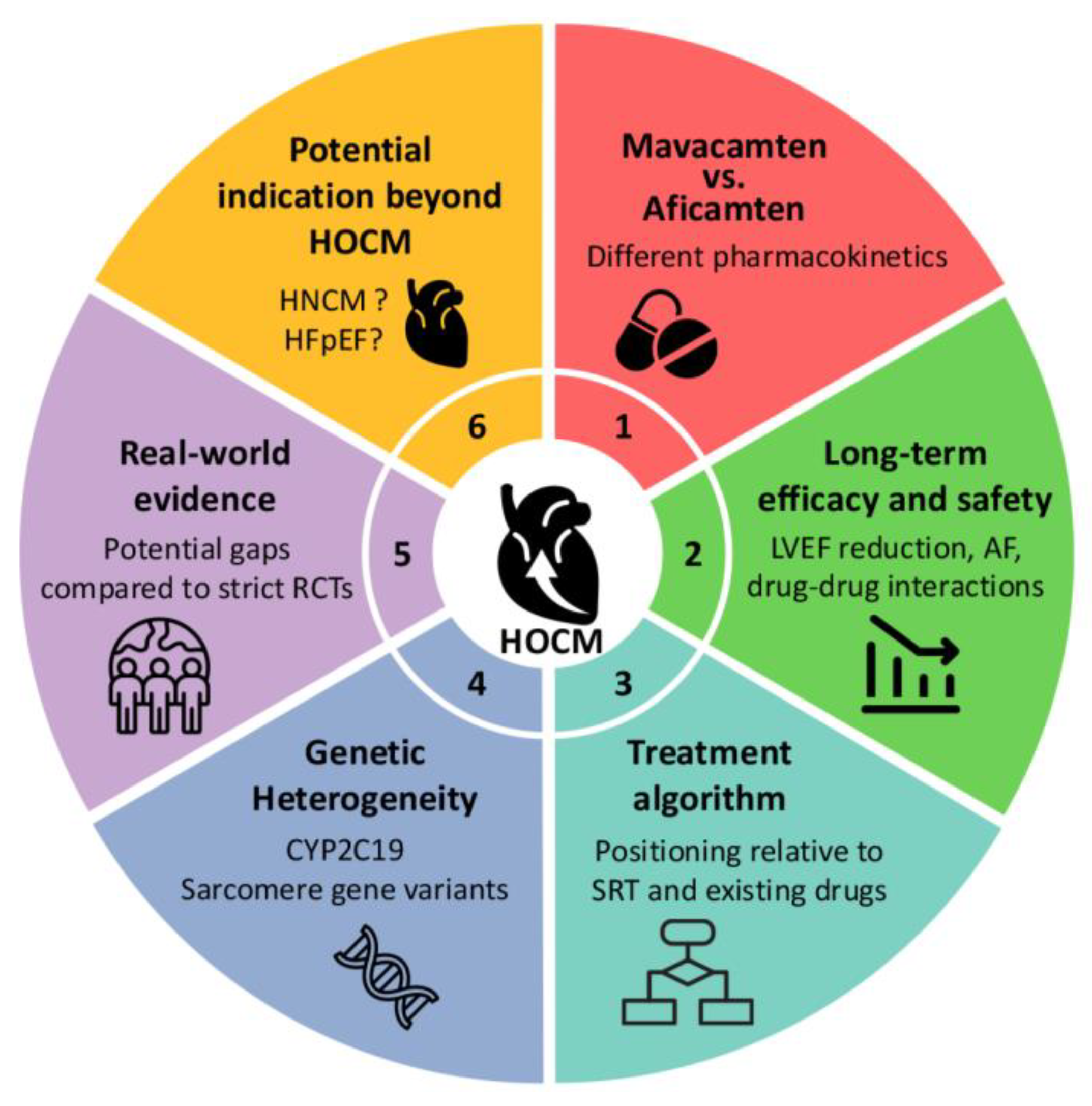
7. Myosin Inhibitors in HCM: Current Landscape and Future Perspectives
7.1. Lack of Long-Term Outcomes of Mavacamten Compared with Septal Reduction Therapies in HOCM
7.2. Myosin Inhibitors vs. Beta Blockers as a First-Line Therapy in HOCM
7.3. Myosin Inhibitors in Non-Obstructive HCM: Promises and Pitfalls
7.4. Pharmacogenomics and Drug Metabolism: CYP2C19 and Clinical Implications
7.5. Genetic Heterogeneity and Treatment Responsiveness
7.6. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| CMI | Cardiac myosin inhibitors |
| CYP | Cytochrome P450 |
| D-HCM | Dilated phase of hypertrophic cardiomyopathy |
| DRX | Disordered relaxed state |
| HCM | Hypertrophic cardiomyopathy |
| HOCM | Hypertrophic obstructive cardiomyopathy |
| IHM | Interacting-heads motif |
| LVOT | Left ventricular outflow tract |
| LVOTO | Left ventricular outflow tract obstruction |
| MRI | Magnetic resonance imaging |
| MYBPC3 | Myosin binding protein C 3 |
| MYH7 | Myosin heavy chain 7 |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| NYHA | New York Heart Association |
| PTSMA | Percutaneous septal myocardial ablation |
| pVO2 | Peak oxygen consumption |
| SM | Surgical myectomy |
| SRT | Septal reduction therapy |
| SRX | Super relaxed state |
References
- Teare, D. Asymmetrical hypertrophy of the heart in young adults. Br. Heart J. 1958, 20, 1–8. [Google Scholar] [CrossRef]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Moore, J.R.; Leinwand, L.; Warshaw, D.M. Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor. Circ. Res. 2012, 111, 375–385. [Google Scholar] [CrossRef]
- Green, E.M.; Wakimoto, H.; Anderson, R.L.; Evanchik, M.J.; Gorham, J.M.; Harrison, B.C.; Henze, M.; Kawas, R.; Oslob, J.D.; Rodriguez, H.M.; et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 2016, 351, 617–621. [Google Scholar] [CrossRef]
- Chuang, C.; Collibee, S.; Ashcraft, L.; Wang, W.; Vander Wal, M.; Wang, X.; Hwee, D.T.; Wu, Y.; Wang, J.; Chin, E.R.; et al. Discovery of Aficamten (CK-274), a Next-Generation Cardiac Myosin Inhibitor for the Treatment of Hypertrophic Cardiomyopathy. J. Med. Chem. 2021, 64, 14142–14152. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Maron, M.S. Global Burden of Hypertrophic Cardiomyopathy. JACC Heart Fail. 2018, 6, 376–378. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef]
- Ikeda, Y.; Yamamoto, T.; Torigoe, M.; Casaes Teixeira, B.; Laurent, T. Prevalence, Patient Characteristics, and Treatment of Patients with Hypertrophic Cardiomyopathy: A Nationwide Payer Database Study. Cardiol. Ther. 2025, 14, 71–86, Erratum in Cardiol. Ther. 2025, 14, 493–494. [Google Scholar] [CrossRef]
- Miura, K.; Nakagawa, H.; Morikawa, Y.; Sasayama, S.; Matsumori, A.; Hasegawa, K.; Ohno, Y.; Tamakoshi, A.; Kawamura, T.; Inaba, Y. Epidemiology of idiopathic cardiomyopathy in Japan: Results from a nationwide survey. Heart 2002, 87, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, A.; Furukawa, Y.; Hasegawa, K.; Sato, Y.; Nakagawa, H.; Morikawa, Y.; Miura, K.; Ohno, Y.; Tamakoshi, A.; Inaba, Y.; et al. Epidemiologic and clinical characteristics of cardiomyopathies in Japan: Results from nationwide surveys. Circ. J. 2002, 66, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Nakamura, K.; Morita, H.; Toh, N.; Nakagawa, K.; Yoshida, M.; Watanabe, A.; Nishii, N.; Miyoshi, T.; Ito, H. New Appearance of Fragmented QRS as a Predictor of Ventricular Arrhythmic Events in Patients with Hypertrophic Cardiomyopathy. Circ. J. 2020, 84, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, H.; Tsutsui, H.; Kubo, T.; Ide, T.; Chikamori, T.; Fukuda, K.; Fujino, N.; Higo, T.; Isobe, M.; Kamiya, C.; et al. JCS/JHFS 2018 Guideline on the Diagnosis and Treatment of Cardiomyopathies. Circ. J. 2021, 85, 1590–1689. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.S.; et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef]
- Kubo, T.; Sugiura, K.; Tokita, Y.; Takano, H.; Takamisawa, I.; Takayama, M.; Doi, Y.L.; Minami, Y.; Shirotani, S.; Ebato, M.; et al. Contemporary clinical characteristics and management patterns in hypertrophic cardiomyopathy: Insights from baseline enrolment data in a nationwide prospective Japanese registry. Heart 2025, 111, 885–892. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Yoshikawa, D.; Ishii, H.; Hirashiki, A.; Okumura, T.; Kubota, A.; Sakai, S.; Harada, K.; Somura, F.; Mizuno, T.; et al. Clinical Characteristics and Long-Term Outcomes of Hypertrophic Cardiomyopathy. Int. Heart J. 2015, 56, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Topriceanu, C.C.; Pereira, A.C.; Moon, J.C.; Captur, G.; Ho, C.Y. Meta-Analysis of Penetrance and Systematic Review on Transition to Disease in Genetic Hypertrophic Cardiomyopathy. Circulation 2024, 149, 107–123. [Google Scholar] [CrossRef]
- Harper, A.R.; Goel, A.; Grace, C.; Thomson, K.L.; Petersen, S.E.; Xu, X.; Waring, A.; Ormondroyd, E.; Kramer, C.M.; Ho, C.Y.; et al. Common genetic variants and modifiable risk factors underpin hypertrophic cardiomyopathy susceptibility and expressivity. Nat. Genet. 2021, 53, 135–142. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef]
- Imai, Y.; Kusano, K.; Aiba, T.; Ako, J.; Asano, Y.; Harada-Shiba, M.; Kataoka, M.; Kosho, T.; Kubo, T.; Matsumura, T.; et al. JCS/JCC/JSPCCS 2024 Guideline on Genetic Testing and Counseling in Cardiovascular Disease. Circ. J. 2024, 88, 2022–2099. [Google Scholar] [CrossRef] [PubMed]
- Spoladore, R.; Maron, M.S.; D’Amato, R.; Camici, P.G.; Olivotto, I. Pharmacological treatment options for hypertrophic cardiomyopathy: High time for evidence. Eur. Heart J. 2012, 33, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Sherrid, M.V.; Barac, I.; McKenna, W.J.; Elliott, P.M.; Dickie, S.; Chojnowska, L.; Casey, S.; Maron, B.J. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 45, 1251–1258. [Google Scholar] [CrossRef]
- Hamada, M.; Ikeda, S.; Ohshima, K.; Nakamura, M.; Kubota, N.; Ogimoto, A.; Shigematsu, Y. Impact of chronic use of cibenzoline on left ventricular pressure gradient and left ventricular remodeling in patients with hypertrophic obstructive cardiomyopathy. J. Cardiol. 2016, 67, 279–286. [Google Scholar] [CrossRef]
- Ommen, S.R.; Maron, B.J.; Olivotto, I.; Maron, M.S.; Cecchi, F.; Betocchi, S.; Gersh, B.J.; Ackerman, M.J.; McCully, R.B.; Dearani, J.A.; et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kimmelstiel, C.; Zisa, D.C.; Kuttab, J.S.; Wells, S.; Udelson, J.E.; Wessler, B.S.; Rastegar, H.; Kapur, N.K.; Weintraub, A.R.; Maron, B.J.; et al. Guideline-Based Referral for Septal Reduction Therapy in Obstructive Hypertrophic Cardiomyopathy Is Associated with Excellent Clinical Outcomes. Circ. Cardiovasc. Interv. 2019, 12, e007673. [Google Scholar] [CrossRef]
- Marian, A.J. Molecular Genetic Basis of Hypertrophic Cardiomyopathy. Circ. Res. 2021, 128, 1533–1553. [Google Scholar] [CrossRef]
- Ostrominski, J.W.; Guo, R.; Elliott, P.M.; Ho, C.Y. Cardiac Myosin Inhibitors for Managing Obstructive Hypertrophic Cardiomyopathy: JACC: Heart Failure State-of-the-Art Review. JACC Heart Fail. 2023, 11, 735–748. [Google Scholar] [CrossRef]
- Kitai, T.; Kohsaka, S.; Kato, T.; Kato, E.; Sato, K.; Teramoto, K.; Yaku, H.; Akiyama, E.; Ando, M.; Izumi, C.; et al. JCS/JHFS 2025 Guideline on Diagnosis and Treatment of Heart Failure. Circ. J. 2025, 89, 1278–1444. [Google Scholar] [CrossRef]
- Maleszewski, J.; Burke, A.; Veinot, J.; Edwards, W. (Eds.) Hypertrophic cardiomyopathy. In Disorders of the Heart and Blood Vessels; AFIP Atlas of Tumor and Non-Tumor Pathology, Series 5; Armed Forces Institute of Pathology: Washington, DC, USA, 2024; Volume 17, pp. 301–322. [Google Scholar]
- Maron, B.J.; Wolfson, J.K.; Ciro, E.; Spirito, P. Relation of electrocardiographic abnormalities and patterns of left ventricular hypertrophy identified by 2-dimensional echocardiography in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 1983, 51, 189–194. [Google Scholar] [CrossRef]
- Braunwald, E.; Morrow, A.G.; Cornell, W.P.; Aygen, M.M.; Hilbish, T.F. Idiopathic hypertrophic subaortic stenosis. Am. J. Med. 1960, 29, 924–945. [Google Scholar] [CrossRef]
- Maron, B.J.; Henry, W.L.; Roberts, W.C.; Epstein, S.E. Comparison of echocardiographic and necropsy measurements of ventricular wall thicknesses in patients with and without disproportionate septal thickening. Circulation 1977, 55, 341–346. [Google Scholar] [CrossRef]
- Kwon, D.H.; Setser, R.M.; Thamilarasan, M.; Popovic, Z.V.; Smedira, N.G.; Schoenhagen, P.; Garcia, M.J.; Lever, H.M.; Desai, M.Y. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart 2008, 94, 1295–1301. [Google Scholar] [CrossRef]
- Perillo, E.F.; Canciello, G.; Borrelli, F.; Todde, G.; Imbriaco, M.; Ordine, L.; Di Napoli, S.; Lombardi, R.; Esposito, G.; Losi, M.A. Diagnosis and Clinical Implication of Left Ventricular Aneurysm in Hypertrophic Cardiomyopathy. Diagnostics 2023, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Nunoda, S.; Genda, A.; Sekiguchi, M.; Takeda, R. Left ventricular endomyocardial biopsy findings in patients with essential hypertension and hypertrophic cardiomyopathy with special reference to the incidence of bizarre myocardial hypertrophy with disorganization and biopsy score. Heart Vessel. 1985, 1, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Matturri, L.; Biondo, B.; Colombo, B.; Lavezzi, A.M.; Rossi, L. Significance of the DNA synthesis in hypertrophic cardiomyopathies. Basic. Res. Cardiol. 1997, 92, 85–89. [Google Scholar] [CrossRef]
- Maron, B.J.; Sato, N.; Roberts, W.C.; Edwards, J.E.; Chandra, R.S. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum. Comparison of fetuses and infants with and without congenital heart disease and patients with hypertrophic cardiomyopathy. Circulation 1979, 60, 685–696. [Google Scholar] [CrossRef]
- Kwon, D.H.; Smedira, N.G.; Rodriguez, E.R.; Tan, C.; Setser, R.; Thamilarasan, M.; Lytle, B.W.; Lever, H.M.; Desai, M.Y. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: Correlation with histopathology and prevalence of ventricular tachycardia. J. Am. Coll. Cardiol. 2009, 54, 242–249. [Google Scholar] [CrossRef]
- Nishikawa, T.; Sekiguchi, M.; Ishibashi-Ueda, H. More than 50 Years after Konno’s Development of the Endomyocardial Biopsy. Int. Heart J. 2017, 58, 840–846. [Google Scholar] [CrossRef]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Diagnostic contribution of left ventricular endomyocardial biopsy in patients with clinical phenotype of hypertrophic cardiomyopathy. Hum. Pathol. 2013, 44, 133–141. [Google Scholar] [CrossRef]
- Tazelaar, H.D.; Billingham, M.E. The surgical pathology of hypertrophic cardiomyopathy. Arch. Pathol. Lab. Med. 1987, 111, 257–260. [Google Scholar]
- Clark, C.E.; Henry, W.L.; Epstein, S.E. Familial prevalence and genetic transmission of idiopathic hypertrophic subaortic stenosis. N. Engl. J. Med. 1973, 289, 709–714. [Google Scholar] [CrossRef]
- Geisterfer-Lowrance, A.A.; Kass, S.; Tanigawa, G.; Vosberg, H.P.; McKenna, W.; Seidman, C.E.; Seidman, J.G. A molecular basis for familial hypertrophic cardiomyopathy: A beta cardiac myosin heavy chain gene missense mutation. Cell 1990, 62, 999–1006. [Google Scholar] [CrossRef]
- Rosenzweig, A.; Watkins, H.; Hwang, D.S.; Miri, M.; McKenna, W.; Traill, T.A.; Seidman, J.G.; Seidman, C.E. Preclinical diagnosis of familial hypertrophic cardiomyopathy by genetic analysis of blood lymphocytes. N. Engl. J. Med. 1991, 325, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Malik, F.I.; Hartman, J.J.; Elias, K.A.; Morgan, B.P.; Rodriguez, H.; Brejc, K.; Anderson, R.L.; Sueoka, S.H.; Lee, K.H.; Finer, J.T.; et al. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science 2011, 331, 1439–1443. [Google Scholar] [CrossRef]
- Heitner, S.B.; Jacoby, D.; Lester, S.J.; Owens, A.; Wang, A.; Zhang, D.; Lambing, J.; Lee, J.; Semigran, M.; Sehnert, A.J. Mavacamten Treatment for Obstructive Hypertrophic Cardiomyopathy: A Clinical Trial. Ann. Intern. Med. 2019, 170, 741–748. [Google Scholar] [CrossRef]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Masri, A.; Nassif, M.E.; Barriales-Villa, R.; Arad, M.; Cardim, N.; Choudhury, L.; Claggett, B.; Coats, C.J.; Dungen, H.D.; et al. Aficamten for Symptomatic Obstructive Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2024, 390, 1849–1861. [Google Scholar] [CrossRef]
- Kitaoka, H.; Ieda, M.; Ebato, M.; Kozuma, K.; Takayama, M.; Tanno, K.; Komiyama, N.; Sakata, Y.; Maekawa, Y.; Minami, Y.; et al. Phase 3 Open-Label Study Evaluating the Efficacy and Safety of Mavacamten in Japanese Adults with Obstructive Hypertrophic Cardiomyopathy—The HORIZON-HCM Study. Circ. J. 2024, 89, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Lambrew, C.T.; Rockoff, S.D.; Ross, J., Jr.; Morrow, A.G. Idiopathic Hypertrophic Subaortic Stenosis. I. A Description of the Disease Based Upon an Analysis of 64 Patients. Circulation 1964, 30 (Suppl. 4), 3–119. [Google Scholar] [CrossRef]
- Ireland, C.G.; Ho, C.Y. Genetic Testing in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2024, 212S, S4–S13. [Google Scholar] [CrossRef]
- Cooke, R. The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle. Biophys. Rev. 2011, 3, 33–45. [Google Scholar] [CrossRef]
- Stewart, M.A.; Franks-Skiba, K.; Chen, S.; Cooke, R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. USA 2010, 107, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Hooijman, P.; Stewart, M.A.; Cooke, R. A new state of cardiac myosin with very slow ATP turnover: A potential cardioprotective mechanism in the heart. Biophys. J. 2011, 100, 1969–1976. [Google Scholar] [CrossRef]
- Fusi, L.; Huang, Z.; Irving, M. The Conformation of Myosin Heads in Relaxed Skeletal Muscle: Implications for Myosin-Based Regulation. Biophys. J. 2015, 109, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Toepfer, C.N. Cardiac myosin super relaxation (SRX): A perspective on fundamental biology, human disease and therapeutics. Biol. Open 2021, 10, bio057646. [Google Scholar] [CrossRef]
- Barrick, S.K.; Greenberg, M.J. Cardiac myosin contraction and mechanotransduction in health and disease. J. Biol. Chem. 2021, 297, 101297. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Masri, A.; Choudhury, L.; Olivotto, I.; Saberi, S.; Wang, A.; Garcia-Pavia, P.; Lakdawala, N.K.; Nagueh, S.F.; Rader, F.; et al. Phase 2 Study of Aficamten in Patients with Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2023, 81, 34–45. [Google Scholar] [CrossRef]
- Siontis, K.C.; Abreau, S.; Attia, Z.I.; Barrios, J.P.; Dewland, T.A.; Agarwal, P.; Balasubramanyam, A.; Li, Y.; Lester, S.J.; Masri, A.; et al. Patient-Level Artificial Intelligence-Enhanced Electrocardiography in Hypertrophic Cardiomyopathy: Longitudinal Treatment and Clinical Biomarker Correlations. JACC Adv. 2023, 2, 100582. [Google Scholar] [CrossRef]
- Vinogradova, M.V.; Stone, D.B.; Malanina, G.G.; Karatzaferi, C.; Cooke, R.; Mendelson, R.A.; Fletterick, R.J. Ca2+-regulated structural changes in troponin. Proc. Natl. Acad. Sci. USA 2005, 102, 5038–5043. [Google Scholar] [CrossRef] [PubMed]
- Houdusse, A.; Sweeney, H.L. How Myosin Generates Force on Actin Filaments. Trends Biochem. Sci. 2016, 41, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, H.L.; Houdusse, A. Structural and functional insights into the Myosin motor mechanism. Annu. Rev. Biophys. 2010, 39, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.C.; Angert, I.; Kull, F.J.; Jahn, W.; Schroder, R.R. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature 2003, 425, 423–427. [Google Scholar] [CrossRef]
- Wendt, T.; Taylor, D.; Messier, T.; Trybus, K.M.; Taylor, K.A. Visualization of head-head interactions in the inhibited state of smooth muscle myosin. J. Cell Biol. 1999, 147, 1385–1390. [Google Scholar] [CrossRef]
- Wendt, T.; Taylor, D.; Trybus, K.M.; Taylor, K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc. Natl. Acad. Sci. USA 2001, 98, 4361–4366. [Google Scholar] [CrossRef]
- Toepfer, C.N.; Wakimoto, H.; Garfinkel, A.C.; McDonough, B.; Liao, D.; Jiang, J.; Tai, A.C.; Gorham, J.M.; Lunde, I.G.; Lun, M.; et al. Hypertrophic cardiomyopathy mutations in MYBPC3 dysregulate myosin. Sci. Transl. Med. 2019, 11, eaat1199. [Google Scholar] [CrossRef]
- Anderson, R.L.; Trivedi, D.V.; Sarkar, S.S.; Henze, M.; Ma, W.; Gong, H.; Rogers, C.S.; Gorham, J.M.; Wong, F.L.; Morck, M.M.; et al. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc. Natl. Acad. Sci. USA 2018, 115, E8143–E8152. [Google Scholar] [CrossRef]
- Kawas, R.F.; Anderson, R.L.; Ingle, S.R.B.; Song, Y.; Sran, A.S.; Rodriguez, H.M. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J. Biol. Chem. 2017, 292, 16571–16577. [Google Scholar] [CrossRef]
- Rohde, J.A.; Roopnarine, O.; Thomas, D.D.; Muretta, J.M. Mavacamten stabilizes an autoinhibited state of two-headed cardiac myosin. Proc. Natl. Acad. Sci. USA 2018, 115, E7486–E7494. [Google Scholar] [CrossRef]
- Hartman, J.J.; Hwee, D.T.; Robert-Paganin, J.; Chuang, C.; Chin, E.R.; Edell, S.; Lee, K.H.; Madhvani, R.; Paliwal, P.; Pernier, J.; et al. Aficamten is a small-molecule cardiac myosin inhibitor designed to treat hypertrophic cardiomyopathy. Nat. Cardiovasc. Res. 2024, 3, 1003–1016. [Google Scholar] [CrossRef]
- Onoue, K.; Saito, Y. New treatment for myocardial diseases. J. Cardiol. 2021, 77, 620–625. [Google Scholar] [CrossRef]
- Kalinski, J.K.; Xu, B.; Boyd, R.; Tasseff, N.; Rutkowski, K.; Ospina, S.; Smedira, N.; Thamilarasan, M.; Popovic, Z.B.; Desai, M.Y. Novel Cardiac Myosin Inhibitor Therapy for Hypertrophic Cardiomyopathy in Adults: A Contemporary Review. Am. J. Cardiovasc. Drugs 2024, 24, 591–602. [Google Scholar] [CrossRef]
- Tian, Z.; Li, L.; Li, X.; Wang, J.; Zhang, Q.; Li, Z.; Peng, D.; Yang, P.; Ma, W.; Wang, F.; et al. Effect of Mavacamten on Chinese Patients with Symptomatic Obstructive Hypertrophic Cardiomyopathy: The EXPLORER-CN Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 957–965. [Google Scholar] [CrossRef]
- Desai, M.Y.; Owens, A.; Geske, J.B.; Wolski, K.; Naidu, S.S.; Smedira, N.G.; Cremer, P.C.; Schaff, H.; McErlean, E.; Sewell, C.; et al. Myosin Inhibition in Patients with Obstructive Hypertrophic Cardiomyopathy Referred for Septal Reduction Therapy. J. Am. Coll. Cardiol. 2022, 80, 95–108. [Google Scholar] [CrossRef]
- Wu, X.; Chen, N.; Hsu, P.; Sun, J.; Li, W.; Wang, Q.; Samira, M.; Wei, Q.; Yu, J.; Cao, G.; et al. Pharmacokinetics and safety of mavacamten in healthy Chinese participants with different CYP2C19 phenotypes. Clin. Transl. Sci. 2024, 17, e13877. [Google Scholar] [CrossRef] [PubMed]
- Spertus, J.A.; Fine, J.T.; Elliott, P.; Ho, C.Y.; Olivotto, I.; Saberi, S.; Li, W.; Dolan, C.; Reaney, M.; Sehnert, A.J.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): Health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.M.; Lester, S.J.; Solomon, S.D.; Michels, M.; Elliott, P.M.; Nagueh, S.F.; Choudhury, L.; Zemanek, D.; Zwas, D.R.; Jacoby, D.; et al. Effect of Mavacamten on Echocardiographic Features in Symptomatic Patients with Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 2518–2532. [Google Scholar] [CrossRef]
- Saberi, S.; Cardim, N.; Yamani, M.; Schulz-Menger, J.; Li, W.; Florea, V.; Sehnert, A.J.; Kwong, R.Y.; Jerosch-Herold, M.; Masri, A.; et al. Mavacamten Favorably Impacts Cardiac Structure in Obstructive Hypertrophic Cardiomyopathy: EXPLORER-HCM Cardiac Magnetic Resonance Substudy Analysis. Circulation 2021, 143, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Coats, C.J.; Masri, A.; Barriales-Villa, R.; Abraham, T.P.; Brinkley, D.M.; Claggett, B.L.; Hagege, A.; Hegde, S.M.; Ho, C.Y.; Kulac, I.J.; et al. Cardiac biomarkers and effects of aficamten in obstructive hypertrophic cardiomyopathy: The SEQUOIA-HCM trial. Eur. Heart J. 2024, 45, 4464–4478. [Google Scholar] [CrossRef]
- Sherrod Iv, C.F.; Saberi, S.; Nassif, M.E.; Claggett, B.L.; Coats, C.J.; Garcia-Pavia, P.; Januzzi, J.L.; Lewis, G.D.; Ma, C.; Maron, M.S.; et al. Effect of Aficamten on Health Status Outcomes in Obstructive Hypertrophic Cardiomyopathy: Results from SEQUOIA-HCM. J. Am. Coll. Cardiol. 2024, 84, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.M.; Claggett, B.L.; Wang, X.; Jering, K.; Prasad, N.; Roshanali, F.; Masri, A.; Nassif, M.E.; Barriales-Villa, R.; Abraham, T.P.; et al. Impact of Aficamten on Echocardiographic Cardiac Structure and Function in Symptomatic Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2024, 84, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Cardoso, R.N.; Abraham, T.P.; Claggett, B.L.; Coats, C.J.; Hegde, S.M.; Kulac, I.J.; Lee, M.M.Y.; Maron, M.S.; Merkely, B.; et al. Effect of Aficamten on Cardiac Structure and Function in Obstructive Hypertrophic Cardiomyopathy: SEQUOIA-HCM CMR Substudy. J. Am. Coll. Cardiol. 2024, 84, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.J.; Schaff, H.V.; Dearani, J.A.; Nishimura, R.A.; Ommen, S.R. Septal myectomy results in regression of left ventricular hypertrophy in patients with hypertrophic obstructive cardiomyopathy. Ann. Thorac. Surg. 2004, 78, 2118–2122. [Google Scholar] [CrossRef]
- Duan, F.J.; Chen, Y.Z.; Yuan, J.S.; Zhang, Y.; Qiao, S.B. Association between left ventricular reverse remodeling and long-term outcomes after alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Int. J. Cardiovasc. Imaging 2023, 39, 423–432. [Google Scholar] [CrossRef]
- Matsuda, J.; Takano, H.; Imori, Y.; Ishihara, K.; Sangen, H.; Kubota, Y.; Nakata, J.; Miyachi, H.; Hosokawa, Y.; Tara, S.; et al. Long-term clinical outcomes after alcohol septal ablation for hypertrophic obstructive cardiomyopathy in Japan: A retrospective study. Heart Vessel. 2025, 40, 496–508. [Google Scholar] [CrossRef]
- Aimo, A.; Todiere, G.; Barison, A.; Tomasoni, D.; Panichella, G.; Masri, A.; Maron, M.S. Diagnosis and management of hypertrophic cardiomyopathy: European vs. American guidelines. Heart Fail. Rev. 2025, 30, 315–325. [Google Scholar] [CrossRef]
- Davis, B.J.; Volk, H.; Nguyen, O.; Kamna, D.; Chen, H.; Barriales-Villa, R.; Garcia-Pavia, P.; Olivotto, I.; Owens, A.T.; Coats, C.J.; et al. Safety and Efficacy of Mavacamten and Aficamten in Patients with Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2025, 14, e038758. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Oreziak, A.; Masri, A.; Barriales-Villa, R.; Abraham, T.P.; Owens, A.T.; Jensen, M.K.; Wojakowski, W.; Seidler, T.; Hagege, A.; et al. Long-term effect of mavacamten in obstructive hypertrophic cardiomyopathy. Eur. Heart J. 2024, 45, 5071–5083. [Google Scholar] [CrossRef]
- Maurizi, N.; Antiochos, P.; Owens, A.; Lakdwala, N.; Saberi, S.; Russell, M.W.; Fumagalli, C.; Skalidis, I.; Lin, K.Y.; Nathan, A.S.; et al. Long-Term Outcomes After Septal Reduction Therapies in Obstructive Hypertrophic Cardiomyopathy: Insights from the SHARE Registry. Circulation 2024, 150, 1377–1390. [Google Scholar] [CrossRef]
- Hernandez, A.; Masoudi, F.; Mazimba, S.; Saberi, S.; Setoguchi, S.; Spertus, J.; Shen, S.; Balaratnam, G.; Chen, Y.-m.; Afsari, S.; et al. Design of DISCOVER-HCM: A Registry of Real-world Treatment Patterns and Outcomes of Patients with Symptomatic Obstructive Hypertrophic Cardiomyopathy. J. Card. Fail. 2024, 30, S4. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Bilen, O.; Burroughs, M.; Costabel, J.P.; de Barros Correia, E.; Dybro, A.M.; Elliott, P.; Lakdawala, N.K.; Mann, A.; Nair, A.; et al. Aficamten vs Metoprolol for Obstructive Hypertrophic Cardiomyopathy: MAPLE-HCM Rationale, Study Design, and Baseline Characteristics. JACC Heart Fail. 2025, 13, 346–357. [Google Scholar] [CrossRef]
- Ho, C.Y.; Mealiffe, M.E.; Bach, R.G.; Bhattacharya, M.; Choudhury, L.; Edelberg, J.M.; Hegde, S.M.; Jacoby, D.; Lakdawala, N.K.; Lester, S.J.; et al. Evaluation of Mavacamten in Symptomatic Patients with Nonobstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 2649–2660. [Google Scholar] [CrossRef]
- Desai, M.Y.; Nissen, S.E.; Abraham, T.; Olivotto, I.; Garcia-Pavia, P.; Lopes, R.D.; Verheyen, N.; Wever-Pinzon, O.; Wolski, K.; Jaber, W.; et al. Mavacamten in Symptomatic Nonobstructive Hypertrophic Cardiomyopathy: Design, Rationale, and Baseline Characteristics of ODYSSEY-HCM. JACC Heart Fail. 2025, 13, 358–370. [Google Scholar] [CrossRef]
- Masri, A.; Sherrid, M.V.; Abraham, T.P.; Choudhury, L.; Garcia-Pavia, P.; Kramer, C.M.; Barriales-Villa, R.; Owens, A.T.; Rader, F.; Nagueh, S.F.; et al. Efficacy and Safety of Aficamten in Symptomatic Nonobstructive Hypertrophic Cardiomyopathy: Results from the REDWOOD-HCM Trial, Cohort 4. J. Card. Fail. 2024, 30, 1439–1448. [Google Scholar] [CrossRef]
- Chiang, M.; Sychterz, C.; Perera, V.; Merali, S.; Palmisano, M.; Templeton, I.E.; Gaohua, L. Physiologically Based Pharmacokinetic Modeling and Simulation of Mavacamten Exposure with Drug-Drug Interactions from CYP Inducers and Inhibitors by CYP2C19 Phenotype. Clin. Pharmacol. Ther. 2023, 114, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Nguyen, S.; Yang, C.; Witt, L.; Wen, A.; Liao, T.V.; Nguyen, J.; Lin, B.; Altman, R.B.; Palaniappan, L. Pharmacogenomics in Asian Subpopulations and Impacts on Commonly Prescribed Medications. Clin. Transl. Sci. 2020, 13, 861–870. [Google Scholar] [CrossRef]
- Desai, M.Y.; Owens, A.; Saberi, S.; Wang, A.; Wolski, K.; Cremer, P.C.; Lakdawala, N.K.; Tower-Rader, A.; Zenker, M.; Sherrid, M.; et al. Long-Term Effects of Mavacamten on Patients Based on Hypertrophic Cardiomyopathy Pathogenic Genetic Variant Status: Insights from VALOR-HCM Trial. Circ. Genom. Precis. Med. 2025, 18, e005100. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.R.; Ho, C.Y.; Elliott, P.M. Genetics of hypertrophic cardiomyopathy: Established and emerging implications for clinical practice. Eur. Heart J. 2024, 45, 2727–2734. [Google Scholar] [CrossRef]


| Period | Milestone | Reference |
|---|---|---|
| 1958 | Teare reported the first pathological description of asymmetric septal hypertrophy in young sudden cardiac death cases. | [1] |
| 1973 | Familial clustering and autosomal dominant inheritance patterns of HCM were identified. | [44] |
| 1990 | MYH7 mutations were identified as a genetic cause of HCM. | [45,46] |
| 2000s | Hypercontractility and increased ATPase activity were established as key pathogenic mechanisms. | [3] |
| 2011 | Cytokinetics introduced omecamtiv mecarbil, an ATPase activator for systolic heart failure. | [47] |
| 2016 | Mavacamten (MYK-461), a cardiac myosin inhibitor was shown to reverse hypertrophy in mouse models of HCM. | [4] |
| 2019 | PIONEER-HCM trial: Mavacamten improved LVOTO and exercise capacity. | [48] |
| 2020 | EXPLORER-HCM trial: Demonstrated efficacy and safety of mavacamten in patients with HOCM. | [49] |
| 2021 | The cardiac myosin inhibitor, aficamten (CK-274), was discovered. | [5] |
| 2022 | U.S. FDA approved mavacamten for HOCM. | |
| 2024 | SEQUOIA-HCM phase 3 trial showed that aficamten improved peak VO2 in patients with HOCM. | [50] |
| 2024 | HORIZON-HCM trial in Japanese patients showed comparable efficacy. | [51] |
| Trial Name | EXPLORER-HCM [49] | EXPLORER-CN [75] | HORIZON-HCM [51] | VALOR-HCM [76] | SEQUOIA-HCM [50] |
|---|---|---|---|---|---|
| Drug | Mavacamten | Mavacamten | Mavacamten | Mavacamten | Aficamten |
| Trial location | Europe and USA | China | Japan | USA | Europe and USA |
| Eligible patients | NYHA II or III | NYHA II or III | NYHA II or III | Eligible for SRT (NYHA III or IV) | NYHA II or III |
| Number of patients | 251 | 81 | 38 | 110 | 282 |
| Study design | Double-blind randomized | Double-blind randomized | Open-label single-arm | Double-blind randomized | Double-blind randomized |
| Study period | 30 weeks | 30 weeks | 30 weeks | 16 weeks | 24 weeks |
| Primary endpoint | pVO2 and NYHA class | Valsalva LVOT-PG | Post-exercise LVOT-PG | proceeding with SRT or eligible for SRT | pVO2 |
| LVOT-PG | ↓ | ↓ | ↓ | ↓ | ↓ |
| pVO2 | ↑ | Data not collected | Data not collected | Data not collected | ↑ |
| NYHA class | Improved | Improved | Improved | Improved | Improved |
| KCCQ | ↑ (Improved) | ↑ (Improved) | ↑ (Improved) | ↑ (Improved) | ↑ (Improved) |
| NT-proBNP | ↓ | ↓ | ↓ | ↓ | ↓ |
| Cardiac troponin | ↓ | ↓ | ↓ | ↓ | ↓ |
| LVMI | ↓ | ↓ | ↓ | ↓ | ↓ |
| LAVI | ↓ | ↓ | ↓ | ↓ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Okumura, T.; Kato, S.; Onoue, K.; Kubo, T.; Kouzu, H.; Yano, T.; Inomata, T. Cardiac Myosin Inhibitors in Hypertrophic Cardiomyopathy: From Sarcomere to Clinic. Int. J. Mol. Sci. 2025, 26, 9347. https://doi.org/10.3390/ijms26199347
Nakamura K, Okumura T, Kato S, Onoue K, Kubo T, Kouzu H, Yano T, Inomata T. Cardiac Myosin Inhibitors in Hypertrophic Cardiomyopathy: From Sarcomere to Clinic. International Journal of Molecular Sciences. 2025; 26(19):9347. https://doi.org/10.3390/ijms26199347
Chicago/Turabian StyleNakamura, Kazufumi, Takahiro Okumura, Seiya Kato, Kenji Onoue, Toru Kubo, Hidemichi Kouzu, Toshiyuki Yano, and Takayuki Inomata. 2025. "Cardiac Myosin Inhibitors in Hypertrophic Cardiomyopathy: From Sarcomere to Clinic" International Journal of Molecular Sciences 26, no. 19: 9347. https://doi.org/10.3390/ijms26199347
APA StyleNakamura, K., Okumura, T., Kato, S., Onoue, K., Kubo, T., Kouzu, H., Yano, T., & Inomata, T. (2025). Cardiac Myosin Inhibitors in Hypertrophic Cardiomyopathy: From Sarcomere to Clinic. International Journal of Molecular Sciences, 26(19), 9347. https://doi.org/10.3390/ijms26199347







