Biomarkers for Personalised Primary or Secondary Prevention in Cardiovascular Diseases: A Rapid Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Protocol
2.2. Data Sources and Search Strategy
2.2.1. Population Concept and Context (PCC) Framework
Population
Concept
Context
2.3. Eligibility Criteria
2.4. Screening Process
2.5. Data Extraction
2.6. Data Analysis
3. Results
3.1. Biomarker Research Landscape
3.2. Interaction with Risk Factors
3.3. Role of Artificial Intelligence Technologies
4. Discussion
4.1. CVD Research Activity and Gaps
4.2. Genetic Biomarkers
4.3. Biochemical, Imaging, and Physiological Markers
4.4. Risk Prediction Models
4.5. Integration with AI
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Aortic Aneurysm |
| AF | Atrial Fibrillation and Atrial Flutter |
| AI | Artificial Intelligence |
| BMI | Body Mass Index |
| CAD | Coronary Artery Disease |
| CAC | Coronary Artery Calcium |
| CKD | Chronic Kidney Disease |
| CT | Computed Tomography |
| CVE | Cardiovascular Event |
| CVD | Cardiovascular Disease |
| ECG | Electrocardiogram |
| EEG | Electroencephalogram |
| EGMs | Evidence Gap Maps |
| EU | European Union |
| GEO | Gene Expression Omnibus |
| GWAS | Genome-Wide Association Study |
| hs-CRP | High-Sensitivity C-Reactive Protein |
| IARC | International Agency for Research on Cancer |
| IHD | Ischemic Heart Disease |
| lncRNA | Long Non-Coding RNA |
| MACCE | Major Adverse Cardiac and Cerebrovascular Event |
| MACE | Major Acute Cardiac Event |
| MEGASTROKE | Multiancestry Genome-Wide Association Study |
| MRI | Magnetic Resonance Imaging |
| MR | Mendelian Randomisation |
| mtDNA | Mitochondrial DNA |
| NCD | Non-Communicable Disease |
| NICE | National Institute for Health and Care Excellence |
| NRVHD | Nonrheumatic Valvular Heart Disease |
| NT-proBNP | N-terminal Brain Natriuretic Pro-Peptide |
| OSF | Open Science Framework |
| PAD | Peripheral Artery Disease |
| PET | Positron Emission Tomography |
| PCC | Population, Concept, Context |
| PGS | Polygenic Score |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews |
| PROPHET | PeRsOnalised Prevention Roadmap for the Future HEalThcare |
| SNP | Single Nucleotide Polymorphism |
| SPECT | Single-Photon Emission Computed Tomography |
| SRIA | Strategic Research and Innovation Agenda |
| UK | United Kingdom |
| WHO | World Health Organisation |
References
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Antonopoulos, A.S.; Deanfield, J. Imaging residual inflammatory cardiovascular risk. Eur. Heart J. 2020, 41, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Publications Office of the European Union. Council Conclusions on Personalised Medicine for Patients; Publications Office of the European Union: Luxembourg, 2015; Available online: https://op.europa.eu/en/publication-detail/-/publication/f416ce37-a48c-11e5-b528-01aa75ed71a1/language-en (accessed on 20 February 2025).
- Plans-Beriso, E.; Babb-de-Villiers, C.; Petrova, D.; Barahona-López, C.; Diez-Echave, P.; Hernández, O.R.; Fernández-Martínez, N.F.; Turner, H.; García-Ovejero, E.; Craciun, O.; et al. Biomarkers for personalized prevention of chronic diseases. Syst. Rev. 2023, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease 2019 Collaborators. Global mortality from dementia: Application of a new method and results from the Global Burden of Disease Study 2019. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12200. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019 Cancer Collaboration. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021, Erratum in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. [Google Scholar] [CrossRef]
- World Health Organisations. Non Communicable Diseases. 23 December 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 February 2025).
- European Union; The Organisation for Economic Co-operation and Development. Health at a Glance: Europe 2022: State of Health in the EU Cycle; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, J.; Zeng, J.; Pan, H. Global burden of cardiovascular diseases attributed to low physical activity: An analysis of 204 countries and territories between 1990 and 2019. Am. J. Prev. Cardiol. 2024, 17, 100633. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Boccia, S.; Pastorino, R.; Ricciardi, W.; Ádány, R.; Barnhoorn, F.; Boffetta, P.; Cornel, M.C.; De Vito, C.; Gray, M.; Jani, A.; et al. How to Integrate Personalized Medicine into Prevention? Recommendations from the Personalized Prevention of Chronic Diseases (PRECeDI) Consortium. Public Health Genom. 2019, 22, 208–214. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Plans-Beriso, E.; Babb-De-Villiers, C.; Petrova, D.; Barahona-López, C.; Diez-Echave, P.; Hernández, O.R.; Fernández-Martínez, N.F.; Turner, H.; García-Ovejero, E.; Craciun, O.; et al. Biomarkers for personalised prevention of chronic diseases: A common protocol for three rapid scoping reviews. Syst. Rev. 2024, 13, 147. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Glasziou, P.; Del Mar, C.; Bannach-Brown, A.; Stehlik, P.; Scott, A.M. A full systematic review was completed in 2 weeks using automation tools: A case study. J. Clin. Epidemiol. 2020, 121, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res. Synth. Methods 2022, 13, 533–545. [Google Scholar] [CrossRef]
- Nardini, H.G. The Yale MeSH Analyzer; Harvey Cushing John Hay Whitney Medical Library: New Haven, CT, USA, 2015; Available online: https://library.medicine.yale.edu/news/yale-mesh-analyzer (accessed on 19 February 2025).
- Covidence. Covidence—Better Systematic Review Management. Covidence: Melbourne, Australia. 2024. Available online: https://www.covidence.org/ (accessed on 19 February 2025).
- U.S. Food and Drug Administration-National Institutes of Health Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration: Silver Spring, MD, USA, 2016. Available online: http://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 1 June 2023).
- Porta, M. A Dictionary of Epidemiology; Oxford University Press: Oxford, UK, 2016; Available online: https://www.oxfordreference.com/display/10.1093/acref/9780199976720.001.0001/acref-9780199976720 (accessed on 19 February 2025).
- Jaskulski, S.; Nuszbaum, C.; Michels, K.B. Components, prospects and challenges of personalized prevention. Front. Public Health 2023, 11, 1075076. [Google Scholar] [CrossRef]
- Bíró, K.; Dombrádi, V.; Jani, A.; Boruzs, K.; Gray, M. Creating a common language: Defining individualized, personalized and precision prevention in public health. J. Public Health 2018, 40, e552–e559. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 28 April 2023).
- Lin, G.; reactable: Interactive Data Tables for R. R Package Version 0449001. Available online: https://glin.github.io/reactable/ (accessed on 19 February 2025).
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Digital Solution Foundry and EPPI Centre. EPPI-Mapper, Version 2.1.0.; EPPI-Centre University College London Social Research Institute, University College London: London, UK, 2021; Available online: https://eppi.ioe.ac.uk/cms/default.aspx?tabid=3790 (accessed on 28 April 2023).
- Brennan, P.; Davey-Smith, G. Identifying Novel Causes of Cancers to Enhance Cancer Prevention: New Strategies Are Needed. JNCI J. Natl. Cancer Inst. 2021, 114, 353–360. [Google Scholar] [CrossRef]
- Guo, S.; Yin, S.; Tse, G.; Li, G.; Su, L.; Liu, T. Association Between Caliber of Retinal Vessels and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Curr. Atheroscler. Rep. 2020, 22, 16. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Welikala, R.; Barman, S.; Foster, P.J.; Luben, R.; Hayat, S.; Khaw, K.-T.; Whincup, P.; Strachan, D.; Owen, C.G. Artificial intelligence-enabled retinal vasculometry for prediction of circulatory mortality, myocardial infarction and stroke. Br. J. Ophthalmol. 2022, 106, 1722–1729. [Google Scholar] [CrossRef]
- Kocamaz, M.; Karadağ, O.; Onder, S.E. Comparison of choroidal thicknesses in patients with coronary artery disease and patients at risk of coronary artery disease. Int. Ophthalmol. 2021, 41, 2117–2124. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Yang, X.; Jiang, B.; Li, H.; Wang, Y. Multimodal Retinal Imaging for Detection of Ischemic Stroke. Front. Aging Neurosci. 2021, 13, 615813. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, B.; Li, H.; Yang, X.; Cheng, X.; Hong, H.; Wang, Y. Risk Stratification Tool for Ischemic Stroke: A Risk Assessment Model Based on Traditional Risk Factors Combined with White Matter Lesions and Retinal Vascular Caliber. Front. Neurol. 2021, 12, 696986. [Google Scholar] [CrossRef] [PubMed]
- Romero-Trevejo, J.L.; Fernández-Romero, L.; Delgado, J.; Muñoz-García, E.; Sánchez-Pérez, A.; Murri, M.; Gutiérrez-Bedmar, M.; Jiménez-Navarro, M.F. Choroidal thickness and granulocyte colony-stimulating factor in tears improve the prediction model for coronary artery disease. Cardiovasc. Diabetol. 2022, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, C.; Shi, D.; Wang, H.; Shang, X.; Wang, W.; Zhang, X.; Zhang, X.; Hu, Y.; Tang, S.; et al. Integrating oculomics with genomics reveals imaging biomarkers for preventive and personalized prediction of arterial aneurysms. EPMA J. 2023, 14, 73–86. [Google Scholar] [CrossRef]
- Osman, W.; Hassoun, A.; Jelinek, H.F.; Almahmeed, W.; Afandi, B.; Tay, G.K.; Alsafar, H. Genetics of type 2 diabetes and coronary artery disease and their associations with twelve cardiometabolic traits in the United Arab Emirates population. Gene 2020, 750, 144722. [Google Scholar] [CrossRef]
- Burrello, J.; Bolis, S.; Balbi, C.; Burrello, A.; Provasi, E.; Caporali, E.; Gauthier, L.G.; Peirone, A.; D’AScenzo, F.; Monticone, S.; et al. An extracellular vesicle epitope profile is associated with acute myocardial infarction. J. Cell. Mol. Med. 2020, 24, 9945–9957. [Google Scholar] [CrossRef]
- Marei, I.; Chidiac, O.; Thomas, B.; Pasquier, J.; Dargham, S.; Robay, A.; Vakayil, M.; Jameesh, M.; Triggle, C.; Rafii, A.; et al. Angiogenic content of microparticles in patients with diabetes and coronary artery disease predicts networks of endothelial dysfunction. Cardiovasc. Diabetol. 2022, 21, 17. [Google Scholar] [CrossRef]
- Eyileten, C.; Jakubik, D.; Shahzadi, A.; Gasecka, A.; van der Pol, E.; De Rosa, S.; Siwik, D.; Gajewska, M.; Mirowska-Guzel, D.; Kurkowska-Jastrzebska, I.; et al. Diagnostic Performance of Circulating miRNAs and Extracellular Vesicles in Acute Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 4530. [Google Scholar] [CrossRef]
- Lown, M.; Brown, M.; Brown, C.; Yue, A.M.; Shah, B.N.; Corbett, S.J.; Lewith, G.; Stuart, B.; Moore, M.; Little, P.; et al. Machine learning detection of Atrial Fibrillation using wearable technology. PLoS ONE 2020, 15, e0227401. [Google Scholar] [CrossRef]
- Forghani, N.; Maghooli, K.; Dabanloo, N.J.; Farahani, A.V.; Forouzanfar, M. Intelligent Oscillometric System for Automatic Detection of Peripheral Arterial Disease. IEEE J. Biomed. Health Inform. 2021, 25, 3209–3218. [Google Scholar] [CrossRef]
- Lumley, H.A.; Flynn, D.; Shaw, L.; McClelland, G.; Ford, G.A.; White, P.M.; Price, C.I. A scoping review of pre-hospital technology to assist ambulance personnel with patient diagnosis or stratification during the emergency assessment of suspected stroke. BMC Emerg. Med. 2020, 20, 30. [Google Scholar] [CrossRef]
- Diederichsen, S.Z.; Haugan, K.J.; Brandes, A.; Graff, C.; Krieger, D.; Kronborg, C.; Holst, A.G.; Nielsen, J.B.; Køber, L.; Højberg, S.; et al. Incidence and predictors of atrial fibrillation episodes as detected by implantable loop recorder in patients at risk: From the LOOP study. Am. Heart J. 2020, 219, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Toyoda, K.; Iguchi, Y.; Hirano, T.; Metoki, N.; Tomoda, M.; Shiozawa, M.; Koge, J.; Okada, Y.; Terasawa, Y.; et al. Atrial Fibrillation After Ischemic Stroke Detected by Chest Strap-Style 7-Day Holter Monitoring and the Risk Predictors: EDUCATE-ESUS. J. Atheroscler. Thromb. 2021, 28, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Larson, J.; Schoenberg, J.; Sepulveda, A.; Tinker, L.; Wheeler, M.; Albert, C.; Manson, J.E.; Wells, G.; Martin, L.W.; et al. Serial 7-Day Electrocardiogram Patch Screening for AF in High-Risk Older Women by the CHARGE-AF Score. JACC Clin. Electrophysiol. 2022, 8, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Weng, L.C.; Al-Alusi, M.A.; Halford, J.L.; Haimovich, J.S.; Benjamin, E.J.; Trinquart, L.; Ellinor, P.T.; McManus, D.D.; Lubitz, S.A. Accelerometer-derived physical activity and risk of atrial fibrillation. Eur. Heart J. 2021, 42, 2472–2483. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Hu, J.; Guasch-Ferre, M.; Li, J.; Sorond, F.; Zhao, Y.; Shutta, K.H.; Salas-Salvado, J.; Hu, F.; Clish, C.B.; et al. Metabolomic Profiles Associated with Incident Ischemic Stroke. Neurology 2022, 98, e483–e492. [Google Scholar] [CrossRef]
- Guo, F.; Qiu, X.; Zhu, Y.; Tan, Z.; Li, Z.; Ouyang, D. Association between plasma betaine levels and dysglycemia in patients with coronary artery disease. Biosci. Rep. 2020, 40, BSR20200676. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Huang, J.; Liu, Y.; Zhu, Y. Association of genetic variants in lncRNA GAS5/miR-21/mTOR axis with risk and prognosis of coronary artery disease among a Chinese population. J. Clin. Lab. Anal. 2020, 34, e23430. [Google Scholar] [CrossRef]
- Li, J.; Guasch-Ferré, M.; Chung, W.; Ruiz-Canela, M.; Toledo, E.; Corella, D.; Bhupathiraju, S.N.; Tobias, D.K.; Tabung, F.K.; Hu, J.; et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur. Heart J. 2020, 41, 2645–2656. [Google Scholar] [CrossRef]
- Noerman, S.; Kokla, M.; Koistinen, V.M.; Lehtonen, M.; Tuomainen, T.-P.; Brunius, C.; Virtanen, J.K.; Hanhineva, K. Associations of the serum metabolite profile with a healthy Nordic diet and risk of coronary artery disease. Clin. Nutr. 2021, 40, 3250–3262. [Google Scholar] [CrossRef]
- Razquin, C.; Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Guasch-Ferré, M.; García-Gavilán, J.F.; Wittenbecher, C.; Alonso-Gómez, A.; Fitó, M.; Liang, L.; et al. Circulating Amino Acids and Risk of Peripheral Artery Disease in the PREDIMED Trial. Int. J. Mol. Sci. 2022, 24, 270. [Google Scholar] [CrossRef]
- Yin, W.; Li, F.; Tan, X.; Wang, H.; Jiang, W.; Wang, X.; Li, S.; Zhang, Y.; Han, Q.; Wang, Y.; et al. Plasma Ceramides and Cardiovascular Events in Hypertensive Patients at High Cardiovascular Risk. Am. J. Hypertens. 2021, 34, 1209–1216. [Google Scholar] [CrossRef]
- Nicholls, J.K.; Ince, J.; Minhas, J.S.; Chung, E.M.L. Emerging Detection Techniques for Large Vessel Occlusion Stroke: A Scoping Review. Front. Neurol. 2021, 12, 780324. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Pei, D.; Wu, C.-Z.; Kua, H.-C.; Liang, Y.-J.; Chen, Y.-L.; Lin, J.-D. Predictors of abnormality in thallium myocardial perfusion scans for type 2 diabetes. Heart Vessel. 2021, 36, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Nso, N.; Emmanuel, K.; Alshamam, M.; Munira, M.S.; Misra, A. Coronary Artery Calcium Score directed risk stratification of patients with Type-2 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102503. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.-H.; Wu, Y.-L.; Chung, S.-L.; Chen, C.-C.; Chen, W.-C.; Hsu, Y.-C. Coronary Artery Calcium Score Predicts Long-Term Cardiovascular Outcomes in Asymptomatic Patients with Type 2 Diabetes. J. Atheroscler. Thromb. 2021, 28, 1052–1062. [Google Scholar] [CrossRef]
- Wang, X.-R.; Yuan, L.; Shi, R.; Li, H.; Wang, D.-G.; Wu, Y.-G. Predictors of coronary artery calcification and its association with cardiovascular events in patients with chronic kidney disease. Ren. Fail. 2021, 43, 1172–1179. [Google Scholar] [CrossRef]
- Pan, J.; Wu, G.; Yu, J.; Geng, D.; Zhang, J.; Wang, Y. Detecting the Early Infarct Core on Non-Contrast CT Images with a Deep Learning Residual Network. J. Stroke Cerebrovasc. Dis. 2021, 30, 105752. [Google Scholar] [CrossRef]
- Cao, R.; Jiang, Y.; Li, L.; Lu, Y.; Wang, J.; Yu, K.; Chen, M.; Chen, J.; Zeng, X. BNP on Admission Combined with Imaging Markers of Multimodal CT to Predict the Risk of Cardioembolic Stroke. Dis. Markers 2022, 2022, 3327967. [Google Scholar] [CrossRef]
- Sorimachi, T.; Atsumi, H.; Yonemochi, T.; Hirayama, A.; Shigematsu, H.; Srivatanakul, K.; Takizawa, S.; Matsumae, M. Benefits and Risks of CT Angiography Immediately after Emergency Arrival for Patients with Intracerebral Hematoma. Neurol. Med. Chir. 2020, 60, 45–52. [Google Scholar] [CrossRef]
- Himmelreich, J.C.L.; Harskamp, R.E.; Geelhoed, B.; Virdone, S.; Lucassen, W.A.M.; Gansevoort, R.T.; Rienstra, M. Validating risk models versus age alone for atrial fibrillation in a young Dutch population cohort: Should atrial fibrillation risk prediction be expanded to younger community members? BMJ Open 2022, 12, e057476. [Google Scholar] [CrossRef]
- Khurshid, S.; Mars, N.; Haggerty, C.M.; Huang, Q.; Weng, L.-C.; Hartzel, D.N.; Center, R.G.; Lunetta, K.L.; Ashburner, J.M.; Anderson, C.D.; et al. Predictive Accuracy of a Clinical and Genetic Risk Model for Atrial Fibrillation. Circ. Genom. Precis. Med. 2021, 14, e003355. [Google Scholar] [CrossRef]
- Lyu, J.; Li, Z.; Wei, H.; Liu, D.; Chi, X.; Gong, D.-W.; Zhao, Q. A potent risk model for predicting new-onset acute coronary syndrome in patients with type 2 diabetes mellitus in Northwest China. Acta Diabetol. 2020, 57, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Jamthikar, A.D.; Gupta, D.; Puvvula, A.; Khanna, N.N.; Saba, L.; Viskovic, K.; Mavrogeni, S.; Turk, M.; Laird, J.R.; et al. Integration of estimated glomerular filtration rate biomarker in image-based cardiovascular disease/stroke risk calculator: A south Asian-Indian diabetes cohort with moderate chronic kidney disease. Int. Angiol. 2020, 39, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Bodinier, B.; Whitaker, M.; Tzoulaki, I.; Elliott, P.; Chadeau-Hyam, M. Improving cardiovascular risk prediction beyond pooled cohort equations: A prospective cohort of 304,356 participant. medRxiv 2023. [Google Scholar] [CrossRef]
- Buchan, T.A.; Malik, A.; Chan, C.; Chambers, J.; Suk, Y.; Zhu, J.W.; Ge, F.Z.; Huang, L.M.; Vargas, L.A.; Hao, Q.; et al. Predictive models for cardiovascular and kidney outcomes in patients with type 2 diabetes: Systematic review and meta-analyses. Heart Br. Card. Soc. 2021, 107, 1962–1973. [Google Scholar] [CrossRef]
- Devaraj, S.M.; Kriska, A.M.; Orchard, T.J.; Miller, R.G.; Costacou, T. Cardiovascular health in early adulthood predicts the development of coronary heart disease in individuals with type 1 diabetes: 25 year follow-up from the Pittsburgh Epidemiology of Diabetes Complications study. Diabetologia 2021, 64, 571–580. [Google Scholar] [CrossRef]
- Liu, J.; Chou, E.L.; Lau, K.K.; Woo, P.Y.; Li, J.; Chan, K.H.K. Machine learning algorithms identify demographics, dietary features, and blood biomarkers associated with stroke records. J. Neurol. Sci. 2022, 440, 120335. [Google Scholar] [CrossRef]
- Liu, B.; Yu, W.; Wang, J.; Shao, X.; Zhang, F.; Zhou, M.; Shi, Y.; Wang, B.; Xu, Y.; Wang, Y. A model combining rest-only ECG-gated SPECT myocardial perfusion imaging and cardiovascular risk factors can effectively predict obstructive coronary artery disease. BMC Cardiovasc. Disord. 2022, 22, 268. [Google Scholar] [CrossRef]
- Khalil, H.; Jia, R.; Moraes, E.; Munn, Z.; Alexander, L.; Peters, M.; Asran, A.; Godfrey, C.; Tricco, A.; Pollock, D.; et al. Scoping reviews and their role in identifying research priorities. J. Clin. Epidemiol. 2025, 181, 111712. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.-K.; van der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018, 50, 524–537, Erratum in Nat. Genet. 2019, 51, 1192–1193. [Google Scholar] [CrossRef]
- Chief Medical Officer’s Annual Report 2022: Air Pollution. Available online: https://www.gov.uk/government/publications/chief-medical-officers-annual-report-2022-air-pollution (accessed on 19 February 2025).
- Air Pollution. 2024. Available online: https://www.eea.europa.eu/en/topics/in-depth/air-pollution (accessed on 19 February 2025).

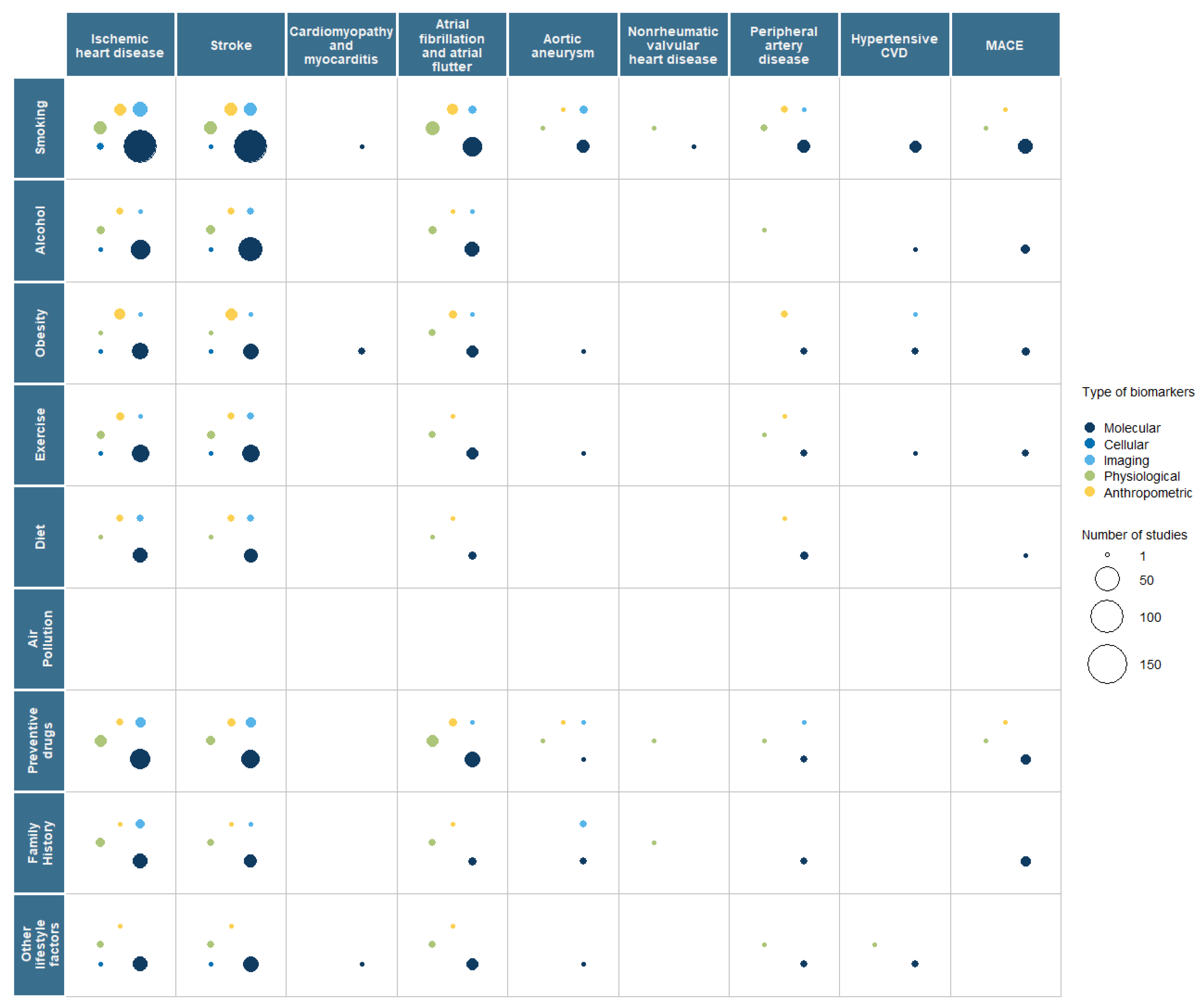
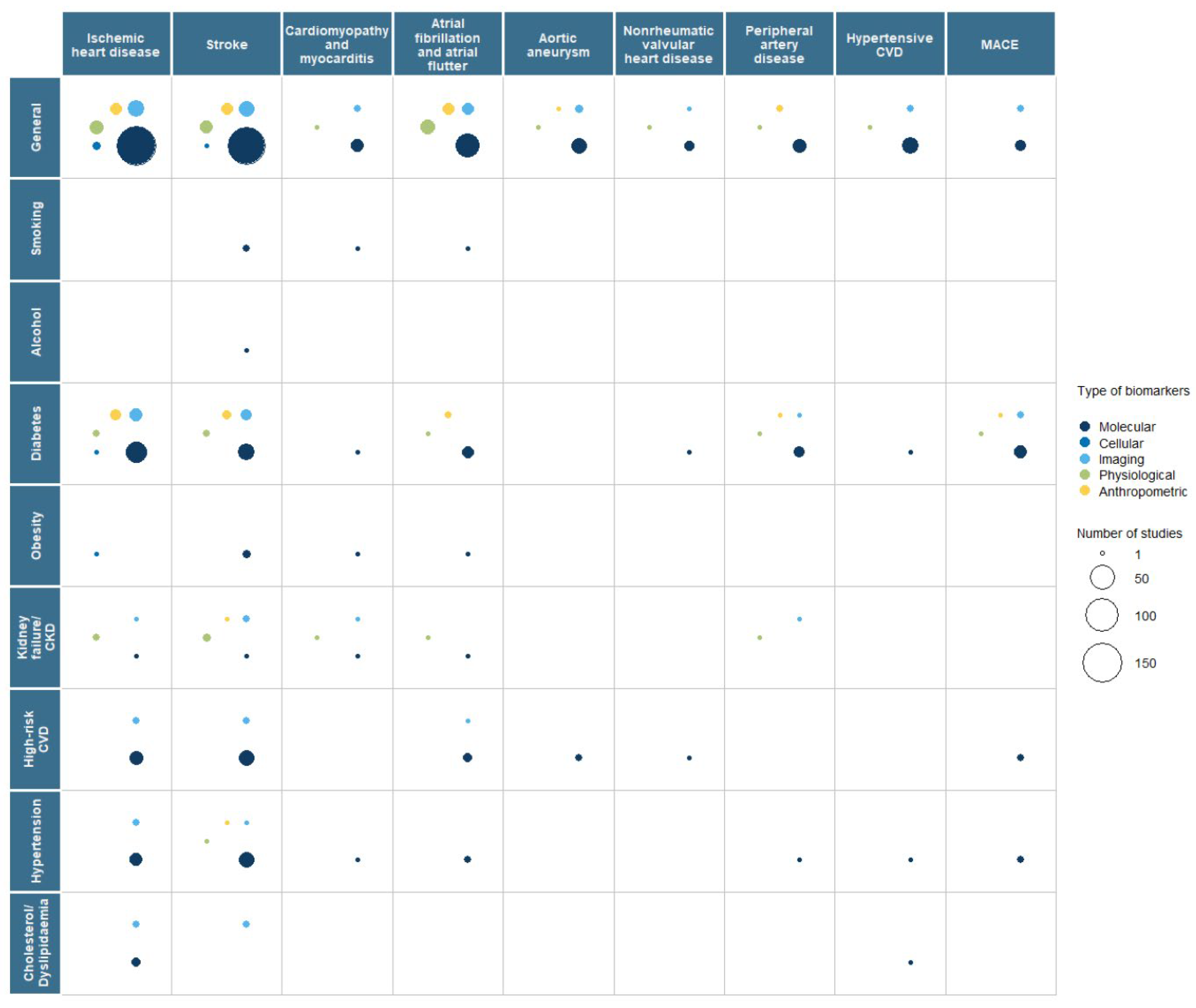
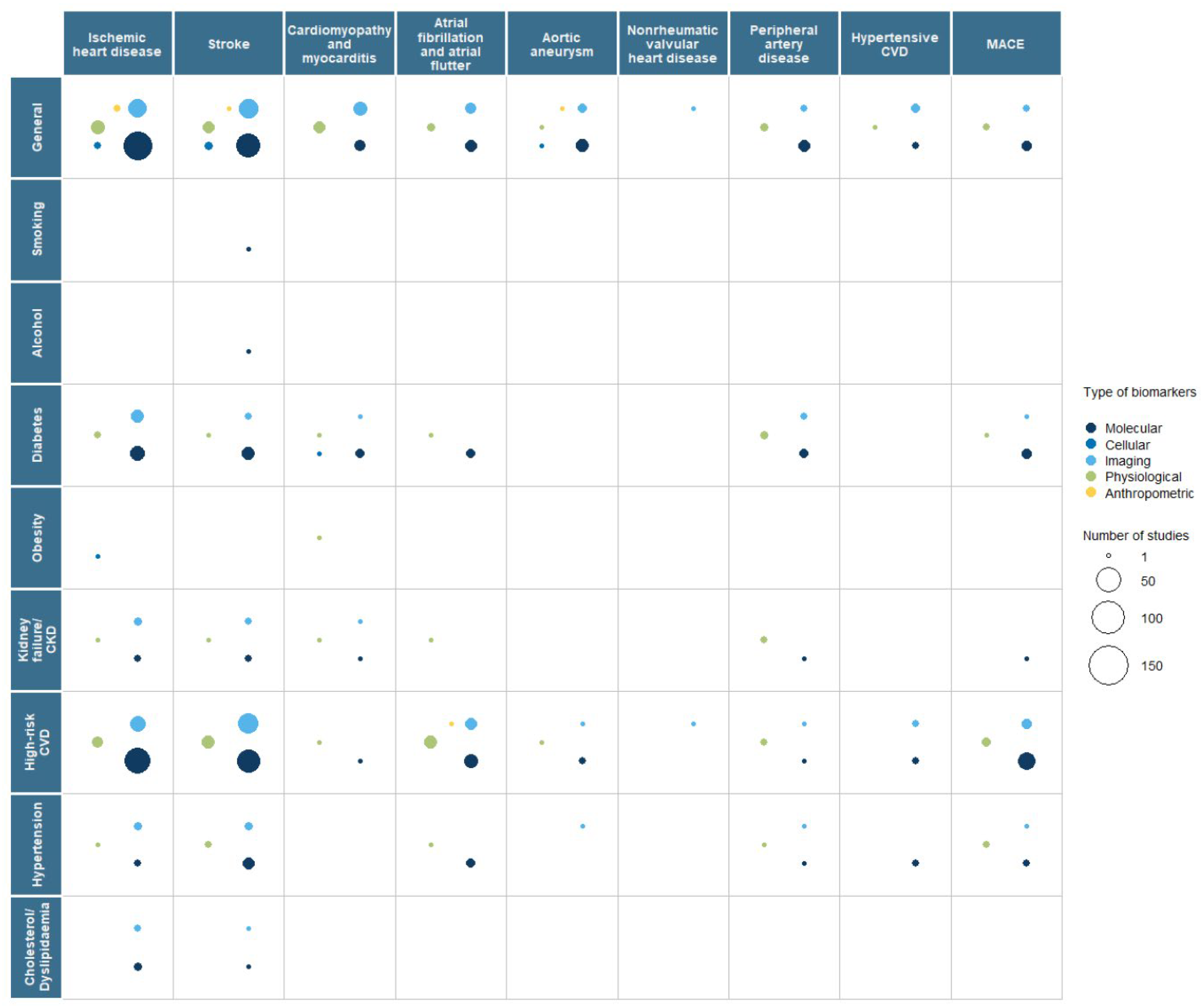
| Primary Prevention | Secondary Prevention | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Total | IHD | Stroke | Cardiomyopathy Myocarditis | AF | AA | NRV HD | PAD | Hypertensive CVD | MACE | Total | IHD | Stroke | Cardiomyopathy Myocarditis | AF | AA | NRV HD | PAD | Hypertensive CVD | MACE |
| Total | 422 | 227 | 198 | 11 | 67 | 19 | 7 | 20 | 21 | 21 | 399 | 191 | 157 | 24 | 43 | 20 | 2 | 22 | 12 | 45 |
| Molecular | 361 | 190 | 172 | 10 | 55 | 17 | 6 | 17 | 20 | 17 | 247 | 135 | 96 | 10 | 22 | 12 | 0 | 13 | 6 | 33 |
| Genetics | 238 | 120 | 117 | 9 | 38 | 35 | 6 | 9 | 16 | 7 | 39 | 20 | 14 | 2 | 2 | 3 | 0 | 2 | 0 | 3 |
| Epigenetics | 7 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Transcriptomics | 26 | 9 | 13 | 0 | 3 | 1 | 1 | 0 | 2 | 0 | 31 | 14 | 10 | 2 | 4 | 3 | 0 | 0 | 1 | 1 |
| Metabolomics | 8 | 4 | 5 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 18 | 11 | 9 | 2 | 0 | 1 | 0 | 0 | 1 | 2 |
| Proteomics | 10 | 5 | 3 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 23 | 11 | 7 | 1 | 1 | 2 | 0 | 3 | 1 | 1 |
| Microbiomics | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biochemical | 119 | 72 | 52 | 1 | 17 | 4 | 0 | 8 | 4 | 11 | 163 | 88 | 69 | 5 | 18 | 7 | 0 | 8 | 4 | 27 |
| Other | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cellular | 3 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 2 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Histology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Cytology | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Imaging | 56 | 31 | 24 | 3 | 8 | 3 | 1 | 2 | 2 | 4 | 134 | 48 | 60 | 13 | 13 | 6 | 2 | 6 | 6 | 9 |
| X rays | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 3 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 1 |
| Ultrasound | 22 | 8 | 8 | 2 | 5 | 1 | 0 | 2 | 1 | 1 | 39 | 10 | 12 | 6 | 5 | 4 | 1 | 3 | 1 | 1 |
| CT scan | 14 | 13 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 45 | 18 | 24 | 1 | 5 | 3 | 0 | 0 | 1 | 4 |
| PET/ SPECT | 5 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 16 | 11 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 1 |
| Spectrometry | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| MRI | 12 | 1 | 6 | 3 | 3 | 1 | 1 | 0 | 1 | 0 | 44 | 6 | 29 | 7 | 6 | 1 | 0 | 1 | 4 | 2 |
| Scintigraphy (Gamma) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mammography | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 7 | 5 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Physiology | 38 | 15 | 14 | 2 | 15 | 1 | 1 | 3 | 1 | 1 | 60 | 21 | 19 | 10 | 13 | 2 | 0 | 7 | 1 | 8 |
| Blood pressure | 16 | 6 | 7 | 0 | 7 | 1 | 0 | 1 | 0 | 1 | 16 | 4 | 7 | 2 | 2 | 1 | 0 | 1 | 0 | 2 |
| Ankle-brachial index | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| ECG | 14 | 3 | 2 | 2 | 9 | 0 | 1 | 0 | 0 | 0 | 30 | 10 | 4 | 8 | 11 | 1 | 0 | 0 | 1 | 2 |
| EEG | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Electromyography | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 11 | 5 | 5 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 11 | 9 | 8 | 2 | 0 | 1 | 0 | 4 | 0 | 4 |
| Anthropometric | 24 | 12 | 11 | 0 | 8 | 1 | 0 | 2 | 0 | 1 | 5 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| BMI | 13 | 7 | 6 | 0 | 5 | 0 | 0 | 1 | 0 | 1 | 3 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Body perimeters | 8 | 5 | 4 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 9 | 4 | 4 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Total | Ischemic Heart Disease | Stroke | Cardiomyopathy and Myocarditis | AF | AA | NVHD | PAD | Hypertensive CVD | MACE | |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary prevention | ||||||||||
| Total | 22 | 10 | 9 | 1 | 8 | 2 | 0 | 0 | 0 | 0 |
| Molecular | 15 | 6 | 4 | 1 | 6 | 2 | 0 | 0 | 0 | 0 |
| Cellular | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Imaging | 9 | 4 | 4 | 1 | 4 | 1 | 0 | 0 | 0 | 0 |
| Physiological | 7 | 1 | 3 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Anthropometric | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Secondary prevention | ||||||||||
| Total | 56 | 24 | 18 | 4 | 8 | 4 | 1 | 3 | 2 | 1 |
| Molecular | 22 | 15 | 7 | 1 | 1 | 2 | 0 | 1 | 0 | 0 |
| Cellular | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Imaging | 28 | 8 | 11 | 2 | 3 | 2 | 1 | 1 | 2 | 1 |
| Physiological | 15 | 4 | 5 | 1 | 5 | 0 | 0 | 1 | 0 | 0 |
| Anthropometric | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villiers, C.B.d.; Plans-Beriso, E.; Erady, C.; Blackburn, L.; Wilson, H.; Turner, H.; Kuhn, I.; Barahona-López, C.; Diez-Echave, P.; Hernández, O.R.; et al. Biomarkers for Personalised Primary or Secondary Prevention in Cardiovascular Diseases: A Rapid Scoping Review. Int. J. Mol. Sci. 2025, 26, 9346. https://doi.org/10.3390/ijms26199346
Villiers CBd, Plans-Beriso E, Erady C, Blackburn L, Wilson H, Turner H, Kuhn I, Barahona-López C, Diez-Echave P, Hernández OR, et al. Biomarkers for Personalised Primary or Secondary Prevention in Cardiovascular Diseases: A Rapid Scoping Review. International Journal of Molecular Sciences. 2025; 26(19):9346. https://doi.org/10.3390/ijms26199346
Chicago/Turabian StyleVilliers, Chantal Babb de, Elena Plans-Beriso, Chaitanya Erady, Laura Blackburn, Hayley Wilson, Heather Turner, Isla Kuhn, Cristina Barahona-López, Paul Diez-Echave, Orlando Romulo Hernández, and et al. 2025. "Biomarkers for Personalised Primary or Secondary Prevention in Cardiovascular Diseases: A Rapid Scoping Review" International Journal of Molecular Sciences 26, no. 19: 9346. https://doi.org/10.3390/ijms26199346
APA StyleVilliers, C. B. d., Plans-Beriso, E., Erady, C., Blackburn, L., Wilson, H., Turner, H., Kuhn, I., Barahona-López, C., Diez-Echave, P., Hernández, O. R., Fernández de Larrea-Baz, N., Petrova, D., Jimenez, R. C., Fernández-Navarro, P., García-Esquinas, E., Rodríguez-Artalejo, F., José Sánchez, M., Moreno, V., Pollán, M., ... Kroese, M. (2025). Biomarkers for Personalised Primary or Secondary Prevention in Cardiovascular Diseases: A Rapid Scoping Review. International Journal of Molecular Sciences, 26(19), 9346. https://doi.org/10.3390/ijms26199346







